Assisted Extraction of Hemp Oil and Its Application to Design Functional Gluten-Free Bakery Foods
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction
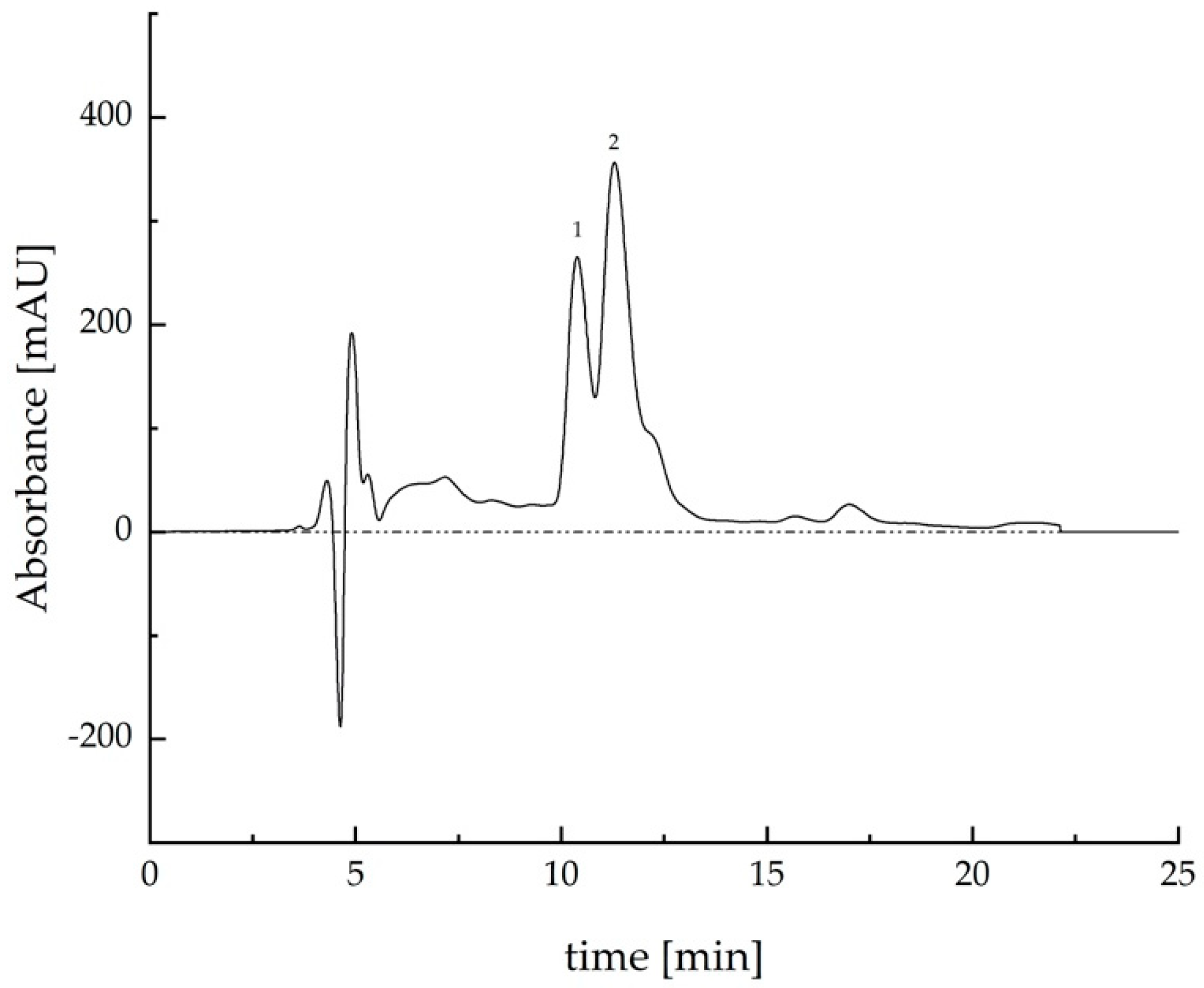
2.2. Extracts Analysis by HPLC
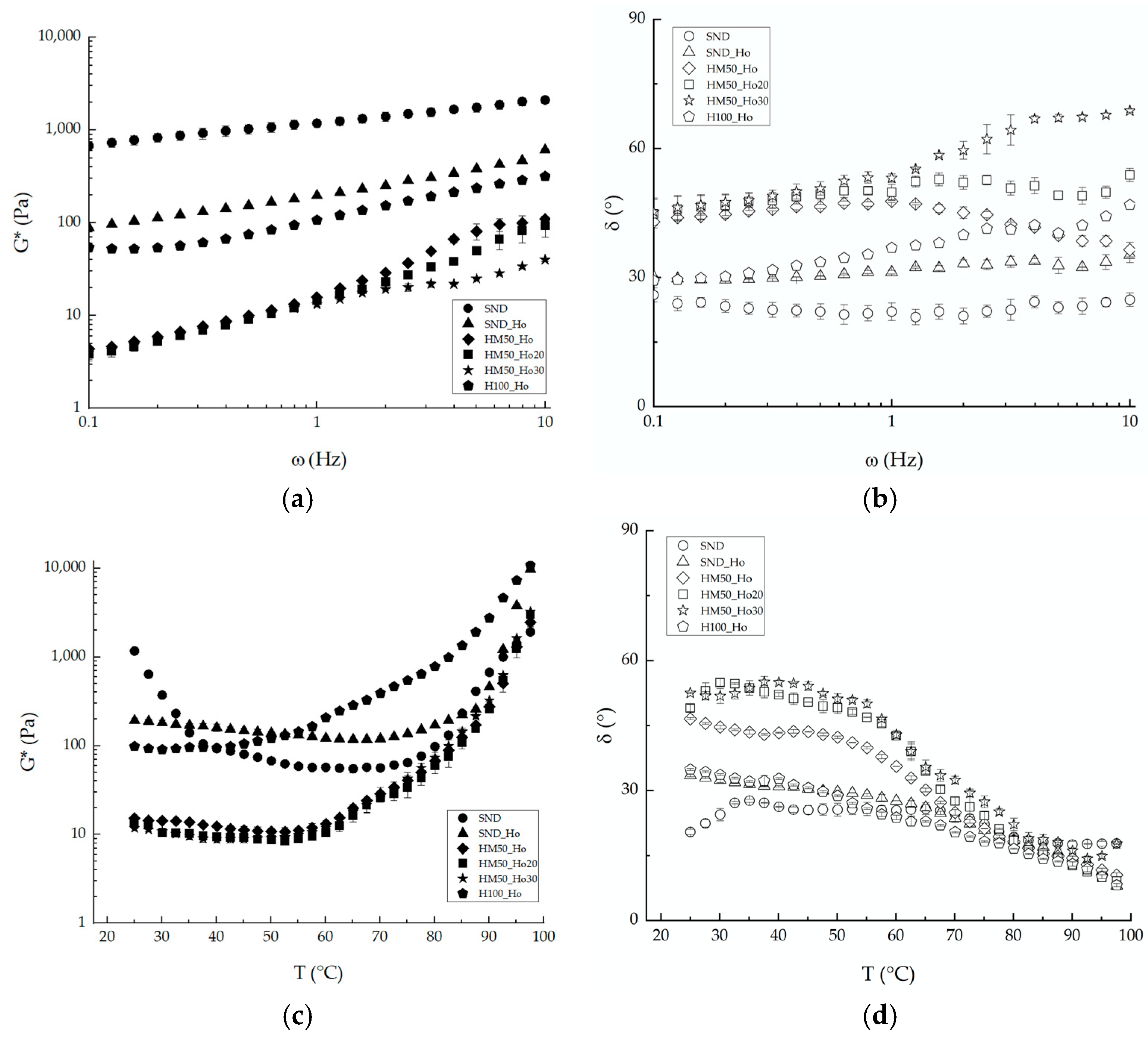
2.3. Rheological Results
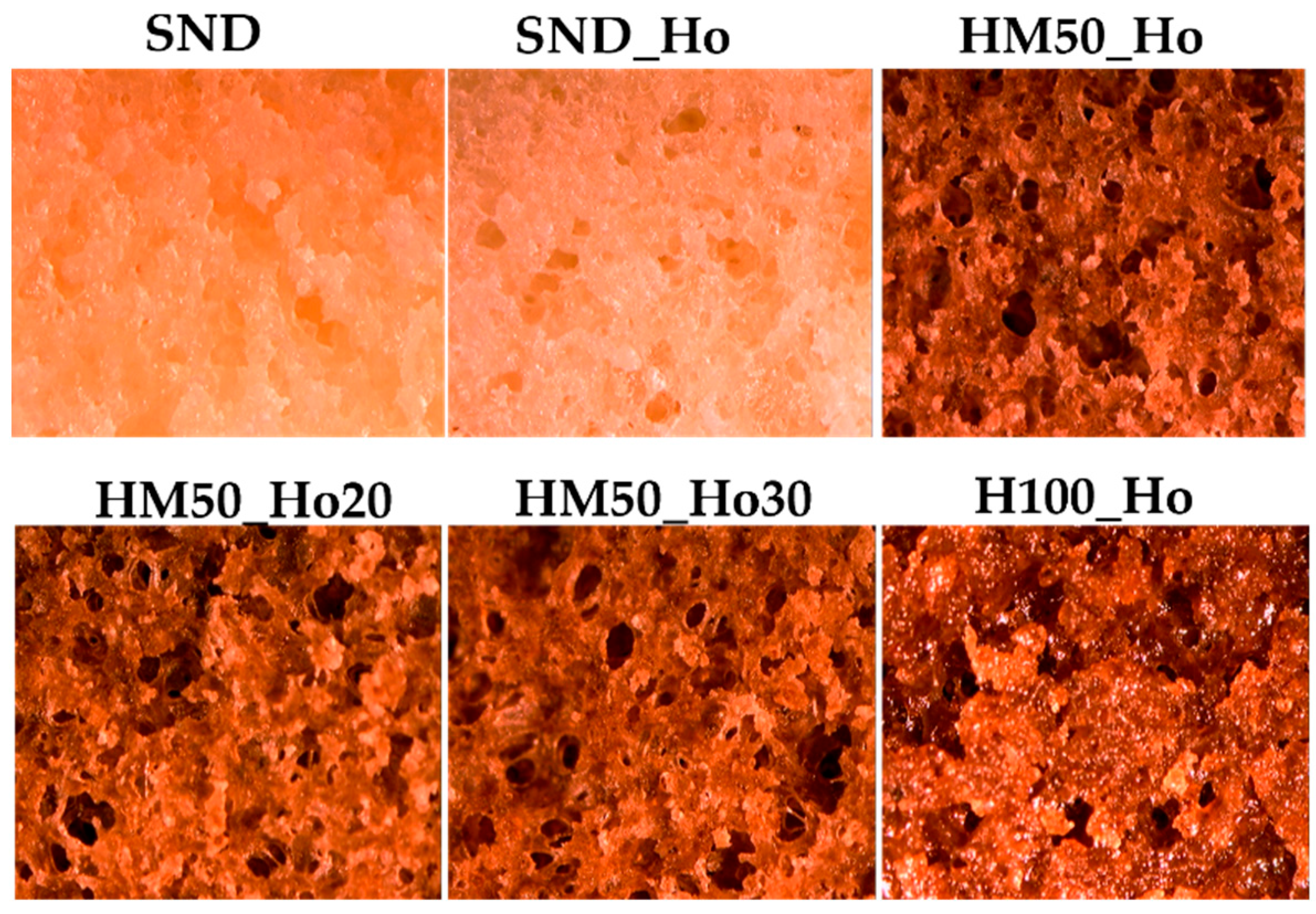
2.4. Baking Results
3. Materials and Methods
3.1. Materials
3.2. Extraction and Extracts Characterization
3.2.1. Extraction
3.2.2. HPLC Analysis
3.3. Cupcake Preparation
3.4. Rheological Dough Measurements
3.5. Cupcake Characterization
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Valizadehderakhshan, M.; Shahbazi, A.; Kazem-Rostami, M.; Todd, M.S.; Bhowmik, A.; Wang, L. Extraction of Cannabinoids from Cannabis sativa L. (Hemp)-Review. Agriculture 2021, 11, 384. [Google Scholar] [CrossRef]
- Yano, H.; Fu, W. Hemp: A Sustainable Plant with High Industrial Value in Food Processing. Foods 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Enarevba, D.R.; Haapala, K.R. The Emerging Hemp Industry: A Review of Industrial Hemp Materials and Product Manufacturing. AgriEngineering 2024, 6, 2891–2925. [Google Scholar] [CrossRef]
- Baldino, N.; Carnevale, I.; Mileti, O.; Aiello, D.; Lupi, F.R.; Napoli, A.; Gabriele, D. Hemp Seed Oil Extraction and Stable Emulsion Formulation with Hemp Protein Isolates. Appl. Sci. 2022, 12, 11921. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, W.; Qin, X.; Hou, C.; Yang, X. Preparation, Modification, Food Application, and Health Effects of Protein and Peptide from Hemp (Cannabis sativa L.) Seed: A Review of the Recent Literature. Foods 2025, 14, 1149. [Google Scholar] [CrossRef] [PubMed]
