The Application of Functional Nanomaterials-Based Electrochemical Biosensors in Detecting Cancer Biomarkers: A Review
Abstract
1. Introduction
2. Electrochemical Biosensors
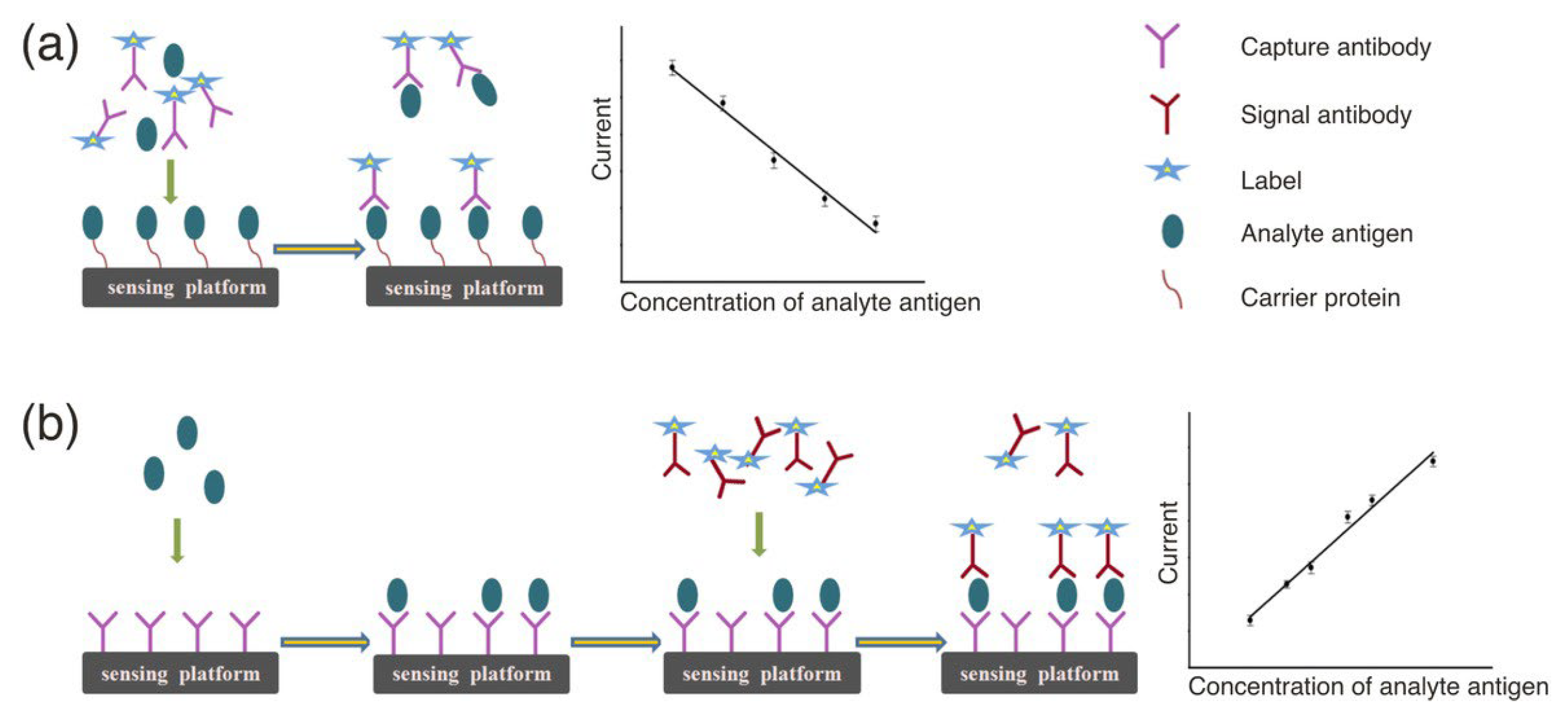
2.1. Electrochemical Immunosensors
2.2. Electrochemical Aptasensors
2.3. Discussion
3. Functional Nanomaterials
3.1. Carbon-Based Nanomaterials
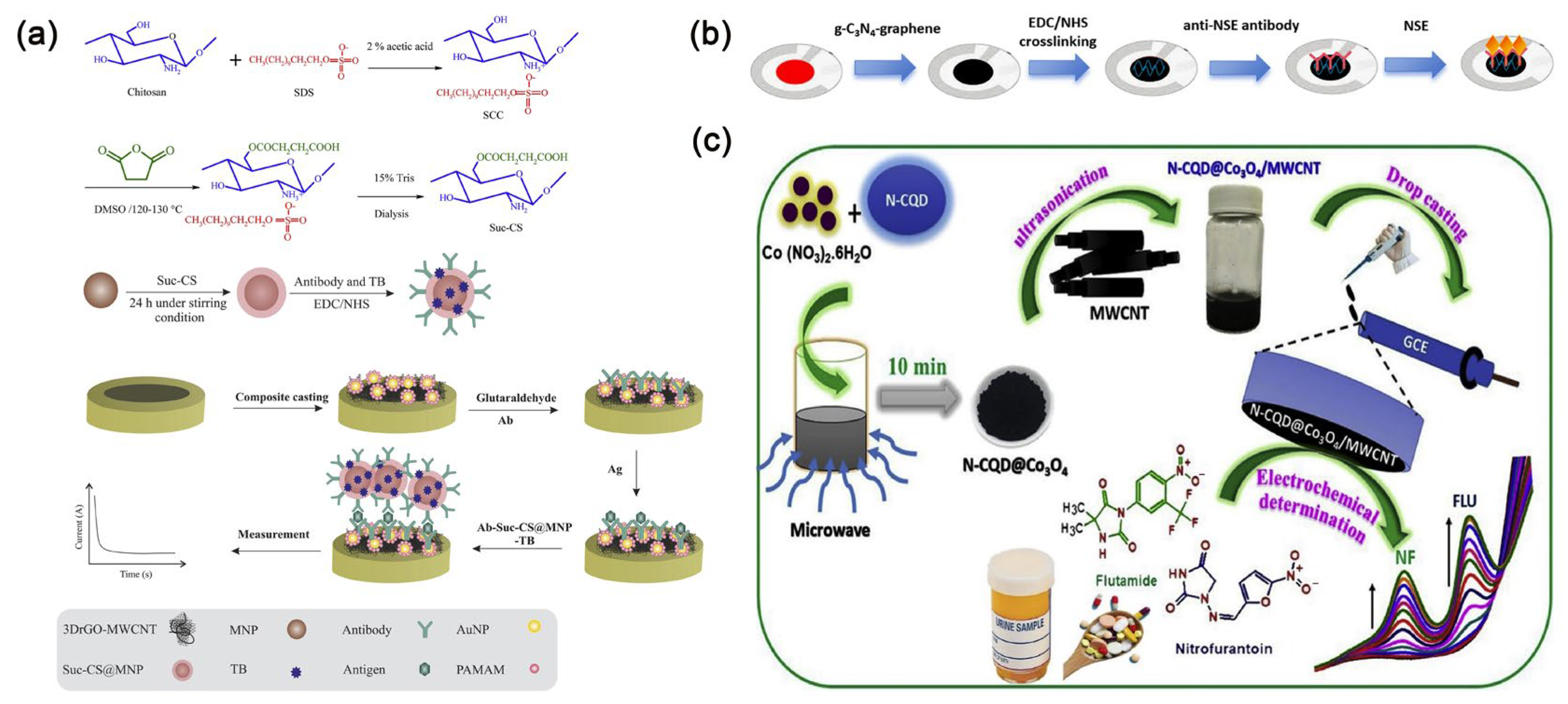
3.2. Metallic Nanomaterials
3.3. Magnetic Nanomaterials
3.4. Metal-Organic Frameworks (MOFs) Nanomaterials

3.5. Discussion
4. The Application of Nanomaterial-Based Electrochemical Biosensors in Detecting Cancer Biomarkers
4.1. Breast Cancer
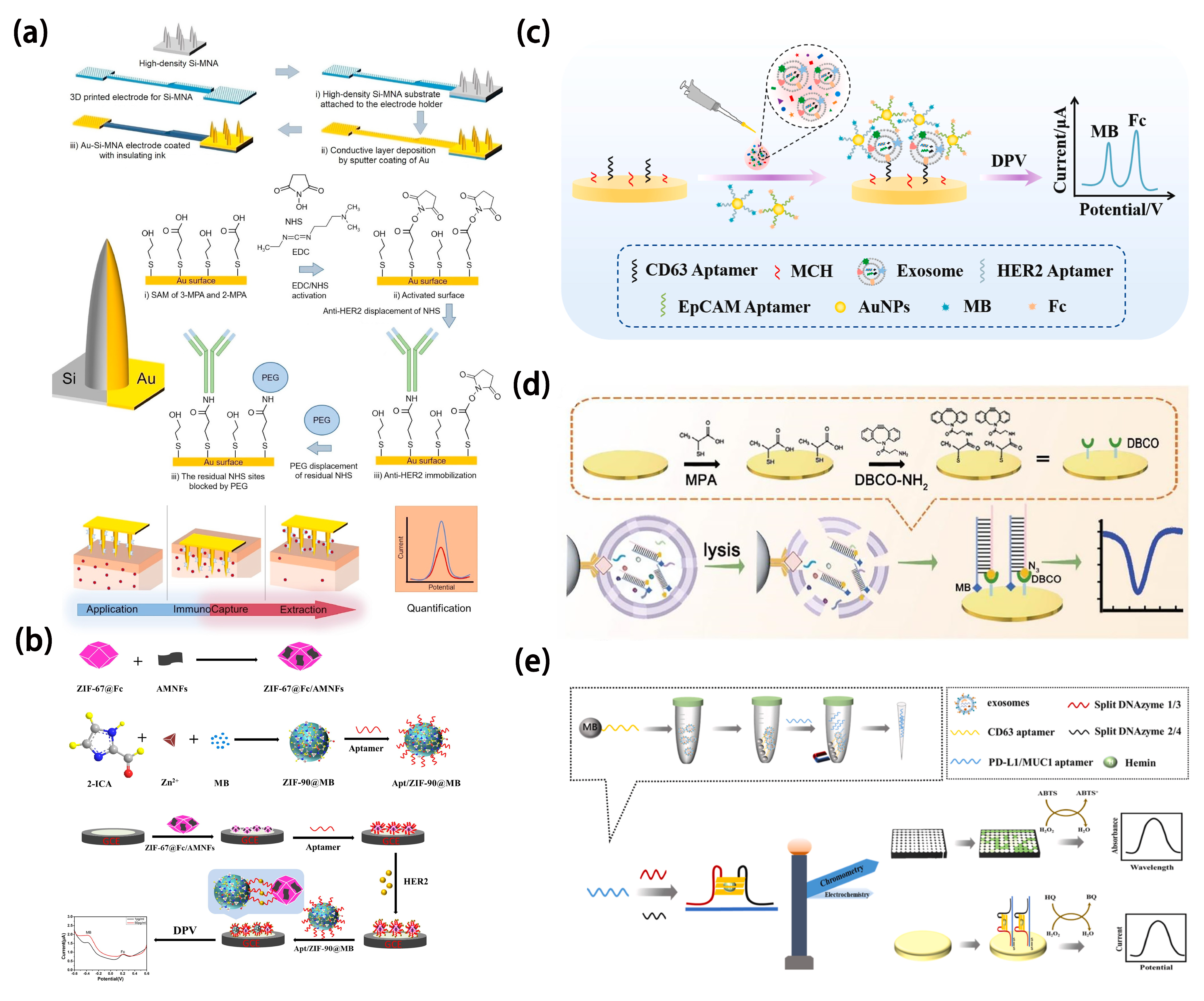
4.2. Cervical Cancer
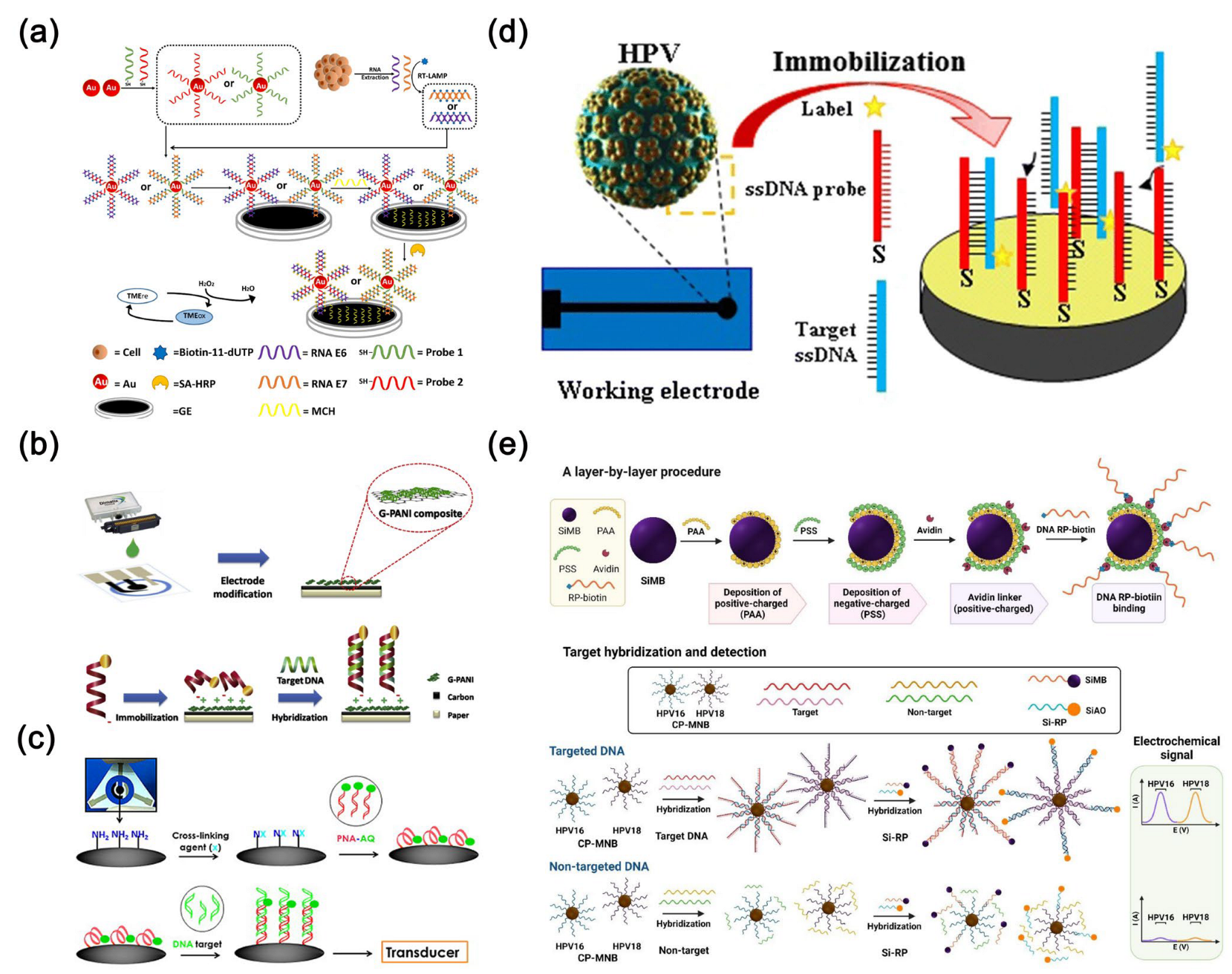
4.3. Lung Cancer

4.4. Gastric Cancer

4.5. Other Cancers
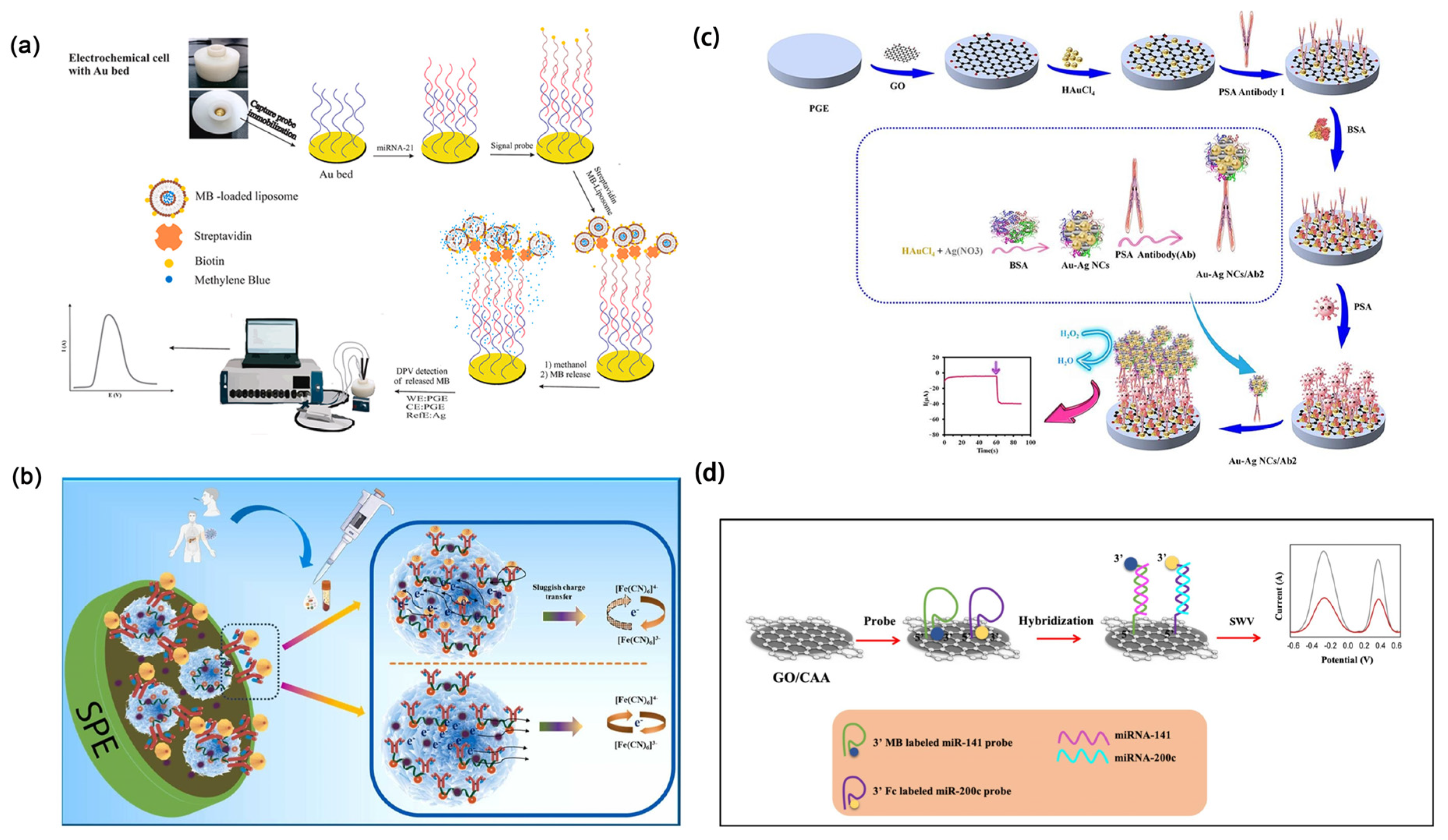
4.6. Discussion
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Mathur, P.; Sathishkumar, K.; Chaturvedi, M.; Das, P.; Sudarshan, K.L.; Santhappan, S.; Nallasamy, V.; John, A.; Narasimhan, S.; Roselind, F.S.; et al. Cancer statistics, 2020: Report from national cancer registry programme, India. JCO Glob. Oncol. 2020, 6, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Kallappa, S.; Kumar, P.; Shukla, S.; Ghosh, R. Simple diagnosis of cancer by detecting CEA and CYFRA 21-1 in saliva using electronic sensors. Sci. Rep. 2022, 12, 15315. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Aatif, M. The footprints of cancer development: Cancer biomarkers. Cancer Treat. Rev. 2009, 35, 193–200. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Sales, M.G.F.; Pereira, C.M. Electrochemistry combined-surface plasmon resonance biosensors: A review. TrAC–Trends Anal. Chem. 2022, 157, 116766. [Google Scholar] [CrossRef]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- Khan, H.; Shah, M.R.; Barek, J.; Malik, M.I. Cancer biomarkers and their biosensors: A comprehensive review. TrAC–Trends Anal. Chem. 2023, 158, 116813. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Du, X.; Liu, M. Application of electrochemical biosensors in tumor cell detection. Thorac. Cancer 2020, 11, 840–850. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Khataee, A.; Karimi, F.; Baghayeri, M.; Fu, L.; Rouhi, J.; Karaman, C.; Karaman, O.; Boukherroub, R. A green and sensitive guanine-based DNA biosensor for idarubicin anticancer monitoring in biological samples: A simple and fast strategy for control of health quality in chemotherapy procedure confirmed by docking investigation. Chemosphere 2022, 291, 132928. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Alizadeh, M.; Orooji, Y.; Karimi, F.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K.; et al. Guanine-based DNA biosensor amplified with Pt/SWCNTs nanocomposite as analytical tool for nanomolar determination of daunorubicin as an anticancer drug: A docking/experimental investigation. Ind. Eng. Chem. Res. 2021, 60, 816–823. [Google Scholar] [CrossRef]
- Yang, M.; Tang, J.; Liu, H.; Zhang, H.; Zhang, H. A novel demodulation method based on spectral clustering for phase-modulated signals interrupted by the plasma sheath channel. IEEE Trans. Plasma Sci. 2020, 48, 3544–3551. [Google Scholar] [CrossRef]
- Marti, A.; Huskens, J. Au nanoparticle-based amplified DNA detection on poly-L-lysine monolayer-functionalized electrodes. Nanomaterials 2022, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Zhang, X.; Nguyen, X.A.; Shi, Q.; Lin, F.; Chauhan, S.; Ge, Z.; Cheng, W. Hierarchically resistive skins as specific and multimetric on-throat wearable biosensors. Nat. Nanotechnol. 2023, 18, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Koman, V.B.; Bakh, N.A.; Jin, X.; Nguyen, F.T.; Son, M.; Kozawa, D.; Lee, M.A.; Bisker, G.; Dong, J.; Strano, M.S. A wavelength-induced frequency filtering method for fluorescent nanosensors in vivo. Nat. Nanotechnol. 2022, 17, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Henzie, J.; Lin, W.C.; Rhodes, C.; Li, Z.; Sartorel, E.; Thorner, J.; Yang, P.; Groves, J.T. Membrane-protein binding measured with solution-phase plasmonic nanocube sensors. Nat. Methods 2012, 9, 1189–1191. [Google Scholar] [CrossRef]
- Han, X.; Li, L.; Wang, C. Synthesis of tin dioxide nanooctahedra with exposed high-index 332 facets and enhanced selective gas sensing properties. Asial J. Chem. 2012, 7, 1572–1575. [Google Scholar] [CrossRef]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-based biosensor and their applications: A review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Cho, I.H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on site analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, C.; Cai, H.; Hu, N.; Zhou, J.; Wang, P. Cell-based biosensors and their application in biomedicine. Chem. Rev. 2014, 114, 6423–6461. [Google Scholar] [CrossRef]
- Turner, A.P. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef]
- Rotariu, L.; Lagarde, F.; Jaffrezic-Renault, N.; Bala, C. Electrochemical biosensors for fast detection of food contaminants—Trends and perspective. TrAC–Trends Anal. Chem. 2016, 79, 80–87. [Google Scholar] [CrossRef]
- Chen, J.; Lou, Y.; Sun, L.; Chia, C.H.; Nilghaz, A.; Tian, J. Play on electrodes. ACS Sens. 2025, 10, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Hammond, J.L.; Formisano, N.; Estrela, P.; Carrara, S.; Tkac, J. Electrochemical biosensors and nanobiosensors. Essays Biochem. 2016, 60, 69–80. [Google Scholar]
- Justino, C.I.L.; Freitas, A.C.; Pereira, R.; Duarte, A.C.; Rocha Santos, T.A.P. Recent developments in recognition elements for chemical sensors and biosensors. TrAC–Trends Anal. Chem. 2015, 68, 2–17. [Google Scholar] [CrossRef]
- Kim, J.; Park, M. Recent progress in electrochemical immunosensors. Biosensors 2021, 11, 360. [Google Scholar] [CrossRef]
- Farka, Z.; Jurik, T.; Kovar, D.; Trnkova, L.; Skladal, P. Nanoparticle-based immunochemical biosensors and assays: Recent advances and challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Huang, R.; Wang, D.; Deng, D.; Zhang, Q.; Luo, L. The applications of electrochemical immunosensors in the detection of disease biomarkers: A review. Molecules 2023, 28, 3605. [Google Scholar] [CrossRef]
