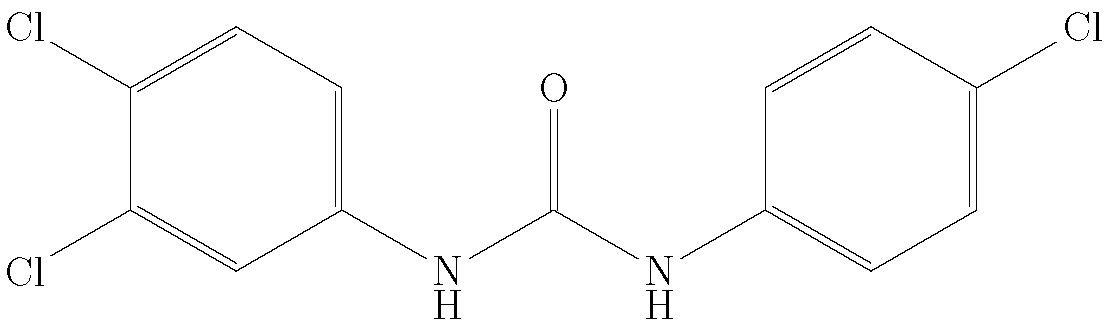
Thermodynamic Study of the Solubility of Triclocarban in Polyethylene Glycol 200 + Water Cosolvent Mixtures at Different Temperatures
Abstract
1. Introduction
2. Results and Discussion
Experimental Solubility ()
3. Solution Thermodynamic Functions of TCC in Cosolvent Mixtures PEG 200 + W
- Sector I: ;
- Sector II: ;
- Sector III: ;
- Sector IV: ;
- Sector V: ;
- Sector VI: ;
- Sector VII: ;
- Sector VIII: .
4. Thermodynamic Functions of Mixing of TCC in Cosolvent Mixtures PEG 200 + W
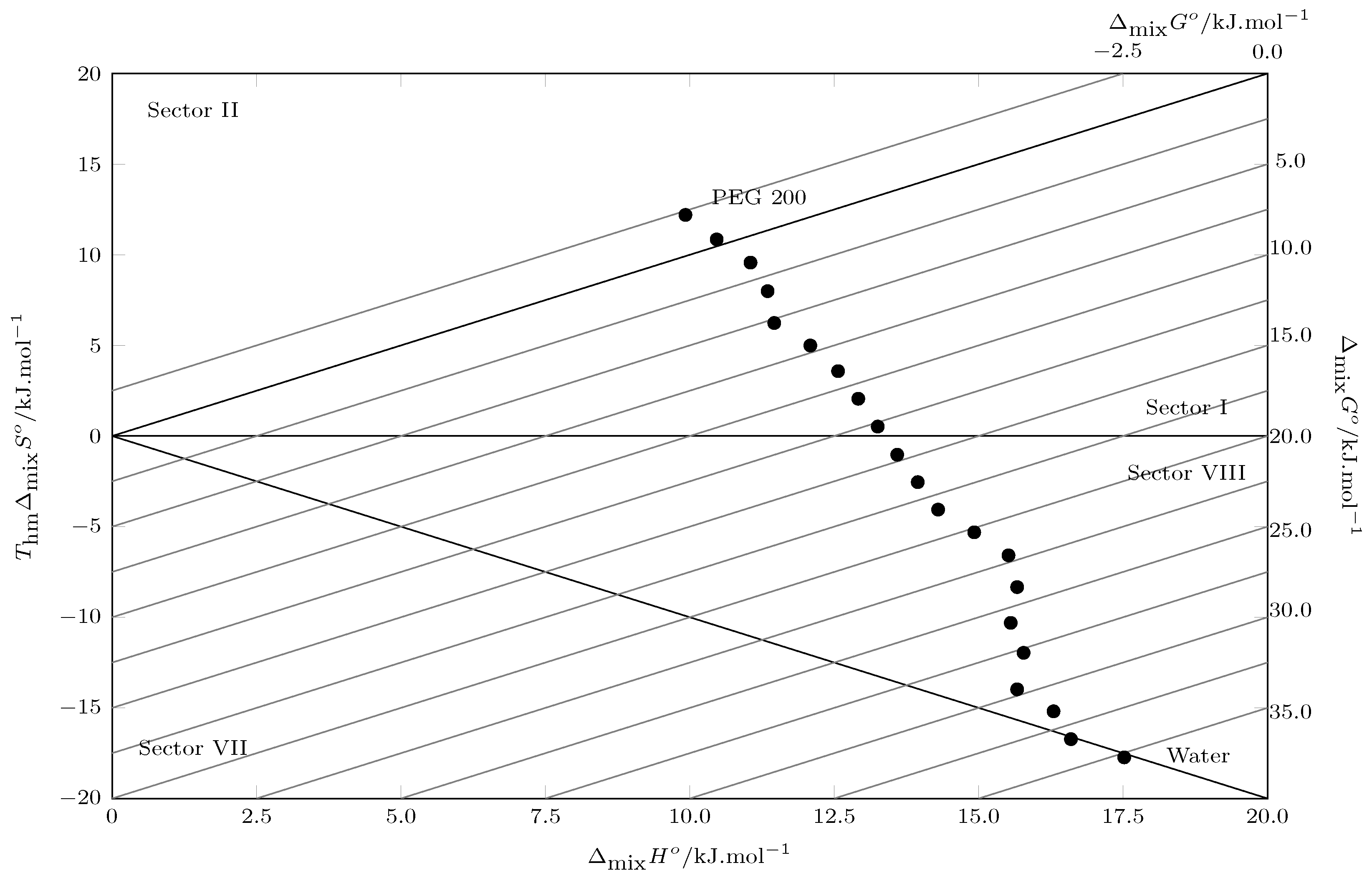
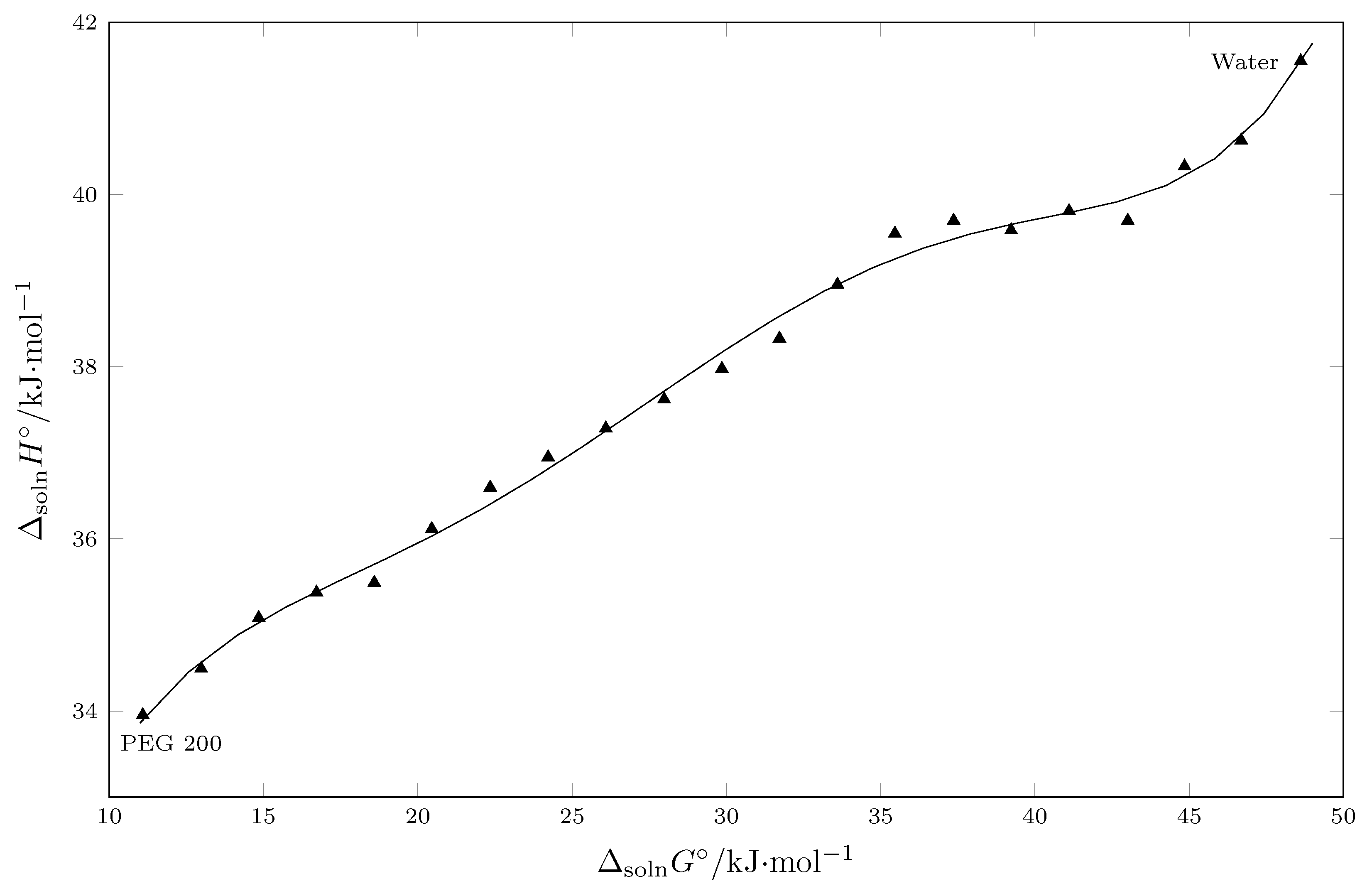
5. Enthalpy–Entropy Compensation (EEC) Analysis
6. Materials and Methods
6.1. Reagents
6.2. Experimental Procedure
6.2.1. Preparation of Cosolvent Mixtures
6.2.2. Addition of Excess Solid Drug
6.2.3. Sealing and Temperature Controlled Stirring
6.2.4. Phase Separation
6.2.5. Quantification
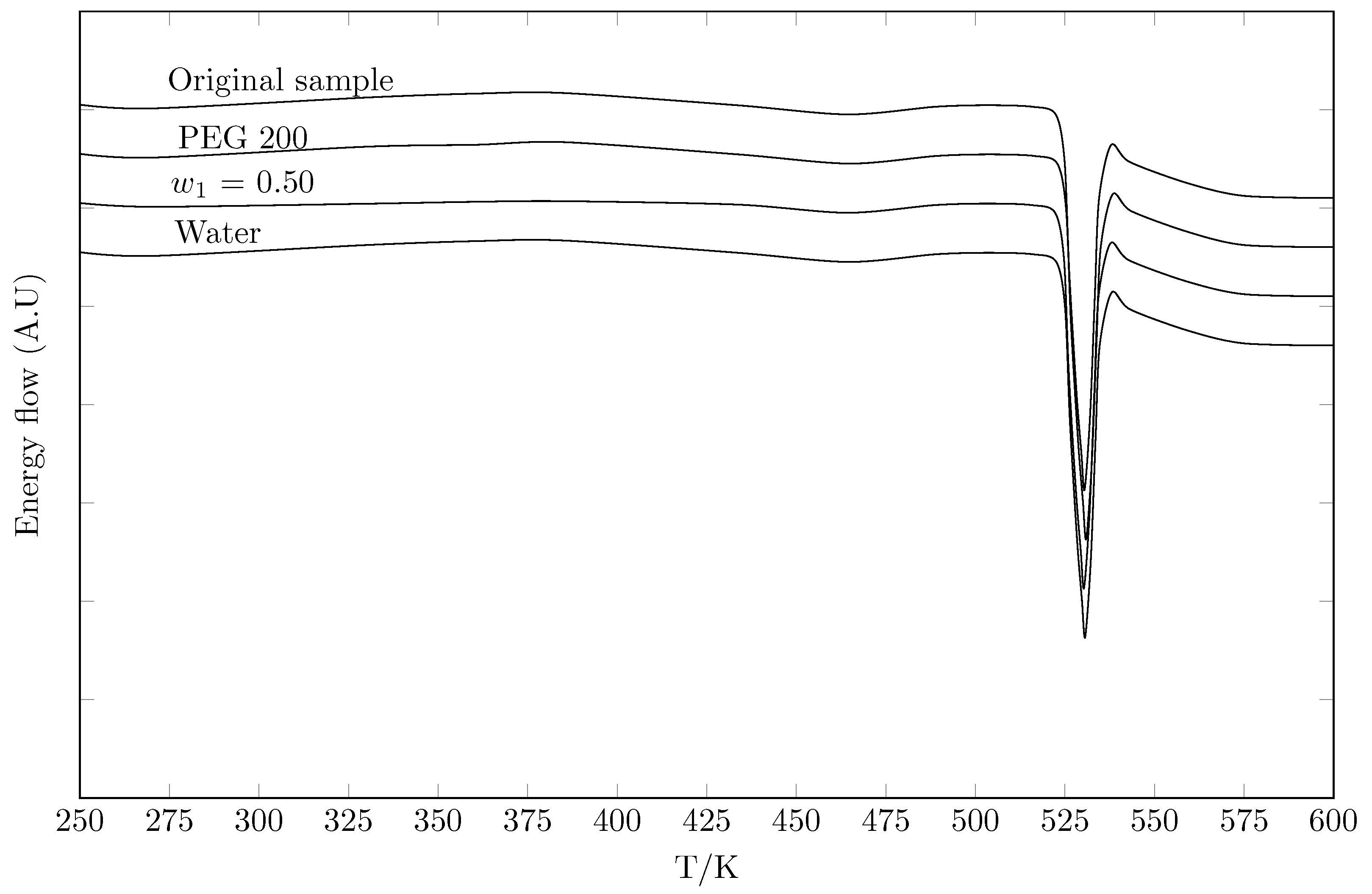
6.2.6. Calorimetric Study
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nyamba, I.; Sombié, C.B.; Yabré, M.; Zimé-Diawara, H.; Yaméogo, J.; Ouédraogo, S.; Lechanteur, A.; Semdé, R.; Evrard, B. Pharmaceutical approaches for enhancing solubility and oral bioavailability of poorly soluble drugs. Eur. J. Pharm. Biopharm. 2024, 204, 114513. [Google Scholar] [CrossRef] [PubMed]
- Yalkowsky, S.H. Solubility and Solubilization in Aqueous Media; ACS personal reference book; American Chemical Society: Washington, DC, USA, 1999. [Google Scholar]
- Yaws, C.L.; Narasimhan, P.K.; Lou, H.H.; Pike, R.W. Solubility of Chemicals in Water. In Water Encyclopedia; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 555–559. [Google Scholar] [CrossRef]
- Martin, A.; Busramante, P.; Chun, A. Physical Pharmacy and Pharmaceutical Sciences, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Harrower, J.; McNaughtan, M.; Hunter, C.; Hough, R.; Zhang, Z.; Helwig, K. Chemical Fate and Partitioning Behavior of Antibiotics in the Aquatic Environment—A Review. Environ. Toxicol. Chem. 2021, 40, 3275–3298. