New Steroids Obtained from Ailanthus altissima Leaves Inhibit the Invasive Bacteria Xanthomonas oryzae pv. oryzae and Pseudomonas syringae pv. maculicola
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Identification of Compounds from the Leaves of Ailanthus Altissima
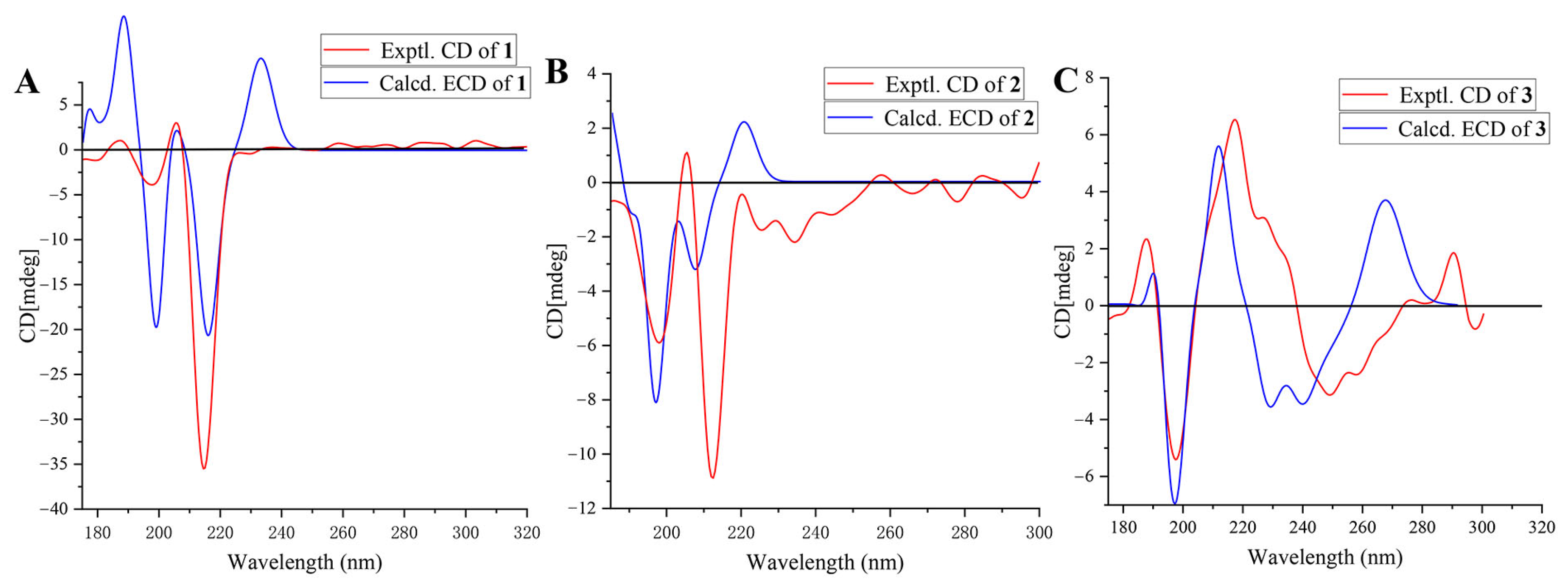