- Hua, X.; Zhou, L.; Feng, L.; Ding, Y.; Shi, H.; Wang, L.; Gee, S.J.; Hammock, B.D.; Wang, M. Competitive and noncompetitive phage immunoassays for the determination of benzothiostrobin. Anal. Chim. Acta 2015, 890, 150–156. [Google Scholar] [CrossRef]
- Huo, X.; Liu, X.; Liu, J.; Sukumaran, P.; Alwarappan, S.; Wong, D.K.Y. Strategic applications of nanomaterials as sensing platforms and signal amplification markers at electrochemical immunosensors. Electroanalysis 2016, 28, 1730–1749. [Google Scholar] [CrossRef]
- Negahdary, M.; Angnes, L. Recent advances in electrochemical nanomaterial-based aptasensors for the detection of cancer biomarkers. Talanta 2023, 259, 124548. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Zhang, C.; Huang, R.; Zhang, W.; Deng, D.; Yan, X.; Zhang, Q.; Luo, L. NiCo2S4 Nanosheets Supported on Cu7S4 Microcubes for the Electrochemical Detection of Glucose in Human Serum. ACS Appl. Nano Mater. 2024, 7, 20905–20912. [Google Scholar] [CrossRef]
- Long, Y.; Zhao, J.; Ma, W.; He, C.; Pei, W.; Hou, J.; Hou, C.; Huo, D. Fe single-atom carbon dots nanozyme collaborated with nucleic acid exonuclease III-driven DNA walker cascade amplification strategy for circulating tumor DNA detection. Anal. Chem. 2024, 96, 4774–4782. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, H.; Huang, R.; Deng, D.; Yan, X.; Luo, L. An origami microfluidic paper device based on core-shell Cu@Cu2S@N-doped carbon hollow nanocubes. Anal. Chim. Acta 2024, 1316, 342828. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, X.; Chen, Y.; Deng, D.; He, H.; Lei, Y.; Luo, L. ZnO-CeO2 Hollow nanospheres for selective determination of dopamine and uric acid. Molecules 2024, 29, 1786. [Google Scholar] [CrossRef]
- Dong, J.; Ouyang, X.; Huo, B.; Deng, D.; Yan, X.; Luo, L. CuO/CoZn-layered double-hydroxide nanowires on carbon cloth as an enzyme-free H2O2 sensor. ACS Appl. Nano Mater. 2024, 7, 6564–6573. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Chen, H.; Song, J.; Deng, D.; Luo, L. Synthesis of quaternary (Ni, Co, Cu)Se2 nanosheet arrays on carbon cloth for non-enzymatic glucose determination. Chemosensors 2023, 11, 530. [Google Scholar] [CrossRef]
- Rosch, J.C.; Balikov, D.A.; Gong, F.; Lippmann, E.S. A systematic evolution of ligands by exponential enrichment workflow with consolidated counterselection to efficiently isolate high-affinity aptamers. Eng. Rep. 2020, 2, e12089. [Google Scholar] [CrossRef]
- Mikaeeli Kangarshahi, B.; Naghib, S.M.; Rabiee, N. DNA/RNA-based electrochemical nanobiosensors for early detection of cancers. Crit. Rev. Clin. Lab. Sci. 2024, 61, 473–495. [Google Scholar] [CrossRef]
- Negahdary, M. Aptamers in nanostructure-based electrochemical biosensors for cardiac biomarkers and cancer biomarkers: A review. Biosens. Bioelectron. 2020, 152, 112018. [Google Scholar] [CrossRef]
- Negahdary, M.; Angnes, L. Electrochemical aptamer-based nanobiosensors for diagnosing Alzheimer’s disease: A review. Mater. Sci. Eng. C-Mater. Biol. Appl. 2022, 135, 112689. [Google Scholar] [CrossRef] [PubMed]
- Atapour, A.; Khajehzadeh, H.; Shafie, M.; Abbasi, M.; Mosleh-Shirazi, S.; Kasaee, S.R.; Amani, A.M. Gold nanoparticle-based aptasensors: A promising perspective for early-stage detection of cancer biomarkers. Mater. Today Commun. 2022, 30, 103181. [Google Scholar] [CrossRef]
- Abd-Ellatief, R.; Abd-Ellatief, M.R. Electrochemical aptasensors: Current satus and future perspectives. Diagnostics 2021, 11, 104. [Google Scholar]
- Zhang, W.; Zhu, S.; Luque, R.; Han, S.; Hu, L.; Xu, G. Recent development of carbon electrode materials and their bioanalytical and environmental applications. Chem. Soc. Rev. 2016, 45, 715–752. [Google Scholar] [CrossRef]
- Chen, A.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, B.; Du, P.; Travas-Sejdic, J. Electrochemical and electrical biosensors for wearable and implantable electronics based on conducting polymers and carbon-based materials. Chem. Rev. 2024, 124, 722–767. [Google Scholar] [CrossRef]
- Wang, L.; Xie, S.; Wang, Z.; Liu, F.; Yang, Y.; Tang, C.; Wu, X.; Liu, P.; Li, Y.; Saiyin, H.; et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 2020, 4, 159–171. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G. Role of CA125 in predicting ovarian cancer survival—A review of the epidemiological literature. J. Ovarian Res. 2009, 2, 13. [Google Scholar] [CrossRef]
- Samadi Pakchin, P.; Fathi, M.; Ghanbari, H.; Saber, R.; Omidi, Y. A novel electrochemical immunosensor for ultrasensitive detection of CA125 in ovarian cancer. Biosens. Bioelectron. 2020, 153, 112029. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukherjee, A.; Bytesnikova, Z.; Ashrafi, A.M.; Richtera, L.; Adam, V. 2D graphene-based advanced nanoarchitectonics for electrochemical biosensors: Applications in cancer biomarker detection. Biosens. Bioelectron. 2024, 250, 116050. [Google Scholar] [CrossRef]
- Andrews, J.P.M.; Joshi, S.S.; Tzolos, E.; Syed, M.B.; Cuthbert, H.; Crica, L.E.; Lozano, N.; Okwelogu, E.; Raftis, J.B.; Bruce, L.; et al. First-in-human controlled inhalation of thin graphene oxide nanosheets to study acute cardiorespiratory responses. Nat. Nanotechnol. 2024, 19, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, Z.; Ttang, Z.; Li, J.Y.; An, P.; Zhang, M.; An, H. Novel electrochemical platform based on C3N4-graphene composite for the detection of neuron-specific enolase as a biomarker for lung cancer. Sci. Rep. 2024, 14, 6350. [Google Scholar]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. A paper-based electrochemical immunosensor with reduced graphene oxide/thionine/gold nanoparticles nanocomposites modification for the detection of cancer antigen 125. Biosens. Bioelectron. 2019, 135, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.A.; Moraes, F.C.; Janegitz, B.C.; Fatibello-Filho, O. Electrochemical biosensors based on nanostructured carbon black: A review. J. Nanomater. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Materon, E.M.; Furuta, R.H.M.; Wilson, D.; do Nascimento, G.F.; Melendez, M.E.; Carvalho, A.L.; Reis, R.M.; Oliveira, O.N.; Gonçalves, D. Screen-printed electrodes modified with carbon black and polyelectrolyte films for determination of cancer marker carbohydrate antigen 19-9. Microchim. Acta 2020, 187, 417. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, M.; Guo, L.; Xie, Y.; Luo, R.; Chen, W.; Cheng, F.; Wang, L. A dual-signal self-checking photoelectrochemical immunosensor based on the sole composite of MIL-101(Cr) and CdSe quantum dots for the detection of alpha-fetoprotein. Biosens. Bioelectron. 2021, 189, 113389. [Google Scholar] [CrossRef]