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Saturnino, C.; Salvagno, L.; Ielo, I.; Drommi, D.; Scali, E.; Plutino, M.R.; Rosace, G.; et al. The Different facets of triclocarban: A Review. Molecules 2021, 26, 2811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, L.; Yang, Y.; Cao, J.; Su, Z.; Zhang, B.; Guo, H.; Wang, Z.; Zhang, P.; Xie, J.; et al. Triclocarban triggers osteoarthritis via DNMT1-mediated epigenetic modification and suppression of COL2A in cartilage tissues. J. Hazard. Mater. 2023, 447, 130747. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, S. A holistic review on triclosan and triclocarban exposure: Epidemiological outcomes, antibiotic resistance, and health risk assessment. Sci. Total Environ. 2023, 872, 162114. [Google Scholar] [CrossRef]
- Kristl, A.; Vesnaver, G. Thermodynamic investigation of the effect of octanol–water mutual miscibility on the partitioning and solubility of some guanine derivatives. J. Chem. Soc. Faraday Trans. 1995, 91, 995–998. [Google Scholar] [CrossRef]
- Chiappetta, D.A.; Degrossi, J.; Teves, S.; D’Aquino, M.; Bregni, C.; Sosnik, A. Triclosan-loaded poloxamine micelles for enhanced topical antibacterial activity against biofilm. Eur. J. Pharm. Biopharm. 2008, 69, 535–545. [Google Scholar] [CrossRef]
- Tarrass, F.; Benjelloun, M. Health and environmental effects of the use of N-methyl-2-pyrrolidone as a solvent in the manufacture of hemodialysis membranes: A sustainable reflexion. Nefrología 2022, 42, 122–124. [Google Scholar] [CrossRef]
- Delgado, D.R.; Holguin, A.R.; Martinez, F. Solution thermodynamics of triclosan and triclocarban in some volatile organic solvents. Vitae 2012, 19, 79–92. [Google Scholar] [CrossRef]
- Lim, J.; Jang, S.; Kim, H.; Cho, H.K.; Shin, M.S. Solubility of triclocarban in pure alkanols at different temperatures. Korean J. Chem. Eng. 2013, 30, 181–186. [Google Scholar] [CrossRef]
- Aragón, D.M.; Sosnik, A.; Martínez, F. Solution thermodynamics of triclocarban in organic solvents of different hydrogen bonding capability. J. Solut. Chem. 2009, 38, 1493–1503. [Google Scholar] [CrossRef]
