Degradation of Folic Acid in the Composition of a Conjugate with Polyvinylpyrrolidone and Fullerene C60 Under UV and E-Beam Irradiation
Abstract
1. Introduction
2. Results and Discussion
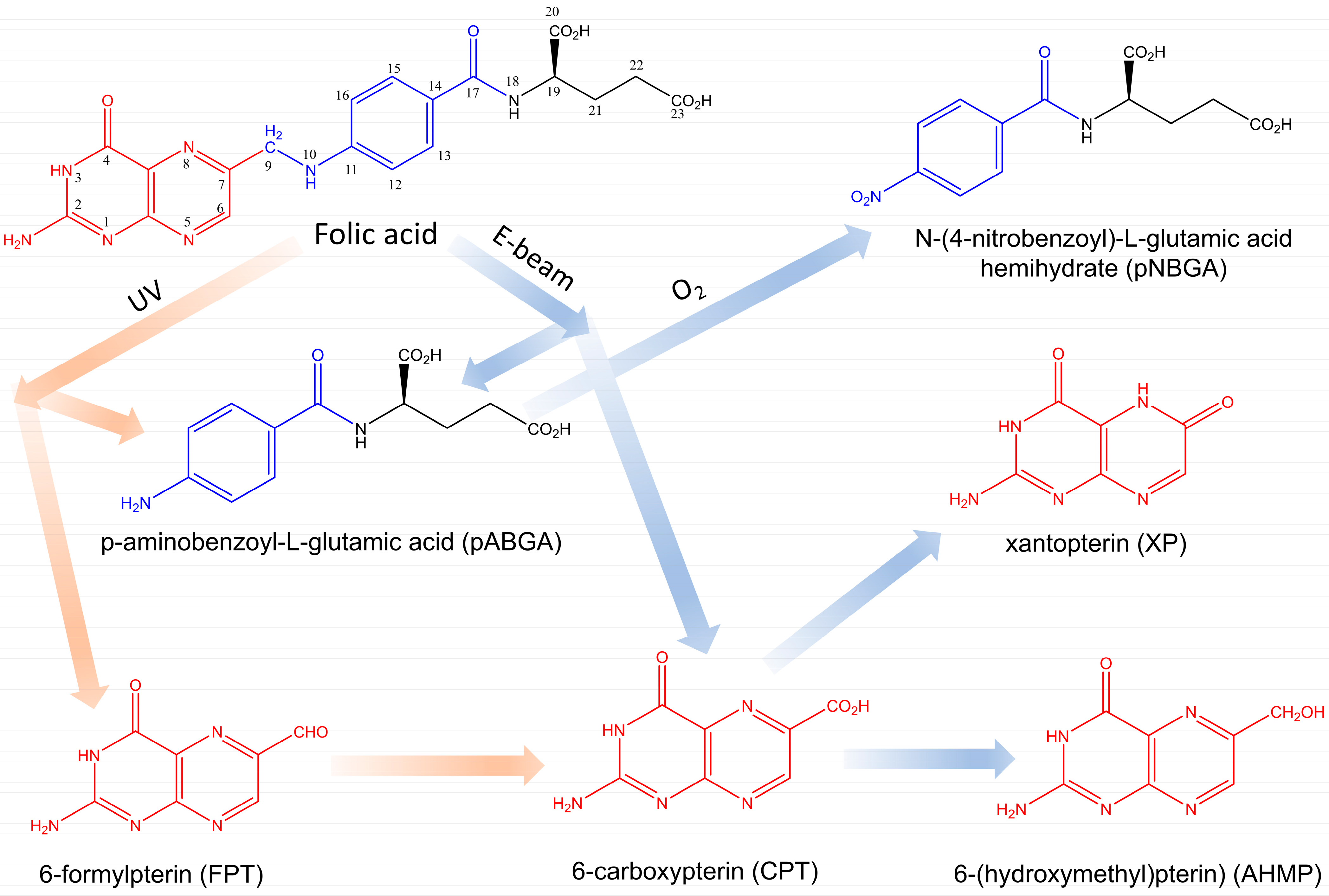
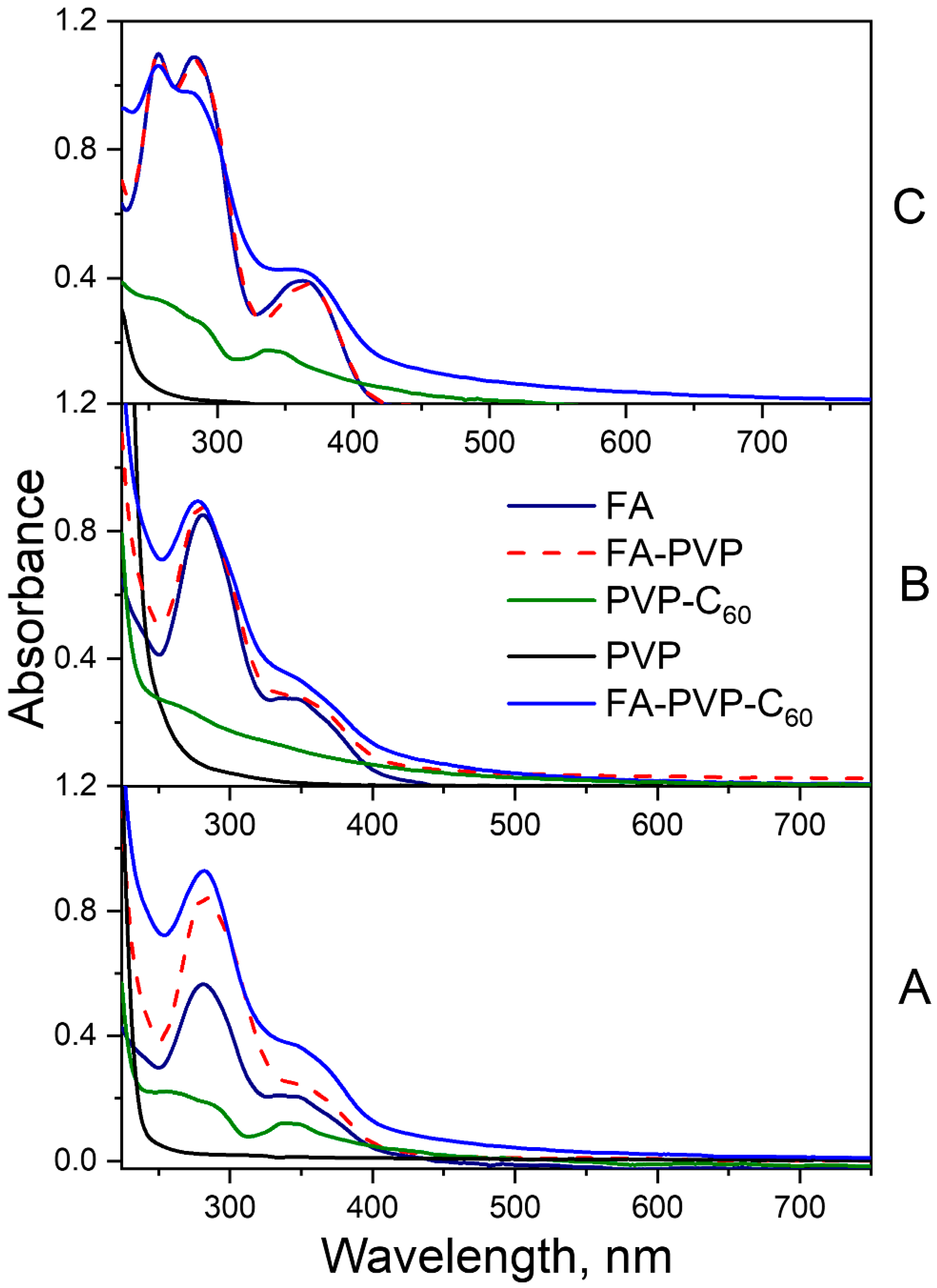
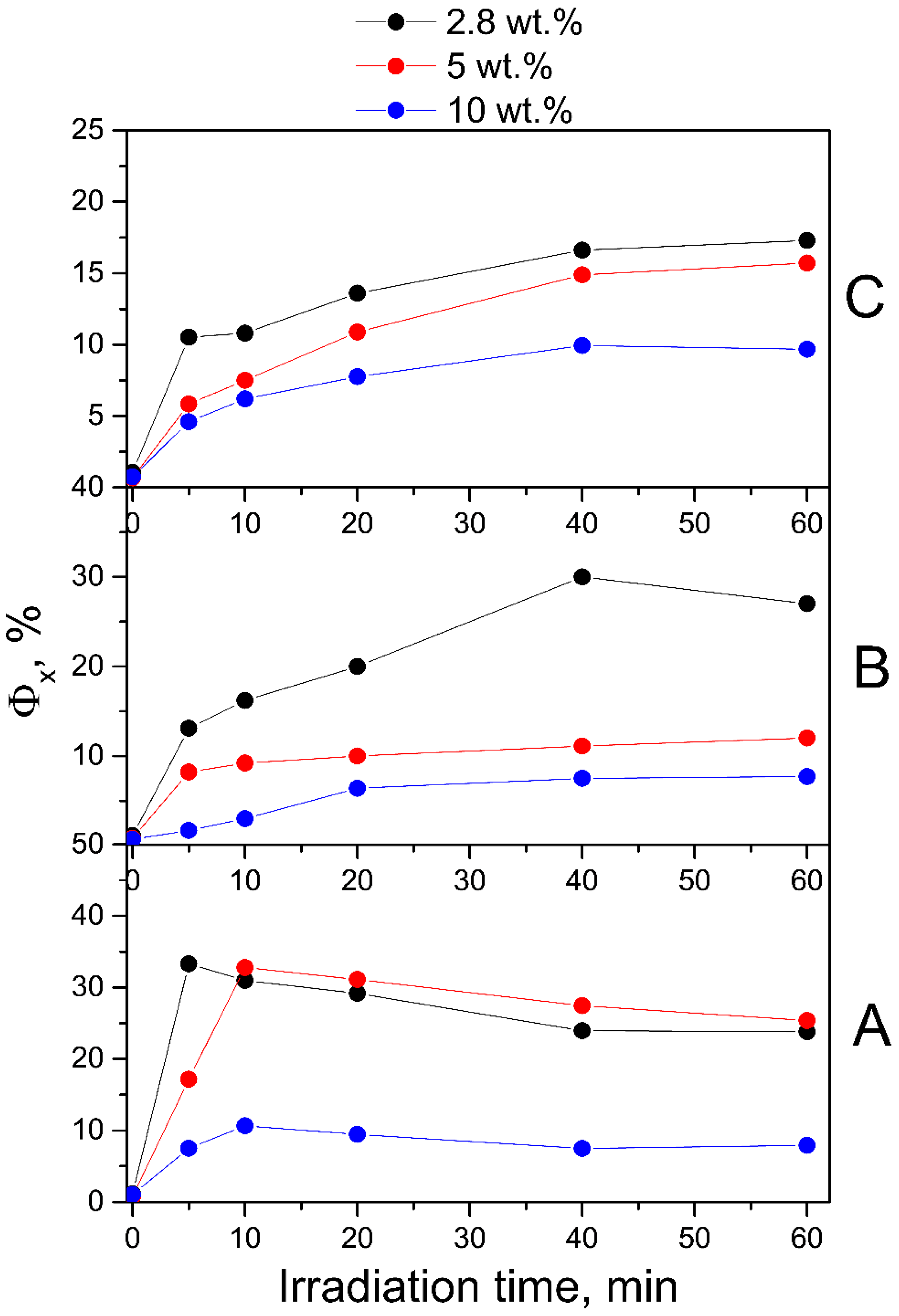
2.1. Photodegradation of FA in FA-PVP-C60
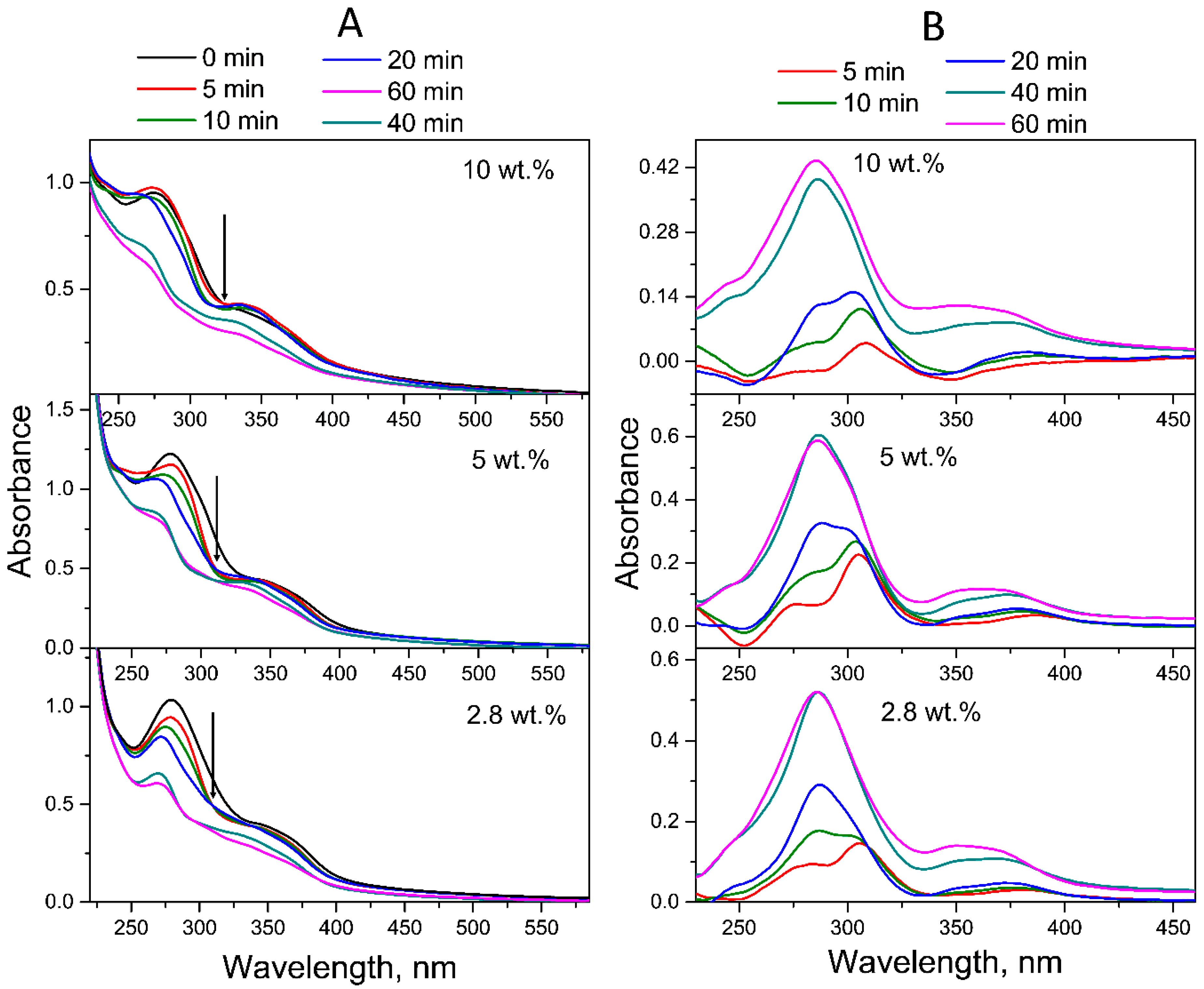
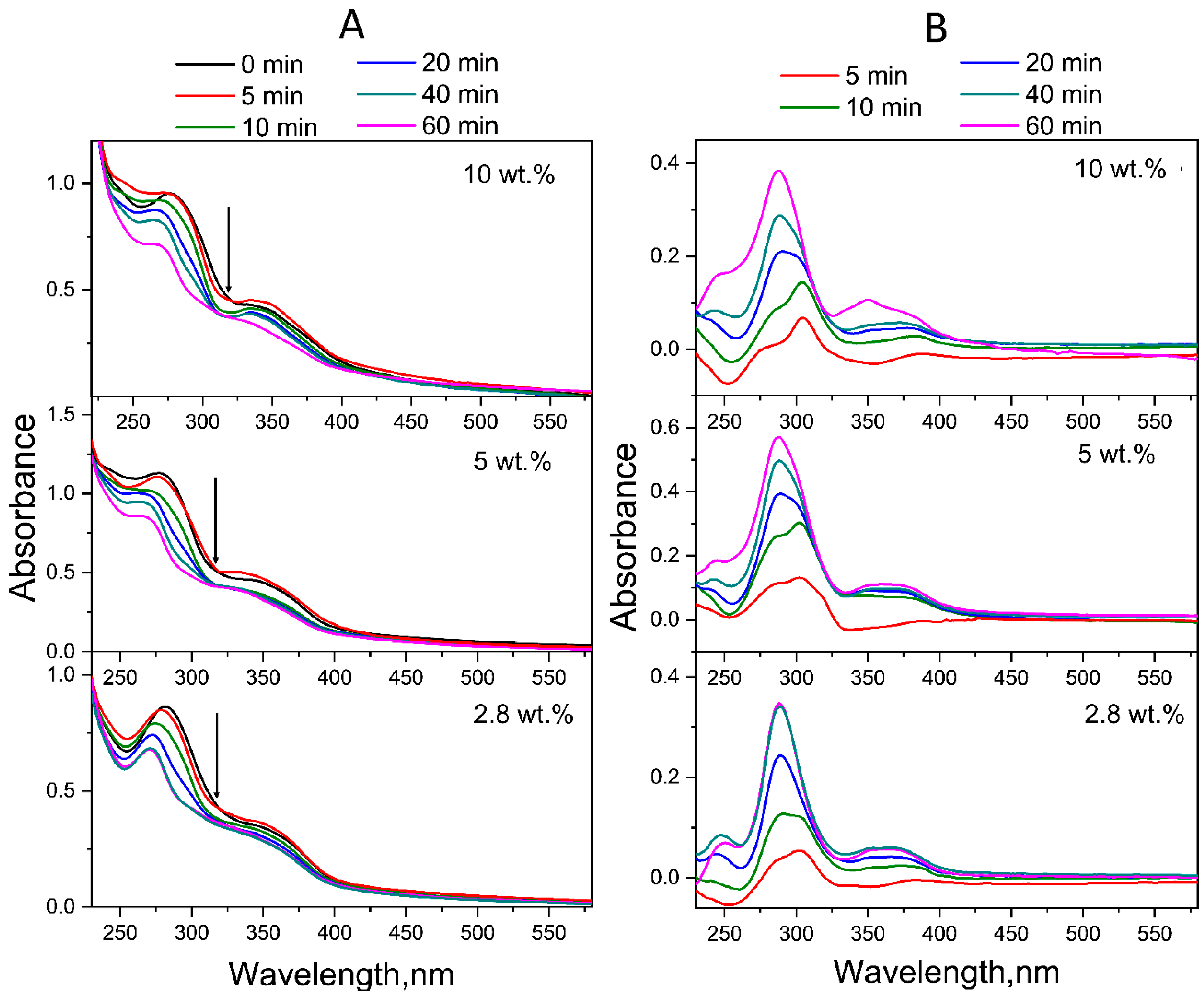
2.1.1. Neutral Aqueous Solutions
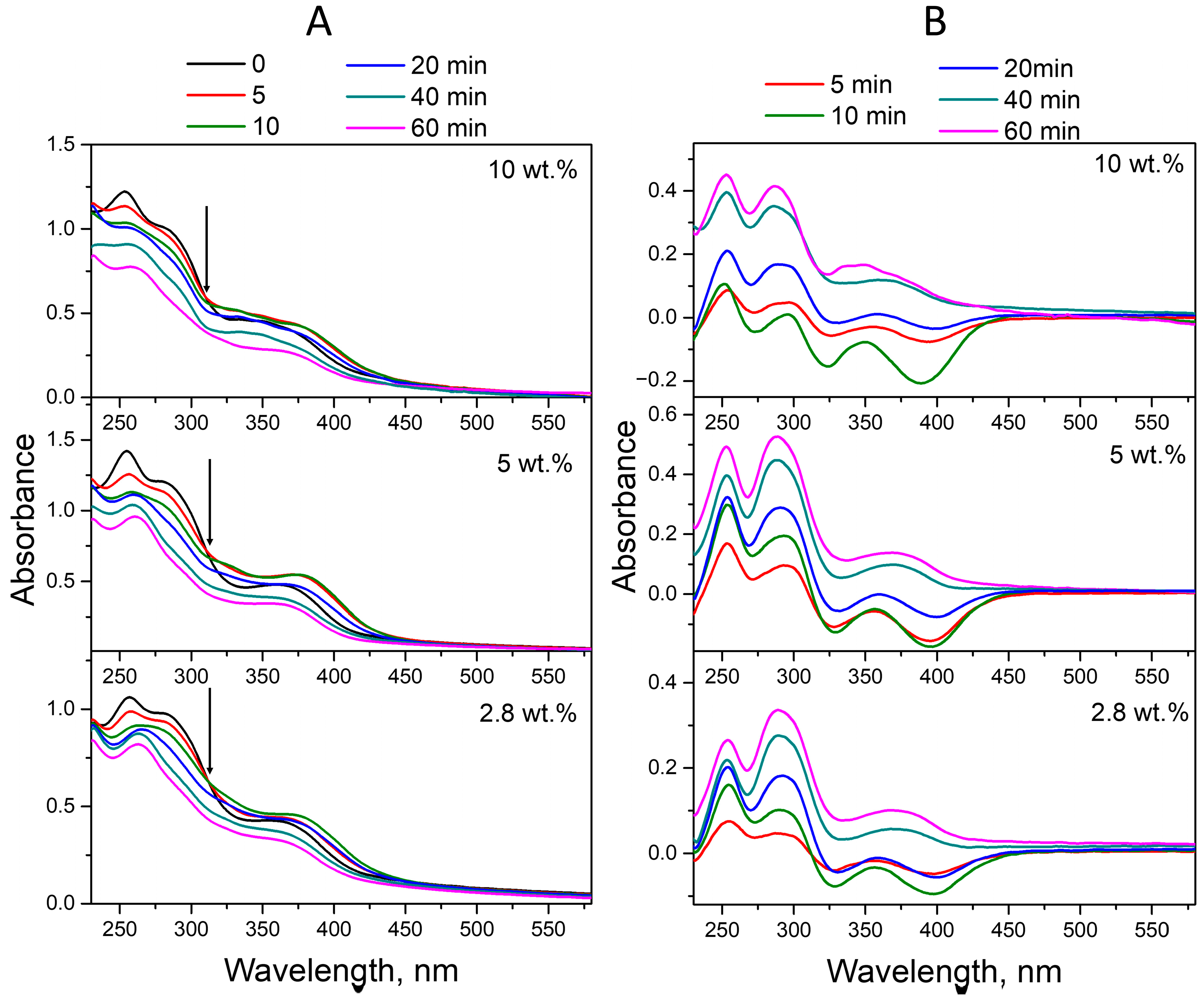
2.1.2. Acid Aqueous Solutions
2.1.3. Alkaline Aqueous Solutions
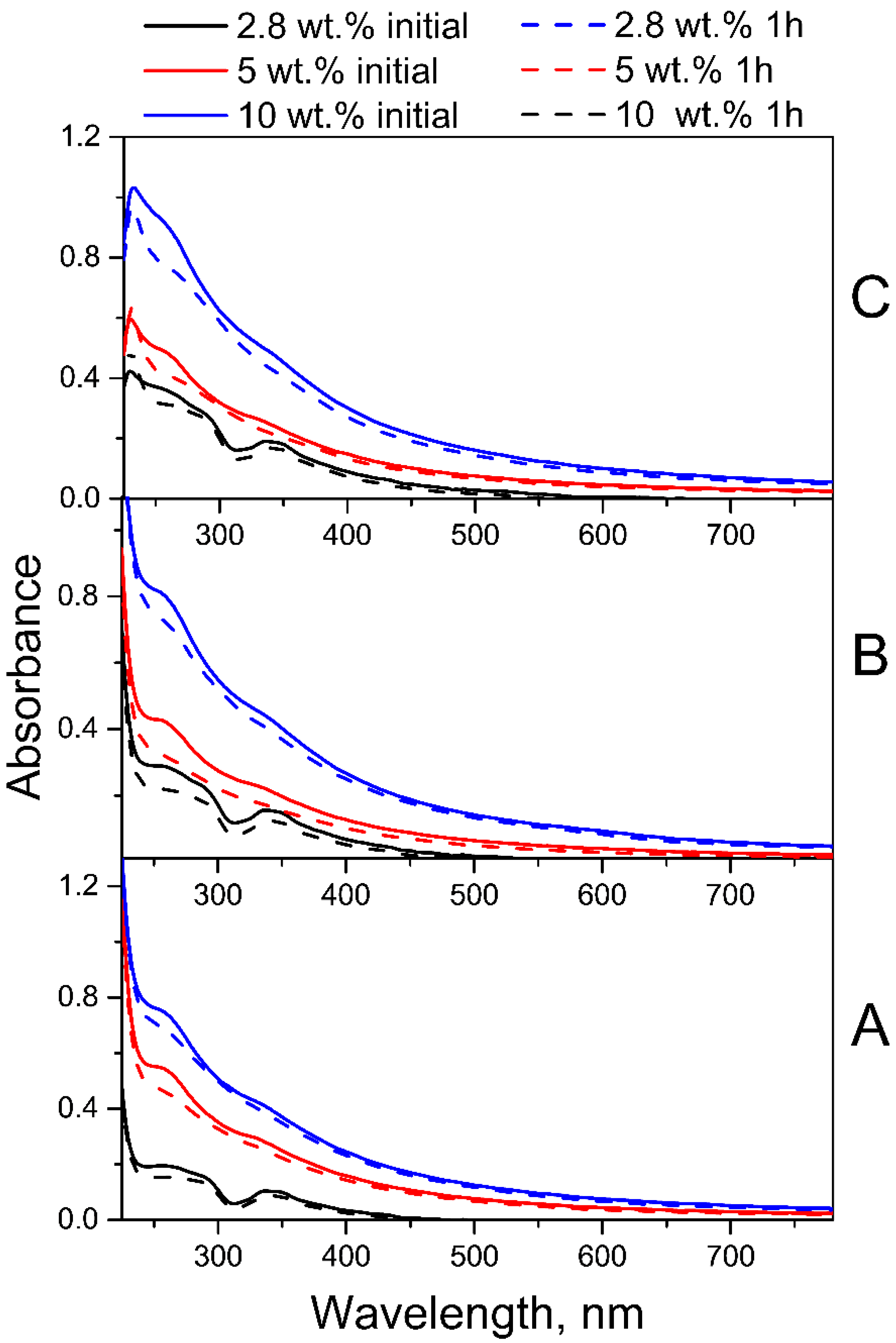
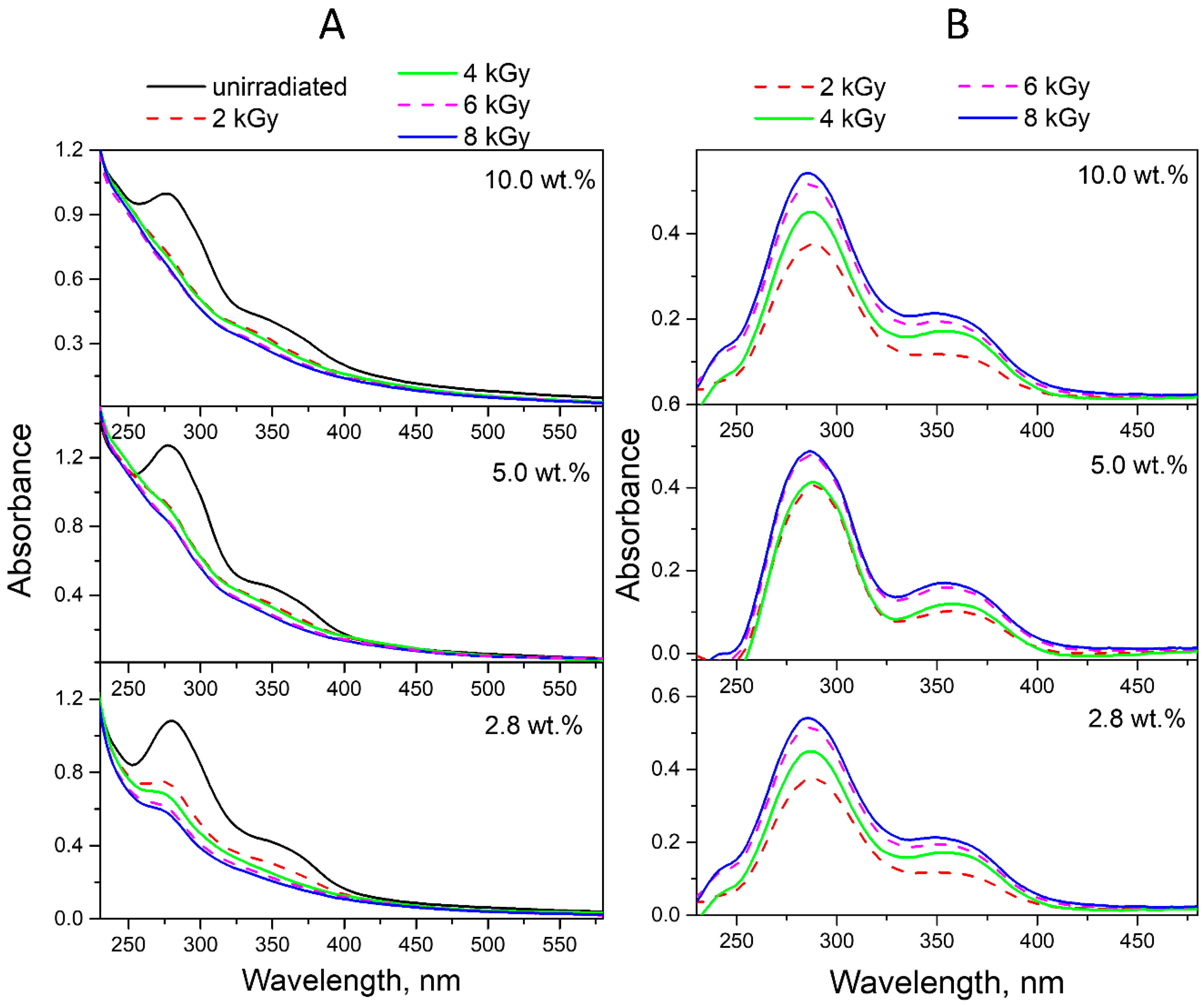
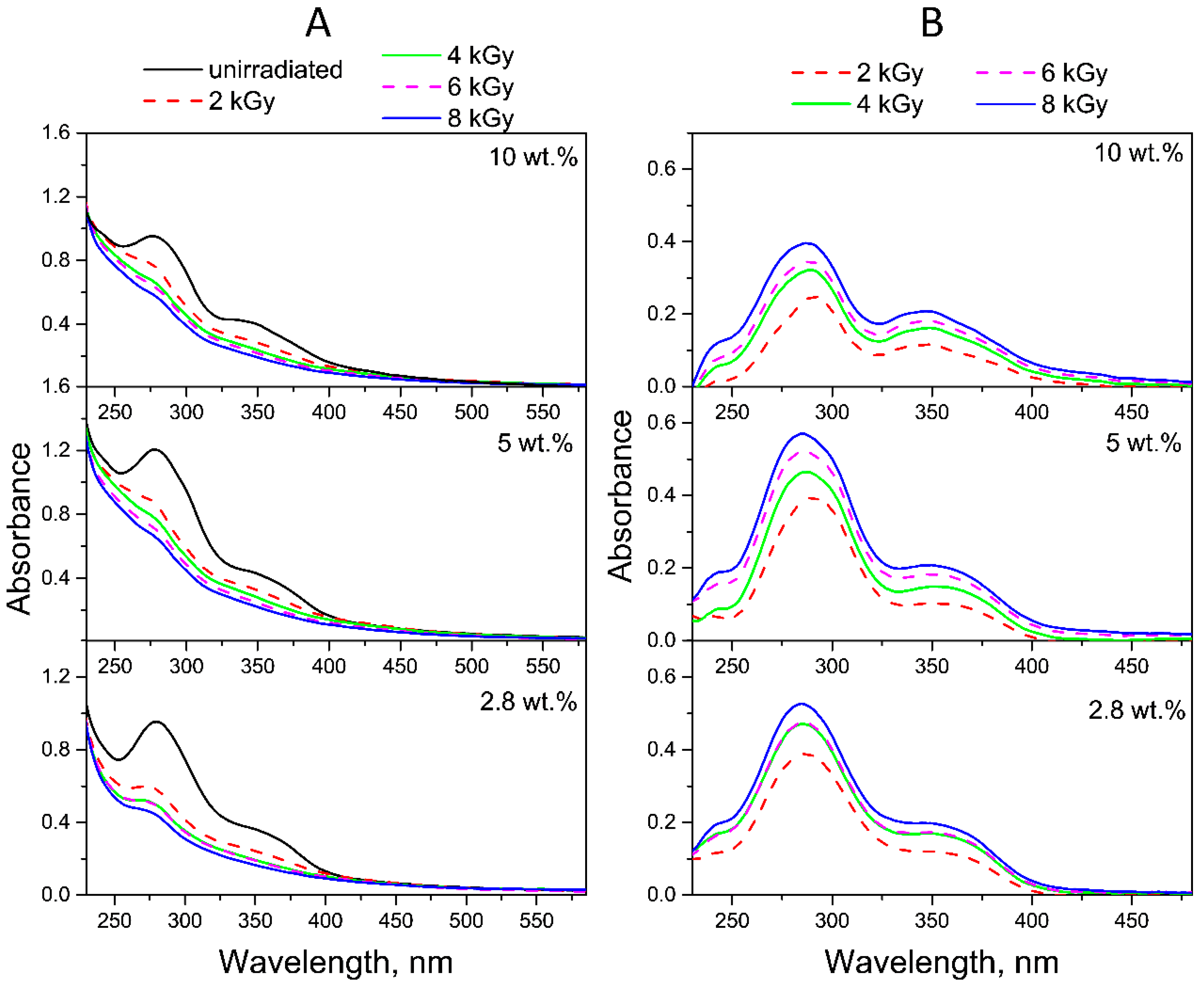
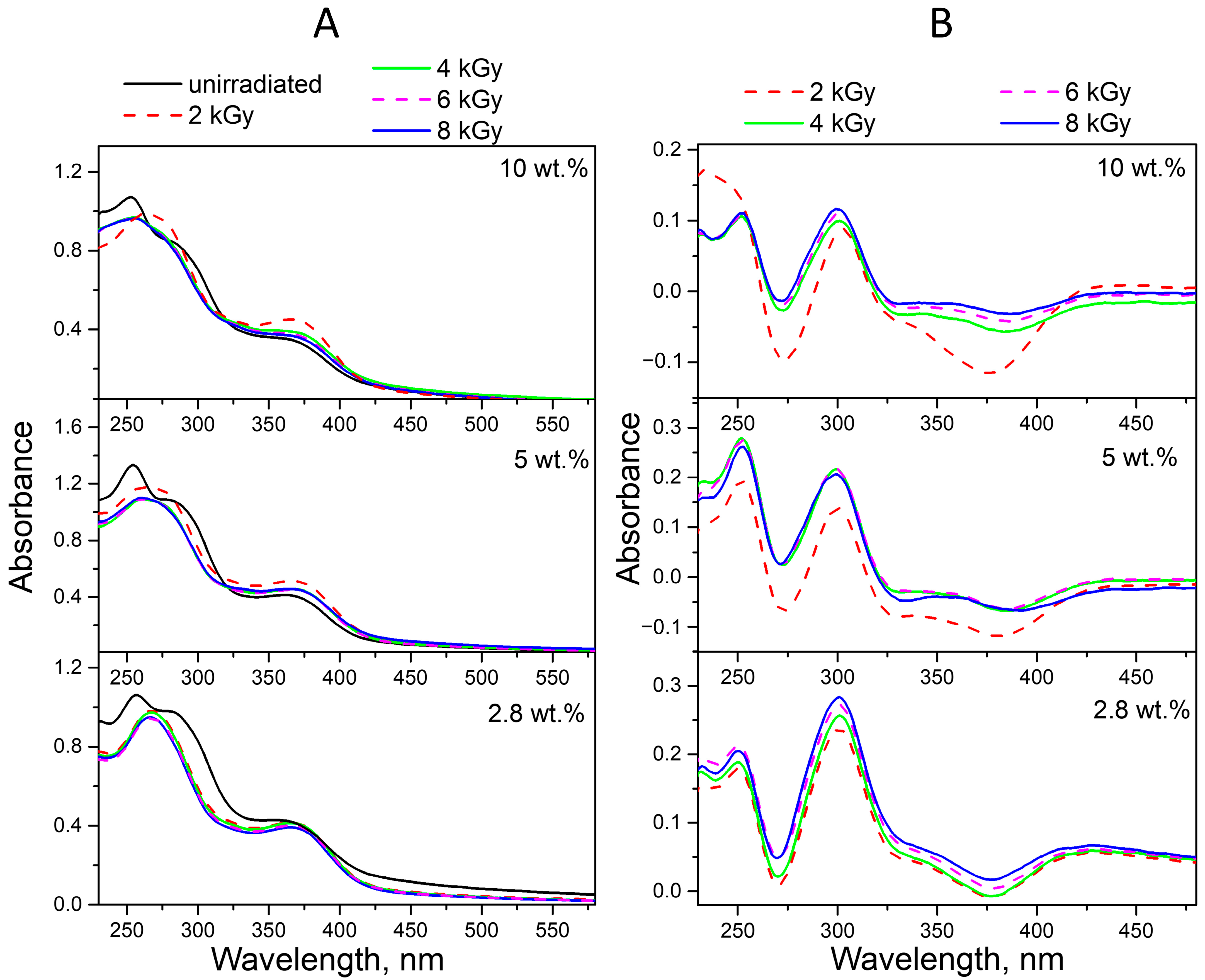
2.2. Radiolysis of FA in FA-PVP-C60
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Characterization of FA-PVP-C60
3.2.2. UV Irradiation of FA-PVP-C60
3.2.3. E-Beam Irradiation of FA-PVP-C60
3.2.4. Absorption and Fluorescence Spectroscopy
3.2.5. Fluorescence Quantum Yield Calculation
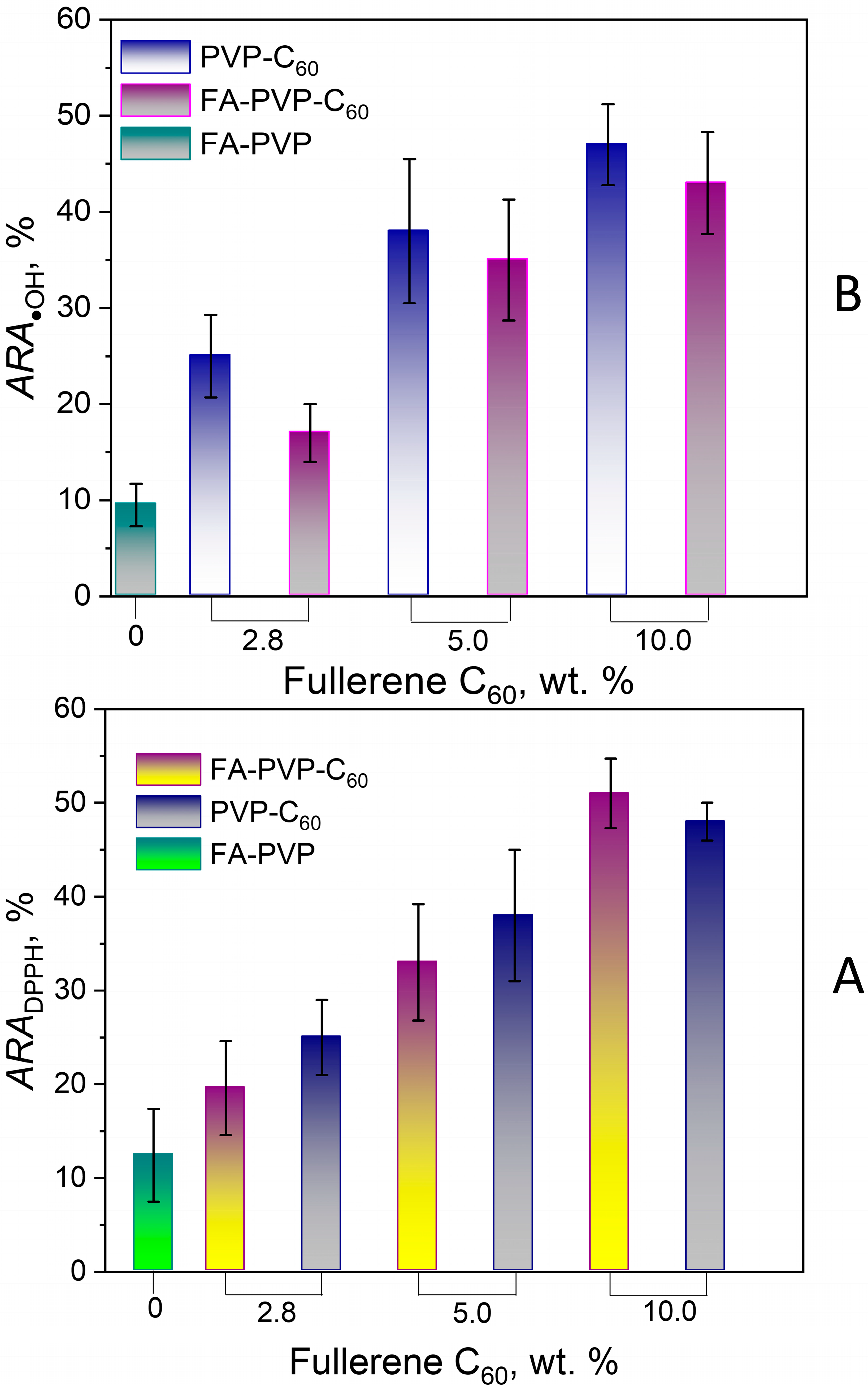
3.2.6. DPPH Scavenging Activity
3.2.7. Hydroxyl Radical Scavenging Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the Folate Receptor α in Oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Schlich, M.; Cryan, J.F.; O’Driscoll, C.M. Targeted Drug Delivery via Folate Receptors for the Treatment of Brain Cancer: Can the Promise Deliver? J. Pharm. Sci. 2017, 106, 3413–3420. [Google Scholar] [CrossRef] [PubMed]
- Dada, S.N.; Babanyinah, G.K.; Tetteh, M.T.; Palau, V.E.; Walls, Z.F.; Krishnan, K.; Croft, Z.; Khan, A.U.; Liu, G.; Wiese, T.E.; et al. Covalent and Noncovalent Loading of Doxorubicin by Folic Acid-Carbon Dot Nanoparticles for Cancer Theranostics. ACS Omega 2022, 7, 23322–23331. [Google Scholar] [CrossRef] [PubMed]
- Clement, S.; Chen, W.; Deng, W.; Goldys, E.M. X-Ray Radiation-Induced and Targeted Photodynamic Therapy with Folic Acid-Conjugated Biodegradable Nanoconstructs. Int. J. Nanomed. 2018, 13, 3553–3570. [Google Scholar] [CrossRef]
- Knežević, N.Z.; Mranović, J.; Borišev, I.; Milenković, S.; Janaćković, O.; Cunin, F.; Djordjevic, A. Hydroxylated Fullerene-Capped, Vinblastine-Loaded Folic Acid-Functionalized Mesoporous Silica Nanoparticles for Targeted Anticancer Therapy. RSC Adv. 2016, 6, 7061–7065. [Google Scholar] [CrossRef]
