Nutritional and Metabolic Consequences of Camelina Seed Oil Compared to Flaxseed Oil in a Rat Diet
Abstract
1. Introduction
2. Results
2.1. Chemical Composition Differences
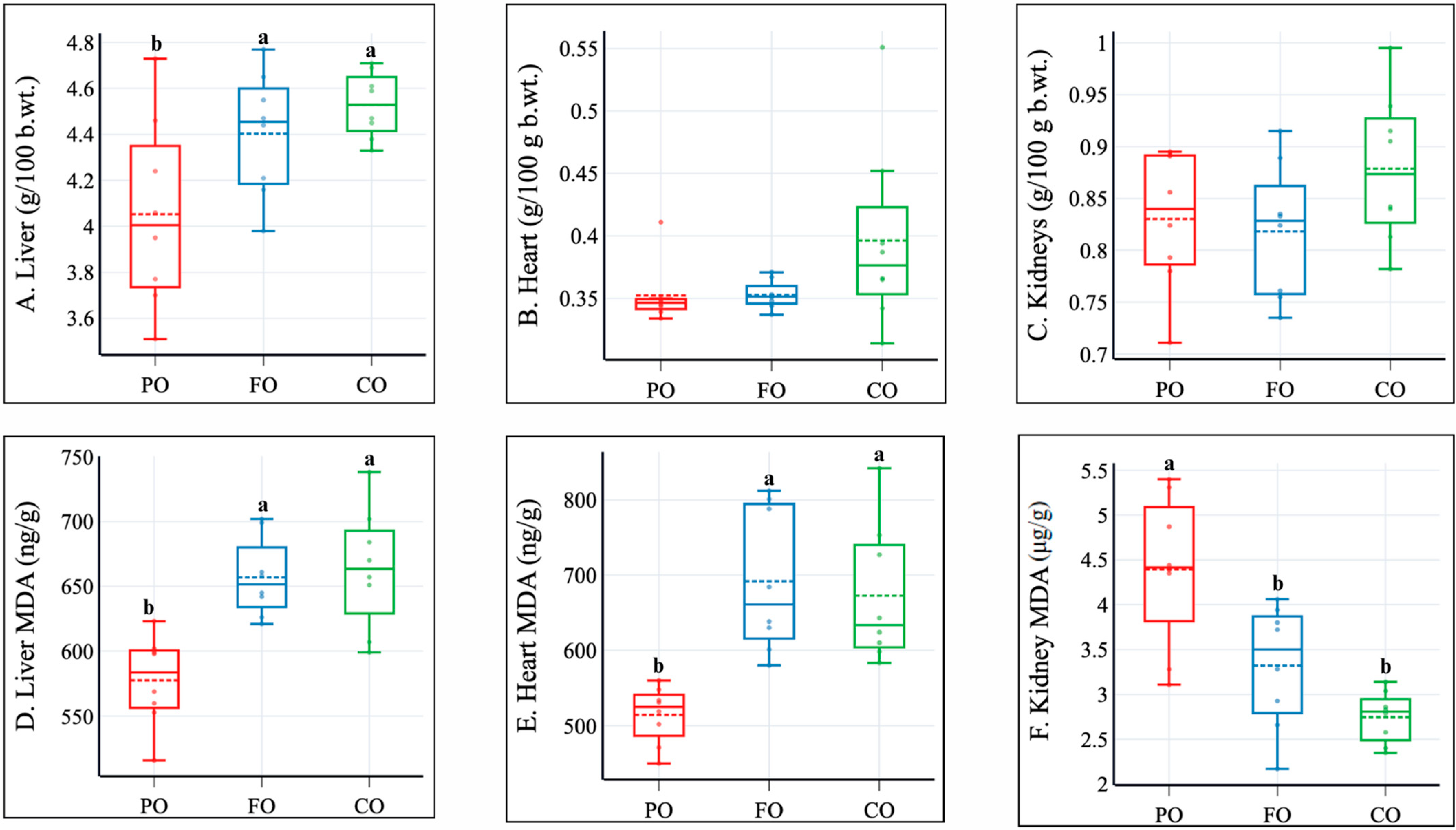
2.2. Dietary Intake, Body Composition, and Internal Organ Weights
2.3. Blood Antioxidant Status and Lipid Profile
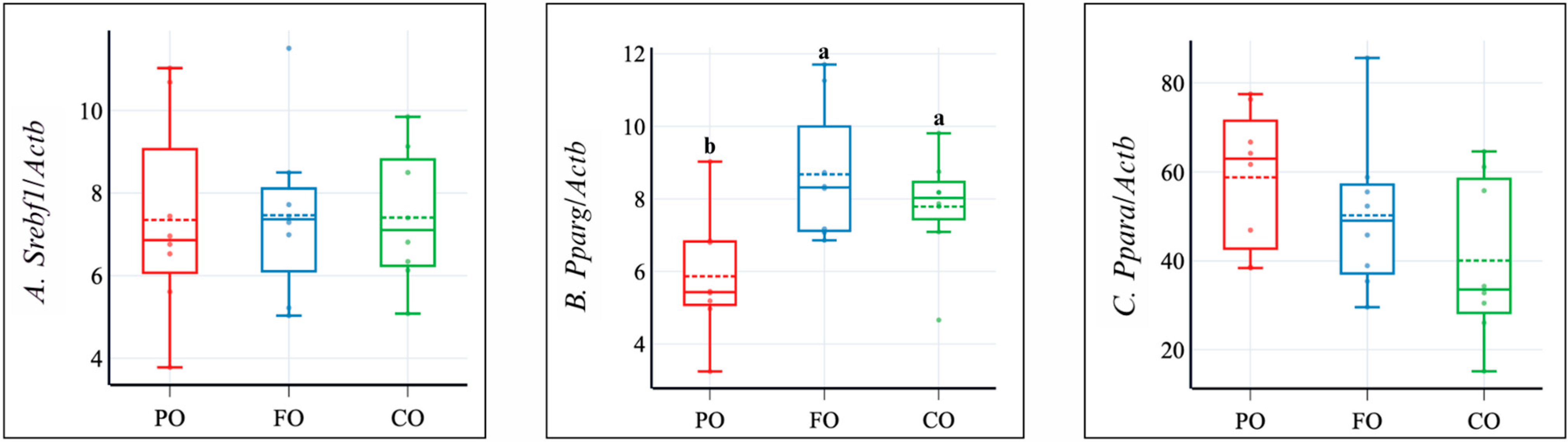
2.4. Liver Lipids, Gene Expression, and Plasma Markers of Liver and Kidney Functions
3. Discussion
4. Materials and Methods
4.1. Chemical Composition of Camelina Oil
4.2. Animals, Diets, and Experimental Design
4.3. Sampling and Analysis of Biological Material
4.4. mRNA Quantification
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PUFAs | Polyunsaturated fatty acids |
| ALA | α-Linolenic acid |
| PO | Palm oil |
| FO | Flaxseed oil |
| CO | Camelina seed oil |
| MDA | Malondialdehyde |
| LA | Linoleic acid |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
| EFSA | European Food Safety Authority |
| FDA | Food and Drug Administration |
| MUFAs | Monounsaturated fatty acids |
| NS | Non-significant |
| ACW | Antioxidant capacity of water-soluble substances |
| ACL | Antioxidant capacity of lipid-soluble substances |
| Srebf1 | Sterol regulatory element-binding protein 1c gene |
| Pparg | Peroxisome proliferator-activated receptor gamma gene |
| Ppara | Peroxisome proliferator-activated receptor alpha gene |
| Actb | β-actin gene |
| AST | Aspartate transaminase |
| ALT | Alanine transaminase |
| ALP | Alkaline phosphatase |
| SFAs | Saturated fatty acids |
| TDI | Tolerable daily intake |
| TRI | Total RNA isolation reagent |
| SEM | Standard error of the mean |
| SD | Standard deviation |
| ANOVA | One-factor analysis of variance |
References