- Mileti, O.; Baldino, N.; Paleologo, M.F.O.; Lupi, F.R.; Marra, M.; Iacopetta, D.; Gabriele, D. Oil Extraction from Hemp Plant as a Potential Source of Cannabidiol for Healthy Protein Foods. Antioxidants 2023, 12, 1950. [Google Scholar] [CrossRef] [PubMed]
- Gigopulu, O.; Geskovski, N.; Stefkov, G.; Poceva Panovska, A.; Piponski, M.; Slaveska Spirevska, I.; Makreski, P. Real-Time Monitoring of CBDA Decarboxylation in Solid State and Cannabis Flowers Using Mid Infrared Spectroscopy Coupled with Multivariate Analysis. Vib. Spectrosc. 2024, 134, 103728. [Google Scholar] [CrossRef]
- Casiraghi, A.; Gentile, A.; Selmin, F.; Gennari, C.G.M.; Casagni, E.; Roda, G.; Pallotti, G.; Rovellini, P.; Minghetti, P. Ultrasound-Assisted Extraction of Cannabinoids from Cannabis Sativa for Medicinal Purpose. Pharmaceutics 2022, 14, 2718. [Google Scholar] [CrossRef]
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a New Extraction Technique and HPLC Method for the Analysis of Non-Psychoactive Cannabinoids in Fibre-Type Cannabis sativa L. (Hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef]
- Formato, M.; Crescente, G.; Scognamiglio, M.; Fiorentino, A.; Pecoraro, M.T.; Piccolella, S.; Catauro, M.; Pacifico, S. (–)-Cannabidiolic Acid, a Still Overlooked Bioactive Compound: An Introductory Review and Preliminary Research. Molecules 2020, 25, 2638. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A.; Decorti, D. Effect of Ultrasound Pre-Treatment of Hemp (Cannabis sativa L.) Seed on Supercritical CO2 Extraction of Oil. J. Food Sci. Technol. 2015, 52, 1748–1753. [Google Scholar] [CrossRef]
