Rich Oxygen Vacancies Induced by Surface Self-Reconstruction in Sandwich-like Hierarchical Structured Electrocatalyst for Boosting Oxygen Evolution Reaction
Abstract
1. Introduction
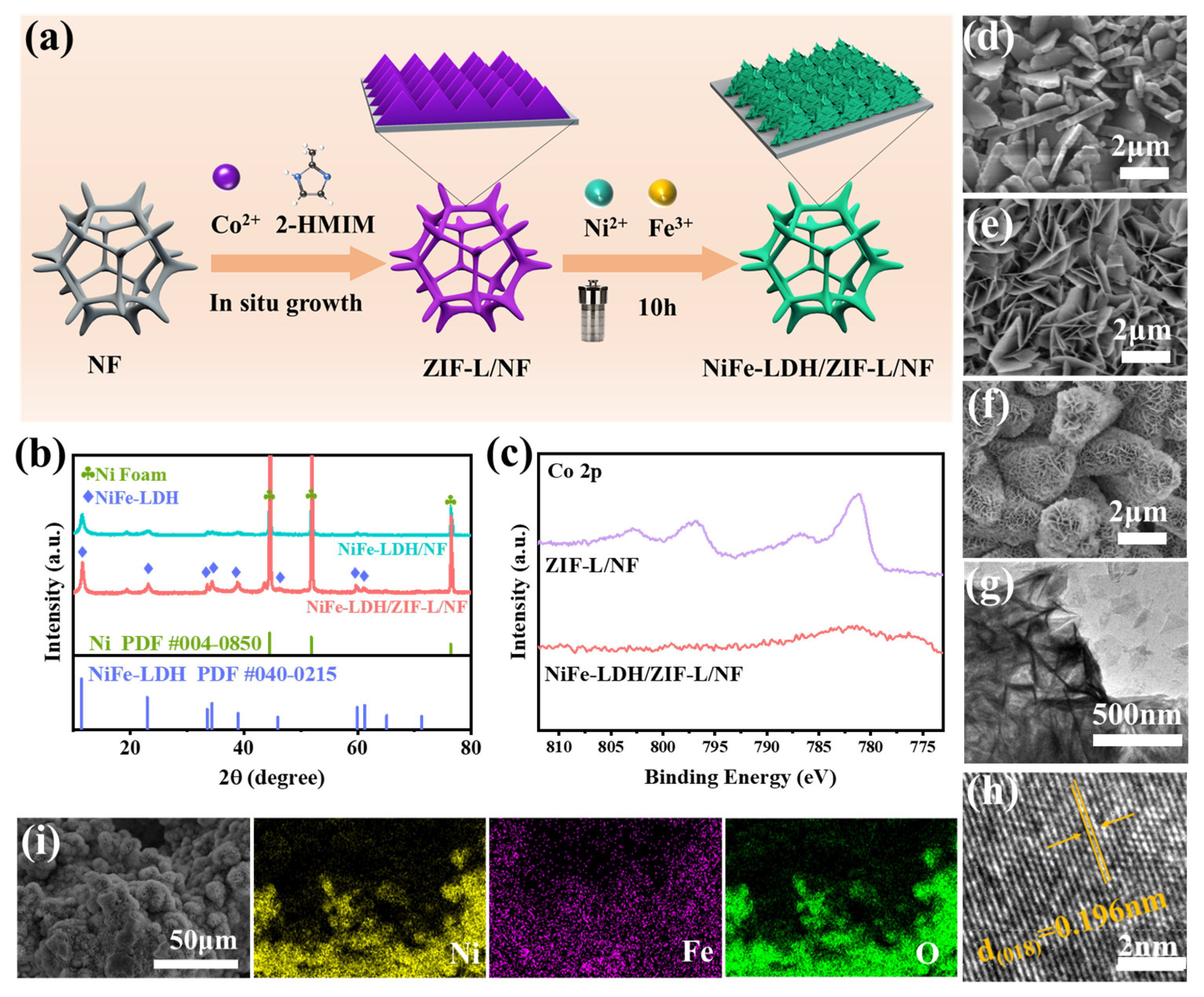
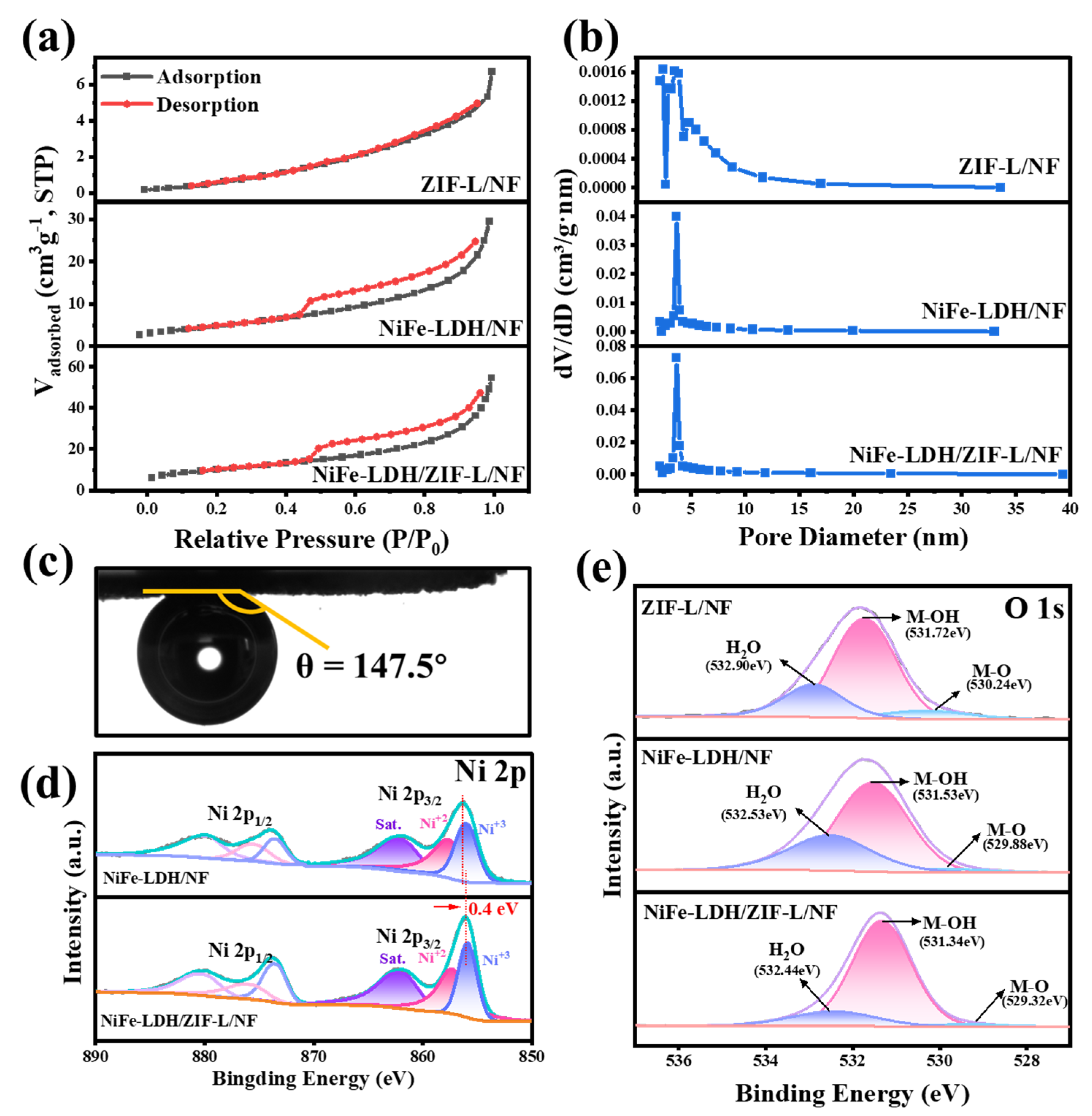
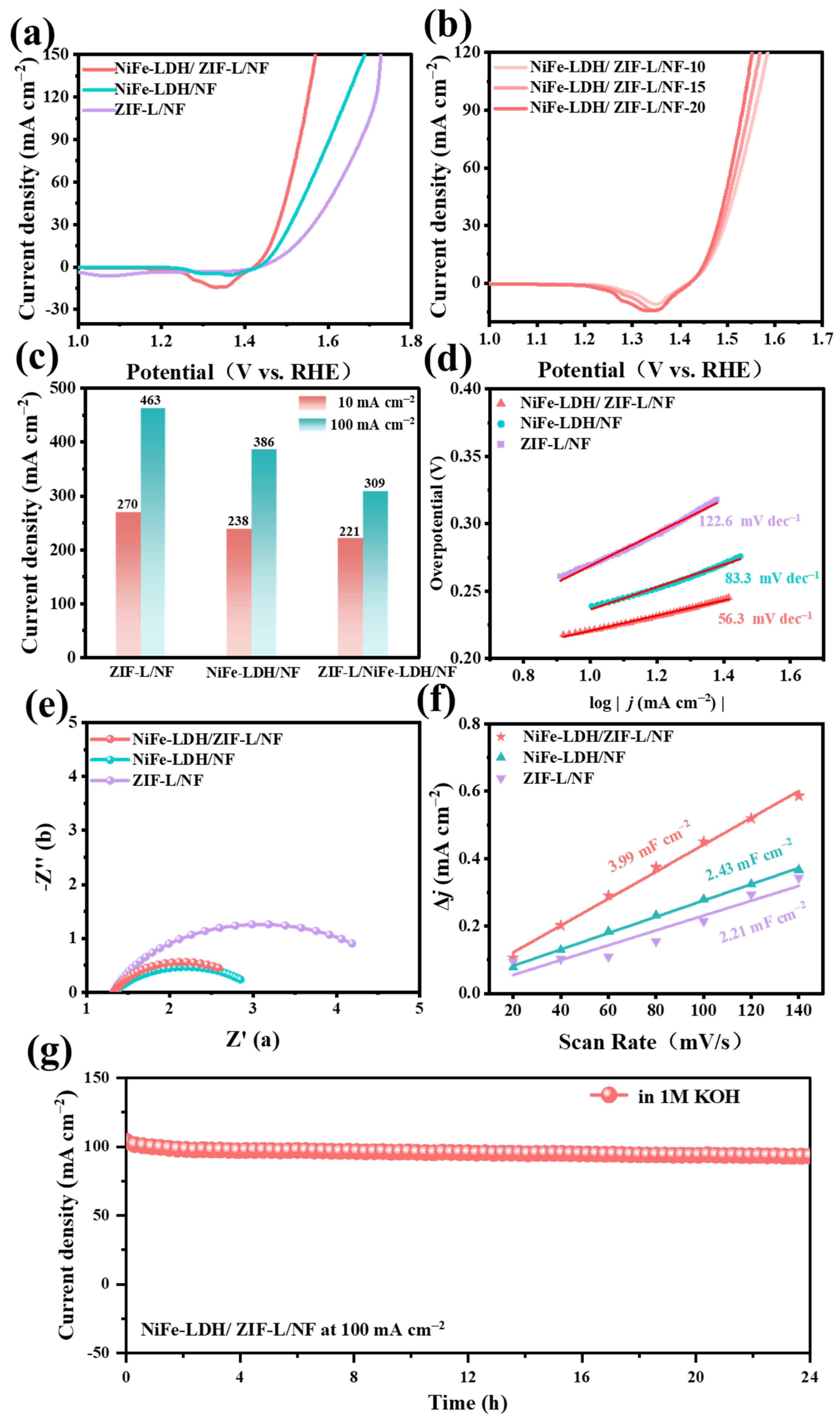
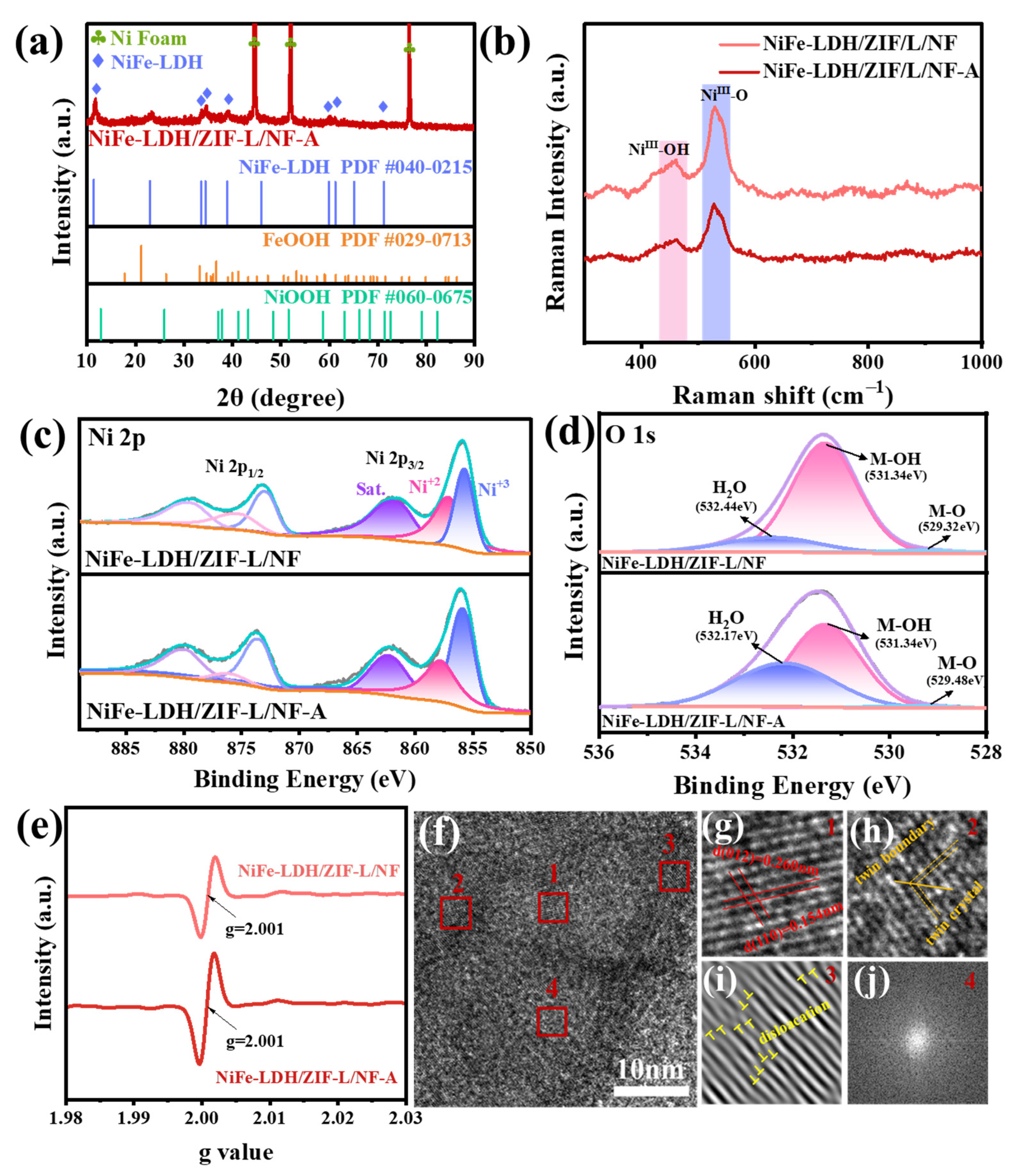
2. Results and Discussion
3. Experimental Setup
3.1. Materials
3.2. Characterization
3.3. Electrochemical Measurements
3.4. Calculation of ECSA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, L.; Ding, Z.; Karkera, G.; Diemant, T.; Chen, D.-H.; Fichtner, M.; Hahn, H.; Aghassi-Hagmann, J.; Breitung, B.; Schweidler, S. Layered high-entropy sulfides: Boosting electrocatalytic performance for hydrogen evolution reaction by cocktail effects. Mater. Futures 2024, 3, 045102. [Google Scholar] [CrossRef]
- Shu, C.; Cao, J.; Gan, Z.; Qiu, P.; Chen, Z.; Guanwu, L.; Chen, Z.; Deng, C.; Tang, W. Synergistic effect between Co single atoms and Pt nanoparticles for efficient alkaline hydrogen evolution. Mater. Futures 2024, 3, 035101. [Google Scholar] [CrossRef]
- Yang, N.; He, T.; Chen, X.; He, Y.; Zhou, T.; Zhang, G.; Liu, Q. TiO2-based heterojunctions for photocatalytic hydrogen evolution reaction. Microstructures 2024, 4, 2024042. [Google Scholar] [CrossRef]
- Milikić, J.; Nastasić, A.; Rakočević, L.; Radinović, K.; Stojadinović, S.; Stanković, D.; Šljukić, B. FeM/rGO (M = Ni and Cu) as bifunctional oxygen electrode. Fuel 2024, 368, 131654. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, D.; Liu, J.; Wang, Z.; Wang, L. Advancing strategies on green H2 production via water electrocatalysis: Bridging the benchtop research with industrial scale-up. Microstructures 2024, 4, 2024020. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Tekalgne, M.; Van Le, Q.; Van Tran, C.; Ahn, S.H.; Kim, S.Y. Recent progress and strategies of non-noble metal electrocatalysts based on MoS2/MOF for the hydrogen evolution reaction in water electrolysis: An overview. Microstructures 2024, 4, 2024046. [Google Scholar] [CrossRef]