- Holguín, A.R.; Delgado, D.R.; Martínez, F. Thermodynamic study of the solubility of triclocarban in ethanol+ propylene glycol mixtures. Quím. Nova 2012, 35, 280–285. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Decision (EU) 2016/110 of 27 January 2016 Not Approving Triclosan as an Existing Active Substance for Use in Biocidal Products for Product-Type 1; Technical report; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- NORMAN Network. Emerging Substances. 2023. Available online: https://www.norman-network.net/?q=node/19 (accessed on 4 May 2025).
- FDA, U.S. Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use-Final Rule, 21 CFR Part 310 Docket No.; Technical report; FDA: Silver Spring, MD, USA, 2016.
- Rubino, J.; Yalkowsky, S. Cosolvency and cosolvent polarity. Pharm. Res. 1987, 4, 220–230. [Google Scholar] [CrossRef]
- Jouyban-Gharamaleki, A.; Valaee, L.; Barzegar-Jalali, M.; Clark, B.; Acree, W. Comparison of various cosolvency models for calculating solute solubility in water–cosolvent mixtures. Int. J. Pharm. 1999, 177, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Paul, S.; Mitra, B.; Pariyar, G.C.; Ghosh, P. PEG-200: A versatile green solvent assisted catalyst-free one-pot three-component synthesis of functionalised N-amino-3-cyano-2-pyridone. Results Chem. 2023, 5, 100871. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R.S. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005, 7, 64–82. [Google Scholar] [CrossRef]
- Caviedes-Rubio, D.I.; Ortiz, C.P.; Martinez, F.; Delgado, D.R. Thermodynamic Assessment of Triclocarban Dissolution Process in N-Methyl-2-pyrrolidone + Water Cosolvent Mixtures. Molecules 2023, 28, 7216. [Google Scholar] [CrossRef]
- Moradi, M.; Sayari, H.; Martinez, F.; Zhao, H.; Hanaee, J.; Rahimpour, E.; Jouyban, A. Dissolution thermodynamic study of naproxen in the mixtures of ethylene glycol and water. J. Mol. Liq. 2021, 333, 115929. [Google Scholar] [CrossRef]
