From Tradition to Innovation: The Role of Sea Fennel in Shaping Kimchi’s Microbial, Chemical, and Sensory Profiles
Abstract
1. Introduction
2. Results
2.1. Measurement of pH
2.2. Determination of Titratable Acidity (TA)
2.3. Organic Acids Quantification
2.4. Microbial Enumeration
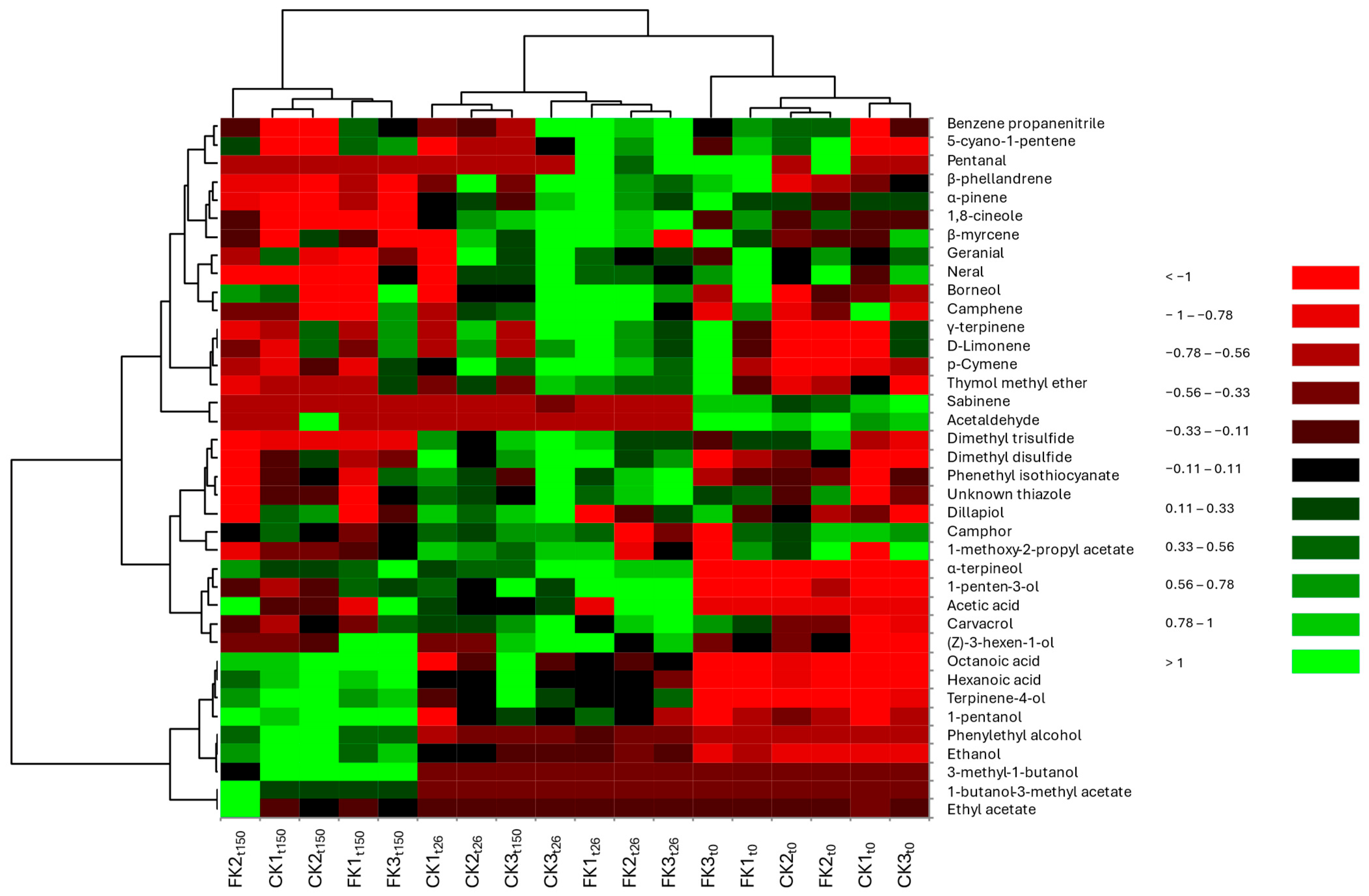
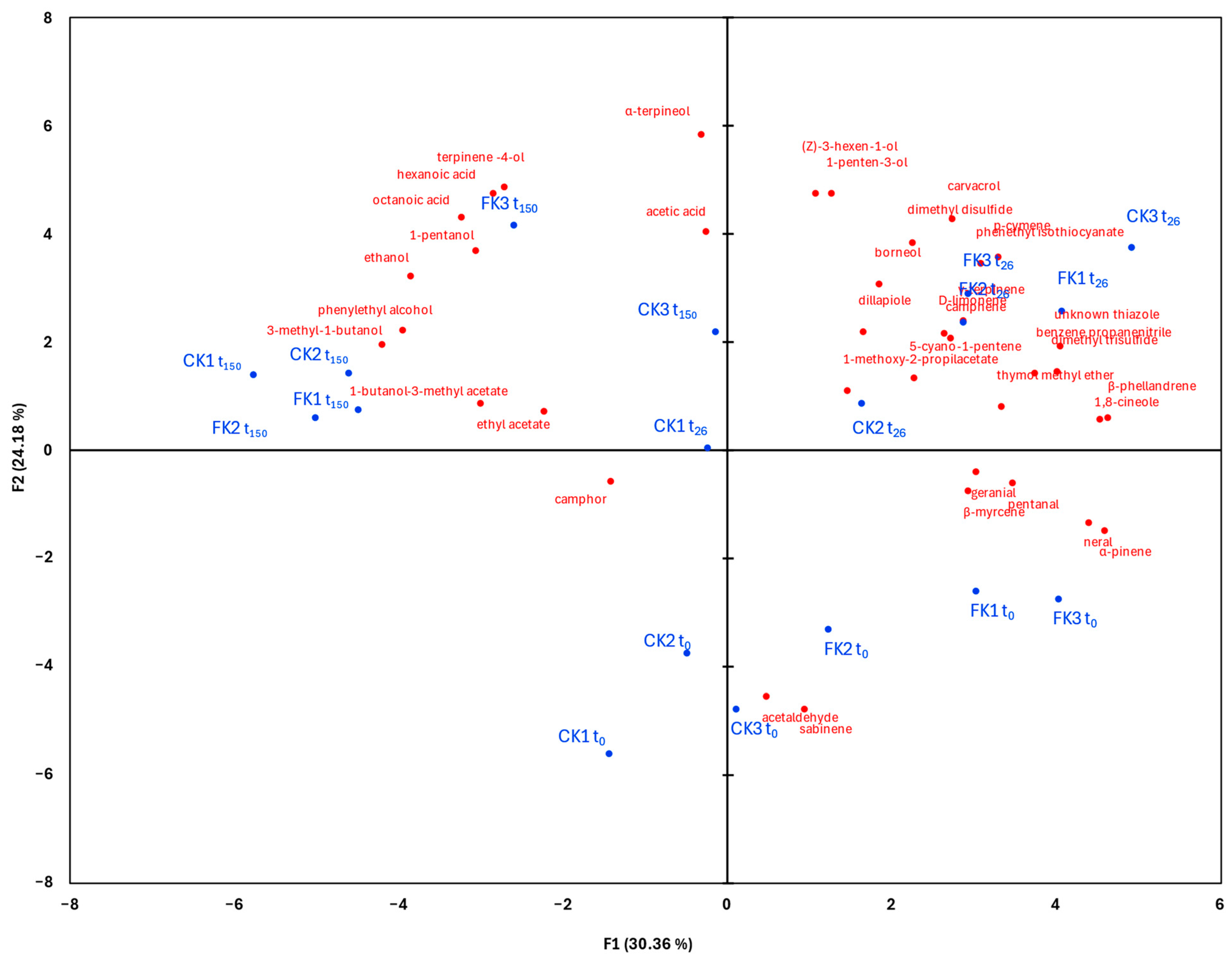
2.5. Volatile Organic Compounds (VOCs) Analysis
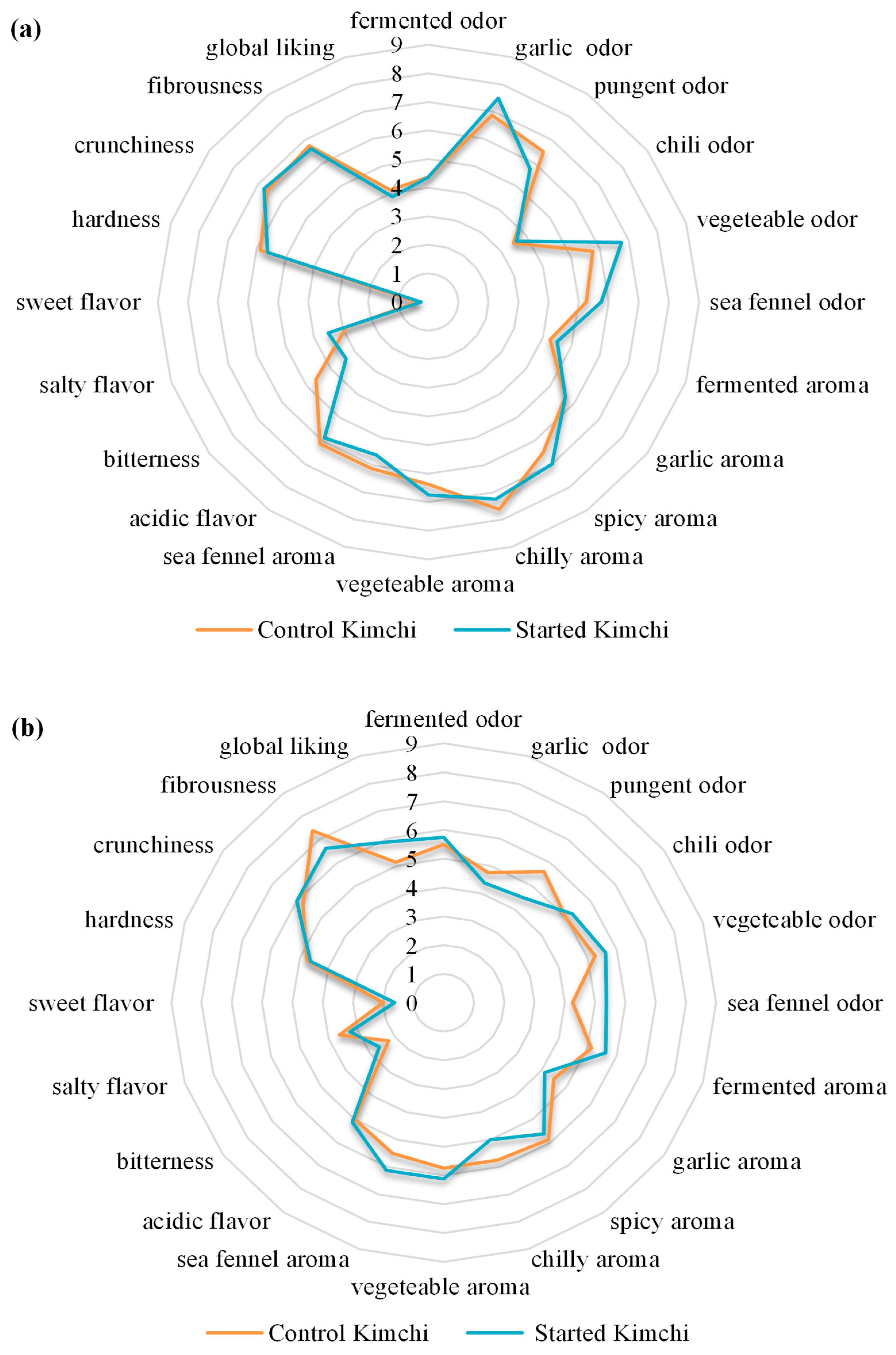
2.6. Sensory Evaluation
3. Discussion
4. Materials and Methods
4.1. Starter Formulation
4.2. Sea Fennel Pre-Treatment
4.3. Kimchi Manufacturing
4.4. Physicochemical Analyses
4.5. Microbiological Analyses
4.6. Determination of Volatile Compounds via Headspace Solid-Phase MicroExtraction–Gas Chromatography/Mass Spectrometry (HS/SPME-GC/MS)
4.7. Sensory Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chavan, J.K.; Kadam, S.S.; Beuchat, L.R. Nutritional Improvement of Cereals by Fermentation. Crit. Rev. Food Sci. Nutr. 1989, 28, 349–400. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Cui, F.; Wang, D.; Lv, X.; Li, X.; Li, J. Fermented Vegetables: Health Benefits, Defects, and Current Technological Solutions. Foods 2023, 13, 38. [Google Scholar] [CrossRef]
- Satora, P.; Skotniczny, M.; Strnad, S.; Piechowicz, W. Chemical Composition and Sensory Quality of Sauerkraut Produced from Different Cabbage Varieties. LWT 2021, 136, 110325. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Sarengaowa; Ji, Y.; Guan, Y.; Feng, K. Microbial Dynamics and Volatilome Profiles during the Fermentation of Chinese Northeast Sauerkraut by Leuconostoc Mesenteroides ORC 2 and Lactobacillus Plantarum HBUAS 51041 under Different Salt Concentrations. Food Res. Int. 2020, 130, 108926. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, Y.; Li, T.; Yang, Y.; Zeng, F.; Wang, H.; Suo, H.; Song, J.; Zhang, Y. Microbial Composition and Correlation between Microbiota and Quality-Related Physiochemical Characteristics in Chongqing Radish Paocai. Food Chem. 2022, 369, 130897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.; Liu, D. Biochemical Changes and Microbial Community Dynamics during Spontaneous Fermentation of Zhacai, a Traditional Pickled Mustard Tuber from China. Int. J. Food Microbiol. 2021, 347, 109199. [Google Scholar] [CrossRef]
- Lee, S.H.; Whon, T.W.; Roh, S.W.; Jeon, C.O. Unraveling Microbial Fermentation Features in Kimchi: From Classical to Meta-Omics Approaches. Appl. Microbiol. Biotechnol. 2020, 104, 7731–7744. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Paramithiotis, S.; Shin, H.-S. Kimchi and Other Widely Consumed Traditional Fermented Foods of Korea: A Review. Front. Microbiol. 2016, 7, 1493. [Google Scholar] [CrossRef]
- Lee, H.-W.; Yoon, S.-R.; Kim, S.-J.; Lee, H.M.; Lee, J.Y.; Lee, J.-H.; Kim, S.H.; Ha, J.-H. Identification of Microbial Communities, with a Focus on Foodborne Pathogens, during Kimchi Manufacturing Process Using Culture-Independent and -Dependent Analyses. LWT-Food Sci. Technol. 2017, 81, 153–159. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.H.; Jeon, C.O. Kimchi Microflora: History, Current Status, and Perspectives for Industrial Kimchi Production. Appl. Microbiol. Biotechnol. 2014, 98, 2385–2393. [Google Scholar] [CrossRef]
- Surh, J.; Lee, Y.-K.K.; Kwo, H. Korean Fermented Foods: Kimchi and Doenjang. In Handbook of Fermented Functional Foods; Farnworth, E.R., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 333–353. [Google Scholar]
- Maoloni, A.; Ferrocino, I.; Milanović, V.; Cocolin, L.; Corvaglia, M.R.; Ottaviani, D.; Bartolini, C.; Talevi, G.; Belleggia, L.; Cardinali, F.; et al. The Microbial Diversity of Non-Korean Kimchi as Revealed by Viable Counting and Metataxonomic Sequencing. Foods 2020, 9, 1568. [Google Scholar] [CrossRef]
- Chung, H.-K.; Yang, H.J.; Shin, D.; Chung, K.R. Aesthetics of Korean Foods: The Symbol of Korean Culture. J. Ethn. Foods 2016, 3, 178–188. [Google Scholar] [CrossRef]
