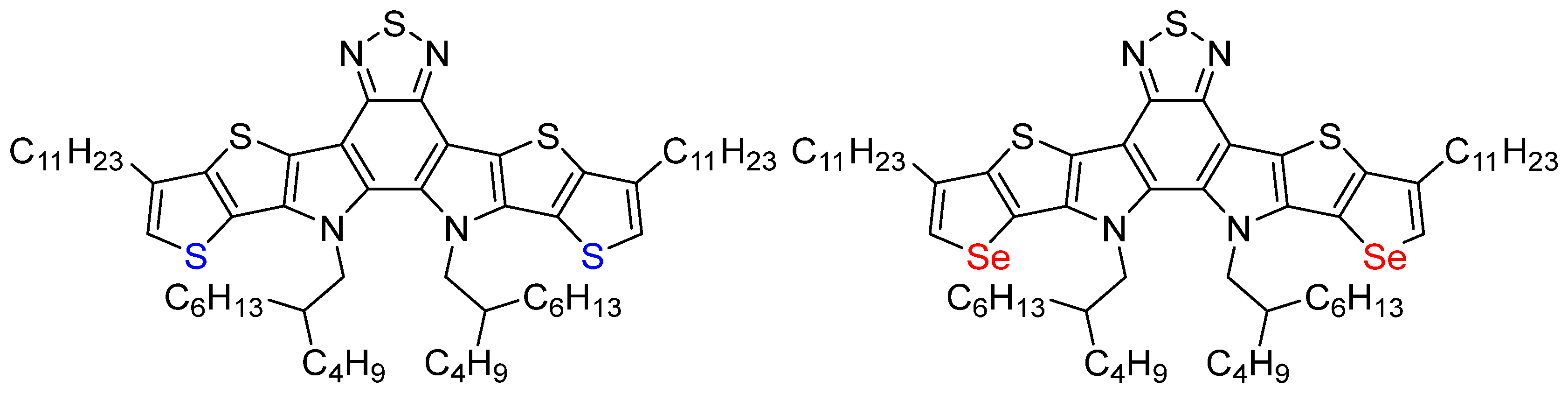
Enhanced Nonlinear Optical Absorption in Fused-Ring Aromatic Donor–Acceptor–Donor Core Units of Y6 Derivatives
Abstract
1. Introduction
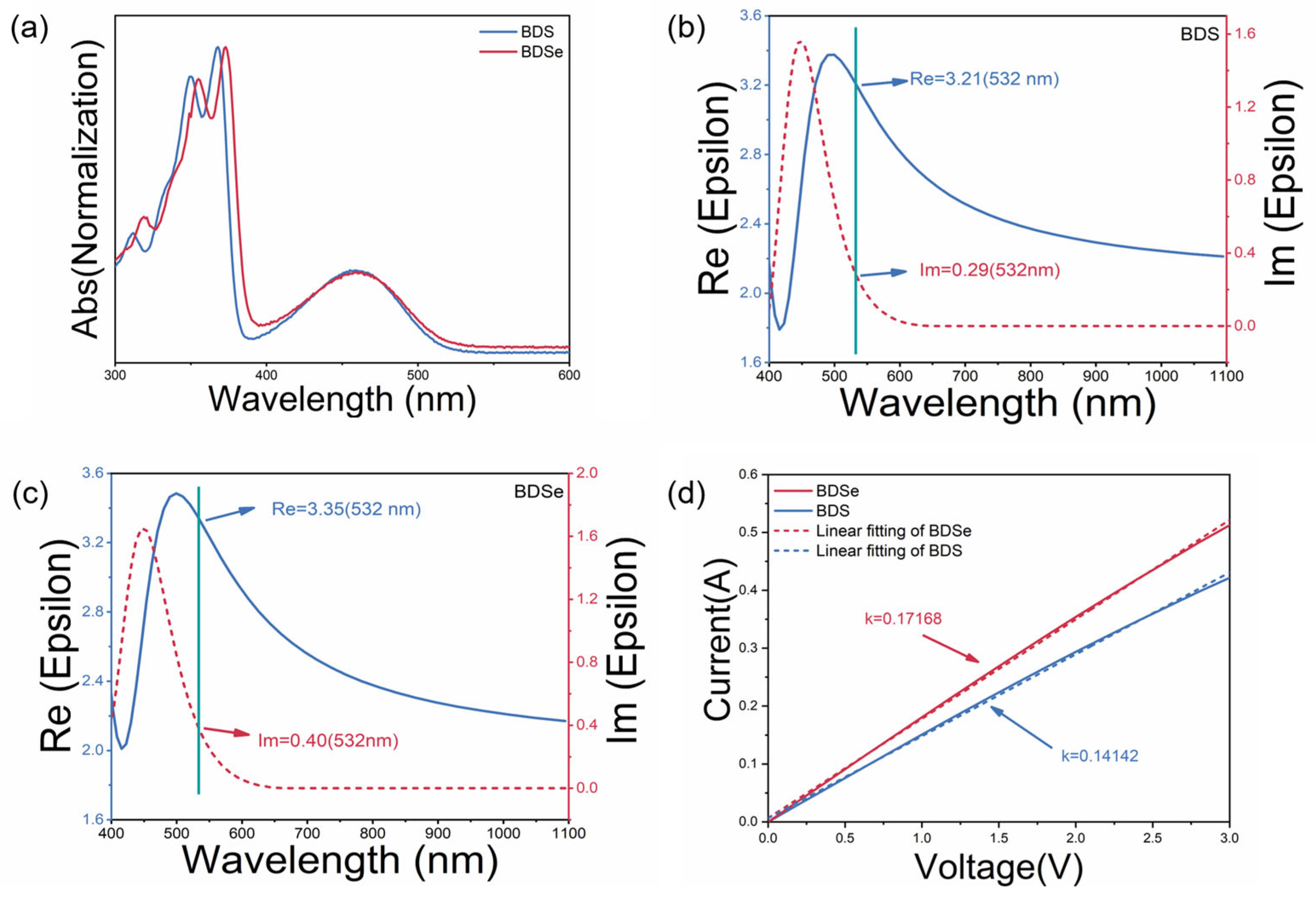
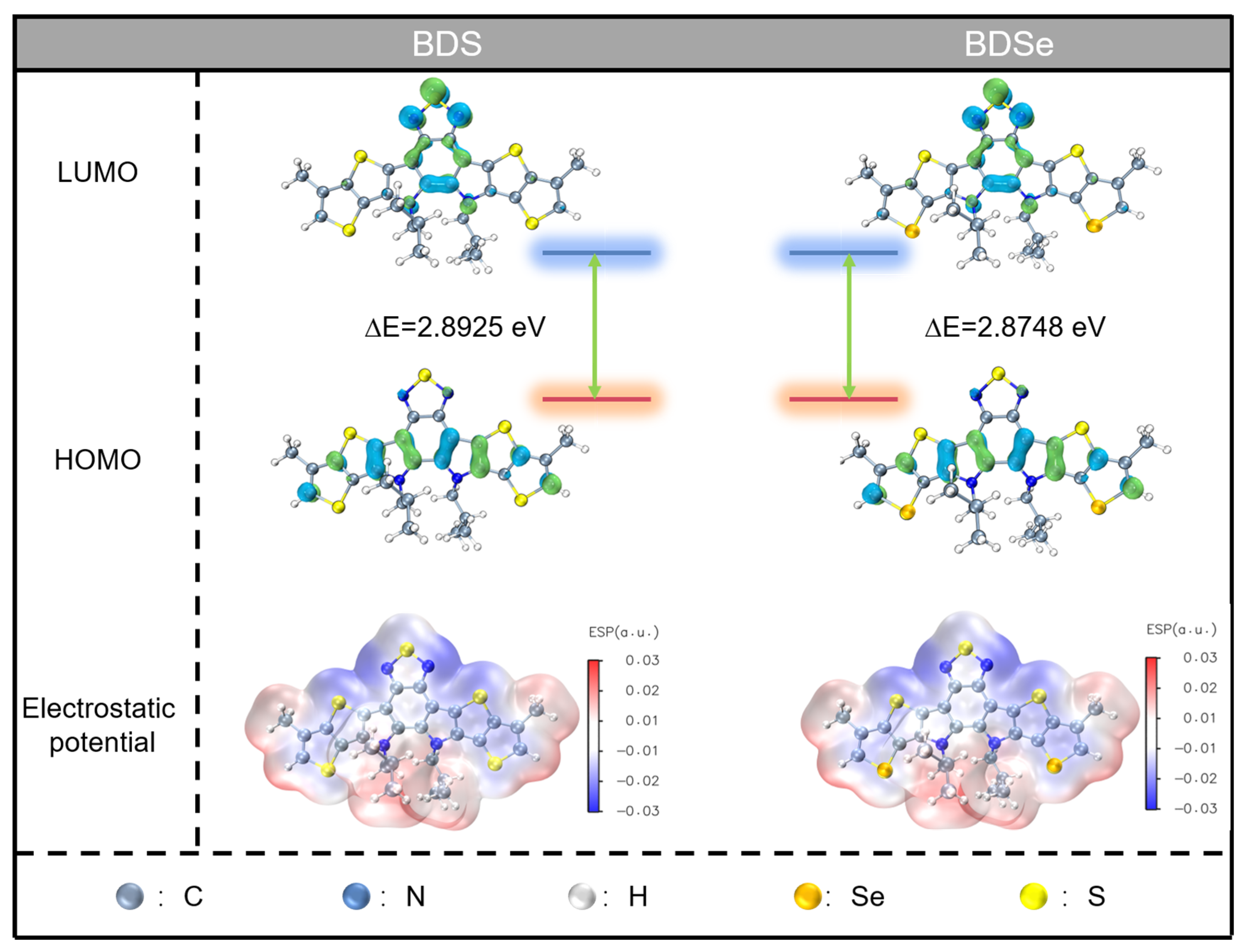
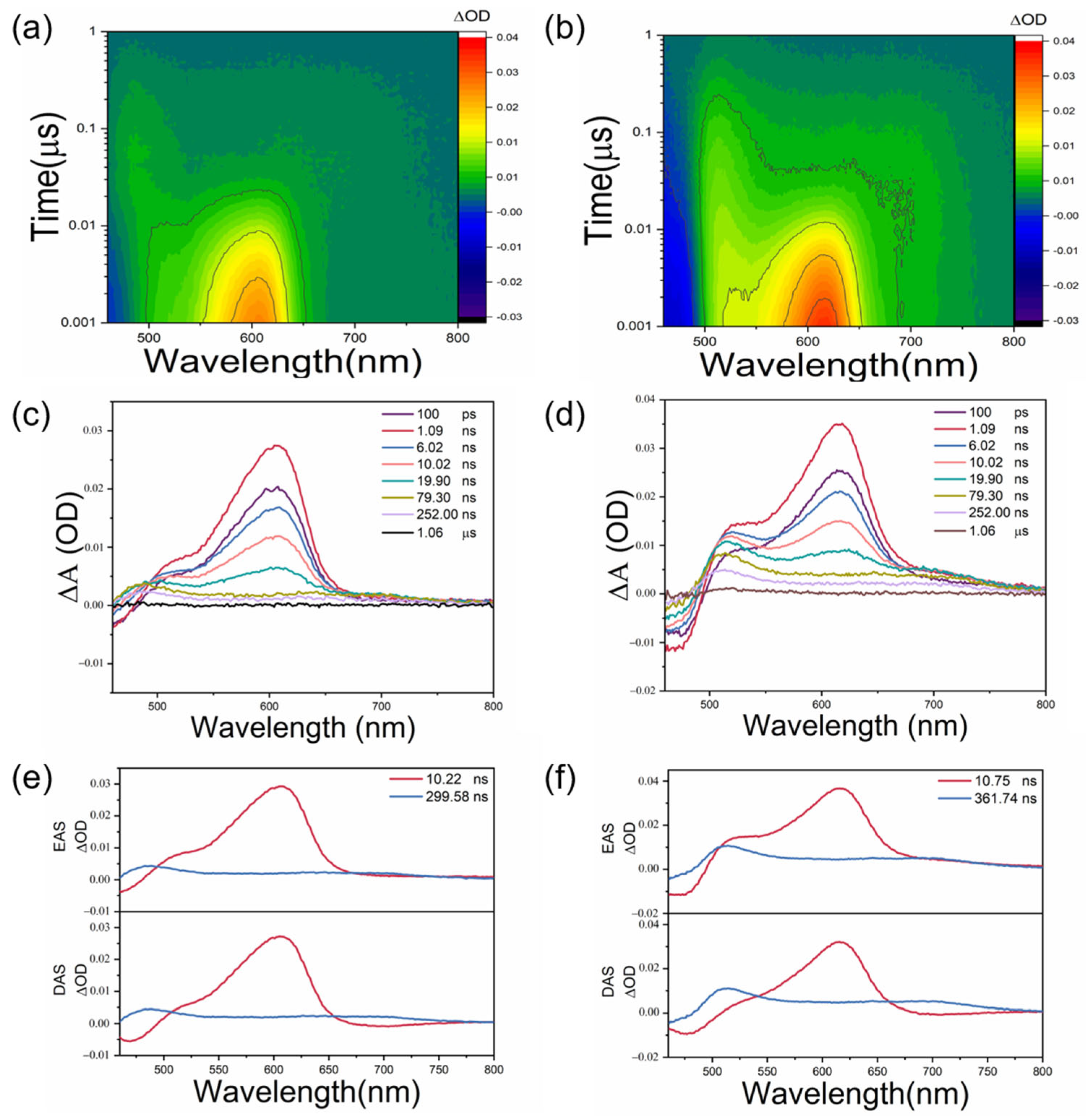
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.2.1. UV-Vis
3.2.2. The Fluorescence Decay Curve
3.2.3. Transient Absorption
3.2.4. Z-Scan Technology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Q.B.; Qi, X.Z.; Zhang, L.S.; Gao, M.; Hu, S.L.; Zhou, W.J.; Zang, W.J.; Zhao, X.X.; Wang, J.Y.; Yan, B.M.; et al. Ultrathin quantum light source with van der Waals NbOCl2 crystal. Nature 2023, 613, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Situ, G. Image encryption using spatial nonlinear optics. eLight 2022, 2, 3. [Google Scholar] [CrossRef]
- Hani, S.U.; Ahmed, S.; Alam, T.I.; Saleque, A.M.; Ivan, M.N.A.S.; Saha, S.; Xu, L.; Tsang, Y.H. Germanium-based ternary chalcogenide: An emerging low-dimensional material for nonlinear optics and ultrafast photonics. Appl. Mater. Today 2025, 42, 102555. [Google Scholar] [CrossRef]
- Xu, J.; Boyd, R.W.; Fischer, G.L. Nonlinear Optical Materials. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Biswal, B.P.; Valligatla, S.; Wang, M.; Banerjee, T.; Saad, N.A.; Mariserla, B.M.K.; Chandrasekhar, N.; Becker, D.; Addicoat, M.; Senkovska, I.; et al. Nonlinear Optical Switching in Regioregular Porphyrin Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2019, 58, 6896–6900. [Google Scholar] [CrossRef]
