Effect of Alkyl Chain Length on Dissolution and Regeneration Behavior of Cotton in 1-Alkyl-3-methylimidazolium Acetate Ionic Liquids
Abstract
1. Introduction
2. Results
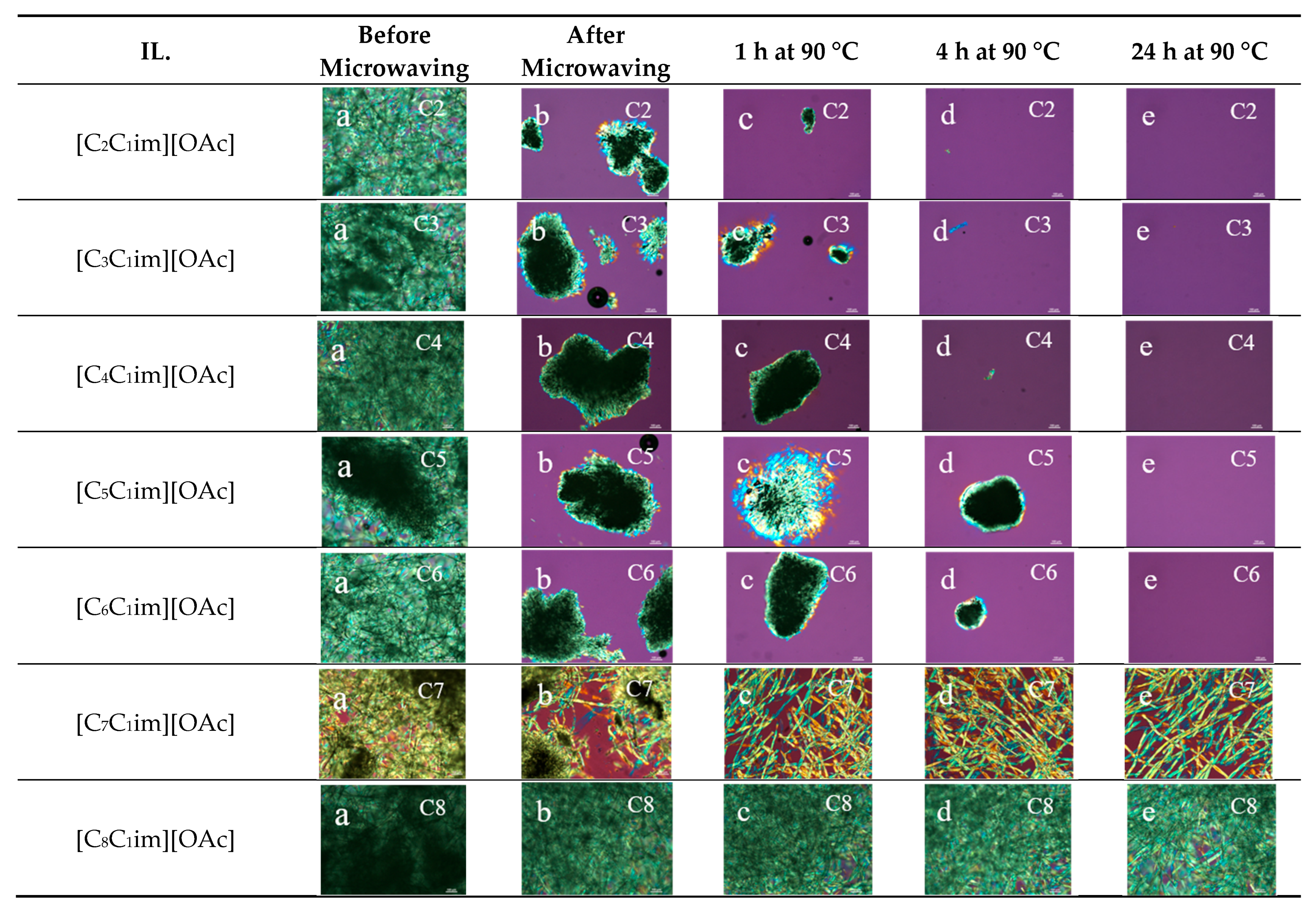
2.1. Polarized Light Microscopy of Cellulose Dissolution
2.2. Characterization of Regenerated Cellulose
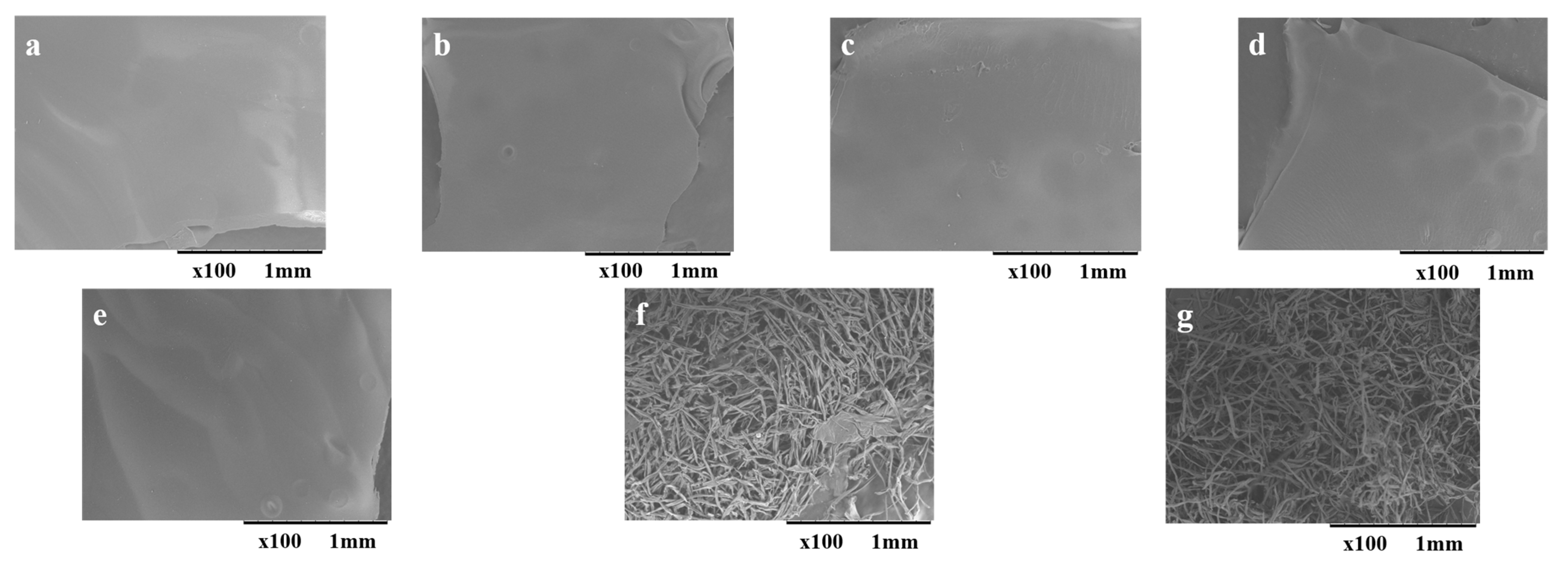
2.2.1. Scanning Electron Microscopic Images of Regenerated Cellulose
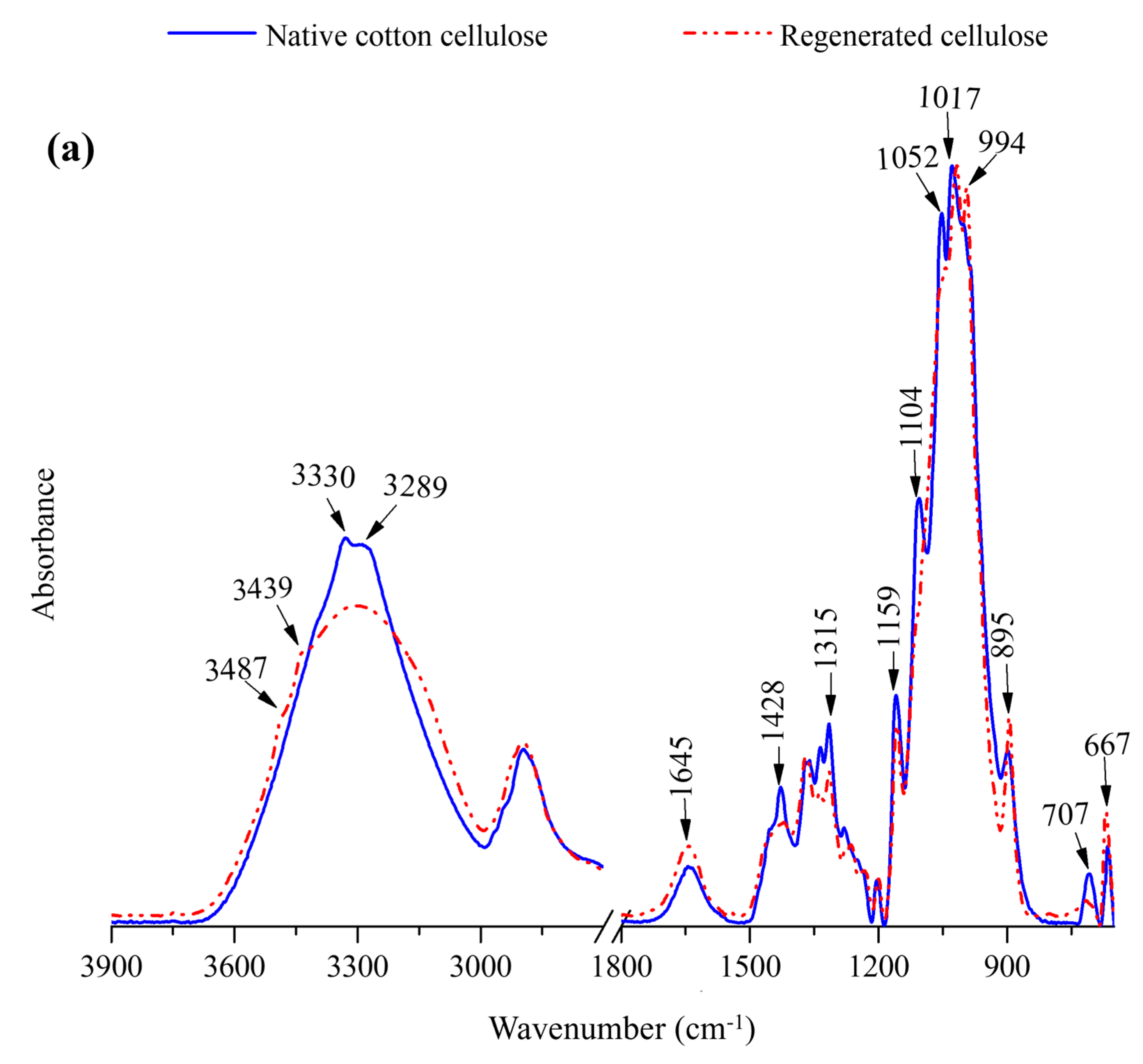
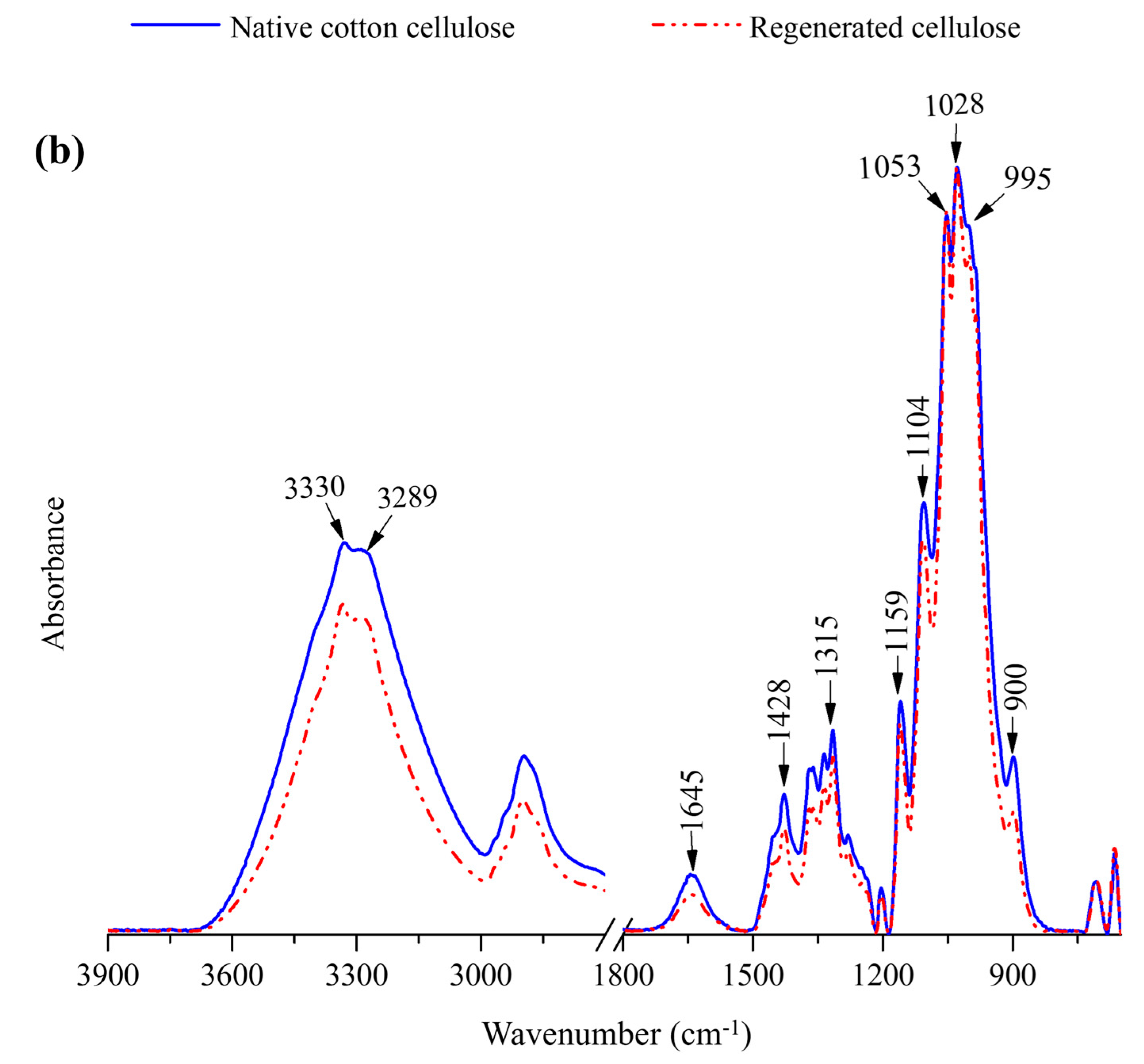
2.2.2. FTIR Spectra of Regenerated Cellulose
3. Discussion
4. Materials and Methods
4.1. Cotton Cellulose
4.1.1. Preparation of Cotton for Dissolution
4.1.2. Dissolution of Ground Cotton Fibers in ILs
4.1.3. Polarized Light Microscopy of Cellulose/IL Mixtures
4.1.4. Regeneration of Cellulose from Each Cellulose/IL Mixture
4.1.5. Drying the Regenerated Cellulose
4.1.6. Characterization of Regenerated Cellulose—Scanning Electron Microscopy (SEM)
4.1.7. Characterization of Regenerated Cellulose—Fourier-Transform Infrared (FTIR) Spectroscopy
4.2. Ionic Liquids
4.2.1. Synthesis of ILs
4.2.2. General Synthesis of 1-Alkyl-3-methylimidazolium Acetate Ionic Liquids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ververis, C.; Georghiou, K.; Christodoulakis, N.; Santas, P.; Santas, R. Fiber dimensions, lignin and cellulose content of various plant materials and their suitability for paper production. Ind. Crops Prod. 2004, 19, 245–254. [Google Scholar] [CrossRef]
- Haigler, C.H.; Betancur, L.; Stiff, M.R.; Tuttle, J.R. Cotton fiber: A powerful single-cell model for cell wall and cellulose research. Front. Plant Sci. 2012, 3, 104. [Google Scholar] [CrossRef]
