Bioplastic Production Using Natural Extracts with Cellulose Assisted by Experimental and Computational Screening
Abstract
1. Introduction
2. Results
2.1. Chemical Characterization of Plant Extracts
2.1.1. FTIR
2.1.2. Phytochemical Screening
2.1.3. Thin-Layer Chromatography Analysis
2.1.4. Column Chromatography
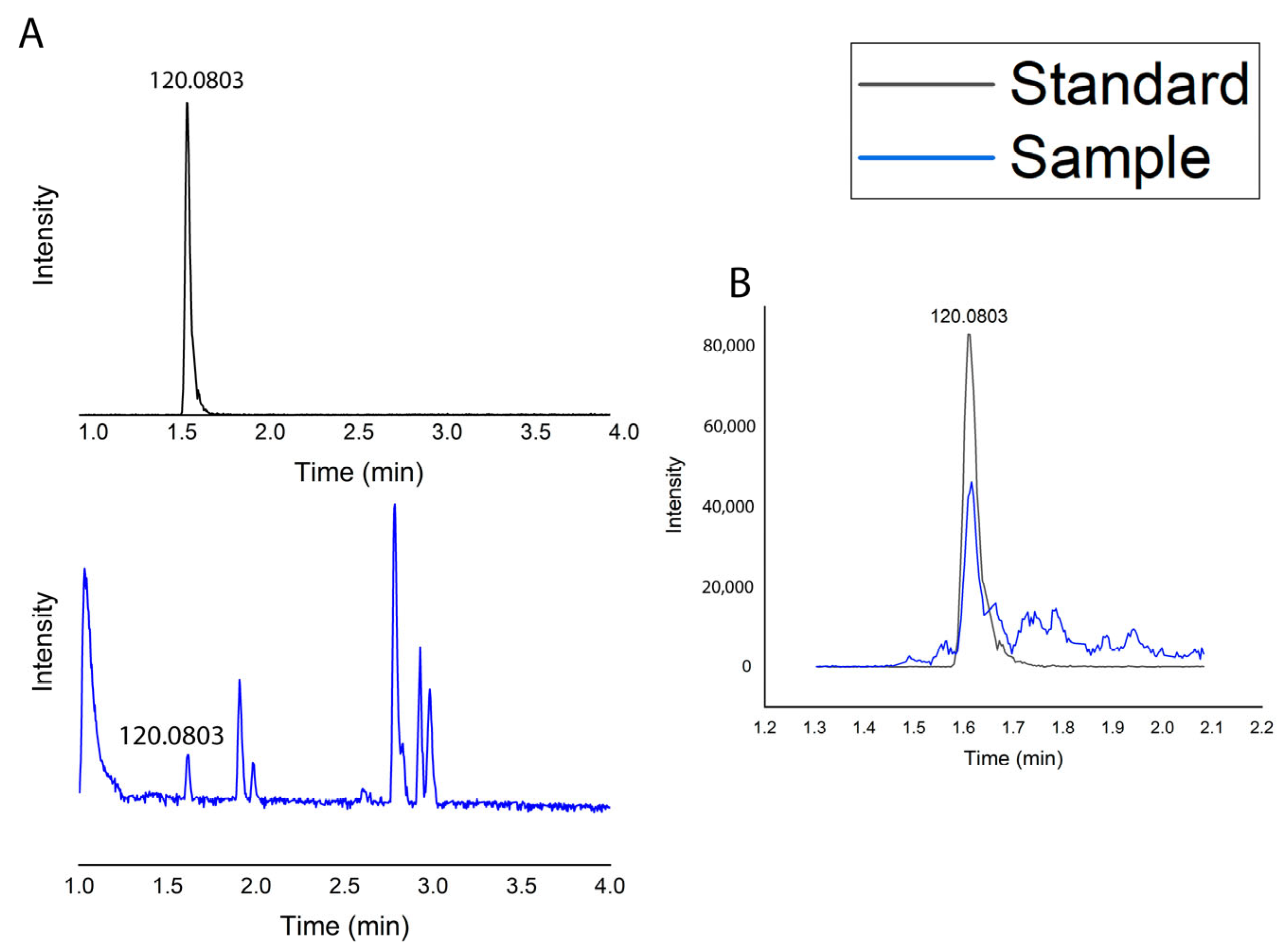
2.1.5. Metabolic Profile
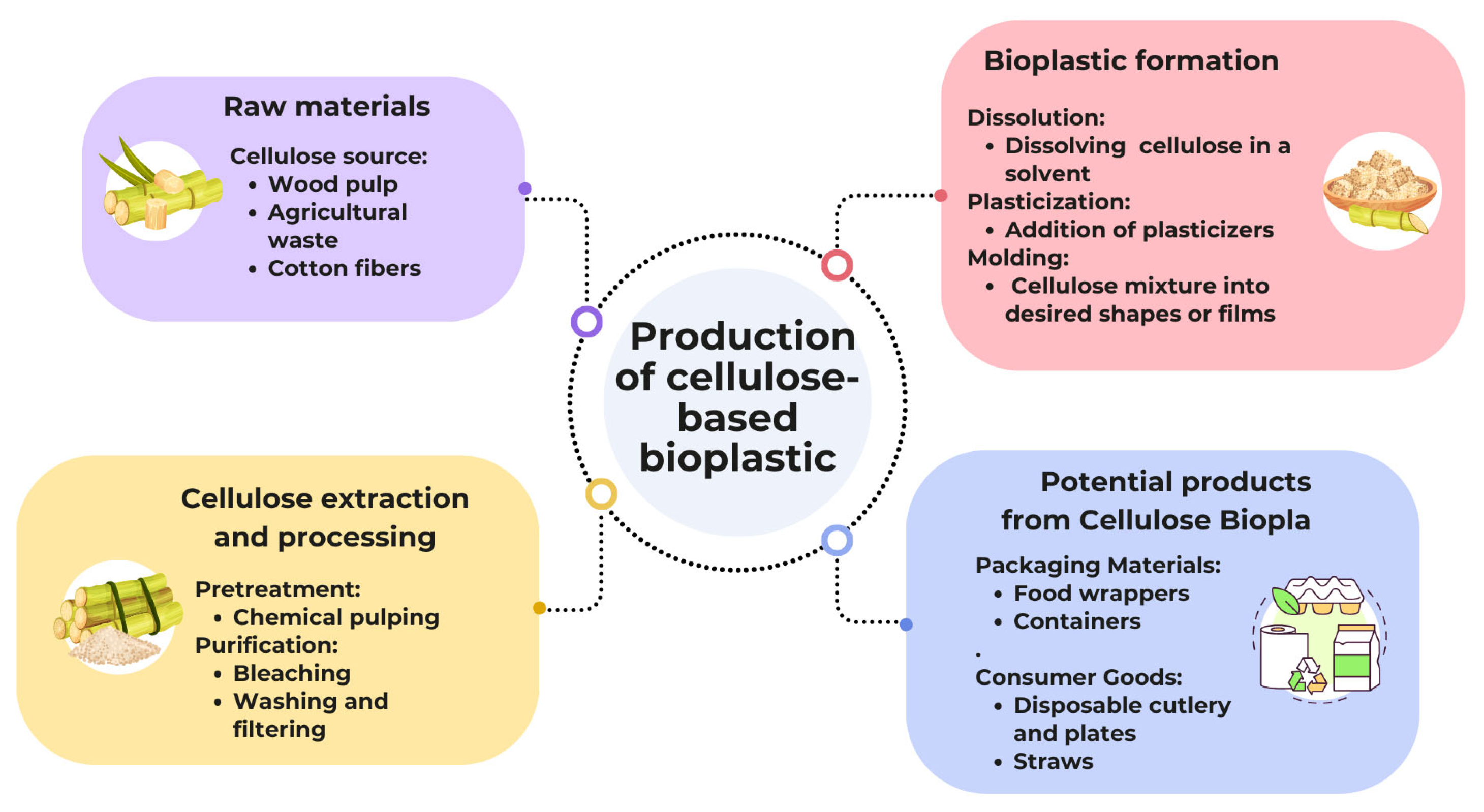
2.2. Preparation of Cellulose-Based Bioplastic Samples
2.3. Chemical Properties of the Bioplastics
2.4. Surface Analysis of Bioplastics
2.5. Biodegradability of Bioplastics
Degradation in Soil and Water
2.6. Water Uptake of Bioplastics
2.7. pH Change in Cause Medium
3. Materials and Methods
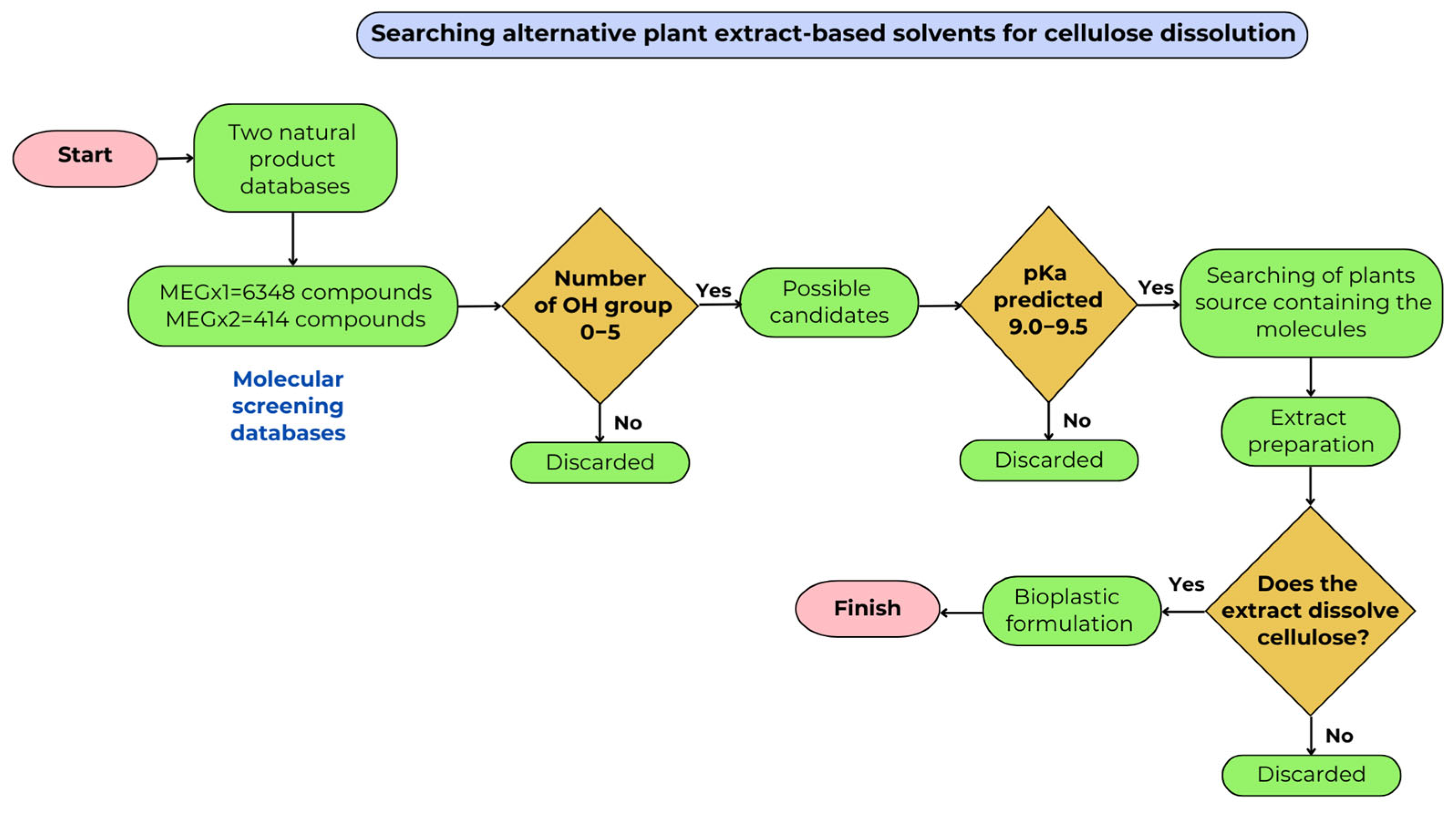
3.1. Computational Screening
3.2. Extract Source Screening
3.3. Other Reagents
3.4. Preparation of Cellulose Bioplastic
3.4.1. Cellulose Extraction
3.4.2. Obtention of Plant Extract
3.4.3. Cellulose Bioplastics
3.5. Characterizations of Plant Extract
3.5.1. Fourier-Transform Infrared Spectroscopy of Plant Extract
3.5.2. Phytochemical Analysis
3.5.3. Thin-Layer Chromatography
3.6. Characterizations of Bioplastics
3.6.1. Fourier-Transform Infrared Spectroscopy of Bioplastics
3.6.2. Scanning Electron Microscope (SEM)
3.6.3. Degradation in Water Test
3.6.4. Absorption of Water Test
3.6.5. Biodegradability Test
3.6.6. Column Chromatography Fractionation
3.6.7. Metabolomic Profile
3.6.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kong, U.; Rawi, N.F.M.; Tay, G.S. The Potential Applications of Reinforced Bioplastics in Various Industries: A Review. Polymers 2023, 15, 2399. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.; Cooper, B.; Posnack, N.G. Bisphenols and phthalates: Plastic chemical exposures can contribute to adverse cardiovascular health outcomes. Birth Defects Res. 2020, 112, 1362–1385. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Narancic, T.; Cerrone, F.; Beagan, N.; O’connor, K.E. Recent Advances in Bioplastics: Application and Biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef]
- Wang, B.-T.; Hu, S.; Yu, X.-Y.; Jin, L.; Zhu, Y.-J.; Jin, F.-J. Studies of Cellulose and Starch Utilization and the Regulatory Mechanisms of Related Enzymes in Fungi. Polymers 2020, 12, 530. [Google Scholar] [CrossRef]
- Acharya, S.; Liyanage, S.; Parajuli, P.; Rumi, S.S.; Shamshina, J.L.; Abidi, N. Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications. Polymers 2021, 13, 4344. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L. Solubility of Cellulose in NaOH/Urea Aqueous Solution. Polym. J. 2000, 32, 866–870. [Google Scholar] [CrossRef]
