Rapid and Effective Recovery of Oleanolic and Maslinic Acids from Olive Leaves Using SFE and pH-Zone Centrifugal Partition Chromatography
Abstract
1. Introduction
2. Results
2.1. Recovery of Enriched Fraction in OA and MA Using SFE
2.2. Purification of Triterpenic Acids Using Refining pH-Zone CPC
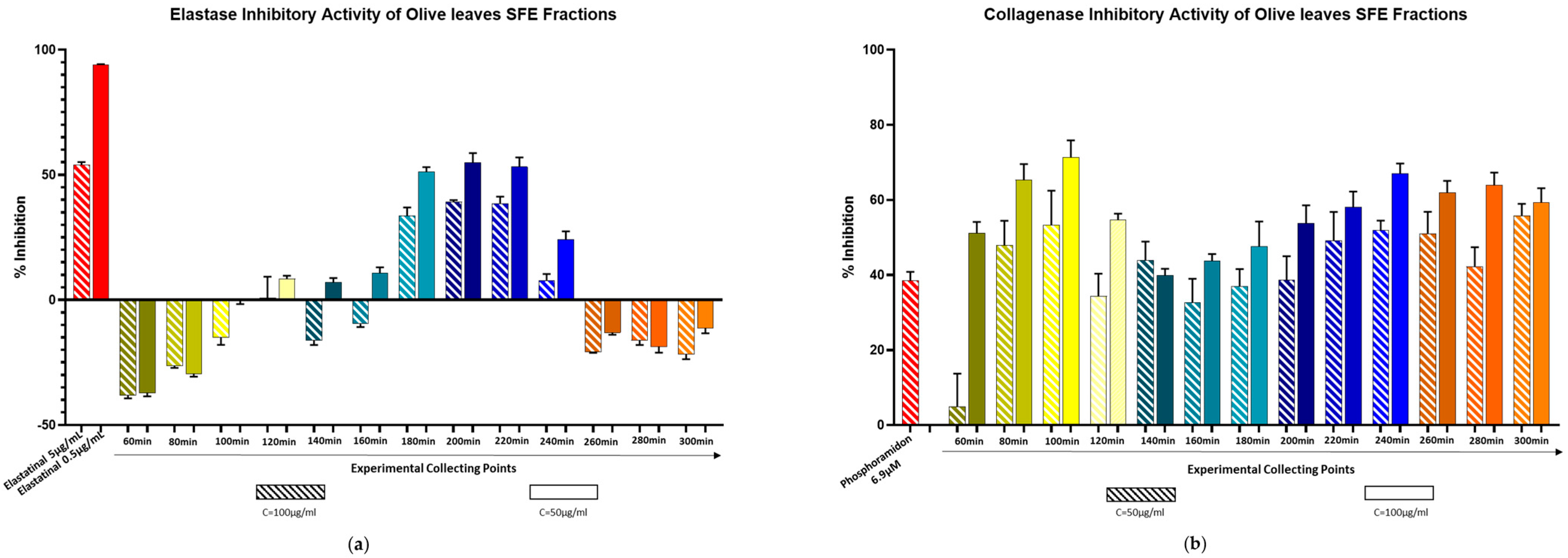
2.3. Biological Evaluation Against Elastase and Collagenase Enzymes
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction of Olive Leaves Using Laboratory SFE
3.3. Fractionation of Triterpenic Acids Using Refining pH-Zone CPC
3.4. HPLC-DAD-ELSD, HPTLC and NMR Profiling
3.5. Enzymatic Assays
3.5.1. Elastase Assay
3.5.2. Collagenase Assay
3.5.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erbay, Z.; Icier, F. The Importance and Potential Uses of Olive Leaves. Food Rev. Int. 2010, 26, 319–334. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Japón-Luján, R. State-of-the-Art and Trends in the Analysis of Oleuropein and Derivatives. TrAC Trends Anal. Chem. 2006, 25, 501–510. [Google Scholar] [CrossRef]
- Bianchi, G.; Pozzi, N.; Vlahov, G. Pentacyclic Triterpene Acids in Olives. Phytochemistry 1994, 37, 205–207. [Google Scholar] [CrossRef]
- Kanakis, P.; Termentzi, A.; Michel, T.; Gikas, E.; Halabalaki, M.; Skaltsounis, A.-L. From Olive Drupes to Olive Oil. An HPLC-Orbitrap-Based Qualitative and Quantitative Exploration of Olive Key Metabolites. Planta Med. 2013, 79, 1576–1587. [Google Scholar] [CrossRef]
- Seo, S.; Yoshimura, Y.; Uomori, A.; Takeda, K.; Seto, H.; Ebizuka, Y.; Sankawa, U. Biosynthesis of Triterpenes, Ursolic Acid and Oleanolic Acid in Tissue Cultures of Rabdosia Japonica Hara Fed [5-13C2H2]Mevalonolactone and [2-13C2H3]Acetate. J. Am. Chem. Soc. 1988, 110, 1740–1745. [Google Scholar] [CrossRef]
- Dewick, P. Medicinal Natural Products A Biosynthetic Approach, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2002; ISBN 0 471 49640 5. [Google Scholar]
- Subbaramaiah, K.; Michaluart, P.; Sporn, M.B.; Dannenberg, A.J. Ursolic Acid Inhibits Cyclooxygenase-2 Transcription in Human Mammary Epithelial Cells. Cancer Res. 2000, 60, 2399–2404. [Google Scholar] [PubMed]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological Activities of Natural Triterpenoids and Their Therapeutic Implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Astudillo, L.; Schmeda-Hirschmann, G. Oleanolic Acid Promotes Healing of Acetic Acid-Induced Chronic Gastric Lesions in Rats. Pharmacol. Res. 2003, 48, 291–294. [Google Scholar] [CrossRef]
