Abstract
In this work, the influences of the Ar flow-rate and sublimation temperature on the phase composition and morphological structure of the sublimation products of analytical reagent MoO3 are investigated. The results show that the sublimation products are always composed of thermodynamically stable orthorhombic molybdenum trioxide (α-MoO3) and metastable monoclinic molybdenum trioxide (β-MoO3) under different reaction conditions, among which the proportion of β-MoO3 gradually increases with the increase in Ar flow-rate and the decrease in sublimation temperature. The formation temperature of α-MoO3 is mainly between 780 K and 847 K, with the particles exhibiting an obvious sheet-like morphology. This work also finds that β-MoO3 is mainly generated below 500 K; however, due to the co-actions of the deposition of gaseous MoO3 molecules, the adsorption of Ar molecules, and the collision effect between the different particles, the newly formed β-MoO3 is more inclined to take a spherical-shaped morphology in order to maintain its lowest energy state.
1. Introduction
Molybdenum trioxide (MoO3) possesses multiple excellent physicochemical properties; hence, it is widely used in numerous contexts, such as batteries [1,2], catalysts [3,4], sensors [5,6,7], and supercapacitors [8,9]. Additionally, as an important intermediate material, MoO3 is usually used to prepare ultrafine Mo powder [10,11], molybdenum carbide [12], and molybdenum disulfide [13,14,15]; thus, its study has attracted significant attention in recent years [16,17]. In general, MoO3 has four kinds of crystal types, and the different types have different crystal structures and physicochemical properties. It has been reported [18] that the metastable monoclinic molybdenum trioxide (β-MoO3) usually has a better catalytic efficiency and electrochemical performance than that of the thermodynamically stable orthorhombic molybdenum trioxide (α-MoO3). Therefore, the large-scale preparation of β-MoO3 has received increasing attention in recent decades [19,20,21].
β-MoO3 was first prepared by McCarron via a solution method [22]; since then, numerous preparation methods have been proposed [23,24]; however, the issues of high cost and route complexity still exist. The physical vapor deposition (PVD) method has the advantages of low cost and process simplicity and has thus gradually become a common method of preparing micro- and nano-sized powder materials. In view of this, the PVD method has also been used in the preparation of micro-/nano-sized β-MoO3 in previous studies [25,26,27], in which water vapor, oxygen, and air atmospheres were adopted, respectively.
As a common gas, Ar is widely used in various industrial fields; however, there have been few studies concerning the preparation of β-MoO3. The present study is intended to fill this gap; in this paper, the influences of the Ar flow-rate and sublimation temperature on the phase composition and morphological structure of the sublimation products are illustrated.
The chemical vapor deposition (CVD) reaction between gaseous MoO3 and H2 for the purpose of preparing ultrafine Mo powder had been reported in our previous work [28]. The results indicated that pure Mo powder can be obtained when the carrier gas (Ar) and reducing gas (H2) are 300 and 500 mL/min, respectively; however, under other conditions, ultrafine MoO2 powder can also be obtained. Herein, the formation of MoO2 was understood to result from the chemical vapor reaction. In our preliminary experiment, carried out before this work, the sublimation–condensation process of MoO3 under 1273 K and Ar atmosphere conditions was conducted, and the results indicated that MoO2 can also be generated under an extremely high Ar flow-rate. Therefore, in order to further clarify the formation mechanism of different ultrafine powders during the CVD process, the authors carried out the following study.
2. Results
2.1. XRD for Phase Analysis
The phase identification of the sublimation products obtained at 1273 K under different Ar flow-rates are mainly analyzed by “Search-Match 2.1.1.0” software, with the corresponding results shown in Figure 1. The results show that the sublimation products always consist of α-MoO3 (PDF card No. 5-508; space group: Pbnm; lattice parameter: a = 3.962 Å, b = 13.858 Å, and c = 3.697 Å) and β-MoO3 (PDF card No. 47-1081; space group: P21/c; lattice parameter: a = 7.118 Å, b = 5.366 Å, and c = 5.568 Å), with a sharp and strong diffraction peak, indicating that both have an excellent crystallinity. It can be observed that the peak intensity of β-MoO3 increases with the increasing Ar flow-rate, which suggests that the proportion of β-MoO3 in the mixed MoO3 crystals gradually increases. Herein, the proportion of β-MoO3 is obtained by calculating the ratio of the peak area of β-MoO3 to that of both α-MoO3 and β-MoO3 according to the XRD pattern, using the common “Origin” software for the calculation. To be more specific, when the Ar flow-rates are 300, 500, 700, 900, 1100, 1500, and 2000 mL/min, the proportion of β-MoO3 in the mixed MoO3 crystals is 4.35, 6.29, 27.69, 19.73, 35.53, 33.18, and 49.96%, respectively; that is, the proportion of β-MoO3 gradually increases with the increase in the Ar flow-rate, even if some deviations exist, as illustrated in Figure 1h. Because a partial overlap exists between the diffraction peaks of the two MoO3 crystals, the calculation results of their respective peak areas may not be very accurate; therefore, the relationship between the proportion of β-MoO3 and the Ar flow-rate does not seem to indicate a strong correlation. Even so, the calculated deviation parameter R2 is still up to 0.8198, and the fitting equation can be written as y = −0.2924 + 0.0255x, where y denotes the proportion of β-MoO3 in the mixed MoO3 crystals(%), and x presents the Ar flow-rate (mL/min).