- Baracaldo-Santamaría, D.; Calderon-Ospina, C.A.; Ortiz, C.P.; Cardenas-Torres, R.E.; Martinez, F.; Delgado, D.R. Thermodynamic Analysis of the Solubility of Isoniazid in (PEG 200 + Water) Cosolvent Mixtures from 278.15 K to 318.15 K. Int. J. Mol. Sci. 2022, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, H.; Jouyban, A. Solubility of Sildenafil Citrate in Polyethylene Glycol 200 + Water Mixtures at 293.2-313.2 K. Pharm. Sci. 2025, 31, 1. [Google Scholar] [CrossRef]
- Panahi-Azar, V.; Ahmadian, S.; Martínez, F.; Acree, W.E.; Jouyban, A. Thermodynamic studies of fluphenazine decanoate solubility in PEG 200+water mixtures. Fluid Phase Equilibria 2012, 330, 36–43. [Google Scholar] [CrossRef]
- Shu, L.; Jegatheesan, L.; Jegatheesan, V.; Li, C.Q. The structure of water. Fluid Phase Equilibria 2020, 511, 112514. [Google Scholar] [CrossRef]
- Chaplin, M.F. Structure and Properties of Water in its Various States. In Water Encyclopedia; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2009; pp. 1–19. [Google Scholar] [CrossRef]
- Zwier, T.S. Squeezing the Water Out of HCl(aq). Science 2009, 324, 1522–1523. [Google Scholar] [CrossRef] [PubMed]
- Agredo-Collazos, J.J.; Ortiz, C.P.; Cerquera, N.E.; Cardenas-Torres, R.E.; Delgado, D.R.; Peña, M.A.; Martínez, F. Equilibrium Solubility of Triclocarban in (Cyclohexane + 1,4-Dioxane) Mixtures: Determination, Correlation, Thermodynamics and Preferential Solvation. J. Solut. Chem. 2021, 51, 1603–1625. [Google Scholar] [CrossRef]
- Cruz-González, A.M.; Vargas-Santana, M.S.; de Jesus Polania-Orozco, S.; Ortiz, C.P.; Cerquera, N.E.; Martínez, F.; Delgado, D.R.; Jouyban, A.; Acree, W.E. Thermodynamic analysis of the solubility of triclocarban in ethylene glycol + water mixtures. J. Mol. Liq. 2021, 325, 115222. [Google Scholar] [CrossRef]
- Delgado, D.R.; Mogollon, E.; Ortiz, C.P.; Peña, M.A.; Almanza, O.A.; Martínez, F.; Jouyban, A. Enthalpy-entropy compensation analysis of the triclocarban dissolution process in some 1,4-dioxane (1) + water (2) mixtures. J. Mol. Liq. 2018, 271, 522–529. [Google Scholar] [CrossRef]
- Perrenot, B.; Widmann, G. Polymorphism by differential scanning calorimetry. Thermochim. Acta 1994, 234, 31–39. [Google Scholar] [CrossRef]
