Processing Suitability of Physical Modified Non-GMO High-Amylose Wheat Flour as a Resistant Starch Ingredient in Cookies
Abstract
1. Introduction
2. Results and Discussion
2.1. Particle Size of Wheat Flours
2.2. Moisture, Amylose and Starch Damage Content of Wheat Flours
2.3. Solvent Retention Capacity (SRC) of Wheat Flours
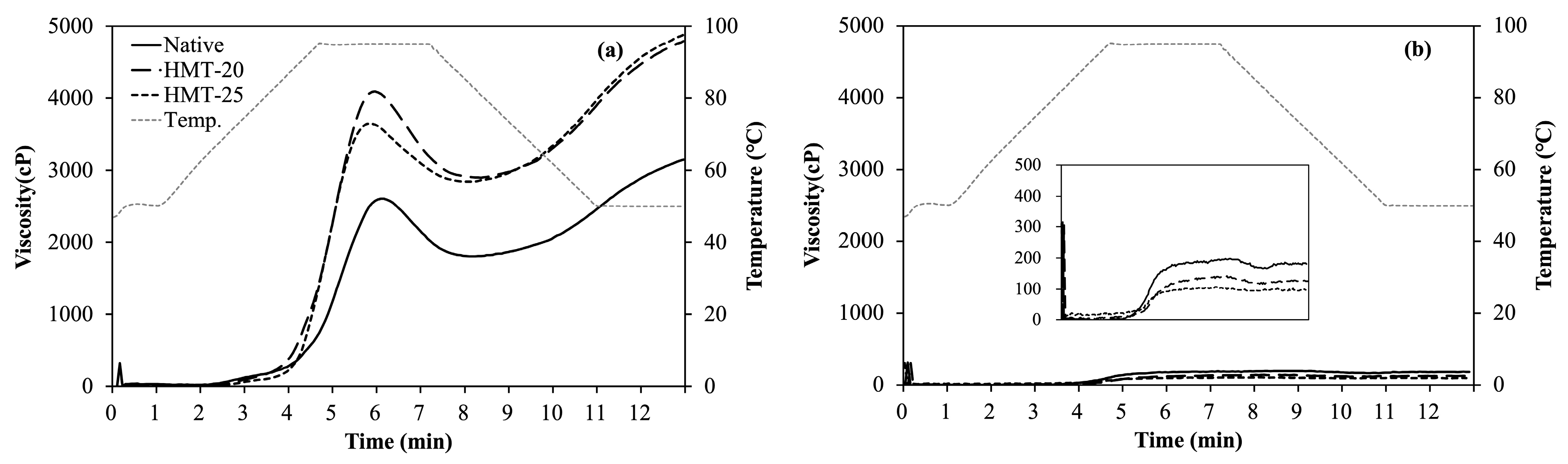
2.4. Pasting Properties of Wheat Flours
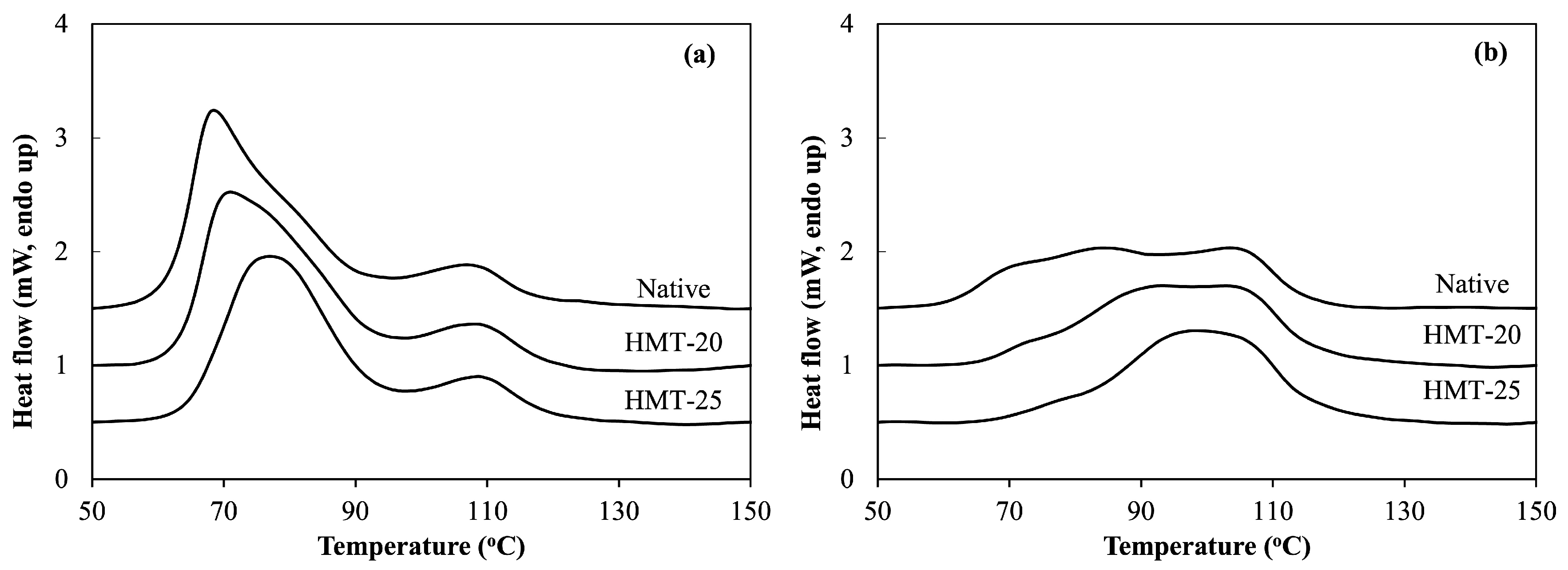
2.5. Thermal Properties of Wheat Flours
2.6. Crystallinity of Wheat Flours
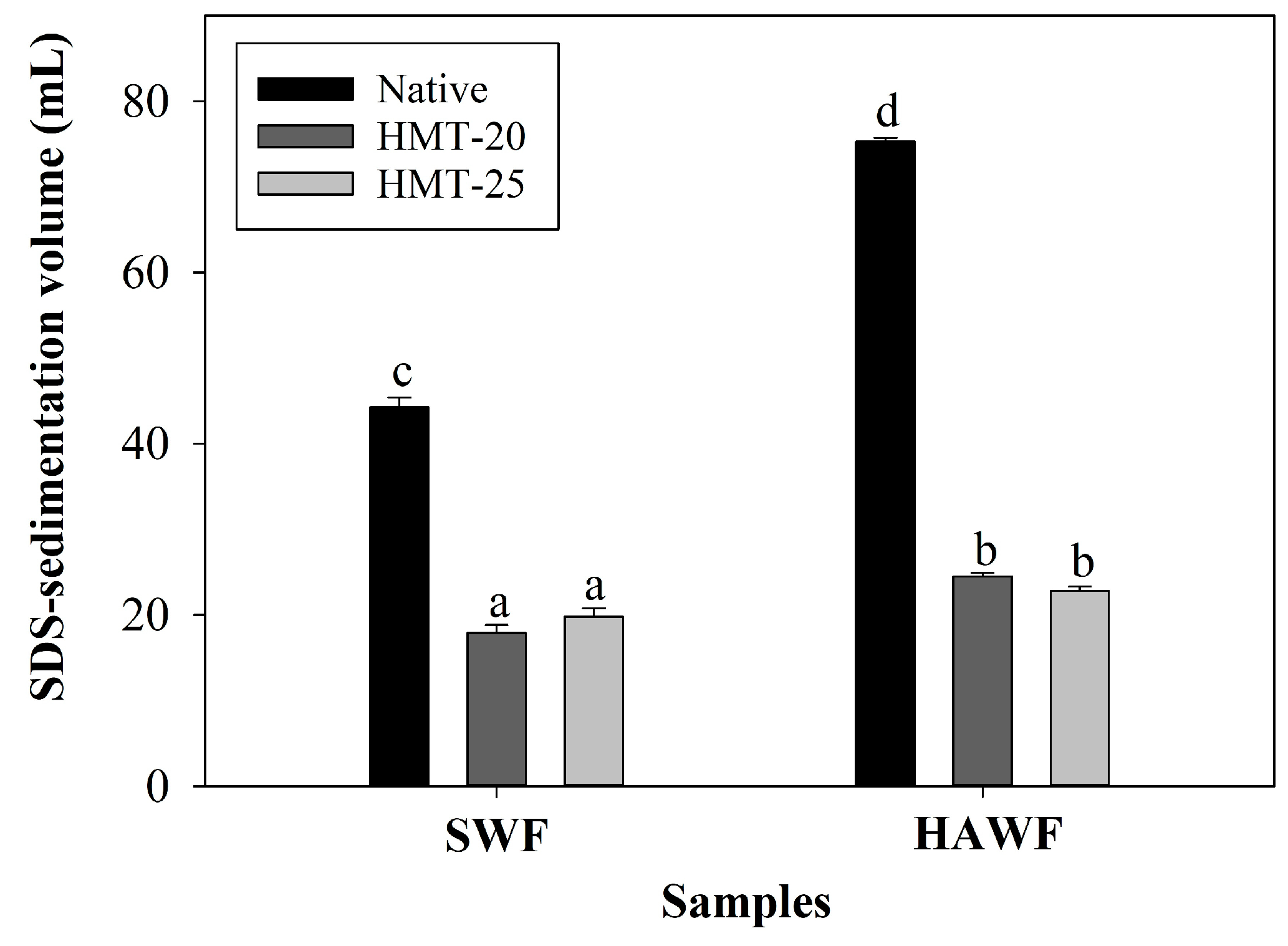
2.7. SDS-Sedimentation Volume of Wheat Flours
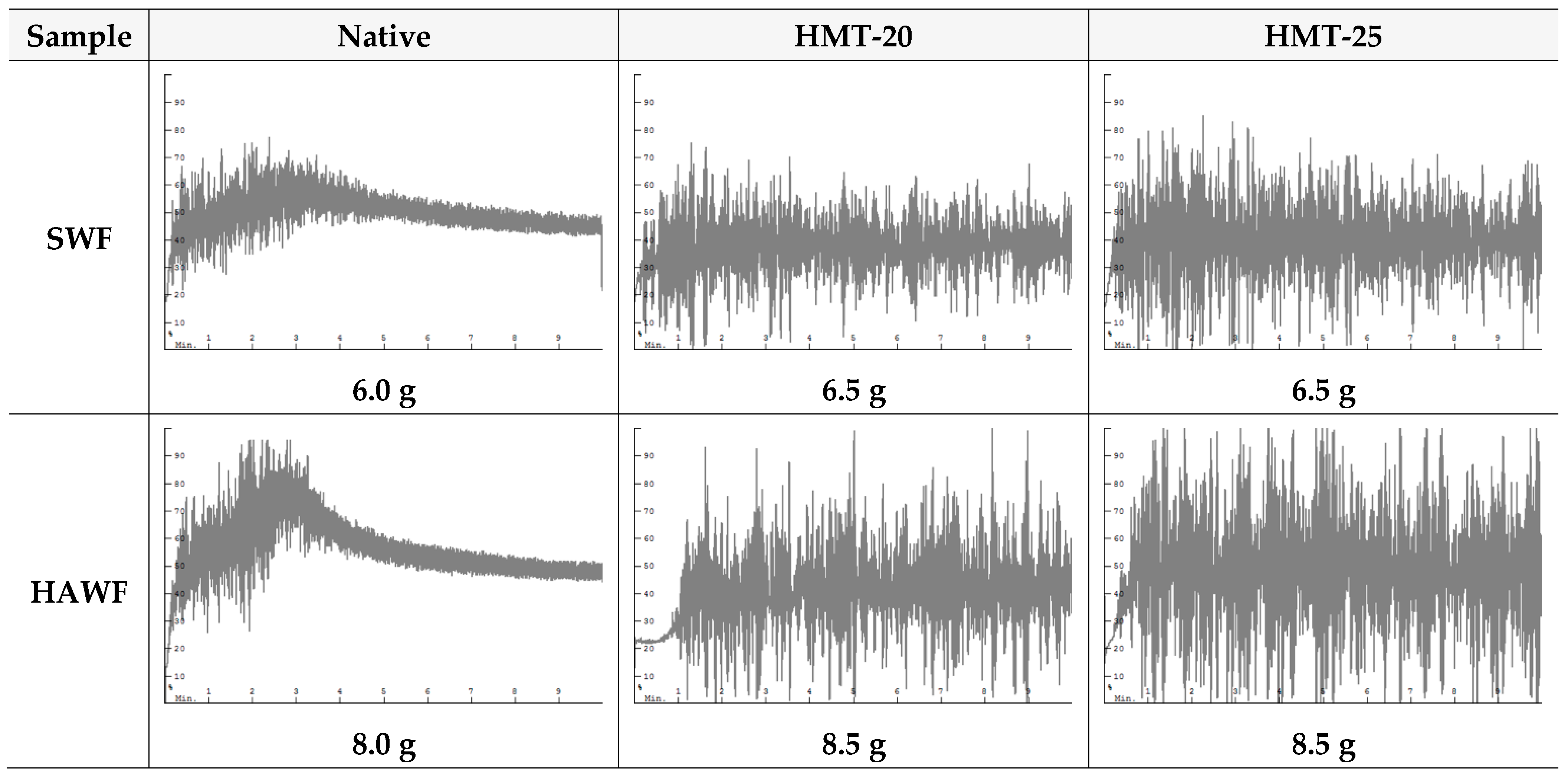
2.8. Dough-Mixing Property of Wheat Flours
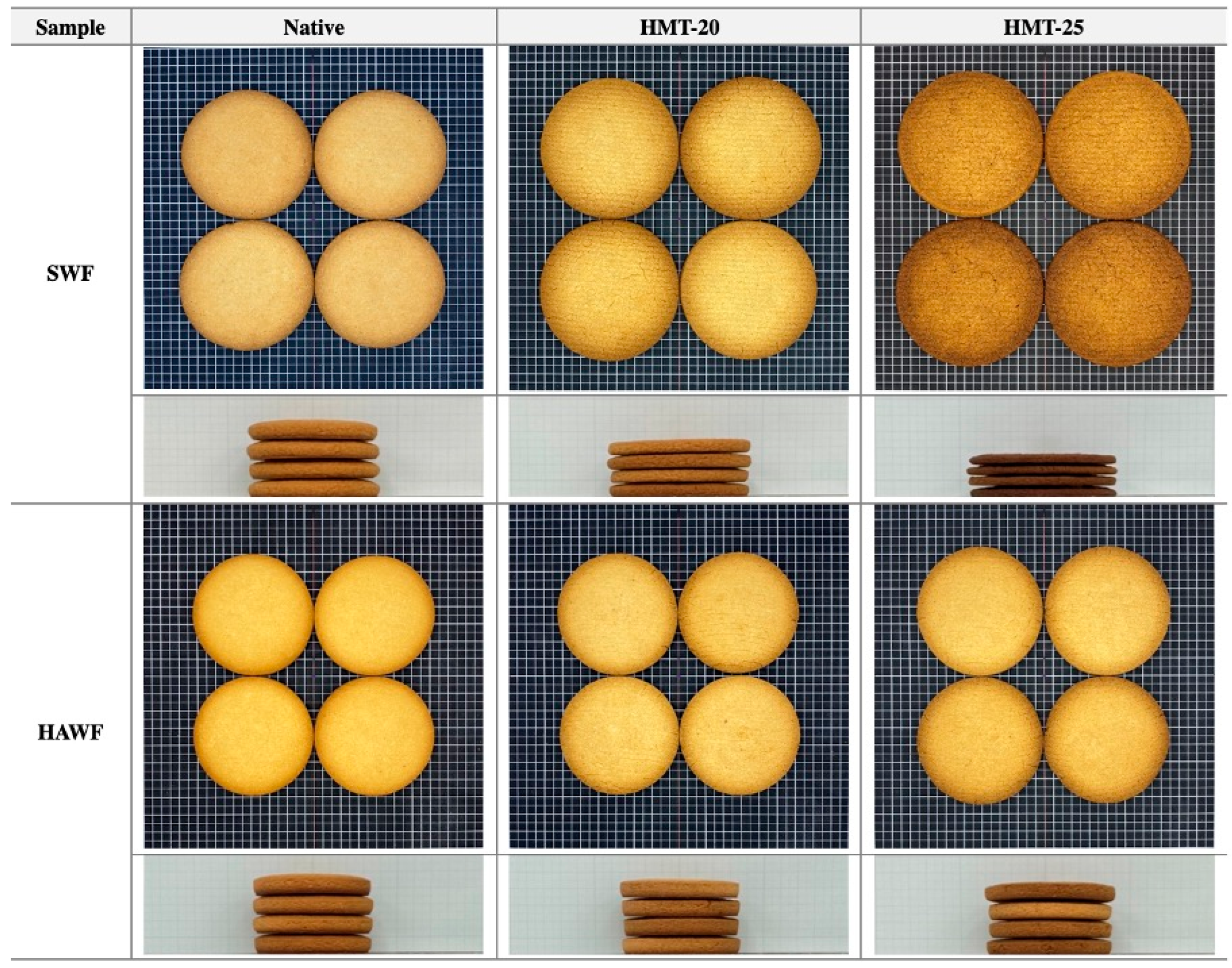
2.9. Quality Characteristics of Cookies
2.10. In Vitro Digestibility of Defatted Cookie Samples
3. Materials and Methods
3.1. Materials
3.2. HMT of Wheat Flours
3.3. Particle Size Analysis of Wheat Flours
3.4. Analysis of Moisture, Amylose, and Damaged Starch Content of Wheat Flours
3.5. Analysis of SRC of Wheat Flours
3.6. Analysis of Pasting Properties of Wheat Flours
3.7. Analysis of Thermal Properties of Wheat Flours
3.8. Analysis of Crystallinity of Wheat Flours
3.9. Measurement of SDS-Sedimentation Volume of Wheat Flours
3.10. Measurement of Mixed Dough Property of Wheat Flours
3.11. Preparation of Cookies with Wheat Flours
3.12. Analysis of Quality of Cookies
3.13. Analysis of In Vitro Digestibility of Wheat Flours and Cookies
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HMT | Heat-moisture treatment |
| SRC | Solvent-retention capacity |
| SDS | Sodium dodecyl sulfate |
| RVA | Rapid viscosity analyzer |
| DSC | Differential scanning calorimetry |
| RS | Resistant starch |
| TS | Total starch |
| RDS | Rapidly digestible starch |
| TDS | Total digestible starch |
References
- Korean Flour Millers Industrial Association. Annual Report on Wheat Consumption in Korea; Korean Flour Millers Industrial Association: Seoul, Republic of Korea, 2020. [Google Scholar]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S33–S50. [Google Scholar]
- Ahuja, G.; Jaiswal, S.; Chibbar, R.N. Starch biosynthesis in relation to resistant starch in wheat. J. Cereal Sci. 2013, 58, 380–386. [Google Scholar]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef]