- Gericke, M.; Fardim, P.; Heinze, T. Ionic Liquids—Promising but Challenging Solvents for Homogeneous Derivatization of Cellulose. Molecules 2012, 17, 7458–7502. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Vitz, J.; Erdmenger, T.; Haensch, C.; Schubert, U.S. Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem. 2009, 11, 417–424. [Google Scholar] [CrossRef]
- Mccormick, C.L.; Callais, P.A.; Hutchinson, B.H. Solution Studies of Cellulose in Lithium Chloride and N, N-Dimethylacetamide. Macromolecules 1985, 18, 2394–2401. [Google Scholar] [CrossRef]
- Medronho, B.; Lindman, B. Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv. Colloid Interface Sci. 2015, 222, 502–508. [Google Scholar] [CrossRef]
- Hu, Y.; Thalangamaarachchige, V.D.; Acharya, S.; Abidi, N. Role of low-concentration acetic acid in promoting cellulose dissolution. Cellulose 2018, 25, 4389–4405. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, R.; Cheng, T.; Guo, J.; Xian, M.; Liu, H. Imidazolium-based ionic liquids for cellulose pretreatment: Recent progresses and future perspectives. Appl. Microbiol. Biotechnol. 2017, 101, 521–532. [Google Scholar] [CrossRef]
- Mezzetta, A.; Guglielmero, L.; Mero, A.; Tofani, G.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L. Expanding the Chemical Space of Benzimidazole Dicationic Ionic Liquids. Molecules 2021, 26, 4211. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis: Second Edition; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Lee, J.; He, M.; Yeo, C.D.; Kumar, G.; Hu, Z.; Quitevis, E.L.; Thalangamaarachchige, V.D. Friction and wear of Pd-rich amorphous alloy (Pd43Cu27Ni10P20) under dry and ionic liquid (IL) lubricated conditions. Wear 2018, 408–409, 190–199. [Google Scholar] [CrossRef]
- Cull, S.G.; Holbrey, J.D.; Vargas-Mora, V.; Seddon, K.R.; Lye, G.J. Room-temperature ionic liquids as replacements for organic solvents in multiphase bioprocess operations. Biotechnol. Bioeng. 2000, 69, 227–233. [Google Scholar] [CrossRef]