- San, H.H.M.; Alcantara, K.P.; Bulatao, B.P.I.; Sorasitthiyanukarn, F.N.; Nalinratana, N.; Suksamrarn, A.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Folic Acid-Grafted Chitosan-Alginate Nanocapsules as Effective Targeted Nanocarriers for Delivery of Turmeric Oil for Breast Cancer Therapy. Pharmaceutics 2023, 15, 110. [Google Scholar] [CrossRef]
- Narmani, A.; Rezvani, M.; Farhood, B.; Darkhor, P.; Mohammadnejad, J.; Amini, B.; Refahi, S.; Abdi Goushbolagh, N. Folic Acid Functionalized Nanoparticles as Pharmaceutical Carriers in Drug Delivery Systems. Drug Dev. Res. 2019, 80, 404–424. [Google Scholar] [CrossRef]
- Wen, D.; Zhang, X.; Ding, L.; Wen, H.; Liu, W.; Zhang, C.; Wang, B.; Li, L.; Diao, H. Folic Acid Functionalized Aggregation-Induced Emission Nanoparticles for Tumor Cell Targeted Imaging and Photodynamic Therapy. RSC Adv. 2022, 12, 4484–4489. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, Y.; Sun, L.; Yao, H.; Zhao, B.; Zhang, R.; Zhang, Y. Folic Acid Functionalized γ-Cyclodextrin C60, a Novel Vehicle for Tumor-Targeted Drug Delivery. J. Biomed. Nanotechnol. 2016, 12, 1393–1403. [Google Scholar] [CrossRef]
- Xia, J.M.; Wei, X.; Chen, X.W.; Shu, Y.; Wang, J.H. Folic Acid Modified Copper Nanoclusters for Fluorescent Imaging of Cancer Cells with Over-Expressed Folate Receptor. Microchim. Acta 2018, 185, 1–7. [Google Scholar] [CrossRef]
- Ma, Y.; Fu, S.; Tan, Y.; Zhang, A. Design and Synthesis of Highly Fluorescent and Stable Fullerene Nanoparticles as Probes for Folic Acid Detection and Targeted Cancer Cell Imaging. Nanotechnology 2021, 32, 195501. [Google Scholar] [CrossRef] [PubMed]
- Gruzdev, D.A.; Telegina, A.A.; Levit, G.L.; Solovieva, O.I.; Gusel’nikova, T.Y.; Razumov, I.A.; Krasnov, V.P.; Charushin, V.N. Carborane-Containing Folic Acid Bis-Amides: Synthesis and In Vitro Evaluation of Novel Promising Agents for Boron Delivery to Tumour Cells. Int. J. Mol. Sci. 2022, 23, 13726. [Google Scholar] [CrossRef]
- Borisenkova, A.A.; Bolshakova, O.I.; Titova, A.V.; Ryabokon, I.S.; Markova, M.A.; Lyutova, Z.B.; Sedov, V.P.; Varfolomeeva, E.Y.; Bakhmetyev, V.V.; Arutyunyan, A.V.; et al. Fullerene C60 Conjugate with Folic Acid and Polyvinylpyrrolidone for Targeted Delivery to Tumor Cells. Int. J. Mol. Sci. 2024, 25, 5350. [Google Scholar] [CrossRef]