- Mustafa, M.N.; Hussain, F.; Hussain, M.; Hussain, R.; Ayub, K.; Muhammad, S.; Khan, M.U.; Ehsan, M.; Adnan, M. Elucidating the Potential of Nonlinear Optical Behavior of Azo Dyes for Advanced Laser-Based Technologies. Adv. Theory Simul. 2025, 8, 2401202. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Liu, H.; Tian, M.-Z.; Li, Y. Self-Assembly of Intramolecular Charge-Transfer Compounds into Functional Molecular Systems. Acc. Chem. Res. 2014, 47, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Kamaal, S.; Mehkoom, M.; Ali, A.; Afzal, S.M.; Alam, M.J.; Ahmad, S.; Ahmad, M. Potential Third-Order Nonlinear Optical Response Facilitated by Intramolecular Charge Transfer in a Simple Schiff Base Molecule: Experimental and Theoretical Exploration. Acs Omega 2021, 6, 6185–6194. [Google Scholar] [CrossRef]
- Gao, W.; Yuan, J.; Luo, Z.; Cao, J.; Tang, W.; Zou, Y.; Yang, C. Non-fullerene Small-Molecule Acceptors for Organic Solar Cells. In Organic Solar Cells; Wiley: New York, NY, USA, 2022; pp. 145–214. [Google Scholar]
- Wang, T.; Brédas, J.-L. Organic Solar Cells Based on Non-fullerene Small-Molecule Acceptors: Impact of Substituent Position. Matter 2020, 2, 119–135. [Google Scholar] [CrossRef]
- Dong, T.; Li, J.; Wang, C.; Yu, L.; Song, Y.; Wang, C.; Jiang, L.; Bai, C. Unexpected Third-Order Nonlinear Optical Responses in Two Isomeric Non-Fused Ring A-D-A Electron-Acceptor Molecules. Adv. Opt. Mater. 2023, 11, 2300482. [Google Scholar] [CrossRef]