- Choi, C.Y.; You, H.J.; Jeong, H.G. Nitric Oxide and Tumor Necrosis Factor-Alpha Production by Oleanolic Acid via Nuclear Factor-KappaB Activation in Macrophages. Biochem. Biophys. Res. Commun. 2001, 288, 49–55. [Google Scholar] [CrossRef]
- Liu, J. Pharmacology of Oleanolic Acid and Ursolic Acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Liu, J. Oleanolic Acid and Ursolic Acid: Research Perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Quere, L.; Wenger, T.; Schramm, H.J. Triterpenes as Potential Dimerization Inhibitors of HIV-1 Protease. Biochem. Biophys. Res. Commun. 1996, 227, 484–488. [Google Scholar] [CrossRef]
- Montilla, M.P.; Agil, A.; Navarro, M.C.; Jimenez, M.I.; Garcia-Granados, A.; Parra, A.; Cabo, M.M. Antioxidant Activity of Maslinic Acid, a Triterpene Derivative Obtained from Olea Europaea. Planta Med. 2003, 69, 472–474. [Google Scholar]
- Marquez Martin, A.; de la Puerta Vazquez, R.; Fernandez-Arche, A.; Ruiz-Gutierrez, V. Supressive Effect of Maslinic Acid from Pomace Olive Oil on Oxidative Stress and Cytokine Production in Stimulated Murine Macrophages. Free Radic. Res. 2006, 40, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Fukumitsu, S.; Villareal, M.O.; Fujitsuka, T.; Aida, K.; Isoda, H. Anti-Inflammatory and Anti-Arthritic Effects of Pentacyclic Triterpenoids Maslinic Acid through NF-ΚB Inactivation. Mol. Nutr. Food Res. 2016, 60, 399–409. [Google Scholar] [CrossRef]
- Li, C.; Yang, Z.; Zhai, C.; Qiu, W.; Li, D.; Yi, Z.; Wang, L.; Tang, J.; Qian, M.; Luo, J.; et al. Maslinic Acid Potentiates the Anti-Tumor Activity of Tumor Necrosis Factor Alpha by Inhibiting NF-KappaB Signaling Pathway. Mol. Cancer 2010, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Tena, S.; Reyes-Zurita, F.J.; Diaz-Moralli, S.; Vinardell, M.P.; Reed, M.; Garcia-Garcia, F.; Dopazo, J.; Lupianez, J.A.; Gunther, U.; Cascante, M. Maslinic Acid-Enriched Diet Decreases Intestinal Tumorigenesis in Apc(Min/+) Mice through Transcriptomic and Metabolomic Reprogramming. PLoS ONE 2013, 8, e59392. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; García-Salguero, L.; Peragón, J.; Medina, P.P.; Parra, A.; Cascante, M.; Lupiáñez, J.A. Maslinic Acid, a Natural Triterpene, Induces a Death Receptor-Mediated Apoptotic Mechanism in Caco-2 P53-Deficient Colon Adenocarcinoma Cells. PLoS ONE 2016, 11, e0146178. [Google Scholar] [CrossRef]