- Baldino, N.; Carnevale, I.; Laitano, F.; Lupi, F.; Curcio, S.; Gabriele, D. Formulation of Bread Model Doughs with Resistant Starch, Vegetable Proteins and Transglutaminase. Eur. Food Res. Technol. 2020, 246, 397–408. [Google Scholar] [CrossRef]
- Haji-Moradkhani, A.; Rezaei, R.; Moghimi, M. Optimization of Pulsed Electric Field-Assisted Oil Extraction from Cannabis Seeds. J. Food Process. Eng. 2019, 42, e13028. [Google Scholar] [CrossRef]
- Li, X.; Xiao, R.; Morrell, J.J.; Zhou, X.; Du, G. Improving the Performance of Hemp Hurd/Polypropylene Composites Using Pectinase Pre-Treatments. Ind. Crop. Prod. 2017, 97, 465–468. [Google Scholar] [CrossRef]
- Cravotto, C.; Grillo, G.; Boffa, L.; Fabiano-Tixier, A.-S.; Bartier, M.; Jacques, L.; Tabasso, S. Microwave-Assisted Extraction of Phytochemicals from Cannabis sativa L. Inflorescences with 2-Methyloxolane. Sustain. Chem. Pharm. 2024, 42, 101812. [Google Scholar] [CrossRef]
- Al Ubeed, H.M.S.; Bhuyan, D.J.; Alsherbiny, M.A.; Basu, A.; Vuong, Q.V. A Comprehensive Review on the Techniques for Extraction of Bioactive Compounds from Medicinal Cannabis. Molecules 2022, 27, 604. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cao, X.; Mi, Y.; Sun, W.; Meng, X.; Chen, W.; Xie, X.; Wang, S.; Li, J.; Yang, W.; et al. Genome-Wide Analysis of WRKY Gene Family in High-CBD Hemp (Cannabis sativa L.) and Identification of the WRKY Genes Involved in Abiotic Stress Responses and Regulation Cannabinoid Accumulation. Ind. Crop. Prod. 2024, 210, 118158. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. Hemp (Cannabis sativa subsp. Sativa) Flour and Protein Preparation as Natural Nutrients and Structure Forming Agents in Starch Based Gluten-Free Bread. LWT 2017, 84, 143–150. [Google Scholar] [CrossRef]