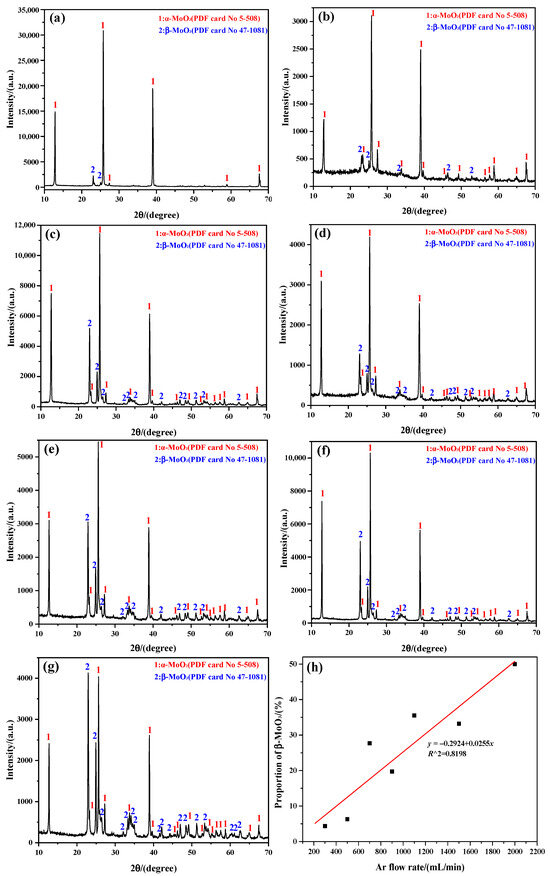
Figure 1.
Phase composition of the sublimation products obtained at 1273 K under different Ar flow-rates: (a) 300 mL/min; (b) 500 mL/min; (c) 700 mL/min; (d) 900 mL/min; (e) 1100 mL/min; (f) 1500 mL/min; (g) 2000 mL/min. (h) The proportion of β-MoO3 in the mixed MoO3 crystals.
When the Ar flow-rate is 1100 mL/min, the influence of the sublimation temperature on the phase composition of the sublimation products is also analyzed. Herein, in order to allow for the diffraction peaks to be seen more clearly, only scanning angles of 10–40° are selected. Meanwhile, the standard PDF card patterns for α-MoO3 (No. 5-508) and β-MoO3 (No. 47-1081) are also provided. The corresponding results are shown in Figure 2. The figure shows that the sublimation products contain both α-MoO3 and β-MoO3, similar to the cases observed under different Ar flow-rates. These results suggest that the change in sublimation temperature has no obvious influence on the phase composition. From Figure 2, it can also be deduced that the crystallinities of the two MoO3 species both gradually increase with the increase in sublimation temperature due to the increasing intensity of the strongest diffraction peak. However, the proportion of β-MoO3 within the sublimation product gradually decreases. In other words, the proportion of α-MoO3 gradually increases with the increase in the sublimation temperature; moreover, due to the relatively strong peaks of the crystal indices of (020), (040), and (060), the newly formed α-MoO3, with a certain anisotropy and preferred growth characteristics, can also be deduced.
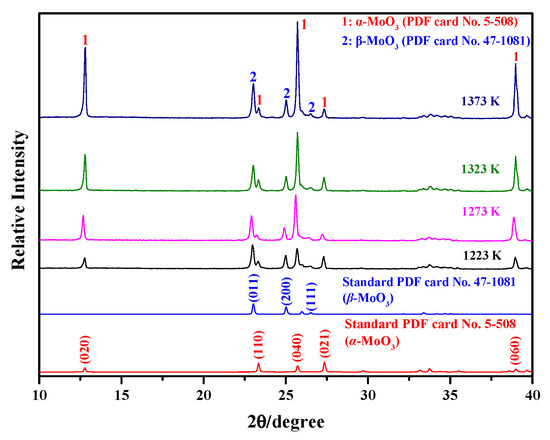
Figure 2.
Phase composition of the sublimation products obtained under different sublimation temperatures (Ar flow-rate: 1100 mL/min).