- Wu, C.; Li, J.; Zhang, Y.; Li, X.; Wang, S.; Li, D. Cellulose Dissolution, Modification, and the Derived Hydrogel: A Review. ChemSusChem 2023, 16, e202300518. [Google Scholar] [CrossRef]
- Gondhalekar, S.C.; Pawar, P.J.; Dhumal, S.S.; Thakre, S.S. Mechanism of xanthation reaction in viscose process. Cellulose 2019, 26, 1595–1604. [Google Scholar] [CrossRef]
- Przypis, M.; Wawoczny, A.; Gillner, D. Biomass and Cellulose Dissolution—The Important Issue in Renewable Materials Treatment. Appl. Sci. 2023, 13, 1055. [Google Scholar] [CrossRef]
- Hidayati, S.; Zulferiyenni; Maulidia, U.; Satyajaya, W.; Hadi, S. Effect of glycerol concentration and carboxy methyl cellulose on biodegradable film characteristics of seaweed waste. Heliyon 2021, 7, e07799. [Google Scholar] [CrossRef] [PubMed]
- Ben, Z.Y.; Samsudin, H.; Yhaya, M.F. Glycerol: Its properties, polymer synthesis, and applications in starch based films. Eur. Polym. J. 2022, 175, 111377. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Lee, C.K.; Nanyan, N.S.M.; Tay, G.S. A systematic review on utilization of biodiesel-derived crude glycerol in sustainable polymers preparation. Int. J. Biol. Macromol. 2024, 261, 129536. [Google Scholar] [CrossRef]
- Benitez, J.J.; Florido-Moreno, P.; Porras-Vázquez, J.M.; Tedeschi, G.; Athanassiou, A.; Heredia-Guerrero, J.A.; Guzman-Puyol, S. Transparent, plasticized cellulose-glycerol bioplastics for food packaging applications. Int. J. Biol. Macromol. 2024, 273, 132956. [Google Scholar] [CrossRef]
- Santana, R.F.; Bonomo, R.C.F.; Gandolfi, O.R.R.; Rodrigues, L.B.; Santos, L.S.; Pires, A.C.d.S.; de Oliveira, C.P.; Fontan, R.d.C.I.; Veloso, C.M. Characterization of starch-based bioplastics from jackfruit seed plasticized with glycerol. J. Food Sci. Technol. 2018, 55, 278–286. [Google Scholar] [CrossRef]
- Fauziyah, S.N.; Mubarak, A.S.; Pujiastuti, D.Y. Application of glycerol on bioplastic based carrageenan waste cellulose on biodegradability and mechanical properties bioplastic. IOP Conf. Ser. Earth Environ. Sci. 2021, 679, 012005. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; Dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Amarakoon, M.; Alenezi, H.; Homer-Vanniasinkam, S.; Edirisinghe, M. Environmental Impact of Polymer Fiber Manufacture. Macromol. Mater. Eng. 2022, 307, 2200356. [Google Scholar] [CrossRef]
- Norgren, M.; Costa, C.; Alves, L.; Eivazi, A.; Dahlström, C.; Svanedal, I.; Edlund, H.; Medronho, B. Perspectives on the Lindman Hypothesis and Cellulose Interactions. Molecules 2023, 28, 4216. [Google Scholar] [CrossRef]
- Kihlman, M.; Medronho, B.F.; Romano, A.L.; Germgård, U.; Lindman, B. Cellulose Dissolution in an Alkali Based Solvent: Influence of Additives and Pretreatments. J. Braz. Chem. Soc. 2013, 24, 295–303. [Google Scholar] [CrossRef]