- Off, M.K.; Steindal, A.E.; Porojnicu, A.C.; Juzeniene, A.; Vorobey, A.; Johnsson, A.; Moan, J. Ultraviolet Photodegradation of Folic Acid. J. Photochem. Photobiol. B 2005, 80, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Gazzali, A.M.; Lobry, M.; Colombeau, L.; Acherar, S.; Azaïs, H.; Mordon, S.; Arnoux, P.; Baros, F.; Vanderesse, R.; Frochot, C. Stability of Folic Acid under Several Parameters. Eur. J. Pharm. Sci. 2016, 93, 419–430. [Google Scholar] [CrossRef]
- Attaf, S.E.; Hasan, H.M.A. Effect of Uv Irradiation on Folic Acid Drug. World J. Pharm. Pharm. Sci. 2019, 8, 103–114. [Google Scholar]
- Dántola, M.L.; Denofrio, M.P.; Zurbano, B.; Gimenez, C.S.; Ogilby, P.R.; Lorente, C.; Thomas, A.H. Mechanism of Photooxidation of Folic Acid Sensitized by Unconjugated Pterins. Photochem. Photobiol. Sci. 2010, 9, 1604–1612. [Google Scholar] [CrossRef]
- Tsyupka, D.V.; Mordovina, E.A.; Sindeeva, O.A.; Sapelkin, A.V.; Sukhorukov, G.B.; Goryacheva, I.Y. High-Fluorescent Product of Folic Acid Photodegradation: Optical Properties and Cell Effect. J. Photochem. Photobiol. A Chem. 2021, 407, 113045. [Google Scholar] [CrossRef]
- Araújo, M.M.; Marchioni, E.; Villavicencio, A.L.C.H.; Zhao, M.; di Pascoli, T.; Kuntz, F.; Bergaentzle, M. Mechanism of Folic Acid Radiolysis in Aqueous Solution. LWT 2015, 63, 599–603. [Google Scholar] [CrossRef]
- Araújo, M.M.; Marchioni, E.; Zhao, M.; Kuntz, F.; Di Pascoli, T.; Villavicencio, A.L.C.H.; Bergaentzle, M. LC/MS/MS Identification of Some Folic Acid Degradation Products after E-Beam Irradiation. Radiat. Phys. Chem. 2012, 81, 1166–1169. [Google Scholar] [CrossRef]
- Araújo, M.M.; Marchioni, E.; Bergaentzle, M.; Zhao, M.; Kuntz, F.; Hahn, E.; Villavicencio, A.L.C.H. Irradiation Stability of Folic Acid in Powder and Aqueous Solution. J. Agric. Food Chem. 2011, 59, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- El-Dessouky, M.M.; Abd-Elwahab, B.M.; Turk, S.A. Effect of Gamma Radiation on Folic Acid and Its Cobalt Complex Solutions. J. Radioanal. Nucl. Chem. Artic. 1988, 125, 255–263. [Google Scholar] [CrossRef]
