1. Introduction
Metabolic remodelling is a requirement for proliferating cancer cells, and it has received increased focus during the last decade [
1]. Cancer cells, in general, have demonstrated enhanced consumption of glucose and lactate production even under normoxic circumstances [
2,
3,
4,
5,
6]. The increased glucose consumption rate is undoubtedly a metabolic hallmark of cancer cells; however, oxidative phosphorylation (OXPHOS) occurs concomitantly with glycolysis using substrates other than glucose [
7]. On the other hand, glycolysis is primarily a precursor-supplying route for different metabolic biosynthetic pathways [
7]. Thus, cancer cells use intermediates of the glycolytic pathway as precursors for synthesizing amino acids, nucleic acids, and lipids, as well as to sustain the tumour cell’s antioxidant defences against hostile tumour microenvironments (TMEs) and chemotherapeutic agents [
8].
The impact of systemic metabolism and diet on tumour evolution is still not fully comprehended, necessitating new experiments exploring the influence of systemic metabolic controllers such as insulin and glucagon. The effect of insulin has been rather exploited in cancer, but glucagon has been passed over. Glucagon is a peptide hormone produced by the α-cells of the pancreatic Langerhans islets upon hypoglycaemia [
9] and exerts its effects by binding to the glucagon receptor (GCGR), a G-protein coupled receptor (GPCR) expressed primarily in liver cells [
10] and certain cancer cell types, including pancreatic neuroendocrine tumours (pNETs) [
11]. Upon binding, the GCGR activates intracellular signalling pathways such as the second messenger cyclic AMP-dependent protein kinase A (cAMP-PKA) pathway, which triggers glycogenolysis and gluconeogenesis in hepatocytes to increase blood glucose levels [
10,
12,
13].
In cancer cells, particularly pNETs, GCGR activation may also support malignant features by upregulating pro-survival and proliferation pathways, such as mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK), commonly associated with cell proliferation and survival, especially under metabolic stress conditions [
14,
15].
Therefore, the role of glucagon in human glucose homeostasis is unveiled, although at the cellular level, especially in cancer cells, much information is yet to be explored. Although insulin’s impact has been addressed in different cancer models, the impact of glucagon and hyperglucagonemia (>100 pmol/mL) on the TME and consequent effect on cancer metabolic remodelling is not well understood.
pNETs are a subtype of neuroendocrine neoplasms constituting 1–2% of all pancreatic neoplasms [
16,
17,
18], and they are derived from the islet of Langerhans cells with various morphologies and behaviours, including metastatic potential [
19]. The incidence of pNETs is rising in the western world [
20], and these tumours are divided into functioning and non-functioning types, ranging from indolent to aggressive, depending on histology and the production of active hormones [
21], including glucagon [
22]. The majority of pNETs (about 60%) are non-functioning [
22], and patients present obscure symptoms or are asymptomatic. Therefore, about 80% of pNETs are metastatic at diagnosis and the 5-year survival rate is 40–60% [
16,
23]. In addition, some studies in patients and animal models indicate pancreatic α-cell hyperplasia (ACH) as an important differential diagnosis of hyperglucagonemia upon inactivating mutations in the GCGR [
11,
24,
25,
26]. Furthermore, ACH is considered a preneoplastic lesion in the stepwise trajectory of pNET carcinogenesis [
25,
27]. This evidence reinforces the need for more studies to understand pNET biology further.
In addition to the GCGR, the glucagon-like peptide-1 receptor (GLP-1R) plays a role in modulating glucagon’s effects within the pancreatic microenvironment, being primarily known for inhibiting glucagon exocytosis by α-cells [
28]. Thus, the binding of glucagon to the glucagon-like peptide-1 receptor (GLP-1R) may subvert the system and contribute to hyperglucagonemia and the activation of signalling that may favour cancer. The subversive loop is influenced by glucagon levels in the tumour and organ microenvironment (TOME), as well as the mutational profile and expression levels of the GCGR and GLP-1R [
29].
In this study, the main goal is to investigate the role of glucagon as a component of the pancreatic microenvironment in the metabolic remodelling of pNETs, given that glucagon’s influence on cellular signalling pathways could be a driving factor in pNET malignancy. Considering that all pancreatic pNETs arise in the Langerhans islets, whose microenvironment is glucagon-rich [
30], and understanding how glucagon promotes tumour survival and metabolic remodelling in pNETs may provide insights into new therapeutic targets.
Specifically, we aim to explore the impact of glucagon on pNET cell features, depending on glucose bioavailability. Additionally, we attempt to explore the potential utility of the GCGR and GLP-1R (the receptor of GLP-1, which is the repressor of glucagon exocytosis by α-cells) [
12,
13], as prognosis markers and/or therapeutic targets. The role of glucagon as a cancer regulator, and the usefulness of the GCGR and GLP1-R as biomarkers are underexplored.
2. Materials and Methods
2.1. Cell Culture
Two pancreatic neuroendocrine tumour (pNET) cell lines, BON-1 (CVCL-3985; JCRB cell Bank, Ibaraki City, Osaka, Japan), derived from a lymph node metastasis of a pancreatic serotonin-producing carcinoid [
31], and QGP-1 (CVCL-3143; JCRB cell Bank), derived from a somatostatinoma [
32], were used in this study. Additionally, a non-malignant immortalized α-cell line from pancreatic islets of Langerhans, α -TC1 clone 6 (CRL-2934™, ATCC, Manassas, VA, USA), was used as a control for glucagon stimulus on cell features and metabolism, since glucagon’s function in mice and humans is conserved [
33,
34]. BON-1 cells were cultured in Dulbecco’s modified Eagle medium/nutrient mixture F-12 (Ham) 1× (DMEM/F-12) (11330-032, Gibco, Life Technologies, Waltham, MA, USA), while QGP-1 cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 Medium 1× (12-167F, Lonza, Bioscience, Basel, Switzerland) supplemented with L-glutamine. α-TC1 clone 6 cells were cultured in Dulbecco’s modified Eagle medium 1× (DMEM) (41965-039, Gibco, Life Technologies, MA, USA) containing 4.5 g/L of D-glucose and 0.58 g/L L-glutamine. No-glucose experiments were performed using DMEM (P04-01549, PAN Biotec, Aidenbach, Germany). All cell culture media were supplemented with 10% foetal bovine serum (FBS; S 0615, Merck, Rahway, NJ, USA), 1% antibiotic-–antimycotic (AA; P06-07300, PAN Biotech) and 50 µg/mL gentamicin (15750-060, Gibco, Life Technologies, MA, USA). Cells were plated on gelatin-coated T75 flasks (75 cm
2) and cultured until they reached 75–100% optical confluency. The detachment of cells was achieved using 0.05% trypsin-EDTA 1× (25300-054, Invitrogen, Waltham, MA, USA) at room temperature (RT), for 3–5 minutes (min). Cells were maintained under 37 °C, 5% CO
2, in a humidified environment. The concentrations of glucagon (G1774, Sigma-Aldrich, St. Louis, MO, USA) were selected in order to simulate physiological serum concentrations (50 and 100 pg/mL) and hyperglucagonemia, at concentrations compatible with glucagonoma syndrome (250 and 500 pg/mL) [
35]. For each assay, the cell number was determined in a Bürker counting chamber. Before any in vitro experiment, cells were synchronized by overnight starvation in 1% FBS culture medium.
