Experimental Research on Quarry Wastewater Purification Using Flocculation Process
Abstract
1. Introduction
2. Materials and Methods
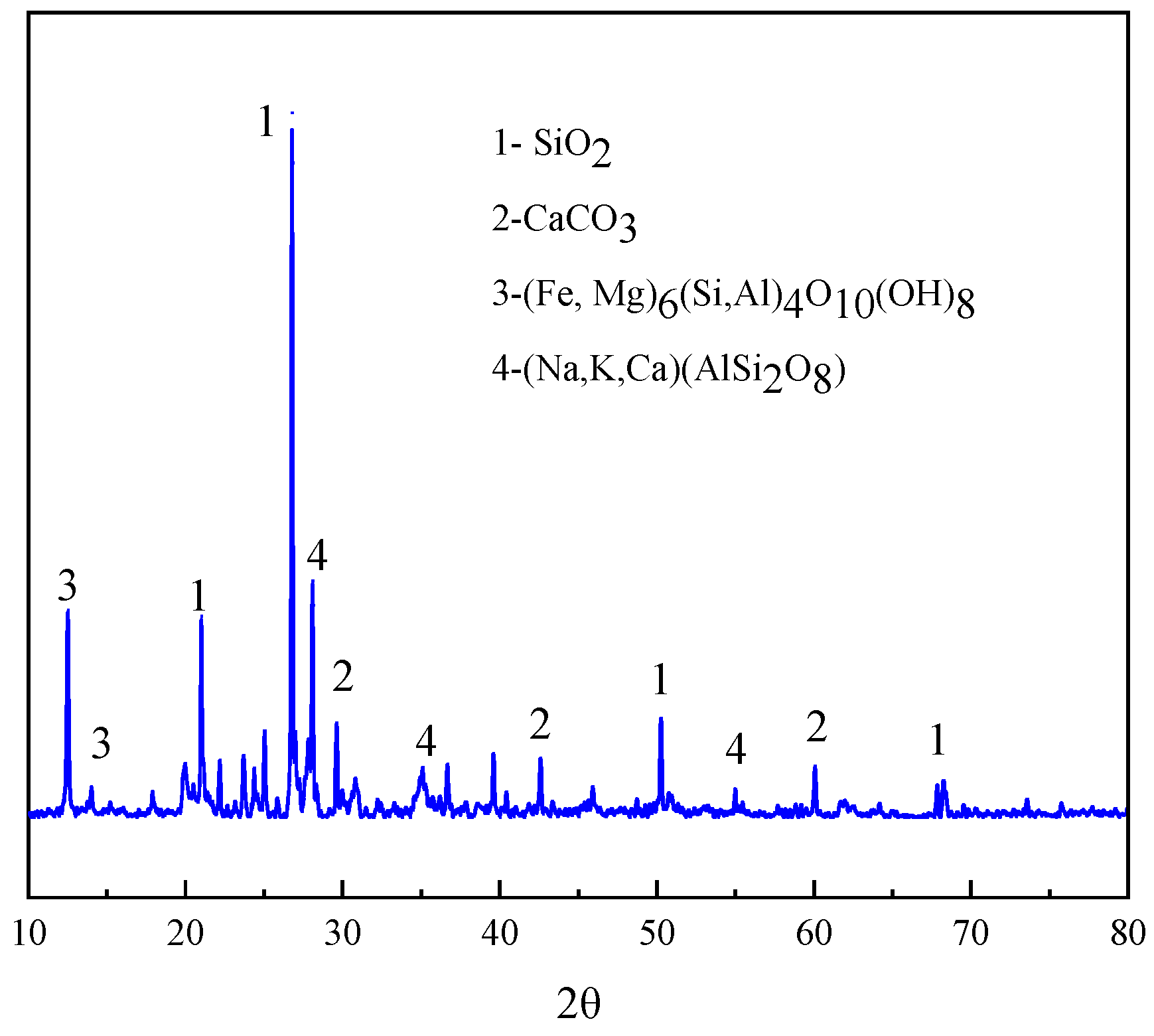
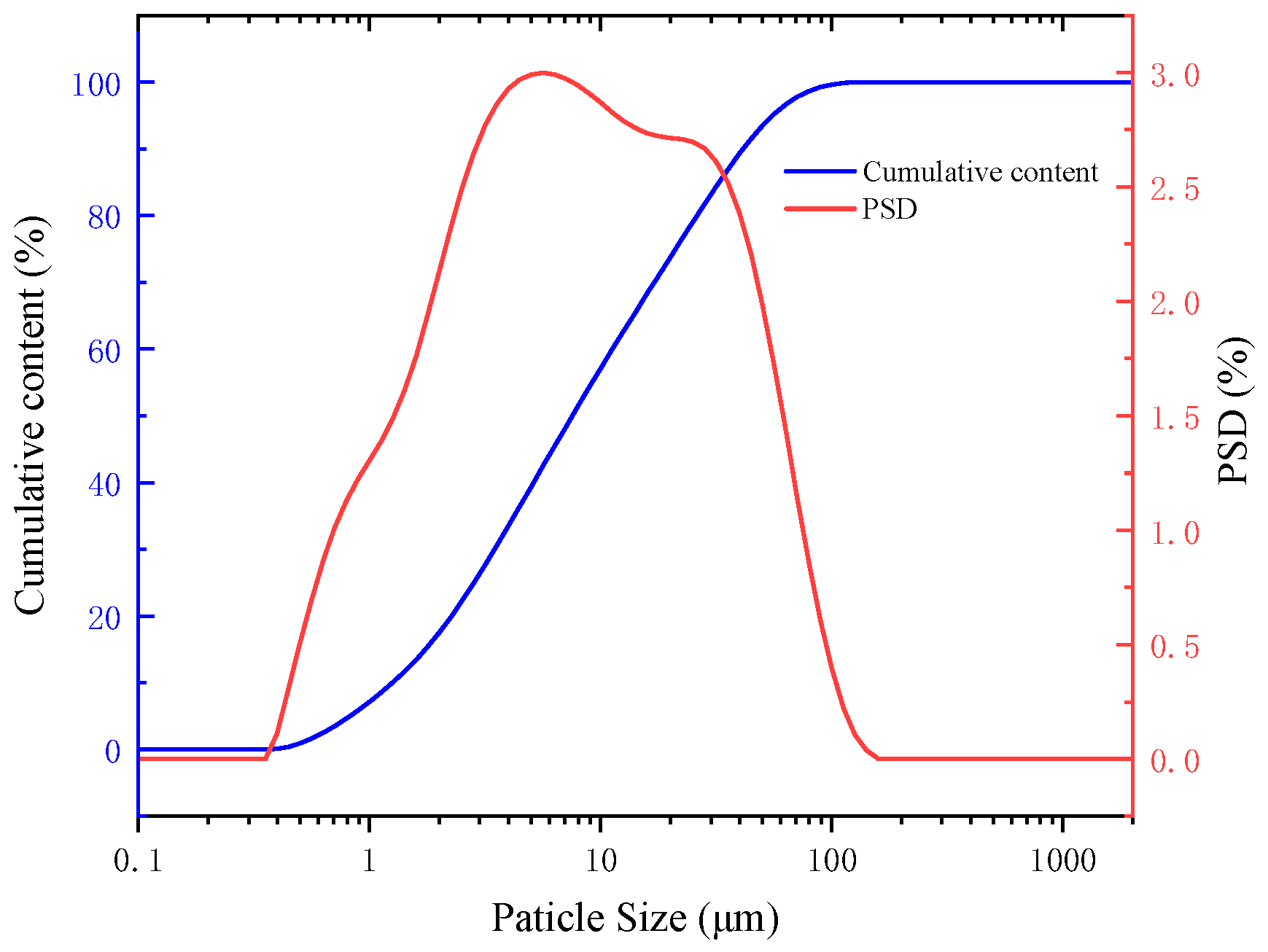
2.1. Materials
2.2. Methods
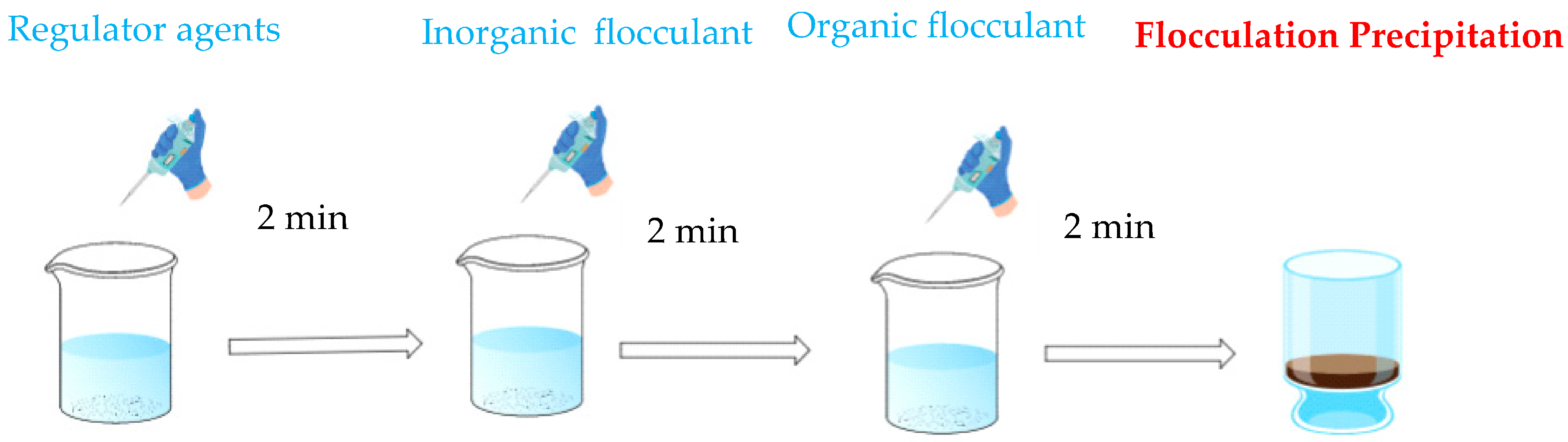
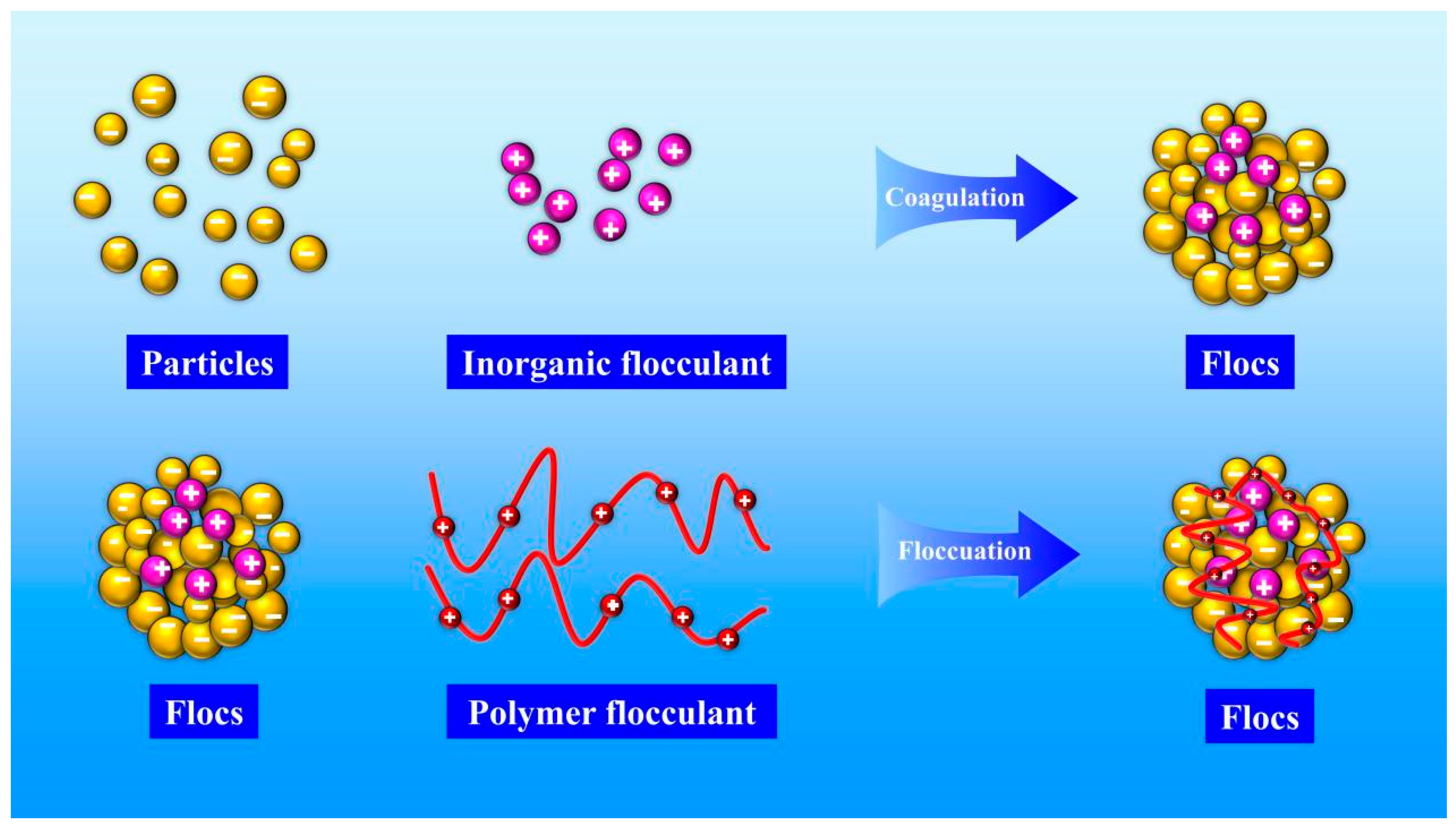
2.2.1. Flocculation Experiment
2.2.2. Zeta Potential
2.2.3. Optical Microscope
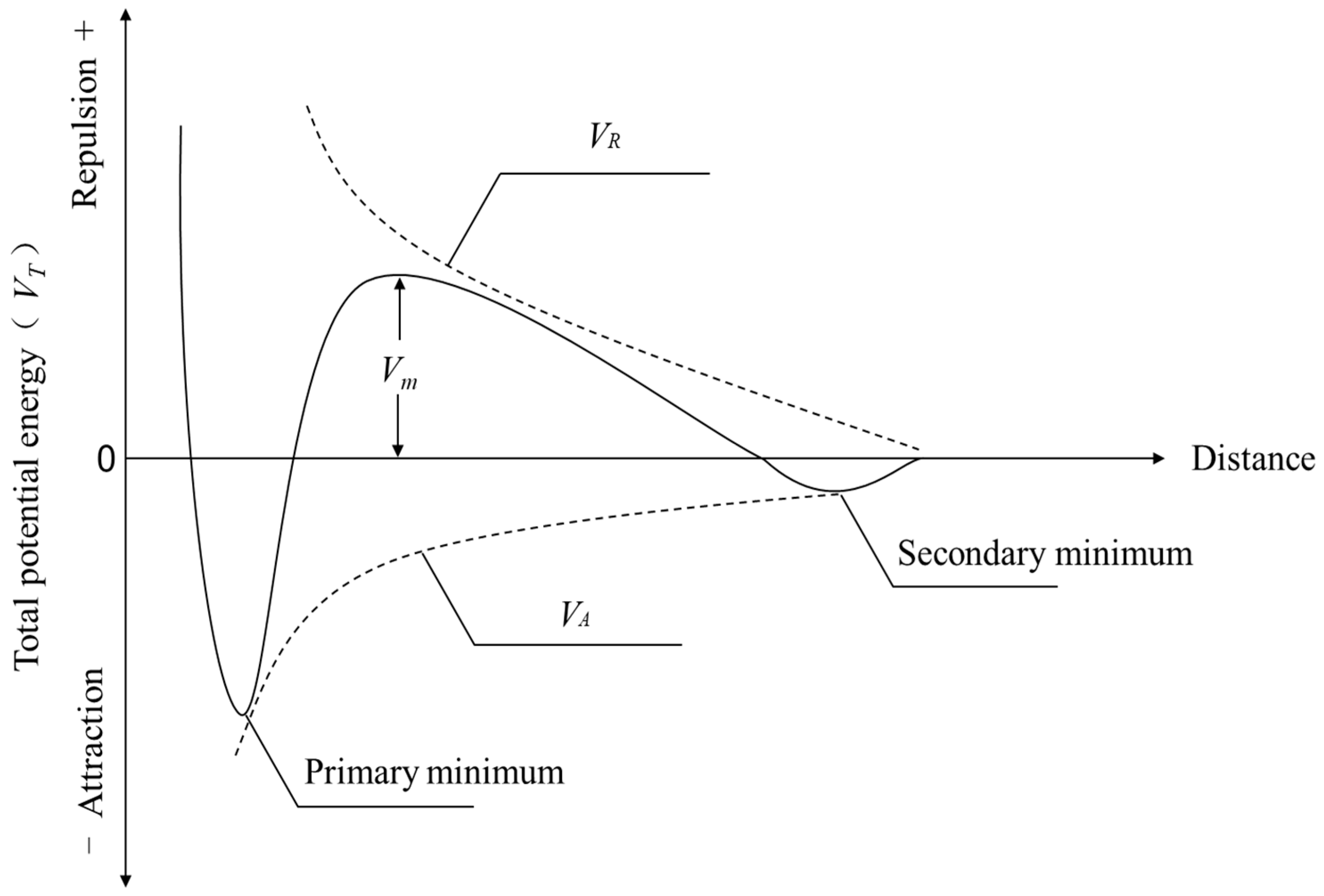
2.2.4. DLVO Theory
3. Results and Discussion
3.1. Wastewater Flocculation Experiment
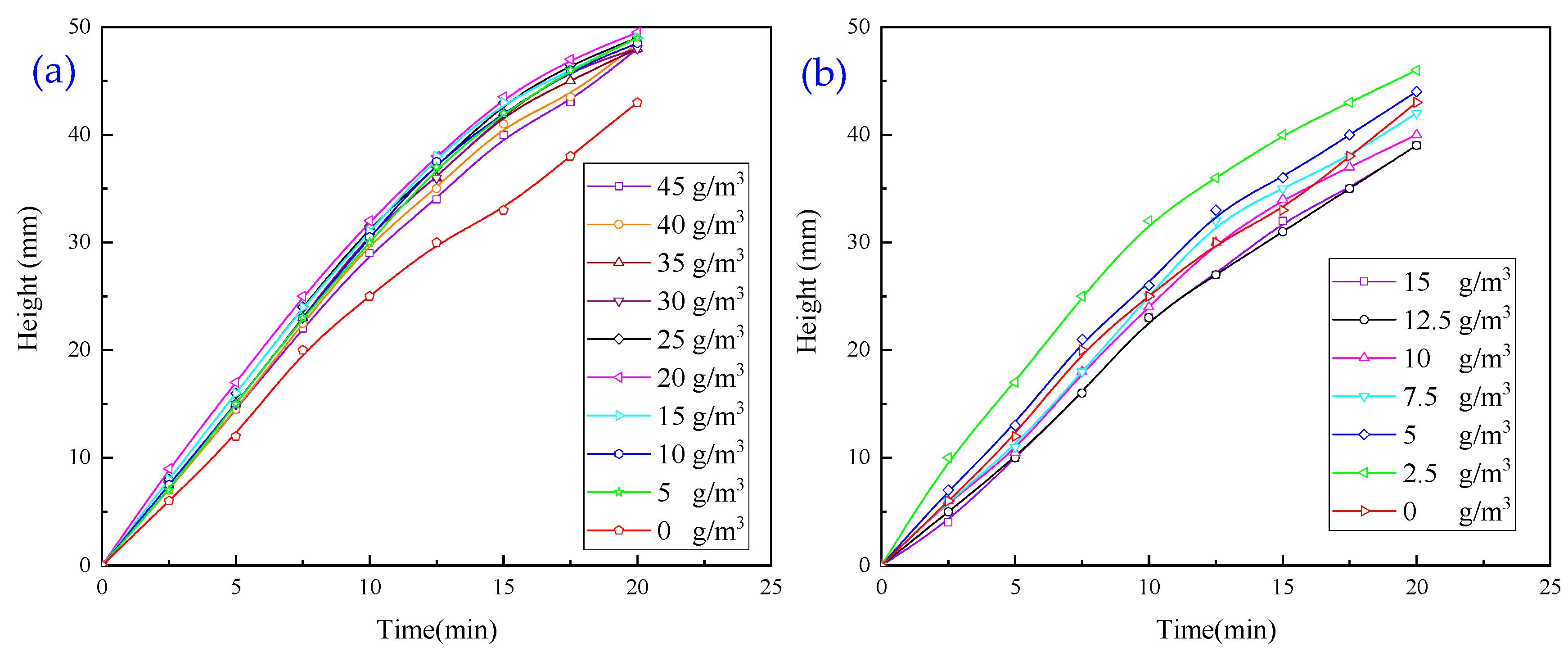
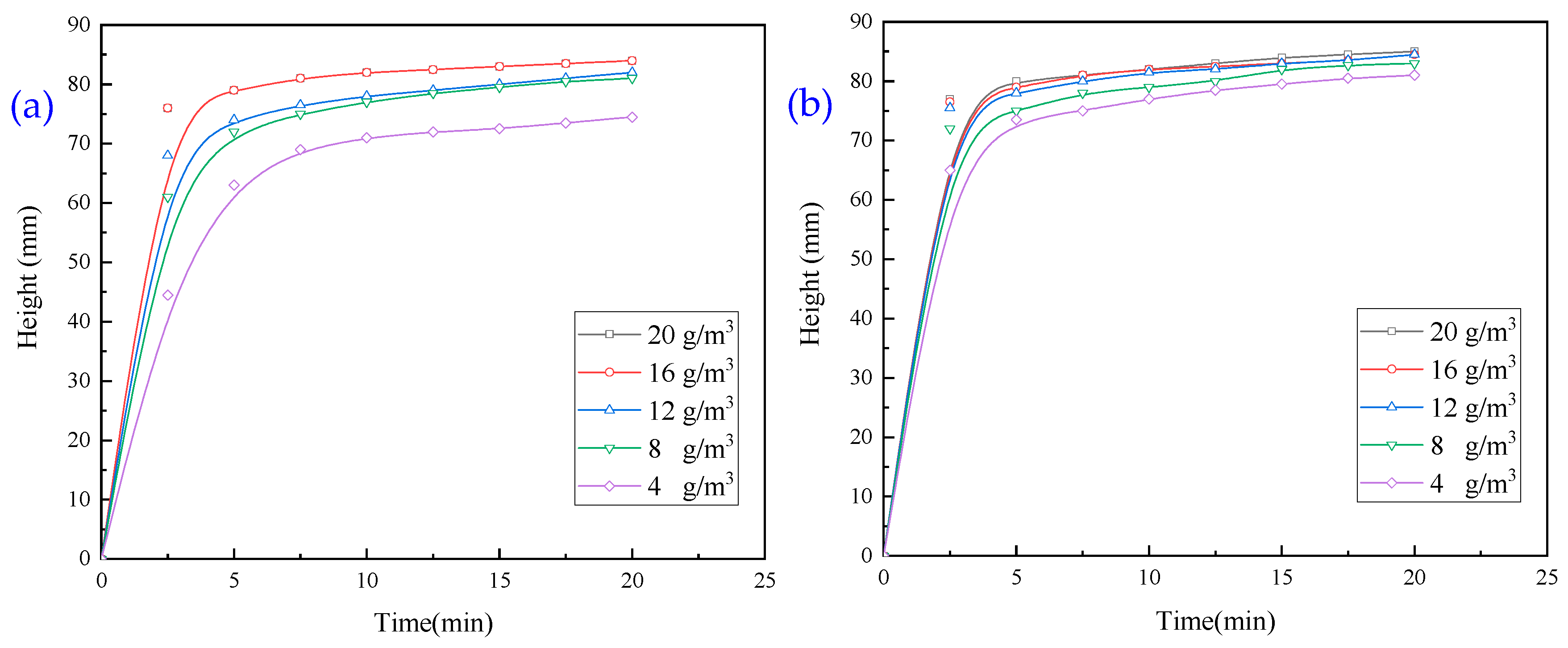
3.1.1. Experiments with Single Inorganic Flocculant
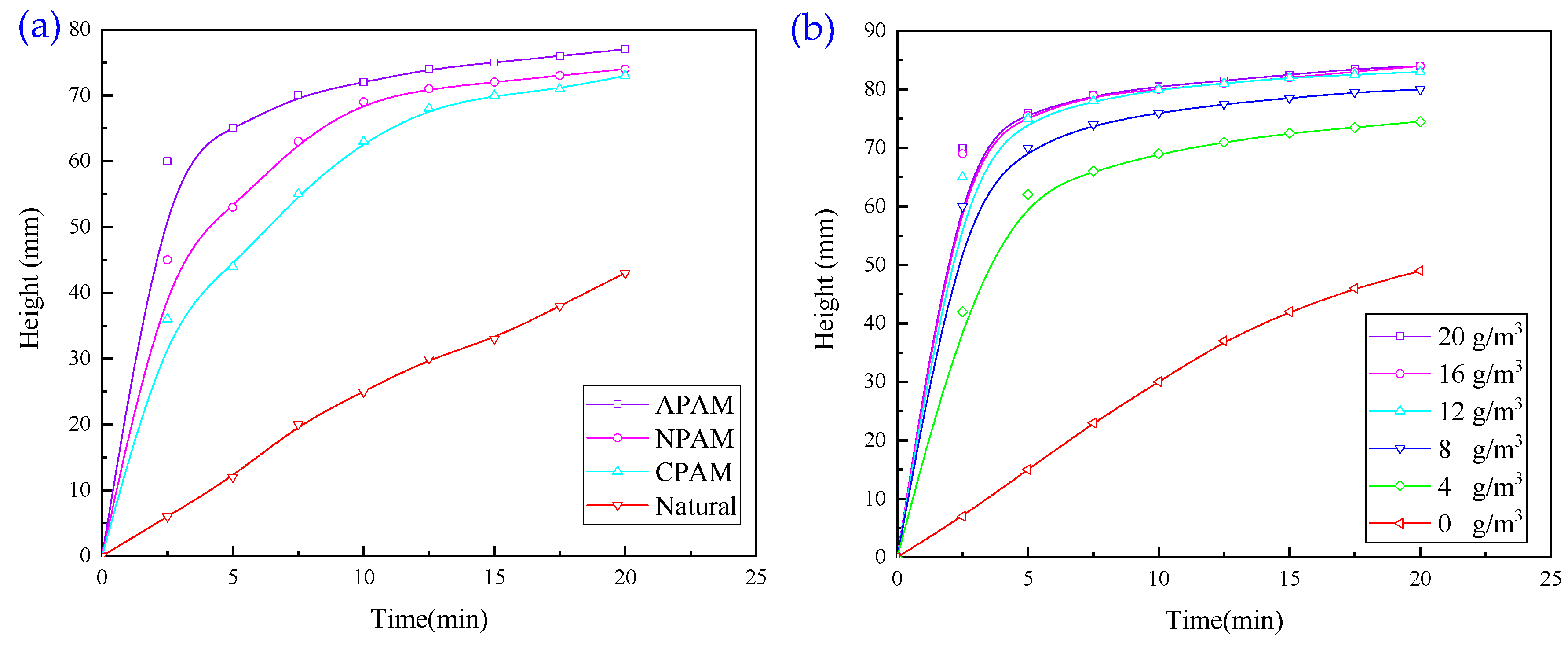
