Modulating D-Band Center of SrTiO3 by Co Doping for Boosted Peroxymonosulfate (PMS) Activation Under Visible Light
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure and Composition Characterization of Photocatalysts
2.2. Photocatalytic Performance and Durability
2.3. Photo-Electrochemical Properties
2.4. Reaction Mechanism
2.5. Reaction Pathways and Toxicity of Products
3. Materials and Methods
3.1. Chemical Materials
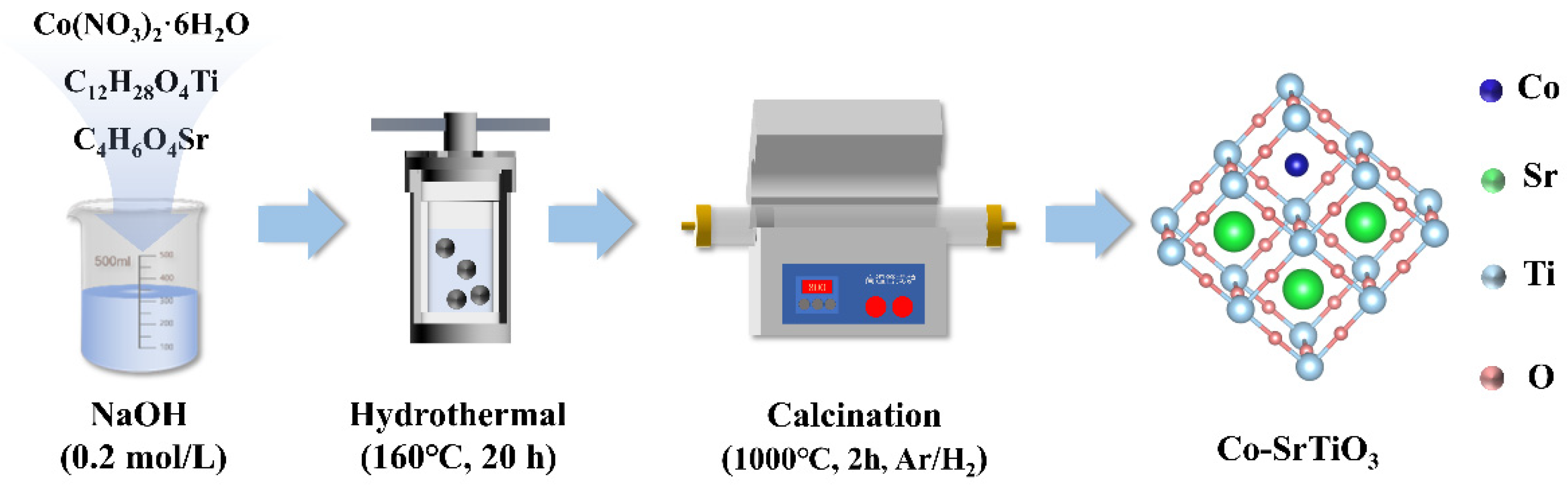
3.2. Photocatalyst Preparation
3.3. Materials Characterization
3.4. Measurement of Photocatalytic Activity
3.5. Theory Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, S.; Li, X.; Gong, Z.; Fan, B.; Hu, J.; Peng, J.; Lu, K.; Gao, S. A new insight into the mechanism in Fe3O4@CuO/PMS system with low oxidant dosage. Chem. Eng. J. 2022, 438, 135474. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.; Che, H.; Gao, X.; Ao, Y.; Wang, P. Boosting 2e− oxygen reduction reaction in garland carbon nitride with carbon defects for high-efficient photocatalysis-self-Fenton degradation of 2,4-dichlorophenol. Appl. Catal. B Environ. Energy 2022, 307, 121185. [Google Scholar] [CrossRef]
- Ma, Y.; Han, Y.; Yao, Y.; Zhou, T.; Sun, D.; Liu, C.; Che, G.; Hu, B.; Valtchev, V.; Fang, Q. A cobalt-modified covalent organic framework enables highly efficient degradation of 2,4-dichlorophenol in high concentrations through peroxymonosulfate activation. Chem. Sci. 2024, 15, 12488–12495. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Guan, R.; Chen, Y.; Sun, Y.; Shang, Q. The unique TiO2(B)/BiOCl0.7I0.3-P Z-scheme heterojunction effectively degrades and mineralizes the herbicide fomesafen. Chem. Eng. J. 2022, 431, 134021. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Sunarso, J.; Zhong, Y.; Shao, Z. Phosphorus-Doped Perovskite Oxide as Highly Efficient Water Oxidation Electrocatalyst in Alkaline Solution. Adv. Funct. Mater. 2016, 26, 5862–5872. [Google Scholar] [CrossRef]
- Wei, K.; Faraj, Y.; Yao, G.; Xie, R.; Lai, B. Strategies for improving perovskite photocatalysts reactivity for organic pollutants degradation: A review on recent progress. Chem. Eng. J. 2021, 414, 128783. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, R.; Wu, Z.; Yang, F.; Luo, M.; Yao, G.; Ao, Z.; Lai, B. Cobalt-doped boosted the peroxymonosulfate activation performance of LaFeO3 perovskite for atrazine degradation. Chem. Eng. J. 2023, 452, 139427. [Google Scholar] [CrossRef]
- Li, Z.; Xu, H.; Zheng, Y.; Liu, L.; Li, L.; Lou, Z.; Wang, L. A reconfigurable heterostructure transistor array for monocular 3D parallax reconstruction. Nat. Electron. 2025, 8, 46–55. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, Y.; He, Q.; Xu, R.; Chen, D.; Xu, X.; Hu, H. Review of doping SrTiO3 for photocatalytic applications. Bull. Mater. Sci. 2022, 46, 6. [Google Scholar] [CrossRef]