2.2. FESEM for Morphology Observation
Figure 3 illustrates the morphological structure of the sublimation products obtained at 1273 K under different Ar flow-rates. It shows that the sublimation products mainly include two different morphologies—one is spherical, and the other is platelet-shaped. The results also show that the amount of the spherical particles gradually increases, while that of the platelet-shaped particles gradually decreases with the increase in Ar flow-rate. Combining the results shown in Figure 1 and those in the literature [27], it can be inferred that the spherical particle is β-MoO3 and the platelet-shaped particle is α-MoO3. The diameter of the spherical β-MoO3 particle is also measured, and the results indicate that it is in the range of 1–5 μm, regardless of the Ar flow-rate. In our previous work [27], the diameter of the spherical β-MoO3 was found to gradually decrease with the increase in the pumping speed of the vacuum pump, with the average diameter being about 0.25 μm. The deviation may result from the difference in the flow-rate of the gaseous molecules. In our previous work, the flow-rate of the gaseous molecules was as high as 40 L/min; while in the current work, the Ar flow-rate is only in the range of 300–2000 mL/min. Due to the small and narrow range of the Ar flow-rate, the particle diameter of the spherical β-MoO3 prepared in this work seems to be unchanged. Combining the previous and current results, this work suggests that the reasonable regulation of the Ar flow-rate during the sublimation process is important for controlling the particle dimension of the spherical β-MoO3 powder.
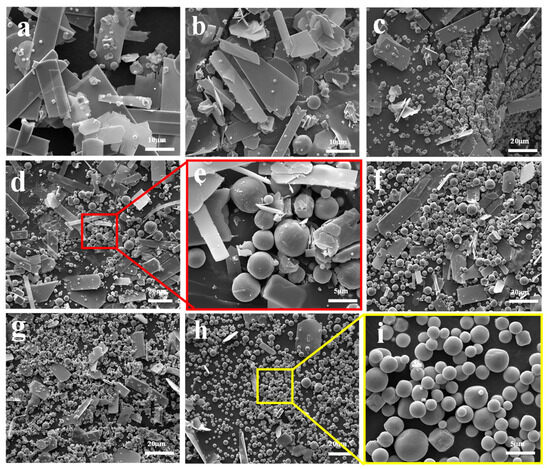
Figure 3.
Morphological structure of the sublimation products obtained at 1273 K under different Ar flow-rates: (a) 300 mL/min; (b) 500 mL/min; (c) 700 mL/min; (d) 900 mL/min; (e) The enlarge image showed in (d) marked with red area; (f) 1100 mL/min; (g) 1500 mL/min; (h) 2000 mL/min; (i) The enlarge image showed in (h) marked with yellow area.
Figure 4 presents the morphological structure of the sublimation products obtained under different sublimation temperatures. It shows that the sublimation products also exhibit two distinct morphologies, namely, platelet-shaped α-MoO3 and spherical β-MoO3. Similar to the results observed under different Ar flow-rates, the diameter of the spherical particles is also in the range of 1–5 μm; however, due to the fast growth rate of particles at a higher temperature, the number of spherical particles with large dimensions gradually increases with the increase in the sublimation temperature.
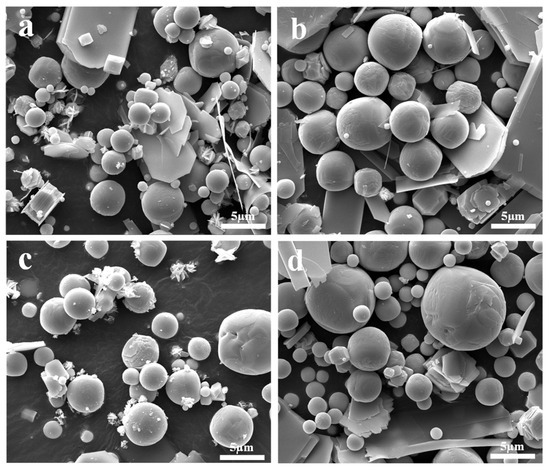
Figure 4.
Morphological structure of the sublimation products obtained under different sublimation temperatures: (a) 1223 K; (b) 1273 K; (c) 1323 K; (d) 1373 K. (Ar flow-rate: 1100 mL/min).