- Atabaev, T.S. Doped carbon dots for sensing and bioimaging applications: A minireview. Nanomaterials 2018, 8, 342. [Google Scholar] [CrossRef]
- Nekoueian, K.; Amiri, M.; Sillanpaa, M.; Marken, F.; Boukherroub, R.; Szunerits, S. Carbon-based quantum particles: An electroanalytical and biomedical perspective. Chem. Soc. Rev. 2019, 48, 4281–4316. [Google Scholar] [CrossRef]
- Wang, Y.H.; Huang, K.J.; Wu, X. Recent advances in transition-metal dichalcogenides based electrochemical biosensors: A review. Biosens. Bioelectron. 2017, 97, 305–316. [Google Scholar] [CrossRef]
- Muthusankar, G.; Rajkumar, C.; Chen, S.-M.; Karkuzhali, R.; Gopu, G.; Sangili, A.; Sengottuvelan, N.; Sankar, R. Sonochemical driven simple preparation of nitrogen-doped carbon quantum dots/SnO2 nanocomposite: A novel electrocatalyst for sensitive voltammetric determination of riboflavin. Sens. Actuators B Chem. 2019, 281, 602–612. [Google Scholar] [CrossRef]
- Muthusankar, G.; Devi, R.K.; Gopu, G. Nitrogen-doped carbon quantum dots embedded Co3O4 with multiwall carbon nanotubes: An efficient probe for the simultaneous determination of anticancer and antibiotic drugs. Biosens. Bioelectron. 2020, 150, 111947. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.D.; Takemura, K.; Li, T.C.; Suzuki, T.; Park, E.Y. Electrical pulse-induced electrochemical biosensor for hepatitis E virus detection. Nat. Commun. 2019, 10, 3737. [Google Scholar] [CrossRef] [PubMed]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef]
- Jie, G.; Jie, G. Sensitive electrochemiluminescence detection of cancer cells based on a CdSe/ZnS quantum dot nanocluster by multibranched hybridization chain reaction on gold nanoparticles. RSC Adv. 2016, 6, 24780–24785. [Google Scholar] [CrossRef]
- Scatena, R. Advances in Cancer Biomarkers: From Biochemistry to Clinic for a Critical Revision; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Vaisocherova-Lisalova, H.; Visova, I.; Ermini, M.L.; Springer, T.; Song, X.C.; Mrazek, J.; Lamacova, J.; Scott Lynn, N., Jr.; Sedivak, P.; Homola, J. Low-fouling surface plasmon resonance biosensor for multi-step detection of foodborne bacterial pathogens in complex food samples. Biosens. Bioelectron. 2016, 80, 84–90. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Zhang, S.; Qiu, W.; Gao, Y. Silver Enhancement of Gold Nanoparticles for Biosensing: From Qualitative to Quantitative. Appl. Spectrosc. Rev. 2013, 49, 121–138. [Google Scholar] [CrossRef]
- Wan, J.; Ai, J.; Zhang, Y.; Geng, X.; Gao, Q.; Cheng, Z. Signal-off impedimetric immunosensor for the detection of Escherichia coli O157:H7. Sci. Rep. 2016, 6, 19806. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Y.; Hou, Q.; Xu, Q.; Ding, C. Magnetic antifouling material based ratiometric electrochemical biosensor for the accurate detection of CEA in clinical serum. Biosens. Bioelectron. 2022, 208, 114216. [Google Scholar] [CrossRef]
- Zanoli, L.M.; D’Agata, R.; Spoto, G. Functionalized gold nanoparticles for ultrasensitive DNA detection. Anal. Bioanal. Chem. 2012, 402, 1759–1771. [Google Scholar] [CrossRef]
- Chen, H.; Song, J.; Li, Y.; Deng, D.; Song, Y.; Zhu, X.; Luo, L. Cascade signal amplifying strategy for ultrasensitive detection of tumor biomarker by DNAzyme cleaving mediated HCR. Sens. Actuators B Chem. 2024, 420, 136466. [Google Scholar] [CrossRef]
- Upan, J.; Youngvises, N.; Tuantranont, A.; Karuwan, C.; Banet, P.; Aubert, P.-H.; Jakmunee, J. A simple label-free electrochemical sensor for sensitive detection of alpha-fetoprotein based on specific aptamer immobilized platinum nanoparticles/carboxylated-graphene oxide. Sci. Rep. 2021, 11, 13969. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Deng, D.; Chen, Z.; Qi, J.; Li, Y.; Han, B.; Luo, L. A sensitive amperometric immunosensor for the detection of carcinoembryonic antigen using ZnMn2O4@reduced graphene oxide composites as signal amplifier. Sens. Actuators B Chem. 2021, 339, 129852. [Google Scholar] [CrossRef]
- Liu, F.; Chen, H.; Deng, D.; Fan, X.; Li, Y.; Madrakian, T.; Luo, L. An ultrasensitive immunosensor based on cellulose nanofibrils/polydopamine/Cu-Ag nanocomposite for the detection of AFP. Bioelectrochemistry 2022, 147, 108200. [Google Scholar] [CrossRef] [PubMed]
- Masud, M.K.; Na, J.; Younus, M.; Hossain, M.S.A.; Bando, Y.; Shiddiky, M.J.A.; Yamauchi, Y. Superparamagnetic nanoarchitectures for disease-specific biomarker detection. Chem. Soc. Rev. 2019, 48, 5717–5751. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Bishop, G.W.; Bhakta, S.; El-Sawy, A.; Suib, S.L.; Rusling, J.F. Fe3O4 nanoparticles on graphene oxide sheets for isolation and ultrasensitive amperometric detection of cancer biomarker proteins. Biosens. Bioelectron. 2017, 91, 359–366. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, N.; Fan, D.; Liu, L.; Yan, T.; Yang, X.; Ding, C.; Wei, Q.; Ju, H. Magnetic electrode-based electrochemical immunosensor using amorphous bimetallic sulfides of CoSnSx as signal amplifier for the NT pro BNP detection. Biosens. Bioelectron. 2019, 131, 250–256. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, N.; Luo, X.; Ding, S.N. Patchy gold coated Fe3O4 nanospheres with enhanced catalytic activity applied for paper-based bipolar electrode-electrochemiluminescence aptasensors. Biosens. Bioelectron. 2018, 114, 44–51. [Google Scholar] [CrossRef]
- Shirazi, H.; Daneshpour, M.; Kashanian, S.; Omidfar, K. Synthesis, characterization and in vitro biocompatibility study of Au/TMC/Fe3O4 nanocomposites as a promising, nontoxic system for biomedical applications. Beilstein J. Nanotechnol. 2015, 6, 1677–1689. [Google Scholar] [CrossRef]
