Influences of Ar Flow-Rate and Sublimation Temperature on the Sublimation Product of Analytical Reagent MoO3
Abstract
1. Introduction
2. Results
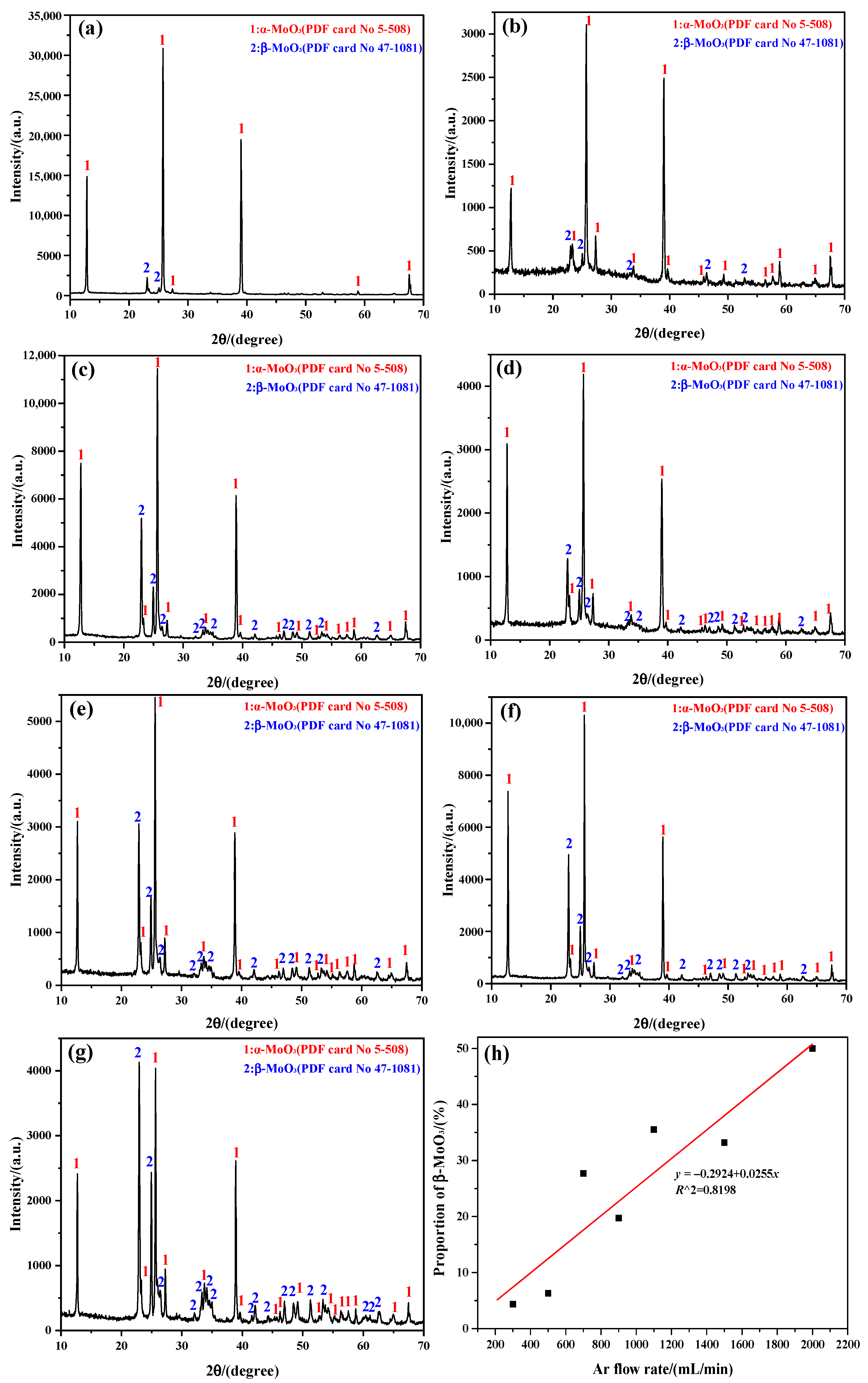
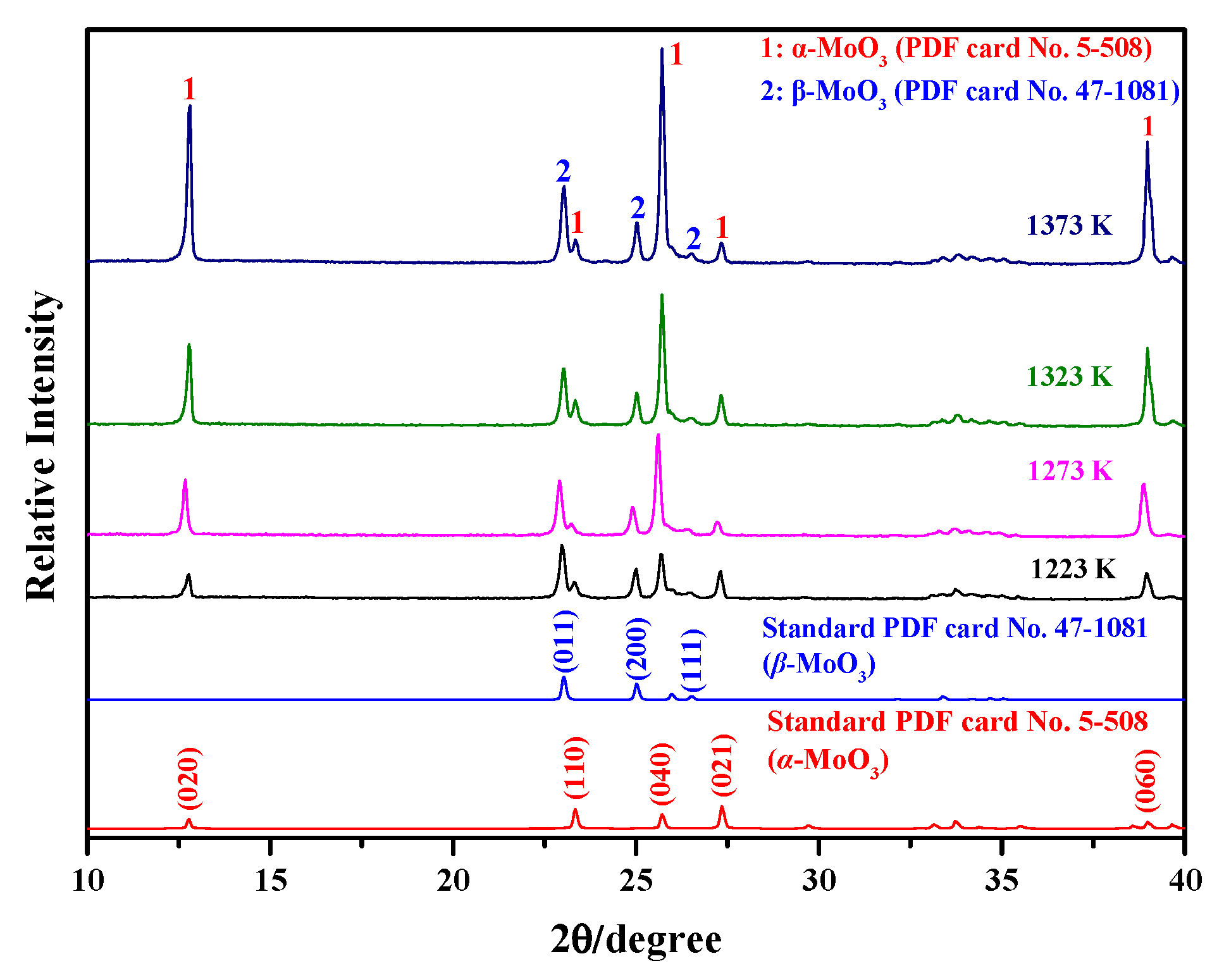
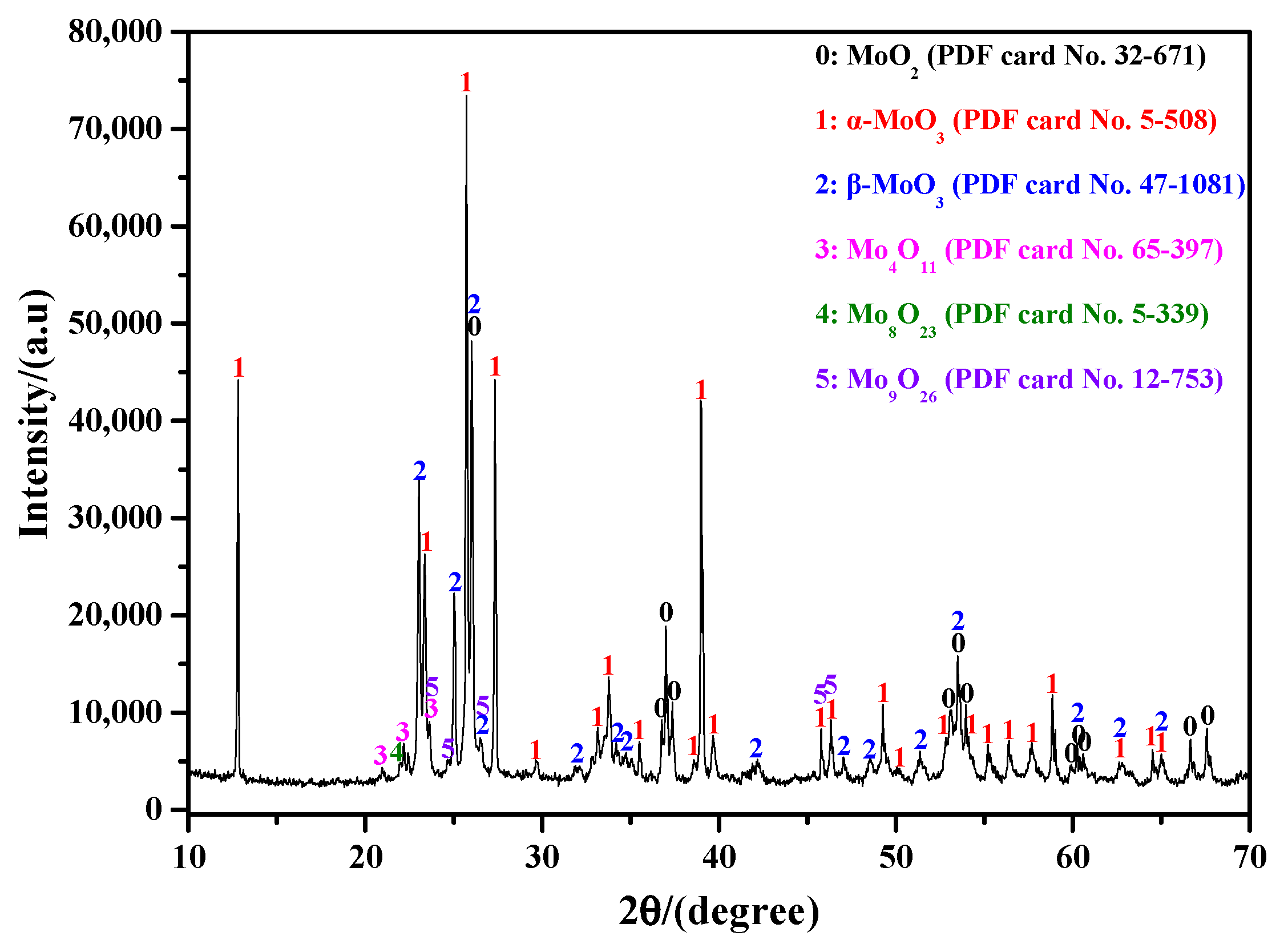
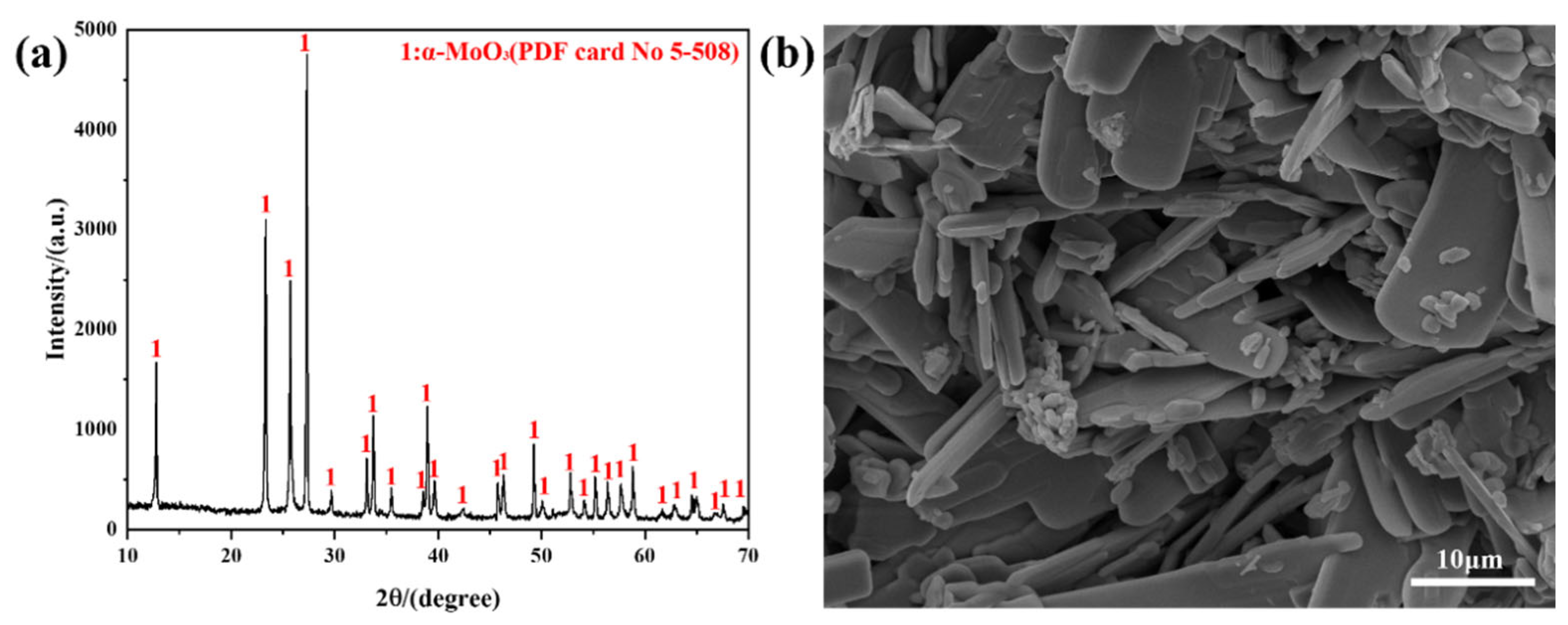
2.1. XRD for Phase Analysis
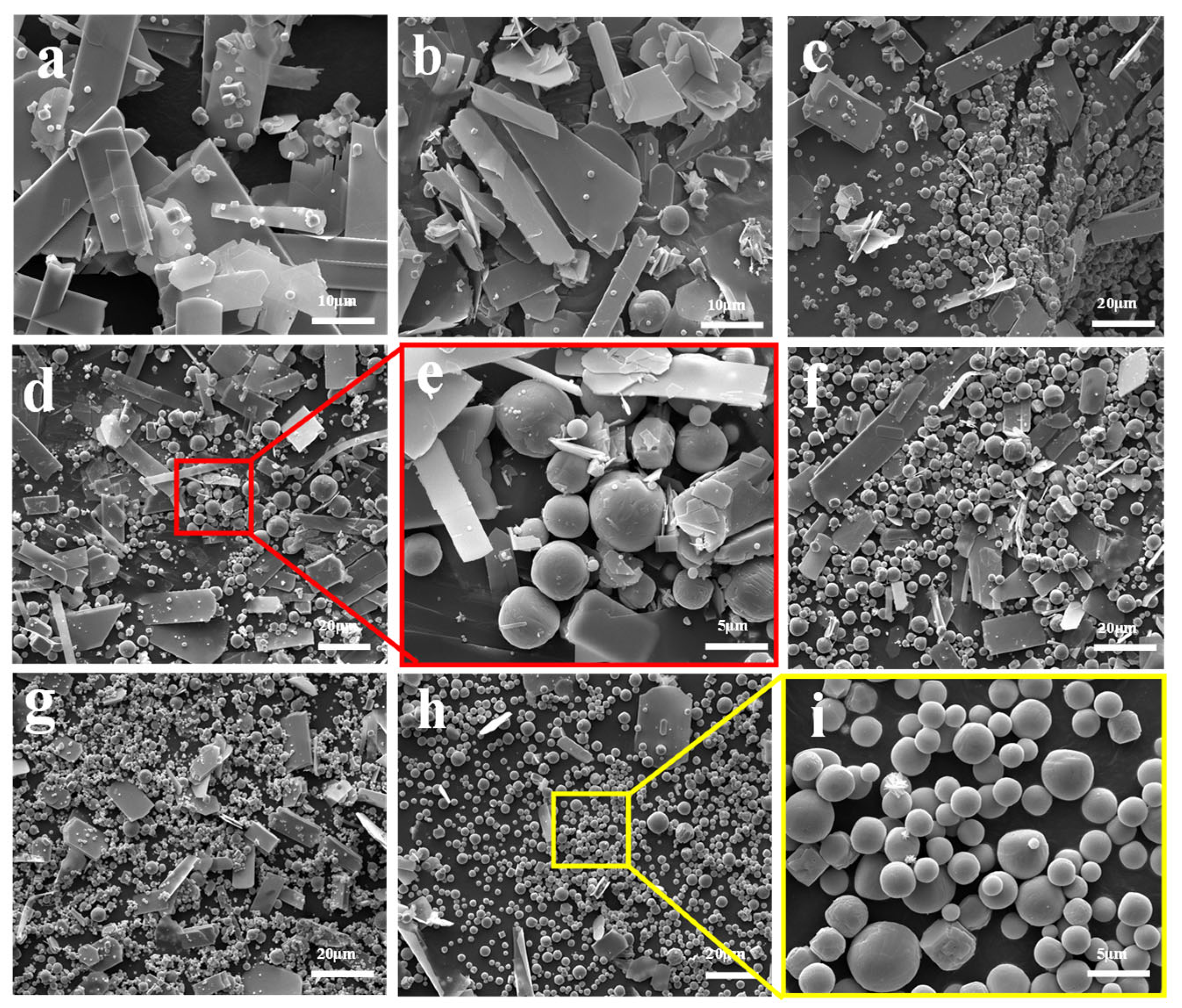
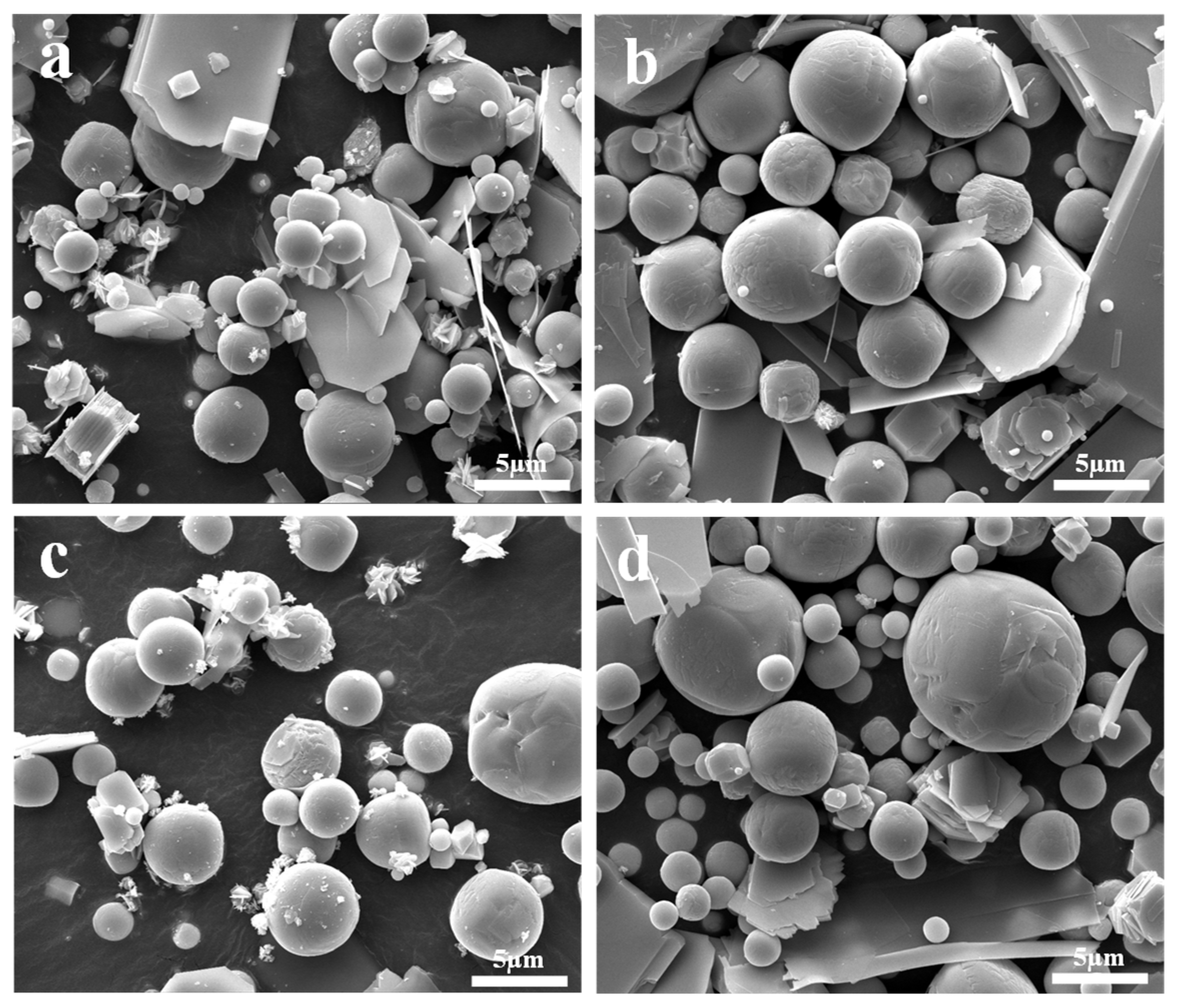
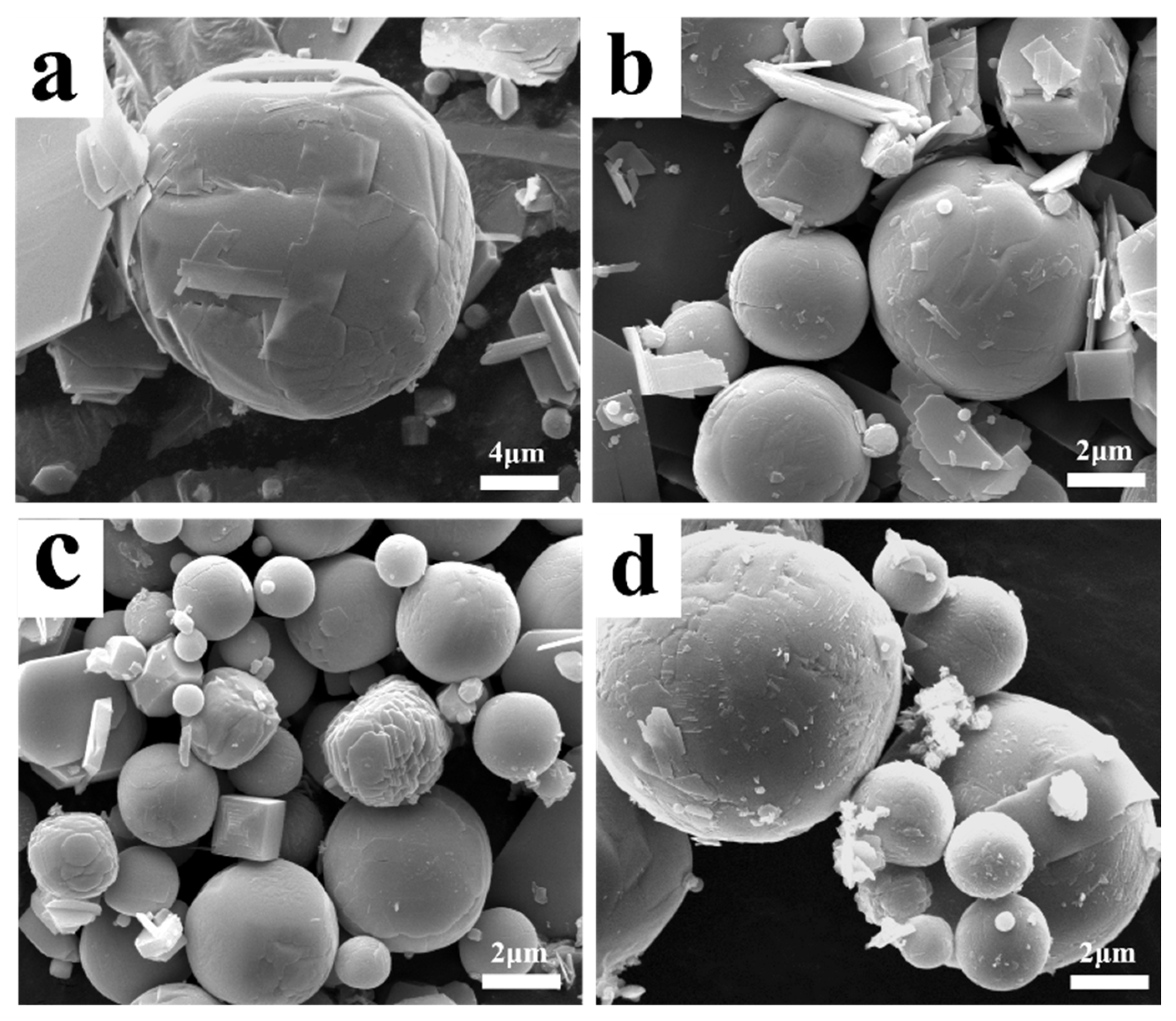
2.2. FESEM for Morphology Observation
3. Discussion
4. Materials and Experimental Procedures
4.1. Materials
4.2. Experimental Procedures
4.3. Product Characterization
5. Conclusions
- (1)
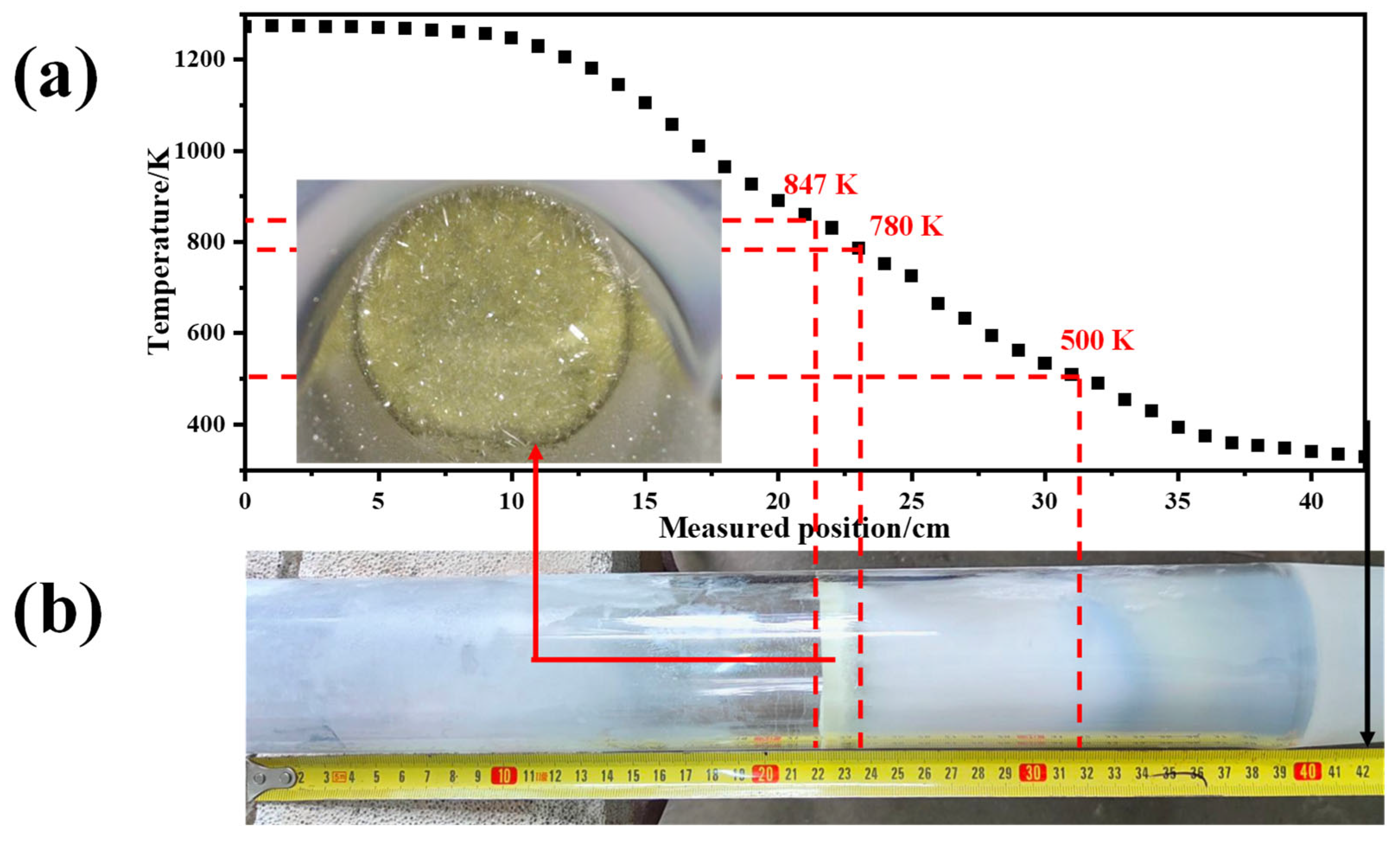
- The sublimation products obtained under different Ar flow-rates and sublimation temperatures are always composed of both α-MoO3 and β-MoO3, among which the proportion of β-MoO3 gradually increases with the increase in Ar flow-rate and the decrease in sublimation temperature.
- (2)
- Platelet-shaped α-MoO3 is usually generated in the range of 780 K to 847 K, while spherical-shaped β-MoO3 forms below 500 K. The diameter of β-MoO3 is determined to be in the range of 1–5 μm and has no obvious relationship with the Ar flow-rate due to its low value and narrow range.
- (3)
- Due to the co-actions of the deposition of gaseous MoO3 molecules, the adsorption of Ar molecules, and the particle collision between different particles, the newly formed β-MoO3 particles usually exhibit a spherical-shaped morphology in order