Effect of Hottentotta judaicus Scorpion Venom on Nociceptive Response and Inflammatory Cytokines in Mice Using Experimental Hyperalgesia
Abstract
1. Introduction
2. Results
2.1. Establishing the Study Sublethal Dose Through Observed Behavioral Effects
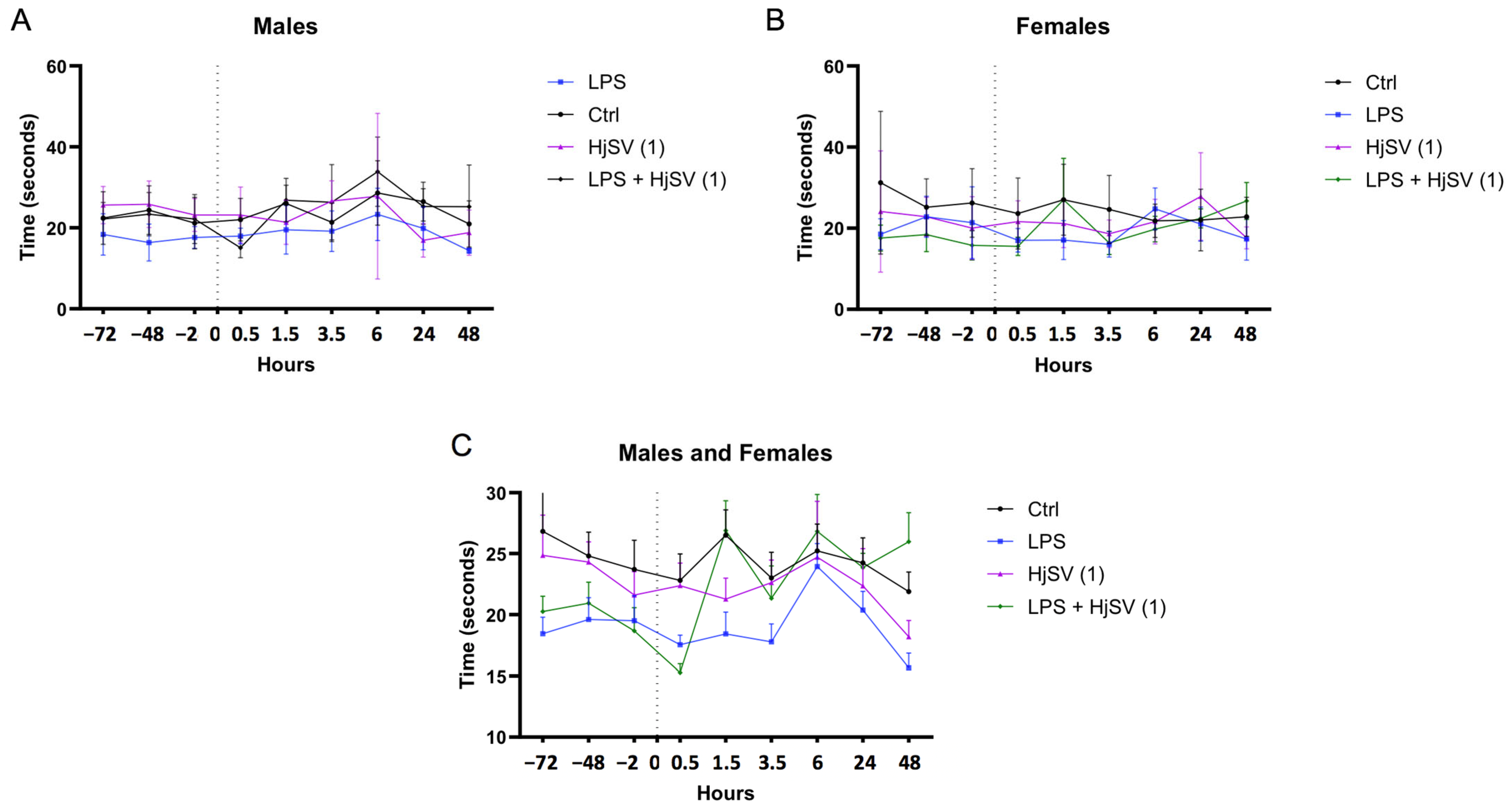
2.2. The Effect of HjSV on Pain Sensitivity in Hyperalgesia
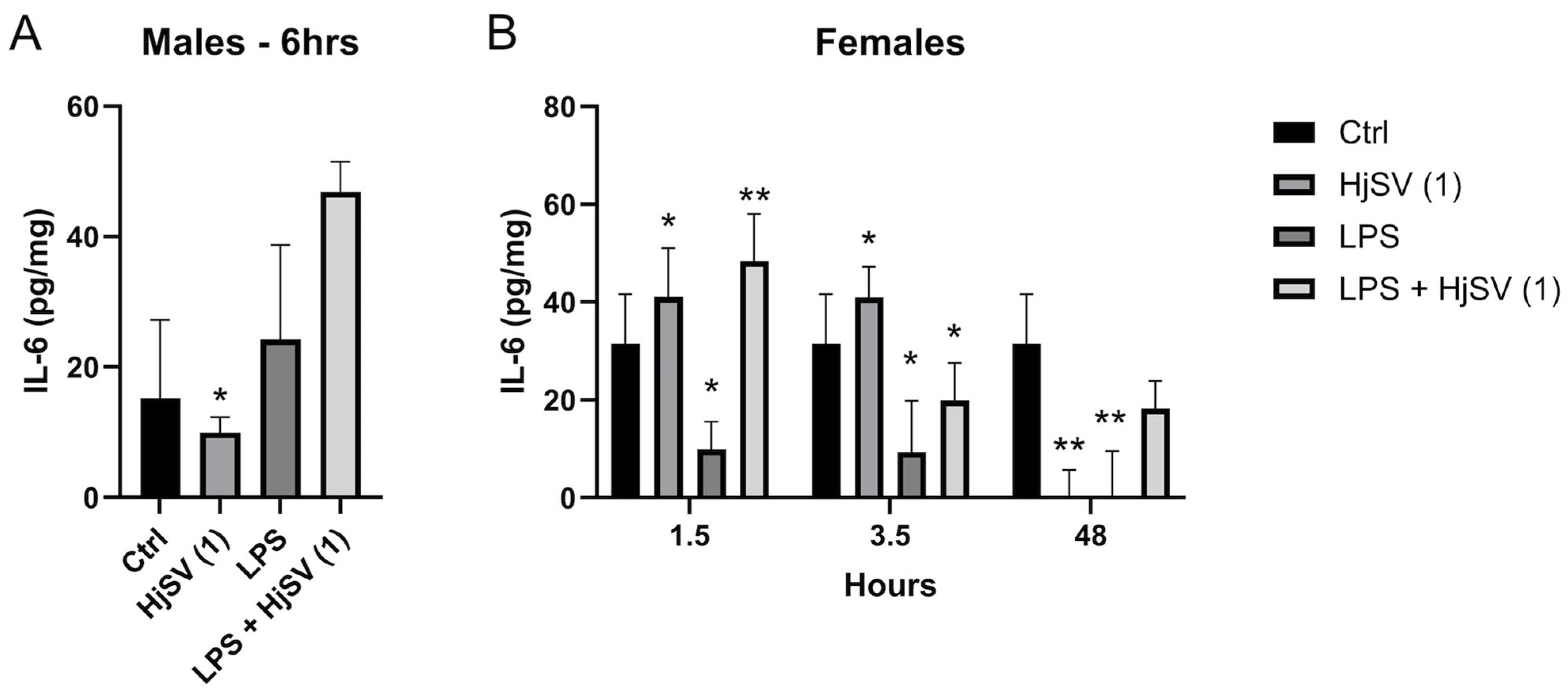
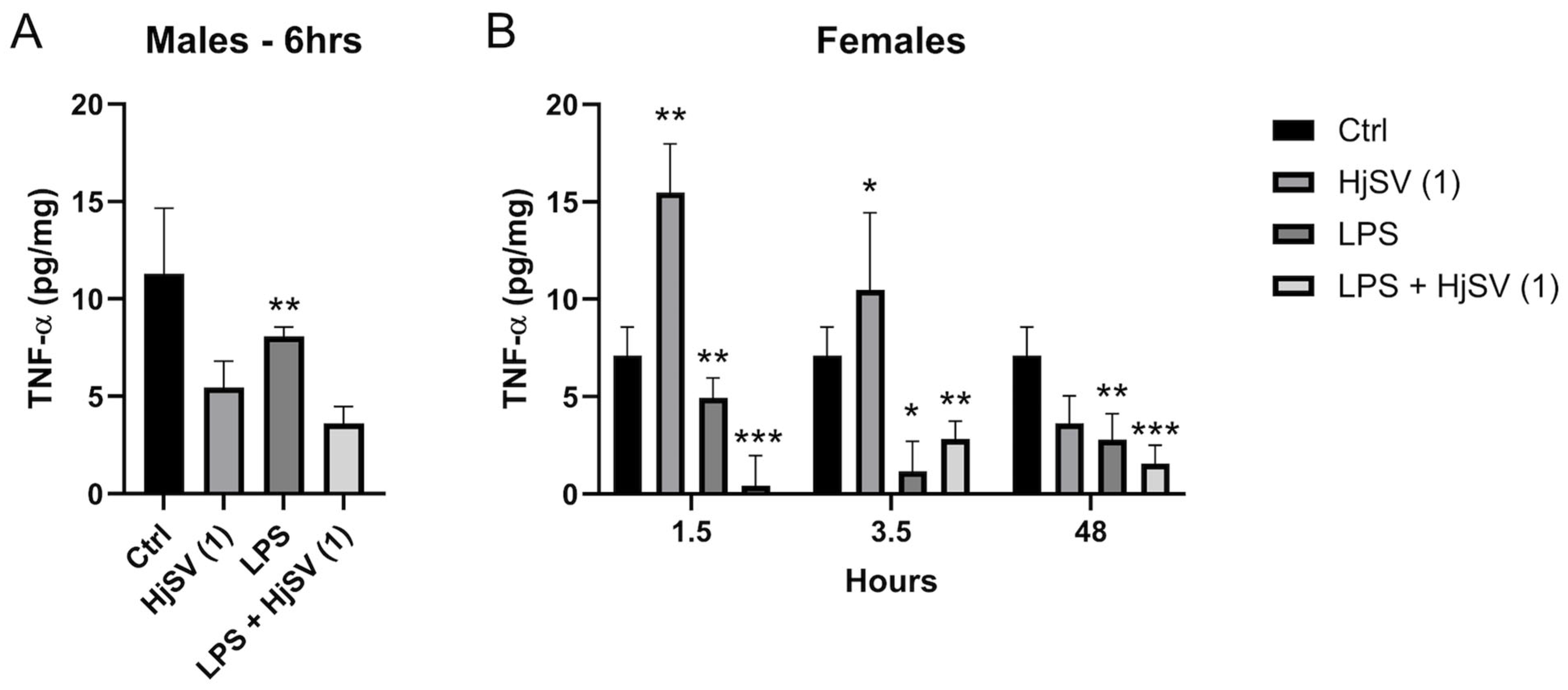
2.3. Effect of the HjSV on IL-4, IL-10, INF-γ, IL-6, and TNF-α in the Spleens of Different Groups Treated with LPS and/or HjSV (1)
3. Discussion
4. Materials and Methods
4.1. HjSV Preparation
4.2. Mice and Their Handling
4.3. Sublethal Dose Determination
4.4. Hot Plate Test
4.5. Injection of a New Batch of Mice for Dissection
4.6. Dissection and Organ Extraction
4.7. Homogenization of Organs
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HjSV | Hottentotta judaicus scorpion venom |
| LPS | Lipopolysaccharide |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| IL-4 | Interleukin 4 |
| IL-10 | Interleukin 10 |
| IL-6 | Interleukin 6 |
| IFN-γ | Interferon gamma |
| TNF-α | Tumor necrosis factor alpha |
| IL-17A | Interleukin 17A |
| TLR4 | Toll-like receptor type 4 |
| MyD88 | Myeloid differentiation primary response 88 |
| PBS | Phosphate-buffered saline |
| RhoA | Ras homolog gene family member A |
| ROCK | Rho-associated coiled-coil-containing protein kinase |
| NO | Nitric oxide |
References
- Ahmadi, S.; Knerr, J.M.; Argemi, L.; Bordon, K.C.F.; Pucca, M.B.; Cerni, F.A.; Arantes, E.C.; Çalışkan, F.; Laustsen, A.H. Scorpion Venom: Detriments and Benefits. Biomedicines 2020, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Bakır, F.; Ozkan, O.; Alcigir, M.E.; Yagmur, E.A. The Lethality, Histological, Haematological and Biochemical Alterations in Mice Envenomated with Aegaeobuthus Nigrocinctus Venom. Toxicon 2021, 200, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Petricevich, V.L. Scorpion Venom and the Inflammatory Response. Mediat. Inflamm. 2010, 2010, 903295. [Google Scholar] [CrossRef]