- Henke, B.R.; Sparks, S.M. Glycogen Phosphorylase Inhibitors. Mini Rev. Med. Chem. 2006, 6, 845–857. [Google Scholar] [CrossRef]
- Liu, J.; Sun, H.; Duan, W.; Mu, D.; Zhang, L. Maslinic Acid Reduces Blood Glucose in KK-Ay Mice. Biol. Pharm. Bull. 2007, 30, 2075–2078. [Google Scholar] [CrossRef]
- Parra, A.; Rivas, F.; Lopez, P.E.; Garcia-Granados, A.; Martinez, A.; Albericio, F.; Marquez, N.; Muñoz, E. Solution- and Solid-Phase Synthesis and Anti-HIV Activity of Maslinic Acid Derivatives Containing Amino Acids and Peptides. Bioorganic Med. Chem. 2009, 17, 1139–1145. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.; Liu, J.; Kim, D.H.; Kai, G. Exploitation of Apple Pomace towards Extraction of Triterpenic Acids, Antioxidant Potential, Cytotoxic Effects, and Inhibition of Clinically Important Enzymes. Food Chem. Toxicol. 2019, 131, 110563. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Gegechkori, V.; Mohammed, E.U.R.; Ku, H.; Morton, D.W. Isolation of Bioactive Pentacyclic Triterpenoid Acids from Olive Tree Leaves with Flash Chromatography. Appl. Sci. 2022, 12, 996. [Google Scholar] [CrossRef]
- Huang, X.Y.; Zhang, X.; Pei, D.; Liu, J.F.; Gong, Y.; Aisa, H.A.; Di, D.L. Continuous Separation of Maslinic and Oleanolic Acids from Olive Pulp by High-Speed Countercurrent Chromatography with Elution-Extrusion Mode. J. Sep. Sci. 2019, 42, 2080–2088. [Google Scholar] [CrossRef] [PubMed]
- Cláudio, A.F.M.; Cognigni, A.; de Faria, E.L.P.; Silvestre, A.J.D.; Zirbs, R.; Freire, M.G.; Bica, K. Valorization of Olive Tree Leaves: Extraction of Oleanolic Acid Using Aqueous Solutions of Surface-Active Ionic Liquids. Sep. Purif. Technol. 2018, 204, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Manganaris, G.A. Towards an Efficient Protocol for the Determination of Triterpenic Acids in Olive Fruit: A Comparative Study of Drying and Extraction Methods. Phytochem. Anal. 2012, 23, 444–449. [Google Scholar] [CrossRef]
- De Hierro, A.G.-N.L. Process for the Industrial Recovery of Oleanolic and Maslinic Acids Contained in the Olive Milling Subproducts. EP0894517A1, 3 February 1999. [Google Scholar]
- Kuno, N.; Shinohara, G. Method for the Preparation of Oleanolic Acid and/or Maslinic Acid. U.S. Patent US6740778, 25 May 2004. [Google Scholar]