3. Discussion
MoO3 has a strong sublimation property, and its sublimation rate can be very large, even at 973 K [29,30,31]. When solid MoO3 raw material is in the high-temperature-zone of the Si-C electronic furnace, it will sublimate into gaseous forms, in which the gaseous (MoO3)n (n = 2, 3, 4, and 5) species dominate [32,33]. Due to the continuous introduction of carrier gas (Ar), the newly formed gaseous MoO3 species will be rapidly carried through the quartz tube toward the collection bottle. During this process, the temperature of the gaseous MoO3 gradually decreases, which can be seen in the temperature distribution diagram in the quartz tube, as shown in Figure 5a. It has been reported [22,34] that when the temperature is below 723 K, gaseous MoO3 is preferentially condensed into β-MoO3, while when the temperature exceeds that value, α-MoO3 is preferentially formed. Because the temperature of the collection bottle is lower than 373 K, β-MoO3 will form in the collection bottle. The larger the Ar flow-rate, the more gaseous MoO3 there will be, and the higher the relative content of deposited β-MoO3 in the collection bottle will be. Meanwhile, a certain amount of gaseous MoO3 will condense on the wall surface of the quartz tube, which is beneficial for the formation of α-MoO3 due to its relatively high temperature. On the one hand, α-MoO3 has an orthorhombic crystal structure with a double-layered network of [MoO6] octahedral. These layers are stacked along the b-axis (010) through van der Waals forces. Moreover, the structure is beneficial for the formation of sheet-like images [35]. On the other hand, the newly formed α-MoO3 in the quartz tube wall is still easily sublimated, even if its sublimation extent is weakened; in this case, the newly formed gaseous MoO3 will transform to the relatively low-temperature zone and condensate on the edge of the original α-MoO3 crystals; that is, parts of the volatilization–condensation process are still undertaken in this zone. As a result, α-MoO3 is more inclined to form a sheet-like structure at a high temperature. One thing that must be noted is that when the α-MoO3 forms in the high-temperature zone, the Ar airflow will carry parts of the α-MoO3 into the collection bottle. Therefore, the sublimation products in the collection bottle always contain both α-MoO3 and β-MoO3. Even though an increment in the Ar flow-rate would enhance its carrying capacity, this capacity is not sufficient to take away all the sheet-like α-MoO3; that is, most of the α-MoO3 are still adhered to the wall of the quartz tube, as seen in the inset of Figure 5a. For this reason, even if all the analytical reagent MoO3 is completely sublimated, and no residue exists in the alumina crucible, the mass of the sublimation product in the collection bottle will still only be in the range of 1–2 g; that is, the yield is about 20–40%. Herein, the temperature of the α-MoO3 deposited on the quartz tube is determined to be in the range of 780 K to 847 K, and that of the β-MoO3 is lower than 500 K; in other words, the above temperature ranges denote the stable existence intervals of α-MoO3 and β-MoO3, respectively. Due to the use of an Ar atmosphere and the lack of O2 in the reaction system, as well as the unstable characteristics of β-MoO3, a certain number of oxygen vacancies will form in the β-MoO3 crystal during the condensation process [36], and this is the reason why the colors of the β-MoO3 deposited on different sites differ, as shown in Figure 5b.
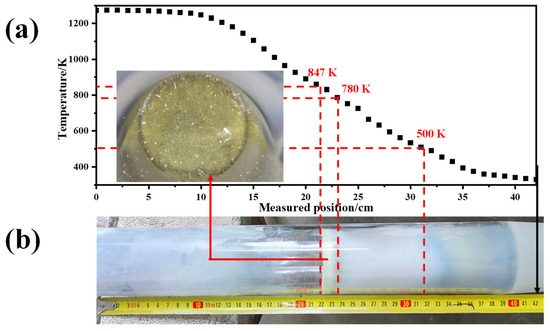
Figure 5.
Macroscopic results of the current experiment: (a) Temperature distribution in the quartz tube at the temperature of 1273 K; (b) Image of the used quartz tube after the experiment.
Figure 6 depicts some special morphologies of the sublimation products obtained under different conditions. Because spherical particles have the lowest surface free energy, and because the rapid reduction in the temperature of gaseous MoO3 would increase its saturated vapor pressure, in order to maintain the lowest energy state, numerous spherical particles will form during the condensation process. However, as shown in Figure 6, some newly formed particles are not perfectly spherical-shaped, and several transition states, such as cubic or elliptic particles, with many high-energy sites on the surface, also exist. In this case, the subsequent gaseous MoO3 molecules can be deposited on these sites to fill the voids and to make the particle spherical-shaped. In addition to gaseous MoO3 molecules, the small volume ratio of Ar molecules may also play important roles. When Ar molecules are adsorbed on the surface of MoO3, they can average the surface energy anisotropy, which may cause any oriented plane to hold the same surface energy and, finally, produce a spherical crystal. This is to say, that the gas molecules’ adsorption is an easy way to manipulate the crystal surface energy [37], which defines the notable Wulff shape of a single crystal. From this figure, it can also be observed that some small or platelet-shaped particles are adsorbed on the surface of the larger ones; in these cases, as time goes on, these small or platelet-shaped particles may gradually disappear and become a part of the larger one, as supported by the clear crystal boundary on the crystal surface, and this process is consistent with the classical Ostwald ripening mechanism [38]. Therefore, due to the co-action of the continuous deposition of gaseous MoO3 molecules, the adsorption of Ar molecules, and the particle collision effect, large spherical particles will form.