- Der-Petrossian, M.; Födinger, M.; Knobler, R.; Hönigsmann, H.; Trautinger, F. Photodegradation of Folic Acid during Extracorporeal Photopheresis. Br. J. Dermatol. 2007, 156, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Fukuwatari, T.; Fujita, M.; Shibata, K. Effects of UVA Irradiation on the Concentration of Folate in Human Blood. Biosci. Biotechnol. Biochem. 2009, 73, 322–327. [Google Scholar] [CrossRef]
- Chen, C.; Ke, J.; Edward Zhou, X.; Yi, W.; Brunzelle, J.S.; Li, J.; Yong, E.L.; Xu, H.E.; Melcher, K. Structural Basis for Molecular Recognition of Folic Acid by Folate Receptors. Nature 2013, 500, 486–489. [Google Scholar] [CrossRef]
- Juzeniene, A.; Tam, T.T.T.; Iani, V.; Moan, J. The Action Spectrum for Folic Acid Photodegradation in Aqueous Solutions. J. Photochem. Photobiol. B 2013, 126, 11–16. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Khan, M.A.; Ahmad, I. Photodegradation of Folic Acid in Aqueous Solution. J. Pharm. Biomed. Anal. 1999, 19, 269–275. [Google Scholar] [CrossRef]
- Jamil Akhtar, M.; Ataullah Khan, M.; Ahmad, I. Identification of Photoproducts of Folic Acid and Its Degradation Pathways in Aqueous Solution. J. Pharm. Biomed. Anal. 2003, 31, 579–588. [Google Scholar] [CrossRef]
- Thomas, A.H.; Suárez, G.; Cabrerizo, F.M.; Martino, R.; Capparelli, A.L. Study of the Photolysis of Folic Acid and 6-Formylpterin in Acid Aqueous Solutions. J. Photochem. Photobiol. A Chem. 2000, 135, 147–154. [Google Scholar] [CrossRef]
- Fonseca, J.L.; Sosa, M.J.; Petroselli, G.; Erra-Balsells, R.; Quindt, M.I.; Bonesi, S.M.; Greer, A.; Greer, E.M.; Thomas, A.H.; Vignoni, M. Synthesis, Characterization and Photocleavage of Bis-Decyl Pteroic Acid: A Folate Derivative with Affinity to Biomembranes†. Photochem. Photobiol. 2023, 99, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Bakry, R.; Vallant, R.M.; Najam-ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal Applications of Fullerenes. Int. J. Nanomed. 2007, 2, 639–649. [Google Scholar]
- Wang, C.; Zhao, M.; Xie, J.; Ji, C.; Leng, Z.; Gu, Z. Fullerenol@nano-Montmorillonite Nanocomposite as an Efficient Radioprotective Agent for Ameliorating Radioactive Duodenal Injury. Chem. Eng. J. 2022, 427, 131725. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, C.; Xie, J.; Ji, C.; Gu, Z. Eco-Friendly and Scalable Synthesis of Fullerenols with High Free Radical Scavenging Ability for Skin Radioprotection. Small 2021, 17, 2102035. [Google Scholar] [CrossRef]