- Laraba-Djebari, F.; Adi-Bessalem, S.; Hammoudi-Triki, D. Scorpion Venoms: Pathogenesis and Biotherapies. In Scorpion Venoms; Gopalakrishnakone, P., Possani, L.D., Schwartz, E.F., Rodríguez de la Vega, R.C., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2020; pp. 1–21. ISBN 978-94-007-6647-1. [Google Scholar]
- Adi-Bessalem, S.; Hammoudi-Triki, D.; Laraba-Djebari, F. Scorpion Venom Interactions with the Immune System. In Scorpion Venoms; Gopalakrishnakone, P., Possani, L.D., Schwartz, E.F., Rodríguez de la Vega, R.C., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2015; pp. 87–107. ISBN 978-94-007-6647-1. [Google Scholar]
- Ait-Lounis, A.; Laraba-Djebari, F. TNF-α Involvement in Insulin Resistance Induced by Experimental Scorpion Envenomation. PLoS Negl. Trop. Dis. 2012, 6, e1740. [Google Scholar] [CrossRef] [PubMed]
- Mingatto, F.E.; Dorta, D.J.; dos Santos, A.B.; Carvalho, I.; da Silva, C.H.T.P.; da Silva, V.B.; Uyemura, S.A.; dos Santos, A.C.; Curti, C. Dehydromonocrotaline Inhibits Mitochondrial Complex I. A Potential Mechanism Accounting for Hepatotoxicity of Monocrotaline. Toxicon 2007, 50, 724–730. [Google Scholar] [CrossRef]
- Sofer, S. Scorpion Envenomation. Intensive Care Med. 1995, 21, 626–628. [Google Scholar] [CrossRef]
- Zoccal, K.F.; da Silva Bitencourt, C.; Secatto, A.; Sorgi, C.A.; Bordon, K.D.C.F.; Sampaio, S.V.; Arantes, E.C.; Faccioli, L.H. Tityus serrulatus Venom and Toxins Ts1, Ts2 and Ts6 Induce Macrophage Activation and Production of Immune Mediators. Toxicon 2011, 57, 1101–1108. [Google Scholar] [CrossRef]
- Rincon, M. Interleukin-6: From an Inflammatory Marker to a Target for Inflammatory Diseases. Trends Immunol. 2012, 33, 571–577. [Google Scholar] [CrossRef]
- Petricevich, V.L. Balance Between Pro- and Anti-Inflammatory Cytokines in Mice Treated with Centruroides Noxius Scorpion Venom. Mediat. Inflamm. 2006, 2006, 054273. [Google Scholar] [CrossRef]
- Petricevich, V.L.; Peña, C.F. The Dynamics of Cytokine d Nitric Oxide Secretion in Mice Injected with Tityus serrulatus Scorpion Venom. Mediat. Inflamm. 2002, 11, 369698. [Google Scholar] [CrossRef]