- Daneshpour, M.; Karimi, B.; Omidfar, K. Simultaneous detection of gastric cancer-involved miR-106a and let-7a through a dual-signal-marked electrochemical nanobiosensor. Biosens. Bioelectron. 2018, 109, 197–205. [Google Scholar] [CrossRef]
- Bai, K.P.; Zhou, L.J.; Yang, G.P.; Cao, M.X.; Wang, Y.Y. Four new metal-organic frameworks based on diverse metal clusters: Syntheses, structures, luminescent sensing and dye adsorption properties. J. Solid State Chem. 2020, 287, 121336. [Google Scholar] [CrossRef]
- Mohan, B.; Modi, K.; Patel, C.; Bhatia, P.; Kumar, P.; Kumar, A.; Sharma, H.K. Selectivity for La3+ ion by synthesized 4-((5-methylfuran-2-yl)methylene)hydrazono)methyl)phenol receptor and its spectral analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Mohan, B.; Kumar, S.; Xi, H.; Ma, S.; Tao, Z.; Xing, T.; You, H.; Zhang, Y.; Ren, P. Fabricated Metal-organic frameworks (MOFs) as luminescent and electrochemical biosensors for cancer biomarkers detection. Biosens. Bioelectron. 2022, 197, 113738. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Yang, Z.; Wang, Y.; Li, R.; Zhai, J. Construction of Metal-organic frameworks (MOFs)–based membranes and their ion transport applications. Small Sci. 2021, 1, 2000035. [Google Scholar] [CrossRef]
- Fu, X.; He, J.; Zhang, C.; Chen, J.; Wen, Y.; Li, J.; Mao, W.; Zhong, H.; Wu, J.; Ji, X.; et al. Trimetallic signal amplification aptasensor for TSP-1 detection based on Ce-MOF@Au and AuPtRu nanocomposites. Biosens. Bioelectron. 2019, 132, 302–309. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Li, D.; Zhao, M.; Wu, H.; Shen, B.; Liu, P.; Ding, S. Electrochemical biosensor for ultrasensitive exosomal miRNA analysis by cascade primer exchange reaction and MOF@Pt@MOF nanozyme. Biosens. Bioelectron. 2020, 168, 112554. [Google Scholar] [CrossRef]
- Hatami, Z.; Jalali, F.; Amouzadeh Tabrizi, M.; Shamsipur, M. Application of metal-organic framework as redox probe in an electrochemical aptasensor for sensitive detection of MUC1. Biosens. Bioelectron. 2019, 141, 111433. [Google Scholar] [CrossRef]
- Zhang, Y.; Vaccarella, S.; Morgan, E.; Li, M.; Etxeberria, J.; Chokunonga, E.; Manraj, S.S.; Kamate, B.; Omonisi, A.; Bray, F. Global variations in lung cancer incidence by histological subtype in 2020: A population-based study. Lancet Oncol. 2023, 24, 1206–1218. [Google Scholar] [CrossRef]
- Tang, J.; Liu, L.; Qin, J.; Lv, X.; Li, J.; Tang, D.; Zhuang, J. Biocatalysis-mediated MOF-to-prussian blue transformation enabling sensitive detection of NSCLC-associated miRNAs with dual-readout signals. Biosens. Bioelectron. 2022, 206, 114139. [Google Scholar] [CrossRef]
- Dezhakam, E.; Vayghan, R.F.; Dehghani, S. Highly efficient electrochemical biosensing platform in breast cancer detection based on MOF-COF@Au core-shell like nanostructure. Sci. Rep. 2024, 14, 29850. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Q.; Huang, Z.; Xiong, H.; Yang, B.; Chen, H.; Kong, J. Aptamer-initiated catalytic hairpin assembly fluorescence assay for universal, sensitive exosome detection. Anal. Chem. 2022, 94, 5723–5728. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Ji, P.; Yang, Y.S.; Xie, S.; Yu, T.J.; Xiao, Y.; Jin, M.L.; Ma, D.; Guo, L.W.; Pei, Y.C.; et al. Metabolic-pathway-based subtyping of triple-negative breast cancer reveals potential therapeutic targets. Cell Metab. 2021, 33, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Britt, K.L.; Cuzick, J.; Phillips, K.A. Key steps for effective breast cancer prevention. Nat. Rev. Cancer 2020, 20, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, J.; Chen, W.; Li, G.; Li, Z.; Lei, J. The updated role of exosomal proteins in the diagnosis, prognosis, and treatment of cancer. Exp. Mol. Med. 2022, 54, 1390–1400. [Google Scholar] [CrossRef]
- Hannafon, B.; Ding, W.Q. Intercellular communication by exosome-derived microRNAs in cancer. Int. J. Mol. Sci. 2013, 14, 14240–14269. [Google Scholar] [CrossRef]
- Dervisevic, M.; Alba, M.; Adams, T.E.; Prieto-Simon, B.; Voelcker, N.H. Electrochemical immunosensor for breast cancer biomarker detection using high-density silicon microneedle array. Biosens. Bioelectron. 2021, 192, 113496. [Google Scholar] [CrossRef]
- Carvajal, S.; Fera, S.N.; Jones, A.L.; Baldo, T.A.; Mosa, I.M.; Rusling, J.F.; Krause, C.E. Disposable inkjet-printed electrochemical platform for detection of clinically relevant HER-2 breast cancer biomarker. Biosens. Bioelectron. 2018, 104, 158–162. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Li, N.; Yang, M.; Xiang, T.; Huo, D.; Qiu, Z.; Yang, L.; Hou, C. An ultra-sensitive dual-signal ratiometric electrochemical aptasensor based on functionalized MOFs for detection of HER2. Bioelectrochemistry 2022, 148, 108272. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Chen, G.; Li, Y.; Xu, W.; Gong, S. Quantum-dot-based theranostic micelles conjugated with an anti-EGFR nanobody for triple-negative breast cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 30297–30305. [Google Scholar] [CrossRef]
- Wang, M.; Liu, W.; Zhang, Y.; Dang, M.; Zhang, Y.; Tao, J.; Chen, K.; Peng, X.; Teng, Z. Intercellular adhesion molecule 1 antibody-mediated mesoporous drug delivery system for targeted treatment of triple-negative breast cancer. J. Colloid Interface Sci. 2019, 538, 630–637. [Google Scholar] [CrossRef]
- Zhang, M.; Xia, L.; Mei, W.; Zou, Q.; Liu, H.; Wang, H.; Zou, L.; Wang, Q.; Yang, X.; Wang, K. One-step multiplex analysis of breast cancer exosomes using an electrochemical strategy assisted by gold nanoparticles. Anal. Chim. Acta 2023, 1254, 341130. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Sha, L.; Yu, X.; Yang, M.; Cao, Y.; Zhao, J. Identification of dual therapeutic targets assisted by in situ automatous DNA assembly for combined therapy in breast cancer. Biosens. Bioelectron. 2021, 176, 112913. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef]
- Curtale, G.; Mirolo, M.; Renzi, T.A.; Rossato, M.; Bazzoni, F.; Locati, M. Negative regulation of Toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc. Natl. Acad. Sci. USA 2013, 110, 11499–11504. [Google Scholar] [CrossRef]
- Drury, R.E.; O’Connor, D.; Pollard, A.J. The clinical application of microRNAs in infectious disease. Front. Immunol. 2017, 8, 1182. [Google Scholar] [CrossRef]