- Hasjim, J.; Li, E.; Dhital, S. Milling of rice grains: Effects of starch/flour structures on gelatinization and pasting properties. Carbohydr. Polym. 2013, 92, 682–690. [Google Scholar] [CrossRef]
- BeMiller, J.N. Physical modification of starch. In Starch in Food, 2nd ed.; Sjöö, M., Nilsson, L., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 223–253. [Google Scholar]
- Zavareze, E.D.R.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Hoover, R. The impact of heat-moisture treatment on molecular structures and properties of starches isolated from different botanical sources. Crit. Rev. Food Sci. Nutr. 2010, 50, 835–847. [Google Scholar] [CrossRef]
- Chung, H.-J.; Liu, Q.; Hoover, R. Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinized corn, pea and lentil starches. Carbohydr. Polym. 2009, 75, 436–447. [Google Scholar] [CrossRef]
- Rocha, T.S.; Cunha, V.A.G.; Jane, J.; Franco, C.M.L. Structural characterization of Peruvian carrot (Arracacia xanthorrhiza) starch and the effect of annealing on its semicrystalline structure. J. Agric. Food Chem. 2011, 59, 4208–4216. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Chen, L.; Li, X. Understanding the structure and digestibility of heat-moisture treated starch. Int. J. Biol. Macromol. 2016, 88, 1–8. [Google Scholar] [CrossRef]
- Lehmann, U.; Robin, F. Slowly digestible starch—Its structure and health implications: A review. Trends Food Sci. Technol. 2007, 18, 346–355. [Google Scholar] [CrossRef]
- Zhang, G.; Hamaker, B.R. Slowly digestible starch: Concept, mechanism, and proposed extended glycemic index. Crit. Rev. Food Sci. Nutr. 2009, 49, 852–867. [Google Scholar] [CrossRef]
- Newberry, M.; Berbezy, P.; Belobrajdic, D.; Chapron, S.; Tabouillot, P.; Regina, A.; Bird, A. High-amylose wheat foods: A new opportunity to meet dietary fiber targets for health. Cereal Foods World 2018, 63, 188–193. [Google Scholar]
- Bird, A.R.; Regina, A. High amylose wheat: A platform for delivering human health benefits. J. Cereal Sci. 2018, 82, 99–105. [Google Scholar] [CrossRef]
- Li, H.; Gidley, M.J.; Dhital, S. High-amylose starches to bridge the “Fiber Gap”: Development, structure, and nutritional functionality. Compr. Rev. Food Sci. Food Saf. 2019, 18, 362–379. [Google Scholar] [CrossRef]