- Malaque, C.M.S.A.; de Bragança, A.C.; Sanches, T.R.; Volpini, R.A.; Shimizu, M.H.; Hiyane, M.I.; Câmara, N.O.S.; Seguro, A.C.; Andrade, L. The Role of Dexamethasone in Scorpion Venom-Induced Deregulation of Sodium and Water Transport in Rat Lungs. Intensive Care Med. Exp. 2015, 3, 28. [Google Scholar] [CrossRef]
- Casella-Martins, A.; Ayres, L.R.; Burin, S.M.; Morais, F.R.; Pereira, J.C.; Faccioli, L.H.; Sampaio, S.V.; Arantes, E.C.; Castro, F.A.; Pereira-Crott, L.S. Immunomodulatory Activity of Tityus serrulatus Scorpion Venom on Human T Lymphocytes. J. Venom. Anim. Toxins Trop. Dis. 2015, 21, 46. [Google Scholar] [CrossRef]
- Gunas, V.; Maievskyi, O.; Synelnyk, T.; Raksha, N.; Vovk, T.; Halenova, T.; Savchuk, O.; Gunas, I. Cytokines and Their Regulators in Rat Lung Following Scorpion Envenomation. Toxicon X 2024, 22, 100198. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, M.M.; Pereira, M.E.S.; Amaral, C.F.S.; Rezende, N.A.; Campolina, D.; Bucaretchi, F.; Gazzinelli, R.T.; Cunha-Melo, J.R. Serum Levels of Cytokines in Patients Envenomed by Tityus serrulatus Scorpion Sting. Toxicon 1999, 37, 1155–1164. [Google Scholar] [CrossRef]
- Petricevich, V.L.; Lebrun, I. Immunomodulatory Effects of the Tityus serrulatus Venom on Murine Macrophage Functions In Vitro. Mediators Inflamm. 2005, 2005, 275876. [Google Scholar] [CrossRef] [PubMed]
- Witzenrath, M.; Ahrens, B.; Schmeck, B.; Kube, S.M.; Hippenstiel, S.; Rosseau, S.; Hamelmann, E.; Suttorp, N.; Schütte, H. Rho-Kinase and Contractile Apparatus Proteins in Murine Airway Hyperresponsiveness. Exp. Toxicol. Pathol. 2008, 60, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Maduwage, K.; Isbister, G.K.; Silva, A.; Bowatta, S.; Mendis, S.; Gawarammana, I. Epidemiology and Clinical Effects of Hump-Nosed Pit Viper (Genus: Hypnale) Envenoming in Sri Lanka. Toxicon 2013, 61, 11–15. [Google Scholar] [CrossRef]