- Chinnappan, M.; Singh, A.K.; Kakumani, P.K.; Kumar, G.; Rooge, S.B.; Kumari, A.; Varshney, A.; Rastogi, A.; Singh, A.K.; Sarin, S.K.; et al. Key elements of the RNAi pathway are regulated by hepatitis B virus replication and HBx acts as a viral suppressor of RNA silencing. Biochem. J. 2014, 462, 347–358. [Google Scholar] [CrossRef]
- Fu, Y.R.; Liu, X.J.; Li, X.J.; Shen, Z.Z.; Yang, B.; Wu, C.C.; Li, J.F.; Miao, L.F.; Ye, H.Q.; Qiao, G.H.; et al. MicroRNA miR-21 attenuates human cytomegalovirus replication in neural cells by targeting Cdc25a. J. Virol. 2015, 89, 1070–1082. [Google Scholar] [CrossRef]
- Ondevilla, N.A.P.; Liu, P.-W.; Huang, W.-T.; Weng, T.-P.; Lee, N.-Y.; Ma, S.-C.; Huang, J.-J.; Wong, T.-W.; Chang, H.-C. A point-of-care electrochemical biosensor for the rapid and sensitive detection of biomarkers in murine models with LPS-induced sepsis. Biosens. Bioelectron. 2024, 254, 116202. [Google Scholar] [CrossRef]
- Hannafon, B.N.; Trigoso, Y.D.; Calloway, C.L.; Zhao, Y.D.; Lum, D.H.; Welm, A.L.; Zhao, Z.J.; Blick, K.E.; Dooley, W.C.; Ding, W.Q. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016, 18, 90. [Google Scholar] [CrossRef]
- Cao, Y.; Yu, X.; Zeng, T.; Fu, Z.; Zhao, Y.; Nie, B.; Zhao, J.; Yin, Y.; Li, G. Molecular characterization of exosomes for subtype-based diagnosis of breast cancer. J. Am. Chem. Soc. 2022, 144, 13475–13486. [Google Scholar] [CrossRef]
- Cheng, W.; Yao, Y.; Li, D.; Duan, C.; Wang, Z.; Xiang, Y. Asymmetrically split DNAzyme-based colorimetric and electrochemical dual-modal biosensor for detection of breast cancer exosomal surface proteins. Biosens. Bioelectron. 2023, 238, 115552. [Google Scholar] [CrossRef] [PubMed]
- Moura, S.L.; Martin, C.G.; Marti, M.; Pividori, M.I. Electrochemical immunosensing of nanovesicles as biomarkers for breast cancer. Biosens. Bioelectron. 2020, 150, 111882. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Clifford, G.; Franceschi, S.; Diaz, M.; Munoz, N.; Villa, L.L. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 2006, 24, 26–34. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef]
- Yang, N.; Liu, P.; Cai, C.; Zhang, R.; Sang, K.; Shen, P.; Huang, Y.; Lu, Y. Triple signal amplification strategy for the ultrasensitive electrochemical detection of human papillomavirus 16 E6/E7 mRNA. Enzym. Microb. Technol. 2021, 149, 109855. [Google Scholar] [CrossRef]
- Teengam, P.; Siangproh, W.; Tuantranont, A.; Henry, C.S.; Vilaivan, T.; Chailapakul, O. Electrochemical paper-based peptide nucleic acid biosensor for detecting human papillomavirus. Anal. Chim. Acta 2017, 952, 32–40. [Google Scholar] [CrossRef]
- Jampasa, S.; Wonsawat, W.; Rodthongkum, N.; Siangproh, W.; Yanatatsaneejit, P.; Vilaivan, T.; Chailapakul, O. Electrochemical detection of human papillomavirus DNA type 16 using a pyrrolidinyl peptide nucleic acid probe immobilized on screen-printed carbon electrodes. Biosens. Bioelectron. 2014, 54, 428–434. [Google Scholar] [CrossRef]
- Mahmoodi, P.; Rezayi, M.; Rasouli, E.; Avan, A.; Gholami, M.; Ghayour Mobarhan, M.; Karimi, E.; Alias, Y. Early-stage cervical cancer diagnosis based on an ultra-sensitive electrochemical DNA nanobiosensor for HPV-18 detection in real samples. J. Nanobiotechnol. 2020, 18, 11. [Google Scholar] [CrossRef]
- Koo Siew Kim, N.S.; Parmin, N.A.; Hashim, U.; Gopinath, S.C.B.; Rejali, Z.; Afzan, A.; Uda, M.N.A.; Afnan Uda, M.N.; Hong, V.C. Electrochemical DNA biosensor based on 30 nM gold nanoparticle modified electrode by electroless deposition for human papillomavirus (HPV) 18 E6 region. IOP Conf. Ser. Mater. Sci. Eng. 2020, 864, 012167. [Google Scholar] [CrossRef]
- Chaibun, T.; Thanasapburachot, P.; Chatchawal, P.; Su Yin, L.; Jiaranuchart, S.; Jearanaikoon, P.; Promptmas, C.; Buajeeb, W.; Lertanantawong, B. A multianalyte electrochemical genosensor for the detection of high-risk HPV genotypes in oral and cervical cancers. Biosensors 2022, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Civit, L.; Fragoso, A.; Holters, S.; Durst, M.; O’Sullivan, C.K. Electrochemical genosensor array for the simultaneous detection of multiple high-risk human papillomavirus sequences in clinical samples. Anal. Chim. Acta 2012, 715, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Tang, C.; Deng, Y.; Huang, H.; He, Z. Recent advances in biosensor for detection of lung cancer biomarkers. Biosens. Bioelectron. 2019, 141, 111416. [Google Scholar] [CrossRef]
- Mahmud, N.; Anik, M.I.; Hossain, M.K.; Khan, M.I.; Uddin, S.; Ashrafuzzaman, M.; Rahaman, M.M. Advances in nanomaterial-based platforms to combat COVID-19: Diagnostics, preventions, therapeutics, and vaccine developments. ACS Appl. Bio Mater. 2022, 5, 2431–2460. [Google Scholar] [CrossRef]
- Arya, S.K.; Bhansali, S. Lung cancer and its early detection using biomarker-based biosensors. Chem. Rev. 2011, 111, 6783–6809. [Google Scholar] [CrossRef]
- Mir, T.A.; Yoon, J.H.; Gurudatt, N.G.; Won, M.S.; Shim, Y.B. Ultrasensitive cytosensing based on an aptamer modified nanobiosensor with a bioconjugate: Detection of human non-small-cell lung cancer cells. Biosens. Bioelectron. 2015, 74, 594–600. [Google Scholar] [CrossRef]
- Reckamp, K.L.; Melnikova, V.O.; Karlovich, C.; Sequist, L.V.; Camidge, D.R.; Wakelee, H.; Perol, M.; Oxnard, G.R.; Kosco, K.; Croucher, P.; et al. A highly sensitive and quantitative test platform for detection of NSCLC EGFR mutations in urine and plasma. J. Thorac. Oncol. 2016, 11, 1690–1700. [Google Scholar] [CrossRef]
- Shoja, Y.; Kermanpur, A.; Karimzadeh, F. Diagnosis of EGFR exon21 L858R point mutation as lung cancer biomarker by electrochemical DNA biosensor based on reduced graphene oxide/functionalized ordered mesoporous carbon/Ni-oxytetracycline metallopolymer nanoparticles modified pencil graphite electrode. Biosens. Bioelectron. 2018, 113, 108–115. [Google Scholar]
- Shin Low, S.; Pan, Y.; Ji, D.; Li, Y.; Lu, Y.; He, Y.; Chen, Q.; Liu, Q. Smartphone-based portable electrochemical biosensing system for detection of circulating microRNA-21 in saliva as a proof-of-concept. Sens. Actuators B Chem. 2020, 308, 127718. [Google Scholar] [CrossRef]