- Cheigh, H. Production, characteristics and health functions of kimchi. Acta Hortic. 1999, 483, 405–420. [Google Scholar] [CrossRef]
- Lee, M.; Song, J.H.; Jung, M.Y.; Lee, S.H.; Chang, J.Y. Large-Scale Targeted Metagenomics Analysis of Bacterial Ecological Changes in 88 Kimchi Samples during Fermentation. Food Microbiol. 2017, 66, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-L.; Oh, C.-K.; Oh, M.-C.; Kim, S.-H. Isolation and Identification of Lactic Acid Bacteria from Commercial Kimchi. J. Korean Soc. Food Sci. Nutr. 2009, 38, 732–741. [Google Scholar] [CrossRef]
- CXS 223-2001; Standard for Kimchi. FAO/WHO Codex Alimentarius: Rome, Italy, 2001.
- Lee, M.-E.; Jang, J.-Y.; Lee, J.-H.; Park, H.-W.; Choi, H.-J.; Kim, T.-W. Starter Cultures for Kimchi Fermentation. J. Microbiol. Biotechnol. 2015, 25, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Shim, J.M.; Kim, D.W.; Yao, Z.; Kim, J.A.; Kim, H.-J.; Kim, J.H. Effects of Different Types of Salts on the Growth of Lactic Acid Bacteria and Yeasts during Kimchi Fermentation. Food Sci. Biotechnol. 2017, 27, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Cheigh, H.; Park, K.; Lee, C.Y. Biochemical, Microbiological, and Nutritional Aspects of Kimchi (Korean Fermented Vegetable Products). Crit. Rev. Food Sci. Nutr. 1994, 34, 175–203. [Google Scholar] [CrossRef]
- Lee, H.; Park, C.S.; Joo, Y.; Kim, S.; Yoon, J.; Park, Y.-H.; Hwang, I.; Ahn, J.; Mheen, T. Identification and Characterization of Bacteriocin-Producing Lactic Acid Bacteria Isolated from Kimchi. J. Microbiol. Biotechnol. 1999, 9, 282–291. [Google Scholar]
- Yun, J.W.; Kang, S.C.; Song, S.K. Mannitol Accumulation during Fermentation of Kimchi. J. Ferment. Bioeng. 1996, 81, 279–280. [Google Scholar] [CrossRef]
- Shim, S.-M.; Kim, J.Y.; Lee, S.M.; Park, J.-B.; Oh, S.-K.; Kim, Y.-S. Profiling of Fermentative Metabolites in Kimchi: Volatile and Non-Volatile Organic Acids. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 463–469. [Google Scholar] [CrossRef]
- Torres, S.; Verón, H.; Contreras, L.; Isla, M.I. An Overview of Plant-Autochthonous Microorganisms and Fermented Vegetable Foods. Food Sci. Hum. Wellness 2020, 9, 112–123. [Google Scholar] [CrossRef]
- Choi, E.A.; Chang, H.C. Cholesterol-Lowering Effects of a Putative Probiotic Strain Lactobacillus Plantarum EM Isolated from Kimchi. LWT-Food Sci. Technol. 2015, 62, 210–217. [Google Scholar] [CrossRef]
- Chang, J.Y.; Chang, H.C. Improvements in the Quality and Shelf Life of Kimchi by Fermentation with the Induced Bacteriocin-Producing Strain, Leuconostoc citreum GJ7 as a Starter. J. Food Sci. 2010, 75, M103–M110. [Google Scholar] [CrossRef] [PubMed]
- Generalić Mekinić, I.; Blažević, I.; Mudnić, I.; Burčul, F.; Grga, M.; Skroza, D.; Jerčić, I.; Ljubenkov, I.; Boban, M.; Miloš, M.; et al. Sea Fennel (Crithmum maritimum L.): Phytochemical Profile, Antioxidative, Cholinesterase Inhibitory and Vasodilatory Activity. J. Food Sci. Technol. 2016, 53, 3104–3112. [Google Scholar] [CrossRef]
- Maoloni, A.; Milanović, V.; Osimani, A.; Cardinali, F.; Garofalo, C.; Belleggia, L.; Foligni, R.; Mannozzi, C.; Mozzon, M.; Cirlini, M.; et al. Exploitation of Sea Fennel (Crithmum maritimum L.) for Manufacturing of Novel High-Value Fermented Preserves. Food Bioprod. Process. 2021, 127, 174–197. [Google Scholar] [CrossRef]
- Kraouia, M.; Nartea, A.; Maoloni, A.; Osimani, A.; Garofalo, C.; Fanesi, B.; Ismaiel, L.; Aquilanti, L.; Pacetti, D. Sea Fennel (Crithmum maritimum L.) as an Emerging Crop for the Manufacturing of Innovative Foods and Nutraceuticals. Molecules 2023, 28, 4741. [Google Scholar] [CrossRef]
- Renna, M.; Gonnella, M. The Use of the Sea Fennel as a New Spice-Colorant in Culinary Preparations. Int. J. Gastron. Food Sci. 2012, 1, 111–115. [Google Scholar] [CrossRef]