to decrease their surface free energy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Liu, X.J.; Wang, R.L.; Mi, R.; Li, S.M.; Cui, Y.H.; Deng, Y.F.; Mei, J.; Liu, H. Coating of α-MoO3 on nitrogen-doped carbon nanotubes by electrodeposition as a high-performance cathode material for lithium-ion batteries. J. Power Sources 2015, 274, 1063–1069. [Google Scholar] [CrossRef]
- Wang, J.Y.; Shi, J.Y.; Shi, B.; Zhang, Y.H. Structural and Electrochemical Investigation of Zinc-Doped Lithiated MoO3 Cathode Materials for Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2020, 15, 9824–9837. [Google Scholar] [CrossRef]
- Chithambararaj, A.; Bose, A.C. Investigation on structural, thermal, optical and sensing properties of meta-stable hexagonal MoO3 nanocrystals of one dimensional structure. Beilstein J. Nanotechnol. 2011, 2, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Li, Z.X.; Zhang, T.; Chen, R.Z.; Zheng, X.Y.; Zhang, G.K. Morphology-dependent catalytic activity of plasmonic MoO3-x for hydrolytic dehydrogenation of ammonia borane. Funct. Mater. Lett. 2017, 10, 1750079. [Google Scholar] [CrossRef]
- Yang, W.M.; Cao, L.L.; Lu, H.J.; Huang, Y.; Yang, W.Q.; Cai, Y.Z.; Li, S.M.; Li, S.Q.; Zhao, J.W.; Xu, W.Z. Custom-printed microfluidic chips using simultaneous ratiometric fluorescence with “Green” carbon dots for detection of multiple antibiotic residues in pork and water samples. J. Food. Sci. 2024, 89, 5980–5992. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, W.T.; Hu, X.T.; Zhang, X.A.; Huang, X.W.; Li, Z.H.; Li, M.Y.; Zou, X.B.; Shi, J.Y. Efficient preparation of dual-emission ratiometric fluorescence sensor system based on aptamer-composite and detection of bis (2-ethylhexyl) phthalate in pork. Food Chem. 2021, 352, 129352. [Google Scholar] [CrossRef]
- Huang, Y.T.; Fan, L.Y.; Liu, Y.F.; Zheng, X.H. High-performance MoO3 nanosheets gas sensors for triethylamine detection: A rapid approach for assessing fish freshness. Ceram. Int. 2025, 51, 9912–9922. [Google Scholar] [CrossRef]
- Sakaray, M.; Chidurala, S.C. Optimization of ternary composite @PANI/MoO3/h-BN and its electrochemical evaluation as an electrode material for supercapacitor application. Synth. Met. 2023, 298, 117448. [Google Scholar] [CrossRef]
- Shakir, I.; Shahid, M.; Cherevko, S. Ultrahigh-energy and stable supercapacitors based on intertwined porous MoO3-MWCNT nanocomposites. Electrochim. Acta. 2011, 58, 76–80. [Google Scholar] [CrossRef]
- Shibata, K.; Tsuchida, K.; Kato, A. Preparation of ultrafine molybdenum powder by vapour phase reaction of the MoO3-H2 system. J. Less Common Met. 1990, 157, L5–L10. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.B.; Zhang, G.H.; Chou, K.C. A novel method for preparing ultrafine molybdenum powder. Int. J. Refract. Met. Hard Mater. 2021, 96, 105491. [Google Scholar] [CrossRef]
- Sun, G.D.; Zhang, G.H.; Yan, B.J.; Chou, K.C. Study on the reduction of commercial MoO3 with carbon black to prepare MoO2 and Mo2C nanoparticles. Int. J. Appl. Ceram Technol. 2020, 17, 917–931. [Google Scholar] [CrossRef]
