Rapid Detection of Chlorpheniramine Maleate in Human Blood and Urine Samples Based on NiCoP/PVP/PAN/CNFs Electrochemiluminescence Sensor
Abstract
1. Introduction
2. Results and Discussion
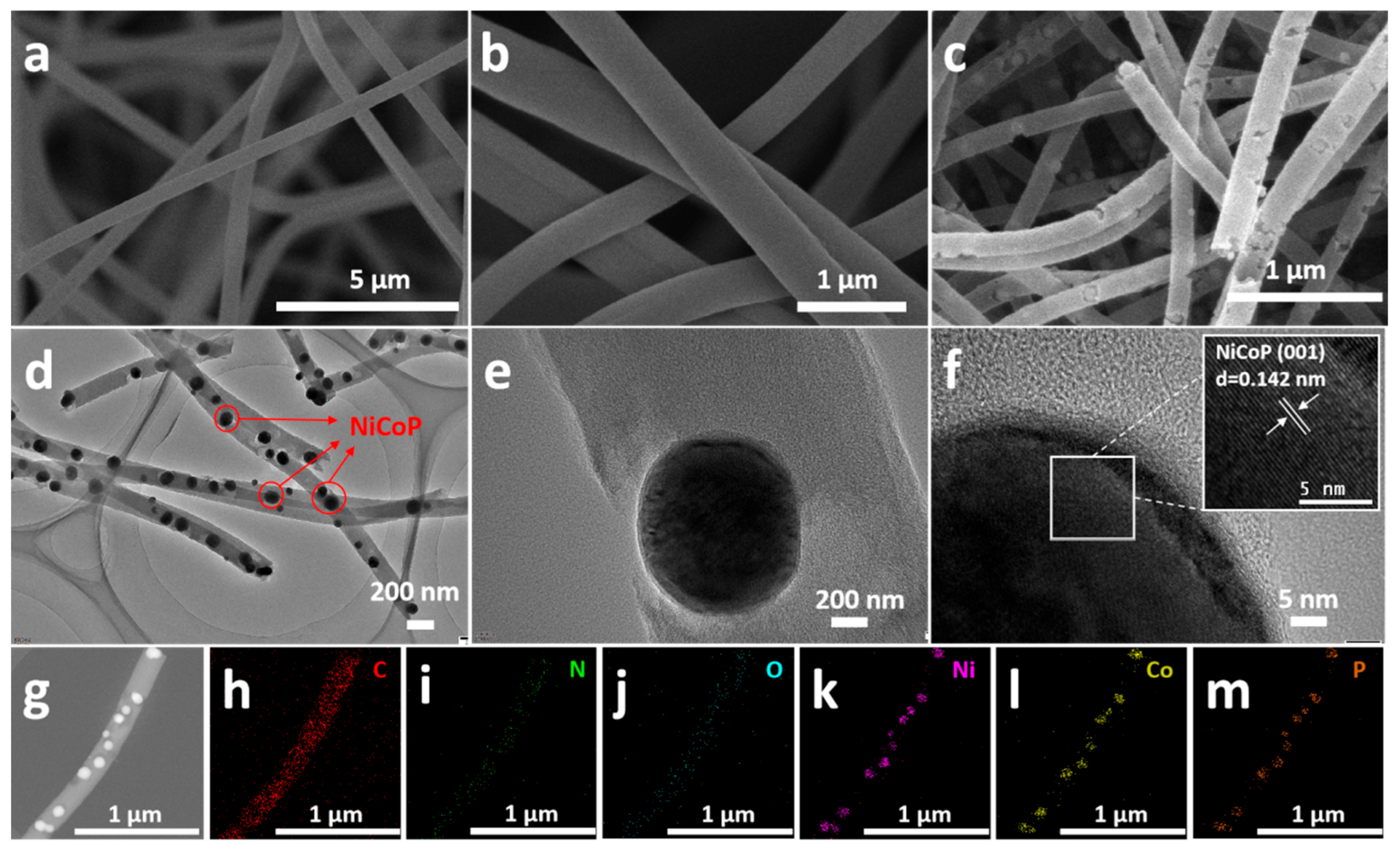
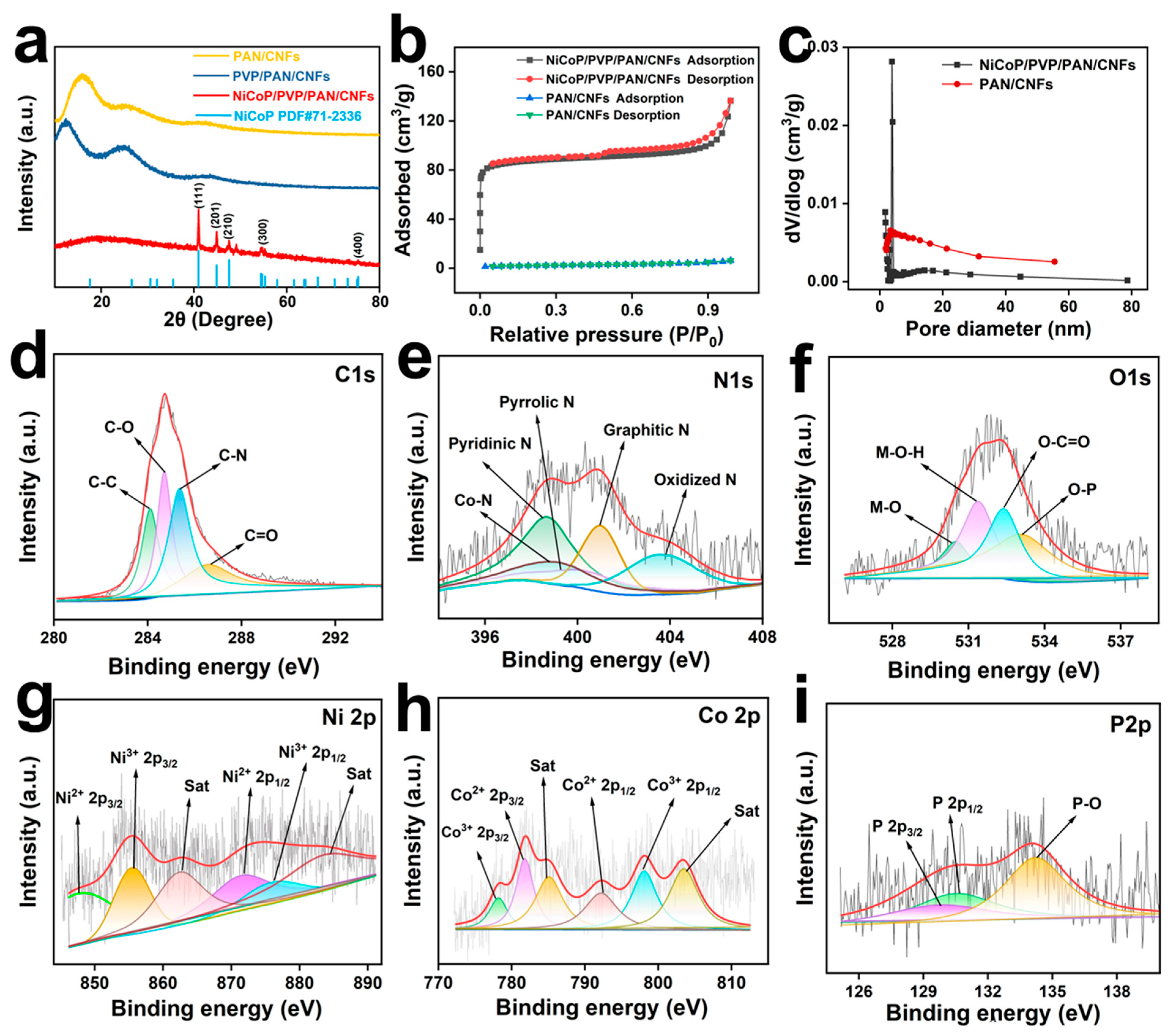
2.1. Characterization of Materials
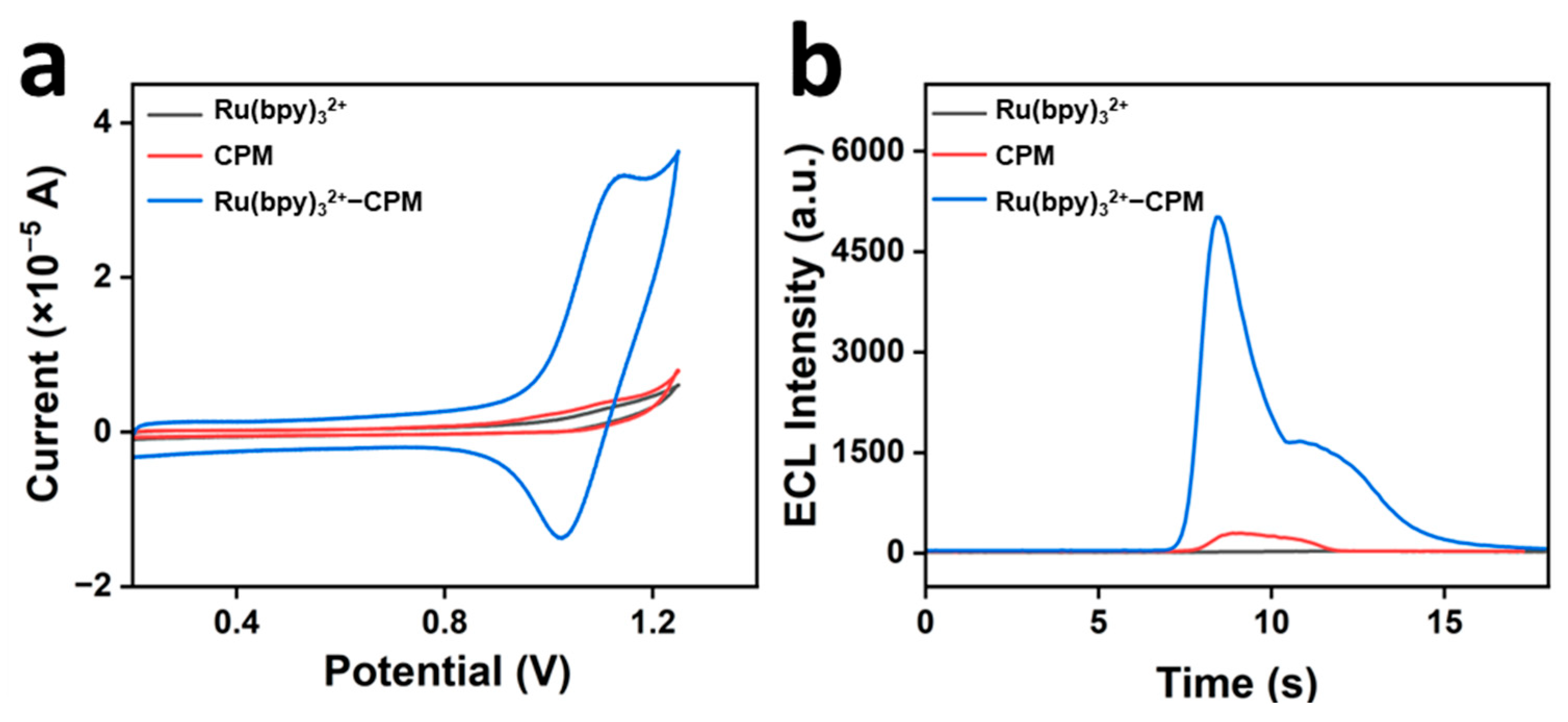
2.2. Electrochemistry and Electrochemiluminescence in the System
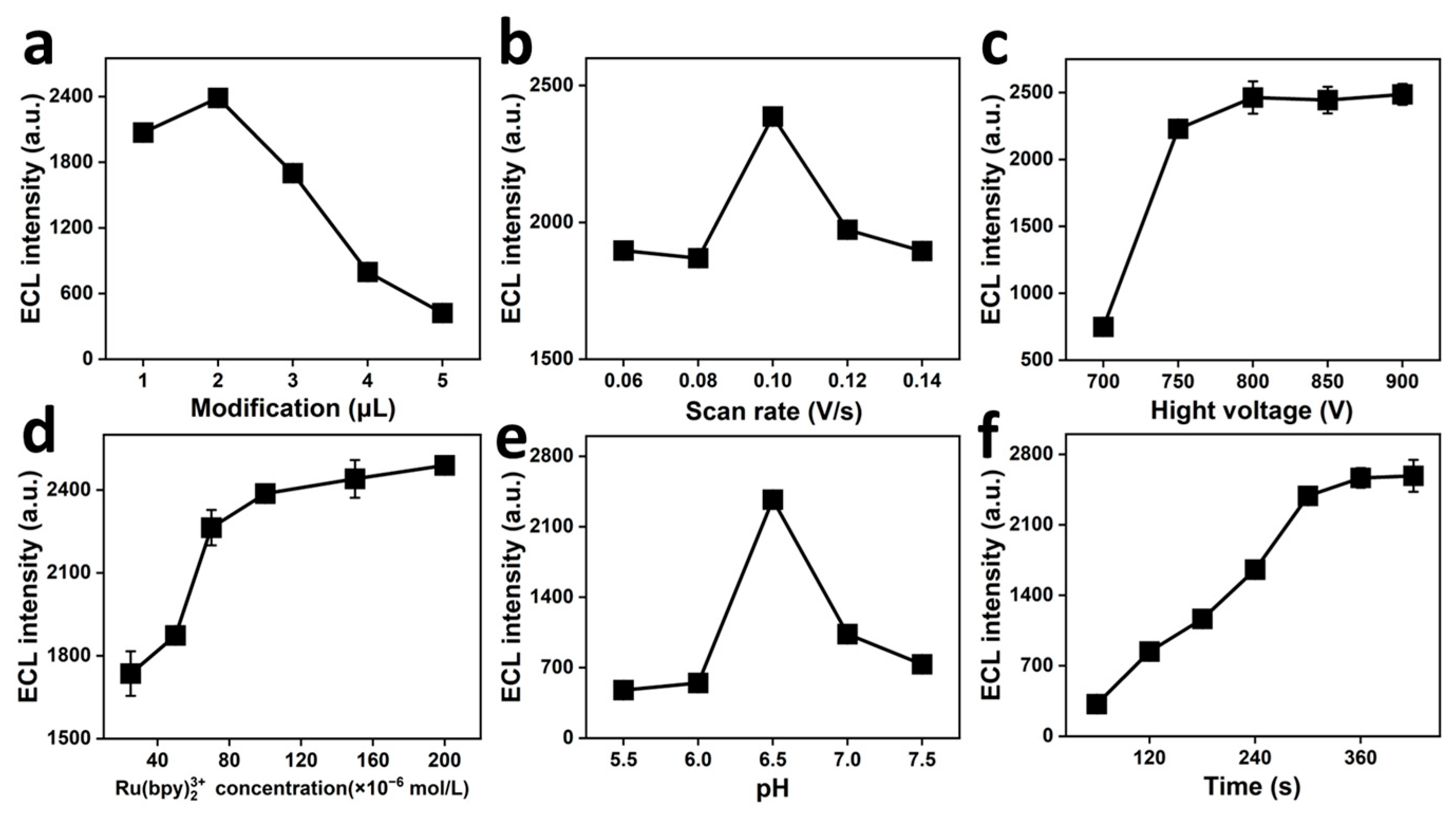
2.3. Experimental Parameter Refinement
2.3.1. Effect of Modification Amount of NiCoP/PVP/PAN/CNFs
2.3.2. Effect of Scan Rate
2.3.3. Effect of Photomultiplying High Voltage
2.3.4. Impact of Ru(bpy)32+ Concentration
2.3.5. Effect of pH Value
2.3.6. Effect of Enrichment Time
2.4. Methodological Evaluations
2.4.1. Establishment of the Linear Equation
2.4.2. Selectivity, Repeatability, and Reproducibility of NiCoP/PVP/PAN/CNFs Modified Electrodes
2.5. Examination of Authentic Samples
3. Experimental Section
3.1. Experimental Reagents
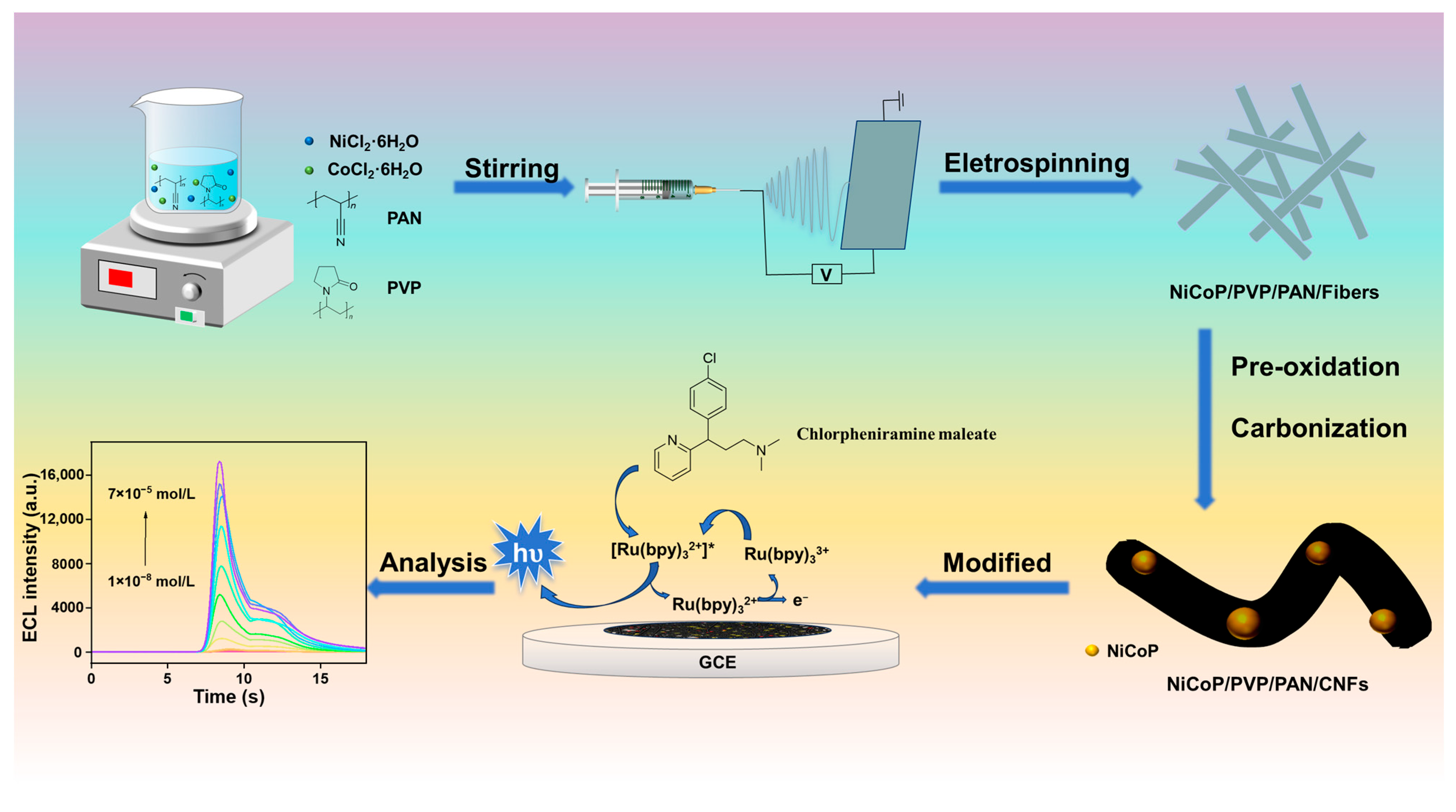
3.2. Preparation of NiCoP/PVP/PAN/CNFs Material
3.3. Preparation of NiCoP/PVP/PAN/CNFs/GCE
3.4. Electrochemical and ECL Analysis
3.5. Handling of Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, J.; Liu, Z.; Guan, T.; Lei, Y.; Pan, L.; Yu, X.; Zhang, S.; Huang, X.-A.; Lei, H.; Chen, J. Antibody Production and Immunoassay Development for Authenticating Chlorpheniramine Maleate Adulteration in Herbal Tea. Foods 2024, 13, 1609. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.E.; Elmaaty, A.A.; El-Sayed, H.M. Determination of six drugs used for treatment of common cold by micellar liquid chromatography. Anal. Bioanal. Chem. 2021, 413, 5051–5065. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A. Stability-Indicating New RP-UPLC Method for Simultaneous Determination of a Quaternary Mixture of Paracetamol, Pseudoephedrine, Chlorpheniramine, and Sodium Benzoate in (Cold–Flu) Syrup Dosage Form. J. AOAC Int. 2022, 105, 703–716. [Google Scholar] [CrossRef]
- Murao, S.; Manabe, H.; Yamashita, T.; Sekikawa, T. Intoxication with Over-the-Counter Antitussive Medication Containing Dihydrocodeine and Chlorpheniramine Causes Generalized Convulsion and Mixed Acidosis. Intern. Med. 2008, 47, 1013–1015. [Google Scholar] [CrossRef][Green Version]
