Linker-Dependent Variation in the Photophysical Properties of Dinuclear 2-Phenylpyridinato(salicylaldiminato)platinum(II) Complexes Featuring NDI Units
Abstract
1. Introduction
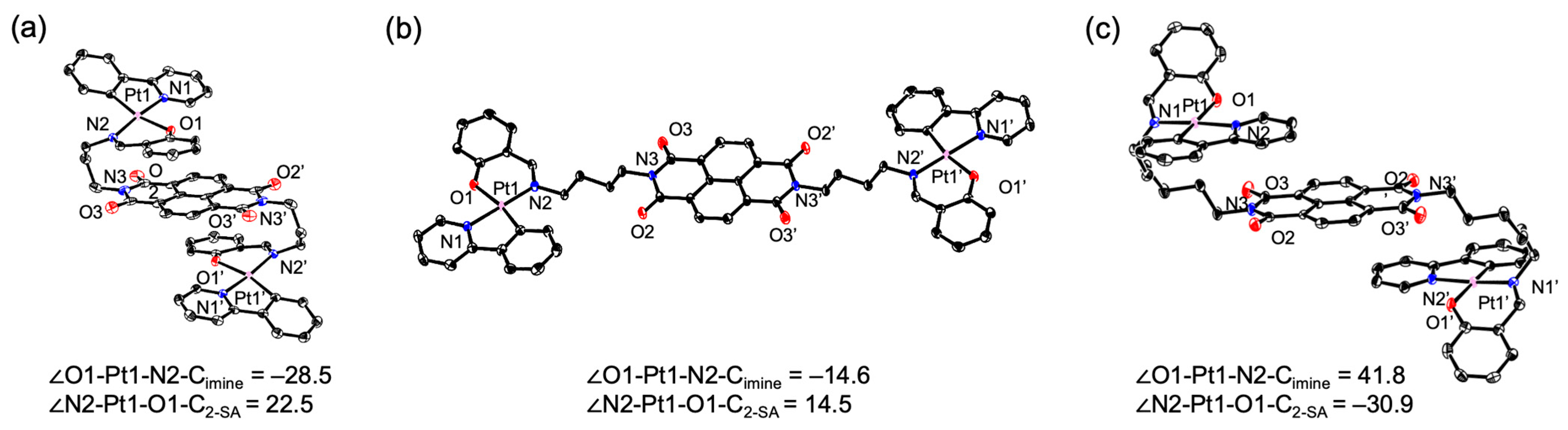
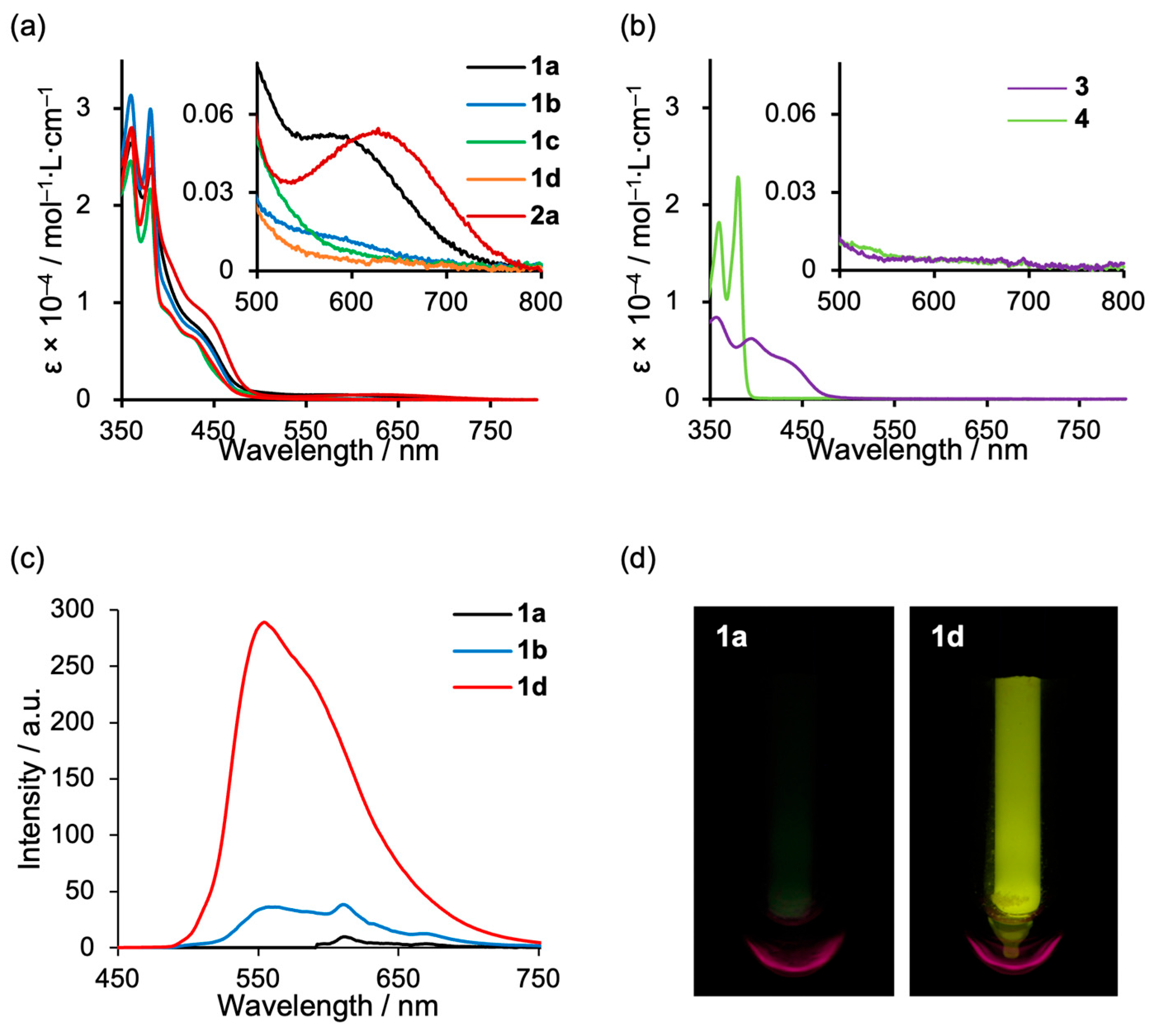
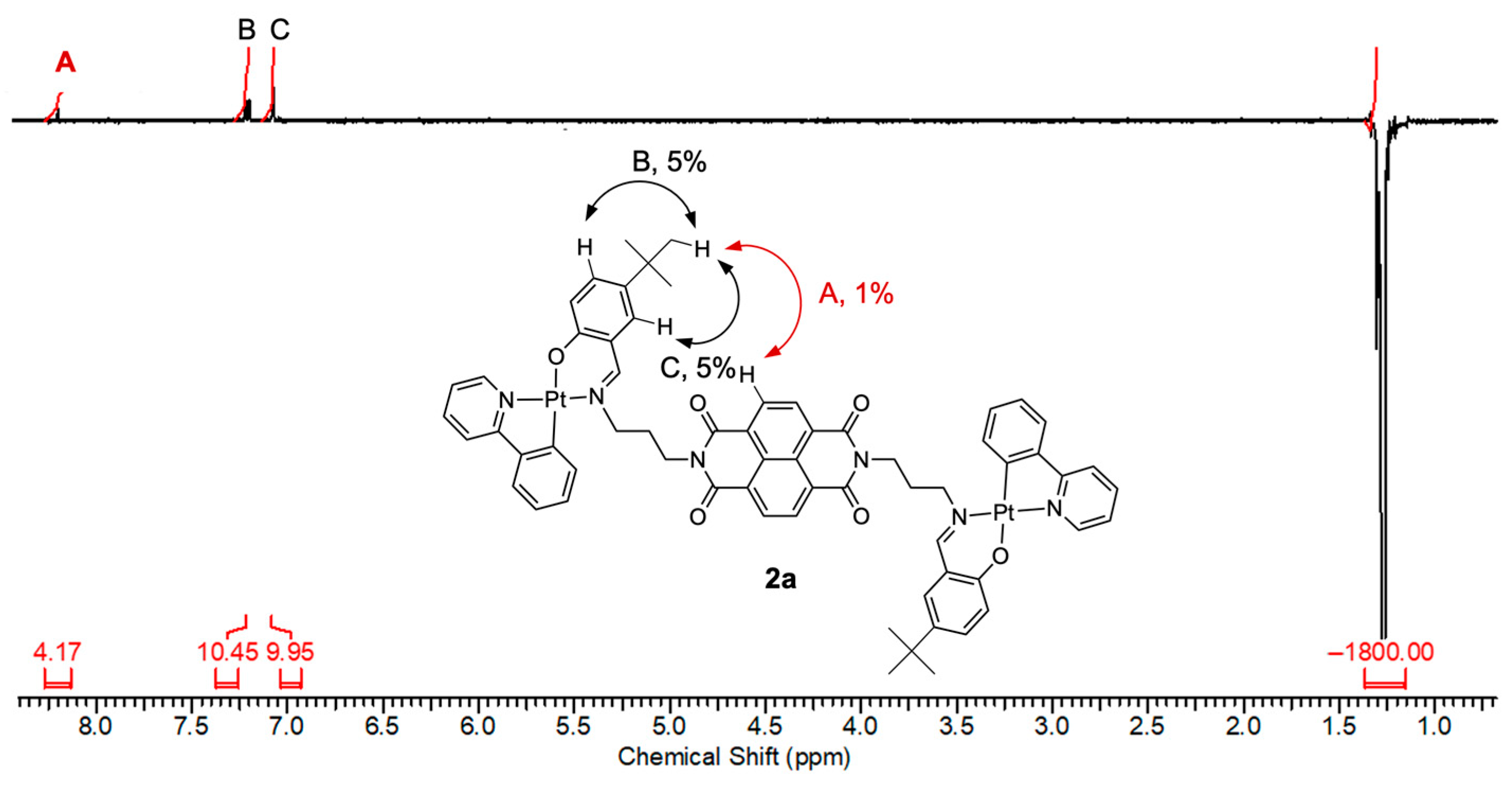
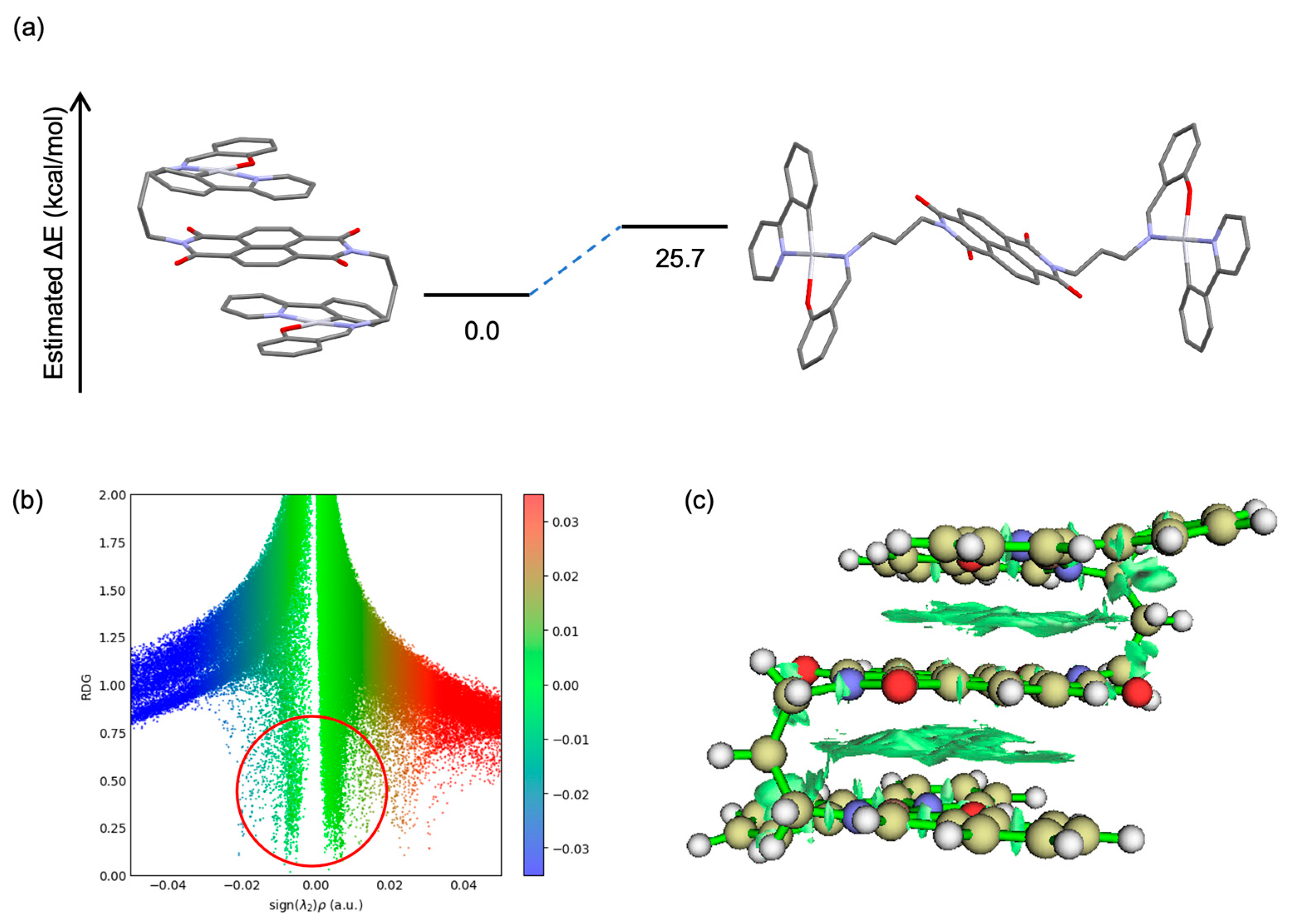
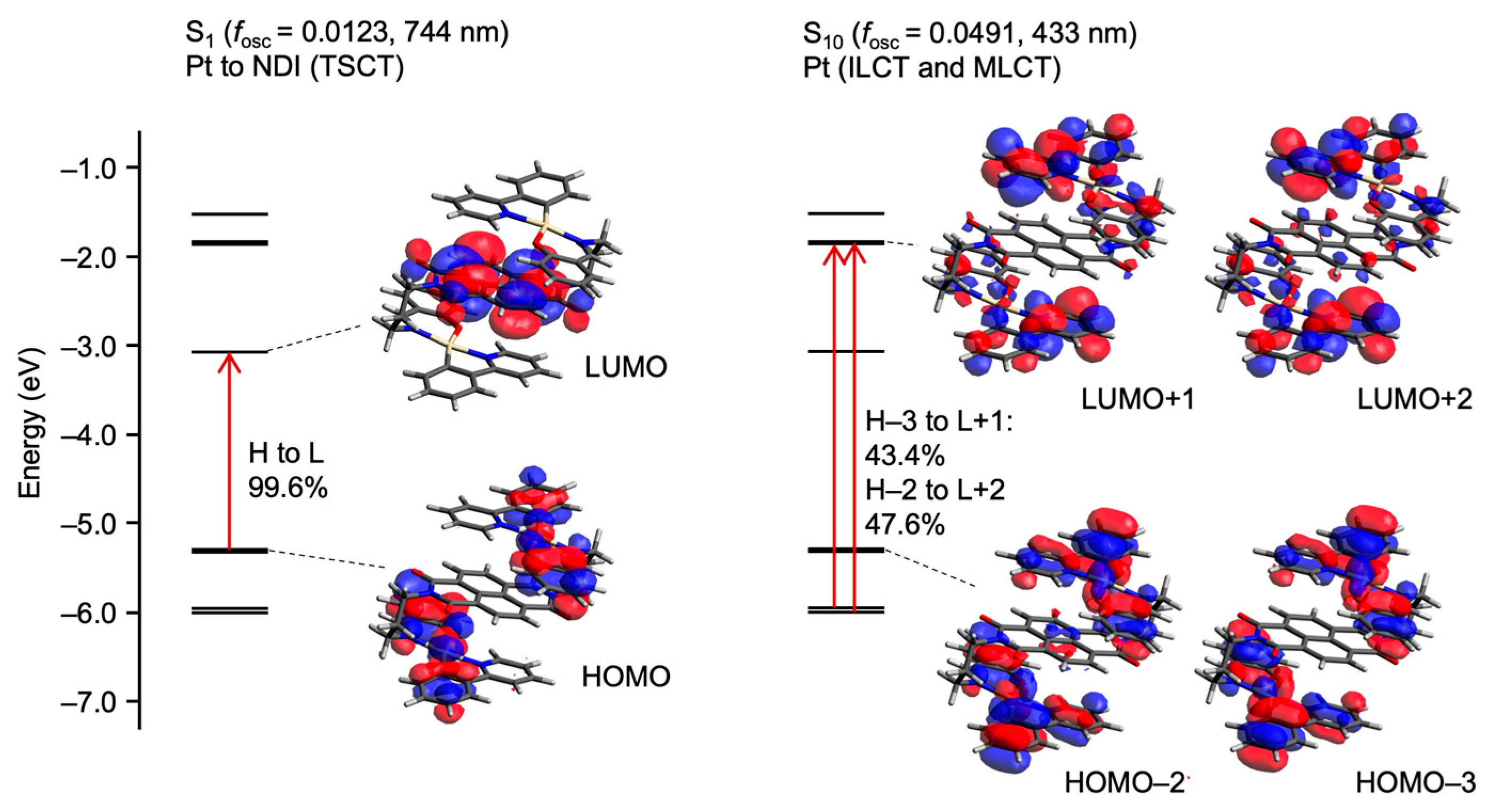
2. Results
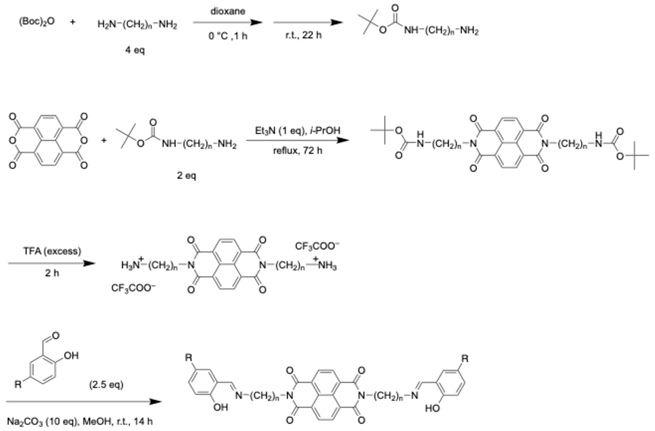
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Shen, P.; Zhao, Z.; Tang, B.Z. Through-Space Conjugation: A Thriving Alternative for Optoelectronic Materials. CCS Chem. 2019, 1, 181–196. [Google Scholar] [CrossRef]
- Shao, S.; Wang, L. Through-space Charge Transfer Polymers for Solution-processed Organic Light-emitting Diodes. Aggregate 2020, 1, 45–56. [Google Scholar] [CrossRef]
- Ye, J.-T.; Qiu, Y.-Q. The Inspiration and Challenge for Through-Space Charge Transfer Architecture: From Thermally Activated Delayed Fluorescence to Non-Linear Optical Properties. Phys. Chem. Chem. Phys. 2021, 23, 15881–15898. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ying, A.; Gong, S. Recent Progress in Thermally Activated Delayed Fluorescence Dendrimers for Solution-processed Organic Light-emitting Diodes. J. Polym. Sci. 2024, 62, 241–265. [Google Scholar] [CrossRef]
- Peng, J.; Hou, L.; Liu, D.; Zhao, Z.; Zhang, J.; Qiu, Z.; Tang, B.Z. Organic Optoelectronic Devices Based on Through-Space Interaction. ACS Appl. Opt. Mater. 2024, 2, 15–27. [Google Scholar] [CrossRef]
- Meng, X.; Lang, X.; Cao, Z. Structure Evolution of Organic Luminescent Molecules Utilizing Through-Space Charge Transfer Mechanism. Chem. Asian J. 2025, 20, e202401488. [Google Scholar] [CrossRef]
- Brown, C.J.; Farthing, A.C. Preparation and Structure of Di-p-Xylylene. Nature 1949, 164, 915–916. [Google Scholar] [CrossRef]
- Cram, D.J.; Steinberg, H. Macro Rings. I. Preparation and Spectra of the Paracyclophanes. J. Am. Chem. Soc. 1951, 73, 5691–5704. [Google Scholar] [CrossRef]
- Cram, D.J.; Allinger, N.L.; Steinberg, H. Macro Rings. VII. The Spectral Consequences of Bringing Two Benzene Rings Face to Face1. J. Am. Chem. Soc. 1954, 76, 6132–6141. [Google Scholar] [CrossRef]
- Jagtap, S.P.; Collard, D.M. Multitiered 2D π-Stacked Conjugated Polymers Based on Pseudo-Geminal Disubstituted [2.2]Paracyclophane. J. Am. Chem. Soc. 2010, 132, 12208–12209. [Google Scholar] [CrossRef]
- Morisaki, Y.; Chujo, Y. Through-Space Conjugated Polymers Consisting of [2.2]Paracyclophane. Polym. Chem. 2011, 2, 1249. [Google Scholar] [CrossRef]
- Bai, M.; Liang, J.; Xie, L.; Sanvito, S.; Mao, B.; Hou, S. Efficient Conducting Channels Formed by the π-π Stacking in Single [2,2]Paracyclophane Molecules. J. Phys. Chem. 2012, 136, 104701. [Google Scholar] [CrossRef]
- Batra, A.; Kladnik, G.; Vázquez, H.; Meisner, J.S.; Floreano, L.; Nuckolls, C.; Cvetko, D.; Morgante, A.; Venkataraman, L. Quantifying Through-Space Charge Transfer Dynamics in π-Coupled Molecular Systems. Nat. Commun. 2012, 3, 1086. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Jagtap, S.P.; Coropceanu, V.; Brédas, J.; Collard, D.M. π-Stacked Oligo(Phenylene Vinylene)s Based on Pseudo-Geminal Substituted [2.2]Paracyclophanes: Impact of Interchain Geometry and Interactions on the Electronic Properties. Angew. Chem. Int. Ed. 2012, 51, 11629–11632. [Google Scholar] [CrossRef]