- Liang, J.K.; Li, H.X.; Chen, L.; Ren, M.N.; Fakayode, O.A.; Han, J.Y.; Zhou, C.S. Efficient hydrogen evolution reaction performance using lignin-assisted chestnut shell carbon-loaded molybdenum disulfide. Ind. Crop. Prod. 2023, 193, 116214. [Google Scholar] [CrossRef]
- Ji, Q.H.; Yu, X.J.; Chen, L.; Yarley, O.P.N.; Zhou, C.S. Facile preparation of sugarcane bagasse-derived carbon supported MoS2 nanosheets for hydrogen evolution reaction. Ind. Crop. Prod. 2021, 172, 114064. [Google Scholar] [CrossRef]
- Liang, N.N.; Hu, X.T.; Li, W.T.; Wang, Y.Y.; Guo, Z.A.; Huang, X.W.; Li, Z.H.; Zhang, X.A.; Zhang, J.K.; Xiao, J.B.; et al. A dual-signal fluorescent sensor based on MoS2 and CdTe quantum dots for tetracycline detection in milk. Food Chem. 2022, 378, 132076. [Google Scholar] [CrossRef]
- Feng, Y.S.; Chen, F.P.; Li, S.B.; Li, X.B.; Li, G.P.; Hong, Z.Y.; Chen, Y. Microstructure of molybdenum target with different rolling deformations and annealing processes. Copper Eng. 2024, 186, 66–72. [Google Scholar]
- Guo, Z.H.; Gong, Y.B. The infuence factors analysis of molybdenum concentrate oxidizing roasting. Copper Eng. 2015, 134, 67–70. [Google Scholar]
- Mizushima, T.; Phuc, N.H.H.; Moriya, Y. Soft chemical transformation of alpha-MoO3 to beta-MoO3 as a catalyst for vapor-phase oxidation of methanol. Catal. Commun. 2011, 13, 10–13. [Google Scholar] [CrossRef]
- Yamamoto, K.; Shimizu, R.; Shiraki, S.; Hitosugi, T. Transparent conducting properties of Re-doped β-MoO3 films. APL Mater. 2016, 4, 096104. [Google Scholar] [CrossRef]