- Maoloni, A.; Cardinali, F.; Milanović, V.; Reale, A.; Boscaino, F.; Di Renzo, T.; Ferrocino, I.; Rampanti, G.; Garofalo, C.; Osimani, A.; et al. Impact of Different Drying Methods on the Microbiota, Volatilome, Color, and Sensory Traits of Sea Fennel (Crithmum maritimum L.) Leaves. Molecules 2023, 28, 7207. [Google Scholar] [CrossRef]
- Maoloni, A.; Cardinali, F.; Milanović, V.; Osimani, A.; Verdenelli, M.C.; Coman, M.M.; Aquilanti, L. Exploratory Study for Probiotic Enrichment of a Sea Fennel (Crithmum maritimum L.) Preserve in Brine. Foods 2022, 11, 2219. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of Vegetables and Fruits through Lactic Acid Fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Go, C.-Y.; Ha, D.-M. Microfloral Changes of the Lactic Acid Bacteria during Kimchi Fermentation and Identification of the Isolates. Microbiol. Biotechnol. Lett. 1992, 20, 102–109. [Google Scholar]
- Jung, J.Y.; Lee, S.H.; Lee, H.J.; Seo, H.-Y.; Park, W.-S.; Jeon, C.O. Effects of Leuconostoc Mesenteroides Starter Cultures on Microbial Communities and Metabolites during Kimchi Fermentation. Int. J. Food Microbiol. 2012, 153, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Mheen, T.-I.; Kwon, T.-W. Effect of Temperature and Salt Concentration on Kimchi Fermentation. Korean J. Food Sci. Technol. 1984, 16, 443–450. [Google Scholar]
- Cho, K.M.; Math, R.K.; Asraful Islam, S.; Lim, W.J.; Hong, S.Y.; Kim, J.M.; Yun, M.G.; Cho, J.J.; Yun, H.D. Novel Multiplex PCR for the Detection of Lactic Acid Bacteria during Kimchi Fermentation. Mol. Cell Probes 2009, 23, 90–94. [Google Scholar] [CrossRef]
- Jung, S.J.; Kim, M.J.; Chae, S.W. Quality and Functional Characteristics of Kimchi Made with Organically Cultivated Young Chinese Cabbage (Olgari-Baechu). J. Ethn. Foods 2016, 3, 150–158. [Google Scholar] [CrossRef]
- Lee, M.-A.; Choi, Y.-J.; Lee, H.; Hwang, S.; Lee, H.J.; Park, S.J.; Chung, Y.B.; Yun, Y.-R.; Park, S.-H.; Min, S.; et al. Influence of Salinity on the Microbial Community Composition and Metabolite Profile in Kimchi. Fermentation 2021, 7, 308. [Google Scholar] [CrossRef]
- Hong, G.-H.; Lee, S.-Y.; Park, E.-S.; Park, K.-Y. Changes in Microbial Community by Salt Content in Kimchi during Fermentation. J. Korean Soc. Food Sci. Nutr. 2021, 50, 648–653. [Google Scholar] [CrossRef]
- Bozoğlu, F.T.; Ray, B. Lactic Acid Bacteria: Current Advances in Metabolism, Genetics and Applications; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1996. [Google Scholar]
- Jay, J.M. Modern Food Microbiology; Springer: lin/Heidelberg, Germany, 1995. [Google Scholar]
- You, S.-Y.; Yang, J.-S.; Kim, S.H.; Hwang, I.M. Changes in the Physicochemical Quality Characteristics of Cabbage Kimchi with Respect to Storage Conditions. J. Food Qual. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Rhee, S.; Lee, J.-E.; Lee, C.-H. Importance of Lactic Acid Bacteria in Asian Fermented Foods. Microb. Cell Fact. 2011, 10, S5. [Google Scholar] [CrossRef]
- Surendran Nair, M.; Amalaradjou, M.A.; Venkitanarayanan, K. Antivirulence Properties of Probiotics in Combating Microbial Pathogenesis. Adv. Appl. Microbiol. 2017, 98, 1–29. [Google Scholar] [PubMed]
- Ryu, J.; Lee, H.; Rhee, H. Changes of Organic Acids and Volatile Flavor Compounds in Kimchis Fermented with Different Ingredients. Korean J. Food Sci. Technol. 1984, 16, 169–174. [Google Scholar]
- Pan, L.; Zhang, C.-J.; Bai, Z.; Liu, Y.-Y.; Zhang, Y.; Tian, W.-Z.; Zhou, Y.; Zhou, Y.-Y.; Liao, A.-M.; Hou, Y.-C.; et al. Effects of Different Strains Fermentation on Nutritional Functional Components and Flavor Compounds of Sweet Potato Slurry. Front. Nutr. 2023, 10, 1241580. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Kim, C.R.; Chang, H.C. Heterofermentative Lactic Acid Bacteria as a Starter Culture to Control Kimchi Fermentation. LWT 2018, 88, 181–188. [Google Scholar] [CrossRef]
- Chung, Y.B.; Park, S.J.; Choi, Y.-J.; Yun, Y.-R.; Lee, M.-A.; Park, S.H.; Min, S.G.; Seo, H.-Y. Metabolic Shift during Fermentation in Kimchi According to Capsaicinoid Concentration. Heliyon 2024, 10, e24441. [Google Scholar] [CrossRef]
