Enhanced Encapsulation of Linalyl Acetate in Cyclodextrin-Based Metal–Organic Frameworks for Improved Stability
Abstract
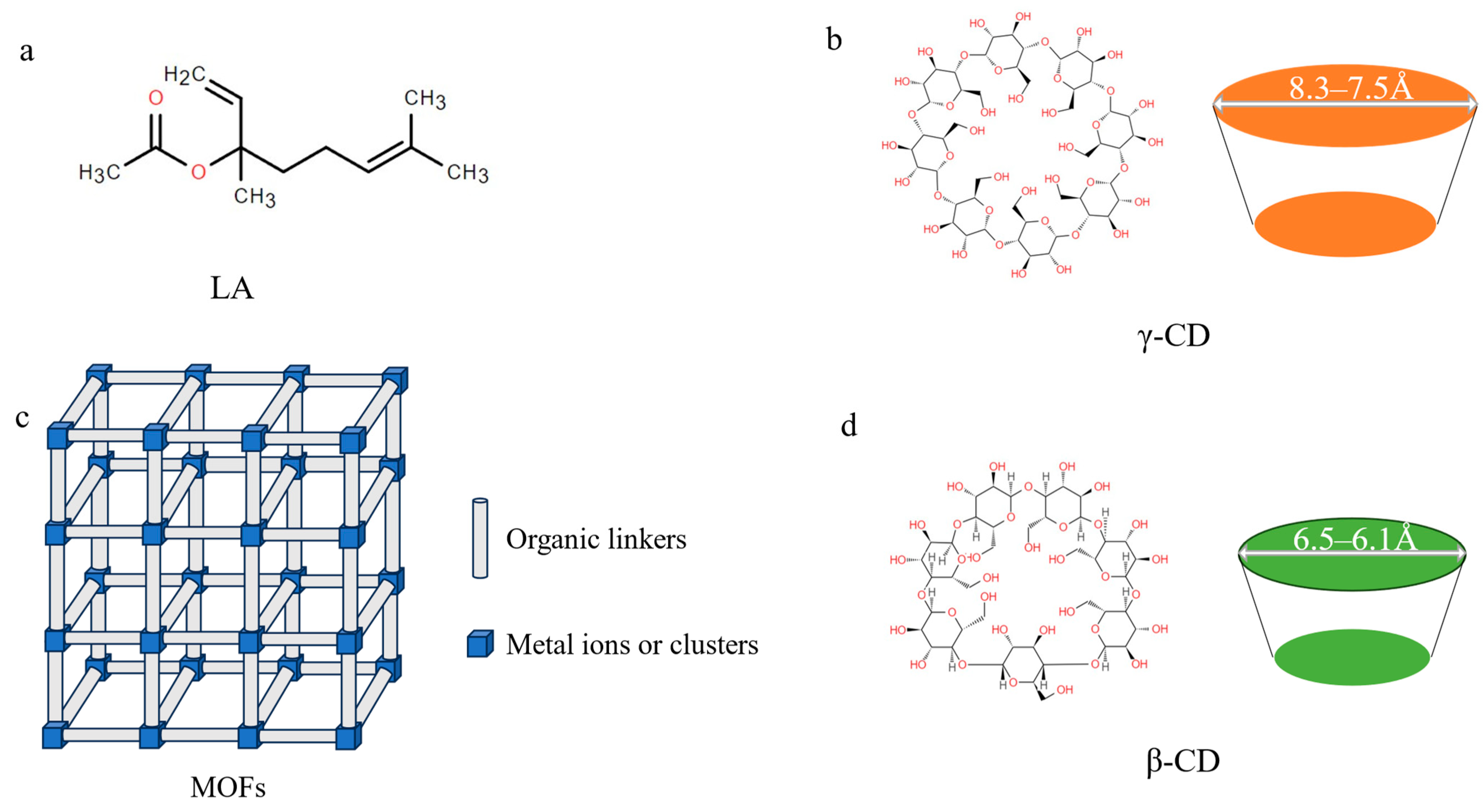
1. Introduction
2. Results and Discussion
2.1. Structural Characterization of CD-MOFs
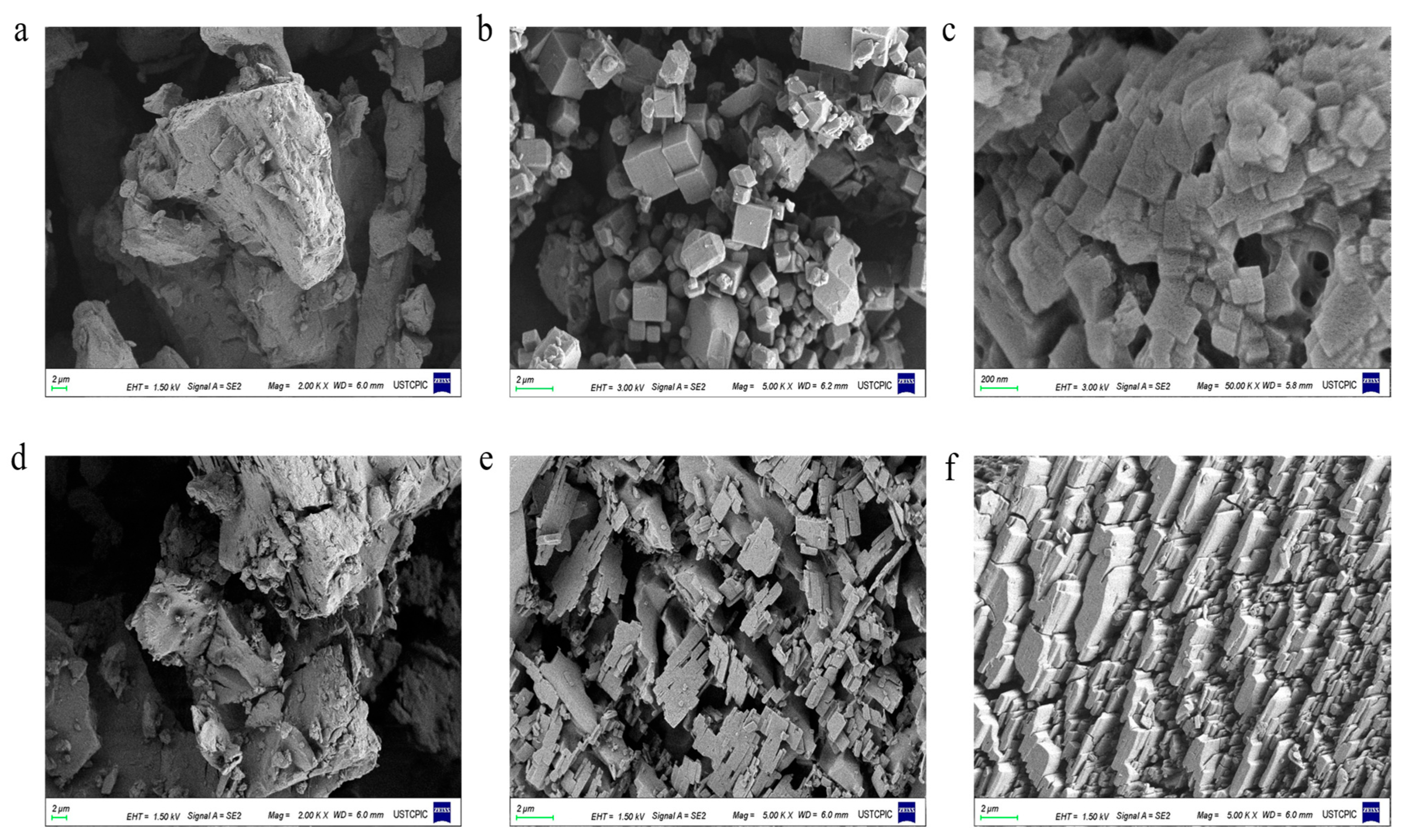
2.1.1. Crystal Morphology
2.1.2. XRD Analysis
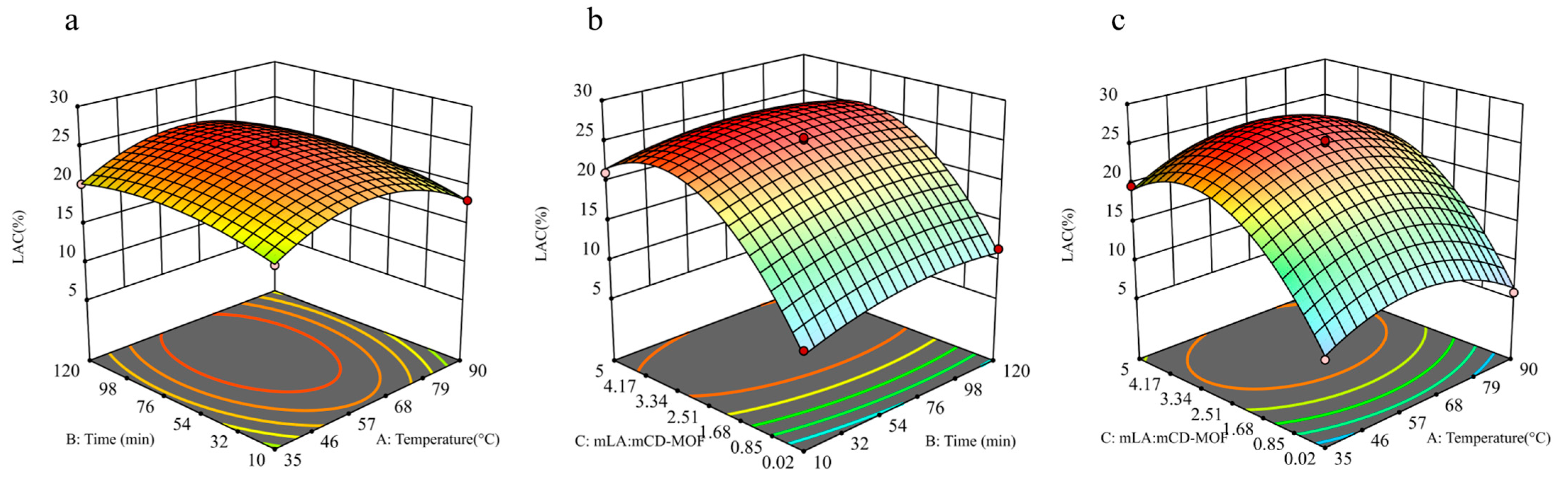
2.2. Optimization of Encapsulation Efficiency
2.3. Characterization of LA-γ-CD-MOF and Intermolecular Interaction
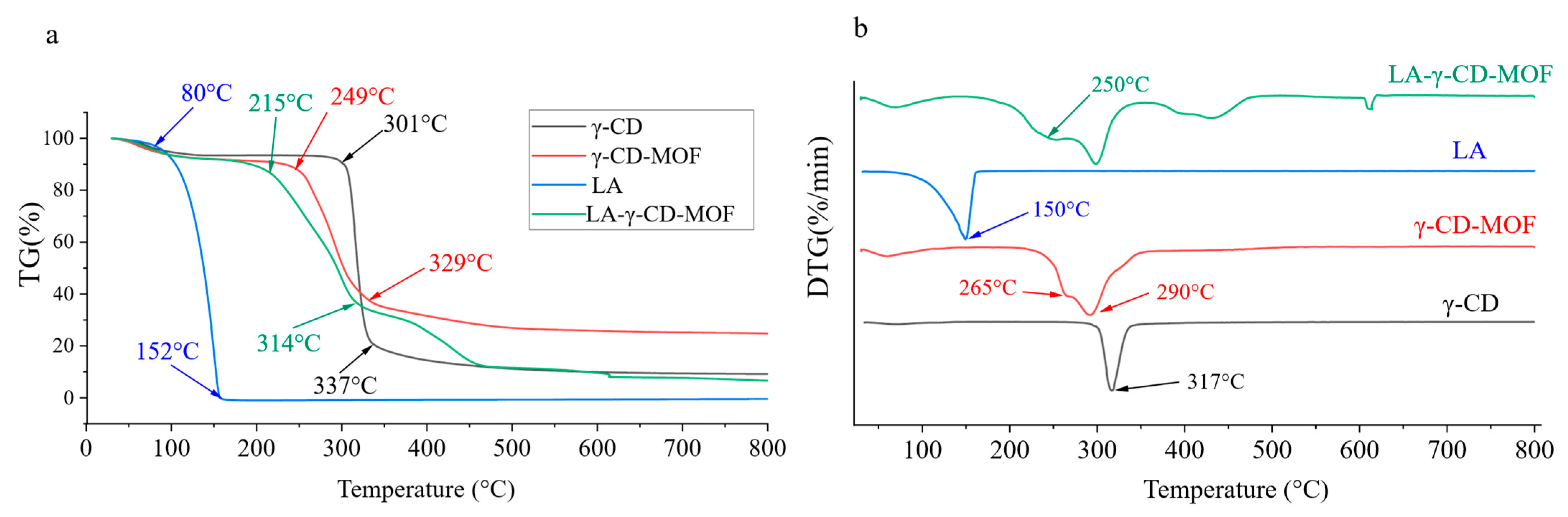
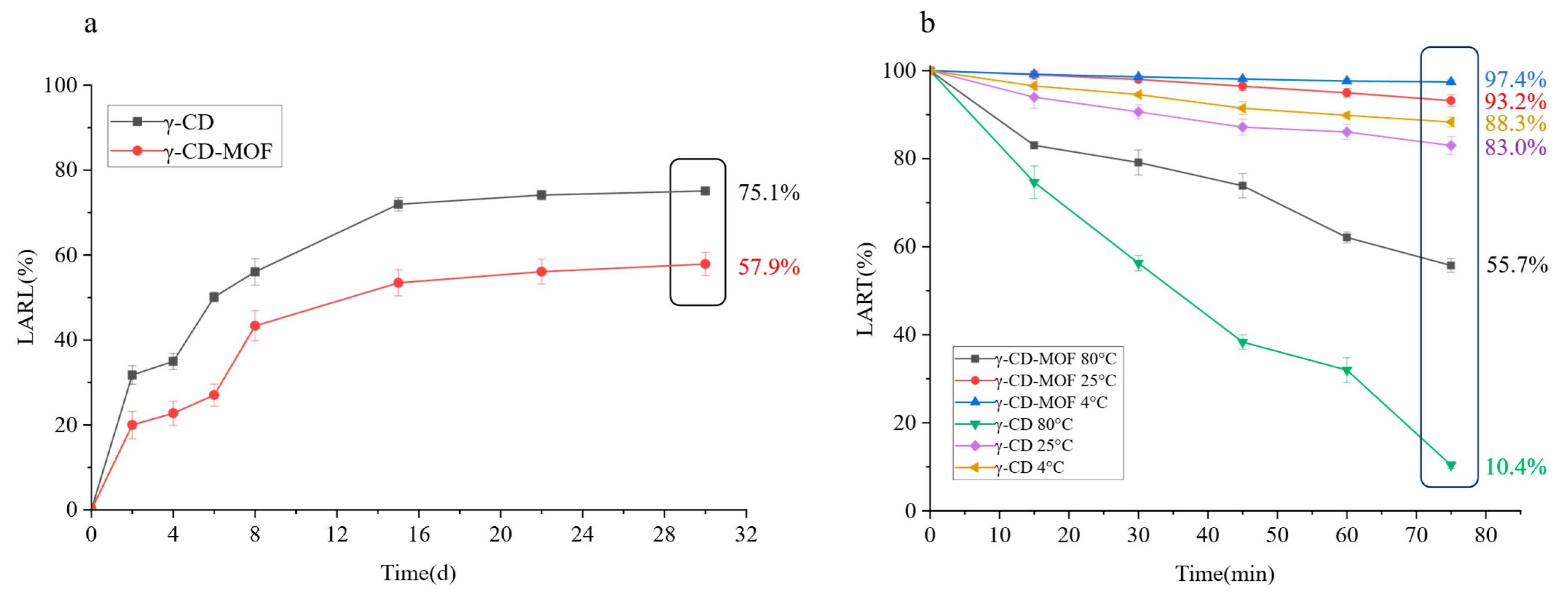
2.4. Thermal Stability Analysis of LA, γ-CD, and Their Inclusion Complex
2.5. Release Behaviors of Combined LA in LA-γ-CD-MOF
3. Materials and Methods
3.1. Materials
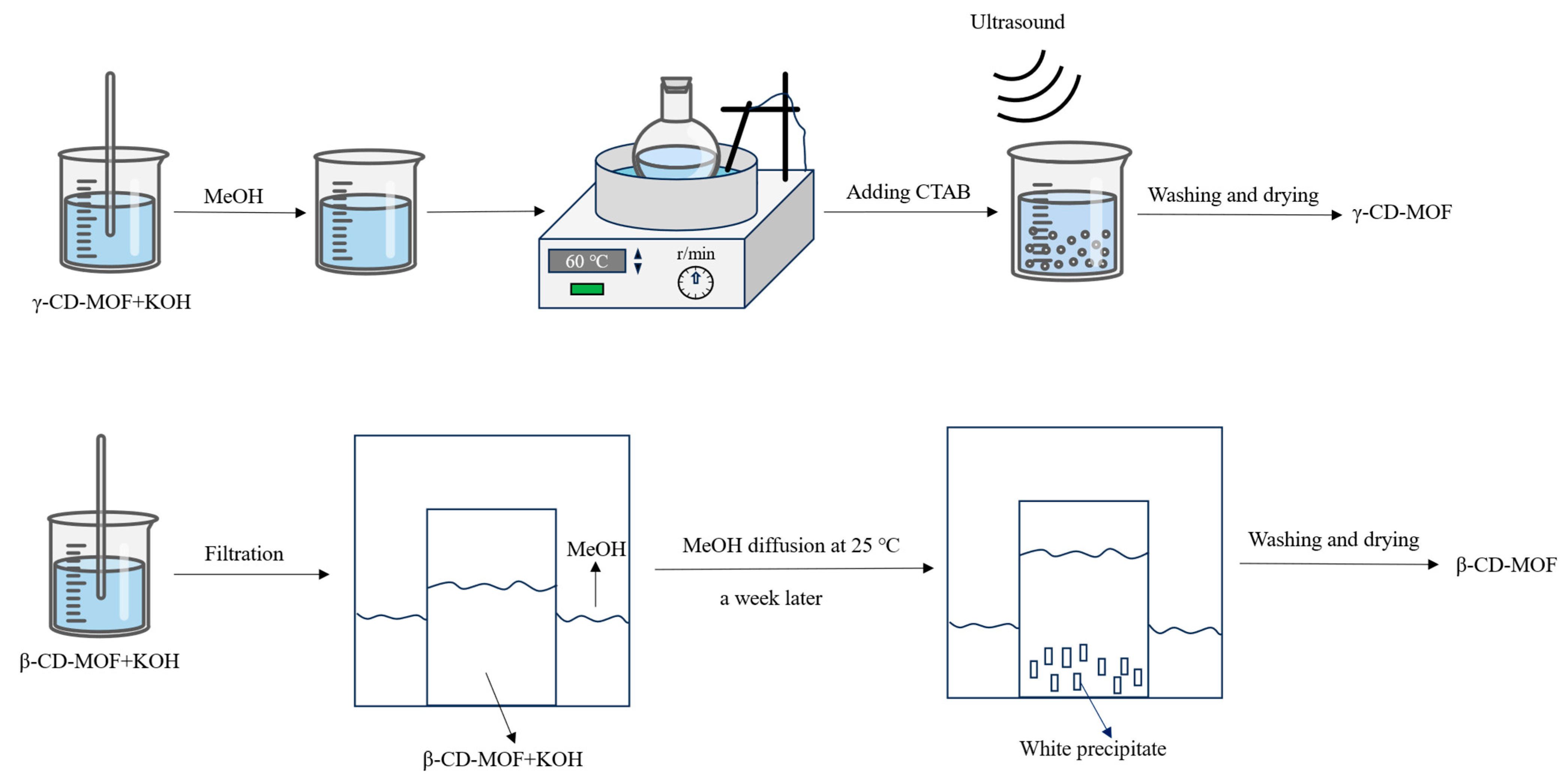
3.2. Preparation of CD-MOFs
3.3. Encapsulation of LA
3.4. Characterization of CD-MOFs and LA-CD-MOFs
3.5. Quantitative Determination of LA Content in Inclusion Complex
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Jin, C.; Wu, Z.; Han, H.; Zhang, Z.; Xie, Y.; Zhang, D. Toxicity and physiological effects of nine lamiaceae essential oils and their major compounds on Reticulitermes dabieshanensis. Molecules 2023, 28, 2007. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mnayer, D.; Ozcelik, B.; Altin, G.; Kasapoglu, K.N.; Daskaya-Dikmen, C.; Sharifi-Rad, M.; Selamoglu, Z.; Acharya, K.; Sen, S.; et al. Plants of the genus Lavandula: From farm to pharmacy. Nat. Prod. Commun. 2018, 13, 1385–1402. [Google Scholar] [CrossRef]
- Alqahtani, A.; Abdelhameed, M.F.; Abdou, R.; Ibrahim, A.M.; Dawoud, M.; Alasmari, S.M.; Raey, M.A.E.; Attia, H.G. Mechanistic action of linalyl acetate: Acyclic monoterpene isolated from bitter orange leaf as anti-inflammatory, analgesic, antipyretic agent: Role of TNF-α, IL1β, PGE2, and COX-2. Ind. Crop. Prod. 2023, 203, 117131. [Google Scholar] [CrossRef]