- Lee, S.-H.; Lee, Y.; Woo, S.-D.; Doo, K.-E.; Ha, C.-Y.; Lee, Y.-H.; Ye, Y.-M. Chlorpheniramine-induced anaphylaxis. Medicine 2019, 98, e18369. [Google Scholar] [CrossRef]
- Hammouda, M.E.A.; Salem, Y.A.; El-Ashry, S.M.; Abu El-Enin, M.A. Inclusive study for sustainable enantioseparation of racemic chlorpheniramine and caffeine by HPLC using dual cyclodextrin system as chiral mobile phase additive: Assessment with AGREE and Complex-GAPI approaches. Sustain. Chem. Pharm. 2023, 35, 101201. [Google Scholar] [CrossRef]
- Bachute, M.T.; Shanbhag, S.V.; Turwale, S.L. Simultaneous determination of four active pharmaceuticals in tablet dosage form by reversed-phase high performance liquid chromatography. Trop. J. Pharm. Res. 2021, 18, 2161–2166. [Google Scholar] [CrossRef]
- Ioutsi, A.N.; Ioutsi, V.A.; Shapovalova, E.N.; Shpigun, O.A. Determination of Pharmacologically Active Nitrogen-Containing Compounds on Silica Doubly Modified with 6,10-Ionene and Dextran Sulphate by High-Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Anal. Chem. 2020, 75, 930–935. [Google Scholar] [CrossRef]
- Papadouli, K.; Ntorkou, M.; Manousi, N.; Kabir, A.; Furton, K.G.; Zacharis, C.K. Poly(tetrahydrofuran)-based fabric phase sorptive extraction combined with HPLC-UV for the determination of antihistamines in human urine. Microchem. J. 2023, 192, 108951. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Qu, N.; Gu, J. Electrochemiluminescence for high-performance Chiral recognition of enantiomers: Recent advances and future perspectives. Org. Electron. 2023, 122, 106902. [Google Scholar] [CrossRef]
- Geng, L.; Huang, J.; Fang, M.; Wang, H.; Liu, J.; Wang, G.; Hu, M.; Sun, J.; Guo, Y.; Sun, X. Recent progress of the research of metal-organic frameworks-molecularly imprinted polymers (MOFs-MIPs) in food safety detection field. Food Chem. 2024, 458, 140330. [Google Scholar] [CrossRef] [PubMed]
- Sradha, S.A.; George, L.P.K.; Varghese, A. Recent advances in electrochemical and optical sensing of the organophosphate chlorpyrifos: A review. Crit. Rev. Toxicol. 2022, 52, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, X.; Gao, G.; Wang, X. Flexible carbonaceous silica nanofibers with superior toughness and strength for oil-in-water emulsions separation. Sep. Purif. Technol. 2025, 358, 130487. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Assadpour, E.; Tabarestani, H.S.; Falsafi, S.R.; Jafari, S.M. Electrospinning approach for nanoencapsulation of bioactive compounds; recent advances and innovations. Trends Food Sci. Technol. 2020, 100, 190–209. [Google Scholar] [CrossRef]