- Wusigale; Hu, L.; Cheng, H.; Gao, Y.; Liang, L. Mechanism for Inhibition of Folic Acid Photodecomposition by Various Antioxidants. J. Agric. Food Chem. 2020, 68, 340–350. [Google Scholar] [CrossRef]
- Wusigale; Fang, Z.; Hu, L.; Gao, Y.; Li, J.; Liang, L. Protection of Resveratrol against the Photodecomposition of Folic Acid and Photodecomposition-Induced Structural Change of Beta-Lactoglobulin. Food Res. Int. 2017, 102, 435–444. [Google Scholar] [CrossRef]
- Aceituno-Medina, M.; Mendoza, S.; Lagaron, J.M.; López-Rubio, A. Photoprotection of Folic Acid upon Encapsulation in Food-Grade Amaranth (Amaranthus hypochondriacus L.) Protein Isolate—Pullulan Electrospun Fibers. LWT 2015, 62, 970–975. [Google Scholar] [CrossRef]
- Wusigale; Fu, X.; Yin, X.; Ji, C.; Cheng, H.; Liang, L. Effects of Folic Acid and Caffeic Acid on Indirect Photo-Oxidation of Proteins and Their Costabilization under Irradiation. J. Agric. Food Chem. 2021, 69, 12505–12516. [Google Scholar] [CrossRef]
- Luo, S.; Wang, M.; Zhao, W.; Wang, Y. Interactions between Surfactants and Folic Acid and the Effects of Surfactants on the Photodegradation of Folic Acid. Wuli Huaxue Xuebao Acta Phys. Chim. Sin. 2019, 35, 766–774. [Google Scholar] [CrossRef]
- Fu, X.; Wusigale; Cheng, H.; Fang, Z.; Liang, L. Mechanism for Improved Protection of Whey Protein Isolate against the Photodecomposition of Folic Acid. Food Hydrocoll. 2018, 79, 439–449. [Google Scholar] [CrossRef]
- Bourassa, P.; Hasni, I.; Tajmir-Riahi, H.A. Folic Acid Complexes with Human and Bovine Serum Albumins. Food Chem. 2011, 129, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhang, J.; Zhou, P.; Subirade, M. Protective Effect of Ligand-Binding Proteins against Folic Acid Loss Due to Photodecomposition. Food Chem. 2013, 141, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Fani, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Photo-Protection and Controlled Release of Folic Acid Using Edible Alginate/Chitosan Nanolaminates. J. Food Eng. 2018, 229, 72–82. [Google Scholar] [CrossRef]
- Hamblin, M.R. Fullerenes as Photosensitizers in Photodynamic Therapy: Pros and Cons. Photochem. Photobiol. Sci. 2018, 17, 1515–1533. [Google Scholar] [CrossRef]