- Yan, J.; Wu, R.; Jin, G.; Jia, L.; Feng, G.; Tong, X. The hybrid Pt nanoclusters/Ru nanowires catalysts accelerating alkaline hydrogen evolution reaction. Adv. Powder Mater. 2024, 3, 100214. [Google Scholar] [CrossRef]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I. Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar]
- Zhao, Y.; Tian, Z.; Wang, W.; Deng, X.; Tseng, J.-C.; Wang, G. Size-dependent activity of Fe-N-doped mesoporous carbon nanoparticles towards oxygen reduction reaction. Green Carbon 2024, 2, 221–230. [Google Scholar] [CrossRef]
- Liu, G.; Xu, Y.; Yang, T.; Jiang, L. Recent advances in electrocatalysts for seawater splitting. Nano Mater. Sci. 2023, 5, 101–116. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, W.; Yang, B.; Liao, C.; Kang, Q.; Chen, G.; Liu, M.; Liu, X.; Ma, R.; Zhang, N. Tuned d-band states over lanthanum doped nickel oxide for efficient oxygen evolution reaction. Nano Mater. Sci. 2023, 5, 228–236. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, F.; Liu, Y.; Liu, W. Preparation of Cu(OH)2/Cu2S arrays for enhanced hydrogen evolution reaction. Battery Energy 2024, 3, 20230060. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Tekalgne, M.; Nguyen, T.P.; Van Le, Q.; Ahn, S.H.; Kim, S.Y. Electrocatalysts based on MoS2 and WS2 for hydrogen evolution reaction: An overview. Battery Energy 2023, 2, 20220057. [Google Scholar] [CrossRef]
- Vij, V.; Sultan, S.; Harzandi, A.M.; Meena, A.; Tiwari, J.N.; Lee, W.-G.; Yoon, T.; Kim, K.S. Nickel-based electrocatalysts for energy-related applications: Oxygen reduction, oxygen evolution, and hydrogen evolution reactions. Acs Catal. 2017, 7, 7196–7225. [Google Scholar] [CrossRef]
- Milikić, J.; Nastasić, A.; Knežević, S.; Rakočević, L.; Stojadinović, S.; Stanković, D.; Šljukić, B. Efficient nano-size ZnM/rGO (M= Ni, Cu, and Fe) electrocatalysts for oxygen electrode reactions in alkaline media. Int. J. Hydrogen Energy 2025, 97, 247–258. [Google Scholar] [CrossRef]
- Amin, H.M.; Attia, M.; Tetzlaff, D.; Apfel, U.P. Tailoring the Electrocatalytic Activity of Pentlandite FexNi9-XS8 Nanoparticles via Variation of the Fe: Ni Ratio for Enhanced Water Oxidation. ChemElectroChem 2021, 8, 3863–3874. [Google Scholar] [CrossRef]