- Sanes, J.; Carrión, F.J.; Bermúdez, M.D.; Martínez-Nicolás, G. Ionic liquids as lubricants of polystyrene and polyamide 6-steel contacts. Preparation and properties of new polymer-ionic liquid dispersions. Tribol. Lett. 2006, 21, 121–133. [Google Scholar] [CrossRef]
- Le Bideau, J.; Gaveau, P.; Bellayer, S.; Néouze, M.A.; Vioux, A. Effect of confinement on ionic liquids dynamics in monolithic silica ionogels: 1H NMR study. Phys. Chem. Chem. Phys. 2007, 9, 5419–5422. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Ohno, Y.; Kobayashi, Y.; Miyashiro, H.; Usami, A.; Mita, Y.; Tokuda, H.; Watanabe, M.; Hayamizu, K.; Tsuzuki, S.; et al. Imidazolium-based room-temperature ionic liquid for lithium secondary batteries—Effects of lithium salt concentration. J. Electrochem. Soc. 2007, 154, 173–177. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Exnarb, I.; Grätzel, M. High efficiency dye-sensitized nanocrystalline solar cells based on ionic liquidpolymer gel electrolyte. Chem. Commun. 2002, 24, 2972–2973. [Google Scholar] [CrossRef]
- Gu, W.; Chen, H.; Tung, Y.-C. Multiplexed Hydraulic Value Actuation Using Ionic Liquid Filled Soft Channels and Braille Displays. Appl. Phys. Lett. 2007, 90, 033505. [Google Scholar] [CrossRef]
- Thakurathi, M.; Gurung, E.; Cetin, M.M.; Thalangamaarachchige, V.D.; Mayer, M.F.; Korzeniewski, C.; Quitevis, E.L. The Stokes-Einstein equation and the diffusion of ferrocene in imidazolium-based ionic liquids studied by cyclic voltammetry: Effects of cation ion symmetry and alkyl chain length. Electrochim. Acta 2018, 259, 245–252. [Google Scholar] [CrossRef]
- Lee, J.; Yeo, C.-D.; Hu, Z.; Thalangama-Arachchige, V.D.; Kaur, J.; Quitevis, E.L.; Kumar, G.; Koh, Y.P.; Simon, S. Friction and wear of pd-rich amorphous alloy (Pd43cu27ni10p20) with ionic liquid (IL) as lubricant at high temperatures. Metals 2019, 9, 1180. [Google Scholar] [CrossRef]