- Morisaki, Y.; Kawakami, N.; Nakano, T.; Chujo, Y. Energy-Transfer Properties of a [2.2]Paracyclophane-Based Through-Space Dimer. Chem. Eur. J. 2013, 19, 17715–17718. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, Y.; Kawakami, N.; Shibata, S.; Chujo, Y. Through-Space Conjugated Molecular Wire Comprising Three π-Electron Systems. Chem. Asian J. 2014, 9, 2891–2895. [Google Scholar] [CrossRef] [PubMed]
- Zafra, J.L.; Molina Ontoria, A.; Mayorga Burrezo, P.; Peña-Alvarez, M.; Samoc, M.; Szeremeta, J.; Ramírez, F.J.; Lovander, M.D.; Droske, C.J.; Pappenfus, T.M.; et al. Fingerprints of Through-Bond and Through-Space Exciton and Charge π-Electron Delocalization in Linearly Extended [2.2]Paracyclophanes. J. Am. Chem. Soc. 2017, 139, 3095–3105. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kobayakawa, K.; Matsuzawa, H.; Nishinaga, T.; Hirose, T.; Sako, K.; Mazaki, Y. Macrocyclic Oligothiophene with Stereogenic [2.2]Paracyclophane Scaffolds: Chiroptical Properties from π-Transannular Interactions. Chem. Eur. J. 2017, 23, 3267–3271. [Google Scholar] [CrossRef]
- Morisaki, Y.; Shibata, S.; Chujo, Y. [2.2]Paracyclophane-Based Single Molecular Wire Consisting of Four π-Electron Systems. Can. J. Chem. 2017, 95, 424–431. [Google Scholar] [CrossRef]
- Gon, M.; Morisaki, Y.; Chujo, Y. Optically Active Phenylethene Dimers Based on Planar Chiral Tetrasubstituted [2.2]Paracyclophane. Chem. Eur. J. 2017, 23, 6323–6329. [Google Scholar] [CrossRef]
- Spuling, E.; Sharma, N.; Samuel, I.D.W.; Zysman-Colman, E.; Bräse, S. (Deep) Blue through-Space Conjugated TADF Emitters Based on [2.2]Paracyclophanes. Chem. Commun. 2018, 54, 9278–9281. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Yoshizawa, M.; Akita, M.; Fujita, M. Engineering Double to Quintuple Stacks of a Polarized Aromatic in Confined Cavities. J. Am. Chem. Soc. 2010, 132, 960–966. [Google Scholar] [CrossRef]
- Morisaki, Y.; Sawamura, T.; Murakami, T.; Chujo, Y. Synthesis of Anthracene-Stacked Oligomers and Polymer. Org. Lett. 2010, 12, 3188–3191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Lam, J.W.Y.; Chan, C.Y.K.; Chen, S.; Liu, J.; Lu, P.; Rodriguez, M.; Maldonado, J.; Ramos-Ortiz, G.; Sung, H.H.Y.; et al. Stereoselective Synthesis, Efficient Light Emission, and High Bipolar Charge Mobility of Chiasmatic Luminogens. Adv. Mater. 2011, 23, 5430–5435. [Google Scholar] [CrossRef] [PubMed]
- Rios, C.; Salcedo, R. Computational Study of Electron Delocalization in Hexaarylbenzenes. Molecules 2014, 19, 3274–3296. [Google Scholar] [CrossRef]
- Wu, Y.; Frasconi, M.; Gardner, D.M.; McGonigal, P.R.; Schneebeli, S.T.; Wasielewski, M.R.; Stoddart, J.F. Electron Delocalization in a Rigid Cofacial Naphthalene-1,8:4,5-bis(Dicarboximide) Dimer. Angew. Chem. Int. Ed. 2014, 53, 9476–9481. [Google Scholar] [CrossRef]