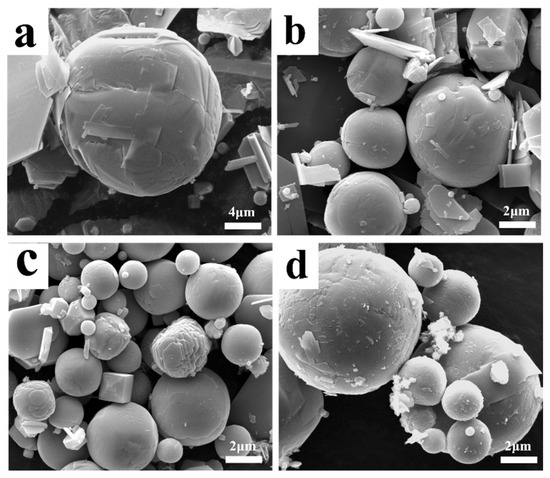
Figure 6.
Some special morphologies of the sublimation product obtained under different conditions: (a) 1273 K, 500 mL/min Ar; (b) 1273 K, 900 mL/min Ar; (c) 1223 K, 1100 mL/min Ar; (d) 1323 K, 1100 mL/min Ar.
Table 1 lists some preparation methods for β-MoO3 powder. It shows that many parameters, such as Mo source, sublimation temperature, and reaction atmosphere, may have close relationships with the microstructure of β-MoO3. However, it is also the case that β-MoO3 is entirely formed at a relatively low temperature [25,26,27]. Since the Ar gas is used in this work, many oxygen vacancies form inside the MoO3 species. When the Ar flow-rate is not very high, metastable β-MoO3 can be obtained, while when the Ar flow-rate is sufficiently high, the oxygen content in the reaction system will be extremely low; meanwhile, the condensation rate of the gaseous MoO3 will rapidly increase, which will lead to the formation of more oxygen vacancies. In this situation, the newly formed metastable β-MoO3 will be decomposed into numerous sub-oxides, such as MoO2, Mo4O11, and other oxides; this is the reason why many other sub-oxides appeared in our preliminary finding, as shown in Figure 7. Therefore, based on the current results, and those reported in our previous study, we propose that increasing the sublimation temperature appropriately may be a good option for improving the preparation efficiency of ultrafine β-MoO3 powder; moreover, the reasonable control of the reaction atmosphere and its gas flow-rate are also very important.

Table 1.
Different preparation methods for ultrafine β-MoO3 powder.
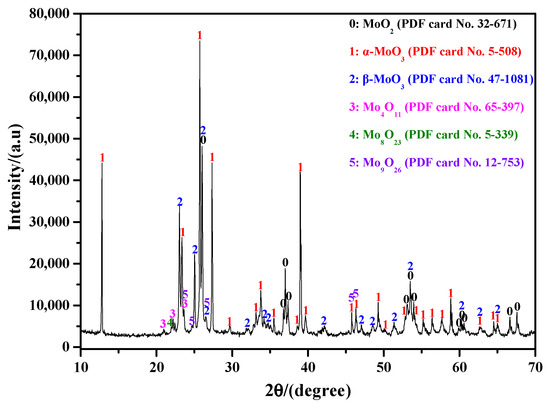
Figure 7.
XRD result of the sublimation product of analytical reagent MoO3 under the Ar atmosphere with an extremely high flow-rate (the condition was realized via the vacuum pump).
4. Materials and Experimental Procedures
4.1. Materials
Analytical reagent MoO3 (AR, 99.5%), purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., is used as a raw material. The XRD result shows that the raw material belongs to α-MoO3, and FESEM imaging shows that the raw material exhibits a sheet-like morphology, as observed in Figure 8. The carrier gas used in the work is high-purity Ar (the amount of impurities < 5 ppm).
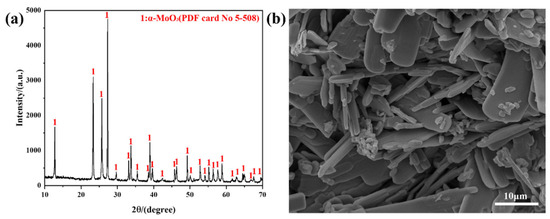
Figure 8.
Raw material used in the work: (a) XRD pattern; (b) FESEM micrograph.