- Chu, N.M.; Hieu, N.D.; Do, T.M.D.; Nakayama, T.; Niihara, K.; Suematsu, H. Synthesis of β-MoO3 nanowhiskers from core/shell molybdenum/molybdenum oxide wire by pulsed wire discharge. Int. J. Appl. Ceram. Technol. 2021, 18, 889–901. [Google Scholar] [CrossRef]
- Chu, N.M.; Hieu, N.D.; Do, T.M.D.; Nakayama, T.; Niihara, K.; Suematsu, H. Synthesis of metastable monoclinic beta molybdenum trioxide nanoparticles by pulsed wire discharge. Jpn. J. Appl. Phys. 2020, 59, SCCC02. [Google Scholar] [CrossRef]
- McCarron, E.M. β-MoO3: A metastable analogue of WO3. J. Chem. Soc. Chem. Commun. 1986, 1, 336–338. [Google Scholar] [CrossRef]
- Maiti, P.; Guha, P.; Singh, R.; Dash, J.K.; Satyam, P.V. Optical band gap, local work function and field emission properties of MBE grown β-MoO3 nanoribbons. Appl. Surf. Sci. 2019, 476, 691–700. [Google Scholar] [CrossRef]
- Pham, T.T.P.; Nguyen, P.H.D.; Vo, T.T.; Luu, C.L.; Nguyen, H.H.P. Preparation of NO-doped β-MoO3 and its methanol oxidation property. Mater. Chem. Phys. 2016, 184, 5–11. [Google Scholar] [CrossRef]
- Sun, H.; Li, G.H.; Yu, J.J.; Luo, J.; Rao, M.J.; Peng, Z.W.; Zhang, Y.B.; Jiang, T. Preparation of high purity MoO3 through volatilization of technical-grade Mo calcine in water vapor atmosphere. Int. J. Refract. Met. Hard Mater. 2018, 77, 1–7. [Google Scholar] [CrossRef]