- Ohno, H.; Fukaya, Y. Task Specific Ionic Liquids for Cellulose Technology. Chem. Lett. 2009, 38, 2–7. [Google Scholar] [CrossRef]
- Du, H.; Qian, X. The effects of acetate anion on cellulose dissolution and reaction in imidazolium ionic liquids. Carbohydr. Res. 2011, 346, 1985–1990. [Google Scholar] [CrossRef]
- Payal, R.S.; Bejagam, K.K.; Mondal, A.; Balasubramanian, S. Dissolution of cellulose in room temperature ionic liquids: Anion dependence. J. Phys. Chem. B 2015, 119, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Remsing, R.C.; Swatloski, R.P.; Rogers, R.D.; Moyna, G. Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3-methylimidazolium chloride: A 13C and 35/37Cl NMR relaxation study on model systems. Chem. Commun. 2006, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Bodachivskyi, I.; Page, C.J.; Kuzhiumparambil, U.; Hinkley, S.F.R.; Sims, I.M.; Williams, D.B.G. Dissolution of Cellulose: Are Ionic Liquids Innocent or Noninnocent Solvents? ACS Sustain. Chem. Eng. 2020, 8, 10142–10150. [Google Scholar] [CrossRef]
- Owens, C.E.; Du, J.; Sánchez, P.B. Understanding the Dynamics of Cellulose Dissolved in an Ionic Liquid Solvent Under Shear and Extensional Flows. Biomacromolecules 2022, 23, 1958–1969. [Google Scholar] [CrossRef]
- Dignani, M.T.; Bioni, T.A.; Paixão, T.R.L.C.; El Seoud, O.A. Cellulose Dissolution in Mixtures of Ionic Liquids and Dimethyl Sulfoxide: A Quantitative Assessment of the Relative Importance of Temperature and Composition of the Binary Solvent. Molecules 2020, 25, 5975. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Yin, D.; Zhang, J.; Mi, Q.; Lu, H.; Liang, D.; Zhang, J. The solution state and dissolution process of cellulose in ionic-liquid-based solvents with different hydrogen-bonding basicity and microstructures. Green Chem. 2022, 24, 3824–3833. [Google Scholar] [CrossRef]