- Ramesh Gawali, C.; Daweshar, E.; Kolhe, A.; Kumar, S. Recent advances in nanostructured conducting polymer electrospun for application in electrochemical biosensors. Microchem. J. 2024, 200, 110326. [Google Scholar] [CrossRef]
- Mohite, D.D.; Chavan, S.S.; Dubal, S.; Karandikar, P.B. Electrospun polyacrylonitrile (PAN) carbon nanofibers (CFNs) as electrode material for supercapacitors: A comprehensive review of synthesis, characterization, and electrochemical performance. AIP Adv. 2023, 13, 110326. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, Y.; Jin, X.; Sun, Y.; Lu, B.; Wang, H.; Fang, J.; Shao, H.; Lin, T. Unexpectedly high piezoelectricity of electrospun polyacrylonitrile nanofiber membranes. Nano Energy 2019, 56, 588–594. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, Z.-H.; Kang, F. Electrospun preparation of microporous carbon ultrafine fibers with tuned diameter, pore structure and hydrophobicity from phenolic resin. Carbon 2014, 66, 705–712. [Google Scholar] [CrossRef]
- Roy, S.; Panda, P.; Barman, S. Electrospun highly porous carbon nitride-carbon nanofibers for high performance supercapacitor application. J. Energy Storage 2024, 91, 112007. [Google Scholar] [CrossRef]
- Yang, S.-H.; Fu, W.-Q.; Cui, Y.-W.; Cao, B.-Q. PVP/PAN-derived porous carbon fiber for zinc-ion hybrid supercapacitors. Rare Metals 2024, 43, 3066–3073. [Google Scholar] [CrossRef]
- Heckova, M.; Streckova, M.; Orinakova, R.; Hovancova, J.; Guboova, A.; Sopcak, T.; Kovalcikova, A.; Plesingerova, B.; Medved, D.; Szabo, J.; et al. Porous carbon fibers for effective hydrogen evolution. Appl. Surf. Sci. 2020, 506, 144955. [Google Scholar] [CrossRef]
- Ruiz-Montoya, J.G.; Quispe-Garrido, L.V.; Gómez, J.C.; Baena-Moncada, A.M.; Gonçalves, J.M. Recent progress in and prospects for supercapacitor materials based on metal oxide or hydroxide/biomass-derived carbon composites. Sustain. Energy Fuels 2021, 5, 5332–5365. [Google Scholar] [CrossRef]