- Zubr, J. Dietary fatty acids and amino acids of Camelina sativa seed. J. Food Qual. 2003, 26, 451–462. [Google Scholar] [CrossRef]
- Berhow, M.A.; Polat, U.; Glinski, J.A.; Glensk, M.; Vaughn, S.F. Evaluating the phytochemical potential of Camelina: An emerging new crop of Old World origin. In Phytochemicals—Biosynthesis, function, and application; Jetter, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 44, pp. 129–148. [Google Scholar] [CrossRef]
- Karpaviciene, B.; Marsalkiene, N.; Žilėnaitė, L. Oil content and composition in seeds of Camelina sativa and Crambe abyssinica cultivars. J. Elem. 2020, 25, 1399–1412. [Google Scholar] [CrossRef]
- Rabiej-Kozioł, D.; Król, A.; Starzak, M.; Chmurzyńska, A.; Kowalska, D.; Dereń, K. Health benefits, antioxidant activity, and sensory attributes of selected cold-pressed oils. Molecules 2023, 28, 5484. [Google Scholar] [CrossRef] [PubMed]
- Günç Ergönül, P.; Aksoylu Özbek, Z. Identification of bioactive compounds and total phenol contents of cold pressed oils from safflower and camelina seeds. J. Food Meas. Charact. 2018, 12, 2313–2323. [Google Scholar] [CrossRef]
- Al-Madhagy, S.; Alsuhaibani, A.M.; Alqumber, M.A.; Alharthi, S.E.; Ahmed, H.G.; Alsharari, S.D. A comprehensive review of the health benefits of flaxseed oil in relation to its chemical composition and comparison with other omega-3-rich oils. Eur. J. Med. Res. 2023, 28, 240. [Google Scholar] [CrossRef]
- Bernacchia, R.; Preti, R.; Vinci, G. Chemical composition and health benefits of flaxseed. Austin J. Nutr. Food Sci. 2014, 2, 1045. [Google Scholar]
- Rafalowski, R.; Zegarska, Z.; Kuncewicz, A.; Borejszo, Z. Fatty acid composition, tocopherols and beta-carotene content in Polish commercial vegetable oils. Pak. J. Nutr. 2008, 7, 278–282. [Google Scholar] [CrossRef]