- Meng, D.; Zheng, R.; Zhao, Y.; Zhang, E.; Dou, L.; Yang, Y. Near-Infrared Materials: The Turning Point of Organic Photovoltaics. Adv. Mater. 2022, 34, 2107330. [Google Scholar] [CrossRef]
- Zhai, Y.; Ma, J.; Liu, X.; Xuan, F.; Hu, K.; Wang, S.; Feng, X.; Cao, L.; Teng, B. Structural design and characterization of a new type of organic nonlinear optical flavanone crystal. J. Mol. Struct. 2025, 1322, 140305. [Google Scholar] [CrossRef]
- Blanco-Acuña, E.F.; Texon-García, I.A.; Vázquez-López, L.A.; Pérez-Gamboa, A.; García-Ortega, H. Tuning the photophysical and NLO properties of β-diketonates derived from coumarin-triphenylamine-chalcones: Effect of the BF2 group. Tetrahedron 2024, 168, 134338. [Google Scholar] [CrossRef]
- Shang, H.; Song, G.; Zhou, W.; Zhang, T.; Zhang, X.; Wang, Y.; Xiao, J.; Song, Y. Synthesis, Photophysical, and Optical Limiting Properties of Twistacene-Functionalized Arenes. J. Phys. Chem. B 2024, 128, 5481–5488. [Google Scholar] [CrossRef]
- Chaharmahali, R.; Fattah-alhosseini, A.; Karbasi, M. Unveiling Enhanced Photocatalytic Behavior: Plasma Electrolytic Oxidation for TiO2/Bi2WO6 heterojunction coatings to enhance the photocatalytic degradation of methylene blue under visible light. Ceram. Int. 2025, 51, 11255–11266. [Google Scholar] [CrossRef]
- Chang, C.-J.; Wang, Y.-C.; Yu, Y.-H.; Pu, Y.-C.; Kan, W.-L. Morphology control and enhanced activity of (Cu–S)nMOF@ZnS heterostructures for photocatalytic H2 production. J. Colloid Interface Sci. 2025, 683, 166–181. [Google Scholar] [CrossRef]