- Dissanayake, N.; Thalangamaarachchige, V.D.; Troxell, S.; Quitevis, E.L.; Abidi, N. Substituent effects on cellulose dissolution in imidazolium-based ionic liquids. Cellulose 2018, 25, 6887–6900. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhang, S.; Yao, Y.; Yao, X.; Xu, J.; Lu, X. Dissolving process of a cellulose bunch in ionic liquids: A molecular dynamics study. Phys. Chem. Chem. Phys. 2015, 17, 17894–17905. [Google Scholar] [CrossRef]
- Lu, B.; Xu, A.; Wang, J. Cation does matter: How cationic structure affects the dissolution of cellulose in ionic liquids. Green Chem. 2014, 16, 1326–1335. [Google Scholar] [CrossRef]
- Zhao, B.; Greiner, L.; Leitner, W. Cellulose solubilities in carboxylate-based ionic liquids. RSC Adv. 2012, 2, 2476–2479. [Google Scholar] [CrossRef]
- Gupta, K.M.; Jiang, J. Cellulose dissolution and regeneration in ionic liquids: A computational perspective. Chem. Eng. Sci. 2015, 121, 180–189. [Google Scholar] [CrossRef]
- Olsson, C.; Hedlund, A.; Idström, A.; Westman, G. Effect of methylimidazole on cellulose/ionic liquid solutions and regenerated material therefrom. J. Mater. Sci. 2014, 49, 3423–3433. [Google Scholar] [CrossRef]
- Meenatchi, B.; Renuga, V.; Manikandan, A. Cellulose dissolution and regeneration using various imidazolium based protic ionic liquids. J. Mol. Liq. 2017, 238, 582–588. [Google Scholar] [CrossRef]
- Ferreira, D.C.; Oliveira, M.L.; Bioni, T.A.; Nawaz, H.; King, A.W.; Kilpeläinen, I.; Hummel, M.; Sixta, H.; El Seoud, O.A. Binary mixtures of ionic liquids-DMSO as solvents for the dissolution and derivatization of cellulose: Effects of alkyl and alkoxy side chains. Carbohydr. Polym. 2019, 212, 206–214. [Google Scholar] [CrossRef]