- Dziki, D.; Krajewska, A.; Findura, P. Particle size as an indicator of wheat flour quality: A review. Processes 2024, 12, 2480. [Google Scholar] [CrossRef]
- Kweon, M.; Slade, L.; Levine, H. Solvent retention capacity (SRC) testing of wheat flour: Principles and value in predicting flour functionality in different wheat-based food processes and in wheat breeding—A review. Cereal Chem. 2011, 88, 537–552. [Google Scholar] [CrossRef]
- Bartz, J.; Zavareze, E.R.; Dias, A.R.G. Study of heat–moisture treatment of potato starch granules by chemical surface gelatinization. J. Sci. Food Agric. 2017, 97, 3114–3123. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Zheng, X. Recent Advances in heat-moisture modified cereal starch: Structure, functionality and its applications in starchy food systems. Food Chem. 2021, 344, 128700. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, S.; Yan, J.; Sun, B.; Wang, X. Effect of heat–moisture treatment on the physicochemical properties, structure, morphology, and starch digestibility of highland barley (Hordeum vulgare L. var. nudum hook. f) flour. Foods 2022, 11, 3511. [Google Scholar] [CrossRef]
- Du, M.; Cao, T.; Yu, M.; Zhang, C.; Xu, W. Effect of heat-moisture treatment on physicochemical properties of chickpea starch. Food Sci. Technol. 2023, 43, e108822. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, S. Influence of heat-moisture treatment (HMT) on physicochemical and functional properties of starches from different Indian oat (Avena sativa L.) cultivars. Int. J. Biol. Macromol. 2019, 122, 312–319. [Google Scholar] [CrossRef]
- Wang, H.; Ding, J.; Xiao, N.; Liu, X.; Zhang, Y.; Zhang, H. Insights into the hierarchical structure and digestibility of starch in heat-moisture treated adlay seeds. Food Chem. 2020, 318, 126489. [Google Scholar] [CrossRef]
- Bian, L.; Chung, H.-J. Molecular structure and physicochemical properties of starch isolated from hydrothermally treated brown rice flour. Food Hydrocoll. 2016, 60, 345–352. [Google Scholar] [CrossRef]
- Liu, C.; Song, M.; Liu, L.; Hong, J.; Guan, E.; Bian, K.; Zheng, X. Effect of heat-moisture treatment on the structure and physicochemical properties of ball mill damaged starches from different botanical sources. Int. J. Biol. Macromol. 2020, 156, 403–410. [Google Scholar] [CrossRef]