- Cho, J.; Lee, D.; Yang, C.; Jeon, J.; Kim, J.; Han, H. Microbial Population Dynamics of Kimchi, a Fermented Cabbage Product. FEMS Microbiol. Lett. 2006, 257, 262–267. [Google Scholar] [CrossRef]
- Osimani, A.; Belleggia, L.; Botta, C.; Ferrocino, I.; Milanović, V.; Cardinali, F.; Haouet, M.N.; Garofalo, C.; Mozzon, M.; Foligni, R.; et al. Journey to the Morpho-Textural Traits, Microbiota, and Volatilome of Ciauscolo PGI Salami. Food Biosci. 2023, 53, 102582. [Google Scholar] [CrossRef]
- Park, S.-H.; Itoh, K.; Kikuchi, E.; Niwa, H.; Fujisawa, T. Identification and Characteristics of Nisin Z-Producing Lactococcus Lactis Subsp. Lactis Isolated from Kimchi. Curr. Microbiol. 2003, 46, 385–388. [Google Scholar] [CrossRef]
- Fadda, S.; López, C.; Vignolo, G. Role of Lactic Acid Bacteria during Meat Conditioning and Fermentation: Peptides Generated as Sensorial and Hygienic Biomarkers. Meat Sci. 2010, 86, 66–79. [Google Scholar] [CrossRef]
- Park, S.-E.; Seo, S.-H.; Kim, E.-J.; Byun, S.; Na, C.-S.; Son, H.-S. Changes of Microbial Community and Metabolite in Kimchi Inoculated with Different Microbial Community Starters. Food Chem. 2019, 274, 558–565. [Google Scholar] [CrossRef]
- Kim, J.; Bang, J.; Beuchat, L.R.; Kim, H.; Ryu, J.-H. Controlled Fermentation of Kimchi Using Naturally Occurring Antimicrobial Agents. Food Microbiol. 2012, 32, 20–31. [Google Scholar] [CrossRef]
- Song, H.S.; Lee, S.H.; Ahn, S.W.; Kim, J.Y.; Rhee, J.-K.; Roh, S.W. Effects of the Main Ingredients of the Fermented Food, Kimchi, on Bacterial Composition and Metabolite Profile. Food Res. Int. 2021, 149, 110668. [Google Scholar] [CrossRef]
- Maoloni, A.; Cardinali, F.; Milanović, V.; Osimani, A.; Garofalo, C.; Ferrocino, I.; Corvaglia, M.R.; Cocolin, L.; Aquilanti, L. Microbial Dynamics and Key Sensory Traits of Laboratory-Scale Co-Fermented Green Olives (Olea europaea L. cv. Ascolana tenera) and Sea Fennel (Crithmum maritimum L.). Food Biosci. 2022, 50, 102077. [Google Scholar] [CrossRef]
- Zaika, L.L.; Kissinger, J.C.; Wasserman, A.E. Inhibition of Lactic Acid Bacteria by Herbs. J. Food Sci. 1983, 48, 1455–1459. [Google Scholar] [CrossRef]
- Hong, S.P.; Lee, E.J.; Kim, Y.H.; Ahn, D.U. Effect of Fermentation Temperature on the Volatile Composition of Kimchi. J. Food Sci. 2016, 81, 114355. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Lee, J.H.; Min, S.; Min, D.B. Changes of Volatile Compounds, Lactic Acid Bacteria, PH, and Headspace Gases in Kimchi, a Traditional Korean Fermented Vegetable Product. J. Food Sci. 2003, 68, 849–854. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Yong, S.; Lee, M.J.; Park, S.J.; Yun, Y.-R.; Park, S.-H.; Lee, M.-A. Changes in Volatile and Non-Volatile Compounds of Model Kimchi through Fermentation by Lactic Acid Bacteria. LWT 2019, 105, 118–126. [Google Scholar] [CrossRef]
- Lee, G.; Heo, S.; Park, J.; Lee, J.-S.; Jeong, D.-W. Effects of Jogi, Micropogonias Undulatus, Addition on the Production of Volatile Compounds in Baechu-Kimchi. PLoS ONE 2024, 19, e0312441. [Google Scholar] [CrossRef]
- Wooderck, S. Hawer A Study on the Analysis of Volatile Flavour of Kimchee. Anal. Sci. Technol. 1994, 7, 125–132. [Google Scholar]
- Pavela, R.; Maggi, F.; Lupidi, G.; Cianfaglione, K.; Dauvergne, X.; Bruno, M.; Benelli, G. Efficacy of Sea Fennel (Crithmum maritimum L., Apiaceae) Essential Oils against Culex Quinquefasciatus Say and Spodoptera Littoralis (Boisd.). Ind. Crops Prod. 2017, 109, 603–610. [Google Scholar] [CrossRef]
- Raffo, A.; Aquilanti, L.; Baiamonte, I.; Casavecchia, S.; Melini, V.; Nardo, N.; Nartea, A.; Pacetti, D.; Civitelli, E.S.; Sforza, L. An Insight into the Sea Fennel (Crithmum maritimum L.) Volatile Fraction: Diversity in Wild Populations of Different Geographical Origin and Identification of Key Odorants. Food Biosci. 2025, 65, 106137. [Google Scholar] [CrossRef]