- Lee, S.C.; Liu, S.; Shinde, P.A.; Chung, K.Y.; Chan Jun, S. A systematic approach to achieve high energy density hybrid supercapacitors based on Ni–Co–Fe hydroxide. Electrochim. Acta 2020, 353, 136578. [Google Scholar] [CrossRef]
- Sun, M.; Ren, X.; Gan, Z.; Liu, M.; Sun, Y.; Shen, W.; Li, Z.; Fu, Y. Nanostructured binary transition-metal-sulfides and nanocomposites as high-performance electrodes for hybrid supercapacitors. Appl. Phys. Rev. 2024, 11, 021318. [Google Scholar] [CrossRef]
- Peng, H.; Zhou, J.; Chen, Z.; Zhao, R.; Liang, J.; Wang, F.; Ma, G.; Lei, Z. Integrated carbon nanosheet frameworks inlaid with nickel phosphide nanoparticles by substrate-free chemical blowing and phosphorization for aqueous asymmetric supercapacitor. J. Alloys Compd. 2019, 797, 1095–1105. [Google Scholar] [CrossRef]
- Cui, M.; Meng, X. Overview of transition metal-based composite materials for supercapacitor electrodes. Nanoscale Adv. 2020, 2, 5516–5528. [Google Scholar] [CrossRef]
- Lera, I.L.; Khasnabis, S.; Wangatia, L.M.; Olu, F.E.; Ramamurthy, P.C. Insights into the Electrochemical Behavior and Kinetics of NiP@PANI/rGO as a High-Performance Electrode for Alkaline Urea Oxidation. Electrocatalysis 2022, 13, 283–298. [Google Scholar] [CrossRef]
- He, G.; Wang, L. Conductive Ni2P nanosheet arrays-carbon nanofibers as binder-free pseudocapacitive electrode materials. J. Electroanal. Chem. 2022, 907, 116054. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, Y.; Zhou, Y.; Du, Y.; Wang, C. In situ electrodeposition of CoP nanoparticles on carbon nanomaterial doped polyphenylene sulfide flexible electrode for electrochemical hydrogen evolution. Appl. Surf. Sci. 2018, 442, 1–11. [Google Scholar] [CrossRef]
- Huang, F.; Jia, Y.; Yang, S.; Long, Y.; Dong, Y.; Du, P. Hierarchical carbon layer coated NiCoP nanoparticles embedded on nitrogen-doped carbon nanotubes for high-performance supercapacitors. J. Ind. Eng. Chem. 2023, 128, 420–431. [Google Scholar] [CrossRef]
- Li, P.; Han, Y.; Yan, F.; Yan, L.; Huang, H.; Zhou, W. Engineering NiCoP arrays by cross-linked nanowires and nanosheets as advanced materials for hybrid supercapacitors. J. Energy Storage 2021, 38, 102503. [Google Scholar] [CrossRef]