- Amin, H.M.; Apfel, U.P. Metal-Rich chalcogenides as sustainable electrocatalysts for oxygen evolution and reduction: State of the art and future perspectives. Eur. J. Inorg. Chem. 2020, 2020, 2679–2690. [Google Scholar] [CrossRef]
- Mu, Y.; Pei, X.; Zhao, Y.; Dong, X.; Kou, Z.; Cui, M.; Meng, C.; Zhang, Y. In situ confined vertical growth of Co2.5Ni0.5Si2O5(OH)4 nanoarrays on rGO for an efficient oxygen evolution reaction. Nano Mater. Sci. 2023, 5, 351–360. [Google Scholar] [CrossRef]
- Marquez, R.A.; Kalokowski, E.; Espinosa, M.; Bender, J.T.; Son, Y.J.; Kawashima, K.; Chukwuneke, C.E.; Smith, L.A.; Celio, H.; Dolocan, A. Transition metal incorporation: Electrochemical, structure, and chemical composition effects on nickel oxyhydroxide oxygen-evolution electrocatalysts. Energy Environ. Sci. 2024, 17, 2028–2045. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Zhang, Z.; Li, Y.; Liu, J.; Yang, Y.; Xue, B.; Li, F. Self-adaptively electrochemical reconstruction of NiFe-layered double hydroxide on Ni foam for high-performance water splitting. J. Alloys Compd. 2023, 934, 167846. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Q.; Zeng, X. Construction of multiple heterogeneous interfaces and oxygen evolution reaction of hollow CoFe bimetallic phosphides derived from MOF template. Prog. Nat. Sci. Mater. Int. 2024, 34, 913–920. [Google Scholar] [CrossRef]
- Mane, R.S.; Mane, S.; Somkuwar, V.; Thombre, N.V.; Patwardhan, A.V.; Jha, N. A novel hierarchically hybrid structure of MXene and bi-ligand ZIF-67 based trifunctional electrocatalyst for zinc-air battery and water splitting. Battery Energy 2023, 2, 20230019. [Google Scholar] [CrossRef]
- Yu, X.; Chen, Y.; Wu, Y.; Chu, X.; Liu, B.; Hu, B.; Che, G.; Jiang, W.; Liu, C. Self-reconstructed ZIF-L/Co(OH)2 heterointerface modulates electronic structure of Ru and boosts electrocatalytic hydrogen evolution. Chem. Eng. J. 2024, 490, 151926. [Google Scholar] [CrossRef]