- Kweon, M.; Levine, H.; Gannon, D. Cookie- versus cracker-baking—What’s the difference? Flour functionality requirements explored by SRC and Alveography. Crit. Rev. Food Sci. Nutr. 2014, 54, 115–138. [Google Scholar] [CrossRef]
- Duyvejonck, A.E.; Lagrain, B.; Dornez, E.; Delcour, J.A.; Courtin, C.M. Suitability of solvent retention capacity tests to assess the cookie and bread making quality of European wheat flours. LWT—Food Sci. Technol. 2012, 47, 56–63. [Google Scholar] [CrossRef]
- Gaines, C.S. Prediction of sugar-snap cookie diameter using sucrose solvent retention capacity, milling softness, and flour protein content. Cereal Chem. 2004, 81, 549–552. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Zhang, Y.; He, Z.; Peña, R.J. Effects of solvent retention capacities, pentosan content, and dough rheological properties on sugar snap cookie quality in Chinese soft wheat genotypes. Crop Sci. 2007, 47, 656–662. [Google Scholar] [CrossRef]
- Keppler, S.; Bakalis, S.; Leadley, E.; Sahi, S.S.; Fryer, P.J. Evaluation of dry heat treatment of soft wheat flour for the production of high ratio cakes. Food Res. Int. 2018, 107, 360–370. [Google Scholar] [CrossRef]
- Hong, T.; Ma, Y.; We, F.; Jin, Y.; Xu, D.; Xu, X. Understanding the effects of dry heat treatment on wheat flour pasting: Insight from protein and starch structural changes. J. Cereal Sci. 2023, 113, 103740. [Google Scholar] [CrossRef]
- Van Steertegem, B.; Pareyt, B.; Slade, L.; Levein, H.; Brijs, K.; Delcour, J.A. Impact of heat treatment on wheat flour solvent retention capacity (SRC) profiles. Cereal Chem. 2013, 90, 608–610. [Google Scholar] [CrossRef]
- Ungureanu-Iuga, M.; Mironeasa, S. Changes induced by heat moisture treatment in wheat flour and pasta rheological, physical and starch digestibility properties. Gels 2023, 9, 449. [Google Scholar] [CrossRef]
- Tester, R.F.; Morrison, W.R. Swelling and gelatinization of cereal starches. I. Effects of amylopectin, amylose, and lipids. Cereal Chem. 1990, 67, 551–557. [Google Scholar]
- Li, C.; Dhital, S.; Gilbert, R.G.; Gidley, M.J. High-amylose wheat starch: Structural basis for water absorption and pasting properties. Carbohydr. Polym. 2020, 245, 116557. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, G.E.; Vermeylen, R.; Geeroms, J.; Delcour, J.A. Rice starches. III. Structural aspects provide insight in amylopectin retrogradation properties and gel texture. J. Cereal Sci. 2003, 38, 61–68. [Google Scholar] [CrossRef]