- Ticona Chambi, J.; Fandaruff, C.; Cuffini, S.L. Identification and quantification techniques of polymorphic forms—A review. J. Pharm. Biomed. Anal. 2024, 242, 116038. [Google Scholar] [CrossRef] [PubMed]
- Moffat, A.C.; Osselton, M.D.; Widdop, B.; Watts, J. Clarke’s Analysis of Drugs and Poisons; Pharmaceutical Press: London, UK, 2011; Volume 3. [Google Scholar]
- Krug, R.R.; Hunter, W.G.; Grieger, R.A. Enthalpy-entropy compensation. 1. Some fundamental statistical problems associated with the analysis of van’t Hoff and Arrhenius data. J. Phys. Chem. 1976, 80, 2335–2341. [Google Scholar] [CrossRef]
- Krug, R.R.; Hunter, W.G.; Grieger, R.A. Enthalpy-entropy compensation. 2. Separation of the chemical from the statistical effect. J. Phys. Chem. 1976, 80, 2341–2351. [Google Scholar] [CrossRef]
- Silbey, R.J.; Alberty, R.A.; Papadantonakis, G.A.; Bawendi, M.G. Physical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Hildebrand, J.H.; Prausnitz, J.M.; Scott, R.L. Regular and Related Solutions: The Solubility of Gases, Liquids, and Solids; Van Nostrand Reinhold: Cleveland, OH, USA, 1970. [Google Scholar]
- Perlovich, G.L.; Kurkov, S.V.; Kinchin, A.N.; Bauer-Brandl, A. Thermodynamics of solutions III: Comparison of the solvation of (+)-naproxen with other NSAIDs. Eur. J. Pharm. Biopharm. 2004, 57, 411–420. [Google Scholar] [CrossRef]
- Yalkowsky, S.; Wu, M. Estimation of the ideal solubility (crystal–liquid fugacity ratio) of organic compounds. J. Pharm. Sci. 2010, 99, 1100–1106. [Google Scholar] [CrossRef]
- Bustamante, P.; Romero, S.; Peña, A.; Escalera, B.; Reillo, A. Enthalpy-entropy compensation for the solubility of drugs in solvent mixtures: Paracetamol, acetanilide, and nalidixic acid in dioxane–water. J. Pharm. Sci. 1998, 87, 1590–1596. [Google Scholar] [CrossRef]
- Bustamante, C.; Bustamante, P. Nonlinear enthalpy–entropy compensation for the solubility of phenacetin in dioxane–water solvent mixtures. J. Pharm. Sci. 1996, 85, 1109–1111. [Google Scholar] [CrossRef]
- Ryde, U. A fundamental view of enthalpy–entropy compensation. Med. Chem. Commun. 2014, 5, 1324–1336. [Google Scholar] [CrossRef]