3.1.2. Experiments with Single Organic Flocculant
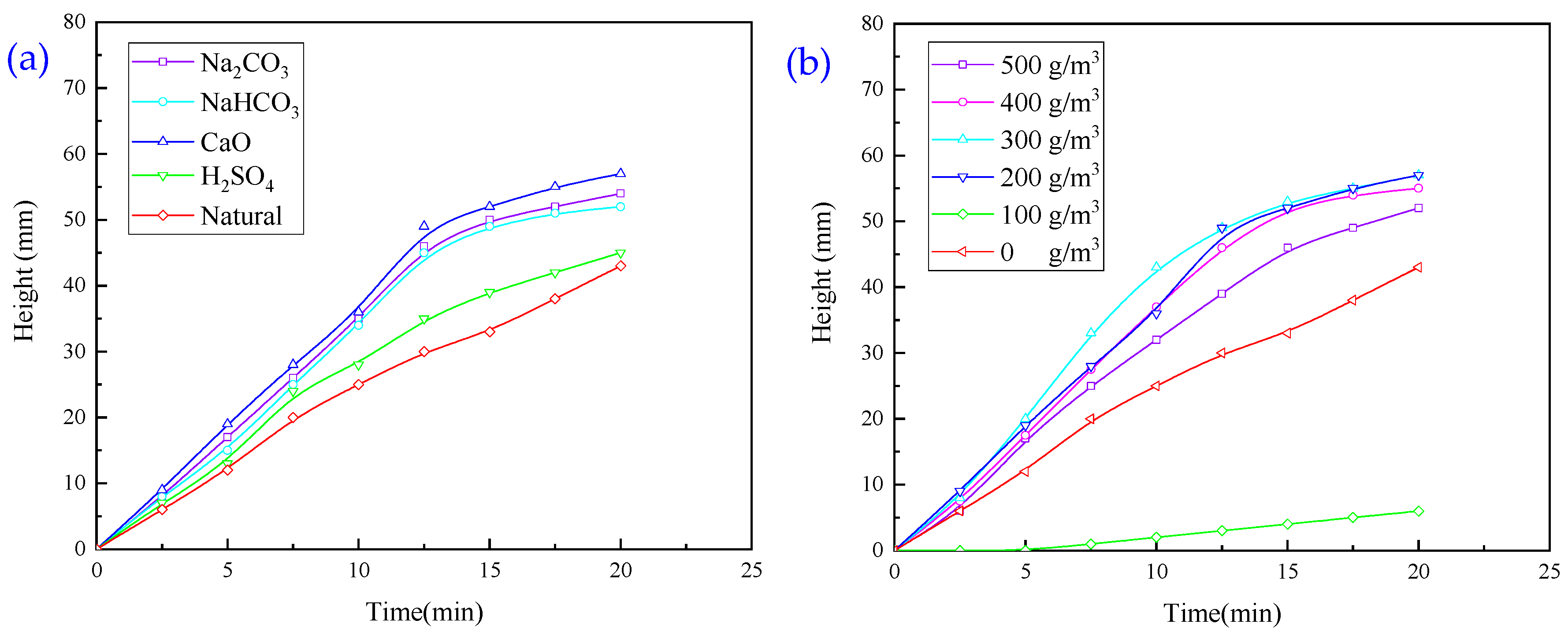
3.1.3. Experiments with Single Regulator Agent
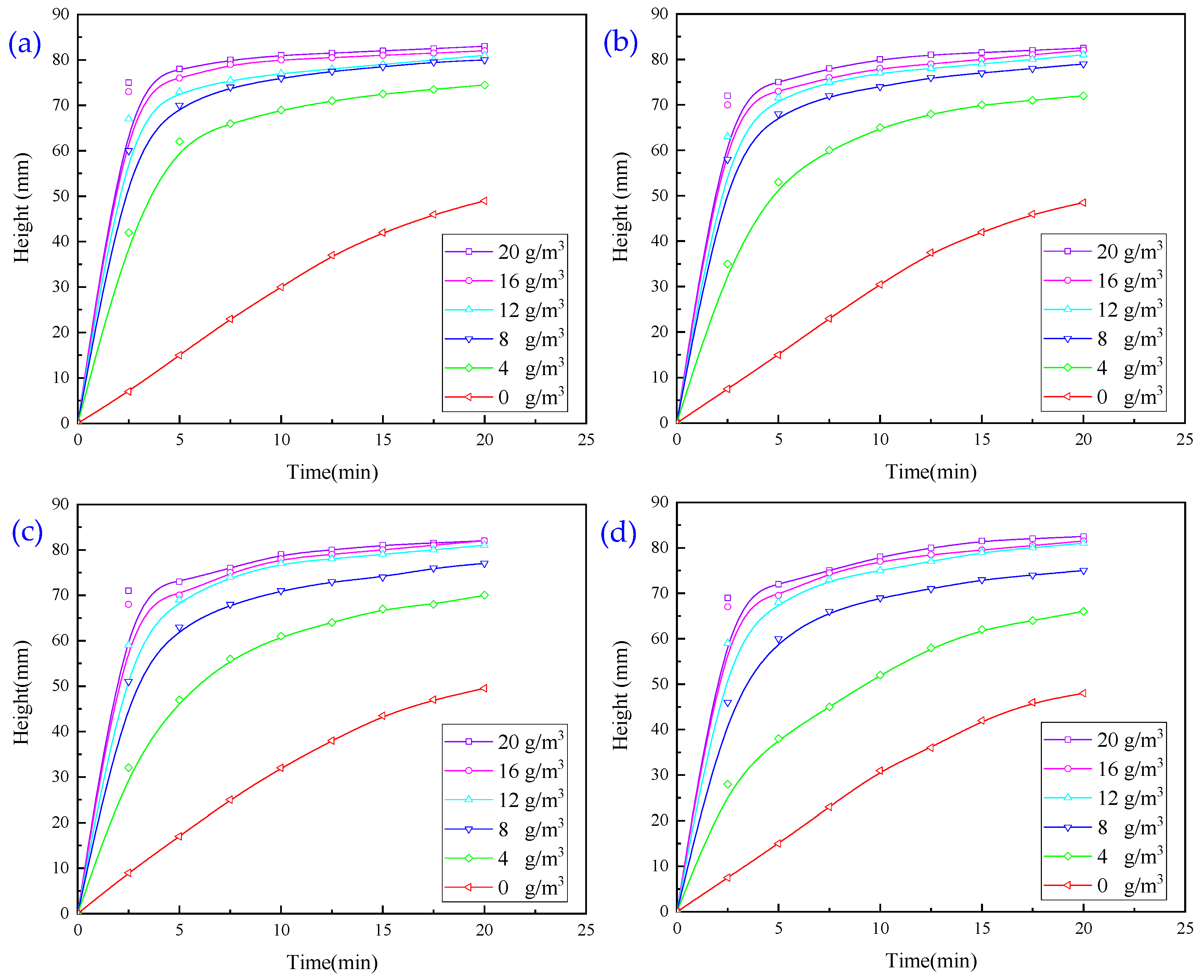
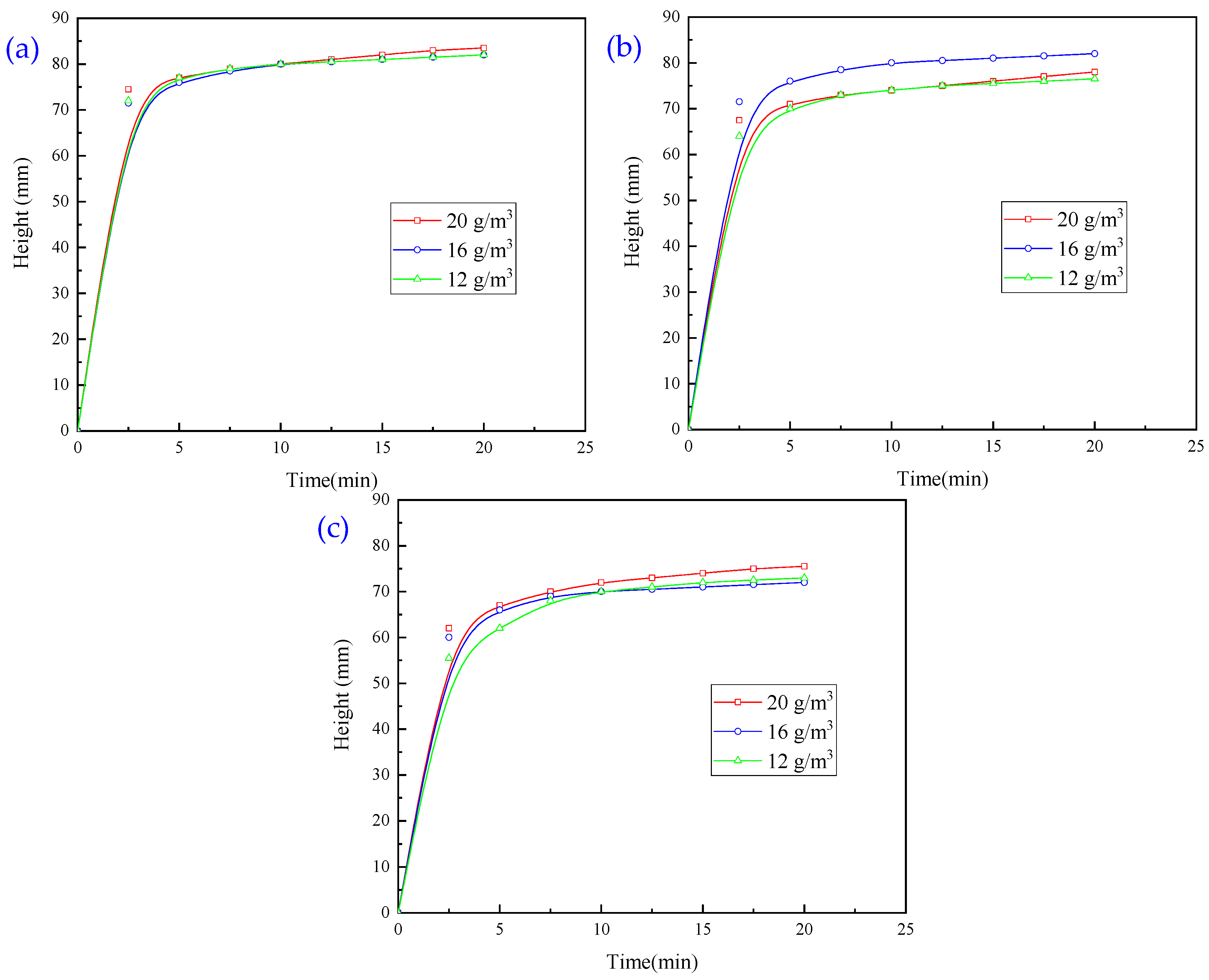
3.1.4. Experiments with Flocculation Conditions Using a Combination of Two Agents
3.1.5. Experiments with Flocculation Conditions Using a Combination of Three Agents
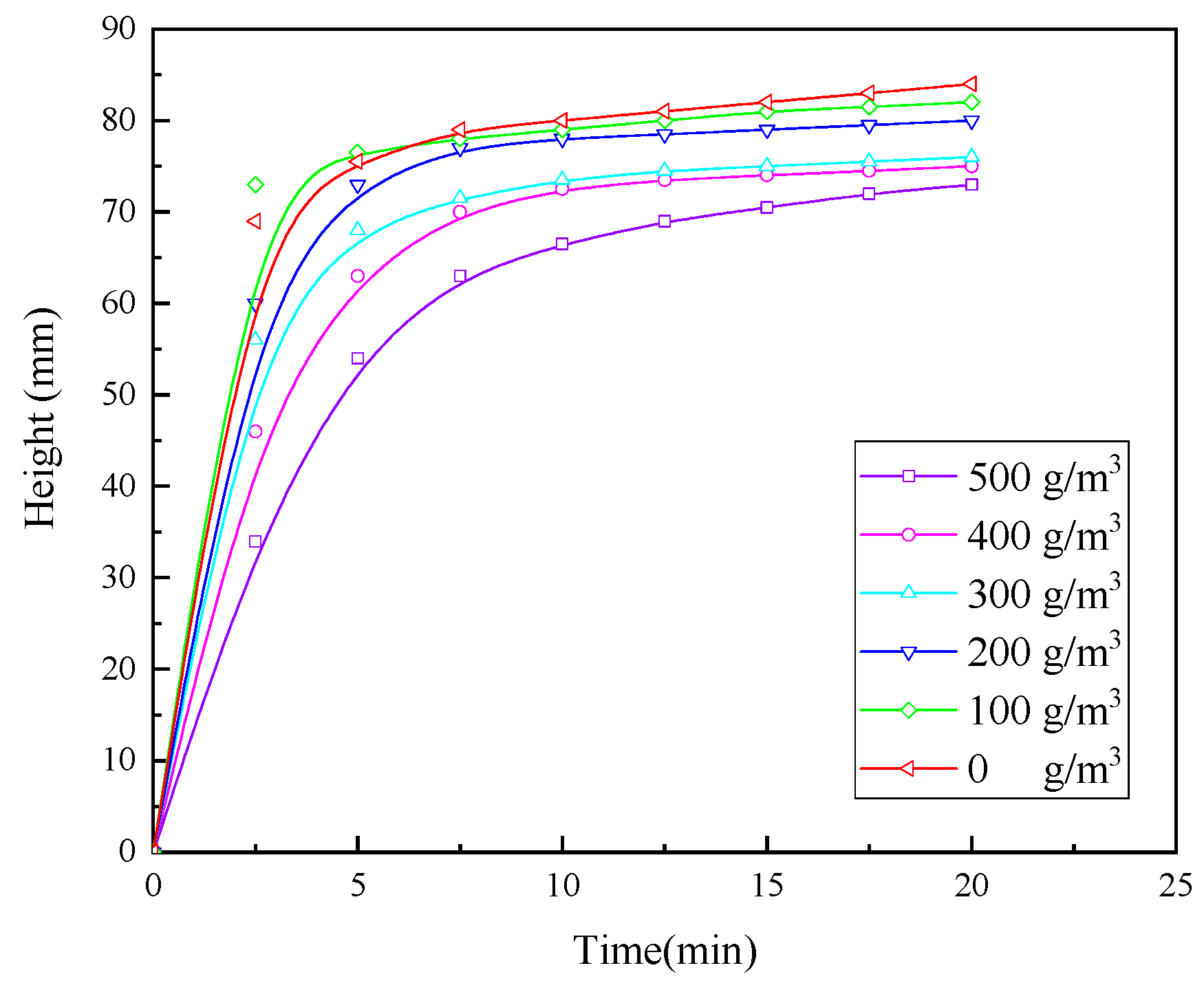
3.1.6. Experiments with Optimised Conditions of Flocculating Agents
3.2. Zeta Potential Measurement
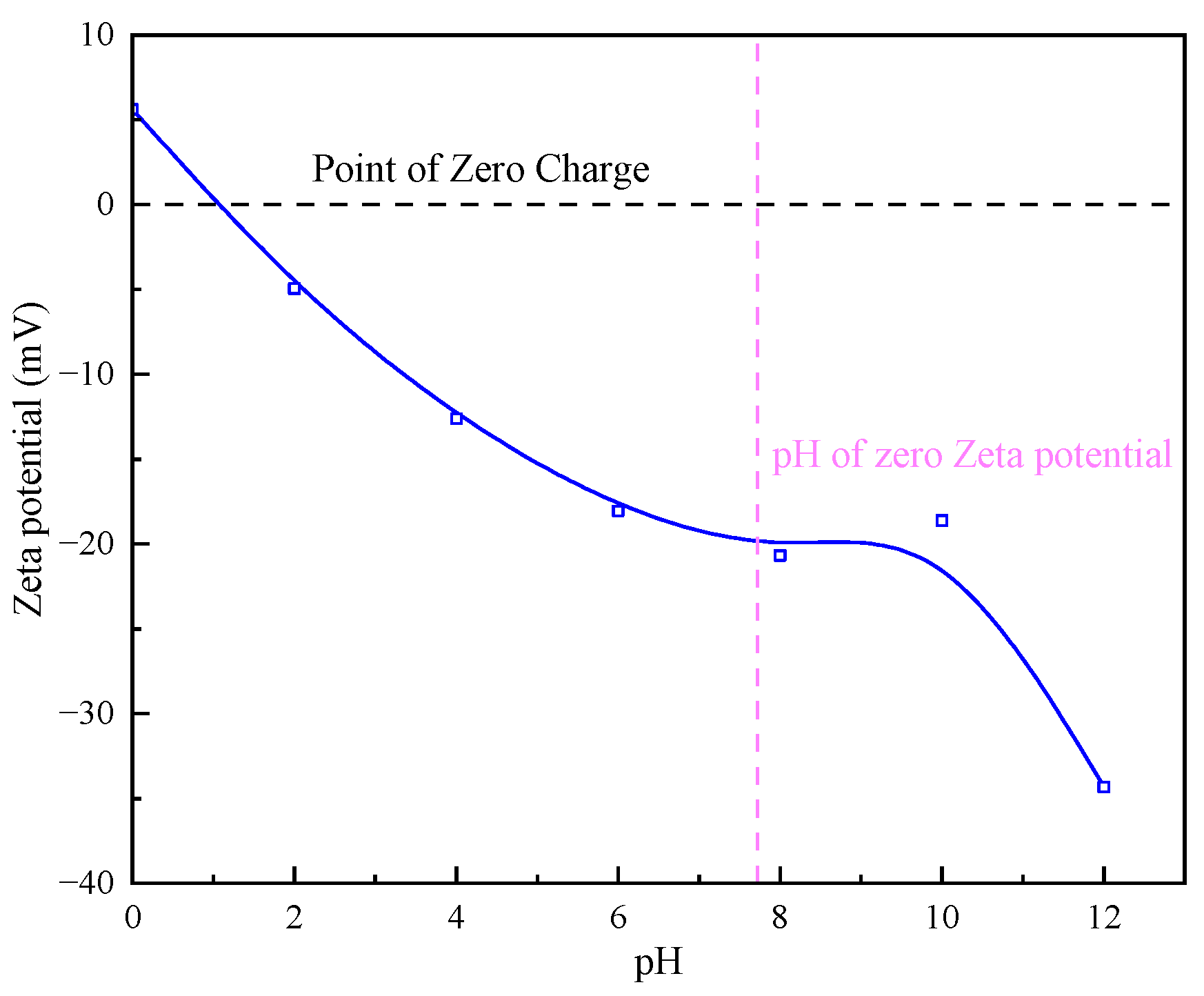
3.2.1. Sediment Surface Zeta Potential Detection
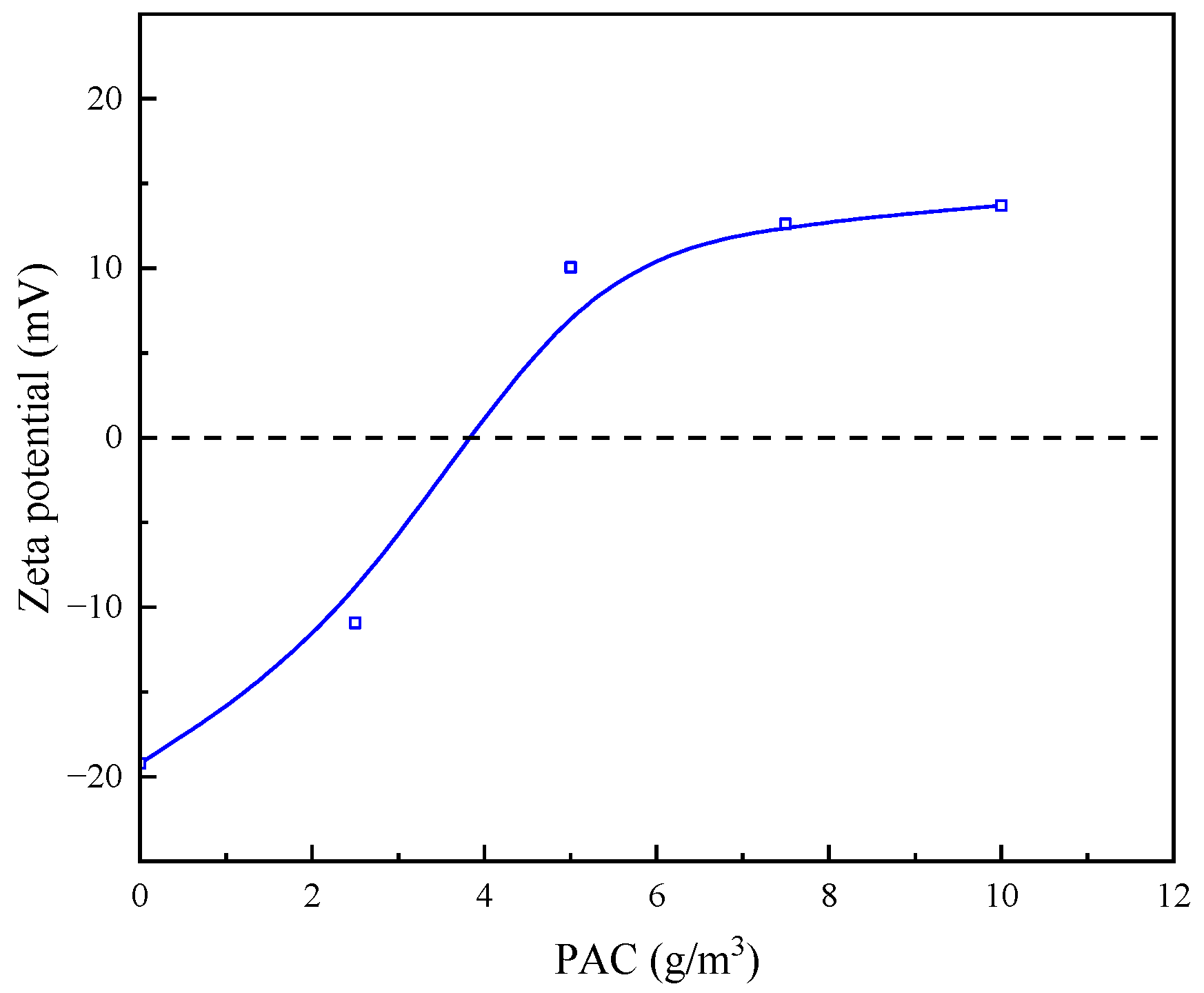