2.2. Cell Viability
For cell viability, cells were plated in 96-well plates, and depending on the cell line, different numbers of cells were used: BON-1 and QGP-1 cell lines (6 × 103 cells/well/100 µL), and α-TC1 clone 6 (2 × 104 cells/well/100 µL). After starvation, cells were cultured in experimental conditions for 24 and 48 h. Cell viability was assessed using Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Gaithersburg, MD, USA), according to the manufacturer’s instructions, to evaluate the effect of different concentrations of glucagon (50, 100, 250, and 500 pg/mL; G1774, Sigma-Aldrich, St. Louis, MO, USA) on malignant features of pNET cells and non-malignant α-TC1 cells. Briefly, after treatment conditions, the CCK-8 solution was added to the culture medium and incubated at 37 °C for 2 h. The absorbance related to the conversion of the tetrazolium salt (WST-8) into a water-soluble formazan dye was measured at 450 nm with gentle shaking in an iMark Microplate Absorbance Reader (1681130, Bio-Rad, Hercules, CA, USA). Cell viability was determined using the formula (experimental group absorbance value/control group absorbance value) × 100%, and presented as a fold change to the control.
2.3. Proliferation Curves
For cell proliferation assays, cells were plated in 96-well plates, and depending on the cell line, different numbers of cells were used: BON-1 and QGP-1 cell lines (6 × 103 cells/well/100 µL), and α-TC1 clone 6 (2 × 104 cells/well/100 µL). After starvation, cells were cultured in experimental conditions for 24 and 48 h. Cells in suspension were collected to an Eppendorf and adherent cells were washed with PBS (1×), and harvested using 200 µL of 0.05% trypsin-EDTA; then, cells were added to the respective supernatant (conditioned culture media) and centrifuged at 150× g for 3 min. The total cell number per mL was calculated at different time points (0, 24 and 48 h) using a Neubauer counting chamber.
2.4. Immunofluorescence
For the immunofluorescence assay to detect and evaluate the expression of the glucagon receptor (GCGR) and GLP-1 receptor (GLP-1R), cells (1 × 10
5 cells/ well/500 µL) were seeded on a 24-well plate on the top of coverslips coated with 0.2% gelatin (G-1890, Sigma Aldrich). After 24 h of experimental conditions, cells were fixed with 200 µL of 2% paraformaldehyde for 15 min at RT. Then, cells were rinsed with PBS (1×) and incubated with 50 mM of NH4Cl in PBS (1×). First, 0.5% BSA-0.1% saponin-PBS (
w/
v/
v) was used for 15 min at room temperature (RT) to block and permeabilize cells. Cells were then incubated with primary antibodies (rabbit anti-human GCGR, ab75240, Abcam; rabbit anti-human GLP-1R, 1R1J8, Invitrogen), diluted 1:500 and 1:300, respectively, in 0.5% BSA0.1% saponin-PBS (
w/
v/
v) overnight, at 4 °C with slow agitation. Cells were washed 3 × with 0.5% BSA-0.1% saponin-PBS (
w/
v/
v) and incubated with Alexa Fluor
® 488 goat anti-rabbit (A11034, Invitrogen) secondary antibody, diluted 1: 1000 in 0.5% BSA-0.1% saponin-PBS (
w/
v/
v) for 2 h at RT. To validate the specificity of the secondary antibody, negative controls without primary antibodies were used. Cells were washed with 0.5% BSA-0.1% saponin-PBS (
w/
v/
v) and then rinsed with PBS (1×). The coverslips were mounted in slides with VECTASHIELD media with DAPI (4′-6-diamidino-2-phenylindole) (H-1200, VectorLabs). The analysis was performed by standard fluorescence microscopy using a Zeiss Imajer.Z1 AX10 microscope. Images were acquired and processed with CytoVision v.7.1 software and quantified using Image J v.1.53 software (
rsb.info.nih.gov/ij/).
2.5. Relative Quantifying Real-Time Polymerase Chain Reaction (RQ-PCR)
For gene expression analysis, mRNA was quantified by RT plus RQ-PCR. For this, 2 × 10
5 cells/wells/1 mL were cultured in 12-well plates. After starvation, cells were cultured under experimental conditions for 48 h. RNA extraction was performed using the RNeasy Mini Kit Qiagen
® (74104, Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The concentration of RNA samples was measured at 260 nm using a NanoDrop 2000 (ND-2000, Thermo Scientific, Waltham, MA, USA). For cDNA synthesis, 0.5 µg of total RNA was incubated at 70 °C for 10 min with random primers (11034731001, Roche, Basel, Switzerland) and water to achieve a final volume of 8 µL. Subsequently, cDNA was synthesized using SuperScript™ II reverse transcriptase (18080-44, Invitrogen), RNAse OUT™ (10777-019, Invitrogen), and a mix of deoxynucleotides (dNTPs) (10 mM; 28-4065-22V, 28-4065-02V, 28-4065-12V, and 28-4065-32V, GE Healthcare) following the manufacturer’s protocol. The influence of glucagon on the expression of metabolic key genes was evaluated by RQ-PCR using specific primers for human genes (
Table 1) and SYBR
® Green Master Mix (04707516001, Roche, Basel, Switzerland) according to the manufacturer’s instructions. The reactions and quantification were conducted using a Lightcycler
® 480 System instrument (05015243001, Roche). The hypoxanthine-guanine phosphoribosyltransferase 1 gene (HPRT1) served as a reference or housekeeping gene for normalization purposes.
2.6. Wound Healing Assay
For the wound-healing assay, 3.5 × 105 cells/well/1 mL were plated in 12-well plates and cultured onto gelatin until they formed a confluent monolayer. To ensure the accuracy of the wound healing progression, cell proliferation was inhibited by the addition of mitomycin-C, an effective antimitotic agent, (M4287, Sigma), followed by incubation for 3 h. Next, a linear scratch, simulating a wound, was created across the diameter of each well using a precise P200 pipette tip, creating a distinct gap in the cell monolayer. After the scratch, the media was replaced to eliminate debris and suspended cells, exposing the cells to the experimental conditions (control, 50, 250, and 500 pg/µL of glucagon) and control conditions. Bright-field images of each well were captured at specific timepoints (0, 10, 24, and 48 h) using the Olympus IX53 Inverted Microscope. ImageJ v.1.53 software was utilized to analyse and quantify the obtained images.