- Yan, M.; Chen, S.; Wu, S.; Zhou, X.; Fu, S.; Wang, D.; Kübel, C.; Hahn, H.; Lan, S.; Feng, T. Enhanced activity and durability of FeCoCrMoCBY nanoglass in acidic hydrogen evolution reaction. J. Mater. Sci. Technol. 2024, 170, 212–220. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Liu, M.; Zhang, L.T.; Lu, N.; Wang, X.; Li, Z.G.; Zhang, X.H.; Li, N.; Bu, X.H. Strategic Defect Engineering Enabled Efficient Oxygen Evolution Reaction in Reconstructed Metal-Organic Frameworks. Adv. Funct. Mater. 2025, 35, 2412406. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, F.; Yang, X.; Zhu, J.; Yang, S.; Chen, L.; Zhao, P.; Wang, Q.; Zhang, Q. Constructing interlaced network structure by grain boundary corrosion methods on CrCoNiFe alloy for high-performance oxygen evolution reaction and urea oxidation reaction. J. Mater. Sci. Technol. 2024, 203, 97–107. [Google Scholar] [CrossRef]
- Amin, H.M.; Baltruschat, H.; Wittmaier, D.; Friedrich, K.A. A highly efficient bifunctional catalyst for alkaline air-electrodes based on a Ag and Co3O4 hybrid: RRDE and online DEMS insights. Electrochim. Acta 2015, 151, 332–339. [Google Scholar] [CrossRef]
- Ding, J.; Guo, D.; Wang, N.; Wang, H.F.; Yang, X.; Shen, K.; Chen, L.; Li, Y. Defect engineered metal–organic framework with accelerated structural transformation for efficient oxygen evolution reaction. Angew. Chem. Int. Ed. 2023, 62, e202311909. [Google Scholar] [CrossRef]
- Gu, P.; Song, Y.; Fan, Y.; Meng, X.; Liu, J.; Wang, G.; Li, Z.; Sun, H.; Zhao, Z.; Zou, J. Crystalline-Amorphous Interface-Triggered Electron Redistribution on Copper (II) Sulfide@ Metal (Ni, Co, and Fe) Oxyhydroxides for Ultra-Efficient Overall Water/Seawater Splitting. Adv. Energy Mater. 2025, 15, 2403657. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Zhang, G.; Yu, X.; Zhao, Z.; Zhang, Y.; Shi, Y.; Zhu, H.-B.; Xiao, X. Efficient electrocatalytic overall water splitting of porous 3D CoPt3/a-FCWO-NS heterostructures: Morphology modulation and interfacial engineering for the formation of crystalline-amorphous nanosheets. Appl. Catal. B Environ. 2024, 342, 123387. [Google Scholar] [CrossRef]
- Liao, Y.; He, R.; Pan, W.; Li, Y.; Wang, Y.; Li, J.; Li, Y. Lattice distortion induced Ce-doped NiFe-LDH for efficient oxygen evolution. Chem. Eng. J. 2023, 464, 142669. [Google Scholar] [CrossRef]