- Mader, W.J.; Higuchi, T. Phase solubility analysis. C R C Crit. Rev. Anal. Chem. 1970, 1, 193–215. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K. Advances in Analytical Chemistry and Instrumentation; Interscience Publishers, Inc.: New York, NY, USA, 1965. [Google Scholar]
- Dittert, L.W.; Higuchi, T.; Reese, D.R. Phase solubility technique in studying the formation of complex salts of triamterene. J. Pharm. Sci. 1964, 53, 1325–1328. [Google Scholar] [CrossRef]
- Walfish, S. Analytical Methods: A Statistical Perspective on the ICH Q2A and Q2B Guidelines for Validation of Analytical Methods. BioPharm Int. 2006, 19, 40–45. [Google Scholar]
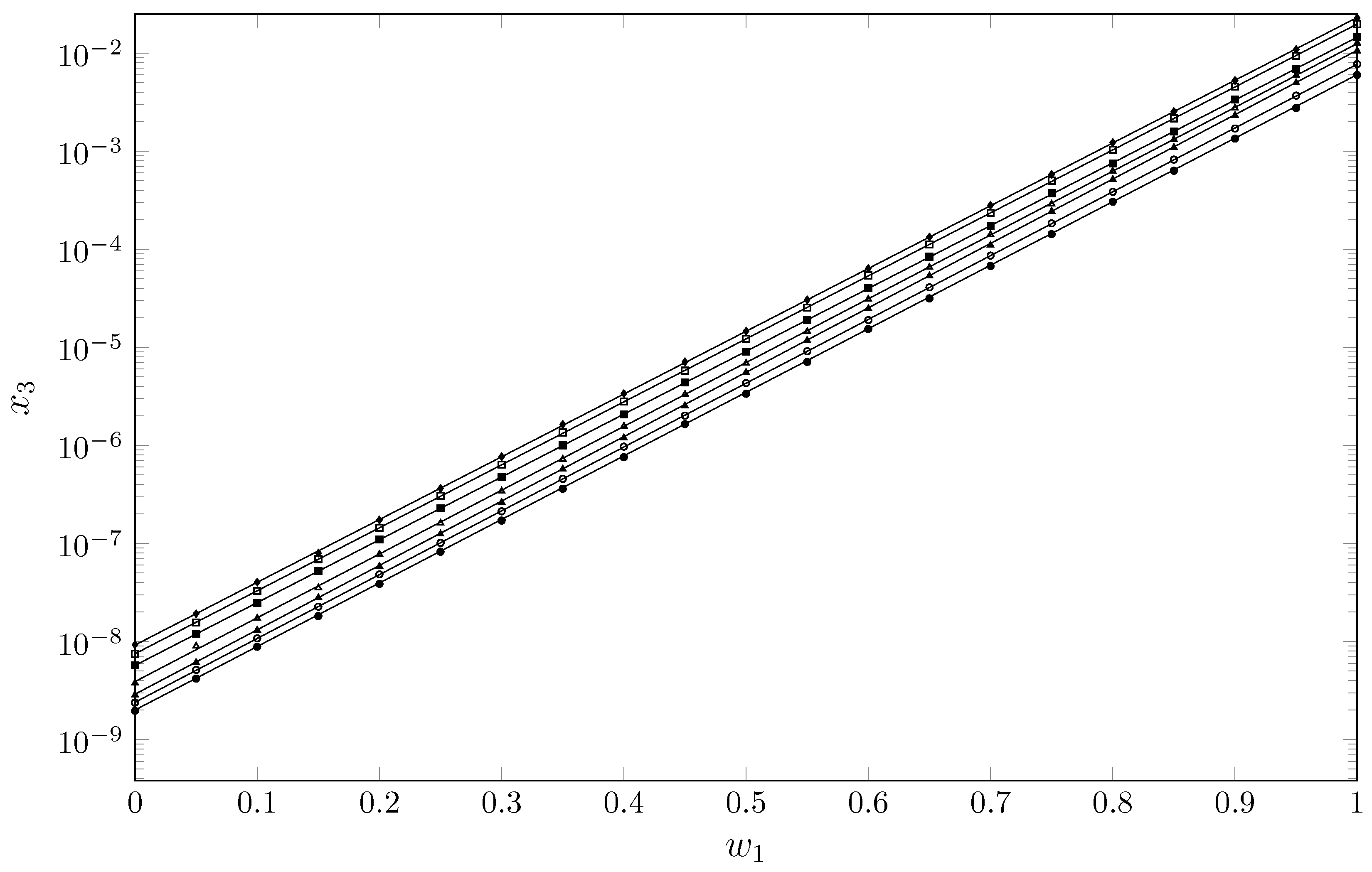

| w1 | Temperatures | ||||||
|---|---|---|---|---|---|---|---|
| 288.15 K | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | 318.15 K | |
| 0.00 | 1.96 | 2.38 | 2.85 | 3.78 | 5.72 | 7.48 | 9.28 |
| 0.05 | 4.19 | 5.13 | 6.11 | 9.00 | 1.20 | 1.56 | 1.92 |
| 0.10 | 8.84 | 1.08 | 1.31 | 1.73 | 2.47 | 3.28 | 4.04 |
| 0.15 | 1.82 | 2.27 | 2.80 | 3.53 | 5.23 | 6.92 | 7.98 |
| 0.20 | 3.86 | 4.84 | 5.84 | 7.77 | 1.10 | 1.44 | 1.73 |
| 0.25 | 8.23 | 1.01 | 1.25 | 1.62 | 2.29 | 3.07 | 3.64 |
| 0.30 | 1.71 | 2.13 | 2.61 | 3.44 | 4.77 | 6.37 | 7.67 |
| 0.35 | 3.61 | 4.55 | 5.72 | 7.21 | 1.00 | 1.35 | 1.64 |
| 0.40 | 7.58 | 9.71 | 1.19 | 1.57 | 2.07 | 2.80 | 3.39 |
| 0.45 | 1.65 | 2.01 | 2.52 | 3.32 | 4.38 | 5.78 | 7.10 |
| 0.50 | 3.36 | 4.32 | 5.56 | 6.88 | 9.02 | 1.22 | 1.46 |
| 0.55 | 7.10 | 9.12 | 1.17 | 1.45 | 1.90 | 2.55 | 3.05 |
| 0.60 | 1.54 | 1.90 | 2.47 | 3.10 | 4.03 | 5.41 | 6.37 |
| 0.65 | 3.16 | 4.09 | 5.34 | 6.54 | 8.37 | 1.12 | 1.34 |
| 0.70 | 6.77 | 8.62 | 1.10 | 1.41 | 1.72 | 2.35 | 2.81 |
| 0.75 | 1.43 | 1.84 | 2.44 | 2.90 | 3.73 | 5.00 | 5.80 |
| 0.80 | 3.05 | 3.85 | 5.14 | 6.26 | 7.50 | 1.03 | 1.22 |
| 0.85 | 6.31 | 8.21 | 1.09 | 1.32 | 1.59 | 2.17 | 2.54 |
| 0.90 | 1.34 | 1.70 | 2.32 | 2.76 | 3.37 | 4.56 | 5.27 |
| 0.95 | 2.76 | 3.69 | 4.98 | 5.93 | 6.92 | 9.38 | 1.09 |
| 1.00 | 5.99 | 7.72 | 1.05 | 1.27 | 1.47 | 1.98 | 2.28 |
| Sample | Enthalpy of Fusion, | Melting Point, | Ref. |
|---|---|---|---|
| Original sample | This work | ||