2.7. Western Blotting
For Western blotting, 5 × 105 cells/well/2 mL were plated in a 6-well plate. After starvation, the cells were cultured for 48 h in the presence of 250 pg/mL of glucagon, with or without glucose, and control conditions. To assess the effect of the activation of MAPK signalling pathway induced by glucagon, the levels of phosphorylated extracellular signal-regulated kinase1/2 (pERK1/2) were analysed using the Western blotting technique. Cell pellets were lysed in 3-(N-morpholino)propanesulfonic acid (MOPS) buffer and stored at −20 °C. The protein concentration was determined using the Bradford method and spectrophotometric quantification at 595 nm with the Bio-Rad protein assay reagent. Samples were prepared by adding 5× loading buffer with 10% β-mercaptoethanol to each cell lysate, followed by boiling at 95–100 °C for 10 min. After centrifugation, the denatured protein samples were loaded into a 12% polyacrylamide gel and separated by electrophoresis (PAGE) using the MINI-PROTEAN Tetra Electrophoresis System. Electrophoresis was conducted in 1× TGS buffer at 140 V. Subsequently, the proteins were transferred from the gel to an Immuno-Blot PVDF membrane using the Trans-Blot Turbo Blotting system. The membrane was blocked with 5% BSA in 1× TBS containing 0.1% Tween 20 for 2 h, at RT. For protein detection, the membrane was incubated overnight at 4 °C with anti-p ERK1/2 (anti-phospho p44/42 MAPK, 9101, Cell Signalling Technology, Danvers, MA, USA, at 1:1000 concentration), in 3% BSA in 1× TBS containing 0.1% Tween 20, overnight. After washing off the unbound primary antibodies, the membrane was incubated with a secondary antibody IgG-conjugated horseradish peroxidase (HRP; anti-rabbit, 1:5000, 31460, Thermo Scientific) for 2 h, at RT. Following additional washes, the immunoreactive bands on the membrane were developed using an ECL chemiluminescent substrate and captured using a ChemiDoc XRS System with Image Lab v2.0.1 software. Total ERK1/2 was determined using the anti-p44/42 MAPK (Erk1/2; 137F5, Cell Signalling Technology, at 1:1000 concentration), in 3% BSA in 1× TBS containing 0.1% Tween 20, overnight. After washing off the unbound primary antibodies, the membrane was incubated with a secondary antibody IgG-conjugated horseradish peroxidase (HRP; anti-rabbit, 1:5000, 31460, Thermo Scientific) for 2 h, at RT. To normalize the protein levels, the membrane was subsequently probed with a mouse anti-human α-tubulin antibody (1:4000; clone B-5-1-2, T5168, Merck KGaA) followed by a secondary antibody IgG-conjugated horseradish peroxidase (HRP; anti-mouse, 1:5000, 31430, Thermo Scientific). The bands were quantified using Image J software.
2.8. Nuclear Magnetic Resonance (NMR) Spectroscopy
For nuclear magnetic resonance (NMR) spectroscopy, 1 × 105 cells/well/500 µL were cultured in 24-well plates, and the supernatant was collected, cleared upon centrifugation (150× g, 5 min) and stored at −80 °C until NMR analysis. After starvation, cells were cultured in the experimental conditions for 48 h in the presence of 250 pg/mL of glucagon, without or with glucose (4.5 g/L). NMR spectroscopy was performed to define the exometabolome (metabolic profiles of conditioned culture media) under the influence of glucagon in the absence or presence of glucose in pNET cell lines and in the non-malignant α-TC1 cell line. The supernatants were collected, and centrifuged 150× g for 3 min, and stored at −80 °C to preserve their integrity. For sample preparation, 60 µL of a solution containing 0.022 mM trimethylsilyl propionate-d4 (TSP) and 0.04% (v/v) sodium azide, both in D2O, was added in a 1:1 proportion to each supernatant. TSP serves as an internal standard, providing a reference point for concentration measurements. 1H-NMR spectra were acquired in a 500 MHz magnetic field in the 500 UltraShieldTM Spectrometer (Bruker) using a 5 mm TCI-z (5 mm), using the noesypr1d pulse program, at 25 °C. Acquisition parameters were as follows: 128 scans, a spectral width of 11.7616 ppm, and a free induction decay (FID) of 0.244711 Hz. To process and analyse the obtained spectra, the TopSpin 4.1 software (Bruker) was used. Spectral processing techniques, such as baseline correction and referencing, were applied to enhance the quality of the spectra. Additionally, spectral assignments were performed to identify and characterise the metabolites present in the samples by resorting to spectral databases: Chenomx NMR Suite 8.11 and Human Metabolome (HMDB). Results are expressed as the net concentration of organic compounds in the conditioned culture media. The initial concentration of the same compounds in the fresh (naïve) media were considered to discuss either the augment/production or reduction/consumption upon cell culture at the different conditions.
2.9. Immunohistochemistry (IHC)
The expression of the GCGR and GLP-1R was analysed in 60 paired tumour and normal tissue samples. These pNET specimens were obtained from a cohort of patients (23 men and 37 women; 50% were 24–55 years old and 50% were 55–85 years old) at the Pathology Department of Hospital Curry Cabral from the Centro Hospitalar Universitário Lisboa Central (CHULC). The retrospective study evaluated surgical samples collected from 2013 and 2020 and received approval from the Ethical Committee of CHULC (REF: 1264/2022). Immunodetection was performed in 4 µm thick formalin-fixed, paraffin-embedded tissue samples, after deparaffinization and rehydration according to standard protocols [
36]. The evaluation of the GCGR and GLP-1R expression in tumours and normal pancreas tissue provided information on the distribution and abundance of the GCGR and GLP-1R and on their dynamics upon malignancy. Specific primary antibodies against the GCGR and GLP-1R were used (rabbit anti-human GCGR, 1:500, ab75240, Abcam, and rabbit anti-human GLP-1R, 1:300, 1R1J8, Invitrogen), for 16 min. Pretreatment (CC1—24 min; Ventana Medical Systems, Tucson, AZ, USA) with appropriate positive and negative controls samples was conducted using the BenchMark ULTRA IHC/ISH Automatic staining platform (Ventana Medical Systems, Tucson, AZ, USA) and the OptiView DAB IHC Detection Kit with diaminobenzidine as the chromogen to detect antigen expression. Tissue sections were counterstained with Mayer’s haematoxylin before mounting. Image acquisition was performed using a Digital Microimaging Device Leica DMD108 (version 1.15 Build 704, Leica Microsystems, Wetzlar, Germany). The histopathological analysis was performed by two independent pathologists and a detection score was defined (0—absent, 1—weak, 2—moderate, and 3—strong), as represented in
Figure S1.
2.10. Statistical Analysis
All results were analysed by GraphPad Prism 7.00 software (
www.graphpad.com/), using a student’s
t-test, one-way ANOVA, or two-way ANOVA to evaluate the statistical significance of results. The relationship between GCGR/GLP1R expression and clinicopathological features was analysed using univariate analysis (two-tailed t-test) and a binomial exact test on SPSS 24.0 software. The assays were performed with, at least, 3 replicates per condition and the differences were considered statistically significant at
p < 0.05. A multivariate statistical analysis of
1H NMR data was performed on MetaboAnalyst 5.0 (assessed on 4 August 2023) using metabolite concentrations as inputs and scaled using pareto-scaling.
1H-NMR data were subjected to unbiased metabolic profiling using principal component analysis (PCA), and for the maximization of the metabolic differences between different groups, a partial least square analysis (PLS-DA) was performed, which can be classified as supervised multivariate statistical analysis. Heatmaps representing the univariate analysis of the extracellular levels of the different metabolites detected by NMR were created for each cell line. The parameters that were used for the analysis were the Euclidean distance measure and the Ward cluster algorithm, using MetaboAnalyst 5.0 software.
