Anti-Amyloid Aggregation Effects of Gobaishi (Galla chinensis) and Its Active Constituents
Abstract
1. Introduction
2. Results
2.1. Identification and Characterization of Major Compounds in Gobaishi
2.2. Isolation of Aβ Aggregation Inhibitors from Gobaishi
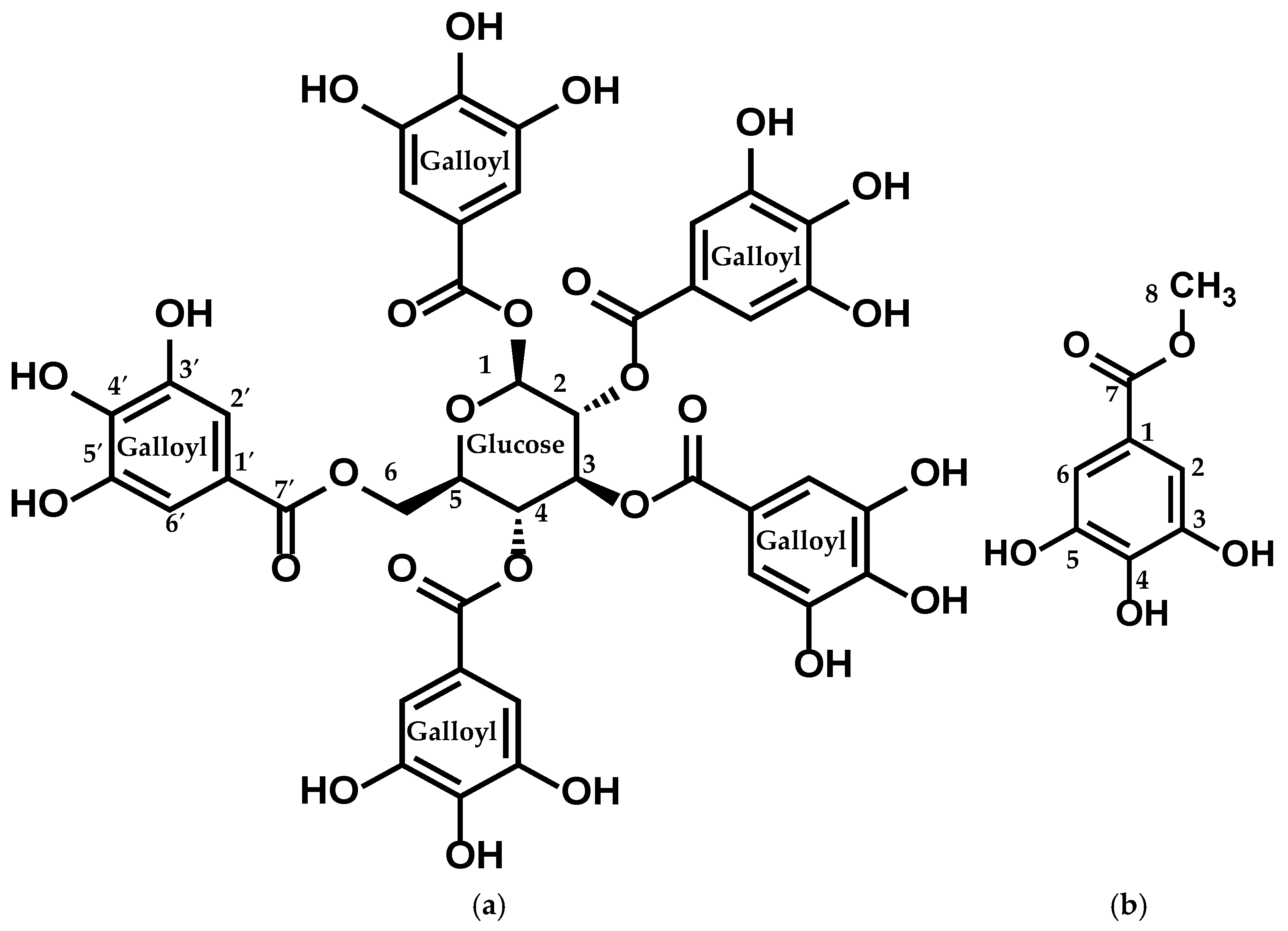
2.3. Structure Elucidation of the Active Compounds
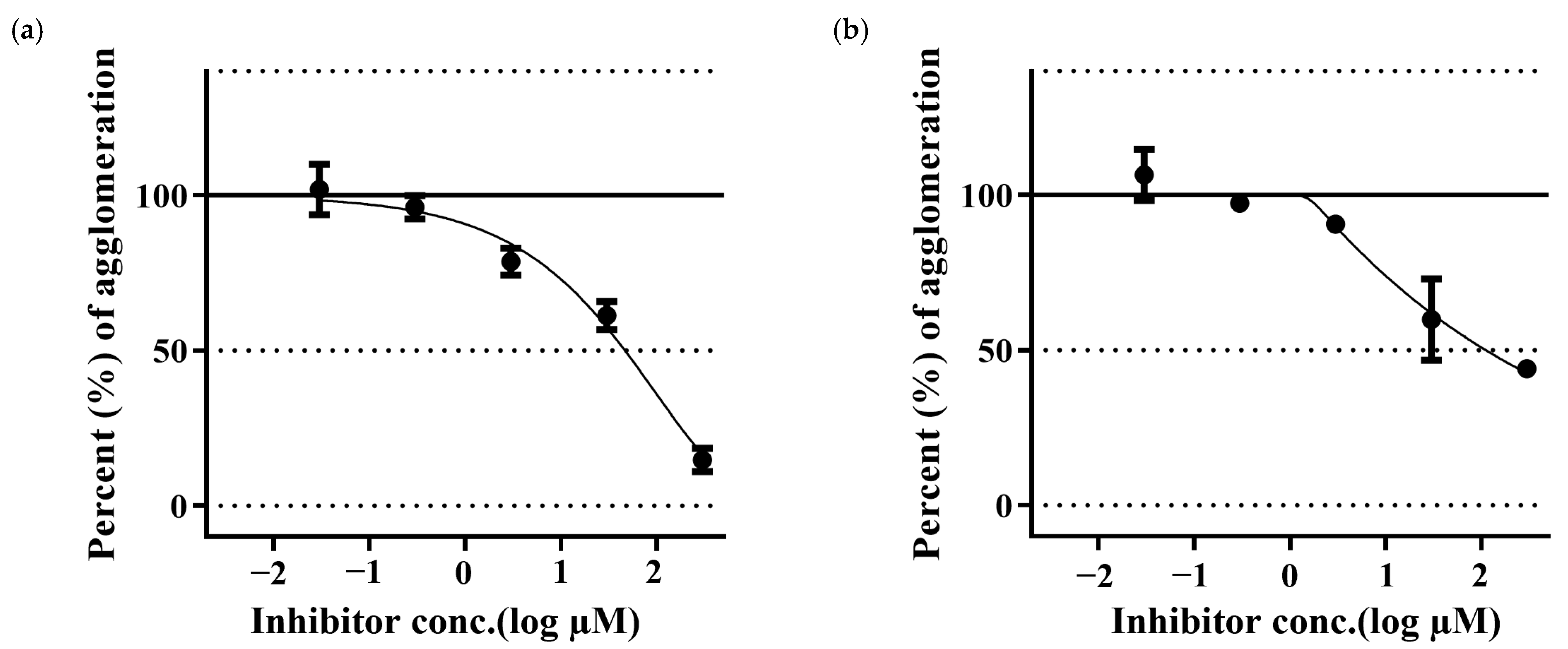
2.4. Aβ42 Aggregation Inhibitory Activity Monitored by ThT Assay
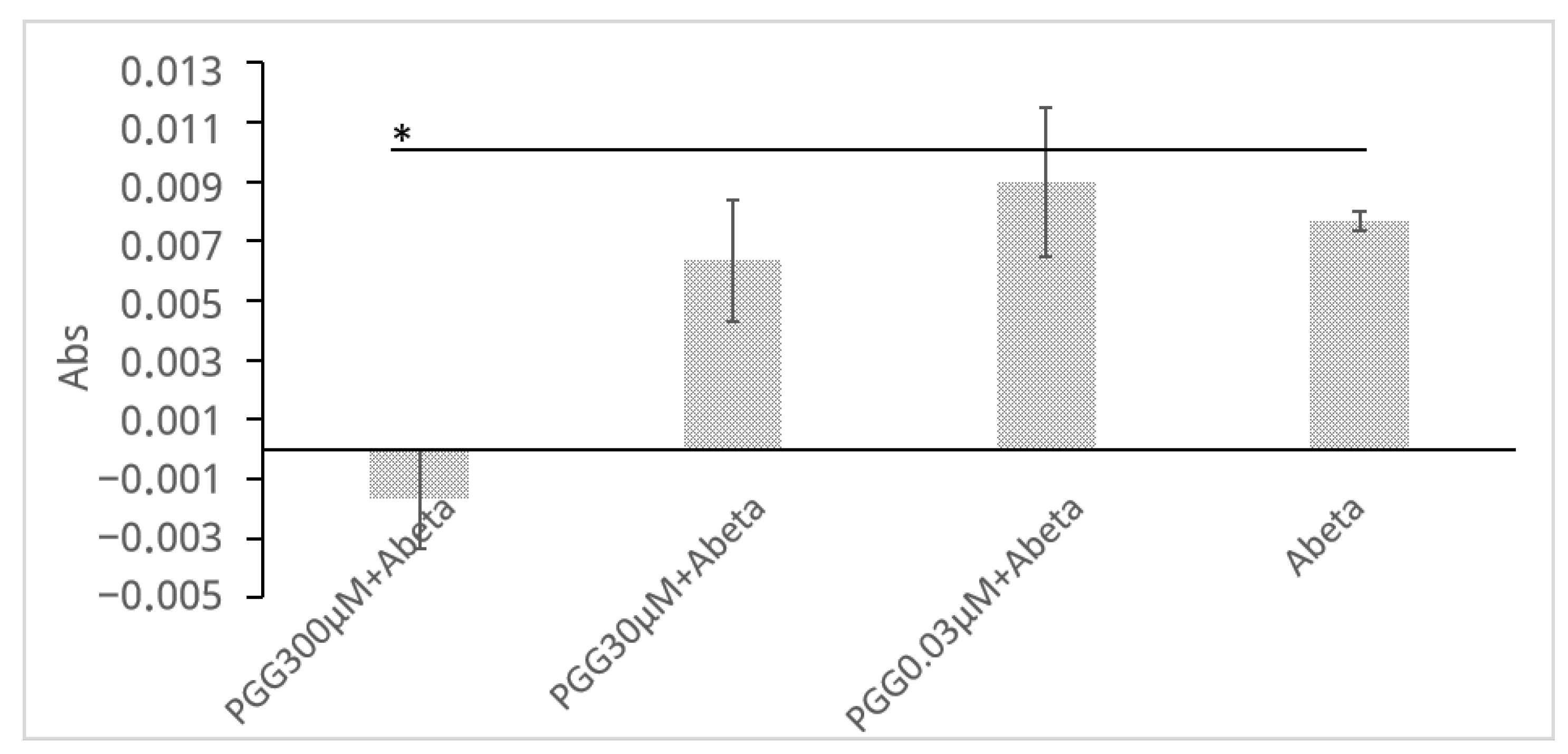
2.5. Confirmation of Aβ Aggregate Formation
2.6. DPPH Radical Scavenging Activity of Isolated Compounds
2.7. Inhibition of Aβ-Induced Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Reagents and Instruments
4.2. Preparation of Plant Extract
4.3. Metabolite Analysis
4.4. Bioassay-Guided Fractionation
4.5. NMR Spectroscopy
4.6. LC-MS/MS Analysis
4.7. Bioactivity Assays
4.7.1. Thioflavin T (ThT) Assay
4.7.2. Turbidity Assay
4.7.3. DPPH Assay
4.7.4. Apoptosis Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer′s Disease |
| Aβ | Amyloid Beta |
| PGG | Pentagalloyl Glucose |
| MG | Methyl Gallate |
| ThT | Thioflavin T |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| NMR | Nuclear Magnetic Resonance |
| HMQC | Heteronuclear Multiple Quantum Correlation |
| HMBC | Heteronuclear Multiple Bond Correlation |
| COSY | Homonuclear Correlation Spectroscopy |
| DEPT | Distortionless Enhancement by Polarization Transfer |
| UHPLC | Ultra-High Performance Liquid Chromatography |
| ESI | Electrospray Ionization |
| MS | Mass Spectrometry |
| AChE | Acetylcholinesterase |
| BChE | Butyrylcholinesterase |
| BACE1 | Beta-Site APP Cleaving Enzyme 1 |
| ROS | Reactive Oxygen Species |
| RSA | Radical Scavenging Activity |
| RA | Rosmarinic Acid |
References
- Khoba, K.; Kumar, S.; Chatterjee, S.; Purty, R.S. Isolation, characterization, and in silico interaction studies of bioactive compounds from Caesalpinia bonducella with target proteins involved in alzheimer′s disease. Appl. Biochem. Biotechnol. 2023, 195, 2216–2234. [Google Scholar] [CrossRef] [PubMed]
- 2024 Alzheimer′s disease facts and figures. Alzheimer′s Dementia 2024, 20, 3708–3821. [CrossRef] [PubMed]
- World Health Organization. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 17 October 2024).
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer′s disease. Neurotherapeutics 2023, 19, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.J.; Renton, A.E.; Fulton-Howard, B.; Podlesny-Drabiniok, A.; Marcora, E.; Goate, A.M. The complex genetic architecture of Alzheimer′s disease: Novel insights and future directions. EBioMedicine 2023, 90, 104511. [Google Scholar] [CrossRef]