- Mathew, S.; Crandall, L.A.; Ziegler, C.J.; Hartley, C.S. Enhanced Helical Folding of Ortho -Phenylenes through the Control of Aromatic Stacking Interactions. J. Am. Chem. Soc. 2014, 136, 16666–16675. [Google Scholar] [CrossRef]
- Hirose, T.; Tsunoi, Y.; Fujimori, Y.; Matsuda, K. Fluorescence Enhancement of Covalently Linked 1-Cyano-1,2-diphenylethene Chromophores with Naphthalene-1,8-diyl Linker Units: Analysis Based on Kinetic Constants. Chem. Eur. J. 2015, 21, 1637–1644. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; He, B.; Nie, H.; Hu, R.; Huang, F.; Qin, A.; Zhou, X.; Zhao, Z.; Tang, B.Z. Multichannel Conductance of Folded Single-Molecule Wires Aided by Through-Space Conjugation. Angew. Chem. Int. Ed. 2015, 54, 4231–4235. [Google Scholar] [CrossRef]
- He, B.; Nie, H.; Chen, L.; Lou, X.; Hu, R.; Qin, A.; Zhao, Z.; Tang, B.Z. High Fluorescence Efficiencies and Large Stokes Shifts of Folded Fluorophores Consisting of a Pair of Alkenyl-Tethered, π-Stacked Oligo- p -Phenylenes. Org. Lett. 2015, 17, 6174–6177. [Google Scholar] [CrossRef]
- Zhuang, Z.; Shen, P.; Ding, S.; Luo, W.; He, B.; Nie, H.; Wang, B.; Huang, T.; Hu, R.; Qin, A.; et al. Synthesis, Aggregation-Enhanced Emission, Polymorphism and Piezochromism of TPE-Cored Foldamers with through-Space Conjugation. Chem. Commun. 2016, 52, 10842–10845. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schmidt, H.C.; Spulber, M.; Neuburger, M.; Palivan, C.G.; Meuwly, M.; Wenger, O.S. Charge Transfer Pathways in Three Isomers of Naphthalene-Bridged Organic Mixed Valence Compounds. J. Org. Chem. 2016, 81, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Sinnwell, M.A.; MacGillivray, L.R. Halogen-Bond-Templated [2+2] Photodimerization in the Solid State: Directed Synthesis and Rare Self-Inclusion of a Halogenated Product. Angew. Chem. Int. Ed. 2016, 55, 3477–3480. [Google Scholar] [CrossRef] [PubMed]
- Hartley, C.S. Folding of Ortho-Phenylenes. Acc. Chem. Res. 2016, 49, 646–654. [Google Scholar] [CrossRef]
- Shen, P.-C.; Zhuang, Z.-Y.; Zhao, Z.-J.; Tang, B.Z. Recent Advances of Folded Tetraphenylethene Derivatives Featuring Through-Space Conjugation. Chin. Chem. Lett. 2016, 27, 1115–1123. [Google Scholar] [CrossRef]
- Vij, V.; Bhalla, V.; Kumar, M. Hexaarylbenzene: Evolution of Properties and Applications of Multitalented Scaffold. Chem. Rev. 2016, 116, 9565–9627. [Google Scholar] [CrossRef]
- He, B.; Luo, W.; Hu, S.; Chen, B.; Zhen, S.; Nie, H.; Zhao, Z.; Tang, B.Z. Synthesis and Photophysical Properties of New Through-Space Conjugated Luminogens Constructed by Folded Tetraphenylethene. J. Mater. Chem. C 2017, 5, 12553–12560. [Google Scholar] [CrossRef]
- Hayashi, S.; Yamamoto, S.; Koizumi, T. A Cyclic Compound Based on Xanthene-Linked π-Stacked Dimer via Direct Arylation. Chem. Lett. 2017, 46, 200–203. [Google Scholar] [CrossRef]
- Vemuri, G.N.; Pandian, R.R.; Spinello, B.J.; Stopler, E.B.; Kinney, Z.J.; Hartley, C.S. Twist Sense Control in Terminally Functionalized Ortho-Phenylenes. Chem. Sci. 2018, 9, 8260–8270. [Google Scholar] [CrossRef]
- Zhen, S.; Mao, J.-C.; Chen, L.; Ding, S.; Luo, W.; Zhou, X.-S.; Qin, A.; Zhao, Z.; Tang, B.Z. Remarkable Multichannel Conductance of Novel Single-Molecule Wires Built on Through-Space Conjugated Hexaphenylbenzene. Nano Lett. 2018, 18, 4200–4205. [Google Scholar] [CrossRef]