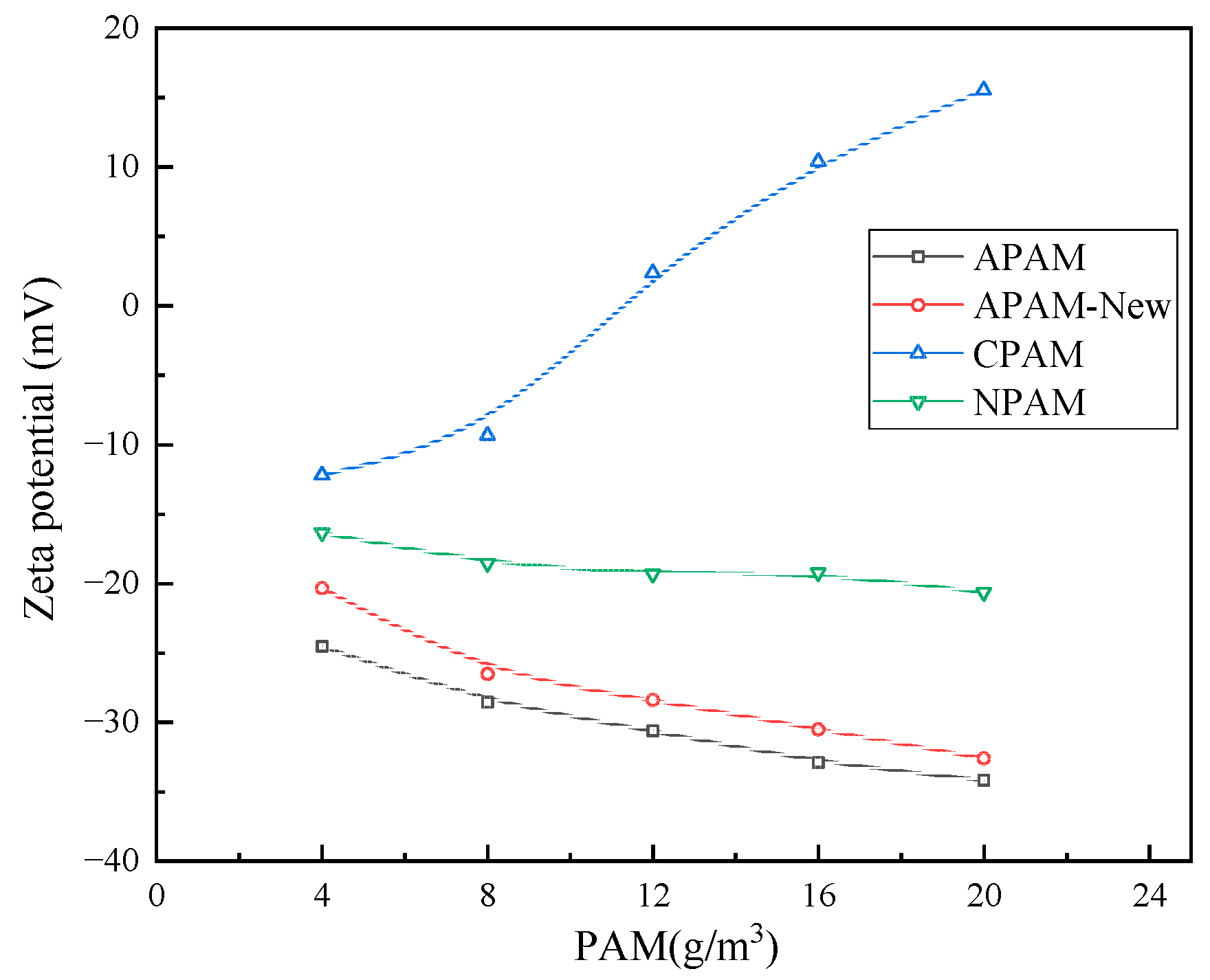
3.2.2. Effect of Single Flocculant Dosage on Zeta Point Position of Quarry Wastewater Particles
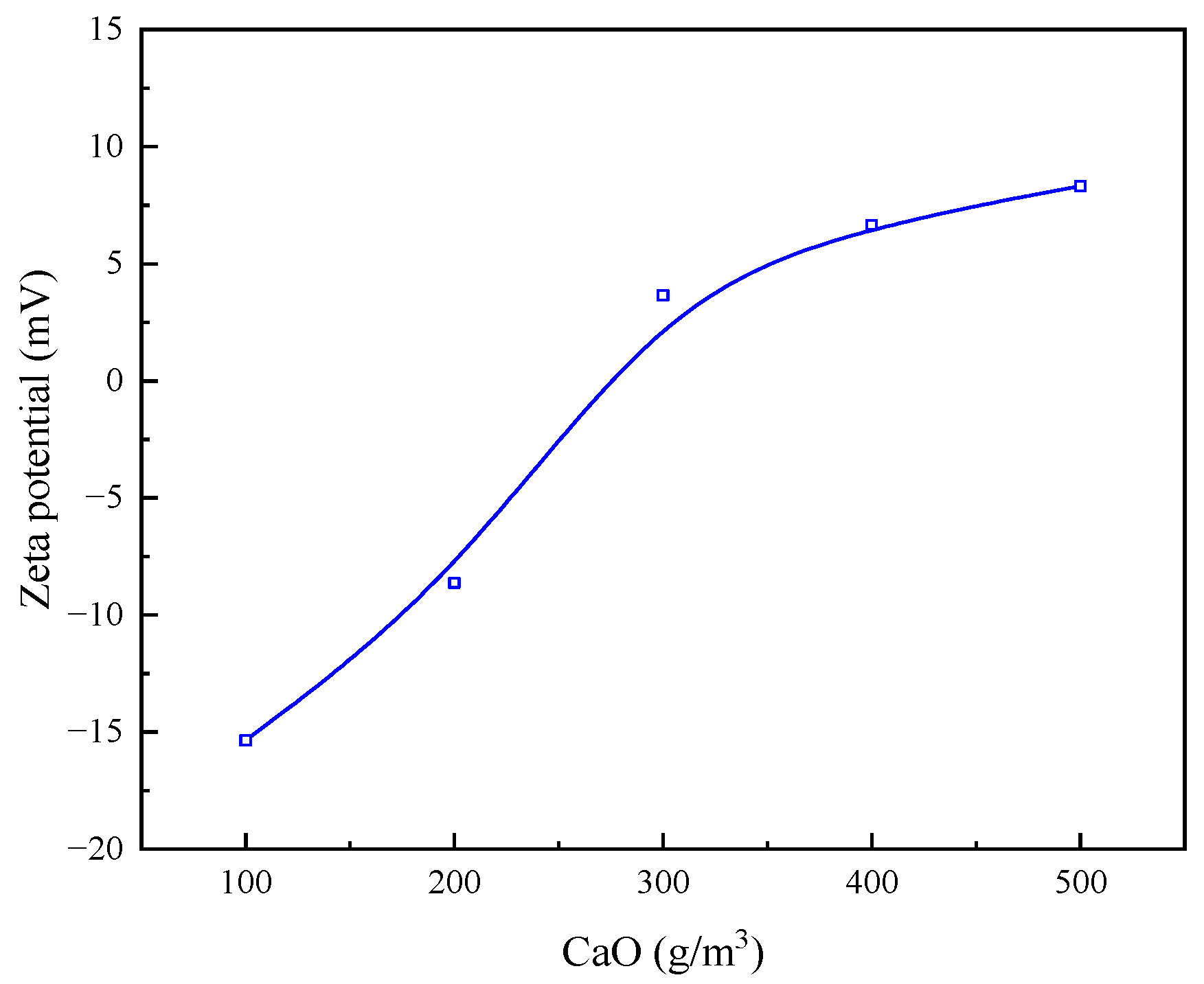
3.2.3. Effect of Optimum Flocculating Agent Dosage on Zeta Potential of Quarry Wastewater Particles
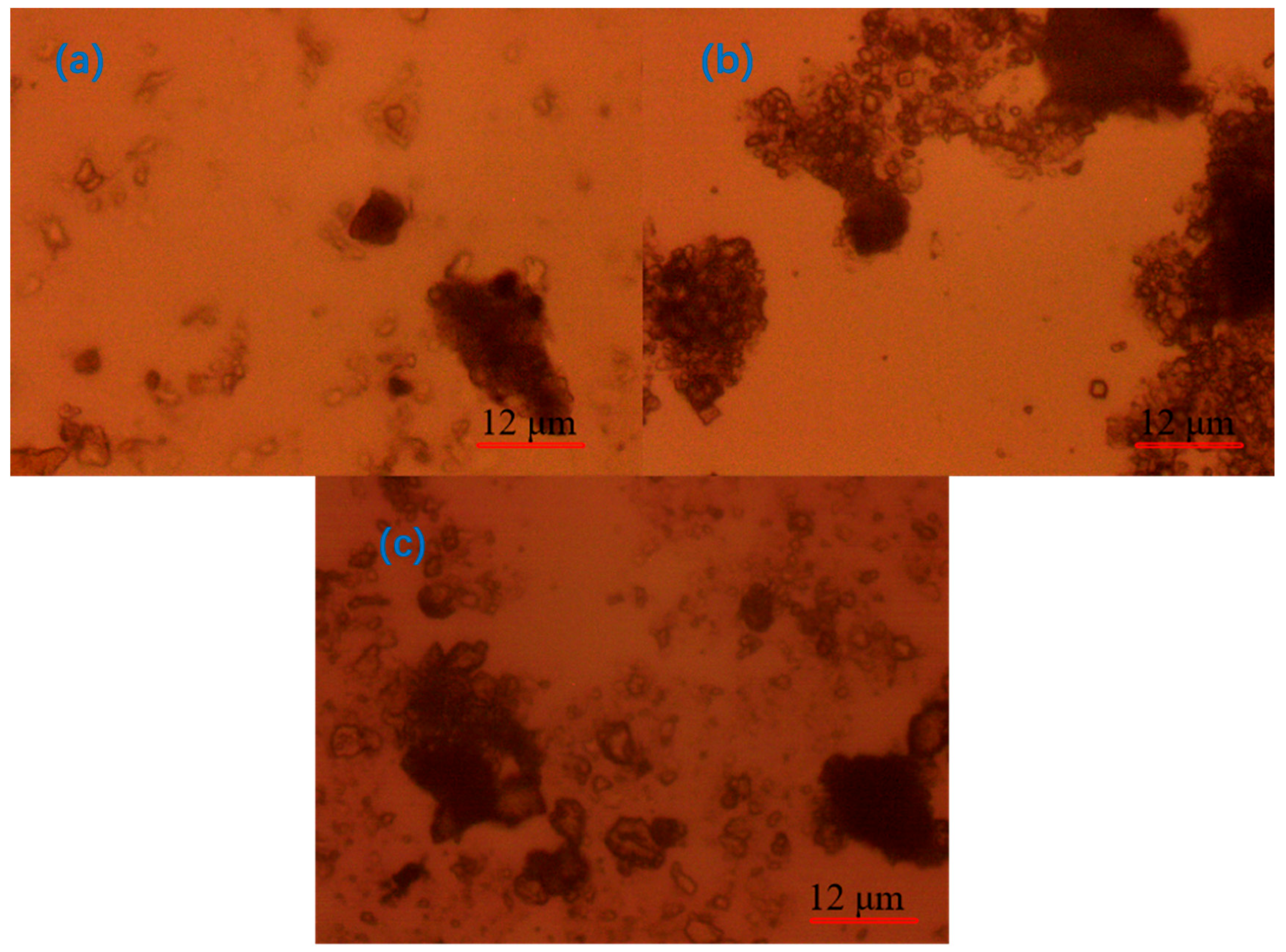
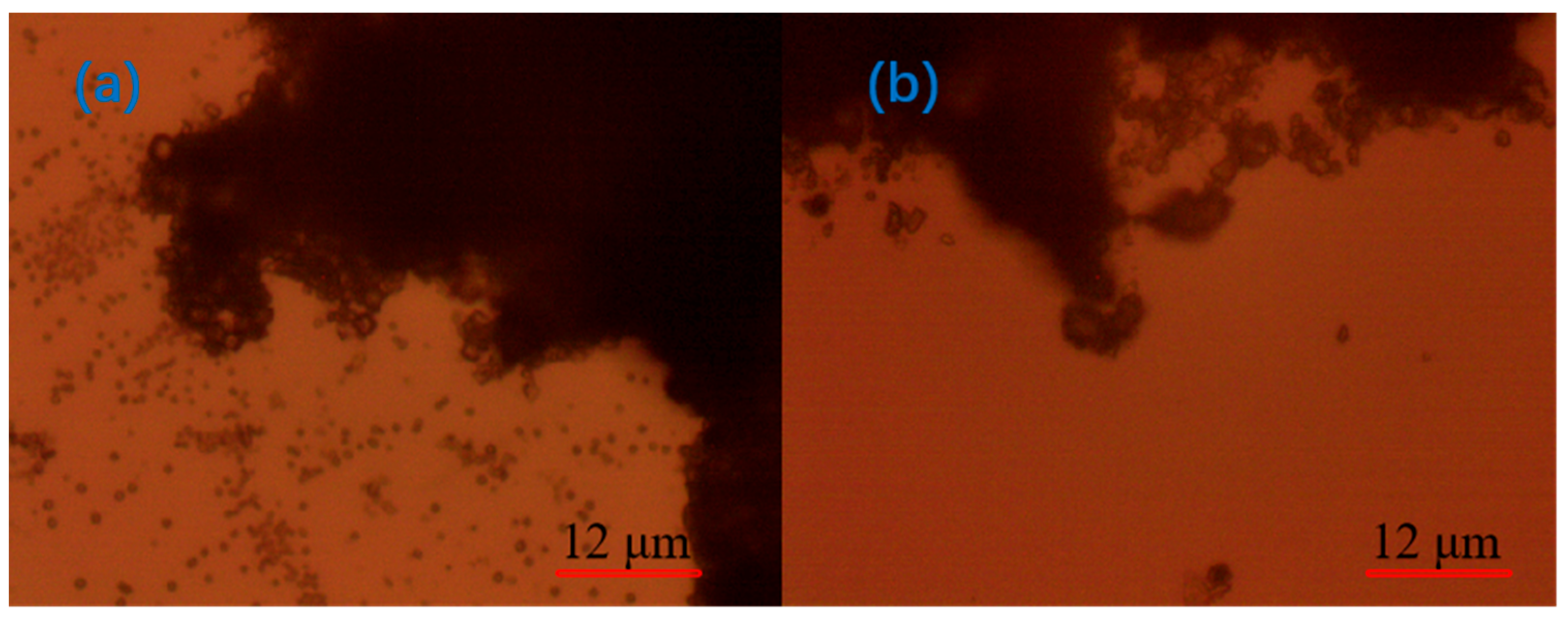
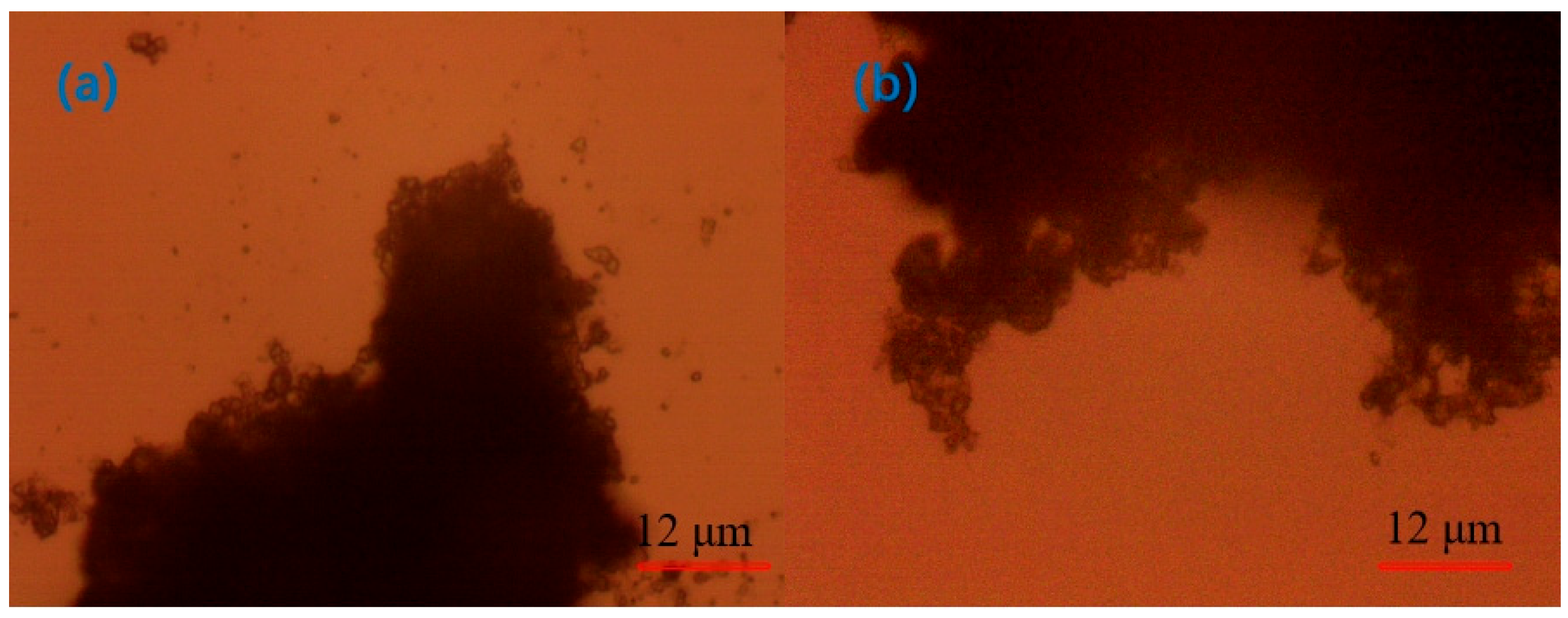
3.3. Optical Microscope Analysis
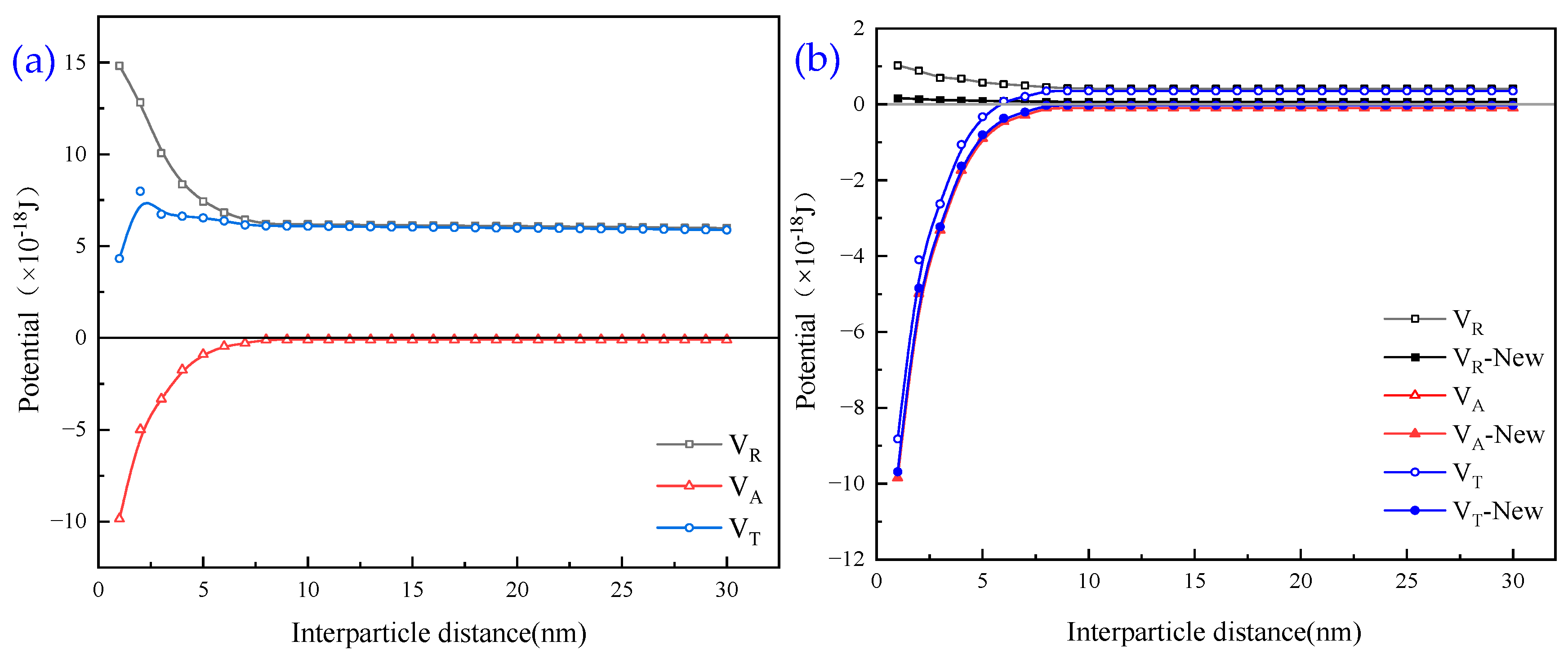
3.4. Theoretical Analysis of DLVO of Wastewater Particles
4. Conclusions
- (1)
- When exploring the effect of flocculants added individually on the flocculation effect of quarry wastewater, the regulator agent has the