- Bhakta, H.K.; Park, C.H.; Yokozawa, T.; Tanaka, T.; Jung, H.A.; Choi, J.S. Potential anti-cholinesterase and β-site amyloid precursor protein cleaving enzyme 1 inhibitory activities of cornuside and gallotannins from Cornus officinalis fruits. Arch. Pharmacal Res. 2017, 40, 836–853. [Google Scholar] [CrossRef]
- Kubo, K.; Watanabe, H.; Kumeta, H.; Aizawa, T.; Seki, C.; Nakano, H.; Tokuraku, K.; Uwai, K. Chemical analysis of amyloid β aggregation inhibitors derived from Geranium thunbergii. Bioorg. Med. Chem. 2022, 68, 116840. [Google Scholar] [CrossRef]
- Huang, L.-K.; Chao, S.-P.; Hu, C.-J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020, 27, 18. [Google Scholar] [CrossRef]
- Sekiguchi, M.; Horiuchi, M.; Tozawa, Y.; Shigemori, H. Seven undescribed meroterpenoids from Sargassum siliquastrum and their inhibitory activity against amyloid β aggregation. J. Nat. Med. 2025, 79, 73–81. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Zhang, X.; Li, Y.; Wang, D.; Sun, F.; Wang, C.; Shi, Z.; Yang, X.; Yang, Z.; et al. Di-caffeoylquinic acid: A potential inhibitor for amyloid-beta aggregation. J. Nat. Med. 2024, 78, 1029–1043. [Google Scholar] [CrossRef]
- Djakpo, O.; Yao, W. Rhus chinensis and Galla Chinensis—Folklore to modern evidence: Review. Phytother. Res. 2010, 24, 1739–1747. [Google Scholar] [CrossRef]
- Wang, H.; Cui, K.; Shao, S.; Liu, J.; Chen, H.; Wang, C.; Wu, H.; Yang, Z.; Lu, Q.; King-Jones, K. Molecular response of gall induction by aphid Schlechtendalia chinensis (Bell) attack on Rhus chinensis Mill. J. Plant Interact. 2017, 12, 465–479. [Google Scholar] [CrossRef]
- Liu, P.; Yang, Z.X.; Chen, X.M.; Yang, P. RNA-Seq-based transcriptome and the reproduction-related genes for the aphid Schlechtendalia chinensis (Hemiptera, Aphididae). Genet. Mol. Res. 2017, 16, 16019448. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-L.; Liu, M.-D.; Li, J.-Y.; Zhou, X.-D.; Ten Cate, J.M. Chemical composition of Galla chinensis extract and the effect of its main component (s) on the prevention of enamel demineralization in vitro. Int. J. Oral Sci. 2012, 4, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Li, B.; Ji, B.; Zhang, G.; Luo, Y. Identification and structure–activity relationship of gallotannins separated from Galla chinensis. LWT-Food Sci. Technol. 2009, 42, 1289–1295. [Google Scholar] [CrossRef]
- Tiwari, D.; Rawat, S.; Bhatt, I.D. Rhus chinensis Mill. In Himalayan Fruits and Berries; Academic Press: Cambridge, MA, USA, 2023; pp. 341–356. [Google Scholar]