- Ngo, C.M.; Nguyen, H.D.; Saito, N.; Do, T.M.D.; Nakayama, T.; Niihara, K.; Suematsu, H. Synthesis of β-MoO3 whiskers by the thermal evaporation method with flowing oxygen gas. J. Am. Ceram. Soc. 2022, 105, 1622–1628. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.H. Modified monoclinic metastable β-MoO3 submicrospheres: Controllable preparation, phase identification, and their formation mechanisms. Ceram. Int. 2023, 49, 18756–18769. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.H.; Chou, K.C. Synthesis of nanocrystalline molybdenum powder by hydrogen reduction of industrial grade MoO3. Int. J. Refract. Met. Hard Mater. 2016, 59, 100–104. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Wang, L.; Xue, Z.L. Volatilization behavior of high-purity MoO3 in the range of 600 °C to 750 °C: Influences of water-vapor concentration and reaction temperature. Int. J. Refract. Met. Hard Mater. 2025, 132, 107264. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.Q.; Liu, X.; Cao, Y.L. Research progress on the preparation methods and fundamental principles of ultrafine Mo powder. Int. J. Refract. Met. Hard Mater. 2025, 132, 107281. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.C.; Xue, Z.L.; Zhang, G.H.; Huang, A. Sublimation behavior of industrial grade molybdenum trioxide. Trans. Indian. Inst. Met. 2021, 74, 1469–1477. [Google Scholar] [CrossRef]
- Berkowitz, J.; Inghram, M.G.; Chupka, W. Polymeric gaseous species in the sublimation of molybdenum trioxide. J. Chem. Phys. 1957, 26, 842–846. [Google Scholar] [CrossRef]