4.2. Experimental Procedures
Figure 9 shows the schematic diagram of the experimental device. In each of the experiments, 5 g of MoO3 raw material was weighed and placed into an alumina crucible (50 mm × 20 mm × 20 mm). Then, the MoO3-containing crucible was placed on the right end of the inner quartz tube (inner diameter: 30 mm; outer diameter: 38 mm). After the Si-C electronic furnace reaching the specified temperature, the inner quart tube was inserted into the external quartz tube (inner diameter: 50 mm; outer diameter: 60 mm), with the MoO3 raw material placed into the constant-temperature zone of the furnace. Meanwhile, Ar gas with a certain flow-rate was introduced from the left end of the inner quartz tube to carry the gaseous MoO3 towards the collection bottle and accelerate its condensation rate. After 1 h of reaction time, we turned off the Ar gas and then collected the sublimation product in the collection bottle for further testing. In order to ensure parity with our previous work [28] and make a further contribution under the same study conditions, Ar flow-rates of 300, 500, 700, 900, 1100, 1500, and 2000 mL/min and sublimation temperatures of 1223, 1273, 1323, and 1373 K were selected for the current experiments.
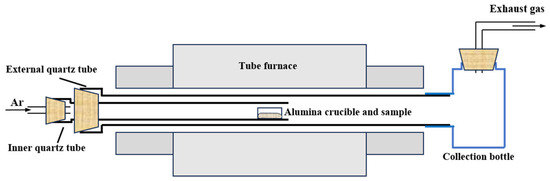
Figure 9.
Schematic diagram of the experimental device.
4.3. Product Characterization
The phase composition of the sublimation product was analyzed by an X-ray diffraction analyzer (XRD; D8 Advance, AXS Corporation, Bruker, Germany; operation voltage: 30 kV; operation current: 30 mA; scanning speed: 10°/min; scanning angle: 7–90°; Cu-Kα1 radiation with the wavelength of 1.54056 Å), and its morphological structure was observed via field emission scanning electron microscopy (FESEM; Nova 400 Nano SEM, FEI Corporation, Hillsboro, OR, USA; Operation voltage: 15 kV).
5. Conclusions
In this work, the phase composition and morphological structure of the sublimation products of analytical reagent MoO3 are investigated. The following conclusions are drawn.
- (1)
- The sublimation products obtained under different Ar flow-rates and sublimation temperatures are always composed of both α-MoO3 and β-MoO3, among which the proportion of β-MoO3 gradually increases with the increase in Ar flow-rate and the decrease in sublimation temperature.
- (2)
- Platelet-shaped α-MoO3 is usually generated in the range of 780 K to 847 K, while spherical-shaped β-MoO3 forms below 500 K. The diameter of β-MoO3 is determined to be in the range of 1–5 μm and has no obvious relationship with the Ar flow-rate due to its low value and narrow range.
- (3)
- Due to the co-actions of the deposition of gaseous MoO3 molecules, the adsorption of Ar molecules, and the particle collision between different particles, the newly formed β-MoO3 particles usually exhibit a spherical-shaped morphology in order to decrease their surface free energy.
Author Contributions
F.-J.D., formal analysis and writing—review and editing; J.-J.Y., investigation, writing—original draft, validation, and data curation; J.-G.L., writing—review and editing and funding acquisition; L.W., conceptualization, methodology, funding acquisition, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support for this work from the Nanping Science and Technology Commissioner Resource Industry Science and Technology Innovation Joint Funding Project (N2024Z006), the Fujian Natural Science Foundation (2024J01911), and the National Natural Science Foundation of China (52104310).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data can be provided upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, H.; Liu, X.J.; Wang, R.L.; Mi, R.; Li, S.M.; Cui, Y.H.; Deng, Y.F.; Mei, J.; Liu, H. Coating of α-MoO3 on nitrogen-doped carbon nanotubes by electrodeposition as a high-performance cathode material for lithium-ion batteries. J. Power Sources 2015, 274, 1063–1069. [Google Scholar] [CrossRef]
- Wang, J.Y.; Shi, J.Y.; Shi, B.; Zhang, Y.H. Structural and Electrochemical Investigation of Zinc-Doped Lithiated MoO3 Cathode Materials for Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2020, 15, 9824–9837. [Google Scholar] [CrossRef]