- Xie, M.; Liu, Q.; Liu, J.; Yu, J.; Chen, R.; Zhu, J.; Li, R.; Wang, J. Copper-doped nano-cubic SrTiO3 photocatalyst efficiently removes U(VI) from uranium mine pit water. Sep. Purif. Technol. 2024, 346, 127338. [Google Scholar] [CrossRef]
- Moss, B.; Wang, Q.; Butler, K.T.; Grau-Crespo, R.; Selim, S.; Regoutz, A.; Hisatomi, T.; Godin, R.; Payne, D.J.; Kafizas, A.; et al. Linking in situ charge accumulation to electronic structure in doped SrTiO3 reveals design principles for hydrogen-evolving photocatalysts. Nat. Mater. 2021, 20, 511–517. [Google Scholar] [CrossRef]
- Feng, B.; Sun, K.; Che, G.; Yang, T.; Zhang, Y.; He, Q.; Chang, T.; Wang, L.; Guan, R. Efficient PDS activation and carrier extraction by Gd2O3 decorated SrTiO3 for Fenton-like photocatalysis. Chem. Eng. J. 2024, 502, 157880. [Google Scholar] [CrossRef]
- Wu, X.; Chen, J.; Yang, X.; Zheng, H.; Ma, Y.; Li, Y. Synergistic activation of peroxymonosulfate for tetracycline hydrochloride degradation with SrTiO3/Ti3C2Tx photocatalyst. Appl. Surf. Sci. 2025, 680, 161317. [Google Scholar] [CrossRef]
- Murthy, D.H.K.; Nandal, V.; Furube, A.; Seki, K.; Katoh, R.; Lyu, H.; Hisatomi, T.; Domen, K.; Matsuzaki, H. Origin of Enhanced Overall Water Splitting Efficiency in Aluminum-Doped SrTiO3 Photocatalyst. Adv. Energy Mater. 2023, 13, 2302064. [Google Scholar] [CrossRef]
- Cheng, X.; Guan, R.; Chen, Y.; Qian, Y.; Shang, Q.; Sun, Y. Adsorption and photocatalytic degradation process of oxytetracycline using mesoporous Fe-TiO2 based on high-resolution mass spectrometry. Chem. Eng. J. 2023, 460, 141618. [Google Scholar] [CrossRef]
- Gonçalves, N.P.F.; Paganini, M.C.; Armillotta, P.; Cerrato, E.; Calza, P. The effect of cobalt doping on the efficiency of semiconductor oxides in the photocatalytic water remediation. J. Environ. Chem. Eng. 2019, 7, 103475. [Google Scholar] [CrossRef]
- Li, M.; Guan, R.; Li, J.; Zhao, Z.; Zhang, J.; Qi, Y.; Zhai, H.; Wang, L. Photocatalytic Performance and Mechanism Research of Ag/HSTiO2 on Degradation of Methyl Orange. ACS Omega 2020, 5, 21451–21457. [Google Scholar] [CrossRef]
- Li, R.; Takata, T.; Zhang, B.; Feng, C.; Wu, Q.; Cui, C.; Zhang, Z.; Domen, K.; Li, Y. Criteria for Efficient Photocatalytic Water Splitting Revealed by Studying Carrier Dynamics in a Model Al-doped SrTiO3 Photocatalyst. Angew. Chem. Int. Ed. 2023, 62, e202313537. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, Y.; Yao, S.; Li, Y.; Fu, Y.; Zhou, Q. Interfacial interaction-induced shift of d-band center promotes photocatalytic antibiotics mineralization. Appl. Catal. B Environ. Energy 2024, 352, 123998. [Google Scholar] [CrossRef]
- Liu, H.-Z.; Shu, X.-X.; Huang, M.; Wu, B.-B.; Chen, J.-J.; Wang, X.-S.; Li, H.-L.; Yu, H.-Q. Tailoring d-band center of high-valent metal-oxo species for pollutant removal via complete polymerization. Nat. Commun. 2024, 15, 2327. [Google Scholar] [CrossRef]