- Ashim Kumar, B.; Prem, C. Dielectric Properties of Materials. In Ferroelectrics: Principles and Applications; Wiley: New York, NY, USA, 2017; pp. 1–18. [Google Scholar]
- Yan, W.; Wang, Z.; Gong, Y.; Guo, S.; Jiang, J.; Chen, J.; Tang, C.; Xia, R.; Huang, W.; Xin, H. Naphthalene-diimide selenophene copolymers as efficient solution-processable electron-transporting material for perovskite solar cells. Org. Electron. 2019, 67, 208–214. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01; Gaussian: Wallingford, CT, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Rekhate, P.N.; Patil, P.S.; Thakare, T.G.; Devatkar, S.N.; Sekar, N. Influence of central heteroatom and donor or acceptor groups on the linear and nonlinear optical properties of epindolidione derivatives using DFT study. Chem. Phys. 2025, 591, 112593. [Google Scholar] [CrossRef]
- Slama, T.; Mazouz, Z.; Abdelaziz, B.; Bouazizi, S.; Cherif, I.; Ghabi, A.; Gassoumi, B.; Kaur, H.; Boubaker, T.; Ayachi, S. Multi-faceted spectroscopic, computational, and nonlinear optical characterization of 2-(pyrrolidin-1-yl)-3,5-dinitropyridine. J. Mol. Liq. 2025, 418, 126722. [Google Scholar] [CrossRef]
- Sheik-Bahae, M.; Said, A.A.; Wei, T.H.; Hagan, D.J.; Stryland, E.W.V. Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef]