- Hong, T.; Xu, D.; Jin, Y.; Wu, F.; Huang, G.; Zhong, X.; Zhang, J.; Xu, X. Gluten Protein Transformations during Bread Processing: Molecular and Microstructural Analysis. LWT 2025, 217, 117341. [Google Scholar] [CrossRef]
- Wiedemair, V.; Gruber, K.; Knöpfle, N.; Bach, K.E. Technological Changes in Wheat-Based Breads Enriched with Hemp Seed Press Cakes and Hemp Seed Grit. Molecules 2022, 27, 1840. [Google Scholar] [CrossRef]
- Mohammadi, A.; Saberi, F.; Rostami, O.; Kamali Rousta, L.; Hashemi, H. Impact of Fat Profile on the Rheological and Structural Characteristics of Cup Cake. LWT 2024, 211, 116922. [Google Scholar] [CrossRef]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; de Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Addo, P.W.; Sagili, S.U.K.R.; Bilodeau, S.E.; Gladu-Gallant, F.A.; MacKenzie, D.A.; Bates, J.; McRae, G.; MacPherson, S.; Paris, M.; Raghavan, V.; et al. Cold Ethanol Extraction of Cannabinoids and Terpenes from Cannabis Using Response Surface Methodology: Optimization and Comparative Study. Molecules 2022, 27, 8780. [Google Scholar] [CrossRef] [PubMed]
- Bagherian, H.; Zokaee Ashtiani, F.; Fouladitajar, A.; Mohtashamy, M. Comparisons between Conventional, Microwave- and Ultrasound-Assisted Methods for Extraction of Pectin from Grapefruit. Chem. Eng. Process. Process Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Christaki, M.; Verboven, P.; Van Dyck, T.; Nicolaï, B.; Goos, P.; Claes, J. The Predictive Power of Batter Rheological Properties on Cake Quality—The Effect of Pregelatinized Flour, Leavening Acid Type and Mixing Time. J. Cereal Sci. 2017, 77, 219–227. [Google Scholar] [CrossRef]