- Shen, P.; Zhuang, Z.; Jiang, X.-F.; Li, J.; Yao, S.; Zhao, Z.; Tang, B.Z. Through-Space Conjugation: An Effective Strategy for Stabilizing Intramolecular Charge-Transfer States. J. Phys. Chem. Lett. 2019, 10, 2648–2656. [Google Scholar] [CrossRef]
- Song, Y.; Tian, M.; Yu, R.; He, L. Through-Space Charge-Transfer Emitters Developed by Fixing the Acceptor for High-Efficiency Thermally Activated Delayed Fluorescence. ACS Appl. Mater. Interfaces 2021, 13, 60269–60278. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, C.; Li, W.; Zhang, Y.; Yuan, J.; Chen, R. Promoting Through-Space Charge Transfer-Based TADF via Rational Alignment of Quasi-Planar Donor and Acceptor in Solid State. Sci. China Mater. 2023, 66, 3958–3967. [Google Scholar] [CrossRef]
- Dos Santos, J.M.; Hall, D.; Basumatary, B.; Bryden, M.; Chen, D.; Choudhary, P.; Comerford, T.; Crovini, E.; Danos, A.; De, J.; et al. The Golden Age of Thermally Activated Delayed Fluorescence Materials: Design and Exploitation. Chem. Rev. 2024, 124, 13736–14110. [Google Scholar] [CrossRef]
- Liao, P.; Huang, J.; Yan, Y.; Tang, B.Z. Clusterization-Triggered Emission (CTE): One for All, All for One. Mater. Chem. Front. 2021, 5, 6693–6717. [Google Scholar] [CrossRef]
- Zhang, J.; Alam, P.; Zhang, S.; Shen, H.; Hu, L.; Sung, H.H.Y.; Williams, I.D.; Sun, J.; Lam, J.W.Y.; Zhang, H.; et al. Secondary Through-Space Interactions Facilitated Single-Molecule White-Light Emission from Clusteroluminogens. Nat. Commun. 2022, 13, 3492. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hong, Y.-L.; Fang, X.-Q.; Wang, C.; Liu, C.-M. Fluorescent Phosphine Oxide-Containing Hyperbranched Polyesters: Design, Synthesis and Their Application for Fe3+ Detection. J. Mater. Chem. C 2023, 11, 1927–1936. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, Z.; Zhang, H.; Tang, B.Z. Emergent Clusteroluminescence from Nonemissive Molecules. Nat. Commun. 2025, 16, 3910. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, B.Z. Through-Space Interactions in Clusteroluminescence. JACS Au 2021, 1, 1805–1814. [Google Scholar] [CrossRef]
- Ruduss, A.; Turovska, B.; Belyakov, S.; Stucere, K.A.; Vembris, A.; Traskovskis, K. Carbene–Metal Complexes As Molecular Scaffolds for Construction of through-Space Thermally Activated Delayed Fluorescence Emitters. Inorg. Chem. 2022, 61, 2174–2185. [Google Scholar] [CrossRef]
- Zhan, L.; Chen, T.; Zhong, C.; Cao, X.; Zhang, Y.; Zou, Y.; Bin, Z.; You, J.; Zhang, D.; Duan, L.; et al. Luminescent Gold(III) Exciplexes Enable Efficient Multicolor Electroluminescence. Sci. China Chem. 2023, 66, 3213–3222. [Google Scholar] [CrossRef]
- Chen, T.; Xu, Y.; Ying, A.; Yang, C.; Lin, Q.; Gong, S. Through-Space Charge-Transfer Organogold(III) Complexes Enable High-Performance X-ray Scintillation and Imaging. Angew. Chem. Int. Ed. 2024, 63, e202401833. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wu, Y.; Huang, M.; Cheng, L.; Pan, Y.; Wu, C.; Yeh, C.; Li, J.; Lin, Y.; Chi, Y.; et al. Iridium(III) Carbene Complexes Featuring Either Metal-to-Ligand Charge Transfer (MLCT) or Through-Space Charge Transfer (TSCT) Blue Luminescence. Angew. Chem. Int. Ed. 2025, 64, e202424694. [Google Scholar] [CrossRef]