- Bozova, B.; Golukcu, M.; Giuffre, A.M. The effect of different hydrodistillation times on the composition and yield of bergamot (Citrus bergamia Risso) peel essential oil and a comparison of the cold-pressing method. Flavour Frag. J. 2024, 39, 263–270. [Google Scholar] [CrossRef]
- Aihaiti, A.; Zhao, L.; Maimaitiyiming, R.; Wang, L.; Liu, R.; Mu, Y.; Chen, K.; Wang, Y. Changes in volatile flavors during the fermentation of tomato (Solanum lycopersicum L.) Juice and its storage stabilization. Food Chem. 2025, 463, 141077. [Google Scholar] [CrossRef]
- Zhu, T.; Han, Y.; Liu, S.; Yuan, B.; Liu, Y.; Ma, H. Porous materials confining single atoms for catalysis. Front. Chem. 2021, 9, 717201. [Google Scholar] [CrossRef]
- Guo, Z.; Xiao, Y.; Wu, W.; Zhe, M.; Yu, P.; Shakya, S.; Li, Z.; Xing, F. Metal-organic framework-based smart stimuli-responsive drug delivery systems for cancer therapy: Advances, challenges, and future perspectives. J. Nanobiotechnol. 2025, 23, 157. [Google Scholar] [CrossRef]
- Hou, K.; Gu, H.; Yang, Y.; Lam, S.S.; Li, H.; Sonne, C.; Ouyang, H.; Chen, X. Recent progress in advanced covalent organic framework composites for environmental remediation. Adv. Compos. Hybrid Mater. 2023, 6, 199. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, G.; Duan, G.; Wu, Y.; Zhao, X.; Gong, X. Structural design of carbon dots/porous materials composites and their applications. Chem. Eng. J. 2021, 421, 127743. [Google Scholar] [CrossRef]
- Gandhi, R.; Chopade, N.; Deshmukh, P.K.; Ingle, R.G.; Harde, M.; Lakade, S.; More, M.P.; Tade, R.S.; Bhadane, M.S. Unveiling cyclodextrin conjugation as multidentate excipients: An exploratory journey across industries. Carbohydr. Res. 2025, 549, 109357. [Google Scholar] [CrossRef]