- Chithambararaj, A.; Bose, A.C. Investigation on structural, thermal, optical and sensing properties of meta-stable hexagonal MoO3 nanocrystals of one dimensional structure. Beilstein J. Nanotechnol. 2011, 2, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Li, Z.X.; Zhang, T.; Chen, R.Z.; Zheng, X.Y.; Zhang, G.K. Morphology-dependent catalytic activity of plasmonic MoO3-x for hydrolytic dehydrogenation of ammonia borane. Funct. Mater. Lett. 2017, 10, 1750079. [Google Scholar] [CrossRef]
- Yang, W.M.; Cao, L.L.; Lu, H.J.; Huang, Y.; Yang, W.Q.; Cai, Y.Z.; Li, S.M.; Li, S.Q.; Zhao, J.W.; Xu, W.Z. Custom-printed microfluidic chips using simultaneous ratiometric fluorescence with “Green” carbon dots for detection of multiple antibiotic residues in pork and water samples. J. Food. Sci. 2024, 89, 5980–5992. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, W.T.; Hu, X.T.; Zhang, X.A.; Huang, X.W.; Li, Z.H.; Li, M.Y.; Zou, X.B.; Shi, J.Y. Efficient preparation of dual-emission ratiometric fluorescence sensor system based on aptamer-composite and detection of bis (2-ethylhexyl) phthalate in pork. Food Chem. 2021, 352, 129352. [Google Scholar] [CrossRef]
- Huang, Y.T.; Fan, L.Y.; Liu, Y.F.; Zheng, X.H. High-performance MoO3 nanosheets gas sensors for triethylamine detection: A rapid approach for assessing fish freshness. Ceram. Int. 2025, 51, 9912–9922. [Google Scholar] [CrossRef]
- Sakaray, M.; Chidurala, S.C. Optimization of ternary composite @PANI/MoO3/h-BN and its electrochemical evaluation as an electrode material for supercapacitor application. Synth. Met. 2023, 298, 117448. [Google Scholar] [CrossRef]
- Shakir, I.; Shahid, M.; Cherevko, S. Ultrahigh-energy and stable supercapacitors based on intertwined porous MoO3-MWCNT nanocomposites. Electrochim. Acta. 2011, 58, 76–80. [Google Scholar] [CrossRef]
- Shibata, K.; Tsuchida, K.; Kato, A. Preparation of ultrafine molybdenum powder by vapour phase reaction of the MoO3-H2 system. J. Less Common Met. 1990, 157, L5–L10. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.B.; Zhang, G.H.; Chou, K.C. A novel method for preparing ultrafine molybdenum powder. Int. J. Refract. Met. Hard Mater. 2021, 96, 105491. [Google Scholar] [CrossRef]
- Sun, G.D.; Zhang, G.H.; Yan, B.J.; Chou, K.C. Study on the reduction of commercial MoO3 with carbon black to prepare MoO2 and Mo2C nanoparticles. Int. J. Appl. Ceram Technol. 2020, 17, 917–931. [Google Scholar] [CrossRef]
- Liang, J.K.; Li, H.X.; Chen, L.; Ren, M.N.; Fakayode, O.A.; Han, J.Y.; Zhou, C.S. Efficient hydrogen evolution reaction performance using lignin-assisted chestnut shell carbon-loaded molybdenum disulfide. Ind. Crop. Prod. 2023, 193, 116214. [Google Scholar] [CrossRef]
- Ji, Q.H.; Yu, X.J.; Chen, L.; Yarley, O.P.N.; Zhou, C.S. Facile preparation of sugarcane bagasse-derived carbon supported MoS2 nanosheets for hydrogen evolution reaction. Ind. Crop. Prod. 2021, 172, 114064. [Google Scholar] [CrossRef]
- Liang, N.N.; Hu, X.T.; Li, W.T.; Wang, Y.Y.; Guo, Z.A.; Huang, X.W.; Li, Z.H.; Zhang, X.A.; Zhang, J.K.; Xiao, J.B.; et al. A dual-signal fluorescent sensor based on MoS2 and CdTe quantum dots for tetracycline detection in milk. Food Chem. 2022, 378, 132076. [Google Scholar] [CrossRef]
- Feng, Y.S.; Chen, F.P.; Li, S.B.; Li, X.B.; Li, G.P.; Hong, Z.Y.; Chen, Y. Microstructure of molybdenum target with different rolling deformations and annealing processes. Copper Eng. 2024, 186, 66–72. [Google Scholar]
- Guo, Z.H.; Gong, Y.B. The infuence factors analysis of molybdenum concentrate oxidizing roasting. Copper Eng. 2015, 134, 67–70. [Google Scholar]
- Mizushima, T.; Phuc, N.H.H.; Moriya, Y. Soft chemical transformation of alpha-MoO3 to beta-MoO3 as a catalyst for vapor-phase oxidation of methanol. Catal. Commun. 2011, 13, 10–13. [Google Scholar] [CrossRef]
- Yamamoto, K.; Shimizu, R.; Shiraki, S.; Hitosugi, T. Transparent conducting properties of Re-doped β-MoO3 films. APL Mater. 2016, 4, 096104. [Google Scholar] [CrossRef]