- Bekkari, N.; Martin-Eauclaire, M.-F.; Laraba-Djebari, F. Complement System and Immunological Mediators: Their Involvements in the Induced Inflammatory Process by Androctonus australis Hector Venom and Its Toxic Components. Exp. Toxicol. Pathol. 2015, 67, 389–397. [Google Scholar] [CrossRef]
- Vasconcelos, J.F.; Teixeira, M.M.; Barbosa-Filho, J.M.; Lúcio, A.S.S.C.; Almeida, J.R.G.S.; de Queiroz, L.P.; Ribeiro-dos-Santos, R.; Soares, M.B.P. The Triterpenoid Lupeol Attenuates Allergic Airway Inflammation in a Murine Model. Int. Immunopharmacol. 2008, 8, 1216–1221, Erratum in Int. Immunopharmacol. 2008, 8, 1714. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Gilhen, J.; Russell, R.W.; Krysko, K.L.; Melaun, C.; Kurz, A.; Kauferstein, S.; Kordis, D.; Mebs, D. Variability of Tetrodotoxin and of Its Analogues in the Red-Spotted Newt, Notophthalmus viridescens (Amphibia: Urodela: Salamandridae). Toxicon 2012, 59, 257–264. [Google Scholar] [CrossRef]
- Xia, Z.; He, D.; Wu, Y.; Kwok, H.F.; Cao, Z. Scorpion Venom Peptides: Molecular Diversity, Structural Characteristics, and Therapeutic Use from Channelopathies to Viral Infections and Cancers. Pharmacol. Res. 2023, 197, 106978. [Google Scholar] [CrossRef]
- Xin, K.; Sun, R.; Xiao, W.; Lu, W.; Sun, C.; Lou, J.; Xu, Y.; Chen, T.; Wu, D.; Gao, Y. Short Peptides from Asian Scorpions: Bioactive Molecules with Promising Therapeutic Potential. Toxins 2025, 17, 114. [Google Scholar] [CrossRef]
- Arnon, T.; Potikha, T.; Sher, D.; Elazar, M.; Mao, W.; Tal, T.; Bosmans, F.; Tytgat, J.; Ben-Arie, N.; Zlotkin, E. BjαIT: A Novel Scorpion α-Toxin Selective for Insects—Unique Pharmacological Tool. Insect Biochem. Mol. Biol. 2005, 35, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Grkovich, A.; Johnson, C.A.; Buczynski, M.W.; Dennis, E.A. Lipopolysaccharide-Induced Cyclooxygenase-2 Expression in Human U937 Macrophages Is Phosphatidic Acid Phosphohydrolase-1-Dependent*. J. Biol. Chem. 2006, 281, 32978–32987. [Google Scholar] [CrossRef]