- Guan, R.; Wang, D.; Zhang, Y.; Liu, C.; Xu, W.; Wang, J.; Zhao, Z.; Feng, M.; Shang, Q.; Sun, Z. Enhanced photocatalytic N2 fixation via defective and fluoride modified TiO2 surface. Appl. Catal. B Environ. 2021, 282, 119580. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, E.; Zhu, H.; Wang, H.; Rong, A.; Mao, L. NiCoP as a cocatalyst decorating CdIn2S4 for enhanced photocatalytic performance under visible light: DFT calculation and mechanism insight. Int. J. Hydrogen Energy 2024, 51, 1287–1302. [Google Scholar] [CrossRef]
- Wang, R.; Qiu, Z.; Wan, S.; Wang, Y.; Liu, Q.; Ding, J.; Zhong, Q. Insight into mechanism of divalent metal cations with different d-bands classification in layered double hydroxides for light-driven CO2 reduction. Chem. Eng. J. 2022, 427, 130863. [Google Scholar] [CrossRef]
- Peng, D.; Mao, L.; Sun, J.; Li, X.; Shi, H.; Su, Z. S-scheme graphitic carbon nitride/nickel titanate (g-C3N4/NiTiO3) heterojunction as bifunctional photocatalysts for hydrogen production and pollutants degradation. Int. J. Hydrogen Energy 2025, 114, 60–70. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Jiang, S.; Sun, C.; Song, S. Regulation of d-Band Centers in Localized CdS Homojunctions through Facet Control for Improved Photocatalytic Water Splitting. Angew. Chem. Int. Ed. 2023, 62, e202307808. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Huang, Y.; He, S.; Chen, G.; Liu, X.; He, C.; Du, C.; Chen, Q. Preparation of Co-Fe based Prussian blue analogs loaded nickel foams for Fenton-like degradation of tetracycline. Appl. Catal. A Gen. 2023, 650, 118985. [Google Scholar] [CrossRef]
- Almomani, F.; Al-Jaml, K.L.; Bhosale, R.R. Solar photo-catalytic production of hydrogen by irradiation of cobalt co-doped TiO2. Int. J. Hydrogen Energy 2021, 46, 12068–12081. [Google Scholar] [CrossRef]
- Dong, H.; Zuo, Y.; Song, N.; Hong, S.; Xiao, M.; Zhu, D.; Sun, J.; Chen, G.; Li, C. Bimetallic synergetic regulating effect on electronic structure in cobalt/vanadium co-doped carbon nitride for boosting photocatalytic performance. Appl. Catal. B Environ. 2021, 287, 119954. [Google Scholar] [CrossRef]
- Ning, S.; Sun, Y.; Ouyang, S.; Qi, Y.; Ye, J. Solar light-induced injection of hot electrons and photocarriers for synergistically enhanced photothermocatalysis over Cu-Co/SrTiO3 catalyst towards boosting CO hydrogenation into C2–C4 hydrocarbons. Appl. Catal. B Environ. 2022, 310, 121063. [Google Scholar] [CrossRef]
- Lamhani, M.; Chchiyai, Z.; Elomrani, A.; Manoun, B.; Hasnaoui, A. Enhanced Photocatalytic Water Splitting of SrTiO3 Perovskite through Cobalt Doping: Experimental and Theoretical DFT Understanding. Inorg. Chem. 2023, 62, 13405–13418. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Guo, J.-F.; Wang, H.-T.; Chang, N. Enhancing visible light photocatalytic activity by transformation of Co3+/Co2+ and formation of oxygen vacancies over rationally Co doped ZnO microspheres. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128157. [Google Scholar] [CrossRef]