- Özcan, M.M.; Uslu, N.; Figueredo, G.; Al Juhaimi, F.; Ghafoor, K.; Babiker, E.E.; Alsawmahi, O.N.; Isam, A.M.A. The Effect of Fermentation Process on Bioactive Properties, Essential Oil Composition and Phenolic Constituents of Raw Fresh and Fermented Sea Fennel (Crithmum maritimum L.) Leaves. Indian. J. Tradit. Knowl. 2019, 18, 800–804. [Google Scholar]
- Jeong, H.S.; Ko, Y.T. Major Odor Components of Raw Kimchi Materials and Changes in Odor Components and Sensory Properties of Kimchi during Ripening. J. Korean Soc. Food Cult. 2010, 25, 607–614. [Google Scholar]
- Yu, T.H.; Wu, C.M.; Ho, C.T. Volatile Compounds of Deep-Oil Fried, Microwave-Heated and Oven-Baked Garlic Slices. J. Agric. Food Chem. 1993, 41, 800–805. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, M.-A.; Lee, K.-G. Determination of Compositional Quality and Volatile Flavor Characteristics of Radish-Based Kimchi Suitable for Chinese Consumers and Its Correlation to Consumer Acceptability. Food Sci. Biotechnol. 2018, 27, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.J.; Kim, H.; Cadwallader, K.R. Aroma-Active Compounds in Kimchi during Fermentation. J. Agric. Food Chem. 1998, 46, 1944–1953. [Google Scholar] [CrossRef]
- Lee, J.-J.; Choi, Y.-J.; Lee, M.J.; Park, S.J.; Oh, S.J.; Yun, Y.-R.; Min, S.G.; Seo, H.-Y.; Park, S.-H.; Lee, M.-A. Effects of Combining Two Lactic Acid Bacteria as a Starter Culture on Model Kimchi Fermentation. Food Res. Int. 2020, 136, 109591. [Google Scholar] [CrossRef]
- Jeong, S.H.; Lee, S.H.; Jung, J.Y.; Choi, E.J.; Jeon, C.O. Microbial Succession and Metabolite Changes during Long-Term Storage of Kimchi. J. Food Sci. 2013, 78, M763–M769. [Google Scholar] [CrossRef]
- Taveira, M.; Fernandes, F.; Guedes de Pinho, P.; Andrade, P.B.; Pereira, J.A.; Valentão, P. Evolution of Brassica Rapa Var. Rapa L. Volatile Composition by HS-SPME and GC/IT-MS. Microchem. J. 2009, 93, 140–146. [Google Scholar] [CrossRef]
- Lee, M.; Song, J.H.; Park, J.M.; Chang, J.Y. Bacterial Diversity in Korean Temple Kimchi Fermentation. Food Res. Int. 2019, 126, 108592. [Google Scholar] [CrossRef]
- Jang, S.; Kim, M.; Lim, J.; Hong, J. Cross-Cultural Comparison of Consumer Acceptability of Kimchi with Different Degree of Fermentation. J. Sens. Stud. 2016, 31, 124–134. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Park, S.H.; Park, S.-E.; Kim, E.-J.; Kim, H.-W.; Seo, S.-H.; Cho, K.-M.; Kwon, S.J.; Whon, T.W.; Min, S.G.; et al. Comprehensive Elucidation of the Terroir of Korean Kimchi through the Study of Recipes, Metabolites, Microbiota, and Sensory Characteristics. Food Res. Int. 2023, 166, 112614. [Google Scholar] [CrossRef] [PubMed]
- Fangohoi, L. Variasi pemberian bokashi pada budidaya tanaman sawi caisim (Brassica juncea L) di desa randuagung kecamatan lawang kabupaten malang propinsi jawa timur. J. Triton 2016, 7, 21–26. [Google Scholar]
- Kim, J.-Y.; Kim, B.-S.; Kim, J.-H.; Oh, S.-I.; Koo, J. Development of Dynamic Model for Real-Time Monitoring of Ripening Changes of Kimchi during Distribution. Foods 2020, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Politeo, O.; Ćurlin, P.; Brzović, P.; Auzende, K.; Magné, C.; Generalić Mekinić, I. Volatiles from French and Croatian Sea Fennel Ecotypes: Chemical Profiles and the Antioxidant, Antimicrobial and Antiageing Activity of Essential Oils and Hydrolates. Foods 2024, 13, 695. [Google Scholar] [CrossRef]
- Rampanti, G.; Belleggia, L.; Cardinali, F.; Milanović, V.; Osimani, A.; Garofalo, C.; Ferrocino, I.; Aquilanti, L. Microbial Dynamics of a Specialty Italian Raw Ewe’s Milk Cheese Curdled with Extracts from Spontaneous and Cultivated Onopordum Tauricum Willd. Microorganisms 2023, 11, 219. [Google Scholar] [CrossRef]
- ISO 6888-2:2021; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 2: Technique Using Rabbit Plasma Fibrinogen Agar Medium. International Organization for Standardization: Geneva, Switzerland, 2021.