- Paschos, G.K.; Magkos, F.; Panagiotakos, D.B.; Votteas, V.; Zampelas, A. Dietary supplementation with flaxseed oil lowers blood pressure in dyslipidaemic patients. Eur. J. Clin. Nutr. 2007, 61, 1201–1206. [Google Scholar] [CrossRef]
- Kelley, D.S.; Vemuri, M.; Adkins, Y.; Gill, C.H.; Fedor, D.M. Flaxseed oil prevents trans-10, cis-12-conjugated linoleic acid-induced insulin resistance in mice. Br. J. Nutr. 2008, 101, 701–708. [Google Scholar] [CrossRef]
- Seike, M.; Nakao, Y.; Miyoshi, M.; Suzuki, T.; Kishino, S. Dietary flaxseed oil induces production of adiponectin in visceral fat and prevents obesity in mice. Nutr. Res. 2024, 121, 16–27. [Google Scholar] [CrossRef]
- Wilkinson, P.; Leach, C.; Ah-Sing, E.E.; Hussain, N.; Miller, G.J.; Millward, D.J. Influence of alpha-linolenic acid and fish-oil on markers of cardiovascular risk in subjects with an atherogenic lipoprotein phenotype. Atherosclerosis 2005, 181, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Heijenskjöld, L.; Ernster, L. Studies of the mode of action of erucic acid on heart metabolism. Acta Medica Scand. 1975, 585, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Galanty, A.; Sieniawska, E.; Podolak, I. Erucic acid—Both sides of the story: A concise review on its beneficial and toxic properties. Molecules 2023, 28, 1924. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.-C.; et al. Erucic acid in feed and food. EFSA J. 2016, 14, e04593. [Google Scholar] [CrossRef]
- Poddar, K.H.; Iyer, A.; Jialal, I. Mustard oil and cardiovascular health: Why the controversy? J. Clin. Lipidol. 2022, 16, 13–22. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. (n.d.). Import Alert 26-04. Available online: https://www.accessdata.fda.gov/cms_ia/importalert_89.html (accessed on 10 April 2025).