- Miyata, N.; Yamakoshi, Y.; Nakanishi, I. Reactive Species Responsible for Biological Actions of Photoexcited Fullerenes. Yakugaku Zasshi 2000, 120, 1007–1016. [Google Scholar] [CrossRef]
- Oriana, S.; Aroua, S.; Söllner, J.O.B.; Ma, X.J.; Iwamoto, Y.; Yamakoshi, Y. Water-Soluble C60- and C70-PVP Polymers for Biomaterials with Efficient 1O2 Generation. Chem. Commun. 2013, 49, 9302–9304. [Google Scholar] [CrossRef]
- Thomas, A.H.; Suárez, G.; Cabrerizo, F.M.; García Einschlag, F.S.; Martino, R.; Baiocchi, C.; Pramauro, E.; Capparelli, A.L. Photochemical Behavior of Folic Acid in Alkaline Aqueous Solutions and Evolution of Its Photoproducts. Helv. Chim. Acta 2002, 85, 2300–2315. [Google Scholar] [CrossRef]
- McHedlov-Petrossyan, N.O.; Marfunin, M.O.; Tikhonov, V.A.; Shekhovtsov, S.V. Unexpected Colloidal Stability of Fullerenes in Dimethyl Sulfoxide and Related Systems. Langmuir 2022, 38, 10000–10009. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Suzuki, H.; Hatanaka, M.; Wakabayashi, H.; Kodama, T. Infrared Emission Spectra of Fullerene C60 Thin Films. Phys. Rev. B 2024, 109, 035409. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Seo, Y.H.; Oh, T.H. PVP Assisted Synthesis of Hydroxyapatite Nanorods with Tunable Aspect Ratio and Bioactivity. J. Nanomater. 2015, 2015, 621785. [Google Scholar] [CrossRef]
- Behera, M.; Ram, S. Interaction between Poly(Vinyl pyrrolidone) PVP and Fullerene C60 at the Interface in PVP-C60 Nanofluids-A Spectroscopic Study. IOP Conf. Ser. Mater. Sci. Eng. 2018, 330, 012016. [Google Scholar] [CrossRef]
- El Hotaby, W.; Sherif, H.H.A.; Hemdan, B.A.; Khalil, W.A.; Khalil, S.K.H. Assessment of in Situ-Prepared Polyvinylpyrrolidone-Silver Nanocomposite for Antimicrobial Applications. Acta Phys. Pol. A 2017, 131, 1554–1560. [Google Scholar] [CrossRef]
- Nigmatullina, E.K.; Kibalin, I.A.; Sedov, V.P.; Borisenkova, A.A.; Bykov, A.A.; Golosovsky, I.V. “Phantom” Atoms and Thermal Motion in Fullerene C60revealed by X-Ray and Neutron Diffraction. J. Phys. Condens. Matter 2021, 33, 455401. [Google Scholar] [CrossRef]
- Gatta, A.K.; Chandrashekhar, R.; Udupa, N.; Reddy, M.S.; Mutalik, S.; Josyula, V.R. Strategic Design of Dicer Substrate SiRNA to Mitigate the Resistance Mediated by ABCC1 in Doxorubicinresistant Breast Cancer. Indian J. Pharm. Sci. 2020, 82, 329. [Google Scholar] [CrossRef]