- Lawson, L.; Degenstein, L.M.; Bates, B.; Chute, W.; King, D.; Dolez, P.I. Cellulose Textiles from Hemp Biomass: Opportunities and Challenges. Sustainability 2022, 14, 15337. [Google Scholar] [CrossRef]
- Zamora-Mendoza, L.; Guamba, E.; Miño, K.; Romero, M.P.; Levoyer, A.; Alvarez-Barreto, J.F.; Machado, A.; Alexis, F. Antimicrobial Properties of Plant Fibers. Molecules 2022, 27, 7999. [Google Scholar] [CrossRef] [PubMed]
- Gul, R.; Jan, S.U.; Faridullah, S.; Sherani, S.; Jahan, N. Preliminary Phytochemical Screening, Quantitative Analysis of Alkaloids, and Antioxidant Activity of Crude Plant Extracts from Ephedra intermedia Indigenous to Balochistan. Sci. World J. 2017, 2017, 5873648. [Google Scholar] [CrossRef]
- Fukunaga, Y.; Ogawa, R.; Homma, A.; Okada, T. Thin Layer Chromatography-Freeze Surface-Enhanced Raman Spectroscopy: A Powerful Tool for Monitoring Synthetic Reactions. Chem. A Eur. J. 2023, 29, e202300829. [Google Scholar] [CrossRef]
- Dar, A.; Ahmad, M.N.; Samin, G.; Jahangir, M.M.; Rehman, R.; Anwar, J.; Al-Thagafi, Z.T.; Meraf, Z.; Jaber, M.M.; Dar, A.A. Separation of Amino Acids, Dyes, and Pigments Using Novel Pressurized Circular TLC Assembly for Secure Medical Imaging Applications. Int. J. Anal. Chem. 2023, 2023, 9914633. [Google Scholar] [CrossRef]
- Harish, D.; Solanki, N. Chromatographic methods used for characterization of boswellic acids. MOJ Drug Des. Dev. Ther. 2018, 2, 195–201. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Garrard, I. Counter-current chromatography for the separation of terpenoids: A comprehensive review with respect to the solvent systems employed. Phytochem. Rev. 2014, 13, 547–572. [Google Scholar] [CrossRef]
- Nasser, A.L.M.; Mazzolin, L.P.; Hiruma-Lima, C.A.; Santos, L.S.; Eberlin, M.N.; Brito, A.R.M.d.S.; Vilegas, W. Preparative Droplet Counter-Current Chromatography for the Separation of the New Nor-Seco-Triterpene and Pentacyclic Triterpenoids from Qualea Parviflora. Chromatographia 2006, 64, 695–699. [Google Scholar] [CrossRef]
- Salim, M.F.H.; Nugraha, I.M.A.D.P.; Adilla, F.; Yanti, L.P.D. Chromatography Profiles of Terpenoid Compounds in The Extract of Sambiloto (Andrographis paniculata) Herb from Various Solvents. Walisongo J. Chem. 2021, 4, 74–80. [Google Scholar] [CrossRef]
- Heftmann, E. Chromatography: Fundamentals and Applications of Chromatography and Related Differential Migration Methods, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Annadurai, P. Extraction and Isolation of bioactive compounds from Lantana camara leaves by column Chromatographic techniques. Res. J. Pharm. Technol. 2021, 14, 1607–1611. [Google Scholar] [CrossRef]