- Chang, L.; Xue, X.; Deng, Q.; Xie, X.; Zhang, X.; Cheng, C.; Chai, H.; Huang, Y. Modulating the electronic structure of Co center via MgO@C co-doping for PMS activation to remove levofloxacin. Sep. Purif. Technol. 2023, 321, 124151. [Google Scholar] [CrossRef]
- Liu, B.; Qi, Y.; Qiu, X.; Zou, H.; Lin, X.; Qin, Y. Photoelectrocatalytic Pathway for the Preparation of Power-Effective Aviation Fuel Precursors from Lignin. Adv. Funct. Mater. 2025, 35, 2421552. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Yue, F.; Du, R.; Ma, T.; Bian, Y.; Li, R.; Guo, L.; Wang, D.; Fu, F. Co doping regulating electronic structure of Bi2MoO6 to construct dual active sites for photocatalytic nitrogen fixation. Appl. Catal. B Environ. 2023, 338, 123057. [Google Scholar] [CrossRef]
- Wang, L.; Yang, T.; Xu, X.; Zhang, G.; Liu, Y.; Ju, A.; Zhou, G.; Feng, B.; Che, G.; Zhao, Z. Acid groups decorated bimetal-organic catalyst for advanced oxidation technology at full pH range. J. Alloys Compd. 2023, 969, 172370. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, H.; Kang, L.; Gao, Z.; Ren, F. Fe-based metal-organic frameworks as Fenton-like catalysts for highly efficient degradation of tetracycline hydrochloride over a wide pH range: Acceleration of Fe(II)/Fe(III) cycle under visible light irradiation. Appl. Catal. B Environ. 2020, 263, 118282. [Google Scholar] [CrossRef]
- Filho, M.A.M.; Farmer, W.; Hsiao, C.-L.; dos Santos, R.B.; Hultman, L.; Birch, J.; Ankit, K.; Gueorguiev, G.K. Density Functional Theory-Fed Phase Field Model for Semiconductor Nanostructures: The Case of Self-Induced Core–Shell InAlN Nanorods. Cryst. Growth Des. 2024, 24, 4717–4727. [Google Scholar] [CrossRef]
- Kakanakova-Georgieva, A.; Gueorguiev, G.K.; Yakimova, R.; Janzén, E. Effect of impurity incorporation on crystallization in AlN sublimation epitaxy. J. Appl. Phys. 2004, 96, 5293–5297. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Zhu, Y.; An, Z.; Shu, X.; Song, H.; Wang, W.; Chai, Z.; Shang, C.; Jiang, S.; et al. Photo-splitting of water toward hydrogen production and active oxygen species for methane activation to methanol on Co-SrTiO3. Chem Catal. 2022, 2, 1440–1449. [Google Scholar] [CrossRef]
- Eskandari, N.; Nabiyouni, G.; Masoumi, S.; Ghanbari, D. Preparation of a new magnetic and photo-catalyst CoFe2O4–SrTiO3 perovskite nanocomposite for photo-degradation of toxic dyes under short time visible irradiation. Compos. Part B Eng. 2019, 176, 107343. [Google Scholar] [CrossRef]
- Swathi, S.; Yuvakkumar, R.; Kumar, P.S.; Ravi, G.; Velauthapillai, D. Annealing temperature effect on cobalt ferrite nanoparticles for photocatalytic degradation. Chemosphere 2021, 281, 130903. [Google Scholar] [CrossRef] [PubMed]