| [26] | |||
| [34] | |||
| [35] | |||
| 525 | [36] | ||
| 528.15–529.15 | [39] | ||
| Water | This work | ||
| [26] | |||
| PEG 200 () | This work | ||
| PEG 200 | This work |
/kJ·mol−1 | /kJ·mol−1 | /kJ·K−1·mol−1 | /kJ·mol−1 | |||
|---|---|---|---|---|---|---|
| 0.00 | 48.61 | 41.55 | −23.33 | −7.06 | 0.85 | 0.15 |
| 0.05 | 46.69 | 40.63 | −20.01 | −6.06 | 0.87 | 0.13 |
| 0.10 | 44.85 | 40.33 | −14.94 | −4.52 | 0.90 | 0.10 |
| 0.15 | 43.01 | 39.70 | −10.94 | −3.31 | 0.92 | 0.08 |
| 0.20 | 41.11 | 39.81 | −4.28 | −0.30 | 0.97 | 0.03 |
| 0.25 | 39.23 | 39.59 | 1.18 | 0.36 | 0.99 | 0.01 |
| 0.30 | 37.37 | 39.70 | 7.69 | 2.33 | 0.94 | 0.06 |
| 0.35 | 35.46 | 39.55 | 13.48 | 4.08 | 0.91 | 0.09 |
| 0.40 | 33.60 | 38.95 | 17.68 | 5.35 | 0.88 | 0.12 |
| 0.45 | 31.72 | 38.33 | 21.82 | 6.61 | 0.85 | 0.15 |
| 0.50 | 29.85 | 37.98 | 26.82 | 8.12 | 0.82 | 0.18 |
| 0.55 | 27.98 | 37.62 | 31.82 | 9.64 | 0.80 | 0.20 |
| 0.60 | 26.09 | 37.28 | 36.95 | 11.19 | 0.77 | 0.23 |
| 0.65 | 24.22 | 36.95 | 42.01 | 12.72 | 0.74 | 0.26 |
| 0.70 | 22.35 | 36.60 | 47.05 | 14.25 | 0.72 | 0.28 |
| 0.75 | 20.45 | 36.12 | 51.72 | 15.66 | 0.70 | 0.30 |
| 0.80 | 18.59 | 35.49 | 55.82 | 16.90 | 0.68 | 0.32 |
| 0.85 | 16.72 | 35.38 | 61.62 | 18.66 | 0.65 | 0.35 |
| 0.90 | 14.85 | 35.08 | 66.82 | 20.23 | 0.63 | 0.37 |
| 0.95 | 12.98 | 34.49 | 71.05 | 21.51 | 0.62 | 0.38 |
| 1.00 | 11.09 | 33.95 | 75.51 | 22.87 | 0.60 | 0.40 |
/kJ·mol−1 | /kJ·mol−1 | /kJ·mol−1·K−1 | /kJ·mol−1 | |
|---|---|---|---|---|
| 0.00 | 35.25 | 17.52 | −58.55 | −17.73 |
| 0.05 | 33.32 | 16.60 | −55.23 | −16.73 |
| 0.10 | 31.49 | 16.30 | −50.16 | −15.19 |
| 0.15 | 29.64 | 15.67 | −46.16 | −13.98 |
| 0.20 | 27.74 | 15.78 | −39.51 | −11.96 |
| 0.25 | 25.87 | 15.55 | −34.05 | −10.31 |
| 0.30 | 24.00 | 15.67 | −27.53 | −8.34 |
| 0.35 | 22.10 | 15.52 | −21.74 | −6.58 |
| 0.40 | 20.24 | 14.92 | −17.54 | −5.31 |
| 0.45 | 18.36 | 14.30 | −13.41 | −4.06 |
| 0.50 | 16.49 | 13.95 | −8.41 | −2.55 |
| 0.55 | 14.62 | 13.59 | −3.40 | −1.03 |
| 0.60 | 12.73 | 13.25 | 1.72 | 0.52 |
| 0.65 | 10.86 | 12.92 | 6.79 | 2.06 |