best effect on water quality improvement, followed by inorganic flocculants, and organic flocculants have the worst effect on water quality improvement. Organic flocculants have the fastest flocculation and settling rate, followed by inorganic flocculants, and the regulator agent has the slowest settling rate. Thus, we selected inorganic flocculants with the best combined effect, i.e., PAC and PAM, and the regulator agent CaO as the flocculants for the subsequent experiments.
- (2)
- In the combination experiment of APAM and PAC, the particle settling rate is accelerated, and the upper layer of the clear liquid is more turbid. In the combination experiment of APAM and CaO, the particle settling rate is enhanced to a lesser degree, and the upper layer of the clear liquid is clearer. The combination experiment of CaO, PAM, and PAC found that the particle settling rate is faster, and the upper layer of the clear liquid is clearer, but it still fails to meet the industry standards. Therefore, combined with the actual needs of the site, under the same conditions as those used for CaO and PAC, the settling effect of the PAM-modified agent is better than that of PAM. Therefore, the best results of the experiments are obtained under the following conditions: sequential dosing, an agent action time of 2 min, a stirring intensity of 500 r/min, a CaO dosage of 200 g/m3, a PAC dosage of 5 g/m3, and a PAM-modified agent amount of 12 g/m3. Under these conditions, the wastewater turbidity was 97.30 NTU.
- (3)
- By detecting the zeta potential on the surface of settled particles, it was found that the zeta potential on the surface of the particles increased with the increase in the concentration of the inorganic flocculant PAC and regulator agent CaO, which indicated that PAC and CaO mainly promoted mutual flocculation and precipitation among particles through the effect of electroneutralisation. The zeta potential on the surface of the particles did not change much with the increase in the concentration of the organic flocculant, which indicated that the inorganic flocculant mainly promoted mutual adsorption and precipitation among particles through adsorption and bridging. This suggests that the inorganic flocculant mainly promotes mutual adsorption and precipitation between particles through adsorption and bridging.
- (4)
- Under the optical microscope, it was observed that, after adding CaO to the quarry wastewater, the flocs formed by tiny particles were obvious but not compact; after adding PAC, the flocs formed by tiny particles were smaller; after adding PAM, the flocs were larger but compact; and after the optimal combination of agents was added, the flocs were stable, and the agglomerates were large.