- Moon, Y.; Kweon, M. Physicochemical and molecular structural properties and in vitro digestibility of non-GMO high-amylose wheat starch. Korean J. Cook. Sci. 2022, 38, 269–277. [Google Scholar]
- Ambigaipalan, P.; Hoover, R.; Donner, E.; Liu, Q. Starch chain interactions within the amorphous and crystalline domains of pulse starches during heat-moisture treatment at different temperatures and their impact on physicochemical properties. Food Chem. 2014, 143, 175–184. [Google Scholar] [CrossRef]
- Klein, B.; Pinto, V.Z.; Vanier, N.L.; da Rosa Zavareze, E.; Colussi, R.; do Evangelho, J.A.; Gutkoski, L.C.; Dias, A.R.G. Effect of single and dual heat–moisture treatments on properties of rice, cassava, and pinhao starches. Carbohydr. Polym. 2013, 98, 1578–1584. [Google Scholar] [CrossRef]
- Pranoto, Y.; Rakshit, S.K. Physicochemical properties of heat moisture treated sweet potato starches of selected Indonesian varieties. Int. Food Res. J. 2014, 21, 2031. [Google Scholar]
- Jacobs, H.; Delcour, J.A. Hydrothermal modifications of granular starch, with retention of the granular structure: A review. J. Agric. Food Chem. 1998, 46, 2895–2905. [Google Scholar] [CrossRef]
- Fonseca, L.M.; El Halal, S.L.M.; Dias, A.R.G.; da Rosa Zavareze, E. Physical modification of starch by heat-moisture treatment and annealing and their applications: A review. Carbohydr. Polym. 2021, 274, 118665. [Google Scholar] [CrossRef]
- Bahrani, S.A.; Loisel, C.; Maache-Rezzoug, Z.; Della Valle, D.; Rezzoug, S.A. Rheological and viscoelastic properties of corn starch suspension modified by hydrothermal process: Impacts of process intensification. Chem. Eng. Process. Process Intensif. 2013, 64, 10–16. [Google Scholar] [CrossRef]
- Morris, C.F.; Paszczynska, B.; Bettge, A.D.; King, G.E. A critical examination of the sodium dodecyl sulfate (SDS) sedimentation test for wheat meals. J. Sci. Food Agric. 2007, 87, 607–615. [Google Scholar] [CrossRef]
- Mann, J.; Schiedt, B.; Baumann, A.; Conde-Petit, B.; Vilgis, T.A. Effect of heat treatment on wheat dough rheology and wheat protein solubility. Food Sci. Technol. Int. 2014, 20, 341–351. [Google Scholar] [CrossRef]