- Ren, Y.Y.; Zhang, X.R.; Li, T.N.; Zeng, Y.J.; Wang, J.; Huang, Q.W. Galla Chinensis, a Traditional Chinese Medicine: Comprehensive review of botany, traditional uses, chemical composition, pharmacology and toxicology. J. Ethnopharmacol. 2021, 278, 114247. [Google Scholar] [CrossRef]
- Sun, K.; Song, X.; Jia, R.; Yin, Z.; Zou, Y.; Li, L.; Yin, L.; He, C.; Liang, X.; Yue, G. Evaluation of Analgesic and Anti-Inflammatory Activities of Water Extract of Galla Chinensis In Vivo Models. J. Evid.-Based Complement. Altern. Med. 2018, 1, 6784032. [Google Scholar] [CrossRef]
- Mun, J.G.; Kee, J.Y.; Han, Y.H.; Lee, S.; Park, S.H.; Jeon, H.D.; Hong, S.H. Galla Rhois water extract inhibits lung metastasis by inducing AMPK-mediated apoptosis and suppressing metastatic properties of colorectal cancer cells. Oncol. Rep. 2019, 41, 202–212. [Google Scholar] [CrossRef]
- Huang, X.; Deng, M.; Liu, M.; Cheng, L.; Exterkate, R.; Li, J.; Zhou, X.; Ten Cate, J.M. Comparison of composition and anticaries effect of Galla chinensis extracts with different isolation methods. Open Dent. J. 2017, 11, 447. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, J.E.; Kang, M.J.; Choi, H.J.; Bae, S.J.; Kim, S.H.; Jung, Y.S.; Hong, J.T.; Hwang, D.Y. Anti-oxidant activity of gallotannin-enriched extract of Galla rhois can associate with the protection of the cognitive impairment through the regulation of BDNF signaling pathway and neuronal cell function in the scopolamine-treated ICR mice. Antioxidants 2019, 8, 450. [Google Scholar] [CrossRef]
- Heuer, M.; Baker, C.; Sedlak, M.; Woo, K.J.; Kee, K.H.; Bagchi, D. Clinical evaluation of a novel gallotannin-enriched Galla rhois extract (GRE) on vital cognitive functions in healthy volunteers: A randomized, double-blind, placebo-controlled study. Funct. Foods Health Dis. 2023, 13, 487–504. [Google Scholar] [CrossRef]
- Ha, S.A. Composition for Improving Cognitive Ability and Preventing or Treating Dementia and Attention Deficit Hyperactivity Disorder, Comprising Galla Rhois Extract and Fraxin as Active Ingredients. U.S. Patent 11,253,553, 22 February 2022. [Google Scholar]
- Nilsson, M.R. Techniques to study amyloid fibril formation in vitro. Methods 2004, 34, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Dettweiler, M.; Marquez, L.; Lin, M.; Sweeney-Jones, A.M.; Chhetri, B.K.; Zurawski, D.V.; Kubanek, J.; Quave, C.L. Pentagalloyl glucose from Schinus terebinthifolia inhibits growth of carbapenem-resistant Acinetobacter baumannii. Sci. Rep. 2020, 10, 15340. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, B.J.; Popoola, T.D.; van Heerden, F.R.; Fatokun, A.A. Pentagalloylglucose, isolated from the leaf extract of Anacardium occidentale L., could elicit rapid and selective cytotoxicity in cancer cells. BMC Complement. Med. Ther. 2020, 20, 287. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, E.; García, A.; Avalos-Alanís, F.G.; Rivas-Galindo, V.M.; Delgadillo-Puga, C.; del Rayo Camacho-Corona, M. Nuclear magnetic resonance spectroscopy data of isolated compounds from Acacia farnesiana (L) wild fruits and two esterified derivatives. Data Brief. 2019, 22, 255–268. [Google Scholar] [CrossRef]
- Şen, A.; Tan, A.S.B.; Kültür, Ş.; Bitiş, L. Isolation and characterization of antimicrobial compounds from Cotinus coggygria Scop. ethyl acetate extract. Istanbul J. Pharm. 2020, 50, 272–276. [Google Scholar] [CrossRef]
- Khurana, R.; Coleman, C.; Ionescu-Zanetti, C.; Carter, S.A.; Krishna, V.; Grover, R.K.; Roy, R.; Singh, S. Mechanism of thioflavin T binding to amyloid fibrils. J. Struct. Biol. 2005, 151, 229–238. [Google Scholar] [CrossRef]
- Yajing, M.; Sufang, L.; Qingfeng, Z.; Zhonghua, L.; Zhang, Z.; Bin, Y. Approved drugs and natural products at clinical stages for treating Alzheimer′s disease. Chin. J. Nat. Med. 2024, 22, 699–710. [Google Scholar]
- El Gaamouch, F.; Liu, K.; Lin, H.-y.; Wu, C.; Wang, J. Development of grape polyphenols as multi-targeting strategies for Alzheimer′s disease. Neurochem. Int. 2021, 147, 105046. [Google Scholar] [CrossRef]
- Moreira, J.; Machado, M.; Dias-Teixeira, M.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. The neuroprotective effect of traditional Chinese medicinal plants-A critical review. Acta Pharm. Sin. B 2023, 13, 3208–3237. [Google Scholar] [CrossRef]
- Nie, Q.; Du, X.G.; Geng, M.Y. Small molecule inhibitors of amyloid β peptide aggregation as a potential therapeutic strategy for Alzheimer′s disease. Acta Pharm. Sin. B 2011, 32, 545–551. [Google Scholar] [CrossRef]
- Andarzi Gargari, S.; Barzegar, A.; Tarinejad, A. The role of phenolic OH groups of flavonoid compounds with H-bond formation ability to suppress amyloid mature fibrils by destabilizing β-sheet conformation of monomeric Aβ17-42. PLoS ONE 2018, 13, e0199541. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, S.; Pathak, K.; Devi, M.; Saikia, R.; Das, J.; Kashyap, V.H.; Das, D.; Ahmad, M.Z.; Abdel-Wahab, B.A. Some promising medicinal plants used in Alzheimer′s disease: An ethnopharmacological perspective. Discover Appl. Sci. 2024, 6, 215. [Google Scholar] [CrossRef]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective natural products for Alzheimer′s disease. Cells 2021, 10, 44. [Google Scholar] [CrossRef]