- Blackburn, P.E.; Hoch, M.; Johnston, H.L. The vaporization of molybdenum and tungsten oxides. J. Phys. Chem. 1958, 62, 769–773. [Google Scholar] [CrossRef]
- Ngo, C.M.; Hieu, N.D.; Do, T.M.D.; Nakayama, T.; Niihara, K.; Suematsu, H. Hydration process of β-MoO3 powder prepared by pulsed wire discharge method. Jpn. J. Appl. Phys. 2022, 61, SB1018. [Google Scholar] [CrossRef]
- Shan, X.; Wang, F.; Hu, K.; Wei, J.Q.; Lin, X.; Zhao, X.Y.; Zhou, B.Z.; Zhang, K.L. Recent advances in synthesis and memory computing of large-area α-MoO3. Acta Phys. Sin. 2021, 70, 187–199. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.C.; Zhang, G.H.; Xue, Z.L. Morphology evolution and quantitative analysis of β-MoO3 and α-MoO3. High Temp. Mater. Process. 2020, 39, 620–626. [Google Scholar] [CrossRef]
- Hoinkes, H. The physical interaction potential of gas atoms with single-crystal surfaces, determined from gas-surface diffraction experiments. Rev. Mod. Phys. 1980, 52, 933–970. [Google Scholar] [CrossRef]
- Voorhees, P.W. The theory of Ostwald ripening. J. Stat. Phys. 1985, 38, 231–252. [Google Scholar] [CrossRef]
- Mariotti, D.; Lindström, H.; Bose, A.C.; Ostrikov, K. Monoclinic β-MoO3 nanosheets produced by atmospheric microplasma: Application to lithium-ion batteries. Nanotechnology 2009, 19, 495302. [Google Scholar] [CrossRef]

| References | Preparation Processes | Images | Microstructures |
|---|---|---|---|
| Sun et al. [25] |  | The sample deposited at 973 K, presenting as thin strips, was pure α-MoO3, and that deposited at 473 K took the form of spherical β-MoO3 and thin-strips α-MoO3 | |