- Wu, T.; Chen, W.; Kong, D.; Li, X.; Lu, H.; Liu, S.; Wang, J.; Du, L.; Kong, Q.; Huang, X.; et al. miR-25 targets the modulator of apoptosis 1 gene in lung cancer. Carcinogenesis 2015, 36, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Liu, H.; Cui, J.; Liu, Y.; Pan, P. Recent strategies for electrochemical sensing detection of miRNAs in lung cancer. Anal. Biochem. 2023, 661, 114986. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Zhang, T.; Zhang, S.; Johnston, M.; Zheng, X.; Shan, Y.; Liu, T.; Huang, Z.; Qian, F.; Xie, Z.; et al. A CRISPR/Cas13a-powered catalytic electrochemical biosensor for successive and highly sensitive RNA diagnostics. Biosens. Bioelectron. 2021, 178, 113027. [Google Scholar] [CrossRef]
- Asadzadeh-Firouzabadi, A.; Zare, H.R. Preparation and application of AgNPs/SWCNTs nanohybrid as an electroactive label for sensitive detection of miRNA related to lung cancer. Sens. Actuators B Chem. 2018, 260, 824–831. [Google Scholar] [CrossRef]
- Gao, W.; Xu, J.; Liu, L.; Shen, H.; Zeng, H.; Shu, Y. A systematic-analysis of predicted miR-21 targets identifies a signature for lung cancer. Biomed. Pharmacother. 2012, 66, 21–28. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, H.; Pang, Y.; Wang, M.; Liu, S. UTMD-mediated delivery of miR-21-5p inhibitor suppresses the development of lung cancer. Tissue Cell 2022, 74, 101719. [Google Scholar] [CrossRef]
- Liu, S.; Su, W.; Li, Y.; Zhang, L.; Ding, X. Manufacturing of an electrochemical biosensing platform based on hybrid DNA hydrogel: Taking lung cancer-specific miR-21 as an example. Biosens. Bioelectron. 2018, 103, 1–5. [Google Scholar] [CrossRef]
- Anagnostou, V.K.; Syrigos, K.N.; Bepler, G.; Homer, R.J.; Rimm, D.L. Thyroid transcription factor 1 is an independent prognostic factor for patients with stage I lung adenocarcinoma. J. Clin. Oncol. 2009, 27, 271–278. [Google Scholar] [CrossRef]
- Rekhtman, N.; Ang, D.C.; Sima, C.S.; Travis, W.D.; Moreira, A.L. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod. Pathol. 2011, 24, 1348–1359. [Google Scholar] [CrossRef]
- Gloriane, C.L.H.; Severino Imasa, M.; Juat, N.; Hernandez, K.V.; May Sayo, T.; Cristal-Luna, G.; Marie Asur-Galang, S.; Bellengan, M.; John Duga, K.; Brian Buenaobra, B.; et al. Expression landscapes in non-small cell lung cancer shaped by the thyroid transcription factor 1. Lung Cancer 2023, 176, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tang, H.; Zhou, L.; Li, Z. A novel label-free electrochemical immunosensor for the detection of thyroid transcription factor 1 using ribbon-like tungsten disulfide-reduced graphene oxide nanohybrids and gold nanoparticles. Molecules 2024, 29, 552. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shen, X.; Yan, L.; Qu, R.; Wang, Y.; He, Y.; Zhan, Z.; Chen, P.; Lin, F. Controllable self-assembled DNA nanomachine enable homogeneous rapid electrochemical one-pot assay of lung cancer circulating tumor cells. Biosens. Bioelectron. 2024, 246, 115865. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yan, L.; Zhan, Z.; Qu, R.; Wang, Y.; Zeng, X.; Yang, H.; Feng, P.; Wei, Z.; Chen, P. Biomolecules-mediated electrochemical signals of Cu2+: Y-DNA nanomachines enable homogeneous rapid one-step assay of lung cancer circulating tumor cells. Biosens. Bioelectron. 2024, 249, 116030. [Google Scholar] [CrossRef]
- Megyesfalvi, Z.; Gay, C.M.; Popper, H.; Pirker, R.; Ostoros, G.; Heeke, S.; Lang, C.; Hoetzenecker, K.; Schwendenwein, A.; Boettiger, K.; et al. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. A Cancer J. Clin. 2023, 73, 620–652. [Google Scholar] [CrossRef]
- Gazdar, A.F.; Bunn, P.A.; Minna, J.D. Small-cell lung cancer: What we know, what we need to know and the path forward. Nat. Rev. Cancer 2017, 17, 765. [Google Scholar] [CrossRef]
- Gay, C.M.; Stewart, C.A.; Park, E.M.; Diao, L.; Groves, S.M.; Heeke, S.; Nabet, B.Y.; Fujimoto, J.; Solis, L.M.; Lu, W.; et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 2021, 39, 346–360. [Google Scholar] [CrossRef]
- Haque, A.; Polcyn, R.; Matzelle, D.; Banik, N.L. New insights into the role of neuron-specific enolase in neuro-inflammation, neurodegeneration, and neuroprotection. Brain Sci. 2018, 8, 33. [Google Scholar] [CrossRef]
- Karaman, C.; Bölükbaşı, O.S.; Yola, B.B. Electrochemical neuron-specific enolase (NSE) immunosensor based on CoFe2O4@Ag nanocomposite and AuNPs@MoS2/rGO. Anal. Chim. Acta 2022, 1200, 339609. [Google Scholar] [CrossRef]
- Alberts, S.R.; Cervantes, A.; van de Velde, C.J. Gastric cancer: Epidemiology, pathology and treatment. Ann. Oncol. 2003, 14, ii31–ii36. [Google Scholar] [CrossRef]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. A Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Fock, K.M. Review article: The epidemiology and prevention of gastric cancer. Aliment. Pharmacol. Ther. 2014, 40, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Zhao, C.; Luo, Y.; Pan, C.; Li, J. Electrochemical sensor for the simultaneous detection of CA72-4 and CA19-9 tumor markers using dual recognition via glycosyl imprinting and lectin-specific binding for accurate diagnosis of gastric cancer. Biosens. Bioelectron. 2022, 216, 114672. [Google Scholar] [CrossRef]
- Hashimoto, I.; Oshima, T. Claudins and gastric cancer: An overview. Cancers 2022, 14, 290. [Google Scholar] [CrossRef]
- Kanagavalli, P.; Eissa, S. Exploring various carbon nanomaterials-based electrodes modified with polymelamine for the reagentless electrochemical immunosensing of Claudin18.2. Biosens. Bioelectron. 2024, 259, 116388. [Google Scholar] [CrossRef]
- Cho, E.J.; Kim, H.K.; Jeong, T.D.; Ko, D.H.; Bae, S.E.; Lee, J.S.; Lee, W.; Choe, J.W.; Chun, S.; Jung, H.Y.; et al. Method evaluation of pepsinogen I/II assay based on chemiluminescent immunoassays and comparison with other test methods. Clin. Chim. Acta 2016, 452, 149–154. [Google Scholar] [CrossRef]
- Mansour-Ghanaei, F.; Joukar, F.; Baghaee, M.; Sepehrimanesh, M.; Hojati, A. Only serum pepsinogen I and pepsinogen I/II ratio are specific and sensitive biomarkers for screening of gastric cancer. Biomolelar Concepts 2019, 10, 82–90. [Google Scholar] [CrossRef]