- Zhang, X.; Ji, F.; Li, Y.; He, T.; Han, Y.; Wang, D.; Lin, Z.; Chen, S. Rapid Determination of Two Triterpenoid Acids in Chaenomelis Fructus Using Supercritical Fluid Extraction On-Line Coupled with Supercritical Fluid Chromatography. Anal. Sci. 2018, 34, 407–413. [Google Scholar] [CrossRef]
- Xynos, N.; Papaefstathiou, G.; Gikas, E.; Argyropoulou, A.; Aligiannis, N.; Skaltsounis, A.L. Design Optimization Study of the Extraction of Olive Leaves Performed with Pressurized Liquid Extraction Using Response Surface Methodology. Sep. Purif. Technol. 2014, 122, 323–330. [Google Scholar] [CrossRef]
- Mohamed, A.I.H.K. Supercritical Fluid Extraction of Triterpenes and Aliphatic Hydrocarbons from Olive Tree Derivatives. Arab. J. Chem. 2017, 2, S3967–S3973. [Google Scholar]
- Tabera, J.; Guinda, Á.; Ruiz-Rodríguez, A.; Señoráns, F.J.; Ibáñez, E.; Albi, T.; Reglero, G. Countercurrent Supercritical Fluid Extraction and Fractionation of High-Added-Value Compounds from a Hexane Extract of Olive Leaves. J. Agric. Food Chem. 2004, 52, 4774–4779. [Google Scholar] [CrossRef]
- de Lucasa, A.; Martinez de la Ossab, E.; Rincóna, J.; Blancob, M.A.; Graciaa, I. Supercritical Fluid Extraction of Tocopherol Concentrates from Olive Tree Leaves. J. Supercrit. Fluids 2002, 22, 221–228. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Shahrestani, G.R.; Ghaziaskar, H.S. Experimental and Modeling Investigation of Supercritical Extraction of Mannitol from Olive Leaves. Chem. Eng. Technol. 2009, 32, 45–54. [Google Scholar] [CrossRef]
- Lafka, T.I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic Extracts from Wild Olive Leaves and Their Potential as Edible Oils Antioxidants. Foods 2013, 2, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and Supercritical Fluid Extraction of Bioactive Compounds from Plants, Food-by-Products, Seaweeds and Microalgae—An Update. TrAC Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Zabot, G.L.; Moraes, M.N.; Meireles, M.A.A. Supercritical Fluid Extraction of Bioactive Compounds from Botanic Matrices: Experimental Data, Process Parameters and Economic Evaluation. Recent Pat. Eng. 2012, 6, 182–206. [Google Scholar] [CrossRef]
- Ito, Y. Golden Rules and Pitfalls in Selecting Optimum Conditions for High-Speed Counter-Current Chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef]