- Skuredina, A.A.; Le-Deygen, I.M.; Belogurova, N.G.; Kudryashova, E. Effect of cross-linking on the inclusion complex formation of derivatized β-cyclodextrins with small-molecule drug moxifloxacin. Carbohydr. Res. 2020, 498, 108183. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Yamaguchi, M.S.; Christian-Tabak, L.; Takai, Y.; Tode, C. Quantifying the bitter masking effect of drug-cyclodextrin complexation: Nmr-roesy mixing time approach. Carbohydr. Res. 2024, 537, 109067. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chu, Y.; Gu, L.; Zhou, M.; Ding, C.F. The non-covalent complexes of alpha- or gamma-cyclodextrin with divalent metal cations determined by mass spectrometry. Carbohydr. Res. 2020, 492, 107987. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, T.; Kukkar, D.; Kim, K.; Sohn, J.R.; Deep, A. Cyclodextrin-metal-organic framework (CD-MOF): From synthesis to for applications. J. Ind. Eng. Chem. 2019, 72, 50–66. [Google Scholar] [CrossRef]
- Pan, X. Cyclodextrin MOF Material Loading Thymol and Its Application in Cherry Tomato Preservation. Master’s Thesis, South China University of Technology, Guangzhou, China, 2022. [Google Scholar] [CrossRef]
- Hu, Z.; Li, S.; Wang, S.; Zhang, B.; Huang, Q. Encapsulation of menthol into cyclodextrin metal-organic frameworks: Preparation, structure characterization and evaluation of complexing capacity. Food Chem. 2021, 338, 127839. [Google Scholar] [CrossRef]
- Zhu, H.; Lv, Y.; Xin, F.; Wang, M.; Zhao, X.; Ren, X.; Zhang, J.; Yin, D.; Guo, T.; Wu, L. Enhanced stability and solidification of volatile eugenol by cyclodextrin-metal organic framework for nasal powder delivery. AAPS PharmSciTech 2024, 25, 117. [Google Scholar] [CrossRef]