- Kanagavalli, P.; Eissa, S. Redox probe-free electrochemical immunosensor utilizing electropolymerized melamine on reduced graphene oxide for the point-of-care diagnosis of gastric cancer. Talanta 2024, 270, 125549. [Google Scholar] [CrossRef]
- Huang, R.; He, L.; Xia, Y.; Xu, H.; Liu, C.; Xie, H.; Wang, S.; Peng, L.; Liu, Y.; Liu, Y.; et al. A sensitive aptasensor based on a hemin/G-quadruplex-assisted signal amplification strategy for electrochemical detection of gastric cancer exosomes. Small 2019, 15, e1900735. [Google Scholar] [CrossRef]
- Tsai, M.M.; Wang, C.S.; Tsai, C.Y.; Huang, H.W.; Chi, H.C.; Lin, Y.H.; Lu, P.H.; Lin, K.H. Potential diagnostic, prognostic and therapeutic targets of microRNAs in human gastric cancer. Int. J. Mol. Sci. 2016, 17, 945. [Google Scholar] [CrossRef]
- Oo, S.L.; Venkatesh, S.; Karthikeyan, V.; Arava, C.M.; Pathikonda, S.; Yu, P.K.N.; Lau, T.C.K.; Chen, X.; Roy, V.A.L. Highly sensitive and cost-effective portable sensor for early gastric carcinoma diagnosis. Sensors 2021, 21, 2639. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Wan, H.; Zhang, X. Electrochemical detection of miRNA-100 in the sera of gastric cancer patients based on DSN-assisted amplification. Talanta 2021, 225, 121981. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, S.; Lan, F.; Li, W.; Ji, T.; Zhang, L.; Guo, Y.; Pan, W.; Luo, S.; Xie, R. Sensitive electrochemical biosensor for rapid detection of sEV-miRNA based turbo-like localized catalytic hairpin assembly. Anal. Chim. Acta 2024, 1311, 342704. [Google Scholar] [CrossRef] [PubMed]
- Saputra, H.A.; Karim, M.M. Fundamentals and research progression on electrochemical sensing of colorectal cancer. Microchim. Acta 2025, 192, 355. [Google Scholar] [CrossRef]
- Norouzi, S.; Alipour, E.; Soltani, S.; Hallaj-Nezhadi, S.; Hashemzadeh, S. A novel electrochemical biosensor with liposomal amplification for sensitive detection of colon cancer biomarker: A step toward early cancer diagnosis. Talanta 2025, 293, 128079. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, Z.; Ma, M.; Yang, J.; Liu, R.; Zhang, Y.; Ma, P.; Song, D. Electrochemiluminescence biosensor for specific detection of pancreatic ductal carcinoma through dual targeting of MUC1 and miRNA-196a. Biosens. Bioelectron. 2024, 254, 116241. [Google Scholar] [CrossRef]
- Huynh, T.V.; Tran, H.L.; Anh, N.T.N.; Doong, R.A. Electrochemical sensor for rapid diagnosis and early-stage detection of pancreatic cancer using Er-GQDs decorated MoS2 nanoflowers. Sens. Actuators B Chem. 2024, 413, 135893. [Google Scholar] [CrossRef]
- Dehghani, P.; Salehirozveh, M.; Tajabadi, A.; Yeung, C.C.; Lam, M.; Leung, H.Y.; Roy, V.A.L. Next-gen point-of-care tool for ultra-sensitive detection of urinary spermine for prostate cancer diagnosis. ACS Sensors 2025, 10, 2640–2651. [Google Scholar] [CrossRef]
- Sadeghi, M.; Ehzari, H.; Ghasemi, Y. Ultrahighly sensitive sandwich-type electrochemical immunosensor for selective detection of prostate specific antigen based on functionalized bimetallic Au-Ag nanoclusters as label for signal amplification. Microchem. J. 2025, 208, 112455. [Google Scholar] [CrossRef]
- Oyowvi, M.O.; Babawale, K.H.; Atere, A.D. Emerging nanotechnologies and their role in early ovarian cancer detection, diagnosis and interventions. J. Ovarian Res. 2025, 18, 96. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drug delivery. Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef]
- Kivrak, E.; Kara, P. Simultaneous detection of ovarian cancer related miRNA biomarkers with carboxylated graphene oxide modified electrochemical biosensor platform. Bioelectrochemistry 2025, 161, 108806. [Google Scholar] [CrossRef]



| Nanomaterial Type | Key Features and Advantages | Impact on Sensor Performance | Limitations or Challenges | Feature | Limit of Detection | Ref. |
|---|---|---|---|---|---|---|
| Carbon-based nanomaterials | Excellent electrical conductivity, large surface area, and good biocompatibility. | Enhance electron transfer, stabilize signal output, promote biomolecule immobilization, and improve sensitivity and stability. | Material selection and functionalization need optimization. | Three-dimensional reduced graphene oxide-multiwalled carbon nanotube composite | CA 125 6 μU mL−1 | [49] |
| Graphene-graphitic carbon nitride nanocomposite | NSE 3 pg mL−1 | [52] | ||||
| Reduced graphene oxide/L-cysteine/gold nanoparticles | CA 125 0.01 U mL−1 | [53] | ||||
| Carbon black | CA 19-9 0.07 U mL−1 | [55] | ||||
| Co3O4 with nitrogen-doped carbon quantum dots | Antibiotic nitrofurantoin and anticancer drug fluoxetine 0.044 μM and 0.0169 μM | [61] | ||||
| Metallic nanomaterials | Unique plasmonic properties, superior biocompatibility, strong biomolecular conjugation ability, good catalytic activity, increased active surface area, and bioreceptor loading | Used for signal amplification and labeling, significantly improving sensitivity and selectivity, enhance electrochemical. | High cost, sensitive to storage and handling conditions, other metal nanoparticles (e.g., PtNPs) generally exhibit lower usability and recognition performance compared to AuNPs. | Gold nanoparticles | AFP 15.8 fg mL−1 | [71] |
| Platinum nanoparticles | AFP 1.22 ng mL−1 | [72] | ||||
| ZnMn2O4 | CEA 1.93 pg mL−1 | [73] | ||||
| Magnetic nanomaterials | Magnetic responsiveness enables rapid target capture/separation, and improved conductivity when integrated with electrodes. | Reduce detection time and enhance sensitivity and selectivity. | Complex structures and potential stability concerns. | Fe3O4 | PSA and PSMA 15 fg mL−1 and 4.8 fg mL−1 | [76] |
| Fe3O4 | Brain natriuretic peptide N-terminal prohormone 31.5 fg mL−1 | [77] | ||||
| Fe3O4 | MicroR-106a and let-7a 0.02 fM and 0.06 fM | [80] | ||||
| Metal-organic frameworks (MOFs) nanomaterials | Highly porous structures, abundant functional sites, and reusable. | Efficient target capture, signal amplification, and expanded detection capabilities. | Complex synthesis procedures and reproducibility issues. | MIL-88@Pt@MIL-88 | MicroRNA-21 0.29 fM | [86] |
| Cu MOF | MUC1 0.033 pM | [87] | ||||
| Fe MOF | MicroRNA-21 (temperature and electrochemical readouts) 0.3 fM and 0.32 fM | [88] | ||||