- Liu, Z.; Amin, H.M.; Peng, Y.; Corva, M.; Pentcheva, R.; Tschulik, K. Facet-Dependent Intrinsic Activity of Single Co3O4 Nanoparticles for Oxygen Evolution Reaction. Adv. Funct. Mater. 2023, 33, 2210945. [Google Scholar] [CrossRef]
- Wu, H.; Yang, T.; Du, Y.; Shen, L.; Ho, G.W. Identification of facet-governing reactivity in hematite for oxygen evolution. Adv. Mater. 2018, 30, 1804341. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, H.; Zheng, K.; Zhang, Z.; Jiang, Q.; Li, J. Two-dimensional hydrophilic ZIF-L as a highly-selective adsorbent for rapid phosphate removal from wastewater. Sci. Total Environ. 2021, 785, 147382. [Google Scholar] [CrossRef]
- Nasir, A.M.; Nordin, N.M.; Goh, P.; Ismail, A. Application of two-dimensional leaf-shaped zeolitic imidazolate framework (2D ZIF-L) as arsenite adsorbent: Kinetic, isotherm and mechanism. J. Mol. Liq. 2018, 250, 269–277. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, K.; Wang, M.; Fan, H.; Yan, Z.; Du, X.; Chen, Y. In-situ construction of cation vacancies in amphoteric-metallic element-doped NiFe-LDH as ultrastable and efficient alkaline hydrogen evolution electrocatalysts at 1000 mA cm−2. J. Colloid Interface Sci. 2024, 663, 624–631. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Xu, X.-Y.; Shi, C.-X.; Ye, X.-W.; Sun, P.-C.; Chen, T.-H. Iron–nitrogen co-doped hierarchically mesoporous carbon spheres as highly efficient electrocatalysts for the oxygen reduction reaction. RSC Adv. 2017, 7, 8879–8885. [Google Scholar] [CrossRef]
- Masnica, J.P.; Sibt-e-Hassan, S.; Potgieter-Vermaak, S.; Regmi, Y.N.; King, L.A.; Tosheva, L. ZIF-8-derived Fe-C catalysts: Relationship between structure and catalytic activity toward the oxygen reduction reaction. Green Carbon 2023, 1, 160–169. [Google Scholar] [CrossRef]
- Ma, L.; Wei, Z.; Zhao, C.; Meng, X.; Zhang, H.; Song, M.; Wang, Y.; Li, B.; Huang, X.; Xu, C. Hierarchical superhydrophilic/superaerophobic 3D porous trimetallic (Fe, Co, Ni) spinel/carbon/nickel foam for boosting oxygen evolution reaction. Appl. Catal. B Environ. 2023, 332, 122717. [Google Scholar] [CrossRef]
- Ross, B.; Haussener, S.; Brinkert, K. Impact of Gas Bubble Evolution Dynamics on Electrochemical Reaction Overpotentials in Water Electrolyser Systems. J. Phys. Chem. C 2025, 129, 4383–4397. [Google Scholar] [CrossRef]