- Dummert, S.V.; Saini, H.; Hussain, M.Z.; Yadava, K.; Jayaramulu, K.; Casini, A.; Fischer, R.A. Cyclodextrin metal-organic frameworks and derivatives: Recent developments and applications. Chem. Soc. Rev. 2022, 51, 5175–5213. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, F.; Du, M.; Xie, C.; Xie, X.; Zhang, H.; Meng, X.; Li, A.; Deng, T. Encapsulation of catechin into nano-cyclodextrin-metal-organic frameworks: Preparation, characterization, and evaluation of storage stability and bioavailability. Food Chem. 2022, 394, 133553. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, S.; Chen, J. Preparation and sustained-release study of litsea cubeba essential oil inclusion complex with γ-cyclodextrin-metal-organic frameworks. Chem. Biol. Technol. Agric. 2023, 10, 104. [Google Scholar] [CrossRef]
- Han, Y.; Liu, W.; Huang, J.; Qiu, S.; Zhong, H.; Liu, D.; Liu, J. Cyclodextrin-based metal-organic frameworks (CD-MOFs) in pharmaceutics and biomedicine. Pharmaceutics 2018, 10, 271. [Google Scholar] [CrossRef]
- Nadar, S.S.; Vaidya, L.; Maurya, S.; Rathod, V.K. Polysaccharide based metal organic frameworks (polysaccharide-mof): A review. Coord. Chem. Rev. 2019, 396, 1–21. [Google Scholar] [CrossRef]
- Yu, W.; Wang, G.; Chen, H.; Mu, H.; Niu, B.; Han, Y.; Wang, L.; Chen, H.; Gao, H. Sustained antimicrobial polymer film from γ-CD-MOF humidity switch for fruit and vegetable preservation. Food Chem. 2025, 479, 143856. [Google Scholar] [CrossRef] [PubMed]
- Nong, W.; Luo, H.; Wang, G.; Chen, Q.; Zou, X.; Miao, W.; Wu, J.; Guan, W.; Qu, S. β-CD-MOF-based edible antimicrobial packaging film with humidity-controlled carvacrol release for preserving fresh strawberry. Carbohydr. Polym. 2025, 351, 123133. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Luo, H.; Zhang, P.; Zhang, C.; Li, J.; Jiang, P.; Yuan, W.; Cha, R. CD-MOFs: From preparation to drug delivery and therapeutic application. Carbohydr. Polym. 2024, 323, 121424. [Google Scholar] [CrossRef]