- Fujiwara, H.; Tabuchi, M.; Yamaguchi, T.; Iwasaki, K.; Furukawa, K.; Sekiguchi, K.; Ikarashi, Y.; Kudo, Y.; Higuchi, M.; Saido, T.C.; et al. A traditional medicinal herb Paeonia suffruticosa and its active constituent 1,2,3,4,6-penta-O-galloyl-beta-D-glucopyranose have potent anti-aggregation effects on Alzheimer′s amyloid beta proteins in vitro and in vivo. J. Neurochem. 2009, 109, 1648–1657. [Google Scholar] [CrossRef]
- Sancheti, S.; Um, B.-H.; Seo, S.-Y. 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose: A cholinesterase inhibitor from Terminalia chebula. S. Afr. J. Bot. 2010, 76, 285–288. [Google Scholar] [CrossRef]
- Li, F.-J.; Liu, Y.; Yuan, Y.; Yang, B.; Liu, Z.-M.; Huang, L.-Q. Molecular interaction studies of acetylcholinesterase with potential acetylcholinesterase inhibitors from the root of Rhodiola crenulata using molecular docking and isothermal titration calorimetry methods. Int. J. Biol. Macromol. 2017, 104, 527–532. [Google Scholar] [CrossRef]
- Sylla, T.; Pouységu, L.; Da Costa, G.; Deffieux, D.; Monti, J.P.; Quideau, S. Gallotannins and tannic acid: First chemical syntheses and in vitro inhibitory activity on Alzheimer′s amyloid β-peptide aggregation. Angew. Chem. Int. Ed. 2015, 54, 8217–8221. [Google Scholar] [CrossRef]
- Giordano, C.R.; Terlecky, L.J.; Bollig-Fischer, A.; Walton, P.A.; Terlecky, S.R. Amyloid-beta neuroprotection mediated by a targeted antioxidant. Sci. Rep. 2014, 4, 4983. [Google Scholar] [CrossRef]
- Gomes, L.M.; Mahammed, A.; Prosser, K.E.; Smith, J.R.; Silverman, M.A.; Walsby, C.J.; Gross, Z.; Storr, T. A catalytic antioxidant for limiting amyloid-beta peptide aggregation and reactive oxygen species generation. Chem. Sci. 2019, 10, 1634–1643. [Google Scholar] [CrossRef]
- Abdelwahed, A.; Bouhlel, I.; Skandrani, I.; Valenti, K.; Kadri, M.; Guiraud, P.; Steiman, R.; Mariotte, A.M.; Ghedira, K.; Laporte, F.; et al. Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus: Confirmation by microarray expression profiling. Chem. Biol. Interact. 2007, 165, 1–13. [Google Scholar] [CrossRef]
- Yang, M.; Memon, K.H.; Lateef, M.; Na, D.; Wan, S.; Eric, D.; Zhang, L.; Jiang, T. 1,2,3,4,6-pentakis[-O-(3,4,5-trihydroxybenzoyl)]-α, β-D-glucopyranose (PGG) analogs: Design, synthesis, anti-tumor and anti-oxidant activities. Carbohydr. Res. 2016, 430, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Salazar, V.; Ruiz-Gabarre, D.; García-Escudero, V. Alzheimer′s disease and green tea: Epigallocatechin-3-gallate as a modulator of inflammation and oxidative stress. Antioxidants 2023, 12, 1460. [Google Scholar] [CrossRef] [PubMed]