- Baixauli, R.; Sanz, T.; Salvador, A.; Fiszman, S.M. Muffins with Resistant Starch: Baking Performance in Relation to the Rheological Properties of the Batter. J. Cereal Sci. 2008, 47, 502–509. [Google Scholar] [CrossRef]
- Guiné, R.P.F. Textural Properties of Bakery Products: A Review of Instrumental and Sensory Evaluation Studies. Appl. Sci. 2022, 12, 8628. [Google Scholar] [CrossRef]
- Correia, P.; Guiné, R.; Fonseca, M.; Batista, L. Analysis of Textural Properties of Gluten Free Breads. J. Hyg. Eng. Des. 2021, 34, 108. [Google Scholar]
- Pojić, M.; Dapčević Hadnađev, T.; Hadnađev, M.; Rakita, S.; Brlek, T. Bread Supplementation with Hemp Seed Cake: A By-Product of Hemp Oil Processing. J. Food Qual. 2015, 38, 431–440. [Google Scholar] [CrossRef]
- Mikulec, A.; Kowalski, S.; Sabat, R.; Skoczylas, Ł.; Tabaszewska, M.; Wywrocka-Gurgul, A. Hemp Flour as a Valuable Component for Enriching Physicochemical and Antioxidant Properties of Wheat Bread. LWT 2019, 102, 164–172. [Google Scholar] [CrossRef]
- Ryu, B.R.; Islam, M.J.; Kalam Azad, M.O.; Go, E.J.; Rahman, M.H.; Rana, M.S.; Lim, Y.S.; Lim, J.D. Conversion Characteristics of Some Major Cannabinoids from Hemp (Cannabis sativa L.) Raw Materials by New Rapid Simultaneous Analysis Method. Molecules 2021, 26, 4113. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, D.; de Cindio, B.; D’Antona, P. A Weak Gel Model for Foods. Rheol. Acta 2001, 40, 120–127. [Google Scholar] [CrossRef]
- Hayit, F.; Esra, B.; Levent, Y.; and Bescanlar, S. Investigation of the Usability of Hemp Flour in the Production of Gluten-Free Cakes. J. Culin. Sci. Technol. 2024, 1–17. [Google Scholar] [CrossRef]