- Chen, Y.; Su, J.; Dong, W.; Xu, D.; Cheng, L.; Mao, L.; Gao, Y.; Yuan, F. Cyclodextrin-based metal-organic framework nanoparticles as superior carriers for curcumin: Study of encapsulation mechanism, solubility, release kinetics, and antioxidative stability. Food Chem. 2022, 383, 132605. [Google Scholar] [CrossRef]
- Qiu, C.; Mcclements, D.J.; Jin, Z.; Qin, Y.; Hu, Y.; Xu, X.; Wang, J. Resveratrol-loaded core-shell nanostructured delivery systems: Cyclodextrin-based metal-organic nanocapsules prepared by ionic gelation. Food Chem. 2020, 317, 126328. [Google Scholar] [CrossRef]
- Ates, K.; Yildiz, Z.I. Encapsulation of carvacrol in (3-cyclodextrin metal-organic frameworks: Improved solubility, stability, antioxidant capacity and controlled release of carvacrol. J. Food Eng. 2025, 391, 112445. [Google Scholar] [CrossRef]
- Lv, N.; Guo, T.; Liu, B.; Wang, C.; Singh, V.; Xu, X.; Li, X.; Chen, D.; Gref, R.; Zhang, J. Improvement in thermal stability of sucralose by γ-cyclodextrin metal-organic frameworks. Pharm. Res. 2017, 34, 269–278. [Google Scholar] [CrossRef]
- Hu, Z.; Shao, M.; Zhang, B.; Fu, X.; Huang, Q. Enhanced stability and controlled release of menthol using a β-cyclodextrin metal-organic framework. Food Chem. 2022, 374, 131760. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, H.; Meng, X.; Liu, D.; Liu, Y.; Chen, B.; Zou, Z. γ-cyclodextrin metal-organic frameworks as the promising carrier for pulmonary delivery of cyclosporine A. Biomed. Pharmacother. 2024, 171, 116174. [Google Scholar] [CrossRef]