- Baibarac, M.; Smaranda, I.; Nila, A.; Serbschi, C. Optical Properties of Folic Acid in Phosphate Buffer Solutions: The Influence of PH and UV Irradiation on the UV-VIS Absorption Spectra and Photoluminescence. Sci. Rep. 2019, 9, 14278. [Google Scholar] [CrossRef] [PubMed]
- Tinel, L.; Rossignol, S.; Ciuraru, R.; Dumas, S.; George, C. Photosensitized Reactions Initiated by 6-Carboxypterin: Singlet and Triplet Reactivity. Phys. Chem. Chem. Phys. 2016, 18, 17105–17115. [Google Scholar] [CrossRef]
- Thomas, A.H.; Lorente, C.; Capparelli, A.L.; Pokhrel, M.R.; Braun, A.M.; Oliveros, E. Fluorescence of Pterin, 6-Formylpterin, 6-Carboxypterin and Folic Acid in Aqueous Solution: PH Effects. Photochem. Photobiol. Sci. 2002, 1, 421–426. [Google Scholar] [CrossRef]
- Abramova, A.M.; Kokorina, A.A.; Sindeeva, O.A.; Jolibois, F.; Puech, P.; Sukhorukov, G.B.; Goryacheva, I.Y.; Sapelkin, A.V. Molecular Nature of Breakdown of the Folic Acid under Hydrothermal Treatment: A Combined Experimental and DFT Study. Sci. Rep. 2020, 10, 19668. [Google Scholar] [CrossRef]
- Dántola, M.L.; Urrutia, M.N.; Thomas, A.H. Effect of Pterin Impurities on the Fluorescence and Photochemistry of Commercial Folic Acid. J. Photochem. Photobiol. B 2018, 181, 157–163. [Google Scholar] [CrossRef]
- Suárez, G.; Cabrerizo, F.M.; Lorente, C.; Thomas, A.H.; Capparelli, A.L. Study of the Photolysis of 6-Carboxypterin in Acid and Alkaline Aqueous Solutions. J. Photochem. Photobiol. A Chem. 2000, 132, 53–57. [Google Scholar] [CrossRef]
- ISO 11137-1:2025; Sterilization of Health Care Products—Radiation. Part 1: Requirements for the Development, Validation and Routine Control of a Sterilization Process for Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2025.
- Delipetar-Grudl, A.; Getoff, N. Radiation-Induced Sensitizing Effect of Folic Acid (Vitamin B11) and Its Synergistic Action to Mitomycin C: In Vitro Experiments and Radiolysis. Oncol. Res. 2004, 14, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Adhikari, S.; Patro, B.S.; Chattopadhyay, S.; Mukherjee, T. Free Radical Scavenging Behavior of Folic Acid: Evidence for Possible Antioxidant Activity. Free Radic. Biol. Med. 2001, 30, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Nawara, K.; Waluk, J. Improved