- Marzec, A.; Kowalska, J.; Domian, E.; Galus, S.; Ciurzyńska, A.; Kowalska, H. Characteristics of Dough Rheology and the Structural, Mechanical, and Sensory Properties of Sponge Cakes with Sweeteners. Molecules 2021, 26, 6638. [Google Scholar] [CrossRef]
- Campbell, L.; Euston, S.R.; Ahmed, M.A. Effect of Addition of Thermally Modified Cowpea Protein on Sensory Acceptability and Textural Properties of Wheat Bread and Sponge Cake. Food Chem. 2016, 194, 1230–1237. [Google Scholar] [CrossRef]


| Time of Sonication (min) | HI | DHI |
|---|---|---|
| 5 | 171 ± 6 | 122 ± 1 |
| 10 | 262 ± 6 | 244 ± 5 |
| 15 | 276 ± 2 | 261 ± 2 |
| Power, (W) | HI, 30 s | DHI, 30 s | HI, 60 s | DHI, 60 s |
|---|---|---|---|---|
| 120 | 188 ± 13 | 142 ± 11 | 202 ± 7 | 152 ± 11 |
| 540 | 263 ± 9 | 243 ± 13 | 285 ± 27 | 273 ± 16 |
| 700 | 301 ± 2 | 282 ± 15 | 346 ± 10 | 304 ± 10 |
| Time of Sonication (min) | CBD/CBDA | HI | DHI |
|---|---|---|---|
| 0 | CBD | 13.78 ± 1.38 | 35.46 ± 0.30 |
| CBDA | 26.28 ± 1.82 | 6.57 ± 0.03 | |
| 5 | CBD | 15.19 ± 0.50 | 37.31 ± 1.41 |
| CBDA | 28.19 ± 0.67 | 1.19 ± 0.06 | |
| 10 | CBD | 19.46 ± 1.58 | 50.21 ± 3.17 |
| CBDA | 33.32 ± 3.16 | 1.75 ± 0.16 | |
| 15 | CBD | 22.74 ± 1.06 | 53.21 ± 2.78 |
| CBDA | 37.51 ± 2.19 | 2.46 ± 0.23 |
| Power (W) | CBD/CBDA | HI, 30 s | DHI, 30 s | HI, 60 s | DHI, 60 s |
|---|---|---|---|---|---|
| 120 | CBD | 6.85 ± 0.07 | 23.30 ± 0.93 | 8.59 ± 0.77 | 25.17 ± 0.21 |
| CBDA | 19.53 ± 1.03 | 1.39 ± 0.09 | 25.48 ± 1.78 | 1.32 ± 0.10 | |
| 540 | CBD | 11.78 ± 0.54 | 38.10 ± 0.69 | 13.75 ± 0.46 | 41.27 ± 1.29 |
| CBDA | 29.96 ± 1.36 | 1.48 ± 0.04 | 35.81 ± 2.80 | 2.78 ± 0.15 | |