- Subagyo, R.; Yudhowijoyo, A.; Sholeha, N.A.; Hutagalung, S.S.; Prasetyoko, D.; Birowosuto, M.D.; Arramel, A.; Jiang, J.; Kusumawati, Y. Recent advances of modification effect in Co3O4-based catalyst towards highly efficient photocatalysis. J. Colloid Interface Sci. 2023, 650, 1550–1590. [Google Scholar] [CrossRef]
- Feng, S.; Xie, T.; Wang, J.; Yang, J.; Kong, D.; Liu, C.; Chen, S.; Yang, F.; Pan, M.; Yang, J.; et al. Photocatalytic activation of PMS over magnetic heterojunction photocatalyst SrTiO3/BaFe12O19 for tetracycline ultrafast degradation. Chem. Eng. J. 2023, 470, 143900. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, X.; Zhong, Y.; Ge, L.; Chen, Y.; Veder, J.-P.M.; Guan, D.; O’Hayre, R.; Li, M.; Wang, G.; et al. Direct evidence of boosted oxygen evolution over perovskite by enhanced lattice oxygen participation. Nat. Commun. 2020, 11, 2002. [Google Scholar] [CrossRef]
- Koo, B.; Kim, K.; Kim, J.K.; Kwon, H.; Han, J.W.; Jung, W. Sr Segregation in Perovskite Oxides: Why It Happens and How It Exists. Joule 2018, 2, 1476–1499. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, Q.; Xie, Z.; Wang, Y.; Wang, J.; Peng, Y.; Fang, Y.; Deng, L.; Xie, T.; Xu, L. Enhancement mechanism of photocatalytic activity for MoS2/Ti3C2 Schottky junction: Experiment and DFT calculation. J. Alloys Compd. 2021, 887, 161411. [Google Scholar] [CrossRef]
- Chen, R.; Chen, H.; Zhang, Q.; Wang, D. Efficient activation of peroxomonosulfate (PMS) by co-doped g-C3N4/β-Bi2O3 heterojunction for the degradation of 2,4-dichlorophenol under visible light. J. Water Process Eng. 2025, 70, 107034. [Google Scholar] [CrossRef]
- Guan, R.; Cheng, X.; Chen, Y.; Wu, Z.; Zhao, Z.; Shang, Q.; Sun, Y.; Sun, Z. Wettability control of defective TiO2 with alkyl acid for highly efficient photocatalytic ammonia synthesis. Nano Res. 2023, 16, 10770–10778. [Google Scholar] [CrossRef]
- Vijay, A.; Bairagi, K.; Vaidya, S. Relating the structure, properties, and activities of nanostructured SrTiO3 and SrO–(SrTiO3)n (n = 1 and 2) for photocatalytic hydrogen evolution. Mater. Adv. 2022, 3, 5055–5063. [Google Scholar] [CrossRef]
- Li, X.; Feng, D.; He, X.; Qian, D.; Nasen, B.; Qi, B.; Fan, S.; Shang, J.; Cheng, X. Z-scheme heterojunction composed of Fe doped g-C3N4 and MoS2 for efficient ciprofloxacin removal in a photo-assisted peroxymonosulfate system. Sep. Purif. Technol. 2022, 303, 122219. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Yao, J.; Song, Y.; Li, W.; Xuan, X. Coordination tuning of Fe2+ ions concentration in Fe-doped black phosphorus-carbonized cotton fiber (Fe-BP-CCF) composites to regulate photocatalysis and peroxymonosulfate (PMS) activation towards highly efficient degradation of organic pollutants. Chem. Eng. J. 2024, 483, 149326. [Google Scholar] [CrossRef]