- Santos, H.O.; Price, J.C.; Bueno, A.A.; Macedo, R.C.O. Beyond fish oil supplementation: The effects of alternative plant sources of omega-3 polyunsaturated fatty acids upon lipid indexes and cardiometabolic biomarkers—An overview. Nutrients 2020, 12, 3159. [Google Scholar] [CrossRef]
- Petrova, S.; Dimitrov, P.; Willett, W.; Campos, H. Global availability of n-3 fatty acids. Public Health Nutr. 2011, 14, 1157–1164. [Google Scholar] [CrossRef]
- Jalili, C.; Soltani, S.; Mazloom, Z.; Salehi-Abargouei, A. Effects of Camelina oil supplementation on lipid profile and glycemic control: A systematic review and dose–response meta-analysis of randomized clinical trials. Lipids Health Dis. 2022, 21, 132. [Google Scholar] [CrossRef]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary polyunsaturated fatty acids (PUFAs): Uses and potential health benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef]
- Mashek, D.G.; Wu, C. MUFAs. Adv. Nutr. 2015, 6, 276–277. [Google Scholar] [CrossRef]
- Tian, M.; Bai, Y.; Tian, H.; Zhao, X. The chemical composition and health-promoting benefits of vegetable oils—A review. Molecules 2023, 28, 6393. [Google Scholar] [CrossRef] [PubMed]
- Abramovič, H.; Butinar, B.; Nikolič, V. Changes occurring in phenolic content, tocopherol composition and oxidative stability of Camelina sativa oil during storage. Food Chem. 2007, 104, 903–909. [Google Scholar] [CrossRef]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P. Biological and nutritional properties of palm oil and palmitic acid: Effects on health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef]
- Baynes, H.W.; Mideksa, S.; Ambachew, S. The role of polyunsaturated fatty acids (n-3 PUFAs) on the pancreatic β-cells and insulin action. Adipocyte 2018, 7, 81–87. [Google Scholar] [CrossRef]
- Monnard, C.R.; Dulloo, A.G. Polyunsaturated fatty acids as modulators of fat mass and lean mass in human body composition regulation and cardiometabolic health. Obes. Rev. 2021, 22, e13197. [Google Scholar] [CrossRef]
- Berrougui, H.; Momo, C.N.; Khalil, A. Health benefits of high-density lipoproteins in preventing cardiovascular diseases. J. Clin. Lipidol. 2012, 6, 524–533. [Google Scholar] [CrossRef]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid-regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Han, L.; Shen, W.; Bittner, S.; Hu, C.A.A. PPARs: Regulators of metabolism and as therapeutic targets in cardiovascular disease. Part I: PPAR-α. Future Cardiol. 2017, 13, 259–278. [Google Scholar] [CrossRef]
- Kavyani, Z.; Dehghan, P.; Khani, M.; Khalafi, M.; Rosenkranz, S.K. The effects of Camelina sativa oil and high-intensity interval training on liver function and metabolic outcomes in male type 2 diabetic rats. Front. Nutr. 2023, 10, 1102862. [Google Scholar] [CrossRef]
- Kersten, S. Peroxisome proliferator activated receptors and lipoprotein metabolism. PPAR Res. 2008, 2008, 132960. [Google Scholar] [CrossRef]