| 700 | CBD | 15.25 ± 0.84 | 45.29 ± 1.01 | 19.28 ± 0.98 | 48.21 ± 0.56 |
| CBDA | 36.54 ± 2.29 | 2.51 ± 0.19 | 38.94 ± 0.90 | 5.64 ± 0.42 |
| 25 °C | 40 °C | 60 °C | ||||
|---|---|---|---|---|---|---|
| Sample | A, Pa·s1/z | z, - | A, Pa·s1/z | z, - | A, Pa·s1/z | z, - |
| SND | 1200 ± 130 | 4.30 ± 0.01 | 70 ± 3 | 3.70 ± 0.03 | 48 ± 4 | 4.10 ± 0.04 |
| SND_Ho | 200 ± 10 | 2.70 ± 0.01 | 150 ± 7 | 2.50 ± 0.01 | 110 ± 5 | 3.70 ± 0.02 |
| HM50_Ho | 20 ± 1 | 1.45 ± 0.02 | 13 ± 2 | 2.00 ± 0.01 | 20 ± 2 | 2.10 ± 0.01 |
| HM50_Ho20 | 15 ± 2 | 1.50 ± 0.03 | 10 ± 1 | 2.10 ± 0.01 | 15 ± 1 | 3.10 ± 0.02 |
| HM50_Ho30 | 12 ± 1 | 2.10 ± 0.01 | 11 ± 1 | 1.20 ± 0.01 | 20 ± 1 | 1.60 ± 0.01 |
| H100_Ho | 110 ± 3 | 2.40 ± 0.01 | 110 ± 5 | 2.35 ± 0.01 | 270 ± 15 | 3.20 ± 0.01 |
| Sample | SND | SND_Ho | HM50_Ho | HM50_Ho20 | HM50_Ho30 | H100_Ho |
|---|---|---|---|---|---|---|
| Height | 4.1 ± 0.1 | 3.9 ± 0.2 | 4.1 ± 0.1 | 3.6 ± 0.1 | 3.3 ± 0.1 | 4.1± 0.2 |
| Sample | SND | SND_Ho | HM50_Ho | HM50_Ho20 | HM50_Ho30 | H100_Ho |
|---|---|---|---|---|---|---|
| EI | 0.40 ± 0.05 | 0.20 ± 0.02 | 0.22 ± 0.02 | 0.18 ± 0.01 | 0.14 ± 0.01 | 0.19 ± 0.01 |
| Hardness | 2.75 ± 0.03 | 2.25 ± 0.03 | 1.80 ± 0.03 | 1.60 ± 0.03 | 1.50 ± 0.03 | 1.90 ± 0.01 |
| L* | a* | b* | ||||
|---|---|---|---|---|---|---|
| Sample | Crumb | Crust | Crumb | Crust | Crumb | Crust |
| SND | 77 ± 1 | 56 ± 2 | −3.2 ± 0.2 | −1.4 ± 1.1 | 19 ± 1 | 35 ± 2 |
| SND_Ho | 79 ± 2 | 63 ± 2 | −3.0 ± 0.3 | −1.3 ± 0.8 | 23 ± 2 | 38 ± 1 |
| HM50_Ho | 29 ± 1 | 32 ± 1 | 2.7 ± 0.3 | 6.5 ± 0.8 | 17 ± 1 | 16 ± 1 |
| HM50_Ho20 | 30 ± 1 | 32 ± 1 | 2.5 ± 0.2 | 7.3 ± 0.4 | 17 ± 1 | 15 ± 1 |
| HM50_Ho30 | 33 ± 1 | 35 ± 1 | 2.7 ± 0.6 | 7.6 ± 0.3 | 16 ± 1 | 16 ± 1 |