- Yap, C.W. PaDEL-descriptor: An open source software to calculate molecular descriptors and fingerprints. J. Comput. Chem. 2011, 32, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Deng, Y.; Wang, B.; Yang, X. Gas chromatography–mass spectrometry method for determination of phenylalanine and tyrosine in neonatal blood spots. J. Chromatogr. B 2002, 780, 407–413. [Google Scholar] [CrossRef]
- Portella, E.H.; Romanzini, D.; Angrizani, C.C.; Amico, S.C.; Zattera, A.J. Influence of Stacking Sequence on the Mechanical and Dynamic Mechanical Properties of Cotton/Glass Fiber Reinforced Polyester Composites. Mater. Res. 2016, 19, 542–547. [Google Scholar] [CrossRef]
- Hanry, E.L.; Redzwan, N.F.M.; Badeges, N.F.A.K.; Surugau, N. Characterization of biofilms developed from alginate extracted from Padina sp. incorporated with calcium chloride (CaCl2). J. Phys. Conf. Ser. 2022, 2314, 012022. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Amritkumar, P. Synthesis and characterization of eco-friendly bioplastic from low-cost plant resources. SN Appl. Sci. 2019, 1, 1432. [Google Scholar] [CrossRef]
- Phosri, S.; Kunjiek, T.; Mukkhakang, C.; Suebthep, S.; Sinsup, W.; Phornsirigarn, S.; Charoeythornkhajhornchai, P. Biodegradability of bioplastic blown film in a marine environment. Front. Mar. Sci. 2022, 9, 917397. [Google Scholar] [CrossRef]
- Oliveros R, D.A.; Machado, R.A.; Mora, J.R. Quantitative structure–property relationship analysis of the spectrochemical series by employing electronic descriptors from DFT calculations. Mol. Phys. 2022, 120, e2040629. [Google Scholar] [CrossRef]
- García-Jacas, C.R.; Marrero-Ponce, Y.; Cortés-Guzmán, F.; Suárez-Lezcano, J.; Martinez-Rios, F.O.; García-González, L.A.; Pupo-Meriño, M.; Martinez-Mayorga, K. Enhancing Acute Oral Toxicity Predictions by using Consensus Modeling and Algebraic Form-Based 0D-to-2D Molecular Encodes. Chem. Res. Toxicol. 2019, 32, 1178–1192. [Google Scholar] [CrossRef]
- Steven, S.; Fauza, A.N.; Mardiyati, Y.; Santosa, S.P.; Shoimah, S.M. Facile Preparation of Cellulose Bioplastic from Cladophora sp. Algae via Hydrogel Method. Polymers 2022, 14, 4699. [Google Scholar] [CrossRef] [PubMed]
- Sayakulu, N.F.; Soloi, S. The Effect of Sodium Hydroxide (NaOH) Concentration on Oil Palm Empty Fruit Bunch (OPEFB) Cellulose Yield. J. Phys. Conf. Ser. 2022, 2314, 012017. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nortjie, E.; Basitere, M.; Moyo, D.; Nyamukamba, P. Extraction Methods, Quantitative and Qualitative Phytochemical Screening of Medicinal Plants for Antimicrobial Textiles: A Review. Plants 2022, 11, 2011. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, J.; Zhang, L.; Xu, M.; Cheng, H.; Han, C.C.; Kuga, S.; Xiao, J.; Xiao, R. A bioplastic with high strength constructed from a cellulose hydrogel by changing the aggregated structure. J. Mater. Chem. A 2013, 1, 6678–6686. [Google Scholar] [CrossRef]