- Foucault, A. Centrifugal Partition Chromatography; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Weisz, A.; Scher, A.L.; Shinomiya, K.; Fales, H.M.; Ito, Y. A New Preparative-Scale Purification Technique: PH-Zone-Refining Countercurrent Chromatography. J. Am. Chem. Soc. 1994, 116, 704–708. [Google Scholar] [CrossRef]
- Ito, Y.; Ma, Y. PH-Zone-Refining Countercurrent Chromatography. J. Chromatogr. A 1996, 753, 1–36. [Google Scholar] [CrossRef]
- Boudesocque, L.; Kapel, R.; Paris, C.; Dhulster, P.; Marc, I.; Renault, J.-H. Concentration and Selective Fractionation of an Antihypertensive Peptide from an Alfalfa White Proteins Hydrolysate by Mixed Ion-Exchange Centrifugal Partition Chromatography. J. Chromatogr. B 2012, 905, 23–30. [Google Scholar] [CrossRef]
- Ma, Y.; Ito, Y.; Sokolosky, E.; Fales, H.M. Separation of Alkaloids by PH-Zone-Refining Counter-Current Chromatography. J. Chromatogr. A 1994, 685, 259–262. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Garrard, I. Counter-Current Chromatography for the Separation of Terpenoids: A Comprehensive Review with Respect to the Solvent Systems Employed. Phytochem. Rev. 2014, 13, 547–572. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. PH-Zone-Refining Counter-Current Chromatography: Origin, Mechanism, Procedure and Applications. J. Chromatogr. A 2013, 1271, 71–85. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Ignatova, S.; Hewitson, P.; Di, D.-L. An Overview of Recent Progress in Elution Mode of Counter Current Chromatography. TrAC Trends Anal. Chem. 2016, 77, 214–225. [Google Scholar] [CrossRef]
- Hamzaoui, M.; Renault, J.-H.; Reynaud, R.; Hubert, J. Centrifugal Partition Extraction in the PH-Zone-Refining Displacement Mode: An Efficient Strategy for the Screening and Isolation of Biologically Active Phenolic Compounds. J. Chromatogr. B 2013, 937, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Zhao, S.; Sun, W.; Tong, S. Preparative Separation of Structural Isomeric Pentacyclic Triterpene Oleanolic Acid and Ursolic Acid from Natural Products by PH-Zone-Refining Countercurrent Chromatography. RSC Adv. 2019, 9, 38860–38866. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; Deng, Y.; Wang, X.; Cheng, J. Enhanced Extraction of Hydroxytyrosol, Maslinic Acid and Oleanolic Acid from Olive Pomace: Process Parameters, Kinetics and Thermodynamics, and Greenness Assessment. Food Chem. 2019, 276, 662–674. [Google Scholar] [CrossRef]