4. Discussion
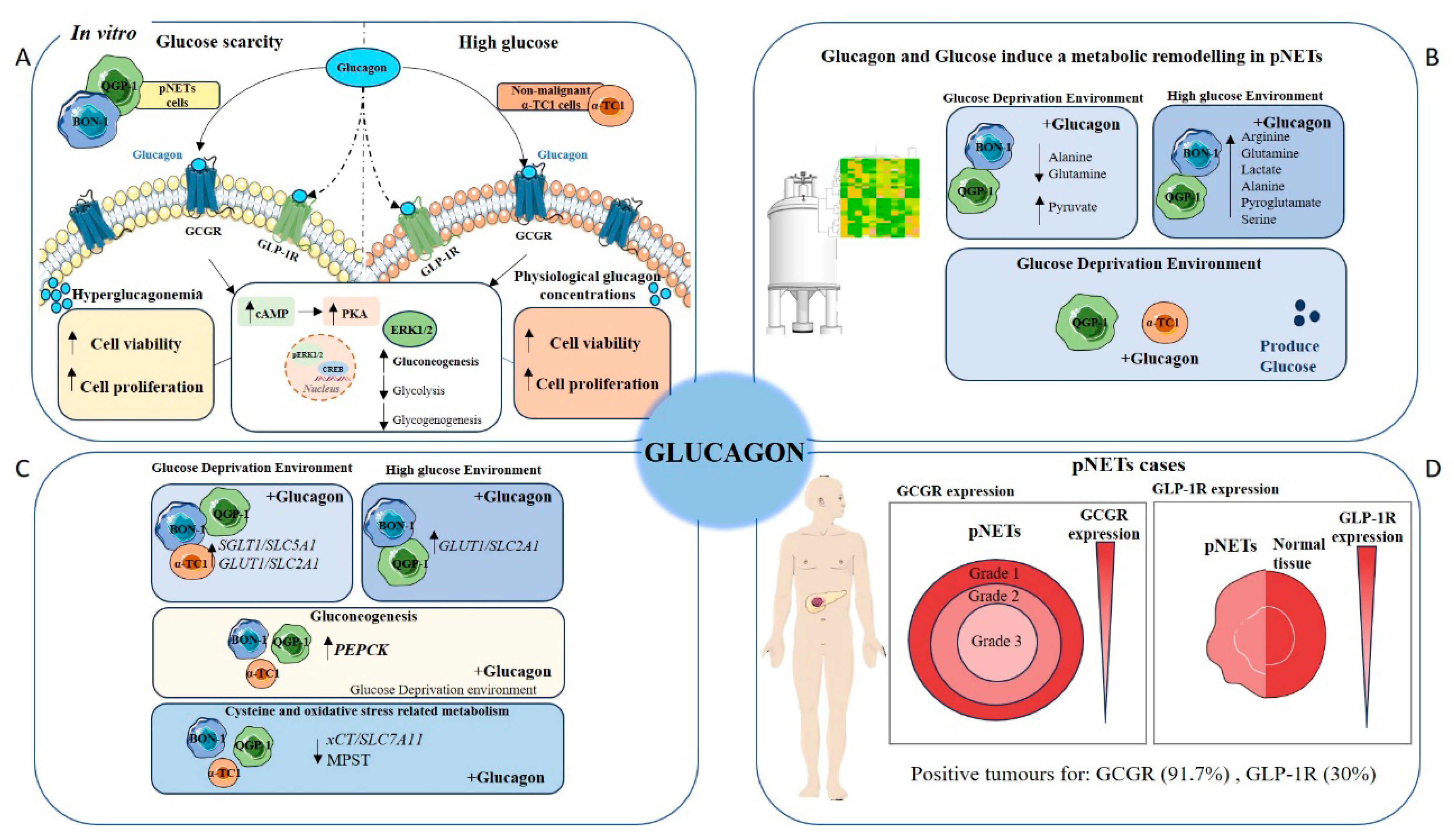
Pancreatic neuroendocrine tumours (pNETs) are challenging to diagnose because most cases are asymptomatic. Therefore, it is crucial to deepen our understanding of the biology of this disease to identify novel biomarkers and therapeutic targets. Given glucagon’s role as a metabolic regulator, it is essential to investigate how systemic metabolism and dietary factors influence tumour progression. Consequently, the role of glucagon in cancer cell survival and tumour progression is underexplored, despite its potential as a regulator of cellular metabolism in predominant organ systems. Our findings contribute to filling this gap, demonstrating that glucagon’s interactions with both the GCGR and GLP-1R add complexity to its role in cancer biology, potentially driving adaptive mechanisms in pNET cells [
40,
41,
42,
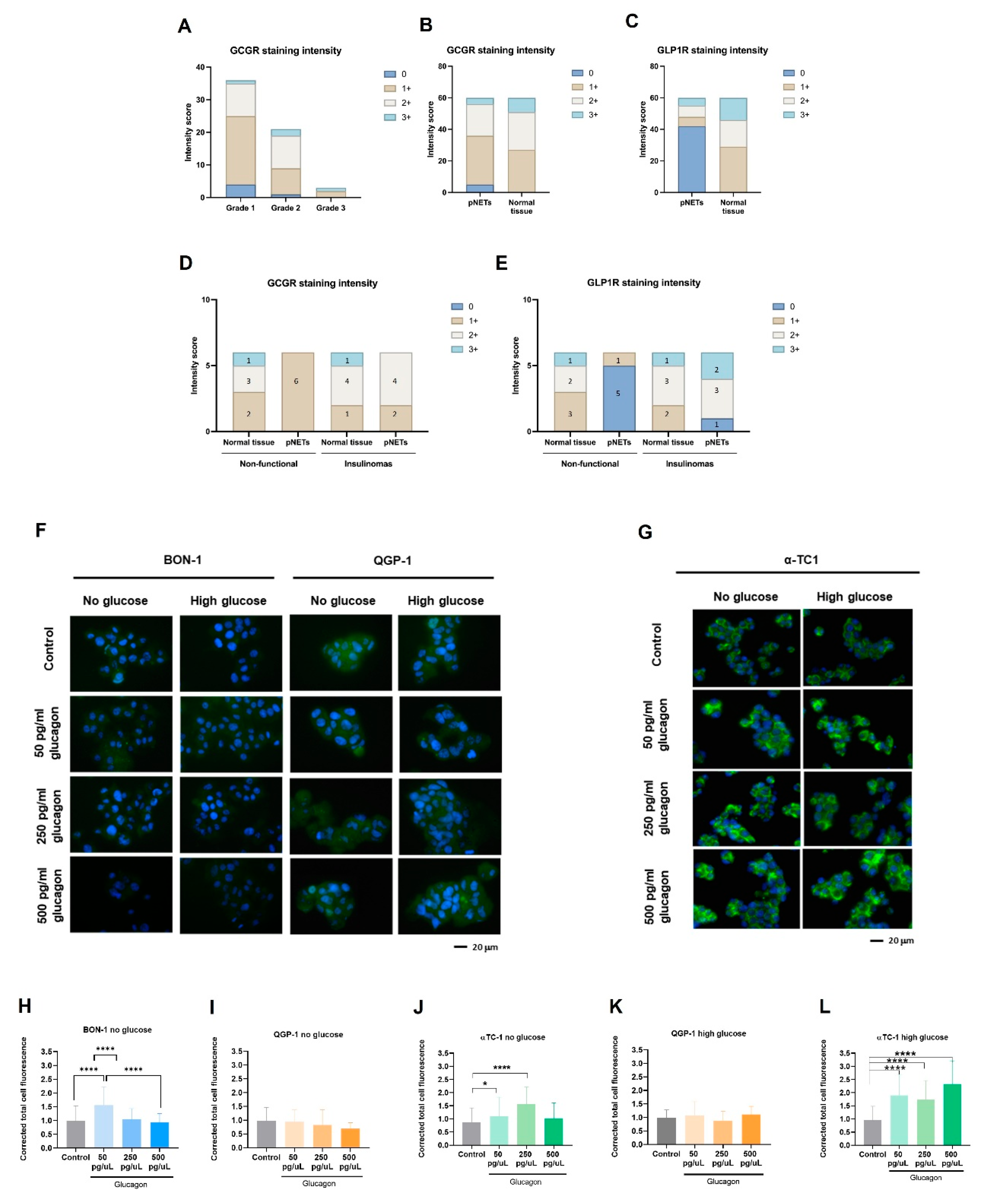
43]. This study used pNETs as a cancer model to explore how glucagon and glucose affect cancer cell metabolism and malignancy. This approach is irrespective of whether a pNET is a glucagonoma, as the majority of pancreatic pNETs develop from hormone-producing cells within the islet of Langerhans, exposing them to a glucagon-rich environment. Therefore, glucagon concentrations reflective of both physiological levels and hyperglucagonemia-related glucagonoma syndrome were tested, providing insights across a wide range of glucagon exposure. Our results confirm GCGR expression in pNET cell lines (BON-1 and QGP-1), with significant upregulation observed under glucose deprivation, especially in the presence of hyperglucagonemia-compatible concentrations (
Figure 1A–E). This suggests a potential compensatory response, whereby GCGR signalling may promote metabolic adaptations under nutrient-limited conditions, helping pNET cells meet their energy needs. Therefore, pNET cells can take advantage of the GCGR function in the regulation of glucose homeostasis and lipid metabolism, as it is described in hepatocytes, adipocytes, skeletal muscle cells, and pancreatic islet β-cells [
44,
45]. We further investigated GCGR expression in non-malignant pancreatic α-TC1 cells, the primary producers of glucagon, and observed significant expression under both high-glucose conditions and the three concentrations of glucagon (