- Li, P.; Chen, Z.; Leung, M.-Y.; Lai, S.-L.; Cheng, S.-C.; Kwok, W.-K.; Ko, C.-C.; Chan, M.-Y.; Yam, V.W.-W. Motivation on Intramolecular Through-Space Charge Transfer for the Realization of Thermally Activated Delayed Fluorescence (TADF)–Thermally Stimulated Delayed Phosphorescence (TSDP) in C^C^N Gold(III) Complexes and Their Applications in Organic Light-Emitting Devices. J. Am. Chem. Soc. 2025, 147, 12092–12104. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; D’Aleo, A.; Inada, K.; Cui, L.; Kim, J.U.; Nakanotani, H.; Adachi, C. Donor–σ–Acceptor Motifs: Thermally Activated Delayed Fluorescence Emitters with Dual Upconversion. Angew. Chem. Int. Ed. 2017, 56, 16536–16540. [Google Scholar] [CrossRef]
- Song, Y.; Yu, R.; Meng, X.; He, L. Donor-σ-Acceptor Molecules with Alkyl σ-Linkers of Different Lengths: Exploration on the Exciplex Emission and Their Use for Efficient Organic Light-Emitting Diodes. Dye. Pigm. 2023, 208, 110876. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, Z.; Zhi Sun, J.; Huang, F.; Zhang, H.; Zhong Tang, B. How the Length of Through-Space Conjugation Influences the Clusteroluminescence of Oligo(Phenylene Methylene)s. Angew. Chem. Int. Ed. 2024, 63, e202318245. [Google Scholar] [CrossRef]
- Tu, W.; Xiong, Z.; Wang, L.; Zhang, J.; Sun, J.Z.; Zhang, H.; Tang, B.Z. The Superiority of Nonconjugated Structures in Fluorescence: Through-Space vs. through-Bond Charge Transfer. Sci. China Chem. 2024, 67, 3121–3130. [Google Scholar] [CrossRef]
- Partanen, I.; Hsu, C.; Shi, E.H.; Maisuls, I.; Eskelinen, T.; Karttunen, A.J.; Saarinen, J.J.; Strassert, C.A.; Belyaev, A.; Chou, P.; et al. Organic Room-Temperature near-IR Phosphorescence Harvested by Intramolecular Through-Space Sensitization in Composite Molecules. Angew. Chem. Int. Ed. 2025, 64, e202503327. [Google Scholar] [CrossRef]
- Komiya, N.; Okada, M.; Fukumoto, K.; Jomori, D.; Naota, T. Highly Phosphorescent Crystals of Vaulted trans-Bis(Salicylaldiminato)Platinum(II) Complexes. J. Am. Chem. Soc. 2011, 133, 6493–6496. [Google Scholar] [CrossRef]
- Komiya, N.; Muraoka, T.; Iida, M.; Miyanaga, M.; Takahashi, K.; Naota, T. Ultrasound-Induced Emission Enhancement Based on Structure-Dependent Homo- and Heterochiral Aggregations of Chiral Binuclear Platinum Complexes. J. Am. Chem. Soc. 2011, 133, 16054–16061. [Google Scholar] [CrossRef] [PubMed]
- Komiya, N.; Okada, M.; Fukumoto, K.; Kaneta, K.; Yoshida, A.; Naota, T. Vaulted trans-Bis(Salicylaldiminato)Platinum(II) Crystals: Heat-Resistant, Chromatically Sensitive Platforms for Solid-State Phosphorescence at Ambient Temperature. Chem. Eur. J. 2013, 19, 4798–4811. [Google Scholar] [CrossRef] [PubMed]
- Komiya, N.; Itami, N.; Naota, T. Solid-State Phosphorescence of trans-Bis(Salicylaldiminato)Platinum(II) Complexes Bearing Long Alkyl Chains: Morphology Control towards Intense Emission. Chem. Eur. J. 2013, 19, 9497–9505. [Google Scholar] [CrossRef]