- ISO 15213:2003; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Sulphite-Reducing Bacteria Growing under Anaerobic Conditions. International Organization for Standardization: Geneva, Switzerland, 2003.
- ISO 11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- Haouet, M.N.; Altissimi, M.S.; Mercuri, M.L.; Baldassarri, C.; Osimani, A.; Clementi, F.; Ortenzi, R. Evaluation of the safety of Milano-type dry fermented sausages produced by a fast drying technology. Ital. J. Food Sci. 2017, 29, 550. [Google Scholar]
- Fu, N.; Woo, M.W.; Selomulya, C.; Chen, X.D. Inactivation of Lactococcus lactis ssp. cremoris cells in a droplet during convective drying. Biochem. Eng. J. 2013, 79, 46–56. [Google Scholar]
- Peryam, D.R.; Pilgrim, F.J. Hedonic Scale Method of Measuring Food Preferences. Food Technol. 1957, 11, 9–14. [Google Scholar]

| Sampling Time (Days) | Started Kimchi | Control Kimchi |
|---|---|---|
| t0 | 4.90 ± 0.08 b,A | 4.87 ± 0.08 c,A |
| t2 | 5.20 ± 0.07 a,A | 5.30 ± 0.17 ab,A |
| t5 | 5.30 ± 0.07 a,B | 5.51 ± 0.13 a,A |
| t7 | 4.90 ± 0.10 b,B | 5.46 ± 0.13 ab,A |
| t9 | 4.70 ± 0.15 bc,B | 5.49 ± 0.07 ab,A |
| t12 | 4.40 ± 0.13 cd,B | 5.16 ± 0.17 bc,A |
| t14 | 4.20 ± 0.03 d,B | 4.97 ± 0.08 c,A |
| t16 | 4.10 ± 0.19 def,B | 4.57 ± 0.08 d,A |
| t19 | 4.00 ± 0.05 ef,B | 4.19 ± 0.08 e,A |
| t22 | 3.90 ± 0.02 f,B | 3.99 ± 0.01 e,A |
| t26 | 3.90 ± 0.02 f,B | 3.97 ± 0.01 e,A |
| Sampling Time (Days) | Started Kimchi | Control Kimchi |
|---|---|---|
| t0 | 0.09 ± 0.01 b,A | 0.09 ± 0.01 b,A |
| t26 | 0.53 ± 0.05 a,A | 0.34 ± 0.03 a,B |
| Sampling Time (Days) | Prototype | Lactic Acid | Acetic Acid | ||
|---|---|---|---|---|---|
| D-Lactic Acid | L-Lactic Acid | Total Lactic Acid | |||
| t0 | Control kimchi | 0.00 ± 0.00 c,A | 0.02 ± 0.00 b,B | 0.02 ± 0.00 b,A | 0.00 ± 0.00 b,C |
| Started kimchi | 0.01 ± 0.00 b,A | 0.02 ± 0.00 b,A | 0.03 ± 0.00 b,A | 0.00 ± 0.00 b,C | |
| t26 | Control kimchi | 0.19 ± 0.01 b,A | 0.38 ± 0.01 a,A | 0.56 ± 0.01 a,A | 0.02 ± 0.01 b,B |
| Started kimchi | 0.14 ± 0.01 a,B | 0.37 ± 0.03 a,A | 0.52 ± 0.04 a,A | 0.10 ± 0.02 a,A | |
| t150 | Control kimchi | 0.21 ± 0.01 a,A | 0.37 ± 0.02 a,A | 0.58 ± 0.03 a,A | 0.10 ± 0.02 a,A |
| Started kimchi | 0.21 ± 0.05 a,A | 0.48 ± 0.11 a,A | 0.69 ± 0.15 a,A | 0.13 ± 0.04 a,A | |
| Microbial Group | Sampling Time (Days) | Started Kimchi | Control Kimchi |
|---|---|---|---|
| Mesophilic lactobacilli | t0 | 7.3 ± 0.1 b,A | 1.9 ± 0.1 d,B |
| t2 | 7.5 ± 0.1 b,A | 3.5 ± 0.3 c,B | |
| t5 | 7.6 ± 0.2 b,A | 5.1 ± 0.5 b,B | |
| t12 | 8.3 ± 0.0 a,A | 8.2 ± 0.1 a,A | |
| t19 | 8.4 ± 0.1 a,A | 8.5 ± 0.1 a,A | |
| t26 | 8.4 ± 0.1 a,A | 8.5 ± 0.0 a,A | |
| Mesophilic lactococci | t0 | 7.3 ± 0.1 b,A | 4.9 ± 0.1 b,B |
| t2 | 7.4 ± 0.0 b,A | 5.9 ± 0.3 a,B | |
| t5 | 7.6 ± 0.2 b,A | 5.8 ± 0.3 a,B | |
| t12 | 8.3 ± 0.0 a,A | 6.6 ± 0.3 a,B | |
| t19 | 8.4 ± 0.1 a,A | 6.0 ± 0.5 a,B | |
| t26 | 8.3 ± 0.1 a,A | 6.1 ± 0.4 a,B | |
| Yeasts | t0 | <1.0 a,A | <1.0 a,A |
| t2 | <1.0 a,A | <1.0 a,A | |
| t5 | <1.0 a,A | <1.0 a,A | |
| t12 | <1.0 a,A | <1.0 a,A | |
| t19 | <1.0 a,A | <1.0 a,A | |
| t26 | <1.0 a,A | <1.0 a,A | |
| Enterobacteriaceae | t0 | 4.4 ± 0.5 a,A | 4.3 ± 0.4 a,A |
| t2 | 5.6 ± 0.2 a,A | 5.7 ± 1.0 a,A | |
| t5 | 5.5 ± 0.8 a,A | 5.1 ± 0.2 a,A | |
| t12 | 4.4 ± 0.8 a,B | 5.7 ± 0.2 a,A | |
| t19 | 1.9 ± 0.7 b,A | 3.1 ± 0.7 b,A | |
| t26 | <1.0 c,A | <1.0 c,A | |
| Mesophilic aerobic bacteria | t0 | 7.2 ± 0.0 c,A | 5.4 ± 0.1 b,B |
| t2 | 7.5 ± 0.1 bc,A | 6.2 ± 0.7 b, B | |
| t5 | 7.6 ± 0.3 b,A | 5.8 ± 0.3 b,B | |
| t12 | 8.3 ± 0.0 a,A | 8.3 ± 0.1 a,A | |
| t19 | 8.4 ± 0.1 a,A | 8.6 ± 0.1 a,A | |
| t26 | 8.4 ± 0.0 a,A | 8.4 ± 0.2 a,A | |
| Pseudomonadaceae | t0 | 5.2 ± 0.3 a,A | 5.2 ± 0.1 a,A |