- Sadeh, P.; Zeinali, S.; Rastegari, B.; Najafipour, I. Functionalization of β-cyclodextrin metal-organic frameworks with gelatin and glutamine for drug delivery of curcumin to cancerous cells. Heliyon 2024, 10, e30349. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lv, N.; Li, X.; Liu, B.; Feng, J.; Ren, X.; Guo, T.; Chen, D.; Stoddart, J.F.; Gref, R.; et al. Composite CD-MOF nanocrystals-containing microspheres for sustained drug delivery. Nanoscale 2017, 9, 7454–7463. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, T.; Zhang, K.; Chen, J.; Wang, C.; Ren, X.; Wang, Q.; Yang, Y.; Liu, C.; Tan, W.; et al. Simultaneous improvement to solubility and bioavailability of active natural compound isosteviol using cyclodextrin metal-organic frameworks. Acta Pharm. Sin. B 2021, 11, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Ye, K.; Li, H.; Wang, C.; Wei, H.; Dang, L. Novel γ-cyclodextrin-based metal-organic frameworks for the effective encapsulation of oregano essential oil and controlled release. New J. Chem. 2023, 47, 10322–10332. [Google Scholar] [CrossRef]
- Oh, J.X.; Murray, B.S.; Mackie, A.R.; Ettelaie, R.; Sadeghpour, A.; Frison, R. γ-cyclodextrin metal-organic frameworks: Do solvents make a difference? Molecules 2023, 28, 6876. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Zhang, Z.; Zhu, H.; Ma, W.; Zhao, X.; Wang, M.; Wang, C.; Chen, W.; Naeem, A.; et al. Solvent-free loading of vitamin a palmitate into β-cyclodextrin metal-organic frameworks for stability enhancement. AAPS PharmSciTech 2023, 24, 136. [Google Scholar] [CrossRef]
- Mihaylova, D.; Gandova, V.; Deseva, I.; Tschuikowa, S.; Schalow, S.; Westphal, G. Arrhenius equation modeling for the oxidative stability evaluation of echium oil enriched with a natural preservative. Eur. J. Lipid Sci. Technol. 2020, 122, 2000118. [Google Scholar] [CrossRef]