- Naito, M.; Souda, H.; Koori, H.; Komiya, N.; Naota, T. Binuclear trans-Bis(Β-iminoaryloxy)Palladium(II) Complexes Doubly Linked with Pentamethylene Spacers: Structure-Dependent Flapping Motion and Heterochiral Association Behavior of the Clothespin-Shaped Molecules. Chem. Eur. J. 2014, 20, 6991–7000. [Google Scholar] [CrossRef]
- Komiya, N.; Okada, M.; Le, N.H.-T.; Kawamorita, S.; Naota, T. Linker-Dependent Chromogenic Control of the Emission of Polymethylene-Vaulted trans-Bis(Salicylaldiminato)Platinum(II) Complexes. J. Lumin. 2015, 161, 363–367. [Google Scholar] [CrossRef]
- Komiya, N.; Okada, M.; Inoue, R.; Kawamorita, S.; Naota, T. Variations in the Emission of Polymethylene-Vaulted trans-Bis(Salicylaldiminato)Platinum(II) Complexes Incorporating Methoxy Functionalities with Linkage Length and Substitution Position. Polyhedron 2015, 98, 75–83. [Google Scholar] [CrossRef]
- Naito, M.; Inoue, R.; Iida, M.; Kuwajima, Y.; Kawamorita, S.; Komiya, N.; Naota, T. Control of Metal Arrays Based on Heterometallics Masquerading in Heterochiral Aggregations of Chiral Clothespin-Shaped Complexes. Chem. Eur. J. 2015, 21, 12927–12939. [Google Scholar] [CrossRef]
- Hashimoto, T.; Fukumoto, K.; Le, N.H.-T.; Matsuoka, T.; Kawamorita, S.; Komiya, N.; Naota, T. Dynamic Neighbouring Participation of Nitrogen Lone Pairs on the Chromogenic Behaviour of trans-Bis(Salicylaldiminato)Pt(II) Coordination Platforms. Dalton Trans. 2016, 45, 19257–19268. [Google Scholar] [CrossRef]
- Inoue, R.; Kawamorita, S.; Naota, T. Single-Point Remote Control of Flapping Motion in Clothespin-Shaped Bimetallic Palladium Complexes Based on the N(Sp2)–N(Sp3) Interconversion in Amide Functionalities. Chem. Eur. J. 2016, 22, 5712–5726. [Google Scholar] [CrossRef]
- Komiya, N.; Nakajima, T.; Hotta, M.; Maeda, T.; Matsuoka, T.; Kawamorita, S.; Naota, T. Kinetic Studies of the Chirality Inversion of Salicylaldiminato–Ruthenium Using Racemic η6-p-Cymene Complexes as a Mechanistic Probe. Eur. J. Inorg. Chem. 2016, 2016, 3148–3156. [Google Scholar] [CrossRef]
- Maeda, T.; Kawamorita, S.; Naota, T. Synthesis, Structure, and Chromogenic Properties of Polymethylene-Vaulted trans-Bis(Salicylaldiminato)Palladium(II) Complexes. Polyhedron 2016, 117, 826–833. [Google Scholar] [CrossRef]
- Matsuoka, T.; Li, Z.; Ikeshita, M.; Kawamorita, S.; Naota, T. Linker Length Dependence of the Chromogenic Properties of Polymethylene-Vaulted trans-Bis(2-Aminotroponato)Palladium(II) Complexes. J. Mol. Struct. 2018, 1165, 217–222. [Google Scholar] [CrossRef]
- Ikeshita, M.; Naota, T. Dynamic Rotational Motions of Vaulted Chiral trans-Bis(Salicylaldiminato)Palladium(II) Complexes Bearing Rigid or Flexible Carbon Chain Linkers. Eur. J. Inorg. Chem. 2018, 2018, 4689–4695. [Google Scholar] [CrossRef]
- Komiya, N.; Hosokawa, T.; Adachi, J.; Inoue, R.; Kawamorita, S.; Naota, T. Regiospecific Remote Pt–H Interactions in Oligomethylene-Vaulted (N^C^N.)-Pincer PtII Complexes. Eur. J. Inorg. Chem. 2018, 2018, 4771–4778. [Google Scholar] [CrossRef]
- Le, N.H.-T.; Inoue, R.; Kawamorita, S.; Komiya, N.; Naota, T. Phosphorescent Molecules That Resist Concentration Quenching in the Solution State: Concentration-Driven Emission Enhancement of Vaulted trans-Bis [2-(Iminomethyl)Imidazolato]Platinum(II) Complexes. Inorg. Chem. 2019, 58, 9076–9084. [Google Scholar] [CrossRef] [PubMed]