- Rabideau, B.D.; Agarwal, A.; Ismail, A.E. The role of the cation in the solvation of cellulose by imidazolium-based ionic liquids. J. Phys. Chem. B 2014, 118, 1621–1629. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Wang, J.; Zhang, S. Effects of cationic structure on cellulose dissolution in ionic liquids: A molecular dynamics study. ChemPhysChem 2012, 13, 3126–3133. [Google Scholar] [CrossRef]
- Rizvi, S.; Gade, H.M. Imidazolium-based ionic liquids as cellulose solvents: Mechanism and molecular insights. Biomass Bioenergy 2025, 196, 107758. [Google Scholar] [CrossRef]
- Kasprzak, D.; Krystkowiak, E.; Stępniak, I.; Galiński, M. Dissolution of cellulose in novel carboxylate-based ionic liquids and dimethyl sulfoxide mixed solvents. Eur. Polym. J. 2019, 113, 89–97. [Google Scholar] [CrossRef]
- Ren, F.; Wang, J.; Yu, J.; Zhong, C.; Xie, F.; Wang, S. Dissolution of Cellulose in Ionic Liquid-DMSO Mixtures: Roles of DMSO/IL Ratio and the Cation Alkyl Chain Length. ACS Omega 2021, 6, 27225–27232. [Google Scholar] [CrossRef]
- Diop, A.; Bouazza, A.H.; Daneault, C.; Montplaisir, D. New ionic liquid for the dissolution of lignin. Bioresources 2013, 8, 4270–4282. [Google Scholar] [CrossRef]
- Erdmenger, T.; Haensch, C.; Hoogenboom, R.; Schubert, U.S. Homogeneous tritylation of cellulose in 1-butyl-3-methylimidazolium chloride. Macromol. Biosci. 2007, 7, 440–445. [Google Scholar] [CrossRef]
- Padmanabhan, J.M.P.S.; Kim, M.; Blanch, H.W. Solubility and rate of dissolution for Miscanthus in hydrophilic ionic liquids. Fluid Phase Equilib. 2011, 309, 89–96. [Google Scholar] [CrossRef]
- Paiva, T.; Echeverria, C.; Godinho, M.H.; Almeida, P.L.; Corvo, M.C. On the influence of imidazolium ionic liquids on cellulose derived polymers. Eur. Polym. J. 2019, 114, 353–360. [Google Scholar] [CrossRef]