- Fuliaş, A.; Ledeţi, I.; Vlase, G.; Vlase, T.; Şoica, C.; Dehelean, C.; Oprean, C.; Bojin, F.; Şuta, L.M.; Bercean, V.; et al. Thermal Degradation, Kinetic Analysis, and Apoptosis Induction in Human Melanoma for Oleanolic and Ursolic Acids. J. Therm. Anal. Calorim. 2016, 125, 759–768. [Google Scholar] [CrossRef]
- Popp, J.R.; Petrakis, E.A.; Angelis, A.; Halabalaki, M.; Bonn, G.K.; Stuppner, H.; Skaltsounis, L.A. Rapid Isolation of Acidic Cannabinoids from Cannabis sativa L. Using PH-Zone-Refining Centrifugal Partition Chromatography. J. Chromatogr. A 2019, 1599, 196–202. [Google Scholar] [CrossRef]
- Dais, P.; Plessel, R.; Williamson, K.; Hatzakis, E. Complete 1H and 13C NMR Assignment and 31P NMR Determination of Pentacyclic Triterpenic Acids. Anal. Methods 2017, 9, 949–957. [Google Scholar] [CrossRef]
- Woessner, J.F. The Family of Matrix Metalloproteinases. Ann. N. Y. Acad. Sci. 1994, 6, 11–21. [Google Scholar] [CrossRef]
- Imokawa, G.; Ishida, K. Biological Mechanisms Underlying the Ultraviolet Radiation-Induced Formation of Skin Wrinkling and Sagging I: Reduced Skin Elasticity, Highly Associated with Enhanced Dermal Elastase Activity, Triggers Wrinkling and Sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F. Potential Role of Natural Compounds against Skin Aging. Curr. Med. Chem. 2015, 22, 1515–1538. [Google Scholar] [CrossRef]
- Aimes, R.T.; Quigley, J.P. Matrix Metalloproteinase-2 Is an Interstitial Collagenase. Inhibitor-Free Enzyme Catalyzes the Cleavage of Collagen Fibrils and Soluble Native Type I Collagen Generating the Specific 3/4- and 1/4-Length Fragments. J. Biol. Chem. 1995, 270, 5872–5876. [Google Scholar] [CrossRef]
- Fanjul-Fernández, M.; Folgueras, A.R.; Cabrera, S.; López-Otín, C. Matrix Metalloproteinases: Evolution, Gene Regulation and Functional Analysis in Mouse Models. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2010, 1803, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Gendron, R.; Grenier, D.; Sorsa, T.; Mayrand, D. Inhibition of the Activities of Matrix Metalloproteinases 2, 8, and 9 by Chlorhexidine. Clin. Diagn. Lab. Immunol. 1999, 6, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Lee, N.H. Antielastase and Free Radical Scavenging Activities of Compounds from the Stems of Cornus Kousa. Phytother. Res. 2007, 21, 1171–1176. [Google Scholar] [CrossRef]