- Adachi, J.; Mori, T.; Inoue, R.; Naito, M.; Le, N.H.; Kawamorita, S.; Hill, J.P.; Naota, T.; Ariga, K. Emission Control by Molecular Manipulation of Double-Paddled Binuclear PtII Complexes at the Air-Water Interface. Chem. Asian J. 2020, 15, 406–414. [Google Scholar] [CrossRef]
- Kawamorita, S.; Ahadito, B.R.; Naota, T. Proximity Effects on the Phosphorescent Properties of Dinuclear Salicylaldiminato Cyclometalated Iridium(III) Complexes Linked with Polymethylene Spacers. Transit. Met. Chem. 2020, 45, 173–186. [Google Scholar] [CrossRef]
- Komiya, N.; Ikeshita, M.; Tosaki, K.; Sato, A.; Itami, N.; Naota, T. Catalytic Enantioselective Rotation of Watermill-Shaped Dinuclear Pd Complexes. Eur. J. Inorg. Chem. 2021, 2021, 1929–1940. [Google Scholar] [CrossRef]
- Inoue, R.; Naota, T.; Ehara, M. Origin of the Aggregation-Induced Phosphorescence of Platinum(II) Complexes: The Role of Metal–Metal Interactions on Emission Decay in the Crystalline State. Chem. Asian J. 2021, 16, 3129–3140. [Google Scholar] [CrossRef]
- Adachi, J.; Naito, M.; Sugiura, S.; Le, N.H.-T.; Nishimura, S.; Huang, S.; Suzuki, S.; Kawamorita, S.; Komiya, N.; Hill, J.P.; et al. Coordination Amphiphile: Design of Planar-Coordinated Platinum Complexes for Monolayer Formation at an Air-Water Interface Based on Ligand Characteristics and Molecular Topology. Bull. Chem. Soc. Jpn. 2022, 95, 889–897. [Google Scholar] [CrossRef]
- Maeda, T.; Mori, T.; Ikeshita, M.; Ma, S.C.; Muller, G.; Ariga, K.; Naota, T. Vortex Flow-controlled Circularly Polarized Luminescence of Achiral Pt(II) Complex Aggregates Assembled at the Air-Water Interface. Small Methods 2022, 6, 2200936. [Google Scholar] [CrossRef]
- Ikeshita, M.; Hara, N.; Imai, Y.; Naota, T. Chiroptical Response Control of Planar and Axially Chiral Polymethylene-Vaulted Platinum(II) Complexes Bearing 1,1′-Binaphthyl Frameworks. Inorg. Chem. 2023, 62, 13964–13976. [Google Scholar] [CrossRef]
- Ikeshita, M.; Ma, S.C.; Muller, G.; Naota, T. Linker-Dependent Control of the Chiroptical Properties of Polymethylene-Vaulted trans-Bis[(β-Iminomethyl)Naphthoxy]Platinum(ii) Complexes. Dalton Trans. 2024, 53, 7775–7787. [Google Scholar] [CrossRef] [PubMed]
- Ikeshita, M.; Takahashi, K.; Hara, N.; Kawamorita, S.; Komiya, N.; Imai, Y.; Naota, T. Ultrasound-induced Circularly Polarized Luminescence Based on Homochiral Aggregation of Clothespin-shaped Pt(II) Complexes. Responsive Mater. 2024, 2, e20240017. [Google Scholar] [CrossRef]
- Takahashi, E.; Takaya, H.; Naota, T. Dynamic Vapochromic Behaviors of Organic Crystals Based on the Open–Close Motions of S-Shaped Donor–Acceptor Folding Units. Chem. Eur. J. 2010, 16, 4793–4802. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, H.; Ma, Y.; Ye, S.; Liu, X.; Zhou, X.; Mou, X.; Wang, L.; Zhao, Q.; Huang, W. Rational Design of Metallophosphors with Tunable Aggregation-Induced Phosphorescent Emission and Their Promising Applications in Time-Resolved Luminescence Assay and Targeted Luminescence Imaging of Cancer Cells. J. Mater. Chem. 2012, 22, 22167. [Google Scholar] [CrossRef]