- Chen, C.; Espinal-Ruiz, M.; Francavilla, A.; Joyce, I.J.; Corrandini, M.G. Morphological changes and color development during cookie baking—Kinetic, heat, and mass transfer considerations. J. Food Sci. 2024, 89, 4331–4344. [Google Scholar] [CrossRef]
- Moiraghi, M.; Vanzetti, L.; Bainotti, C.; Helguera, M.; León, A.; Pérez, G. Relationship between soft wheat flour physicochemical composition and cookie-making performance. Cereal Chem. 2011, 88, 130–136. [Google Scholar] [CrossRef]
- Chung, H.J.; Liu, Q.; Lee, L.; Wei, D. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents. Food Hydrocoll. 2011, 25, 968–975. [Google Scholar] [CrossRef]
- Kweon, M.; Jeon, S.; Cho, J.-H. Molecular structure and thermal properties of the starches from high amylose rice cultivars and their amylose-lipid complex formation with various emulsifiers. Korean J. Food Cook. Sci. 2018, 34, 617–625. [Google Scholar] [CrossRef]
- Li, H.T.; Li, Z.; Fox, G.P.; Gidley, M.J.; Dhital, S. Protein-starch matrix plays a key role in enzymic digestion of high-amylose wheat noodle. Food Chem. 2021, 336, 127719. [Google Scholar] [CrossRef]
- Saito, K.; Ito, T.; Kuribayashi, T.; Mochida, K.; Nakakuki, T.; Shibata, M.; Sugawara, M. Effect of raw and heat-moisture treated high-amylose corn starch on fermentation by the rat cecal bacteria. Starch-Stärke 2001, 53, 424–430. [Google Scholar] [CrossRef]
- Lee, C.S.; Chung, H.J. Enhancing resistant starch content of high amylose rice starch through heat–moisture treatment for industrial application. Molecules 2022, 27, 6375. [Google Scholar] [CrossRef] [PubMed]
- AACC. Approved Method of Analysis, 11th ed.; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]


| Sample | d10 | d25 | d50 | d75 | d90 | |
|---|---|---|---|---|---|---|
| SWF | Native | 7.9 ± 0.4 a | 15.3 ± 0.4 a | 28.1 ± 0.7 a | 70.5 ± 1.0 a | 103.8 ± 0.0 a |
| HMT-20 | 18.5 ± 0.4 b | 33.3 ± 0.7 b | 83.4 ± 1.0 b | 201.3 ± 4.3 b | 381.8 ± 3.2 d | |
| HMT-25 | 46.0 ± 1.5 d | 96.0 ± 1.4 f | 236.3 ± 4.7 e | 426.1 ± 3.0 c | 568.9 ± 2.8 f | |
| HAWF | Native | 18.8 ± 0.1 b | 44.4 ± 0.3 c | 78.7 ± 0.1 b | 114.2 ± 0.3 d | 148.1 ± 1.2 b |
| HMT-20 | 39.7 ± 0.4 c | 65.5 ± 0.4 d | 100.5 ± 0.5 c | 158.6 ± 0.7 e | 321.2 ± 3.8 c | |