- Rahman, M.H.; Nasreen, L.; Habib, K.; Rahman, N. Effects of dietary coconut oil on erucic acid rich rapeseed oil-induced changes of blood serum lipids in rats. Curr. Nutr. Food Sci. 2015, 10, 302–307. [Google Scholar] [CrossRef]
- Loganathan, R.; Tarmizi, A.H.A.; Vethakkan, S.; Teng, K.-T. A review on lipid oxidation in edible oils. Malays. J. Anal. Sci. 2022, 26, 1378–1393. [Google Scholar]
- El-Sayed, M.E.S.Y.; Elsanhoty, R.M.; Ramadan, M.F. Impact of dietary oils and fats on lipid peroxidation in liver and blood of albino rats. Asian Pac. J. Trop. Biomed. 2014, 4, 52–58. [Google Scholar] [CrossRef]
- Islam, F.; Imran, A.; Nosheen, F.; Fatima, M.; Arshad, M.U.; Afzaal, M.; Ijaz, N.; Noreen, R.; Mehta, S.; Biswas, S.; et al. Functional roles and novel tools for improving-oxidative stability of polyunsaturated fatty acids: A comprehensive review. Food Sci. Nutr. 2023, 11, 2471–2482. [Google Scholar] [CrossRef]
- Husein, J.; El-Khayat, Z.; Taha, M.; Morsy, S.; Drees, E.; Khateeb, S. Insulin resistance and oxidative stress in diabetic rats treated with flaxseed oil. J. Med. Plants Res. 2012, 6, 5499–5506. [Google Scholar]
- Miralles-Pérez, B.; Martí-Duran, B.; Torres-Collado, L.; Tresserra-Rimbau, A.; Castañer, O. Effects of a fish oil rich in docosahexaenoic acid on cardiometabolic risk factors and oxidative stress in healthy rats. Mar. Drugs 2021, 19, 555. [Google Scholar] [CrossRef]
- Higdon, J.V.; Liu, J.; Du, S.-H.; Morrow, J.D. Supplementation of postmenopausal women with fish oil rich in eicosapentaenoic acid and docosahexaenoic acid is not associated with greater in vivo lipid peroxidation compared with oils rich in oleate and linoleate as assessed by plasma malondialdehyde and F2-isoprostanes. Am. J. Clin. Nutr. 2000, 72, 714–722. [Google Scholar] [CrossRef]
- Chen, X.; Shang, L.; Deng, S.; Li, P.; Chen, K.; Gao, T.; Zhang, X.; Chen, Z.; Zeng, J. Peroxisomal oxidation of erucic acid suppresses mitochondrial fatty acid oxidation by stimulating malonyl-CoA formation in the rat liver. J. Biol. Chem. 2020, 295, 10168–10179. [Google Scholar] [CrossRef]
- Arrigo, T.; Leonardi, S.; Cuppari, C.; Manti, S.; Lanzafame, A.; D’angelo, G.; Gitto, E.; Marseglia, L.; Salpietro, C. Role of the diet as a link between oxidative stress and liver diseases. World J. Gastroenterol. 2015, 21, 384–395. [Google Scholar] [CrossRef]
- Abdelmagid, S.A.; Clarke, S.E.; Nielsen, D.E.; Badawi, A.; El-Sohemy, A.; Mutch, D.M. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS ONE 2015, 10, e0116195. [Google Scholar] [CrossRef]
- Matsumoto, C.; Ascherio, A.; Rimm, E.B.; Chiuve, S.E. Red blood cell MUFAs and risk of coronary artery disease in the Physicians’ Health Study. Am. J. Clin. Nutr. 2013, 98, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Sczaniecka, A.K.; Yang, W.; Swan, J.; Caan, B.; Slattery, M.L. Dietary intake of specific fatty acids and breast cancer risk among postmenopausal women in the VITAL cohort. Nutr. Cancer 2012, 64, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Kickler, T.S.; Moser, H.W.; Moser, A.B. Effect of erucic acid on platelets in patients with adrenoleukodystrophy. Biochem. Mol. Med. 1996, 57, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Jurgoński, A.; Fotschki, B.; Juśkiewicz, J. Disparate metabolic effects of blackcurrant seed oil in rats fed a basal and obesogenic diet. Eur. J. Nutr. 2015, 54, 991–999. [Google Scholar] [CrossRef]