- Miyake, Y.; Nagata, T.; Tanaka, H.; Yamazaki, M.; Ohta, M.; Kokawa, R.; Ogawa, T. Entropy-Controlled 2D Supramolecular Structures of N,N′-Bis(n-Alkyl)Naphthalenediimides on a HOPG Surface. ACS Nano 2012, 6, 3876–3887. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef]



| 1a | 1b | 1d | |
|---|---|---|---|
| formula | C56H42N6O6Pt2 | C58H46N6O6Pt2 | C64H58Cl4N6O6Pt2 |
| Mr | 1285.16 | 1313.22 | 1539.19 |
| T/K | 113 | 113 | 113 |
| crystal color, habit | gray, block | green, block | Green, chip |
| crystal size/mm | 0.01 × 0.07 × 0.02 | 0.04 × 0.03 × 0.01 | 0.08 × 0.05 × 0.01 |
| crystal system | monoclinic | monoclinic | triclinic |
| space group | P21/c (#14) | P21/n (#14) | P- (#2) |
| a/Å | 12.333 (2) | 12.1907 (14) | 11.075 (2) |
| b/Å | 17.667 (3) | 16.0374 (18) | 11.224 (3) |
| c/Å | 10.1396 (16) | 11.8028 (14) | 13.138 (3) |
| α/° | 90 | 90 | 105.686 (3) |
| β/° | 96.805 (4) | 93.090 (3) | 92.943 (2) |
| γ/° | 90 | 90 | 113.786 (3) |
| V/Å3 | 2193.8 (6) | 2304.2 (5) | 1414.7 (6) |
| Z | 2 | 2 | 1 |
| ρcalcd/g·cm−3 | 1.945 | 1.893 | 1.807 |
| μ (MoKα)/cm−1 | 64.091 | 61.044 | 51.680 |
| F(000) | 1248.00 | 1280.00 | 756.00 |
| 2θmax/° | 54.9 | 55.0 | 55.1 |
| No. of reflns measd | 21,424 | 33,122 | 27,337 |
| No. of obsd reflns | 5000 | 5280 | 6502 |
| No. variables | 316 | 325 | 370 |
| R1 (I > 2σ(I)) a | 0.0281 | 0.0283 | 0.0446 |
| wR2 (all reflns) b | 0.0616 | 0.0533 | 0.0662 |
| Goodness of fit | 0.960 | 1.027 | 1.022 |
| Complex | λabs [nm] a | λem [nm] b | Φ77K b,c |
|---|---|---|---|
| 1a | 363, 382, 442 (sh), 600 | - | - |
| 1b | 360, 381, 442 (sh) | 579 | 0.01 |
| 1c | 360, 381, 436 (sh) | - | - |
| 1d | 360, 381, 429 (sh) | 554 | 0.06 |
| 2a | 361, 382, 451 (sh), 628 | - | - |
| 3 | 444 (sh) | 551 | 0.10 |
| 4 | 360, 380 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawamorita, S.; Matsuoka, T.; Nakamura, K.; Ahadito, B.R.; Naota, T. Linker-Dependent Variation in the Photophysical Properties of Dinuclear 2-Phenylpyridinato(salicylaldiminato)platinum(II) Complexes Featuring NDI Units. Molecules 2025, 30, 2664. https://doi.org/10.3390/molecules30122664
Kawamorita S, Matsuoka T, Nakamura K, Ahadito BR, Naota T. Linker-Dependent Variation in the Photophysical Properties of Dinuclear 2-Phenylpyridinato(salicylaldiminato)platinum(II) Complexes Featuring NDI Units. Molecules. 2025; 30(12):2664. https://doi.org/10.3390/molecules30122664
Chicago/Turabian StyleKawamorita, Soichiro, Tatsuya Matsuoka, Kazuki Nakamura, Bijak Riyandi Ahadito, and Takeshi Naota. 2025. "Linker-Dependent Variation in the Photophysical Properties of Dinuclear 2-Phenylpyridinato(salicylaldiminato)platinum(II) Complexes Featuring NDI Units" Molecules 30, no. 12: 2664. https://doi.org/10.3390/molecules30122664
APA StyleKawamorita, S., Matsuoka, T., Nakamura, K., Ahadito, B. R., & Naota, T. (2025). Linker-Dependent Variation in the Photophysical Properties of Dinuclear 2-Phenylpyridinato(salicylaldiminato)platinum(II) Complexes Featuring NDI Units. Molecules, 30(12), 2664. https://doi.org/10.3390/molecules30122664