- Chu, N.M.; Hieu, N.D.; Do, T.M.D.; Nakayama, T.; Niihara, K.; Suematsu, H. Synthesis of β-MoO3 nanowhiskers from core/shell molybdenum/molybdenum oxide wire by pulsed wire discharge. Int. J. Appl. Ceram. Technol. 2021, 18, 889–901. [Google Scholar] [CrossRef]
- Chu, N.M.; Hieu, N.D.; Do, T.M.D.; Nakayama, T.; Niihara, K.; Suematsu, H. Synthesis of metastable monoclinic beta molybdenum trioxide nanoparticles by pulsed wire discharge. Jpn. J. Appl. Phys. 2020, 59, SCCC02. [Google Scholar] [CrossRef]
- McCarron, E.M. β-MoO3: A metastable analogue of WO3. J. Chem. Soc. Chem. Commun. 1986, 1, 336–338. [Google Scholar] [CrossRef]
- Maiti, P.; Guha, P.; Singh, R.; Dash, J.K.; Satyam, P.V. Optical band gap, local work function and field emission properties of MBE grown β-MoO3 nanoribbons. Appl. Surf. Sci. 2019, 476, 691–700. [Google Scholar] [CrossRef]
- Pham, T.T.P.; Nguyen, P.H.D.; Vo, T.T.; Luu, C.L.; Nguyen, H.H.P. Preparation of NO-doped β-MoO3 and its methanol oxidation property. Mater. Chem. Phys. 2016, 184, 5–11. [Google Scholar] [CrossRef]
- Sun, H.; Li, G.H.; Yu, J.J.; Luo, J.; Rao, M.J.; Peng, Z.W.; Zhang, Y.B.; Jiang, T. Preparation of high purity MoO3 through volatilization of technical-grade Mo calcine in water vapor atmosphere. Int. J. Refract. Met. Hard Mater. 2018, 77, 1–7. [Google Scholar] [CrossRef]
- Ngo, C.M.; Nguyen, H.D.; Saito, N.; Do, T.M.D.; Nakayama, T.; Niihara, K.; Suematsu, H. Synthesis of β-MoO3 whiskers by the thermal evaporation method with flowing oxygen gas. J. Am. Ceram. Soc. 2022, 105, 1622–1628. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.H. Modified monoclinic metastable β-MoO3 submicrospheres: Controllable preparation, phase identification, and their formation mechanisms. Ceram. Int. 2023, 49, 18756–18769. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.H.; Chou, K.C. Synthesis of nanocrystalline molybdenum powder by hydrogen reduction of industrial grade MoO3. Int. J. Refract. Met. Hard Mater. 2016, 59, 100–104. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Wang, L.; Xue, Z.L. Volatilization behavior of high-purity MoO3 in the range of 600 °C to 750 °C: Influences of water-vapor concentration and reaction temperature. Int. J. Refract. Met. Hard Mater. 2025, 132, 107264. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.Q.; Liu, X.; Cao, Y.L. Research progress on the preparation methods and fundamental principles of ultrafine Mo powder. Int. J. Refract. Met. Hard Mater. 2025, 132, 107281. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.C.; Xue, Z.L.; Zhang, G.H.; Huang, A. Sublimation behavior of industrial grade molybdenum trioxide. Trans. Indian. Inst. Met. 2021, 74, 1469–1477. [Google Scholar] [CrossRef]
- Berkowitz, J.; Inghram, M.G.; Chupka, W. Polymeric gaseous species in the sublimation of molybdenum trioxide. J. Chem. Phys. 1957, 26, 842–846. [Google Scholar] [CrossRef]
- Blackburn, P.E.; Hoch, M.; Johnston, H.L. The vaporization of molybdenum and tungsten oxides. J. Phys. Chem. 1958, 62, 769–773. [Google Scholar] [CrossRef]
- Ngo, C.M.; Hieu, N.D.; Do, T.M.D.; Nakayama, T.; Niihara, K.; Suematsu, H. Hydration process of β-MoO3 powder prepared by pulsed wire discharge method. Jpn. J. Appl. Phys. 2022, 61, SB1018. [Google Scholar] [CrossRef]
- Shan, X.; Wang, F.; Hu, K.; Wei, J.Q.; Lin, X.; Zhao, X.Y.; Zhou, B.Z.; Zhang, K.L. Recent advances in synthesis and memory computing of large-area α-MoO3. Acta Phys. Sin. 2021, 70, 187–199. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.C.; Zhang, G.H.; Xue, Z.L. Morphology evolution and quantitative analysis of β-MoO3 and α-MoO3. High Temp. Mater. Process. 2020, 39, 620–626. [Google Scholar] [CrossRef]
- Hoinkes, H. The physical interaction potential of gas atoms with single-crystal surfaces, determined from gas-surface diffraction experiments. Rev. Mod. Phys. 1980, 52, 933–970. [Google Scholar] [CrossRef]
- Voorhees, P.W. The theory of Ostwald ripening. J. Stat. Phys. 1985, 38, 231–252. [Google Scholar] [CrossRef]
- Mariotti, D.; Lindström, H.; Bose, A.C.; Ostrikov, K. Monoclinic β-MoO3 nanosheets produced by atmospheric microplasma: Application to lithium-ion batteries. Nanotechnology 2009, 19, 495302. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