- Campbell, J.N.; Meyer, R.A. Mechanisms of Neuropathic Pain. Neuron 2006, 52, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Gazo Hanna, E.; Younes, K.; Roufayel, R.; Khazaal, M.; Fajloun, Z. Engineering Innovations in Medicine and Biology: Revolutionizing Patient Care through Mechanical Solutions. Heliyon 2024, 10, e26154. [Google Scholar] [CrossRef]
- Tompkins, D.A.; Campbell, C.M. Opioid-Induced Hyperalgesia: Clinically Relevant or Extraneous Research Phenomenon? Curr. Pain Headache Rep. 2011, 15, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F. Production of Scorpion Antivenom. In Handbook of Natural Toxins: Insect Poisons, Allergens and Other Invertebrate Venoms; Tu, A.T., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1984; Volume 2, pp. 577–605. [Google Scholar]
- Calil, I.L.; Zarpelon, A.C.; Guerrero, A.T.G.; Alves-Filho, J.C.; Ferreira, S.H.; Cunha, F.Q.; Cunha, T.M.; Jr, W.A.V. Lipopolysaccharide Induces Inflammatory Hyperalgesia Triggering a TLR4/MyD88-Dependent Cytokine Cascade in the Mice Paw. PLoS ONE 2014, 9, e90013. [Google Scholar] [CrossRef]
- Nürnberger, F.; Ott, D.; Claßen, R.; Rummel, C.; Roth, J.; Leisengang, S. Systemic Lipopolysaccharide Challenge Induces Inflammatory Changes in Rat Dorsal Root Ganglia: An Ex Vivo Study. Int. J. Mol. Sci. 2022, 23, 13124. [Google Scholar] [CrossRef]
- Jain, A.; Hakim, S.; Woolf, C.J. Immune Drivers of Physiological and Pathological Pain. J. Exp. Med. 2024, 221, e20221687. [Google Scholar] [CrossRef]
- Raoof, R.; Willemen, H.L.D.M.; Eijkelkamp, N. Divergent Roles of Immune Cells and Their Mediators in Pain. Rheumatology 2018, 57, 429–440. [Google Scholar] [CrossRef]
- Tahtouh Zaatar, M.; Othman, R.; Abou Samra, E.; Karam, M. Exploring the Link between T-Regulatory Cells and Inflammatory Cytokines in Atherogenesis: Findings from Patients with Stable Angina Pectoris. Ann. Med. Surg. 2024, 86, 4456. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Song, S.; Zhang, Y.; Ge, Y.; Fang, X.; Huang, T.; Du, J.; Gao, J. Inhibition of the Rho/Rho Kinase Pathway Prevents Lipopolysaccharide-Induced Hyperalgesia and the Release of TNF-α and IL-1β in the Mouse Spinal Cord. Sci. Rep. 2015, 5, 14553. [Google Scholar] [CrossRef] [PubMed]