- Fotschki, B.; Opyd, P.; Juśkiewicz, J.; Wiczkowski, W.; Jurgoński, A. Comparative effects of dietary hemp and poppy seed oil on lipid metabolism and the antioxidant status in lean and obese Zucker rats. Molecules 2020, 25, 2921. [Google Scholar] [CrossRef]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Opyd, P.M.; Jurgoński, A. Intestinal, liver and lipid disorders in genetically obese rats are more efficiently reduced by dietary milk thistle seeds than their oil. Sci. Rep. 2021, 11, 20895. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Koza, J.; Jurgoński, A. Partially defatted rather than native poppy seeds beneficially alter lipid metabolism in rats fed a high-fat diet. Sci. Rep. 2023, 13, 14171. [Google Scholar] [CrossRef]
| Fatty Acid | Flaxseed Oil | Camelina Seed Oil |
|---|---|---|
| Palmitic acid (16:0) | 5.40 ± 0.01 | 5.08 ± 0.02 |
| Stearic acid (18:0) | 3.89 ± 0.01 | 2.21 ± 0.01 |
| Oleic acid (18:1 n-9) | 17.6 ± 0.01 | 12.2 ± 0.06 |
| Vaccenic acid (18:1 n-7) | 1.03 ± 0.01 | 1.06 ± 0.01 |
| Erucic acid (22:1 n-9) | – | 2.83 ± 0.01 |
| Linoleic acid (18:2 n-6) | 14.7 ± 0.02 | 17.4 ± 0.01 |
| Arachidic acid (20:0) | 0.11 ± 0.01 | 1.37 ± 0.00 |
| γ-Linolenic acid (C18:3 n-6) | 0.22 ± 0.00 | 0.12 ± 0.00 |
| Gondoic acid (20:1 n-9) | 0.09 ± 0.00 | 12.8 ± 0.03 |
| α-Linolenic acid (18:3 n-3) ALA | 51.9 ± 0.03 | 35.1 ± 0.05 |
| 11,14-Eicosadienoic acid (20:2 n-6) | – | 1.82 ± 0.01 |
| Behenic acid (22:0) | 0.11 ± 0.00 | 0.31 ± 0.00 |
| 11,14,17-Eicosatrienoic acid (20:3 n-3) | – | 1.36 ± 0.01 |
| 13,16-Docosadienoic acid (20:2 n-6) | – | 0.18 ± 0.00 |
| Lignoceric acid (24:0) | – | 0.17 ± 0.00 |
| Nervonic acid (24:1 n-9) | – | 0.62 ± 0.01 |
| Other (unidentified) | 0.25 ± 0.02 | 0.48 ± 0.00 |
| SFAs 2 | 9.51 ± 0.01 | 9.14 ± 0.02 |
| MUFAs 2 | 18.7 ± 0.01 | 29.5 ± 0.02 |
| PUFAs 2 | 66.8 ± 0.01 | 56.0 ± 0.03 |
| n-3 | 51.8 ± 0.02 | 36.5 ± 0.03 |
| n-6 | 14.9 ± 0.01 | 19.5 ± 0.01 |
| Group | ANOVA p Value | |||
|---|---|---|---|---|
| PO | FO | CO | ||
| Initial body weight (g) | 148 ± 2.27 | 146 ± 2.25 | 147 ± 1.65 | NS |
| Initial fat (%) | 20.5 ± 0.704 | 19.0 ± 0.733 | 18.6 ± 0.879 | NS |
| Initial lean (%) | 70.0 ± 0.810 | 72.4 ± 0.943 | 72.7 ± 1.041 | NS |
| Final body weight (g) | 191 ± 4.29 | 198 ± 4.46 | 197 ± 3.10 | NS |
| Dietary intake (g/day) | 13.7 ± 0.318 | 13.5 ± 0.252 | 13.8 ± 0.166 | NS |
| Final fat (%) | 32.6 ± 1.46 a | 27.4 ± 1.21 b | 27.2 ± 1.19 b | <0.05 |
| Final lean (%) | 57.1 ± 1.11 b | 63.6 ± 1.18 a | 63.9 ± 1.32 a | 0.001 |
| Body weight gain (g) | 43.7 ± 2.80 | 51.9 ± 2.70 | 50.1 ± 3.09 | NS |