- Andanson, J.M.; Bordes, E.; Devémy, J.; Leroux, F.; Pádua, A.A.H.; Gomes, M.F.C. Understanding the role of co-solvents in the dissolution of cellulose in ionic liquids. Green Chem. 2014, 16, 2528–2538. [Google Scholar] [CrossRef]
- Clough, M.T.; Geyer, K.; Hunt, P.A.; Son, S.; Vagt, U.; Welton, T. Ionic liquids: Not always innocent solvents for cellulose. Green Chem. 2015, 17, 231–243. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Li, Y.; Lu, Z.; Cao, S.; Fan, M.; Huang, L.; Chen, L. Facile Cellulose Dissolution and Characterization in the Newly Synthesized 1,3-Diallyl-2-ethylimidazolium Acetate Ionic Liquid. Polymers 2017, 9, 526. [Google Scholar] [CrossRef]
- Hawkins, J.E.; Liang, Y.; Ries, M.E.; Hine, P.J. Time temperature superposition of the dissolution of cellulose fibres by the ionic liquid 1-ethyl-3-methylimidazolium acetate with cosolvent dimethyl sulfoxide. Carbohydr. Polym. Technol. Appl. 2021, 2, 100021. [Google Scholar] [CrossRef]
- Le, K.A.; Rudaz, C.; Budtova, T. Phase diagram, solubility limit and hydrodynamic properties of cellulose in binary solvents with ionic liquid. Carbohydr. Polym. 2014, 105, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Zollfrank, C. Ionic liquid aided solution-precipitation method to prepare polymer blends from cellulose with polyesters or polyamide. Eur. Polym. J. 2020, 133, 109743. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Yu, J.; Zhang, X.; He, J.; Zhang, J. Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: State of the art and future trends. Mater. Chem. Front. 2017, 1, 1273–1290. [Google Scholar] [CrossRef]
- Meng, D.J.-M.A.X.; Devemy, J.; Verney, D.V.; Gautier, D.A.; Husson, D.P. Improving Cellulose Dissolution in Ionic Liquids by Tuning the Size of the Ions: Impact of the Length of the Alkyl Chains in Tetraalkylammonium Carboxylate. ChemSusChem 2017, 10, 1749–1760. [Google Scholar] [CrossRef]