- Kumar, S.S.; Manoj, P.; Giridhar, P. Fourier transform infrared spectroscopy (FTIR) analysis, chlorophyll content and antioxidant properties of native and defatted foliage of green leafy vegetables. J. Food Sci. Technol. 2015, 52, 8131–8139. [Google Scholar] [CrossRef]
- Zamora-Mendoza, L.; Vispo, S.N.; De Lima, L.; Mora, J.R.; Machado, A.; Alexis, F. Hydrogel for the Controlled Delivery of Bioactive Components from Extracts of Eupatorium glutinosum Lam. Leaves. Molecules 2023, 28, 1591. [Google Scholar] [CrossRef]
- Barthwal, R.; Mahar, R. Exploring the Significance, Extraction, and Characterization of Plant-Derived Secondary Metabolites in Complex Mixtures. Metabolites 2024, 14, 119. [Google Scholar] [CrossRef]
- Kowalska, T.; Sajewicz, M. Thin-Layer Chromatography (TLC) in the Screening of Botanicals–Its Versatile Potential and Selected Applications. Molecules 2022, 27, 6607. [Google Scholar] [CrossRef]
- Fathanah, U.; Lubis, M.R.; Nasution, F.; Masyawi, M.S. Characterization of bioplastic based from cassava crisp home industrial waste incorporated with chitosan and liquid smoke. IOP Conf. Ser. Mater. Sci. Eng. 2018, 334, 012073. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, J.; Xu, D.; Cai, J.; Qiu, Y.; Ren, J.; Zhang, L. Facile construction of cellulose/montmorillonite nanocomposite biobased plastics with flame retardant and gas barrier properties. Cellulose 2015, 22, 3799–3810. [Google Scholar] [CrossRef]
- Shafqat, A.; Al-Zaqri, N.; Tahir, A.; Alsalme, A. Synthesis and characterization of starch based bioplatics using varying plant-based ingredients, plasticizers and natural fillers. Saudi J. Biol. Sci. 2021, 28, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Gini, T.; Jothi, G.J. Column chromatography and HPLC analysis of phenolic compounds in the fractions of Salvinia molesta mitchell. Egypt. J. Basic Appl. Sci. 2018, 5, 197–203. [Google Scholar] [CrossRef]







| Phytochemical Analysis | Test | D1 W | D2 W | D3 W | D4 W | D5 W | D6 W | Ref. |
|---|---|---|---|---|---|---|---|---|
| Reducing sugars | Benedict’s test | X | X | X | X | X | - | [18] |
| Fehling’s test | X | X | X | - | X | X | [18] | |
| Proteins and amino acids | Ninhydrin test | X | - | X | - | - | X | [19] |
| Xanthoproteic test | X | X | X | X | X | X | [19] | |
| Proteins | Biuret test | - | - | X | - | - | X | [20] |