- Maity, N.; Nema, N.K.; Abedy, M.K.; Sarkar, B.K.; Mukherjee, P.K. Exploring Tagetes Erecta Linn Flower for the Elastase, Hyaluronidase and MMP-1 Inhibitory Activity. J. Ethnopharmacol. 2011, 137, 1300–1305. [Google Scholar] [CrossRef]
- Angelis, A.; Hubert, J.; Aligiannis, N.; Michalea, R.; Abedini, A.; Nuzillard, J.-M.; Gangloff, S.; Skaltsounis, A.-L.; Renault, J.-H. Bio-Guided Isolation of Methanol-Soluble Metabolites of Common Spruce (Picea abies) Bark by-Products and Investigation of Their Dermo-Cosmetic Properties. Molecules 2016, 21, 1586. [Google Scholar] [CrossRef]
- Stamou, P.; Mikropoulou, E.V.; Chalkiadaki, M.; Basdeki, A.; Antoniadi, L.; Poigny, S.; Halabalaki, M. Revealing the Potential of Chios Mastic Gum and Its 2 Constituents for Cosmetic Applications through Chemical 3 Profiling and Biological Evaluation. Cosmetics 2024, 11, 155. [Google Scholar] [CrossRef]
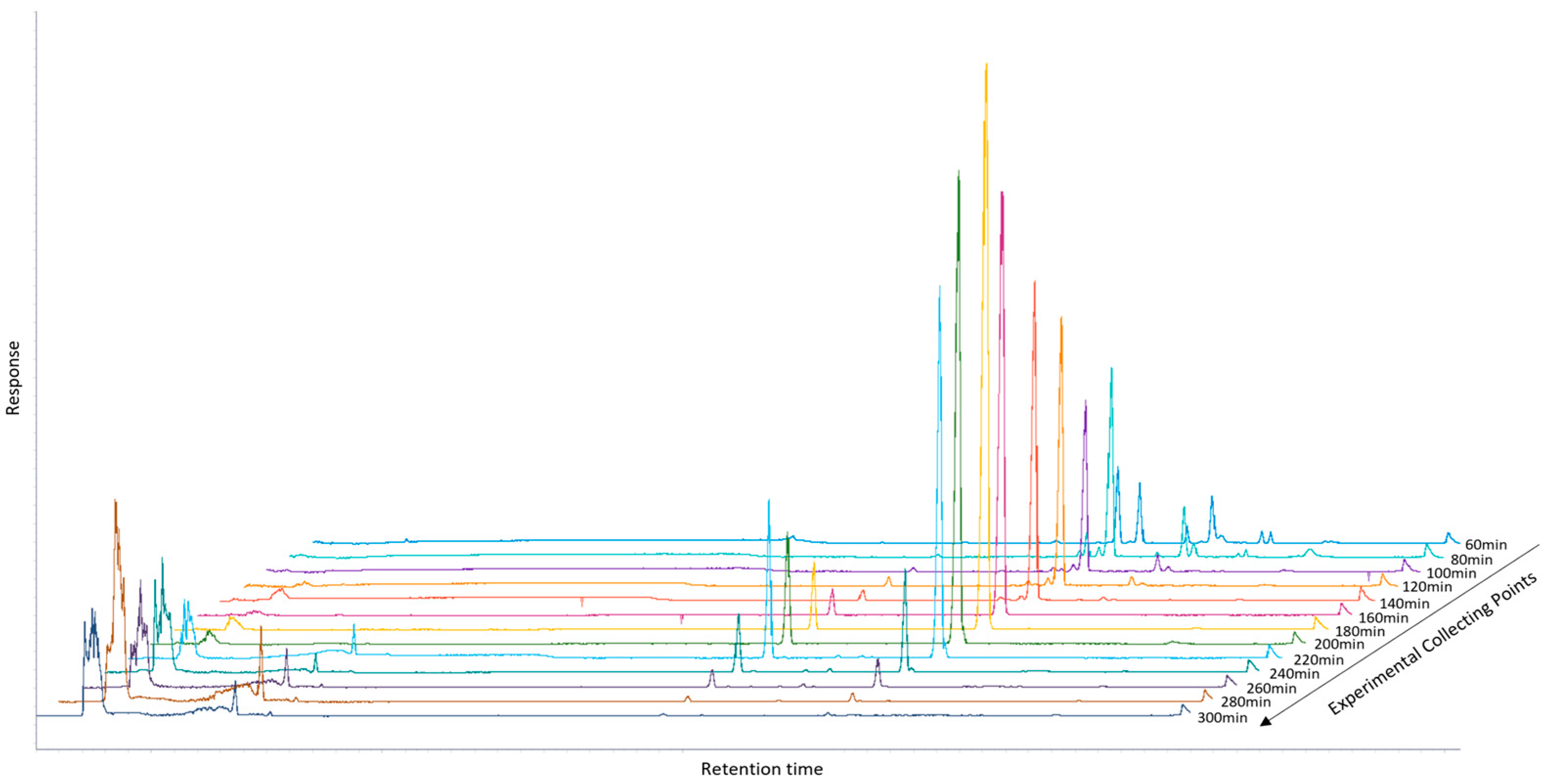
| 20 min | 40 min | 60 min | |
|---|---|---|---|
| CO2 | - | - | 15.5 mg |
| CO2 + 2.5% EtOH | 80.6 mg | 54.4 mg | 32.3 mg |
| CO2 + 5% EtOH | 159.6 mg | 118.8 mg | 101.7 mg |
| CO2 + 7.5% EtOH | 163.7 mg | 109.5 mg | 51.9 mg |
| CO2 + 10% EtOH | 86.2 mg | 79.4 mg | 79.2 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniadi, L.; Angelis, A.; Nikou, T.; Michailidis, D.; Skaltsounis, L.A. Rapid and Effective Recovery of Oleanolic and Maslinic Acids from Olive Leaves Using SFE and pH-Zone Centrifugal Partition Chromatography. Molecules 2025, 30, 2709. https://doi.org/10.3390/molecules30132709
Antoniadi L, Angelis A, Nikou T, Michailidis D, Skaltsounis LA. Rapid and Effective Recovery of Oleanolic and Maslinic Acids from Olive Leaves Using SFE and pH-Zone Centrifugal Partition Chromatography. Molecules. 2025; 30(13):2709. https://doi.org/10.3390/molecules30132709
Chicago/Turabian StyleAntoniadi, Lemonia, Apostolis Angelis, Theodora Nikou, Dimitris Michailidis, and Leandros A. Skaltsounis. 2025. "Rapid and Effective Recovery of Oleanolic and Maslinic Acids from Olive Leaves Using SFE and pH-Zone Centrifugal Partition Chromatography" Molecules 30, no. 13: 2709. https://doi.org/10.3390/molecules30132709
APA StyleAntoniadi, L., Angelis, A., Nikou, T., Michailidis, D., & Skaltsounis, L. A. (2025). Rapid and Effective Recovery of Oleanolic and Maslinic Acids from Olive Leaves Using SFE and pH-Zone Centrifugal Partition Chromatography. Molecules, 30(13), 2709. https://doi.org/10.3390/molecules30132709