- Qin, Y.; Fang, F.; Xie, Z.; Lin, H.; Zhang, K.; Yu, X.; Chang, K. La,Al-Codoped SrTiO3 as a Photocatalyst in Overall Water Splitting: Significant Surface Engineering Effects on Defect Engineering. ACS Catal. 2021, 11, 11429–11439. [Google Scholar] [CrossRef]
- Qin, Y.; Wan, Y.; Xiang, L.; Wang, T.; Guo, D.; Fang, F.; Chang, K. Micro-regulating defect-charge delocalization in Cr-doped SrTiO3 for boosting visible-light-driven overall water splitting. J. Catal. 2024, 437, 115660. [Google Scholar] [CrossRef]
- Ding, L.; Qi, F.; Li, Y.; Lin, J.; Su, Y.; Song, Y.; Wang, L.; Sun, H.; Tong, C. In-situ formation of nanosized 1T-phase MoS2 in B-doped carbon nitride for high efficient visible-light-driven H2 production. J. Colloid Interface Sci. 2022, 614, 92–101. [Google Scholar] [CrossRef]
- Wang, L.; Yang, T.; Feng, B.; Xu, X.; Shen, Y.; Li, Z.; Arramel; Jiang, J. Constructing dual electron transfer channels to accelerate CO2 photoreduction guided by machine learning and first-principles calculation. Chin. J. Catal. 2023, 54, 265–277. [Google Scholar] [CrossRef]
- Song, M.; Wang, L.; Li, J.; Sun, D.; Guan, R.; Zhai, H.; Gao, X.; Li, X.; Zhao, Z.; Sun, Z. Defect density modulation of La2TiO5: An effective method to suppress electron-hole recombination and improve photocatalytic nitrogen fixation. J. Colloid Interface Sci. 2021, 602, 748–755. [Google Scholar] [CrossRef]
- Wang, L.; Guan, R.; Qi, Y.; Zhang, F.; Li, P.; Wang, J.; Qu, P.; Zhou, G.; Shi, W. Constructing Zn-P charge transfer bridge over ZnFe2O4-black phosphorus 3D microcavity structure: Efficient photocatalyst design in visible-near-infrared region. J. Colloid Interface Sci. 2021, 600, 463–472. [Google Scholar] [CrossRef]
- Li, J.; Zou, Y.; Li, Z.; Fu, S.; Lu, Y.; Li, S.; Zhu, X.; Zhang, T. Modulating the Electronic Coordination Configuration and d-Band Center in Homo-Diatomic Fe2N6 Catalysts for Enhanced Peroxymonosulfate Activation. ACS Appl. Mater. Interfaces 2022, 14, 37865–37877. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Ma, X.; Wu, Y.; Wang, Y.; Zhou, L.; Lei, J.; Yamashita, H.; Zhang, J. A-site defect regulates d-band center in perovskite-type catalysts enhancing photo-assisted peroxymonosulfate activation for levofloxacin removal via high-valent iron-oxo species. Appl. Catal. B Environ. Energy 2025, 371, 125273. [Google Scholar] [CrossRef]
- Zhang, H.; An, Q.; Su, Y.; Quan, X.; Chen, S. Co3O4 with upshifted d-band center and enlarged specific surface area by single-atom Zr doping for enhanced PMS activation. J. Hazard. Mater. 2023, 448, 130987. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Zou, Y.; Lin, L.; Li, B.; Li, X.-y. Transition metal single-atom embedded on N-doped carbon as a catalyst for peroxymonosulfate activation: A DFT study. Chem. Eng. J. 2022, 437, 135428. [Google Scholar] [CrossRef]