| MOF-COF@Au | CA15-3 2.6 nU mL−1 | [90] |
| Cancer Type | Nanomaterial (Type) | Key Features and Advantages | Impact on Sensor | Limit of Detection | Ref. |
|---|---|---|---|---|---|
| Breast cancer | Gold and silver nanoparticles (metallic nanomaterials) | Reduce production costs. | below $0.25 per chip. | HER2 12 pg mL−1 | [98] |
| ZIF-67 and ZIF-90 (MOF nanomaterials) | High specific surface area, excellent electrical conductivity, and tunable porosity. | Improved the capture efficiency of HER2 and the signal amplification capability of the sensor. | HER2 155 fg mL−1 | [99] | |
| Gold nanoparticles (metallic nanomaterials) | Excellent electrical conductivity, outstanding electrical conductivity, and good biocompatibility. | Perform efficient signal amplification to enhance sensitivity and accuracy. | HER2 3.4 × 103 particles mL−1 | [102] | |
| Anti-CD44 functionalized immunomagnetic beads (magnetic nanomaterials) | Specific recognition and capture of the target. | Efficient enrichment of breast cancer-derived exosomes and significantly enhancing the specificity and purity of the detection system. | MicroRNA-375 557 particles mL−1 | [111] | |
| Magnetic particles (magnetic nanomaterials) | Specific recognition and capture of the target. | Enhance sensitivity and specificity. | CD24 and CD340 1.94 × 105 exosomes μL−1 and 1.02 × 106 exosomes μL−1 | [113] | |
| Cervical cancer | Gold nanoparticles (metallic nanomaterials) | Large specific surface area, serving as a carrier. | Efficient capture and enrichment of target RNA, thereby enhancing detection sensitivity and specificity. | HPV E6/E7 mRNA 0.08 fM | [118] |
| Graphene (carbon-based nanomaterials) | High specific surface area and excellent electrical conductivity. | Enhance the signal response and detection sensitivity of the paper-based electrochemical DNA biosensor. | HPV type 16 DNA 2.3 nM | [119] | |
| Magnetic silica nanoparticles (magnetic nanomaterials) | Specific recognition and capture of the target. | Achieve signal amplification and enhance sensitivity and specificity. | HPV-16 and HPV-18 22 fM and 20 fM | [123] | |
| Lung cancer | Reduced graphene oxide (Carbon-based nanomaterials) | Excellent electrical conductivity and high specific surface area. | Effectively improve the electrode’s electrochemical reaction efficiency and DNA probe immobilization capacity. | Receptor exon 2-point mutations 120 nM | [131] |
| AgNPs/SWCNTs (metallic Nanomaterials and carbon-based nanomaterials) | Excellent electrical conductivity and high specific surface area. | The synergistic effect of both components enhances the sensor’s sensitivity and selectivity, enabling efficient electrochemical detection of lung cancer-related miRNAs. | MicroRNA-25 3.13 × 10−13 M | [136] | |
| Graphene oxide (carbon-based nanomaterials) | Large specific surface area and abundant functional groups | Facilitate the adsorption of DNA probes and enhance charge transfer. | MicroRNA-21 5 nM | [139] | |
| Reduced graphene oxide (carbon-based nanomaterials) | Highly conductive | Enhance the electrode’s electrochemical reactivity and signal amplification to achieve highly sensitive detection. | TTF-1 0.016 ng mL−1 | [141] | |
| Gastric cancer | Reduced graphene oxide (carbon-based nanomaterials) | Excellent electrical conductivity | Enhance electron transfer efficiency at the electrode and enable highly sensitivity. | Pepsinogen I 9.1 pg·mL−1 | [159] |
| Gold nanoparticles (metallic nanomaterials) | High surface area and excellent conductivity | Enhance the sensitivity and specificity of electrochemical exosome detection. | Gastric cancer exosomes 9.54 × 102 exosomes mL−1 | [160] | |
| Gold nanoparticles (metallic nanomaterials) | High surface area and excellent conductivity | Achieve signal amplification and enhance the sensitivity and specificity of target detection. | MicroRNA-100 100 aM | [163] | |
| Colorectal cancer | Gold nanoparticles (metallic nanomaterials) | High surface area | Achieve signal amplification | MicroRNA-21 85.0 fM | [166] |
| Pancreatic cancer | Er-GQDs and MoS2 nanoflowers (carbon-based nanomaterials and metallic nanomaterials) | The MoS2 nanoflowers provide a large surface area and excellent electrocatalytic activity, while the Er-GQDs serve as functional anchoring sites. | Efficiently immobilize antibodies and enhance signal transduction. | CA 19-9 (0.18–2.95) × 10−4 U mL−1 | [168] |
| Prostate cancer | Au–Ag bimetallic nanoclusters and a reduced graphene oxide–gold nanoparticle composite (carbon-based nanomaterials and metallic nanomaterials) | Au–Ag NCs as signal amplification tags, the rGO–AuNPs substrate offered high conductivity. | Enhance electrocatalytic activity, improve antibody immobilization and electron transfer efficiency. | PSA 30.0 fg/mL | [170] |
| Ovarian cancer | Carboxylated graphene oxide (carbon-based nanomaterials) | Excellent surface functionalization capability. | Enhanced electron transfer properties and stable biointerface. | MicroR-141 and microR-200c 0.029 pM and 0.026 pM | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Song, Y.; Liu, M.; Deng, D.; Zhang, W.; Wang, T.; Luo, L. The Application of Functional Nanomaterials-Based Electrochemical Biosensors in Detecting Cancer Biomarkers: A Review. Molecules 2025, 30, 2708. https://doi.org/10.3390/molecules30132708
Liu M, Song Y, Liu M, Deng D, Zhang W, Wang T, Luo L. The Application of Functional Nanomaterials-Based Electrochemical Biosensors in Detecting Cancer Biomarkers: A Review. Molecules. 2025; 30(13):2708. https://doi.org/10.3390/molecules30132708
Chicago/Turabian StyleLiu, Meiyin, Yuchen Song, Meiru Liu, Dongmei Deng, Wenjiao Zhang, Ting Wang, and Liqiang Luo. 2025. "The Application of Functional Nanomaterials-Based Electrochemical Biosensors in Detecting Cancer Biomarkers: A Review" Molecules 30, no. 13: 2708. https://doi.org/10.3390/molecules30132708
APA StyleLiu, M., Song, Y., Liu, M., Deng, D., Zhang, W., Wang, T., & Luo, L. (2025). The Application of Functional Nanomaterials-Based Electrochemical Biosensors in Detecting Cancer Biomarkers: A Review. Molecules, 30(13), 2708. https://doi.org/10.3390/molecules30132708