- Shi, Y.; Li, M.; Yu, Y.; Zhang, B. Recent advances in nanostructured transition metal phosphides: Synthesis and energy-related applications. Energy Environ. Sci. 2020, 13, 4564–4582. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, W.; Yang, L.; Lu, J.; Cheng, S.; Mai, W.; Tang, Z.; Li, L.; Chen, S. Ultrahigh-Performance Pseudocapacitor Electrodes Based on Transition Metal Phosphide Nanosheets Array via Phosphorization: A General and Effective Approach. Adv. Funct. Mater. 2015, 25, 7530–7538. [Google Scholar] [CrossRef]
- Lan, Y.; Zhao, H.; Zong, Y.; Li, X.; Sun, Y.; Feng, J.; Wang, Y.; Zheng, X.; Du, Y. Phosphorization boosts the capacitance of mixed metal nanosheet arrays for high performance supercapacitor electrodes. Nanoscale 2018, 10, 11775–11781. [Google Scholar] [CrossRef]
- Sun, C.; Xing, X.; Song, Z.; Miao, L.; Lv, Y.; Gan, L.; Liu, M.; Xiong, W. Recent Progress and Outlook on Metal Phosphides for Nitrogen Gas Electrocatalytic Ammonia Synthesis. Energy Fuels 2024, 38, 14925–14943. [Google Scholar] [CrossRef]
- Zhao, Z.; Miao, Y.; Lu, Q. Electrospun nickel cobalt phosphide/carbon nanofibers as high-performance electrodes for supercapacitors. J. Power Sources 2024, 606, 234587. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Y.; Liu, W. Template-assisted synthesized hollow sphere-like NiCoP/carbon nanoparticles composites for high-performance asymmetric supercapacitors. J. Electroanal. Chem. 2021, 880, 114862. [Google Scholar] [CrossRef]
- Shao, Z.; Qi, H.; Wang, X.; Sun, J.; Guo, N.; Huang, K.; Wang, Q. Boosting oxygen evolution by surface nitrogen doping and oxygen vacancies in hierarchical NiCo/NiCoP hybrid nanocomposite. Electrochim. Acta 2019, 296, 259–267. [Google Scholar] [CrossRef]
- Jia, Y.; Zhu, L.; Pan, H.; Liao, Y.; Zhang, Y.; Zhang, X.; Jiang, Z.; Chen, M.; Wang, K. Excellent electrocatalytic hydrogen evolution performance of hexagonal NiCoP porous nanosheets in alkaline solution. Appl. Surf. Sci. 2022, 580, 152314. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Wang, M.; Ma, X.; Sun, X.; Liu, X.; Wang, L.; Wang, W. Hydrochar magnetic adsorbent derived from Chinese medicine industry waste via one-step hydrothermal route: Mechanism analyses of magnetism and adsorption. Fuel 2022, 326, 125110. [Google Scholar] [CrossRef]
- Bläker, C.; Muthmann, J.; Pasel, C.; Bathen, D. Characterization of Activated Carbon Adsorbents—State of the Art and Novel Approaches. ChemBioEng Rev. 2019, 6, 119–138. [Google Scholar] [CrossRef]