| Fat gain (g) | 32.5 ± 4.11 | 26.7 ± 3.31 | 26.3 ± 2.10 | NS |
| Lean gain (g) | 5.58 ± 1.88 b | 19.87 ± 1.78 a | 19.19 ± 2.90 a | <0.001 |
| Group | ANOVA p Value | |||
|---|---|---|---|---|
| PO | FO | CO | ||
| Antioxidant status | ||||
| Uric acid (µmol/L) | 24.9 ± 6.37 | 22.5 ± 1.80 | 22.9 ± 3.83 | NS |
| ACW (µg/mL) | 1.51 ± 0.298 | 1.07 ± 0.107 | 1.60 ± 0.402 | NS |
| ACL (µg/mL) | 11.9 ± 0.860 | 11.6 ± 0.923 | 13.2 ± 0.555 | NS |
| Lipid profile | ||||
| HDL cholesterol (mmol/L) | 0.663 ± 0.037 b | 0.834 ± 0.034 a | 0.813 ± 0.051 a | <0.05 |
| LDL cholesterol (mmol/L) | 0.115 ± 0.008 a | 0.098 ± 0.004 ab | 0.086 ± 0.006 b | 0.01 |
| Cholesterol (mmol/L) | 2.21 ± 0.082 | 2.05 ± 0.094 | 2.24 ± 0.132 | NS |
| Triglycerides (mmol/L) | 3.96 ± 0.606 a | 1.82 ± 0.122 b | 2.14 ± 0.215 ab | <0.01 |
| Group | ANOVA p Value | |||
|---|---|---|---|---|
| PO | FO | CO | ||
| Liver | ||||
| Fat (%) | 8.90 ± 0.289 | 8.62 ± 0.180 | 8.14 ± 0.518 | NS |
| Triglycerides (mg/g) | 6.02 ± 0.566 | 4.59 ± 0.627 | 4.66 ± 0.415 | NS |
| Cholesterol (mg/g) | 1.25 ± 0.033 | 1.20 ± 0.079 | 1.13 ± 0.043 | NS |
| Plasma markers | ||||
| ALT (U/L) | 25.6 ± 1.21 b | 29.9 ± 2.07 ab | 32.5 ± 1.45 a | <0.01 |
| AST (U/L) | 54.4 ± 3.24 | 55.6 ± 3.16 | 54.7 ± 3.11 | NS |
| ALP (U/L) | 175 ± 9.21 b | 240 ± 22.224 a | 265 ± 15.0 a | <0.01 |
| Bile acids (µmol/L) | 15.5 ± 0.739 a | 11.4 ± 0.209 b | 11.3 ± 0.382 b | 0.001 |
| Total bilirubin (µmol/L) | 3.53 ± 0.725 | 3.65 ± 0.850 | 3.23 ± 0.425 | NS |
| Creatinine (µmol/L) | 9.40 ± 1.63 | 10.8 ± 2.19 | 8.70 ± 1.85 | NS |
| Urea (mmol/L) | 6.70 ± 0.257 | 6.36 ± 0.239 | 6.72 ± 0.228 | NS |
| Ingredient (%) | Group 1 | ||
|---|---|---|---|
| PO | FO | CO | |
| Casein 2 | 20.00 | 20.00 | 20.00 |
| DL-methionine | 0.3 | 0.3 | 0.3 |
| Palm oil 3 | 7 | – | – |
| Flaxseed oil 4 | – | 7 | – |
| Camelina seed oil 4 | – | – | 7 |
| Corn starch | 53 | 53 | 53 |
| Sucrose | 10 | 10 | 10 |
| Cellulose | 5 | 5 | 5 |
| Mineral mix 5 | 3.5 | 3.5 | 3.5 |
| Vitamin mix 5 | 1 | 1 | 1 |
| Choline chloride | 0.2 | 0.2 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babu, R.S.; Jurgoński, A. Nutritional and Metabolic Consequences of Camelina Seed Oil Compared to Flaxseed Oil in a Rat Diet. Molecules 2025, 30, 2738. https://doi.org/10.3390/molecules30132738
Babu RS, Jurgoński A. Nutritional and Metabolic Consequences of Camelina Seed Oil Compared to Flaxseed Oil in a Rat Diet. Molecules. 2025; 30(13):2738. https://doi.org/10.3390/molecules30132738
Chicago/Turabian StyleBabu, Reshma Susan, and Adam Jurgoński. 2025. "Nutritional and Metabolic Consequences of Camelina Seed Oil Compared to Flaxseed Oil in a Rat Diet" Molecules 30, no. 13: 2738. https://doi.org/10.3390/molecules30132738
APA StyleBabu, R. S., & Jurgoński, A. (2025). Nutritional and Metabolic Consequences of Camelina Seed Oil Compared to Flaxseed Oil in a Rat Diet. Molecules, 30(13), 2738. https://doi.org/10.3390/molecules30132738