 | |||
| Ngo et al. [26] |  | Composed of large spherical particles with sizes of about 200 nm; some needle-shaped particles also exist | |
| Wang et al. [27] |  | The obtained product is composed of spherical β-MoO3 and platelet-like α-MoO3 particles | |
| Mariotti et al. [39] |  | The obtained β-MoO3 is a nanosheet with a thickness of 50–100 nm | |
| This work |  | The sublimation products include two different species: spherical β-MoO3 and platelet-shaped α-MoO3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, F.-J.; Yu, J.-J.; Liu, J.-G.; Wang, L. Influences of Ar Flow-Rate and Sublimation Temperature on the Sublimation Product of Analytical Reagent MoO3. Molecules 2025, 30, 2751. https://doi.org/10.3390/molecules30132751
Du F-J, Yu J-J, Liu J-G, Wang L. Influences of Ar Flow-Rate and Sublimation Temperature on the Sublimation Product of Analytical Reagent MoO3. Molecules. 2025; 30(13):2751. https://doi.org/10.3390/molecules30132751
Chicago/Turabian StyleDu, Feng-Jiao, Jian-Jun Yu, Jian-Gang Liu, and Lu Wang. 2025. "Influences of Ar Flow-Rate and Sublimation Temperature on the Sublimation Product of Analytical Reagent MoO3" Molecules 30, no. 13: 2751. https://doi.org/10.3390/molecules30132751
APA StyleDu, F.-J., Yu, J.-J., Liu, J.-G., & Wang, L. (2025). Influences of Ar Flow-Rate and Sublimation Temperature on the Sublimation Product of Analytical Reagent MoO3. Molecules, 30(13), 2751. https://doi.org/10.3390/molecules30132751