Method of Fluorescence Quantum Yield Determination. Anal. Chem. 2017, 89, 8650–8655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lloveras, V.; Wu, Y.; Tolosa, J.; García-Martínez, J.C.; Vidal-Gancedo, J. Fluorescent and Magnetic Radical Dendrimers as Potential Bimodal Imaging Probes. Pharmaceutics 2023, 15, 1776. [Google Scholar] [CrossRef]
- Hashemian, S.; Tabatabaee, M.; Gafari, M. Fenton Oxidation of Methyl Violet in Aqueous Solution. J. Chem. 2013, 2013, 509097. [Google Scholar] [CrossRef]

| pH | G(−FA) × 102, Molecules/100 eV | ||
|---|---|---|---|
| Fullerene Content in the Conjugate, wt.% | |||
| 2.8 | 5.0 | 10.0 | |
| 4.5 | 7.0 ± 0.1 | 5.0 ± 0.1 | 4.0 ± 0.2 |
| 7.2 | 6.0 ± 0.1 | 5.8 ± 0.1 | 5.5 ± 0.1 |
| 10.7 | not defined | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borisenkova, A.A.; Baykov, D.V.; Titova, A.V.; Bakhmetyev, V.V.; Markova, M.A.; Lyutova, Z.B.; Popugaev, A.V.; Khaleev, V.S.; Sedov, V.P. Degradation of Folic Acid in the Composition of a Conjugate with Polyvinylpyrrolidone and Fullerene C60 Under UV and E-Beam Irradiation. Molecules 2025, 30, 2718. https://doi.org/10.3390/molecules30132718
Borisenkova AA, Baykov DV, Titova AV, Bakhmetyev VV, Markova MA, Lyutova ZB, Popugaev AV, Khaleev VS, Sedov VP. Degradation of Folic Acid in the Composition of a Conjugate with Polyvinylpyrrolidone and Fullerene C60 Under UV and E-Beam Irradiation. Molecules. 2025; 30(13):2718. https://doi.org/10.3390/molecules30132718
Chicago/Turabian StyleBorisenkova, Alina A., Dmitriy V. Baykov, Anna V. Titova, Vadim V. Bakhmetyev, Maria A. Markova, Zhanna B. Lyutova, Anton V. Popugaev, Vladislav S. Khaleev, and Victor P. Sedov. 2025. "Degradation of Folic Acid in the Composition of a Conjugate with Polyvinylpyrrolidone and Fullerene C60 Under UV and E-Beam Irradiation" Molecules 30, no. 13: 2718. https://doi.org/10.3390/molecules30132718
APA StyleBorisenkova, A. A., Baykov, D. V., Titova, A. V., Bakhmetyev, V. V., Markova, M. A., Lyutova, Z. B., Popugaev, A. V., Khaleev, V. S., & Sedov, V. P. (2025). Degradation of Folic Acid in the Composition of a Conjugate with Polyvinylpyrrolidone and Fullerene C60 Under UV and E-Beam Irradiation. Molecules, 30(13), 2718. https://doi.org/10.3390/molecules30132718