- Zan, L.; Amin, H.M.; Mostafa, E.; Abd-El-Latif, A.A.; Iqbal, S.; Baltruschat, H. Electrodeposited cobalt nanosheets on smooth silver as a bifunctional catalyst for OER and ORR: In situ structural and catalytic characterization. ACS Appl. Mater. Interfaces 2022, 14, 55458–55470. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Humayun, M.; Li, Y.; Zhang, H.; Sun, H.; Wu, Y.; Wang, C. Constructing hierarchical fluffy CoO–Co4N@NiFe-LDH nanorod arrays for highly effective overall water splitting and urea electrolysis. ACS Sustain. Chem. Eng. 2021, 9, 14180–14192. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Xu, J.; Sun, H.; Shi, Z. Constructing surface concave defects on NiFe layered double hydroxides by electrochemical reduction for efficient oxygen evolution reaction. Chem. Eng. J. 2024, 482, 148858. [Google Scholar] [CrossRef]
- Tang, J.; Guan, D.; Xu, H.; Zhao, L.; Arshad, U.; Fang, Z.; Zhu, T.; Kim, M.; Pao, C.-W.; Hu, Z. Undoped ruthenium oxide as a stable catalyst for the acidic oxygen evolution reaction. Nat. Commun. 2025, 16, 801. [Google Scholar] [CrossRef]
- Yin, H.; Su, S.; Yao, D.; Wang, L.; Liu, X.; Isimjan, T.T.; Yang, X.; Cai, D. Ultrathin 2D–2D NiFe LDH/MOF heterojunction nanosheets: An efficient oxygen evolution reaction catalyst for water oxidation. Inorg. Chem. Front. 2024, 11, 2489–2497. [Google Scholar] [CrossRef]
- Chen, J.; Liu, C.; Ren, W.; Sun, J.; Zhang, Y.; Zou, L. Synergistic effect of NF and rGO in preparing 3D NiFe-LDH/rGO@NF composites on electrocatalysts performance. J. Alloys Compd. 2022, 901, 163510. [Google Scholar] [CrossRef]
- Wu, L.; Ning, M.; Xing, X.; Wang, Y.; Zhang, F.; Gao, G.; Song, S.; Wang, D.; Yuan, C.; Yu, L. Boosting oxygen evolution reaction of (Fe, Ni) OOH via defect engineering for anion exchange membrane water electrolysis under industrial conditions. Adv. Mater. 2023, 35, 2306097. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, W.; Wu, Y.; Chu, X.; Liu, B.; Zhou, S.; Liu, C.; Che, G.; Liu, G. Unveiling the Dual Active Sites of Ni/Co(OH)2-Ru Heterointerface for Robust Electrocatalytic Alkaline Seawater Splitting. Small 2025, 21, 2410086. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, Y.; Song, H.; Liu, L. NiFe layered-double-hydroxide nanosheet arrays grown in situ on Ni foam for efficient oxygen evolution reaction. Int. J. Hydrogen Energy 2024, 87, 130–137. [Google Scholar] [CrossRef]
- Cui, W.; Cui, S.; Tang, Y.; Zhou, C.; Li, G.; Han, L. Trimetallic Phosphide Nanostructures Derived from In Situ Coated ZIF-L Film for Overall Water Splitting. ACS Appl. Nano Mater. 2024, 7, 23860–23870. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Yang, J.; Xue, Y.; Dai, L. Ultraviolet/ozone treatment for boosting OER activity of MOF nanoneedle arrays. Chem. Eng. J. 2022, 427, 131498. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Huang, Y.C.; Jing, C.; Tsujimoto, Y.; Xu, X.; Lin, Z.; Tang, J.; Wang, Z.; Sun, X. Operando studies redirect spatiotemporal restructuration of model coordinated oxides in electrochemical oxidation. Adv. Mater. 2025, 37, 2413073. [Google Scholar] [CrossRef]
- Li, J.-G.; Sun, H.; Lv, L.; Li, Z.; Ao, X.; Xu, C.; Li, Y.; Wang, C. Metal–organic framework-derived hierarchical (Co,Ni)Se2@NiFe LDH hollow nanocages for enhanced oxygen evolution. ACS Appl. Mater. Interfaces 2019, 11, 8106–8114. [Google Scholar] [CrossRef]
- Kumar, S.; Raju, S.; Marappa, S.; S, M.; DR, V. Unlocking the Potential of Water Splitting: FeMn-LDH/MoS2 Composite with Enhanced Activity and Durability. ACS Appl. Energy Mater. 2024, 7, 9872–9881. [Google Scholar] [CrossRef]
- Yang, L.; Jin, L.; Wang, K.; Xu, H.; He, G.; Chen, H. Interface coupling induced built-in electric fields boost electrocatalytic oxygen evolution reaction over MOF@ LDHs core-shell nanocones. Colloids Surf. A Physicochem. Eng. Asp. 2023, 672, 131720. [Google Scholar] [CrossRef]
- Karmakar, A.; Jayan, R.; Das, A.; Kalloorkal, A.; Islam, M.M.; Kundu, S. Regulating surface charge by embedding Ru nanoparticles over 2D hydroxides toward water oxidation. ACS Appl. Mater. Interfaces 2023, 15, 26928–26938. [Google Scholar] [CrossRef]