- Han, L.; Wang, X.; Wu, B.; Zhu, S.; Wang, J.; Zhang, Y. In-situ synthesis of zeolite X in foam geopolymer as a CO2 adsorbent. J. Clean. Prod. 2022, 372, 133591. [Google Scholar] [CrossRef]
- Afshan, M.; Kumar, S.; Rani, D.; Pahuja, M.; Ghosh, R.; Siddiqui, S.A.; Riyajuddin, S.; Ghosh, K. Electrodes Based on Se Anchored on NiCoP and Carbon Nanofibers for Flexible Energy Storage Devices. ACS Appl. Nano Mater. 2022, 5, 15328–15340. [Google Scholar] [CrossRef]
- Surendran, S.; Shanmugapriya, S.; Zhu, P.; Yan, C.; Vignesh, R.H.; Lee, Y.S.; Zhang, X.; Selvan, R.K. Hydrothermally synthesised NiCoP nanostructures and electrospun N-doped carbon nanofiber as multifunctional potential electrode for hybrid water electrolyser and supercapatteries. Electrochim. Acta 2019, 296, 1083–1094. [Google Scholar] [CrossRef]
- Gao, H.; Fan, H.; Wang, X.; Li, X.; Song, S.; Zang, J.; Han, W.; Ma, C. NiCoP nanoparticles distributed on N-doped carbon-nanosheets loading on nickel phosphide as self-supporting electrode for efficient overall water splitting. Int. J. Hydrogen Energy 2024, 92, 443–452. [Google Scholar] [CrossRef]
- Liu, X.; Xu, G.; Ding, H.; Zhang, L.; Huang, T. NiCoP nanoparticles embedded in coal-based carbon nanofibers as self-supporting bifunctional electrocatalyst toward water splitting. Int. J. Hydrogen Energy 2023, 48, 35064–35074. [Google Scholar] [CrossRef]
- Zhan, Z.; Gao, P.; Ouyang, C.; Lei, T. Petal-like NiCoP sheets on 3D nitrogen-doped carbon nanofiber network as a robust bifunctional electrocatalyst for water splitting. Int. J. Hydrogen Energy 2022, 47, 34376–34386. [Google Scholar] [CrossRef]
- Wu, H.; Liu, P.; Yin, M.; Hou, Z.; Hu, L.; Dang, J. Surface modification engineering on three-dimensional self-supported NiCoP to construct NiCoOx/NiCoP for highly efficient alkaline hydrogen evolution reaction. J. Alloys Compd. 2020, 835, 155364. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, S.; Zhu, J.; Wang, P.; Wang, X.; Jia, X.; Wågberg, T.; Hu, G. Mesoporous carbon decorated with MIL-100(Fe) as an electrochemical platform for ultrasensitive determination of trace cadmium and lead ions in surface water. Ecotoxicol. Environ. Saf. 2022, 243, 113987. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, C.; Xu, S.; Liu, Y.; Jiang, D.; Zhu, J. Facile synthesis of hierarchical NiCoP nanosheets/NiCoP nanocubes homojunction electrocatalyst for highly efficient and stable hydrogen evolution reaction. Appl. Surf. Sci. 2021, 565, 150537. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.; Guo, X.; Li, X. Design of NiCoP nanorod loaded on cocoon carbon substrate and its non-metal doping for efficient hydrogen evolution. J. Colloid Interface Sci. 2024, 675, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Suntornsuk, L.; Pipitharome, O.; Wilairat, P. Simultaneous determination of paracetamol and chlorpheniramine maleate by micellar electrokinetic chromatography. J. Pharm. Biomed. Anal. 2003, 33, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Sharma, M.C. Validated RP-HPLC method for determining the levels of bromhexine HCl, chlorpheniramine maleate, dextromethorphan HBr and guaiphenesin in their pharmaceutical dosage forms. J. Taibah Univ. Sci. 2018, 10, 38–45. [Google Scholar] [CrossRef]
- Huang, Y.-Q.; You, J.-Q.; Zhang, J.; Sun, W.; Ding, L.; Feng, Y.-Q. Coupling frontal elution paper chromatography with desorption corona beam ionization mass spectrometry for rapid analysis of chlorphenamine in herbal medicines and dietary supplements. J. Chromatogr. A 2011, 1218, 7371–7376. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Ansari, M.T.; Abbas, M. Improved and Validated Quantitative Spectroscopic Analysis of Chlorpheniramine in Pharmaceutical Formulations. Pharm. Chem. J. 2024, 58, 538–545. [Google Scholar] [CrossRef]
- Murugan, E.; Poongan, A.; Kesava, M.; Vinitha, A. Synthesis and characterization of graphene nanosheets for electrochemical quantification of chlorpheniramine maleate drugs using a modified glassy carbon electrode. Indian J. Chem. Technol. (IJCT) 2022, 29, 713–720. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Division of Behavioral and Social Sciences and Education; Division on Earth and Life Studies; Division on Engineering and Physical Sciences; Policy and Global Affairs; Board on Behavioral, Cognitive, and Sensory Sciences; Committee on National Statistics; Nuclear and Radiation Studies Board; Board on Mathematical Sciences and Analytics; Committee on Applied and Theoretical Statistics. Reproducibility and Replicability in Science; National Academies Press: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]