| t2 | 5.0 ± 0.3 a,A | 5.7 ± 0.6 a,A | |
| t5 | 4.9 ± 0.3 a,A | 5.1 ± 0.6 a,A | |
| t12 | 3.4 ± 0.3 b,B | 5.2 ± 0.2 a,A | |
| t19 | 2.5 ± 0.6 bc,A | 2.8 ± 0.3 b,A | |
| t26 | 2.1 ± 0.4 c,A | 2.7 ± 0.6 b,A |
| N° | Retention Index | Volatile Compound | Peak Area (×105) | |||||
|---|---|---|---|---|---|---|---|---|
| Control Kimchi | Started Kimchi | |||||||
| t0 | t26 | t150 | t0 | t26 | t150 | |||
| 1 | 705 | Acetaldehyde | 2.57 ± 0.33 a | 0.00 ± 0.00 b | 1.18 ± 2.05 b | 3.48 ± 0.21 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| 2 | 822 | Ethyl Acetate | 11.39 ± 2.23 b | 23.27 ± 2.61 b | 38.58 ± 18.61 ab | 13.12 ± 1.83 b | 37.69 ± 6.65 ab | 274.85 ± 388.89 a |
| 3 | 950 | Ethanol | 30.19 ± 3.50 c | 78.53 ± 1.95 bc | 175.14 ± 89.02 a | 34.53 ± 4.38 c | 64.34 ± 3.55 c | 124.80 ± 12.66 ab |
| 4 | 987 | Pentanal | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 4.80 ± 1.16 a | 3.80 ± 1.08 a | 0.00 ± 0.00 b |
| 5 | 1029 | α-Pinene | 3.27 ± 0.15 ab | 3.48 ± 0.77 a | 1.28 ± 1.34 bc | 4.19 ± 2.29 a | 4.13 ± 0.81 a | 1.09 ± 1.01 c |
| 6 | 1076 | Camphene | 3.62 ± 1.66 ab | 4.23 ± 1.18 ab | 3.57 ± 1.11 ab | 3.62 ± 1.07 ab | 5.54 ± 1.27 a | 3.32 ± 1.73 b |
| 7 | 1086 | Dimethyl disulfide | 21.22 ± 9.37 c | 59.55 ± 19.50 a | 43.41 ± 7.56 ab | 27.45 ± 10.87 bc | 57.47 ± 17.08 a | 25.71 ± 6.78 bc |
| 8 | 1128 | Sabinene | 30.91 ± 24.26 a | 0.52 ± 0.90 b | 0.00 ± 0.00 b | 20.16 ± 3.41 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| 9 | 1135 | 1-Butanol-3-methyl acetate | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 4.03 ± 3.54 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 18.49 ± 21.32 a |
| 10 | 1172 | β-Myrcene | 2.39 ± 0.99 a | 2.64 ± 2.3 a | 1.77 ± 1.54 a | 3.03 ± 1.29 a | 2.61 ± 2.27 a | 1.31 ± 1.13 a |
| 11 | 1177 | 1-Penten-3-ol | 4.07 ± 1.33 c | 15.21 ± 1.58 b | 13.87 ± 7.26 b | 5.32 ± 1.40 c | 27.83 ± 2.74 a | 15.07 ± 2.60 b |
| 12 | 1209 | D-Limonene | 54.76 ± 31.07 a | 92.47 ± 26.27 a | 73.37 ± 25.59 a | 98.69 ± 64.87 a | 109.32 ± 18.69 a | 83.08 ± 21.47 a |
| 13 | 1220 | β-Phellandrene | 15.34 ± 2.28 ab | 22.30 ± 5.85 a | 12.84 ± 1.79 b | 21.78 ± 7.35 a | 23.92 ± 3.86 a | 12.56 ± 2.02 b |
| 14 | 1226 | 3-Methyl-1-butanol | 0.19 ± 0.33 b | 1.24 ± 2.14 b | 275.84 ± 239.17 a | 0.21 ± 0.36 b | 2.57 ± 2.23 b | 200.60 ± 101.87 a |
| 15 | 1226 | 1,8-Cineol | 11.23 ± 0.67 bc | 18.19 ± 4.91 ab | 7.18 ± 12.43 bc | 16.14 ± 4.70 ab | 23.91 ± 1.98 a | 3.64 ± 6.31 c |
| 16 | 1238 | 1-Methoxy-2-propyl acetate | 74.98 ± 38.38 a | 99.21± 4.15 a | 79.62 ± 11.03 a | 81.61 ± 33.90 a | 80.96 ± 18.71 a | 73.02 ± 9.87 a |
| 17 | 1260 | γ-Terpinene | 86.98 ± 38.29 a | 137.06 ± 35.79 a | 105.65 ± 26.25 a | 125.27 ± 60.17 a | 140.77 ± 17.00 a | 107.74 ± 29.15 a |
| 18 | 1267 | 1-Pentanol | 3.67 ± 3.18 c | 5.60 ± 4.85 c | 12.90 ± 2.08 ab | 4.23 ± 1.39 c | 8.37 ± 3.37 bc | 17.54 ± 1.68 a |
| 19 | 1286 | p-Cymene | 56.97 ± 12.26 c | 109.89 ± 20.52 a | 79.04 ± 17.95 abc | 79.08 ± 33.77 abc | 105.70 ± 8.44 ab | 73.83 ± 14.52 bc |
| 20 | 1367 | 5-Cyano-1-pentene | 39.13 ± 10.03 bc | 38.29 ± 5.87 c | 33.48 ± 3.27 c | 51.90 ± 9.10 a | 58.34 ± 5.05 a | 49.91 ± 3.08 ab |
| 21 | 1402 | (Z)-3-Hexen-1-ol | 29.19 ± 7.54 b | 41.61 ± 7.42 a | 40.86 ± 5.61 a | 39.41 ± 1.57 a | 45.92 ± 4.73 a | 46.20 ± 8.25 a |
| 22 | 1408 | Dimethyl trisulfide | 8.19 ± 4.94 bc | 21.82 ± 11.67 a | 8.98 ± 9.62 bc | 13.73 ± 4.64 abc | 15.36 ± 3.91 ab | 2.68 ± 2.35 c |
| 23 | 1467 | Acetic acid | 1.59 ± 1.40 b | 74.40 ± 2.76 ab | 57.71 ± 12.00 ab | 2.55 ± 0.40 b | 133.76 ± 116.89 a | 120.34 ± 106.02 a |
| 24 | 1556 | Camphor | 49.19 ± 3.98 a | 47.67 ± 2.77 a | 46.47 ± 3.64 a | 35.85 ± 24.45 a | 34.67 ± 12.92 a | 41.68 ± 3.32 a |