- (5)
- The theoretical analysis of DLVO shows that the total potential energy between particles in the quarry wastewater in the natural state is positive, and there is a large repulsive force between these particles, which results in turbid wastewater. After adding the optimal combination of agents, the total potential energy between particles exhibits a negative value, indicating that the particles begin to attract each other and form larger flocs after the addition.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kafu-Quvane, B.; Mlaba, S. Assessing the impact of quarrying as an environmental ethic crisis: A case study of limestone mining in a rural community. Int. J. Environ. Res. Public Health 2024, 21, 458. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, S.; Reddy, R.S. Environmental implications on blasting operations in Indian quarry mines. Int. J. Min. Geo-Eng. 2024, 58, 289–294. [Google Scholar]
- Pająk, M.; Dzieniszewska, A.; Kyzioł-Komosińska, J. Natural (Clinoptilolite) and Synthetic (NaP1) Zeolites in the Adsorption Process for the Removal of Acid Black 1 Dye from Aqueous Solutions. Molecules 2025, 30, 1677. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, W.; Sun, X.; Zhang, Q.; Ji, J.; Yan, Y.; Sun, J. Remediation of Hg-Contaminated Groundwater via Adsorption on Supramolecular Polymers in Batch Process and Column Test. Molecules 2025, 30, 1406. [Google Scholar] [CrossRef]
- Peng, X.; Shi, G.-L.; Zheng, J.; Liu, J.-Y.; Shi, X.-R.; Xu, J.; Feng, Y.-C. Influence of quarry mining dust on PM2.5 in a city adjacent to a limestone quarry: Seasonal characteristics and source contributions. Sci. Total Environ. 2016, 550, 940–949. [Google Scholar] [CrossRef]
- Ambastha, S.K.; Haritash, A.K. Emission of respirable dust from stone quarrying, potential health effects, and its management. Environ. Sci. Pollut. Res. 2022, 29, 6670–6677. [Google Scholar] [CrossRef]
- Svobodova, K.; Plieninger, T.; Sklenicka, P. Place re-making and sense of place after quarrying and social-ecological restoration. Sustain. Dev. 2023, 31, 2240–2255. [Google Scholar] [CrossRef]
- Lesin, Y.; Gogolin, V.; Murko, E.; Markov, S.; Kretschmann, J. The choice of methods of quarry wastewater purifying. E3S Web Conf. 2018, 41, 01039. [Google Scholar] [CrossRef]
- Tyulenev, M.; Lesin, Y.; Litvin, O.; Maliukhina, E.; Abay, A. Increasing the reliability of the work of artificial filtering arrays for the purification of quarry waste water. E3S Web Conf. 2017, 21, 02019. [Google Scholar] [CrossRef]
- Pashkevich, M.A.; Korotaeva, A.E.; Matveeva, V.A. Experimental simulation of a system of swamp biogeocenoses to improve the efficiency of quarry water treatment. J. Min. Inst. 2023, 263, 785–794. [Google Scholar]
- Jermakka, J.; Wendling, L.; Sohlberg, E.; Heinonen, H.; Merta, E.; Laine-Ylijoki, J.; Kaartinen, T.; Mroueh, U.-M. Nitrogen Compounds at Mines and Quarries: Sources, Behaviour and Removal from Mine and Quarry Waters—Literature Study; VTT Technical Research Centre of Finland: Espoo, Finland, 2015. [Google Scholar]
- Su, N. Spherical Polyelectrolyte Brushes as Flocculants and Retention Aids in Wet-End Papermaking. Molecules 2023, 28, 7984. [Google Scholar] [CrossRef]
- Taşdemir, T.; Kurama, H. Fine particle removal from natural stone processing effluent by flocculation. Environ. Prog. Sustain. Energy 2013, 32, 317–324. [Google Scholar] [CrossRef]
- Tyulenev, M.; Zhironkin, S.; Litvin, O. The low-cost technology of quarry water purifying using the artificial filters of overburden rock. Pollut. Res. 2015, 34, 825–830. [Google Scholar]
- Rulyov, N.; Dontsova, T.; Korolyov, V.Y. Separation of finely dispersed sorbents from purified water by ultra-flocculation and turbulent micro-flotation. Int. J. Environ. Pollut. 2007, 30, 345–357. [Google Scholar] [CrossRef]
- Concha, F.; Rulyov, N.; Laskowski, J. Settling velocities of particulate systems 18: Solid flux density determination by ultra-flocculation. Int. J. Miner. Process. 2012, 104, 53–57. [Google Scholar] [CrossRef]
- Wang, Z.; Nan, J.; Ji, X.; Yang, Y. Effect of the micro-flocculation stage on the flocculation/sedimentation process: The role of shear rate. Sci. Total Environ. 2018, 633, 1183–1191. [Google Scholar] [CrossRef]
- Li, C.; Busquets, R.; Moruzzi, R.B.; Campos, L.C. Preliminary study on low-density polystyrene microplastics bead removal from drinking water by coagulation-flocculation and sedimentation. J. Water Process Eng. 2021, 44, 102346. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, X.; Dong, X.; Feng, Z.; Dong, Y. Characterisation of floc size, effective density and sedimentation under various flocculation mechanisms. Water Sci. Technol. 2020, 82, 1261–1271. [Google Scholar] [CrossRef]
- Rulyov, N.; Korolyov, B.; Kovalchuk, N. Ultra-flocculation of quartz suspension: Effects of shear rate, dispersity and solids concentration. Miner. Process. Extr. Metall. 2009, 118, 175–181. [Google Scholar] [CrossRef]
- Wang, D.; Di, S.; Wu, L.; Tan, Y.; Tang, Y. Sedimentation behavior of organic, inorganic, and composite flocculant-treated waste slurry from construction works. J. Mater. Civ. Eng. 2021, 33, 04021134. [Google Scholar] [CrossRef]
- Rulyov, N.N.; Laskowski, J.S.; Concha, F. The use of ultra-flocculation in optimization of the experimental flocculation procedures. Physicochem. Probl. Miner. Process. 2011, 47, 5–16. [Google Scholar]
- Abujazar, M.S.S.; Karaağaç, S.U.; Amr, S.S.A.; Alazaiza, M.Y.; Bashir, M.J. Recent advancement in the application of hybrid coagulants in coagulation-flocculation of wastewater: A review. J. Clean. Prod. 2022, 345, 131133. [Google Scholar] [CrossRef]
- Wang, H.-F.; Hu, H.; Wang, H.-J.; Zeng, R.J. Combined use of inorganic coagulants and cationic polyacrylamide for enhancing dewaterability of sewage sludge. J. Clean. Prod. 2019, 211, 387–395. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, H.; Sun, C.; Yuan, W.; Li, H.; Jiang, W.; Dong, L.; Wang, Y.; Liu, H. Preparation of inorganic–organic composite coagulant and its mechanism in destroying emulsified oil in oilfield sewage. Sep. Purif. Technol. 2024, 330, 125446. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Springer: New York, NY, USA, 2010; pp. 63–70. [Google Scholar]
- López-Maldonado, E.; Oropeza-Guzman, M.; Jurado-Baizaval, J.; Ochoa-Terãn, A. Coagulation–flocculation mechanisms in wastewater treatment plants through zeta potential measurements. J. Hazard. Mater. 2014, 279, 1–10. [Google Scholar] [CrossRef]
- MacIver, M.R.; Pawlik, M. Analysis of in situ microscopy images of flocculated sediment volumes. Chem. Eng. Technol. 2017, 40, 2305–2313. [Google Scholar] [CrossRef]
- Droppo, I.; Ongley, E. The state of suspended sediment in the freshwater fluvial environment: A method of analysis. Water Res. 1992, 26, 65–72. [Google Scholar] [CrossRef]
- Agmo Hernández, V. An overview of surface forces and the DLVO theory. ChemTexts 2023, 9, 10. [Google Scholar] [CrossRef]
- Trefalt, G.; Borkovec, M. Overview of DLVO Theory; Laboratory of Colloid and Surface Chemistry, University of Geneva: Geneva, Switzerland, 2014; Volume 304. [Google Scholar]
- Shi, Z.; Ran, B.; Liu, L. Determining the interaction energy of a quartz–kaolinite system at different pH levels by atomic force microscopy and extended DLVO theory. Powder Technol. 2022, 409, 117842. [Google Scholar] [CrossRef]
- Chang, T.; Zhang, J.L.; Xu, H.X.; Tian, H.; Yin, Y.M.; Cui, J.H. Experimental Study of High Slime Water Settlement Based on EDLVO Theory. Coal Technol. 2022, 41, 220–222. [Google Scholar]
- Wang, Z.; Li, Y.; Xiang, Y.; Yang, X.; Li, W. Influencing Mechanisms of Fine Kaolinite on Pyrite Flotation with Seawater. Met. Mine 2023, 7, 213–218. [Google Scholar]
| Element | Content | Element | Content |
|---|---|---|---|
| O | 47.047 | Mn | 0.285 |
| Si | 28.212 | P | 0.088 |
| Al | 10.723 | S | 0.048 |
| Fe | 4.33 | Cl | 0.029 |
| K | 3.453 | Zr | 0.025 |
| Ca | 3.33 | Sr | 0.023 |
| Na | 1.233 | Rb | 0.021 |
| Mg | 0.585 | Zn | 0.013 |
| Ti | 0.55 | Ga | 0.003 |
| CaO (g/m3) | 0 | 100 | 200 | 300 | 400 | 500 |
| pH | 7.96 | 8.96 | 9.91 | 10.48 | 10.88 | 11.21 |
| Types of Flocculation Chemicals | Blank Test | CaO | PAC | APAM | APAM-New | APAM Combinations | APAM-New Combinations |
|---|---|---|---|---|---|---|---|
| Zeta potential (mV) | −19.83 | −8.63 | −10.94 | −30.65 | −28.42 | −5.35 | −2.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, Y.; Zeng, K.; Yang, H.; Sun, A.; Guan, Q.; Zhou, S.; Peng, W.; Wang, W.; Ge, P.; Yang, Y. Experimental Research on Quarry Wastewater Purification Using Flocculation Process. Molecules 2025, 30, 2761. https://doi.org/10.3390/molecules30132761
Bu Y, Zeng K, Yang H, Sun A, Guan Q, Zhou S, Peng W, Wang W, Ge P, Yang Y. Experimental Research on Quarry Wastewater Purification Using Flocculation Process. Molecules. 2025; 30(13):2761. https://doi.org/10.3390/molecules30132761
Chicago/Turabian StyleBu, Yongjie, Kangjian Zeng, Heng Yang, Aihui Sun, Qingjun Guan, Shuang Zhou, Wenqing Peng, Weijun Wang, Peng Ge, and Yue Yang. 2025. "Experimental Research on Quarry Wastewater Purification Using Flocculation Process" Molecules 30, no. 13: 2761. https://doi.org/10.3390/molecules30132761
APA StyleBu, Y., Zeng, K., Yang, H., Sun, A., Guan, Q., Zhou, S., Peng, W., Wang, W., Ge, P., & Yang, Y. (2025). Experimental Research on Quarry Wastewater Purification Using Flocculation Process. Molecules, 30(13), 2761. https://doi.org/10.3390/molecules30132761