- Baral, P.; Udit, S.; Chiu, I.M. Pain and Immunity: Implications for Host Defence. Nat. Rev. Immunol. 2019, 19, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Zaatar, M.T.; Salman, S.; Hoblos, R.; Roufayel, R.; Fajloun, Z.; Sabatier, J.-M.; Karam, M. Blocking TNF-α Reduces Leishmania Major-Induced Hyperalgesia and Changes the Cytokine Profile in the Paw Skin of BALB/c Mice with a Potential Positive Effect on Parasite Clearance. Microbiol. Res. 2025, 16, 8. [Google Scholar] [CrossRef]
- Tobassum, S.; Tahir, H.M.; Arshad, M.; Zahid, M.T.; Ali, S.; Ahsan, M.M. Nature and applications of scorpion venom: An overview. Toxin Rev. 2018, 39, 214–225. [Google Scholar] [CrossRef]
- Diochot, S. Pain-Related Toxins in Scorpion and Spider Venoms: A Face to Face with Ion Channels. J. Venom. Anim. Toxins Trop. Dis. 2021, 27, e20210026. [Google Scholar] [CrossRef]
- Nasr, S.; Borges, A.; Sahyoun, C.; Nasr, R.; Roufayel, R.; Legros, C.; Sabatier, J.-M.; Fajloun, Z. Scorpion Venom as a Source of Antimicrobial Peptides: Overview of Biomolecule Separation, Analysis and Characterization Methods. Antibiotics 2023, 12, 1380. [Google Scholar] [CrossRef]
- Kania, B.F.; Wrońska, D.; Bracha, U. Pain, Pathophysiological Mechanisms, and New Therapeutic Options for Alternative Analgesic Agents in Sheep: A Review and Investigation. Animals 2021, 11, 909. [Google Scholar] [CrossRef]
- Di Maio, G.; Villano, I.; Ilardi, C.R.; Messina, A.; Monda, V.; Iodice, A.C.; Porro, C.; Panaro, M.A.; Chieffi, S.; Messina, G.; et al. Mechanisms of Transmission and Processing of Pain: A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 3064. [Google Scholar] [CrossRef]
- Cao, B.; Xu, Q.; Shi, Y.; Zhao, R.; Li, H.; Zheng, J.; Liu, F.; Wan, Y.; Wei, B. Pathology of Pain and Its Implications for Therapeutic Interventions. Signal Transduct. Target. Ther. 2024, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Asiri, Y.I.; Moni, S.S.; Ramar, M.; Chidambaram, K. Advancing Pain Understanding and Drug Discovery: Insights from Preclinical Models and Recent Research Findings. Pharmaceuticals 2024, 17, 1439. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The Pro- and Anti-Inflammatory Properties of the Cytokine Interleukin-6. Biochim. Biophys. Acta BBA Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.; Fayjaloun, S.; Roufayel, R.; El Obeid, D.; Fajloun, Z.; Rima, M.; Karam, M. Influence of Apis Mellifera Syriaca Bee Venom on Nociception and Inflammatory Cytokine Profiles in Experimental Hyperalgesia. Toxins 2025, 17, 18. [Google Scholar] [CrossRef]
- Mendoza, J.L.; Escalante, N.K.; Jude, K.M.; Sotolongo Bellon, J.; Su, L.; Horton, T.M.; Tsutsumi, N.; Berardinelli, S.J.; Haltiwanger, R.S.; Piehler, J.; et al. Structure of the IFNγ Receptor Complex Guides Design of Biased Agonists. Nature 2019, 567, 56–60. [Google Scholar] [CrossRef]
- Ferrara, V.; Toti, A.; Ghelardini, C.; Mannelli, L.D.C. Interferon-Gamma and Neuropathy: Balance between Pain and Neuroprotection. Neural Regen. Res. 2022, 17, 2700. [Google Scholar] [CrossRef] [PubMed]
- Aulock, S.V.; Deininger, S.; Draing, C.; Gueinzius, K.; Dehus, O.; Hermann, C. Gender Difference in Cytokine Secretion on Immune Stimulation with LPS and LTA. J. Interferon Cytokine Res. 2006, 26, 887–892. [Google Scholar] [CrossRef]
- Meneses, G.; Rosetti, M.; Espinosa, A.; Florentino, A.; Bautista, M.; Díaz, G.; Olvera, G.; Bárcena, B.; Fleury, A.; Adalid-Peralta, L.; et al. Recovery from an Acute Systemic and Central LPS-Inflammation Challenge Is Affected by Mouse Sex and Genetic Background. PLoS ONE 2018, 13, e0201375. [Google Scholar] [CrossRef]
- Wu, X.-F.; Li, C.; Yang, G.; Wang, Y.-Z.; Peng, Y.; Zhu, D.-D.; Sui, A.-R.; Wu, Q.; Li, Q.-F.; Wang, B.; et al. Scorpion Venom Heat-Resistant Peptide Attenuates Microglia Activation and Neuroinflammation. Front. Pharmacol. 2021, 12, 704715. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27. [Google Scholar] [CrossRef]
- Celik, M.Ö.; Labuz, D.; Keye, J.; Glauben, R.; Machelska, H. IL-4 Induces M2 Macrophages to Produce Sustained Analgesia via Opioids. JCI Insight 2020, 5, e133093. [Google Scholar] [CrossRef] [PubMed]