- Jarero-Basulto, J.J.; Gasca-Martínez, Y.; Rivera-Cervantes, M.C.; Gasca-Martínez, D.; Carrillo-González, N.J.; Beas-Zárate, C.; Gudiño-Cabrera, G. Cytotoxic effect of amyloid-β1-42 oligomers on endoplasmic reticulum and Golgi apparatus arrangement in SH-SY5Y neuroblastoma cells. NeuroSci 2024, 5, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zheng, J.; Chen, H.; Zeng, X.; Wang, S.; Yang, C.; Li, X.; Xiao, Y.; Zheng, L.; Chen, H.; et al. A systematic screening of traditional Chinese medicine identifies two novel inhibitors against the cytotoxic aggregation of amyloid beta. Front. Pharmacol. 2021, 12, 637766. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Wen, C.; Dechsupa, N.; Yu, Z.; Zhang, X.; Liang, S.; Lei, X.; Xu, T.; Gao, X.; Hu, Q.; Innuan, P.; et al. Pentagalloyl glucose: A review of anticancer properties, molecular targets, mechanisms of action, pharmacokinetics, and safety profile. Molecules 2023, 28, 4856. [Google Scholar] [CrossRef]
- Jiamboonsri, P.; Pithayanukul, P.; Bavovada, R.; Saparpakorn, P. Factors influencing oral bioavailability of Thai mango seed kernel extract and its key phenolic principles. Molecules 2015, 20, 21254–21273. [Google Scholar] [CrossRef]
- Pereira, L.M.; Khamto, N.; Chawapun, P.; Siriphong, S.; Innuan, P.; Suwan, A.; Luangsuep, T.; Photilimthana, N.; Maita, W.; Thanacharttanatchaya, R.; et al. Methyl gallate nanomicelles impairs neutrophil accumulation in zymosan-induced arthritis. Colloids Surf. B Biointerfaces 2023, 227, 113351. [Google Scholar] [CrossRef]
- Jiamboonsri, P.; Pithayanukul, P.; Bavovada, R.; Gao, S.; Hu, M. A validated liquid chromatography–tandem mass spectrometry method for the determination of methyl gallate and pentagalloyl glucopyranose: Application to pharmacokinetic studies. J. Chromatogr. B 2015, 986–987, 12–17. [Google Scholar] [CrossRef]
- Scognamiglio, M.; D’Abrosca, B.; Fiumano, V.; Golino, M.; Esposito, A.; Fiorentino, A. Seasonal phytochemical changes in Phillyrea angustifolia L.: Metabolomic analysis and phytotoxicity assessment. Phytochem. Lett. 2014, 8, 163–170. [Google Scholar] [CrossRef]
- Bayaçlı, G.; Patır, İ.; Karkar, B.; Şahin, S. Optimization of the synergistic antioxidant effect of selected phenolic compounds (gallic acid, rosmarinic acid and caffeic acid) and investigation of their ability to prevent formation of DNA base damage. GIDA-J. Food 2024, 49, 777–790. [Google Scholar] [CrossRef]
- Lorca, G.; Ballestero, D.; Langa, E.; Pino-Otín, M.R. Enhancing Antibiotic Efficacy with Natural Compounds: Synergistic activity of tannic acid and nerol with commercial antibiotics against pathogenic bacteria. Plants 2024, 13, 2717. [Google Scholar] [CrossRef] [PubMed]