- Chai, Y.; Liu, X.; Wu, B.; Liu, L.; Wang, Z.; Weng, Y.; Wang, C. In Situ Switching of Photoinduced Electron Transfer Direction by Regulating the Redox State in Fullerene-Based Dyads. J. Am. Chem. Soc. 2020, 142, 4411–4418. [Google Scholar] [CrossRef]
- Cesaretti, A.; Foggi, P.; Fortuna, C.G.; Elisei, F.; Spalletti, A.; Carlotti, B. Uncovering Structure–Property Relationships in Push–Pull Chromophores: A Promising Route to Large Hyperpolarizability and Two-Photon Absorption. J. Phys. Chem. C 2020, 124, 15739–15748. [Google Scholar] [CrossRef]
- Sasikumar, D.; John, A.T.; Sunny, J.; Hariharan, M. Access to the triplet excited states of organic chromophores. Chem. Soc. Rev. 2020, 49, 6122–6140. [Google Scholar] [CrossRef]
- Wang, C.; Wu, B.; Li, Y.; Dong, T.; Chai, Y.; Zhang, Y.; Wang, C. Regioisomeric Benzidine-Fullerenes: Tuning of the Diverse Hole-Distribution to Influence Charge Separation Patterns. Angew. Chem. Int. Ed. 2023, 62, e202300377. [Google Scholar] [CrossRef] [PubMed]
- Gündoğdu, Y. Third-order nonlinear optical properties of fullerene C60 in organic solvents using femtosecond laser z-scan. Indian J. Phys. 2023, 97, 915–922. [Google Scholar] [CrossRef]
- Kumar, M.; Perumbilavil, S.; Vinayakumara, D.R.; Goel, A.; Philip, R.; Kumar, S. Nonlinear Optical Properties of Discotic Hexylthiotruxene Derivatives. ACS Omega 2023, 8, 45961–45969. [Google Scholar] [CrossRef]