Figure 1G,H). This observation suggests a potential feedback loop, wherein increased GCGR expression may regulate glucagon secretion or influence the overall endocrine balance within the pancreatic islet, as demonstrated by Yagi et al. [
14]. These findings collectively highlight the dynamic interplay between glucagon and glucose in modulating cellular responses, reinforcing glucagon’s role as a survival signal under metabolic stress.
The MAPK pathway is prominently stimulated by glucagon, leading to the activation of ERK1/2 [
15,
46], indicating a potential mechanism by which GCGR signalling impacts cellular responses within the pancreatic islet [
47]. It was observed that upon glucagon stimulation, QGP-1 cells presented increased pERK1/2 levels under glucose deprivation with no alterations in the pERK1/2 / total ERK1/2 ratio (
Figure 1I,J). However, when glucose was available, the levels of pERK1/2 tended to reduce upon glucagon exposure in both cell lines, suggesting a regulatory role of glucose in glucagon signalling through the ERK pathway (
Figure 1I,J), which must be explored. This interplay suggests that in nutrient-scarce environments, glucagon signalling could activate pathways that aid survival by enhancing adaptive metabolism [
8]. On the contrary, when glucose is available, cells need less extracellular survival cues, given their existing energy substrate. This is supported by the decreased pERK1/2 response to glucagon in the presence of glucose [
7]. Glucagon plays a role in the metabolic remodelling underlying pNET cell adaptation to stressful conditions.
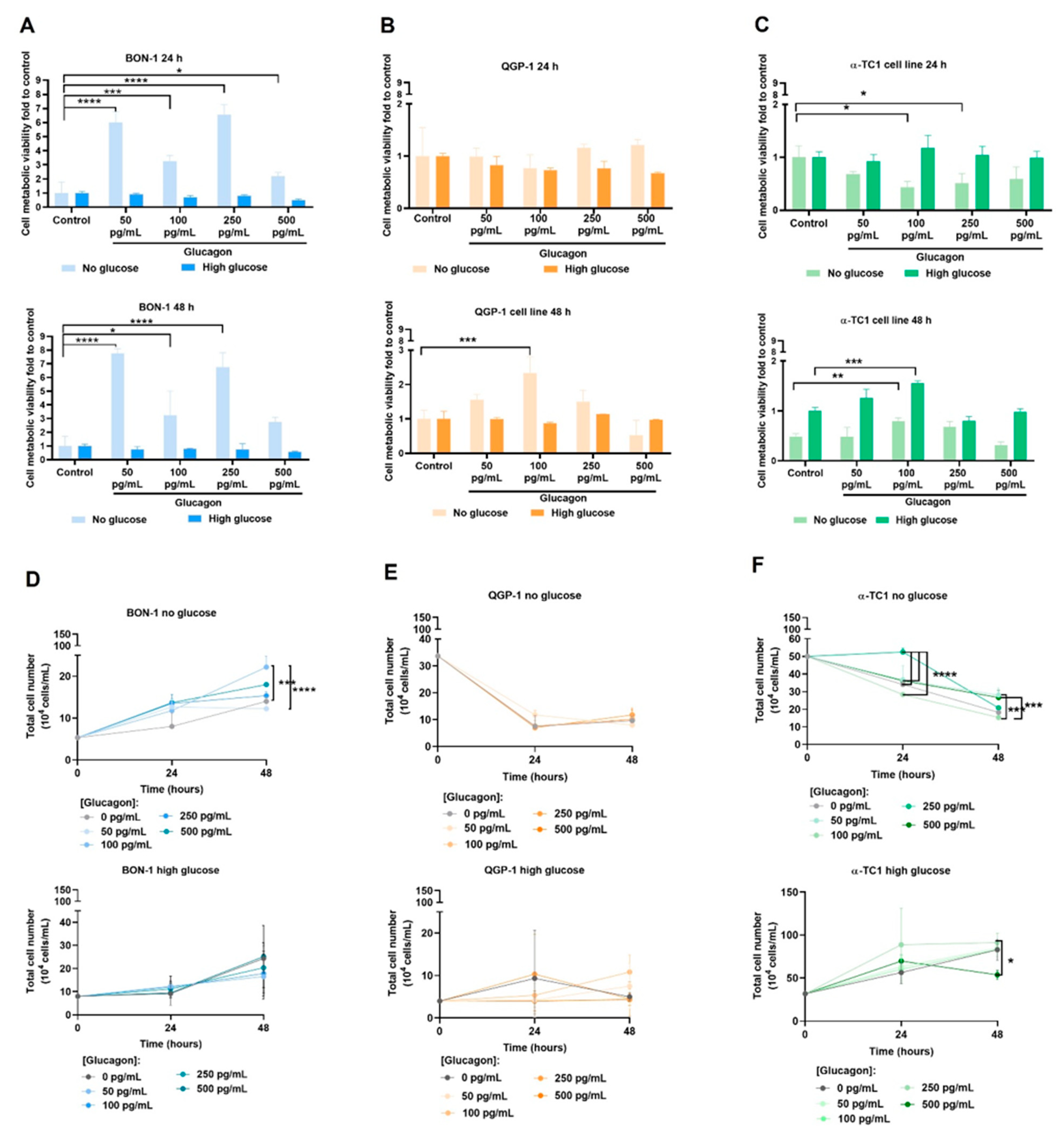
The differential effects of glucagon on cell viability, proliferation, and migration between BON-1 and QGP-1 cells provided further evidence of its role in pNET biology. Upon glucose deprivation, glucagon significantly increased BON-1 cell metabolic viability at all concentrations (
Figure 2A). In the same conditions, BON-1 cell proliferation was increased with glucagon (
Figure 2D), underscoring glucagon’s role as a potential mitogenesis stimulator [
14]. Overall, these findings suggest a glucagon-triggered pro-survival and adaptive mechanism to rescue viability and sustain cell proliferation under glucose deprivation, aligning with the GCGR expression and pERK1/2 levels observed in these conditions, as well as the fact that this cell line is unable to produce glucose in order to compensate its scarcity.