| Terpenoids | Salkowski test | X | X | X | X | X | - | [19] |
| Diterpenes | Copper acetate test | X | X | X | - | X | - | [21] |
| Saponins | Foam test | - | X | X | X | X | X | [19] |
| Glycosides | 10% NaOH test | X | X | - | - | X | - | [21] |
| Keller–Kilani test | - | X | X | - | X | - | [21] | |
| Alkaloids | Dragendroff test | - | - | X | X | X | - | [22] |
| Coumarins and lactones | NaOH test | X | - | - | - | - | - | [23] |
| Flavonoids | Alkaline reagent test | X | X | X | X | - | - | [21] |
| Phenols | Ferric chloride test | X | X | - | X | X | - | [24] |
| Quinones | Bontrager test | - | X | X | X | X | - | [25] |
| Sulfuric acid test | - | X | X | - | - | X | [25] |
| Crude Extract | Mobile Phase | Retention Factor (Rf) | Developer |
|---|---|---|---|
| D1 W | n-butanol/acetic acid/water | 0.65732 | Ninhydrin 2% + ethanol |
| D2 W | n-butanol/acetic acid/water | 0.59523 | Ninhydrin 2% + ethanol |
| D3 W | Toluene/ethyl acetate | 0.40000 | Vanillin sulfuric acid ethanol |
| D4 W | Hexane/acetone | 0.53703 | Iodine |
| D5 W | Chloroform/ethanol | 0.38596 | Iodine |
| D6 W | Toluene/ethyl acetate | 0.78947 | Vanillin sulfuric acid ethanol |
| IL * | RT (min.) | [M + H]+ (m/z) | Molecular Formula | Error (ppm) | MS2 Score * | Identified Metabolite Name |
|---|---|---|---|---|---|---|
| 1 | 1.61 | (120.0803 fragment ion) | C9H11NO2 | −3.33 | - | Phenylalanine |
| 2 | 2.92 | 445.294 [M-2H2O + H]+ | C27H44NO7 | 1.79 | 0.72 | 20-hydroxyecdysone |
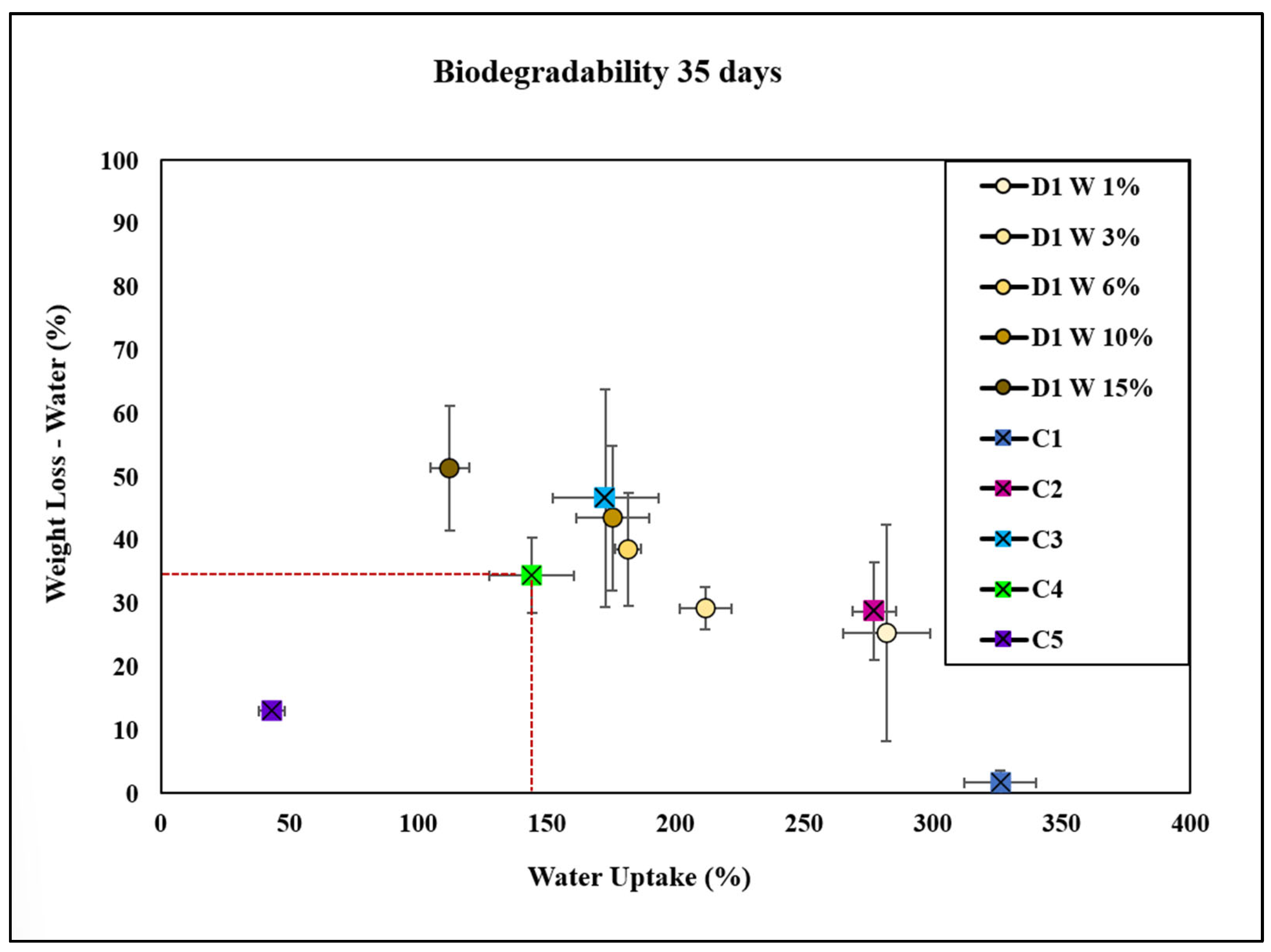
| Bioplastic Type | Composition | Degradation Rate (%) | Water Uptake (%) | Observations |
|---|---|---|---|---|
| C1 | Cellulose (negative control) | Lowest | Highest (325%) | Exhibits the least weight loss in both soil and water, indicating lower biodegradability. Shows the highest water uptake among all groups. |
| C2 | Cellulose + glycerol | Moderate | Moderate | Demonstrates a slower degradation rate compared with C3. Shows higher water uptake than D1 and D6 at 15 wt.%. |
| C3 | Cellulose + NaOH | Moderate | Moderate | Slightly faster degradation than C2. Exhibits higher water uptake compared with C4 but lower than C1. |
| C4 | Cellulose + NaOH + glycerol (commercial control) | Faster | Lower (150%) | Exhibits a faster degradation rate compared with C1, C2, and C3. Lower water uptake compared with C1 but higher than C5. |
| C5 | Bioplastic of avocado | Lower | Lowest | Shows the lowest weight loss among all controls and D1 concentrations, indicating low biodegradability. Water uptake is lower compared with other groups. |
| D1 W 1% | Plant extract D1 W (1 wt.%) | Lower | Higher | At low concentrations, it exhibits reduced biodegradability and higher water uptake compared with D1 W 15%. |