- Hao, L.H.; Dai, E.D.; Ban, Y.Q.; Zhang, Q.Y.; Wang, M.R.; Wang, P.; Zhao, Q.L.; Zhao, Y.J.; Wang, X.; Teng, L.H. Strong nonlinear optical response in star-shaped push–pull triphenylamine derivatives. Indian J. Phys. 2025, 99, 2627–2635. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Han, S.; Li, Y.; Zeng, W.; Sun, J.; Zhou, S. Significant Enhancement of Reverse Saturable Absorption Ability via Pt(II) Incorporation into the Conjugated Backbone of Benzo[1,2-b:4,5-b′]dithiophene-Based Donor–Acceptor Copolymers. Macromolecules 2024, 57, 1030–1037. [Google Scholar] [CrossRef]
- Niu, R.; Chen, S.; Zhou, W.; Wu, X.; Yang, J.; Wang, Y.; Zhang, X.; Song, Y. Investigation of ultrafast broadband optical nonlinearity and intensity-dependent nonlinear absorption in novel hydrazone derivatives. Opt. Laser Technol. 2022, 149, 107798. [Google Scholar] [CrossRef]
- Wen, L.; Fang, Y.; Yang, J.; Han, Y.; Song, Y. Comparison of third-order nonlinear optical properties of benzothiazolium salt and neutral benzothiazole derivative: Broadband absorption response and transient dynamic analysis. Dye. Pigment. 2019, 168, 28–35. [Google Scholar] [CrossRef]
- Niu, R.; Zhou, W.; Han, Y.; Wu, X.; Yang, J.; Chen, S.; Wang, Y.; Zhang, X.; Song, Y. Modulation of naphthanthryl chalcone derivatives by push-pull electronic substituents: Ultrafast nonlinear absorption and transient dynamics. Opt. Mater. 2022, 123, 111898. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Y.; Ruan, R.; Li, X.; Fang, Y.; Chen, Y.; Wu, Q.; Song, Y.; Wu, X. Investigation of transient optical nonlinearity in pyrene-based ferrocene and its optical limiting potential for ultrafast laser absorption. Opt. Mater. 2022, 128, 112467. [Google Scholar] [CrossRef]