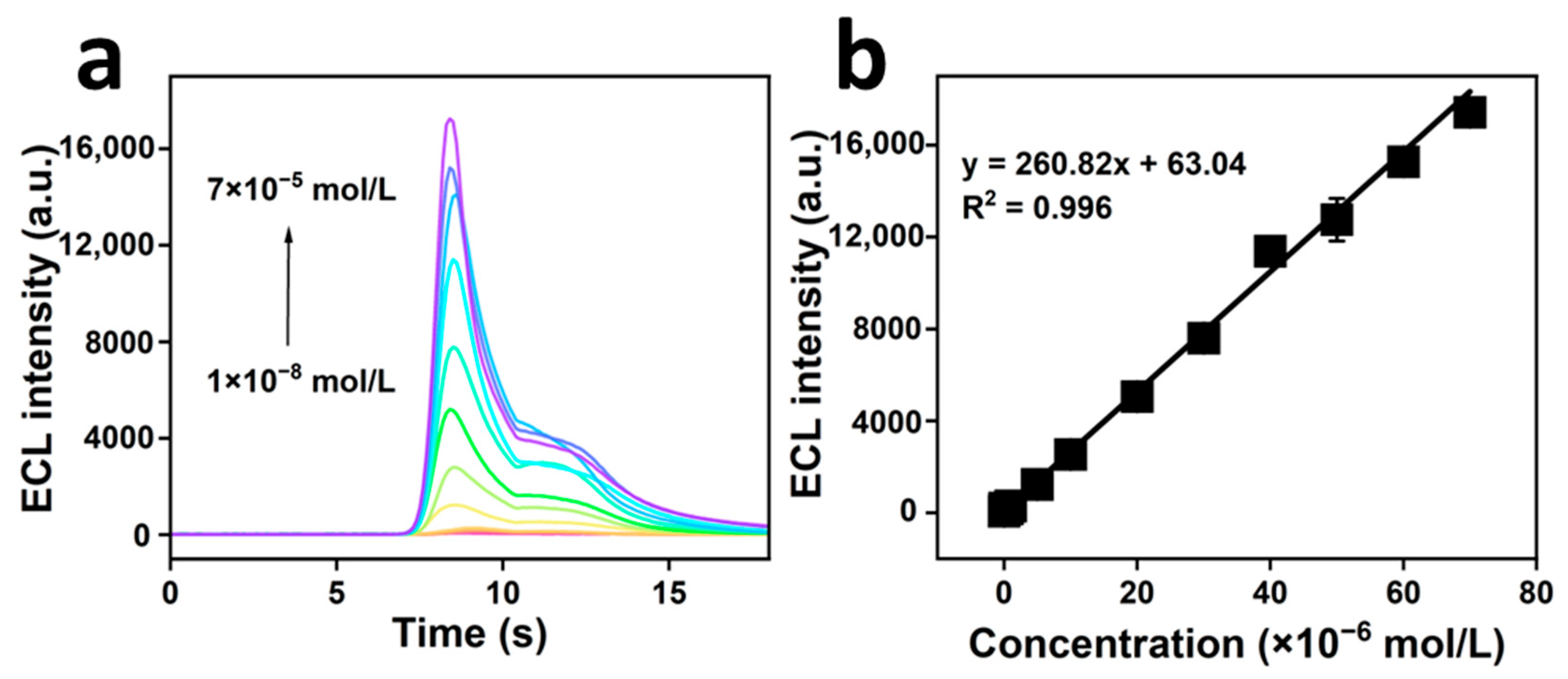

| Method | Linear Range/(mol/L) | LOD/(mol/L) | Reference |
|---|---|---|---|
| UHPLC-MS/MS | 2.5 × 10−5–6.3 × 10−4 | 1.2 × 10−5 | [52] |
| RP-HPLC | 1.2 × 10−5–7.6 × 10−5 | 1.6 × 10−9 | [53] |
| DCBI-MS | 1.2 × 10−5–1.2 × 10−4 | 5.1 × 10−7 | [54] |
| HPLC-UV | 5.1 × 10−8–1.4 × 10−6 | 1.0 × 10−9 | [54] |
| SP | 6.4 × 10−8–3.2 × 10−7 | 3.8 × 10−8 | [55] |
| DPV | 1.0 × 10−5–6.0 × 10−5 | 6.2 × 10−8 | [56] |
| ECL | 1.0 × 10−8–7.0 × 10−5 | 7.8 × 10−10 | This work |
| Detection Methods | Actual Samples | Add/(μmol/L) | Found ± SD/(μmol/L) | Recovery Rate/(%) | RSD/(%, n = 3) |
|---|---|---|---|---|---|
| ECL | Blood serum sample | 0 | - | - | - |
| 1 | 1.03 ± 0.05 | 103.36 | 5.48 | ||
| 11 | 10.46 ± 0.49 | 95.09 | 4.70 | ||
| 31 | 30.22 ± 0.08 | 97.51 | 0.29 | ||
| Urine sample | 0 | - | - | - | |
| 1 | 1.01 ± 0.04 | 101.81 | 4.30 | ||
| 11 | 10.37 ± 0.13 | 94.35 | 1.27 | ||
| 31 | 29.85 ± 0.25 | 96.31 | 0.86 | ||
| HPLC | Blood serum sample | 0 | - | - | - |
| 11 | 10.50 ± 0.07 | 95.45 | 1.23 | ||
| 31 | 31.17 ± 0.32 | 100.54 | 3.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhao, J.; Chen, J.; Tang, T.; Cheng, H. Rapid Detection of Chlorpheniramine Maleate in Human Blood and Urine Samples Based on NiCoP/PVP/PAN/CNFs Electrochemiluminescence Sensor. Molecules 2025, 30, 2603. https://doi.org/10.3390/molecules30122603
Zhang Y, Zhao J, Chen J, Tang T, Cheng H. Rapid Detection of Chlorpheniramine Maleate in Human Blood and Urine Samples Based on NiCoP/PVP/PAN/CNFs Electrochemiluminescence Sensor. Molecules. 2025; 30(12):2603. https://doi.org/10.3390/molecules30122603
Chicago/Turabian StyleZhang, Yi, Jiayu Zhao, Jiaxing Chen, Tingfan Tang, and Hao Cheng. 2025. "Rapid Detection of Chlorpheniramine Maleate in Human Blood and Urine Samples Based on NiCoP/PVP/PAN/CNFs Electrochemiluminescence Sensor" Molecules 30, no. 12: 2603. https://doi.org/10.3390/molecules30122603
APA StyleZhang, Y., Zhao, J., Chen, J., Tang, T., & Cheng, H. (2025). Rapid Detection of Chlorpheniramine Maleate in Human Blood and Urine Samples Based on NiCoP/PVP/PAN/CNFs Electrochemiluminescence Sensor. Molecules, 30(12), 2603. https://doi.org/10.3390/molecules30122603