- Yan, J.; Jia, B.; Liu, B.; Zhang, J. Molecular simulation of coal molecular diffusion properties in chicheng coal mine. Molecules 2023, 28, 6933. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, T.; Li, Y.; Chen, L.; Xu, Y.; Chi, X.; Yu, S.; Wang, W.; Liu, D.; Zhu, B.; et al. Biocompatible hydrophobic cross-linked cyclodextrin-based metal-organic framework as quercetin nanocarrier for enhancing stability and controlled release. Food Chem. 2024, 448, 139167. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Tan, J.; Li, R.; Jiang, Z.; Tang, S. Enhancement of the stabilities and intracellular antioxidant activities of lavender essential oil by metal-organic frameworks based on β-cyclodextrin and potassium cation. Pol. J. Food Nutr. Sci. 2021, 71, 39–50. [Google Scholar] [CrossRef]
- Shen, M. Preparation and Antimicrobial Application of Cyclodextrin Metal-Organic Framework Composites. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2022. [Google Scholar] [CrossRef]
- Ades, H.; Kesselman, E.; Ungar, Y.; Shimoni, E. Complexation with starch for encapsulation and controlled release of menthone and menthol. LWT-Food Sci. Technol. 2012, 45, 277–288. [Google Scholar] [CrossRef]
- Arya, P.; Raghav, N. In-vitro studies of curcumin-β-cyclodextrin inclusion complex as sustained release system. J. Mol. Struct. 2021, 1228, 129774. [Google Scholar] [CrossRef]


| Run | A Temperature/°C | B Time/min | C mLA:mCD-MOF | Y LAC/% |
|---|---|---|---|---|
| 1 | 35 | 65 | 5 | 20.01 |
| 2 | 35 | 10 | 2.51 | 19.42 |
| 3 | 35 | 120 | 2.51 | 20.23 |
| 4 | 62.5 | 65 | 2.51 | 24.96 |
| 5 | 62.5 | 10 | 0.02 | 8.71 |
| 6 | 62.5 | 65 | 2.51 | 25.21 |
| 7 | 90 | 65 | 0.02 | 10.97 |
| 8 | 90 | 65 | 5 | 20.36 |
| 9 | 35 | 65 | 0.02 | 7.47 |
| 10 | 62.5 | 65 | 2.51 | 24.61 |
| 11 | 62.5 | 65 | 2.51 | 25.48 |
| 12 | 90 | 10 | 2.51 | 19.89 |
| 13 | 62.5 | 120 | 0.02 | 11.21 |
| 14 | 62.5 | 10 | 5 | 21.10 |
| 15 | 90 | 120 | 2.51 | 20.38 |
| 16 | 62.5 | 120 | 5 | 21.83 |