- Han, Y.; Xiao, J.; Wu, X.; Zhou, W.; Shen, L.; Zhang, J.; Wang, Y.; Zhang, X.; Song, Y. Tuning Nonlinear Optical Behavior by Incorporation of the Chalcogenophene into Twistacenes. J. Phys. Chem. B 2020, 124, 10766–10775. [Google Scholar] [CrossRef]
- Waszkowska, K.; Cheret, Y.; Zawadzka, A.; Korcala, A.; Strzelecki, J.; El-Ghayoury, A.; Migalska-Zalas, A.; Sahraoui, B. Photoluminescence and nonlinear optical properties of triple stranded helicates based metallo-supramolecular architectures. Dye. Pigment. 2021, 186, 109036. [Google Scholar] [CrossRef]
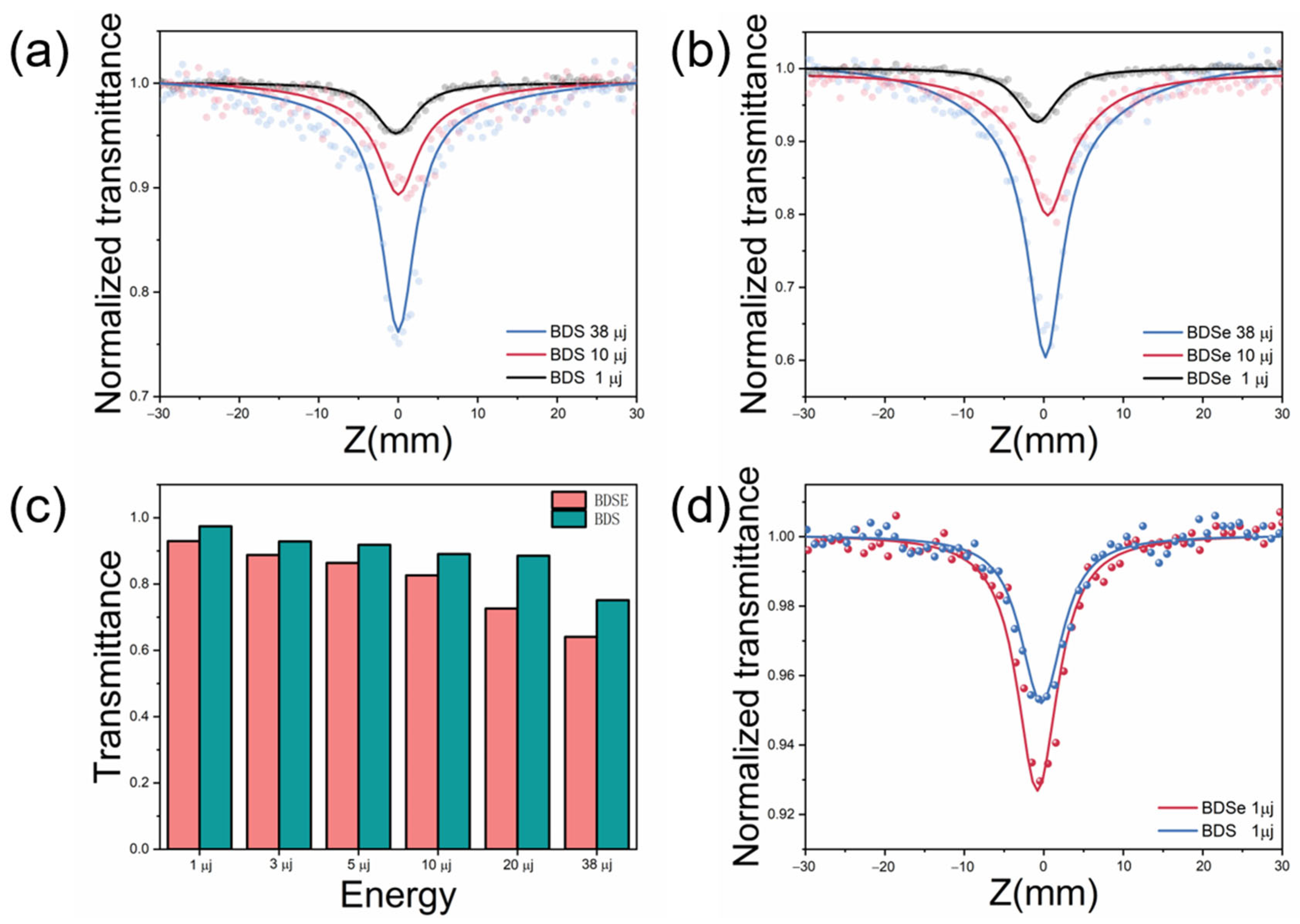
| Laser Energy | 1 μj | 3 μj | 5 μj | 10 μj | 20 μj | 38 μj | |
|---|---|---|---|---|---|---|---|
| BDS | β (m/W) | 2.46 × 10−10 | 1.46 × 10−10 | ||||
| σTPA (GM) | 1655.8 | 982.7 | |||||
| γ (esu) | 2.67 × 10−30 | 1.58 × 10−30 | |||||
| β2 (m/W) | 2.75 × 10−11 | 3.15 × 10−11 | |||||
| Is (W/m2) | 12.7 × 1010 | 9.74 × 1010 | |||||
| BDSe | β (m/W) | 3.32 × 10−10 | 2.21 × 10−10 | ||||
| σTPA (GM) | 2428.2 | 1616.4 | |||||
| γ (esu) | 3.92 × 10−30 | 2.61 × 10−30 | |||||
| β2 (m/W) | 8.14 × 10−11 | 7.26 × 10−11 | |||||
| Is (W/m2) | 18.8 × 1010 | 18.8 × 1010 | |||||
| Sample | Solvent | Β × 10−10 (m/W) | Reference |
|---|---|---|---|
| C60 | Tol | 0.01863 | [29] |
| TrSR1 | IPA | 2 | [30] |
| TrSR2 | IPA | 6.3 | [30] |
| A | CH2Cl2 | −0.443 | [31] |
| B | CH2Cl2 | 1.76 | [31] |
| P1-PT | 1,1,2,2-tetrachloroethane | 4.5 | [32] |
| P2-PT | 1,1,2,2-tetrachloroethane | 7.9 | [32] |