- Villalonga, N.; Ferrere, J.C.; Argiles, J.M.; Condom, E.; Felipe, A. Potassium Channels Are a New Target Field in Anticancer Drug Design. Recent Pat. Anticancer Drug Discov. 2007, 2, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Holaday, S.K.; Martin, B.M.; Fletcher, P.L.; Krishna, N.R. NMR Solution Structure of Butantoxin. Arch. Biochem. Biophys. 2000, 379, 18–27. [Google Scholar] [CrossRef]
- Nelson, M.; Barnes, K.B.; Davies, C.H.; Cote, C.K.; Meinig, J.M.; Biryukov, S.S.; Dyer, D.N.; Frick, O.; Heine, H.; Pfefferle, D.A.; et al. The BALB/c Mouse Model for the Evaluation of Therapies to Treat Infections with Aerosolized Burkholderia Pseudomallei. Antibiotics 2023, 12, 506. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haddad, L.; Chender, A.; Roufayel, R.; Accary, C.; Borges, A.; Sabatier, J.M.; Fajloun, Z.; Karam, M. Effect of Hottentotta judaicus Scorpion Venom on Nociceptive Response and Inflammatory Cytokines in Mice Using Experimental Hyperalgesia. Molecules 2025, 30, 2750. https://doi.org/10.3390/molecules30132750
Haddad L, Chender A, Roufayel R, Accary C, Borges A, Sabatier JM, Fajloun Z, Karam M. Effect of Hottentotta judaicus Scorpion Venom on Nociceptive Response and Inflammatory Cytokines in Mice Using Experimental Hyperalgesia. Molecules. 2025; 30(13):2750. https://doi.org/10.3390/molecules30132750
Chicago/Turabian StyleHaddad, Lara, Amira Chender, Rabih Roufayel, Claudine Accary, Adolfo Borges, Jean Marc Sabatier, Ziad Fajloun, and Marc Karam. 2025. "Effect of Hottentotta judaicus Scorpion Venom on Nociceptive Response and Inflammatory Cytokines in Mice Using Experimental Hyperalgesia" Molecules 30, no. 13: 2750. https://doi.org/10.3390/molecules30132750
APA StyleHaddad, L., Chender, A., Roufayel, R., Accary, C., Borges, A., Sabatier, J. M., Fajloun, Z., & Karam, M. (2025). Effect of Hottentotta judaicus Scorpion Venom on Nociceptive Response and Inflammatory Cytokines in Mice Using Experimental Hyperalgesia. Molecules, 30(13), 2750. https://doi.org/10.3390/molecules30132750