| D1 W 3% | Plant extract D1 W (3 wt.%) | Lower | Higher | Similar to D1 W 1%, with moderate water uptake and degradation rates. Differences in weight loss compared with D1 W 6% and 10% were not consistently significant. |
| D1 W 6% | Plant extract D1 W (6 wt.%) | Higher | Moderate | Higher degradation rate compared with D1 W 1% and 3%. Water uptake is moderate, significantly higher than C1. |
| D1 W 10% | Plant extract D1 W (10 wt.%) | Higher | Moderate | Has a degradation rate similar to D1 W 6%, with higher weight loss compared with C1, C2, and C5. Moderate water uptake. |
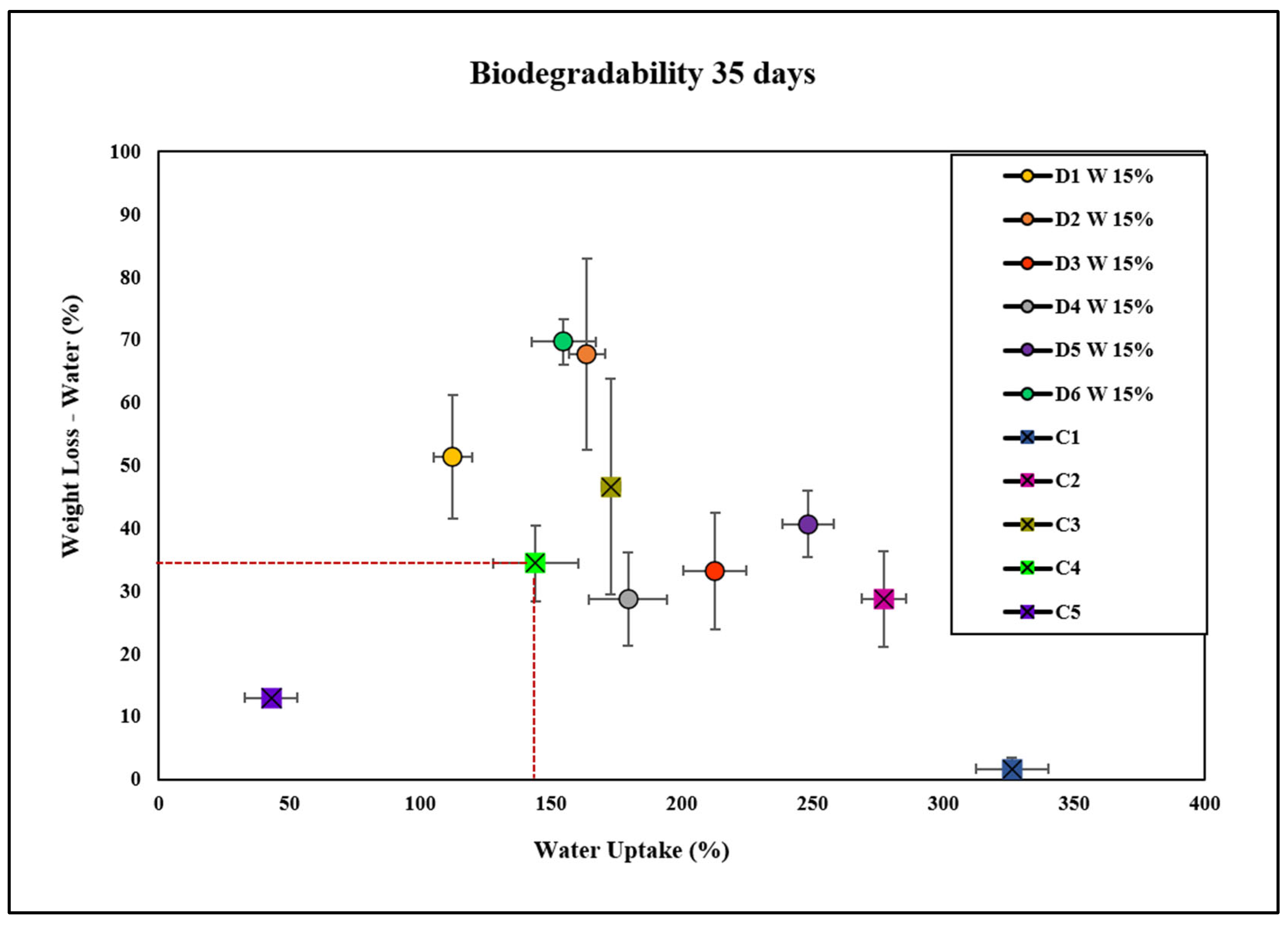
| D1 W 15% | Plant extract D1 W (15 wt.%) | Highest | Lower | Exhibits the highest degradation rates in both soil and water, especially compared with C1. Lower water uptake than C1 and D1 W 1%. |
| D2 W 15% | Plant extract D2 W (15 wt.%) | High | Moderate | High degradation rates in both soil and water. Water uptake is higher than D1 W 15%, D4, D5, D6, and C4, but lower than C1. |
| D3 W 15% | Plant extract D3 W (15 wt.%) | Moderate | High | Exhibits relatively lower weight loss but higher water uptake compared with C4. High water uptake indicates enhanced water absorption capacity without necessarily increasing degradation. |
| D4 W 15% | Plant extract D4 W (15 wt.%) | Lower | High | Lower degradation rates but higher water uptake than C4, suggesting components in D4 W may inhibit degradation while promoting water absorption. |
| D5 W 15% | Plant extract D5 W (15 wt.%) | Variable | High | Show slower degradation in soil but accelerated in water compared with C4. It indicates that D5 W effect is environment dependent. High water uptake but does not necessarily increase degradation. |
| D6 W 15% | Plant extract D6 W (15 wt.%) | High | Moderate | High degradation rates similar to D1 W 15% and D2 W 15%. Water uptake is higher than C4 but lower than D3 W 15%. |
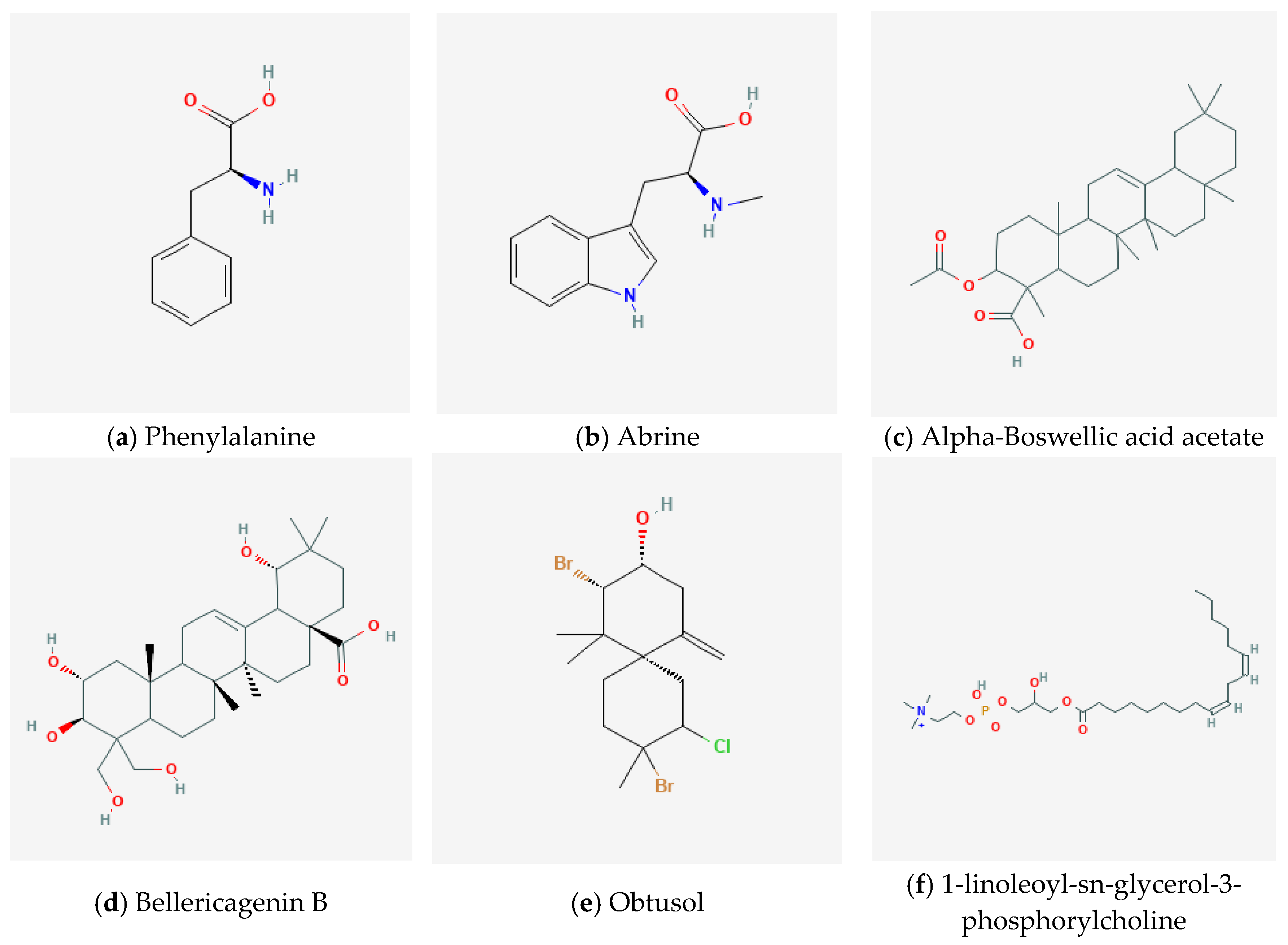
| Code | Compound Name | Chemical Class | Molecular Weight (g/mol) | OH Groups | Predicted pKa | Source |
|---|---|---|---|---|---|---|
| D1 | Phenylalanine | Amino acids and peptides | 165.192 | 0 | 9.48 | Quinoa |
| D2 | Abrine | Amino acids and peptides | 218.256 | 0 | 9.34 | Guayaba |
| D3 | Alpha-Boswellic acid acetate | Terpenoids | 498.748 | 0 | 9.06 | Palo santo |
| D4 | Bellericagenin B | Terpenoids | 520.707 | 5 | 9.02 | Ivory Coast almond |
| D5 | Obtusol | Terpenoids | 442.728 | 2 | 9.01 | Cacalosuchil |
| D6 | 1-linoleoyl-sn-glycerol-3-phosphorylcholine | Aliphatic natural product | 426.729 | 1 | 9.11 | Soya |