| 17 | 62.5 | 65 | 2.51 | 25.43 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 675.93 | 9 | 75.10 | 198.00 | <0.0001 | significant |
| A-Temperature | 0.2888 | 1 | 0.2888 | 0.7614 | 0.4118 | |
| B-Time | 6.48 | 1 | 6.48 | 17.08 | 0.0044 | |
| C-mLA:mCD-MOF | 307.77 | 1 | 307.77 | 811.39 | <0.0001 | |
| AB | 0.1406 | 1 | 0.1406 | 0.3707 | 0.5618 | |
| AC | 1.40 | 1 | 1.40 | 3.70 | 0.0958 | |
| BC | 1.01 | 1 | 1.01 | 2.66 | 0.1467 | |
| A2 | 71.05 | 1 | 71.05 | 187.30 | <0.0001 | |
| B2 | 11.57 | 1 | 11.57 | 30.51 | 0.0009 | |
| C2 | 250.14 | 1 | 250.14 | 659.47 | <0.0001 | |
| Residual | 2.66 | 7 | 0.3793 | |||
| Lack of Fit | 2.14 | 3 | 0.7124 | 5.50 | 0.0665 | not significant |
| Pure Error | 0.5179 | 4 | 0.1295 | |||
| Cor Total | 678.58 | 16 | ||||
| R2 | 0.9961 | |||||
| Adjusted R2 | 0.9911 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, L.; Zhao, M.; Shao, N.; Song, S.; Zhu, X. Enhanced Encapsulation of Linalyl Acetate in Cyclodextrin-Based Metal–Organic Frameworks for Improved Stability. Molecules 2025, 30, 2698. https://doi.org/10.3390/molecules30132698
Zhang C, Zhang L, Zhao M, Shao N, Song S, Zhu X. Enhanced Encapsulation of Linalyl Acetate in Cyclodextrin-Based Metal–Organic Frameworks for Improved Stability. Molecules. 2025; 30(13):2698. https://doi.org/10.3390/molecules30132698
Chicago/Turabian StyleZhang, Cheng, Lirong Zhang, Meiting Zhao, Ning Shao, Shuo Song, and Xiaolan Zhu. 2025. "Enhanced Encapsulation of Linalyl Acetate in Cyclodextrin-Based Metal–Organic Frameworks for Improved Stability" Molecules 30, no. 13: 2698. https://doi.org/10.3390/molecules30132698
APA StyleZhang, C., Zhang, L., Zhao, M., Shao, N., Song, S., & Zhu, X. (2025). Enhanced Encapsulation of Linalyl Acetate in Cyclodextrin-Based Metal–Organic Frameworks for Improved Stability. Molecules, 30(13), 2698. https://doi.org/10.3390/molecules30132698