| HMT-25 | 60.5 ± 1.2 e | 88.9 ± 1.3 e | 141.8 ± 2.4 d | 287.1 ± 7.7 f | 470.5 ± 2.9 e | |
| Sample | Moisture (%) | Amylose (%, dwb) | Starch Damage (%, dwb) | Solvent Retention Capacity (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Water | Lactic Acid | Sodium Carbonate | Sucrose | |||||
| SWF | Native | 13.6 ± 0.0 e | 21.4 ± 0.2 b | 3.4 ± 0.0 e | 51.2 ± 0.3 a | 103.1 ± 1.1 bc | 72.0 ± 0.0 b | 96.4 ± 1.1 c |
| HMT-20 | 10.0 ± 0.0 a | 20.6 ± 0.3 a | 2.0 ± 0.0 c | 65.7 ± 0.3 b | 89.3 ± 1.1 a | 68.9 ± 0.1 a | 88.2 ± 0.3 a | |
| HMT-25 | 10.8 ± 0.0 c | 21.8 ± 0.4 b | 2.8 ± 0.0 d | 68.0 ± 0.1 c | 89.0 ± 0.3 a | 73.7 ± 0.1 c | 91.4 ± 0.8 b | |
| HAWF | Native | 15.2 ± 0.0 f | 46.7 ± 0.1 d | 4.7 ± 0.0 f | 75.5 ± 0.3 d | 118.9 ± 0.4 d | 89.6 ± 0.0 e | 128.5 ± 1.5 d |
| HMT-20 | 10.2 ± 0.0 b | 47.0 ± 0.2 d | 1.5 ± 0.0 a | 99.0 ± 0.6 e | 101.6 ± 0.1 b | 97.7 ± 0.5 f | 155.3 ± 0.6 e | |
| HMT-25 | 11.6 ± 0.0 d | 44.5 ± 0.4 c | 1.6 ± 0.0 b | 99.9 ± 0.4 e | 104.7 ± 0.2 c | 88.1 ± 0.0 d | 159.3 ± 1.2 f | |
| Sample | Peak Viscosity (cP) | Final Viscosity (cP) | Setback Viscosity (cP) | Pasting Temperature (°C) | |
|---|---|---|---|---|---|
| SWF | Native | 2755 ± 8 c | 3294 ± 9 c | 1342 ± 23 b | 64.5 ± 1.1 a |
| HMT-20 | 4241 ± 45 e | 4938 ± 35 d | 1894 ± 1 c | 69.0 ± 0.6 b | |
| HMT-25 | 3803 ± 16 d | 5027 ± 15 e | 2040 ± 54 d | 71.0 ± 0.1 b | |
| HAWF | Native | 368 ± 5 b | 361 ± 1 b | 16 ± 1 a | 81.2 ± 0.7 c |
| HMT-20 | 313 ± 5 ab | 306 ± 8 ab | 12 ± 2 a | 81.9 ± 1.7 c | |
| HMT-25 | 283 ± 0 a | 277 ± 1 a | 7 ± 0 a | 82.3 ± 1.1 c | |
| Gelatinization Peak | Amylose–Lipid Complex Peak | ||||||
|---|---|---|---|---|---|---|---|
| T Onset (°C) | T Peak (°C) | ΔH (J/g) | T Onset (°C) | T Peak (°C) | ΔH (J/g) | ||
| SWF | Native | 61.8 ± 0.1 b | 68.4 ± 0.1 a | 3.74 ± 0.15 a | 97.5 ± 0.6 b | 108.0 ± 0.6 a | 0.41 ± 0.04 a |
| HMT-20 | 63.5 ± 0.1 c | 70.9 ± 0.1 b | 3.72 ± 0.08 a | 95.3 ± 0.4 a | 109.2 ± 0.3 a | 0.44 ± 0.00 a | |
| HMT-25 | 65.3 ± 0.2 d | 76.7 ± 0.0 c | 3.35 ± 0.00 a | 98.8 ± 0.6 b | 109.1 ± 0.0 a | 0.40 ± 0.01 a | |
| HAWF | Native | 60.6 ± 0.1 a | 82.9 ± 0.6 d | 3.54 ± 0.17 a | nd | nd | nd |
| HMT-20 | 70.8 ± 0.3 e | 89.5 ± 0.5 e | 3.58 ± 0.09 a | nd | nd | nd | |
| HMT-25 | 77.5 ± 0.7 f | 94.3 ± 0.5 f | 3.52 ± 0.02 a | nd | nd | nd | |