- Cheng, X.; Guan, R.; Wu, Z.; Sun, Y.; Che, W.; Shang, Q. Establishing carrier transport channels based on Ti-S bonds and enhancing the photocatalytic performance of MXene quantum dots–ZnInS for ammonia synthesis. InfoMat 2024, 6, e12535. [Google Scholar] [CrossRef]
- Liu, C.; Sheng, B.; Zhou, Q.; Xia, Y.; Zou, Y.; Chimtali, P.J.; Cao, D.; Chu, Y.; Zhao, S.; Long, R.; et al. Manipulating d-Band Center of Nickel by Single-Iodine-Atom Strategy for Boosted Alkaline Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2024, 146, 26844–26854. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Xia, M.; Chong, B.; Li, H.; Lin, B.; Yang, G. D-band center modulation of B-mediated FeS2 to activate molecular nitrogen for electrocatalytic ammonia synthesis. Appl. Catal. B Environ. 2024, 343, 123474. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, B.; Li, X.; Fan, X.; Zhang, J.; Yu, Y.; Sun, Y. Anaerobic environment-induced efficient degradation of chloroquine phosphate: Insights into the role of metal-free C3N4 nanotube in visible light-driven peroxymonosulfate activation. Chem. Eng. J. 2023, 457, 141219. [Google Scholar] [CrossRef]
- Hao, M.; Wang, Q.; Yu, F.; Guan, Z.; Zhang, X.; Sun, Y. Efficient degradation of 2,4-dichlorophphenol in groundwater using persulfate activated by nitrogen-doped biochar-supported nano zero-valent iron. J. Clean. Prod. 2024, 458, 142415. [Google Scholar] [CrossRef]
- Shi, H.; Li, Y.; Zhang, L.; Liu, G.; Zhang, Q.; Ru, X.; Zhong, S. Bi2SiO5 nanosheets as piezo-photocatalyst for efficient degradation of 2,4-Dichlorophenol. Appl. Catal. B Environ. Energy 2025, 361, 124581. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Parr, R.G.; Yang, W. Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, K.; Yang, X.; Qi, F.; Liu, Y.; Wang, L.; Feng, B.; Yu, J.; Che, G. Modulating D-Band Center of SrTiO3 by Co Doping for Boosted Peroxymonosulfate (PMS) Activation Under Visible Light. Molecules 2025, 30, 2618. https://doi.org/10.3390/molecules30122618
Sun K, Yang X, Qi F, Liu Y, Wang L, Feng B, Yu J, Che G. Modulating D-Band Center of SrTiO3 by Co Doping for Boosted Peroxymonosulfate (PMS) Activation Under Visible Light. Molecules. 2025; 30(12):2618. https://doi.org/10.3390/molecules30122618
Chicago/Turabian StyleSun, Kaining, Xinyi Yang, Fei Qi, Yingjie Liu, Lijing Wang, Bo Feng, Jiankang Yu, and Guangbo Che. 2025. "Modulating D-Band Center of SrTiO3 by Co Doping for Boosted Peroxymonosulfate (PMS) Activation Under Visible Light" Molecules 30, no. 12: 2618. https://doi.org/10.3390/molecules30122618
APA StyleSun, K., Yang, X., Qi, F., Liu, Y., Wang, L., Feng, B., Yu, J., & Che, G. (2025). Modulating D-Band Center of SrTiO3 by Co Doping for Boosted Peroxymonosulfate (PMS) Activation Under Visible Light. Molecules, 30(12), 2618. https://doi.org/10.3390/molecules30122618