| Source | Amount Biomass (g) | Water (mL) | Time |
|---|---|---|---|
| Quinoa | 200 | 1000 | 1 day |
| Guayaba | 280 | 350 | 1 day |
| Palo santo | 125 | 500 | 7 days |
| Ivory Coast almond | 50 | 500 | 7 days |
| Cacalosuchil | 50 | 500 | 7 days |
| Soya | 200 | 1000 | 1 day |
| Code | Natural Extract | Hemp Cellulose (g) | NaOH (g) | Glycerol (g) | Water (mL) |
|---|---|---|---|---|---|
| D1 W, D2 W, D3 W, D4 W, D5 W, D6 W | 1% (0.1 g), 3% (0.3 g), 6% (0.6 g), 10% (1.0 g), 15% (1.5 g) | 1 | - | 1 | 10 |
| C1 | - | 1 | - | - | 10 |
| C2 | - | 1 | - | 1 | 10 |
| C3 | - | 1 | 0.6 | - | 10 |
| C4 | - | 1 | 0.6 | 1 | 10 |
| Crude Extracts | Mobile Phase | Secondary Metabolite | Solvent System |
|---|---|---|---|
| D1 W | n-butanol/acetic acid/water | Amino acid | 3:3:1 |
| D2 W | n-butanol/acetic acid/water | Amino acid | 3:3:1 |
| D3 W | Toluene/ethyl acetate | Terpenoids | 9.3:0.7 |
| D4 W | Hexane/acetone | Terpenoids | 2:1 |
| D5 W | Chloroform/ethanol | Terpenoids | 10:1 |
| D6 W | Toluene/ethyl acetate | Aliphatic natural product | 9.3:0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamora-Mendoza, L.; Caicho, J.; Mora, J.R.; Negrete-Bolagay, D.; Guerrero, V.H.; Mogollón, N.G.S.; Ochoa-Ocampo, M.; Pastuña-Fasso, J.; Álvarez Barreto, J.F.; Ponce, S.; et al. Bioplastic Production Using Natural Extracts with Cellulose Assisted by Experimental and Computational Screening. Molecules 2025, 30, 2752. https://doi.org/10.3390/molecules30132752
Zamora-Mendoza L, Caicho J, Mora JR, Negrete-Bolagay D, Guerrero VH, Mogollón NGS, Ochoa-Ocampo M, Pastuña-Fasso J, Álvarez Barreto JF, Ponce S, et al. Bioplastic Production Using Natural Extracts with Cellulose Assisted by Experimental and Computational Screening. Molecules. 2025; 30(13):2752. https://doi.org/10.3390/molecules30132752
Chicago/Turabian StyleZamora-Mendoza, Lizbeth, Jhonny Caicho, José R. Mora, Daniela Negrete-Bolagay, Victor H. Guerrero, Noroska G. S. Mogollón, Melanie Ochoa-Ocampo, Jefferson Pastuña-Fasso, José F. Álvarez Barreto, Sebastián Ponce, and et al. 2025. "Bioplastic Production Using Natural Extracts with Cellulose Assisted by Experimental and Computational Screening" Molecules 30, no. 13: 2752. https://doi.org/10.3390/molecules30132752
APA StyleZamora-Mendoza, L., Caicho, J., Mora, J. R., Negrete-Bolagay, D., Guerrero, V. H., Mogollón, N. G. S., Ochoa-Ocampo, M., Pastuña-Fasso, J., Álvarez Barreto, J. F., Ponce, S., Paredes, J., Erazo, H., Pontón, P. I., León, M., & Alexis, F. (2025). Bioplastic Production Using Natural Extracts with Cellulose Assisted by Experimental and Computational Screening. Molecules, 30(13), 2752. https://doi.org/10.3390/molecules30132752