| H100_Ho | 22 ± 1 | 25 ± 2 | 4 ± 0.4 | 8.3 ± 0.5 | 10 ± 2 | 12 ± 1 |
| Component | SND | SND_Ho | HM50_Ho20 | HM50_Ho30 | H100_Ho |
|---|---|---|---|---|---|
| Wheat flour (W) | 22.5 | 22.5 | 0 | 0 | 0 |
| Hemp flour (H) | 0 | 0 | 11.25 | 11.25 | 22.5 |
| Maize starch (M) | 0 | 0 | 11.25 | 11.25 | 0 |
| Sugar (S) | 22.5 | 22.5 | 22.5 | 22.5 | 22.5 |
| Eggs (E) | 22.5 | 22.5 | 22.5 | 22.5 | 22.5 |
| Hemp Oil (HO) | 0 | 15.8 | 12.6 | 11.3 | 15.8 |
| Butter (B) | 22.5 | 0 | 0 | 0 | 0 |
| Milk (M) | 10 | 10 | 10 | 10 | 10 |
| Baking powder (B) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldino, N.; Paleologo, M.F.O.; Chiodo, M.; Mileti, O.; Lupi, F.R.; Gabriele, D. Assisted Extraction of Hemp Oil and Its Application to Design Functional Gluten-Free Bakery Foods. Molecules 2025, 30, 2665. https://doi.org/10.3390/molecules30122665
Baldino N, Paleologo MFO, Chiodo M, Mileti O, Lupi FR, Gabriele D. Assisted Extraction of Hemp Oil and Its Application to Design Functional Gluten-Free Bakery Foods. Molecules. 2025; 30(12):2665. https://doi.org/10.3390/molecules30122665
Chicago/Turabian StyleBaldino, Noemi, Mario F. O. Paleologo, Mariateresa Chiodo, Olga Mileti, Francesca R. Lupi, and Domenico Gabriele. 2025. "Assisted Extraction of Hemp Oil and Its Application to Design Functional Gluten-Free Bakery Foods" Molecules 30, no. 12: 2665. https://doi.org/10.3390/molecules30122665
APA StyleBaldino, N., Paleologo, M. F. O., Chiodo, M., Mileti, O., Lupi, F. R., & Gabriele, D. (2025). Assisted Extraction of Hemp Oil and Its Application to Design Functional Gluten-Free Bakery Foods. Molecules, 30(12), 2665. https://doi.org/10.3390/molecules30122665