| 25 | 1564 | 4-ethyl-5- methlylthiazole | 40.39 ± 10.76 cd | 72.08 ± 15.56 ab | 52.04 ± 2.68 bc | 65.80 ± 5.27 abc | 79.47 ± 13.91 a | 21.66 ± 31.09 d |
| 26 | 1614 | Thymol methyl ether | 63.06 ± 17.06 a | 91.32 ± 17.40 a | 66.80 ± 5.48 a | 108.83 ± 64.53 a | 99.14 ± 6.01 a | 71.04 ± 15.74 a |
| 27 | 1630 | Terpinene-4-ol | 54.12 ± 24.75 d | 239.30 ± 41.02 c | 451.32 ± 20.36 a | 49.23 ± 5.76 d | 249.58 ± 28.29 c | 333.30 ± 15.01 b |
| 28 | 1705 | Neral | 5.00 ± 1.79 ab | 4.30 ± 4.02 ab | 1.63 ± 2.82 b | 8.02 ± 1.92 a | 5.27 ± 0.71 ab | 1.50 ± 2.61 b |
| 29 | 1719 | α-Terpineol | 10.77 ± 2.75 c | 28.95 ± 5.14 ab | 26.32 ± 0.74 b | 11.35 ± 1.65 c | 32.07 ± 0.96 a | 30.62 ± 3.15 ab |
| 30 | 1730 | Borneol | 9.13 ± 1.13 b | 11.18 ± 2.68 ab | 10.43 ± 2.71 ab | 11.30 ± 2.43 ab | 13.58 ± 0.85 a | 11.76 ± 3.81 ab |
| 31 | 1757 | Geranial | 23.81± 2.44 ab | 24.70 ± 14.88 ab | 21.71 ± 7.18 ab | 27.24 ± 6.38 a | 24.14 ± 1.82 ab | 11.94 ± 10.44 b |
| 32 | 1864 | Hexanoic acid | 0.40 ± 0.70 c | 8.59 ± 0.80 b | 17.03 ± 1.74 a | 0.00 ± 0.00 c | 7.69 ± 1.70 b | 17.76 ± 7.04 a |
| 33 | 1946 | Phenylethyl alcohol | 0.38 ± 0.70 c | 3.06 ± 2.65 bc | 37.23 ± 28.23 a | 0.65 ± 0.62 c | 4.86 ± 0.49 bc | 18.98 ± 0.85 ab |
| 34 | 2080 | Octanoic acid | 0.96 ± 1.66 cd | 10.47 ± 9.06 bc | 39.17 ± 5.25 a | 0.00 ± 0.00 d | 16.79 ± 3.03 b | 41.14 ± 9.0 3a |
| 35 | 2083 | Benzene propanenitrile | 21.64 ± 7.55 bc | 25.89 ± 5.36 ab | 14.63 ± 6.85 c | 27.17 ± 2.41 ab | 32.86 ± 2.26 a | 25.34 ± 2.20 ab |
| 36 | 2264 | Carvacrol | 5.47 ± 1.85 b | 8.94 ± 1.73 a | 7.54 ± 1.06 ab | 7.56 ± 0.90 ab | 8.71 ± 1.01 a | 7.49 ± 0.91 ab |
| 37 | 2276 | Phenethyl isothiocyanate | 14.33 ± 3.26 c | 23.74 ± 5.65 a | 17.04 ± 0.77 abc | 15.79 ± 1.30 bc | 23.04 ± 3.96 ab | 13.57 ± 7.24 c |
| 38 | 2385 | Dillapiole | 63.39 ± 19.92 bc | 116.94 ± 28.05 a | 99.47± 6.48 ab | 81.71 ± 20.20 abc | 71.68 ± 18.51 bc | 57.25 ± 19.23 c |
| Ingredient | Weight (g) | % (w/w) |
|---|---|---|
| Chinese cabbage | 3725.34 | 70.53 |
| Onion | 230.62 | 4.37 |
| Garlic | 26.61 | 0.50 |
| Ginger | 26.61 | 0.50 |
| Sea fennel | 372.53 | 7.05 |
| Red pepper | 8.87 | 0.17 |
| Paprika | 97.57 | 1.85 |
| Sugar | 44.35 | 0.84 |
| Salt | 39.91 | 0.76 |
| Water | 709.59 | 13.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraouia, M.; Antonietta, M.; Cardinali, F.; Milanović, V.; Garofalo, C.; Osimani, A.; Raffo, A.; Melini, V.; Nardo, N.; Baiamonte, I.; et al. From Tradition to Innovation: The Role of Sea Fennel in Shaping Kimchi’s Microbial, Chemical, and Sensory Profiles. Molecules 2025, 30, 2731. https://doi.org/10.3390/molecules30132731
Kraouia M, Antonietta M, Cardinali F, Milanović V, Garofalo C, Osimani A, Raffo A, Melini V, Nardo N, Baiamonte I, et al. From Tradition to Innovation: The Role of Sea Fennel in Shaping Kimchi’s Microbial, Chemical, and Sensory Profiles. Molecules. 2025; 30(13):2731. https://doi.org/10.3390/molecules30132731
Chicago/Turabian StyleKraouia, Maryem, Maoloni Antonietta, Federica Cardinali, Vesna Milanović, Cristiana Garofalo, Andrea Osimani, Antonio Raffo, Valentina Melini, Nicoletta Nardo, Irene Baiamonte, and et al. 2025. "From Tradition to Innovation: The Role of Sea Fennel in Shaping Kimchi’s Microbial, Chemical, and Sensory Profiles" Molecules 30, no. 13: 2731. https://doi.org/10.3390/molecules30132731
APA StyleKraouia, M., Antonietta, M., Cardinali, F., Milanović, V., Garofalo, C., Osimani, A., Raffo, A., Melini, V., Nardo, N., Baiamonte, I., Aquilanti, L., & Rampanti, G. (2025). From Tradition to Innovation: The Role of Sea Fennel in Shaping Kimchi’s Microbial, Chemical, and Sensory Profiles. Molecules, 30(13), 2731. https://doi.org/10.3390/molecules30132731