| No. of Carbons in Alkyl Chain | Name of IL | Abbreviation |
|---|---|---|
| 2 | 1-ethyl-3-methylimidazolium acetate | [C2C1im][OAc] |
| 3 | 1-propyl-3-methylimidazolium acetate | [C3C1im][OAc] |
| 4 | 1-butyl-3-methylimidazolium acetate | [C4C1im][OAc] |
| 5 | 1-pentyl-3-methylimidazolium acetate | [C5C1im][OAc] |
| 6 | 1-hexyl-3-methylimidazolium acetate | [C6C1im][OAc] |
| 7 | 1-heptyl-3-methylimidazolium acetate | [C7C1im][OAc] |
| 8 | 1-octyl-3-methylimidazolium acetate | [C8C1im][OAc] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dissanayake, N.; Thalangamaarachchige, V.D.; Quitevis, E.; Abidi, N. Effect of Alkyl Chain Length on Dissolution and Regeneration Behavior of Cotton in 1-Alkyl-3-methylimidazolium Acetate Ionic Liquids. Molecules 2025, 30, 2711. https://doi.org/10.3390/molecules30132711
Dissanayake N, Thalangamaarachchige VD, Quitevis E, Abidi N. Effect of Alkyl Chain Length on Dissolution and Regeneration Behavior of Cotton in 1-Alkyl-3-methylimidazolium Acetate Ionic Liquids. Molecules. 2025; 30(13):2711. https://doi.org/10.3390/molecules30132711
Chicago/Turabian StyleDissanayake, Niwanthi, Vidura D. Thalangamaarachchige, Edward Quitevis, and Noureddine Abidi. 2025. "Effect of Alkyl Chain Length on Dissolution and Regeneration Behavior of Cotton in 1-Alkyl-3-methylimidazolium Acetate Ionic Liquids" Molecules 30, no. 13: 2711. https://doi.org/10.3390/molecules30132711
APA StyleDissanayake, N., Thalangamaarachchige, V. D., Quitevis, E., & Abidi, N. (2025). Effect of Alkyl Chain Length on Dissolution and Regeneration Behavior of Cotton in 1-Alkyl-3-methylimidazolium Acetate Ionic Liquids. Molecules, 30(13), 2711. https://doi.org/10.3390/molecules30132711