- Huo, J.; Wang, Y.; Yan, L.; Xue, Y.; Li, S.; Hu, M.; Jiang, Y.; Zhai, Q.-G. In situ semi-transformation from heterometallic MOFs to Fe–Ni LDH/MOF hierarchical architectures for boosted oxygen evolution reaction. Nanoscale 2020, 12, 14514–14523. [Google Scholar] [CrossRef]
- Huang, S.; Wu, Y.; Fu, J.; Xin, P.; Zhang, Q.; Jin, Z.; Zhang, J.; Hu, Z.; Chen, Z. Hierarchical CoFe LDH/MOF nanorods array with strong coupling effect grown on carbon cloth enables efficient oxidation of water and urea. Nanotechnology 2021, 32, 385405. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, Y.; Li, M.; Fan, G.; Yang, L.; Li, F. A strong coupled 2D metal-organic framework and ternary layered double hydroxide hierarchical nanocomposite as an excellent electrocatalyst for the oxygen evolution reaction. Electrochim. Acta 2019, 307, 275–284. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Liu, X.; Chen, W. 3D nanostructured Ce-doped CoFe-LDH/NF self-supported catalyst for high-performance OER. Dalton Trans. 2023, 52, 12038–12048. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San, X.; Wu, W.; Li, X.; Zhang, L.; Qi, J.; Meng, D. Rich Oxygen Vacancies Induced by Surface Self-Reconstruction in Sandwich-like Hierarchical Structured Electrocatalyst for Boosting Oxygen Evolution Reaction. Molecules 2025, 30, 2632. https://doi.org/10.3390/molecules30122632
San X, Wu W, Li X, Zhang L, Qi J, Meng D. Rich Oxygen Vacancies Induced by Surface Self-Reconstruction in Sandwich-like Hierarchical Structured Electrocatalyst for Boosting Oxygen Evolution Reaction. Molecules. 2025; 30(12):2632. https://doi.org/10.3390/molecules30122632
Chicago/Turabian StyleSan, Xiaoguang, Wanmeng Wu, Xueying Li, Lei Zhang, Jian Qi, and Dan Meng. 2025. "Rich Oxygen Vacancies Induced by Surface Self-Reconstruction in Sandwich-like Hierarchical Structured Electrocatalyst for Boosting Oxygen Evolution Reaction" Molecules 30, no. 12: 2632. https://doi.org/10.3390/molecules30122632
APA StyleSan, X., Wu, W., Li, X., Zhang, L., Qi, J., & Meng, D. (2025). Rich Oxygen Vacancies Induced by Surface Self-Reconstruction in Sandwich-like Hierarchical Structured Electrocatalyst for Boosting Oxygen Evolution Reaction. Molecules, 30(12), 2632. https://doi.org/10.3390/molecules30122632