Regarding QGP-1 cells, no differences were observed due to glucagon exposure, except a significant rescue of cell viability, after 48 h at 100 pg/mL of glucagon (
Figure 2B). These viability findings align with the overall reduced proliferation in the absence of glucose but not in glucose-rich conditions over time (
Figure 2E). Our results indicate that QGP-1 cells possess mechanisms to overcome glucose scarcity, supported in part by their ability to produce glucose (
Figure 3L). This variability between cell lines indicates distinct mechanisms by which BON-1 and QGP-1 take advantage of glucagon stimulus.
The different responses of pNET cell lines to glucagon reflect the imbalance between the need for glucose and the ability to adapt to the lack of glucose. This is managed differently by these two cell lines, which showed metabolic remodelling and different abilities for consuming and producing glucose.
In terms of cell migration, under glucose scarcity, both cell lines were unable to close the wound mainly due to decreased adherent capacity. Despite QGP-1 cells also detaching under glucose-deprived conditions, this occurred later compared to BON-1 cells. Overall, QGP-1 cells exhibited higher migratory capacity than BON-1 cells, especially at hyperglucagonemia-compatible concentrations (
Figure 2J–L). In glucose-rich conditions, BON-1 cells showed increased wound closure at hyperglucagonemia-compatible concentrations (
Figure 2G–I). Given that BON-1 cells exhibit reduced GCGR levels in high-glucose conditions, these results imply that in the presence of scarce receptors, high glucagon concentrations more efficiently trigger a cellular migratory response. While the low migratory capacity of BON-1 and QGP-1 cell lines has been previously described [
26], the role of glucagon in pNET migration has not been addressed until now.
α-TC1 cells showed a direct correlation between viability and glucagon levels in high-glucose conditions (
Figure 2C). This emphasizes glucagon’s physiological role in promoting the survival and function of α-cells, especially when glucose is abundant [
15,
48]. However, the decreased viability in the absence of glucose at 24 h might indicate that these cells are dependent on glucose as a metabolic source (
Figure 2C). The significant increase in cell viability at 48 h, even under glucose deprivation, suggests an adaptive pro-survival response (
Figure 2C) and it can also be related to the adjustments these cells must undergo to produce glucose, as was observed (
Figure 3L). This is in line with the proliferation increase in no-glucose conditions under 500 pg/mL of glucagon (
Figure 2F). In high-glucose conditions, the proliferation of α-TC1 cells increases across most glucagon concentrations, suggesting a synergetic effect of glucose and glucagon in supporting α-cell proliferation (
Figure 2F). However, a decrease in proliferation was observed after 24 h of exposure to 500 pg/mL of glucagon, possibly due to reaching a saturation point or the activation of inhibitory feedback mechanisms [
43,
49]. Regarding migration, α-TC1 cells showed a moderate increase in migration in the presence of 50 pg/mL of glucagon under high-glucose conditions (
Figure 2N,P). This indicates that physiological levels of glucagon, paired with glucose, might slightly increase migration, which fits with the increased proliferation rate observed under the same conditions. Glucagon dynamics have been described as having a great impact on normal α-cell proliferation [
50,
51]. It is possible that, in a normal pancreas, glucagon is a regulator of tissue repair and equilibrium [
52,
53] and proliferation and migration are important phenomena involved.
The metabolic remodelling prompted by glucagon and glucose in pNET cells is evident in the heat maps and PCA and PLS-DA analyses (
Figure 3A–I). The amino acid profiles showed a clear impact of glucagon and glucose availability. Arginine and glutamine were exclusively detected in pNET cell lines under high-glucose conditions (
Figure 3A,B). Since the metabolism of glutamine and arginine is deeply connected, since they can be interconverted to answer cell needs [
54], this suggests that their metabolism is influenced by glucose availability. A synergistic effect between glucose and glucagon in BON-1 cells indicates that glucagon also influences amino acid dynamics (
Figure 4A). Conversely, the production of arginine and glutamine in QGP-1 cells seemed to be glucagon-independent (
Figure 3B). Furthermore, alanine, glutamine, and lactate levels decreased upon glucose scarcity, which may be related to the diversion of these compounds to gluconeogenesis. Conversely, alanine, pyroglutamate, and serine increased in BON-1 and QGP-1 under high-glucose conditions (
Figure 3A,B). Alanine holds a central role in glucose metabolism as a substrate for gluconeogenesis, at the cellular level, and systemically, as a player in the glucose-alanine cycle [
7]. Since pyroglutamate results from the degradation of glutathione [
55], its elevated levels in high-glucose conditions might indicate an increased demand for glutathione and consequently higher turnover, possibly as a way of maintaining the metabolic flow by sustaining redox reactions [
56]. Accordingly, glycine levels were increased in high-glucose conditions for QGP-1 cells (
Figure 3B), and glycine is a component of glutathione [
7]. Serine is pivotal in several pathways like one-carbon metabolism and protein, purine, and pyrimidine synthesis [
7]. Elevated serine levels in high-glucose contexts point to intensified serine synthesis driven by glucose availability. The increase in serine may also imply amplified needs in nucleotide synthesis, to sustain rapid proliferation and survival [
7]. Proline metabolism by proline oxidase promotes the production of ROS and controls cell survival by inducing apoptosis [
57]. The increased levels of proline in the extracellular media may contribute to increased cell viability since the import of proline is possibly reduced in glucose conditions. Contrarily, several amino acids, including isoleucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine, showed decreased levels in high-glucose conditions in QGP-1 cells (
Figure 3B). These amino acids are nutritionally indispensable [
58], so their reduced levels in high-glucose conditions might indicate an increased uptake to further supply metabolic pathways. The consistent observation that most amino acids levels were influenced by glucose conditions, irrespective of glucagon exposure, underscores the dominant role of glucose in dictating the metabolic behaviour of pNET cells, mainly QGP-1 cells.
Regarding organic acids, the increased levels of pyruvate in QGP-1 cells cultured in no-glucose conditions (
Figure 3D) suggest a shift in metabolic priorities, with the activation of pathways also accounting for pyruvate production as fatty acids and cysteine catabolism [
59]. Conversely, the increase in lactate levels in cells exposed to high glucose levels (
Figure 3D,E) suggests an enhanced glycolytic activity. In QGP-1 cells, the contribution of glucagon to this increment might indicate a glucagon-mediated enhancement of glycolysis (
Figure 3E). Accordingly, a glucagon-dependent hyperglycaemia is described as promoting cancer progression and angiogenesis under the activation of HIF1α pathways, which is the main controller of glycolysis [
60]. In both BON-1 and QGP-1, the higher levels of isobutyrate in cells cultured in no-glucose than in glucose conditions (
Figure 3A,D) demonstrates an adaptation to glucose scarcity, involving the activation of fatty acid or amino acid catabolism [
61].