| 0.70 | 8.99 | 12.57 | 11.82 | 3.58 |
| 0.75 | 7.09 | 12.09 | 16.49 | 4.99 |
| 0.80 | 5.23 | 11.46 | 20.59 | 6.24 |
| 0.85 | 3.35 | 11.35 | 26.39 | 7.99 |
| 0.90 | 1.48 | 11.05 | 31.60 | 9.57 |
| 0.95 | −0.38 | 10.46 | 35.82 | 10.85 |
| 1.00 | −2.27 | 9.92 | 40.28 | 12.20 |
| Chemical Name | CAS a | Source | Purity in Mass Fraction | Analytic Technique b |
|---|---|---|---|---|
| Triclocarban | 101-20-2 | Sigma-Aldrich | >0.990 | HPLC |
| Polyethylene glycol 200 | 25322-68-3 | Sigma-Aldrich | 0.998 | GC |
| Water | 7732-18-5 | |||
| Ethanol | 64-17-5 | Sigma-Aldrich | 0.998 | GC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puentes-Lozada, V.; Caviedes-Rubio, D.I.; Rincón-Guio, C.; Cerquera, N.E.; Cardenas-Torres, R.E.; Ortiz, C.P.; Martinez, F.; Delgado, D.R. Thermodynamic Study of the Solubility of Triclocarban in Polyethylene Glycol 200 + Water Cosolvent Mixtures at Different Temperatures. Molecules 2025, 30, 2631. https://doi.org/10.3390/molecules30122631
Puentes-Lozada V, Caviedes-Rubio DI, Rincón-Guio C, Cerquera NE, Cardenas-Torres RE, Ortiz CP, Martinez F, Delgado DR. Thermodynamic Study of the Solubility of Triclocarban in Polyethylene Glycol 200 + Water Cosolvent Mixtures at Different Temperatures. Molecules. 2025; 30(12):2631. https://doi.org/10.3390/molecules30122631
Chicago/Turabian StylePuentes-Lozada, Vanesa, Diego Ivan Caviedes-Rubio, Cristian Rincón-Guio, Nestor Enrique Cerquera, Rossember Edén Cardenas-Torres, Claudia Patricia Ortiz, Fleming Martinez, and Daniel Ricardo Delgado. 2025. "Thermodynamic Study of the Solubility of Triclocarban in Polyethylene Glycol 200 + Water Cosolvent Mixtures at Different Temperatures" Molecules 30, no. 12: 2631. https://doi.org/10.3390/molecules30122631
APA StylePuentes-Lozada, V., Caviedes-Rubio, D. I., Rincón-Guio, C., Cerquera, N. E., Cardenas-Torres, R. E., Ortiz, C. P., Martinez, F., & Delgado, D. R. (2025). Thermodynamic Study of the Solubility of Triclocarban in Polyethylene Glycol 200 + Water Cosolvent Mixtures at Different Temperatures. Molecules, 30(12), 2631. https://doi.org/10.3390/molecules30122631