| Sample | Moisture Loss (%) | Top Surface Color | Diameter (mm) | Height (mm) | Spread Ratio (Dia./Height) | |||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||
| SWF | Native | 14.7 ± 0.0 c | 62.3 ± 1.6 b | 14.4 ± 0.7 a | 33.0 ± 0.4 bc | 74.9 ± 0.0 d | 40.3 ± 0.1 c | 7.4 ± 0.0 d |
| HMT-20 | 17.1 ± 0.0 d | 61.4 ± 1.4 b | 13.7 ± 0.7 a | 31.9 ± 0.6 b | 78.9 ± 0.1 e | 32.4 ± 0.1 b | 9.7 ± 0.0 e | |
| HMT-25 | 17.5 ± 0.0 e | 38.2 ± 4.4 a | 13.8 ± 0.5 a | 22.9 ± 2.5 a | 82.4 ± 0.0 f | 24.0 ± 0.1 a | 13.7 ± 0.0 f | |
| HAWF | Native | 14.2 ± 0.0 a | 65.0 ± 1.1 c | 14.1 ± 0.5 a | 34.8 ± 0.5 d | 66.9 ± 0.0 b | 44.7 ± 0.1 f | 6.0 ± 0.0 a |
| HMT-20 | 14.6 ± 0.0 b | 64.9 ± 1.2 c | 14.3 ± 0.5 a | 33.7 ± 0.5 cd | 66.3 ± 0.0 a | 42.4 ± 0.1 e | 6.3 ± 0.0 b | |
| HMT-25 | 14.6 ± 0.0 b | 61.7 ± 2.3 b | 15.2 ± 0.9 b | 33.5 ± 0.3 c | 70.3 ± 0.0 c | 41.0 ± 0.2 d | 6.9 ± 0.0 c | |
| RDS (%) | SDS (%) | TDS (%) | RS (%) | ||
|---|---|---|---|---|---|
| SWF | Native | 40.9 ± 0.0 e | 8.4 ± 0.2 a | 48.7 ± 0.2 b | 0.3 ± 0.0 a |
| HMT-20 | 39.4 ± 0.2 d | 9.9 ± 0.6 b | 48.9 ± 0.1 b | 0.3 ± 0.0 a | |
| HMT-25 | 37.1 ± 0.4 c | 11.5 ± 0.3 c | 48.9 ± 0.1 b | 0.3 ± 0.0 a | |
| HAWF | Native | 25.3 ± 0.0 b | 9.6 ± 0.2 ab | 35.7 ± 0.0 a | 7.2 ± 0.0 b |
| HMT-20 | 23.6 ± 0.2 a | 10.7 ± 0.3 bc | 35.6 ± 0.2 a | 7.6 ± 0.1 c | |
| HMT-25 | 23.1 ± 0.1 a | 10.5 ± 0.0 bc | 35.8 ± 0.3 a | 8.5 ± 0.0 d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, Y.; Kweon, M. Processing Suitability of Physical Modified Non-GMO High-Amylose Wheat Flour as a Resistant Starch Ingredient in Cookies. Molecules 2025, 30, 2619. https://doi.org/10.3390/molecules30122619
Moon Y, Kweon M. Processing Suitability of Physical Modified Non-GMO High-Amylose Wheat Flour as a Resistant Starch Ingredient in Cookies. Molecules. 2025; 30(12):2619. https://doi.org/10.3390/molecules30122619
Chicago/Turabian StyleMoon, Yujin, and Meera Kweon. 2025. "Processing Suitability of Physical Modified Non-GMO High-Amylose Wheat Flour as a Resistant Starch Ingredient in Cookies" Molecules 30, no. 12: 2619. https://doi.org/10.3390/molecules30122619
APA StyleMoon, Y., & Kweon, M. (2025). Processing Suitability of Physical Modified Non-GMO High-Amylose Wheat Flour as a Resistant Starch Ingredient in Cookies. Molecules, 30(12), 2619. https://doi.org/10.3390/molecules30122619