- Jahić Mujkić, A.; Tušek Žnidarič, M.; Berbić, S.; Žerovnik, E. Synergy of the inhibitory action of polyphenols plus vitamin C on amyloid fibril formation: Case study of human stefin B. Antioxidants 2021, 10, 1471. [Google Scholar] [CrossRef] [PubMed]
- Watrelot, A.A.; Le Guernevé, C.; Hallé, H.; Meudec, E.; Véran, F.D.R.; Williams, P.; Robillard, B.; Garcia, F.O.; Poncet-Legrand, C.L.; Cheynier, V.R. Multimethod approach for extensive characterization of gallnut tannin extracts. J. Agric. Food Chem. 2020, 68, 13426–13438. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications, and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Sano, H.; Kawaguchi, S.; Iimori, T.; Kuragano, M.; Tokuraku, K.; Uwai, K. On-site evaluation of constituent content and functionality of Perilla frutescens var. crispa using fluorescence spectra. Molecules 2023, 28, 7199. [Google Scholar] [CrossRef]
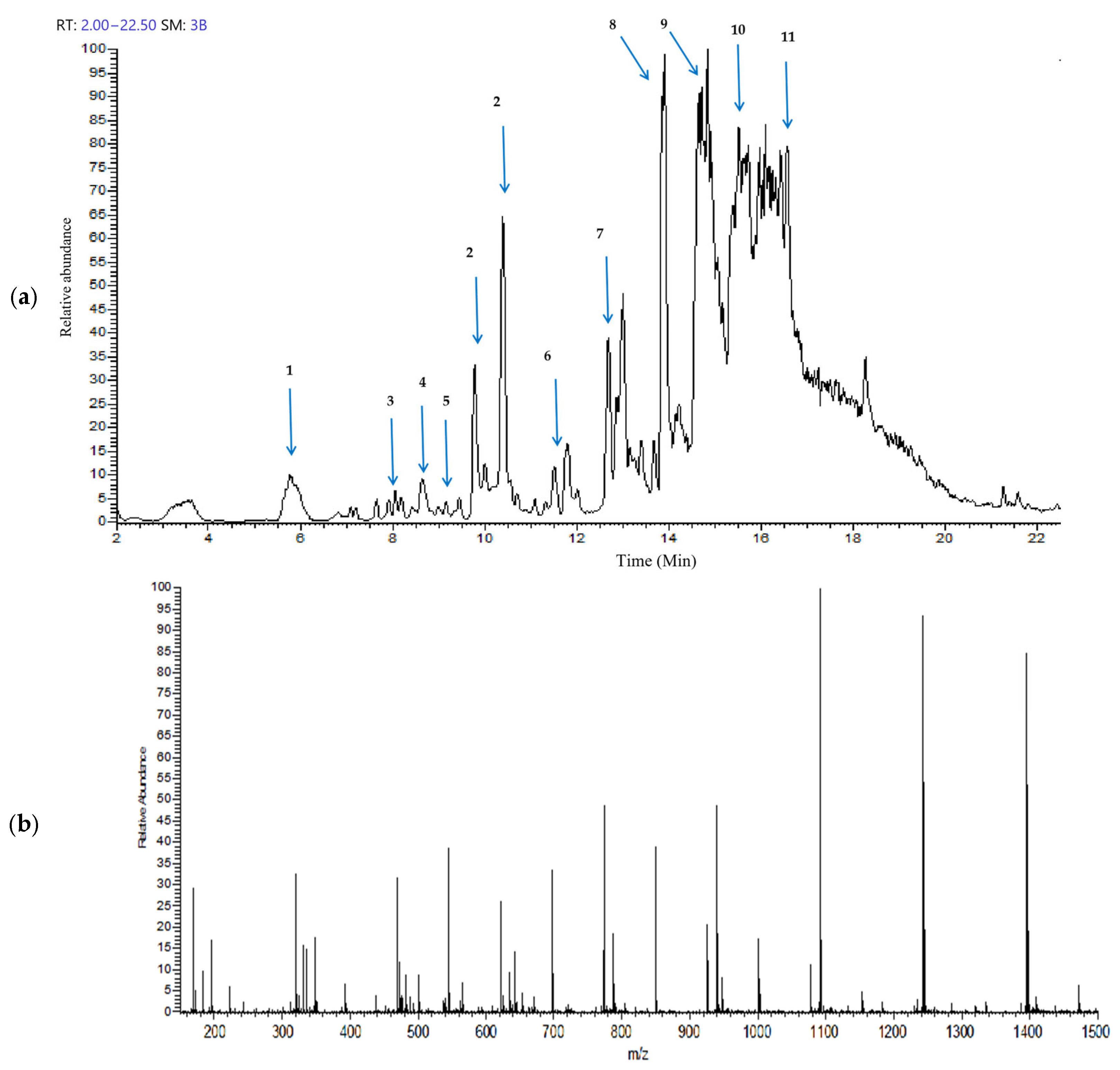


| Peak No. | Compounds | * RT/Min | Fragment Masses (m/z) | Molecular Weight | Molecular Formula |
|---|---|---|---|---|---|
| 1 | Gallic acid | 5.77 | 168.89 | 170.12 | C7H6O5 |
| 2 | Digallic acid | 9.79, 10.40 | 321.1, 169.01 | 322.22 | C14H10O9 |
| 3 | Galloylshikimic acid | 8.05 | 325.04, 305.04, 168.87 | 326.25 | C14H1409 |
| 4 | Monogalloyl Glucose | 8.65 | 331.01, 168.91 | 332.26 | C13H16O10 |
| 5 | Digalloyl Glucose | 9.16 | 483.09, 331.11, 285.07 | 484.4 | C20H20O14 |
| 6 | Trigalloyl Glucose | 11.49 | 635.16, 483.17, 373.15, 168.92 | 636.5 | C27H24O18 |
| 7 | Tetragalloyl Glucose | 12.67 | 787.23, 635.35, 393.24, 168.96 | 788.6 | C34H28O22 |
| 8 | Pentagalloyl Glucose | 13.91 | 939.48, 769.49, 469.36, 169.03 | 940.7 | C41H32O26 |
| 9 | Hexagalloyl Glucose | 14.64 | 1091.36, 939.48, 545.53, 469.63 | 1092.8 | C48H36O30 |
| 10 | Heptagalloyl Glucose | 15.58 | 1243.48, 1091.55, 621.68, 545.59, 469.67 | 1244.9 | C55H40O34 |
| 11 | Octagalloyl Glucose | 16.28 | 1395.45, 1243.32, 697.48, 621.38, 545.38, 469.52 | 1396 | C62H44O38 |
| Fractions | Percentage of Yield (%) | * EC50 (mg/mL ± SD) |
|---|---|---|
| 95% EtOH extract | 58.5 | 1.65 ± 0.11 |
| CHCl3 fraction | 5.4 | n.a. |
| EtOAc fraction | 61.5 | 2.376 ± 0.93 |
| n-Butanol fraction | 29 | 10.89 ± 4.23 |
| Aqueous fraction | 3.9 | n.a. |
| Position | δH | δC | HMBC (H-C) | DEPT |
|---|---|---|---|---|
| Glucose Moiety | ||||
| 1 | 6.24(d, J = 8.6 Hz, 1H) | 93.8 | C3, C7′ (Gal 1) | CH |
| 2 | 5.64–5.57 (m, 2H) | 72.2 | C1, C3, C4, C5, C7′ (Gal 2) | CH |