Nucleotide and nitrogen base metabolism are crucial to sustain cell proliferation, and the detection of hypoxanthine in BON-1 cells (
Figure 3A), and the decreased levels of methylxanthine in all cell lines (
Figure 3G–I) are in agreement with the proliferation results obtained in high-glucose conditions. Hypoxanthine is a purine derivative and is a precursor in the synthesis of nucleic acids [
62] and a by-product of DNA repair mechanisms [
62]. The decreased methylxanthine levels might indicate a reduced catabolism of purines or an altered adenosine metabolism [
63].
In BON-1, glucagon increased the levels of tyramine in no-glucose conditions (
Figure 3I and
Figure S3G), indicating glucagon regulates catecholamine synthesis or secretion [
64,
65].
Regarding glucose itself, our results indicate that QGP-1 cells are the most efficient at consuming glucose, with an impressive 80% consumption rate (
Figure 3L). In contrast, BON-1 cells consumed only 50% of the available glucose (
Figure 3L). The differential consumption rates between these cell lines could be attributed to their distinct metabolic profiles and requirements, as demonstrated by their unique exometabolome. Regarding glucose production, BON-1 cells did not produce detectable levels of glucose, whereas QGP-1 cells evidently produced glucose (
Figure 3J). This ability of QGP-1 cells to produce glucose, together with increased levels of alanine in high-glucose conditions, indicates gluconeogenesis is activated in these cells.
Non-malignant α-TC1 cells presented a metabolic profile different in some aspects from pNET cell lines. The increased levels of arginine in high-glucose conditions (
Figure 3G), suggest an increased synthesis or decreased uptake, possibly driven by the availability of glucose. The observed decrease in lysine levels in high-glucose conditions (
Figure 3G) indicates either increased uptake or catabolism, reflecting adaptive mechanisms in response to glucose availability. The increased levels of isobutyrate and succinate in high-glucose conditions (
Figure S3F), suggest a decrease in TCA cycle activity [
7,
65], accounting for an increased export of these intermediates. The decreased levels of acetate, lactate, and pyruvate in high-glucose conditions (
Figure S3F) indicate a reduced glycolytic activity or enhanced consumption of these intermediates in oxidative phosphorylation. The absence of succinate detection in the presence of glucagon and high glucose levels (
Figure S3F) indicates that the TCA cycle is active, and cells are taking advantage of glucose for energetic purposes [
7,
66]. In α-TC1 cells, glucagon decreased the levels of tyramine in no-glucose conditions (
Figure 3I and
Figure S3G), indicating it impacts catecholamine metabolism [
64,
65]. Interestingly, α-TC1 cells consumed 30% of the available glucose, and glucagon increased glucose consumption by about 10% (
Figure 3L). This suggests an autocrine regulatory role of glucagon in modulating glucose metabolism in α-cells. The observed increase in alanine levels in high-glucose conditions (
Figure 3C) aligns with its role in gluconeogenesis, where alanine serves as a substrate for glucose production [
67]. Importantly, α-TC1 cells were the most efficient glucose producers, with glucagon stimulating a 2-fold increase in production (
Figure 3J). Such an observation is one of the most significant results of our study, underscoring the importance of α-cells in physiological contexts. Pancreatic neuroendocrine α-cells produce glucagon, which, in addition to its systemic role, acts on α-cells in an autocrine fashion to regulate their metabolism. This includes the activation of gluconeogenesis, thereby helping to resolve hypoglycaemia [
48].
Overall, the NMR exometabolome analysis reveals that, in glucose-rich conditions, pNET cells predominantly utilize glycolysis, leading to increased lactate and alanine production. However, under glucagon stimulation, especially under glucose deprivation, pNET cells shift towards gluconeogenesis and amino acid metabolism, using these pathways to sustain their energy needs.
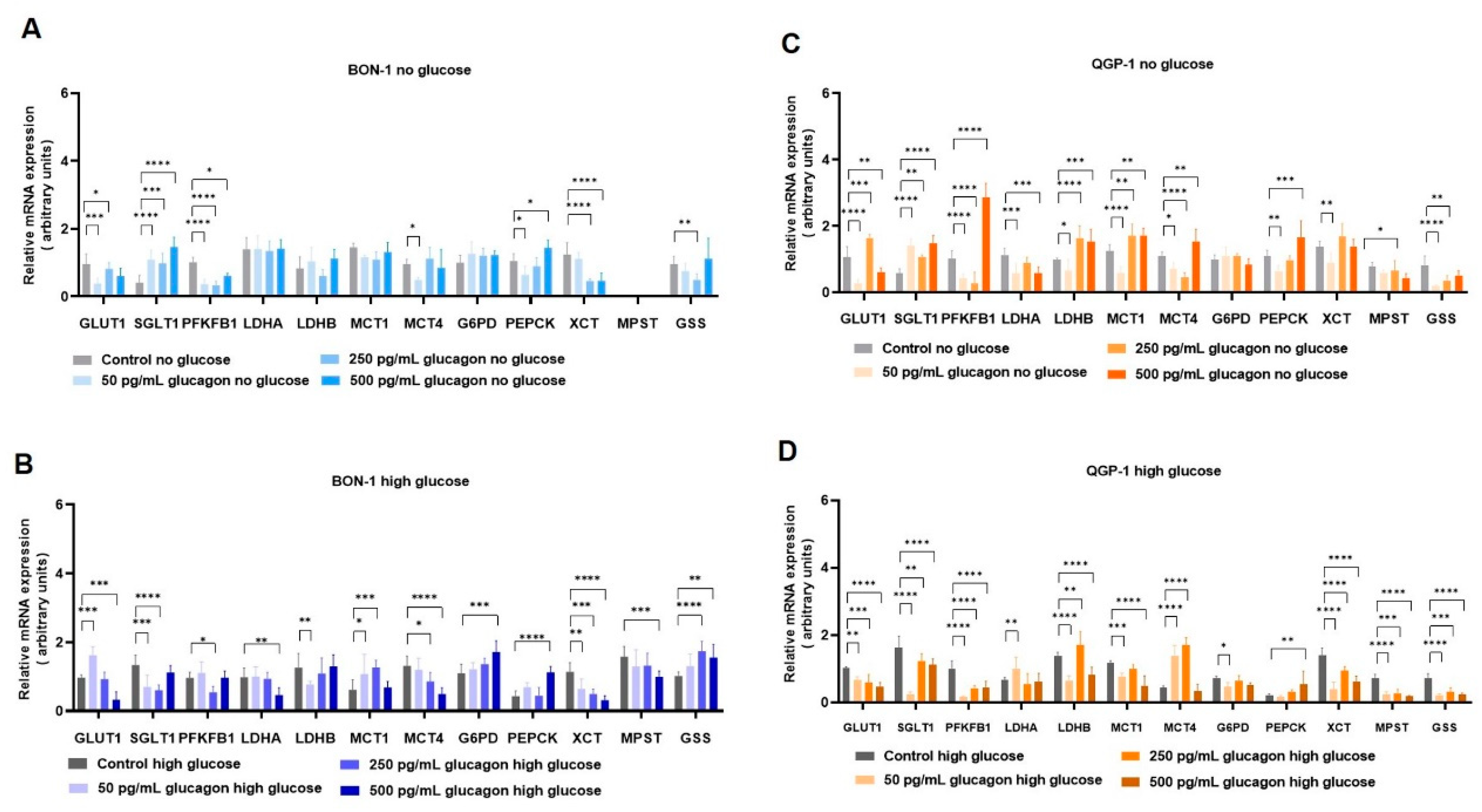
In the complex landscape of pNETs, understanding the genetic mechanisms and regulation underlying the foremost metabolic shifts offers a more comprehensive view (
Figure 6). In deprived glucose conditions, both BON-1 (
Figure 4A) and QGP-1 (
Figure 4C) increased the expression of genes encoding the glucose transporters
GLUT1/SLC2A1 and
SGLT1/SLC5A1, which may constitute a compensatory mechanism. In high-glucose conditions, a contrasting behaviour was observed in pNET cells. BON-1 (
Figure 4B) showed an increased expression of
GLUT1/SLC2A1 and
SGLT1/SLC5A1, while in QGP-1 (
Figure 4D) the expression of both genes decreased. This shows a decreased reliance on glucose supplementation in QGP-1 compared to BON-1 cells, which fits with QGP-1 cells’ efficiency in producing glucose.