| BTH-1 | DMSO | 0.0016 | [33] |
| BTH-2 | DMSO | 0.012 | [33] |
| C25H15NS | DMF | 0.0011 | [34] |
| Nan-1 | DMSO | 0.00054 | [35] |
| Py-Fc | CH2Cl2 | 0.0079 | [36] |
| Py-PF | Tol | 0.0345 | [37] |
| HelFe | ACN | 0.0352 | [38] |
| NOC6F-1 | Tol | 0.057 | [11] |
| NOC6F-2 | Tol | 0.010 | [11] |
| BDS | Tol | 2.46 | This Work |
| BDSe | Tol | 3.32 | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Dong, T.; Wu, X.; Xu, J.; Shi, X.; Song, Y.; Wang, C.; Jiang, L. Enhanced Nonlinear Optical Absorption in Fused-Ring Aromatic Donor–Acceptor–Donor Core Units of Y6 Derivatives. Molecules 2025, 30, 2748. https://doi.org/10.3390/molecules30132748
Wen X, Dong T, Wu X, Xu J, Shi X, Song Y, Wang C, Jiang L. Enhanced Nonlinear Optical Absorption in Fused-Ring Aromatic Donor–Acceptor–Donor Core Units of Y6 Derivatives. Molecules. 2025; 30(13):2748. https://doi.org/10.3390/molecules30132748
Chicago/Turabian StyleWen, Xingyuan, Tianyang Dong, Xingzhi Wu, Jiabei Xu, Xiaofeng Shi, Yinglin Song, Chunru Wang, and Li Jiang. 2025. "Enhanced Nonlinear Optical Absorption in Fused-Ring Aromatic Donor–Acceptor–Donor Core Units of Y6 Derivatives" Molecules 30, no. 13: 2748. https://doi.org/10.3390/molecules30132748
APA StyleWen, X., Dong, T., Wu, X., Xu, J., Shi, X., Song, Y., Wang, C., & Jiang, L. (2025). Enhanced Nonlinear Optical Absorption in Fused-Ring Aromatic Donor–Acceptor–Donor Core Units of Y6 Derivatives. Molecules, 30(13), 2748. https://doi.org/10.3390/molecules30132748