| 3 | 5.91 (t, J = 9.7 Hz, 1H) | 74.1 | C2, C4, C7′ (Gal 3) | CH |
| 4 | 5.64–5.57 (m, 2H), | 69.8 | C1, C2, C3, C5, C6, C7′ (Gal 4) | CH |
| 5 | 4.43–4.36 (m, 2H) | 74.4 | C3, C4 | CH |
| 6a/6b | 4.43–4.36 (m, 2H), 4.51 (d, J = 10.3 Hz, 1H) | 63.1 | C4, C7′ (Gal 5) | CH2 |
| Galloyl moiety 1 | ||||
| 1′ | 121 | |||
| 2′/6′ | 7.05 (s, 2H) | 110.6 | C1′, C3′, C4′, C5′, C7′ (Gal 1) | CH |
| 3′/5′ | 146.5 | |||
| 4′ | 140 | |||
| 7′ | 166.2 | |||
| Galloyl moiety 2 | ||||
| 1′ | 120.3 | |||
| 2′/6′ | 6.90 (s, 2H) | 110.4 | C1′, C3′, C4′, C5′, C7′ (Gal 2) | CH |
| 3′/5′ | 146.5 | |||
| 4′ | 140.3 | |||
| 7′ | 167.3 | |||
| Galloyl moiety 3 | ||||
| 1′ | 120.2 | |||
| 2′/6′ | 6.98 (s, 2H) | 110.4 | C1′, C3′, C4′, C5′, C7′ (Gal 3) | CH |
| 3′/5′ | 146.4 | |||
| 4′ | 140.3 | |||
| 7′ | 166.9 | |||
| Galloyl moiety 4 | ||||
| 1′ | 120.2 | |||
| 2′/6′ | 6.95 (s, 2H) | 110.4 | C1′, C3′, C4′, C5′, C7′ (Gal 4) | CH |
| 3′/5′ | 146.4 | |||
| 4′ | 140.1 | |||
| 7′ | 166.9 | |||
| Galloyl moiety 5 | ||||
| 1′ | 119.7 | |||
| 2′/6′ | 7.11 (s, 2H) | 110.3 | C1′, C3′, C4′, C5′, C7′ (Gal 5) | CH |
| 3′/5′ | 146.3 | |||
| 4′ | 140 | |||
| 7′ | 167.9 | |||
| Position | δH | δC | HMBC (H → C) | DEPT |
|---|---|---|---|---|
| C1 | 120.8 | |||
| C2/C6 | 6.99 (s, 2H) | 109.9 | C1, C3, C4, C5, C7 | CH |
| C3/C5 | 144.6 | |||
| C4 | 138.1 | |||
| C7 | 169.0 | |||
| C8 | 3.74 (s, 3H) | 52.5 | C7 | CH3 |
| Test Sample | EC50 (µM ± SD) * | p-Value |
|---|---|---|
| PGG | 1.16 ± 0.025 | 9.99 × 10−8 |
| MG | 6.44 ± 0.13 | 7.65 × 10−6 |
| α-tocopherol | 3.19 ± 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, S.; Tohge, T.; Ananda, S.H.; Kuragano, M.; Tokuraku, K.; Uwai, K. Anti-Amyloid Aggregation Effects of Gobaishi (Galla chinensis) and Its Active Constituents. Molecules 2025, 30, 2720. https://doi.org/10.3390/molecules30132720
Akter S, Tohge T, Ananda SH, Kuragano M, Tokuraku K, Uwai K. Anti-Amyloid Aggregation Effects of Gobaishi (Galla chinensis) and Its Active Constituents. Molecules. 2025; 30(13):2720. https://doi.org/10.3390/molecules30132720
Chicago/Turabian StyleAkter, Sharmin, Takayuki Tohge, Sahithya Hulimane Ananda, Masahiro Kuragano, Kiyotaka Tokuraku, and Koji Uwai. 2025. "Anti-Amyloid Aggregation Effects of Gobaishi (Galla chinensis) and Its Active Constituents" Molecules 30, no. 13: 2720. https://doi.org/10.3390/molecules30132720
APA StyleAkter, S., Tohge, T., Ananda, S. H., Kuragano, M., Tokuraku, K., & Uwai, K. (2025). Anti-Amyloid Aggregation Effects of Gobaishi (Galla chinensis) and Its Active Constituents. Molecules, 30(13), 2720. https://doi.org/10.3390/molecules30132720