Exploring gluconeogenesis, both BON-1 and QGP-1 cells, upon glucose scarcity, upregulated
PEPCK gene expression in response to glucagon stimulus (
Figure 4A,C), aligning with literature that assigns
PEPCK as a pivotal enzyme in pNETs [
68,
69,
70]. Considering cysteine and oxidative stress-related metabolism, BON-1 and QGP-1 cells decreased
xCT/SLC7A11 expression in response to glucagon, independent of glucose availability (
Figure 4A–D). In BON-1 (
Figure 4A), this corresponds with the decreased expression of
MPST, encoding an enzyme central to cysteine catabolism with the consequent production of pyruvate [
71], which fits with the decreased levels of pyruvate in no-glucose conditions. These results indicate glucagon promotes adjustments in redox control metabolism, given the role of
xCT/SLC7A11 in the uptake of cysteine for glutathione synthesis [
7,
71], which is reinforced by the decreased
GSS expression in response to glucagon exposure in BON-1 and QGP-1 cells.
In the context of pNET pathology, the expression of the GCGR presents a promising pathophysiological marker or therapeutic target (
Figure 6). Our study found that GCGR expression was inversely associated with tumour grade (
Figure 5A,B), suggesting possible differences in glucagon signalling between early-stage and more advanced tumours. Therefore, further investigations with a larger sample size and more grade 3 tumours will clarify this correlation. Nonetheless, 91.7% of cases were positive for GCGR expression suggesting a biological role for GCGR in disease that deserves to be investigated (
Table 4). Another interesting observation is the decreased expression of GLP-1R in pNETs compared to normal tissue (
Table 3 and
Figure 5C), which may reflect a disrupted insulin–glucagon axis. This is supported by the negative correlation between the two receptors (
Table 4) and the fact that 92.8% of GLP-1R-negative cases are positive for GCGR expression. Considering the role of GLP-1R in glucose homeostasis [
29,
72,
73], its diminished expression in pNETs could indicate a dysregulation in the insulin–glucagon axis [
28]. This is reinforced by the maintenance of high levels of GLP-1R in insulinomas (
Figure 5E), highlighting again the need for a systematic functional characterization of pNETs. Our in vitro analyses further corroborate the observed differences in GLP-1R expression between pNETs and healthy tissue (
Figure 5F–L); nevertheless, pNET cell lines used in this study are not insulinoma-derived, and the lack of pNET representative cell lines is a technical difficulty. Clinically, this inverse relationship could serve as a prognostic indicator, as high GCGR and low GLP-1R expression may signal more aggressive pNET phenotypes. Future studies could investigate the prognosis and therapeutic power of GCGR/GLP-1R ratio in pNETs.
5. Conclusions
Pancreatic neuroendocrine tumours (pNETs) present significant diagnostic challenges due to their often asymptomatic nature, underscoring the urgent need for deeper biological insights to identify novel biomarkers and therapeutic targets. Our study highlights glucagon’s pivotal role as a metabolic regulator in pNET biology, demonstrating that its interaction with the GCGR and GLP-1R orchestrates complex adaptive responses, particularly under conditions of glucose deprivation.
The differential responses of BON-1, QGP-1, and α-TC1 cells to glucagon highlight the complex and heterogeneous nature of pNET biology, as well as the physiological specificity of glucagon action in normal α-cells. pNETs present significant diagnostic challenges due to their often asymptomatic nature, emphasizing the urgent need for a deeper understanding of their underlying biology to identify novel biomarkers and therapeutic targets. Our study demonstrates that glucagon, through its interaction with GCGR and/or GLP-1R, plays a pivotal role in orchestrating metabolic and adaptive responses in both tumour and normal endocrine cells, particularly under conditions of glucose deprivation.
In BON-1 cells, glucagon significantly enhanced metabolic viability and proliferation under glucose-deprived conditions, suggesting a strong reliance on glucagon-triggered survival pathways. This effect is likely due to BON-1’s limited ability to produce glucose endogenously, necessitating external signals to sustain cell survival. The upregulation of GCGR expression and activation of the MAPK/ERK pathway further support glucagon’s role as a compensatory mitogenic and pro-survival factor in this context. These findings point to a glucagon-mediated metabolic remodelling in BON-1 cells, confirmed by NMR and involving the activation of pathways related to gluconeogenesis and amino acid utilization.
In contrast, QGP-1 cells exhibited a more restrained response to glucagon. While a modest rescue in cell viability was observed after 48 h of glucagon exposure, no significant proliferation increase occurred under glucose-deprived conditions. This muted response appears to stem from QGP-1’s intrinsic capacity for glucose production, allowing the cells to adapt more efficiently to nutrient scarcity without heavily relying on glucagon signalling. These contrasting behaviours between BON-1 and QGP-1 cells reflect the metabolic heterogeneity within pNETs and underscore how distinct adaptive strategies may influence their response to hormonal stimuli and potentially to therapeutic interventions.
The α-TC1 cell line, representative of normal pancreatic α-cells, displayed a more physiologically predictable response to glucagon. In high-glucose conditions, glucagon stimulated both viability and proliferation, in line with its known role in supporting α-cell function. Under glucose-deprived conditions, α-TC1 cells initially showed reduced viability, but a significant recovery was observed at 48 h, indicating an adaptive pro-survival response, likely tied to endogenous glucose production mechanisms. Additionally, α-TC1 cells exhibited a moderate increase in migration in response to glucagon, aligning with their proliferative behaviour and suggesting a potential role for glucagon in islet tissue maintenance and repair.
Together, these findings reveal that glucagon promotes cell viability, proliferation, and migration in a cell type- and context-dependent manner. In pNET cells, glucagon’s effects are closely tied to each cell line’s metabolic capabilities and receptor expression profiles. Clinically, the high prevalence of GCGR expression in pNET tissues and its inverse correlation with tumour grade, alongside the reduced GLP-1R expression, point to disrupted insulin–glucagon signalling in tumour progression. This receptor expression profile holds promise as a prognostic marker and potential therapeutic target.
In summary, this study expands our understanding of glucagon’s role in pNET pathophysiology, emphasizing the significance of metabolic regulation in tumour adaptation and highlighting GCGR as a key candidate for future biomarker development and targeted therapy. Further research into the insulin–glucagon axis and metabolic signalling pathways could pave the way for improved diagnostic and treatment strategies in pNET management.