2.2. The Structural Elucidation of New Compounds
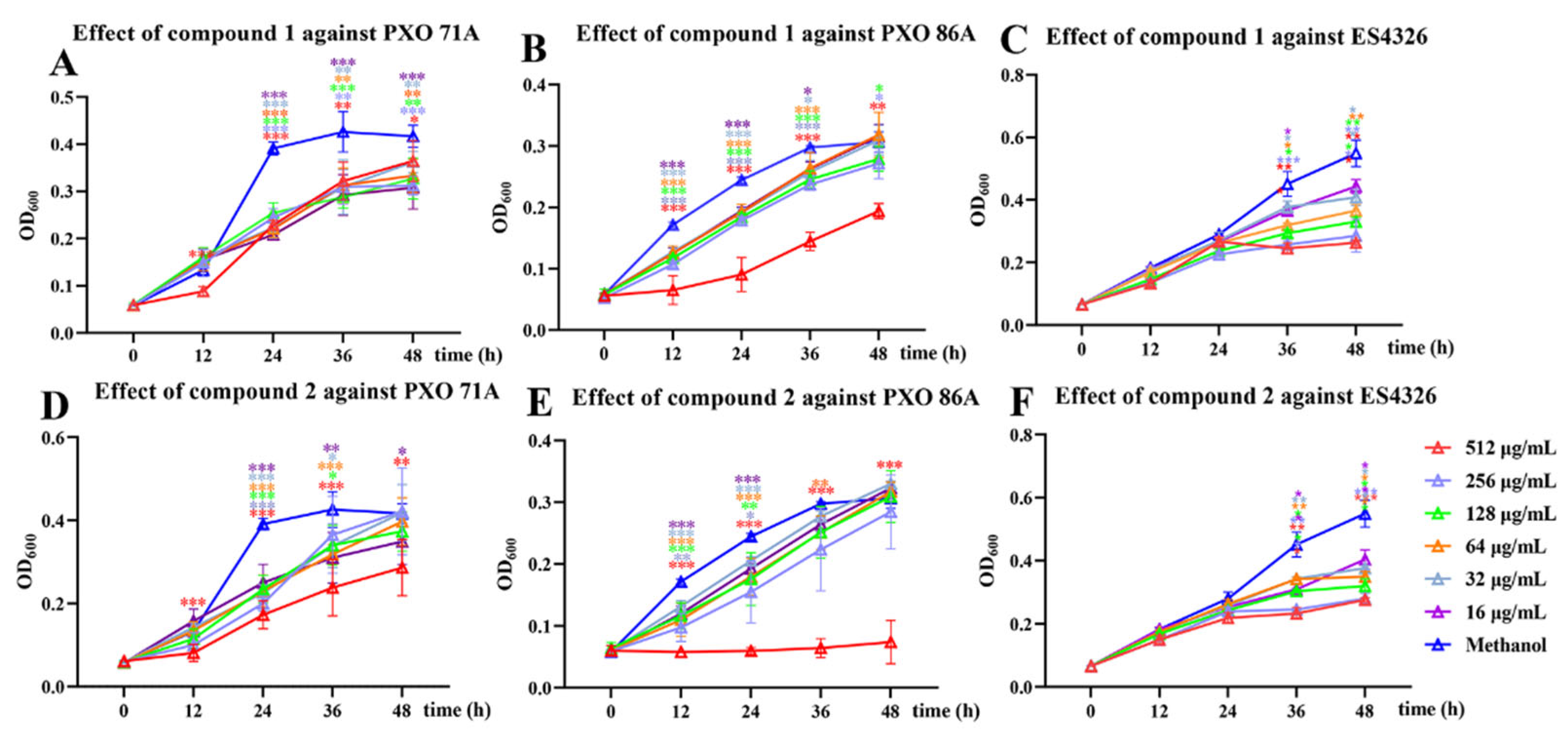
2.3. Growth Inhibition Activity Screening of Compounds Against X. oryzae pv. oryzae PXO 71A and PXO 86A and P. syringae pv. maculicola ES4326
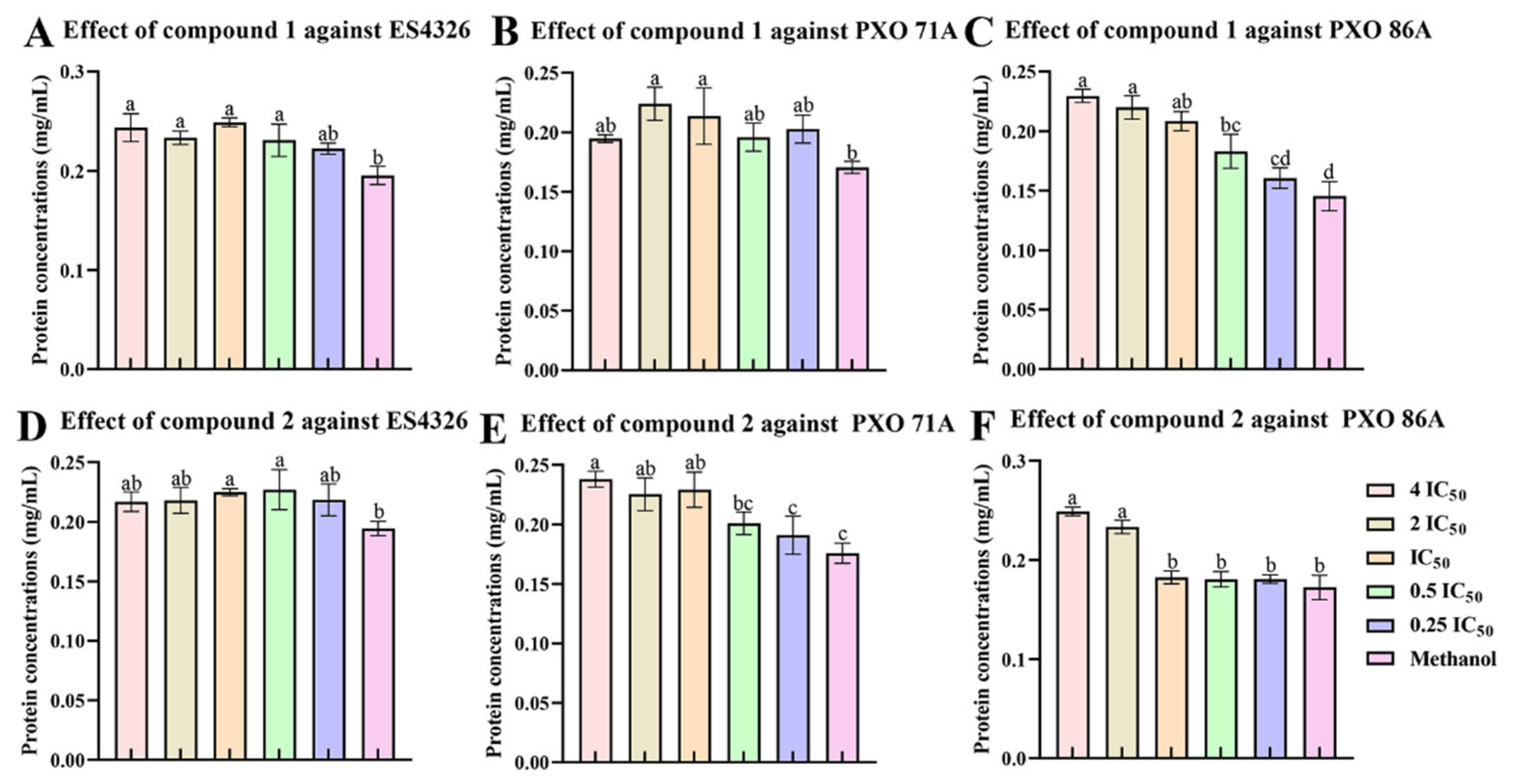
2.4. The Ailanstigols A and B Lead to Intracellular Protein Leakage
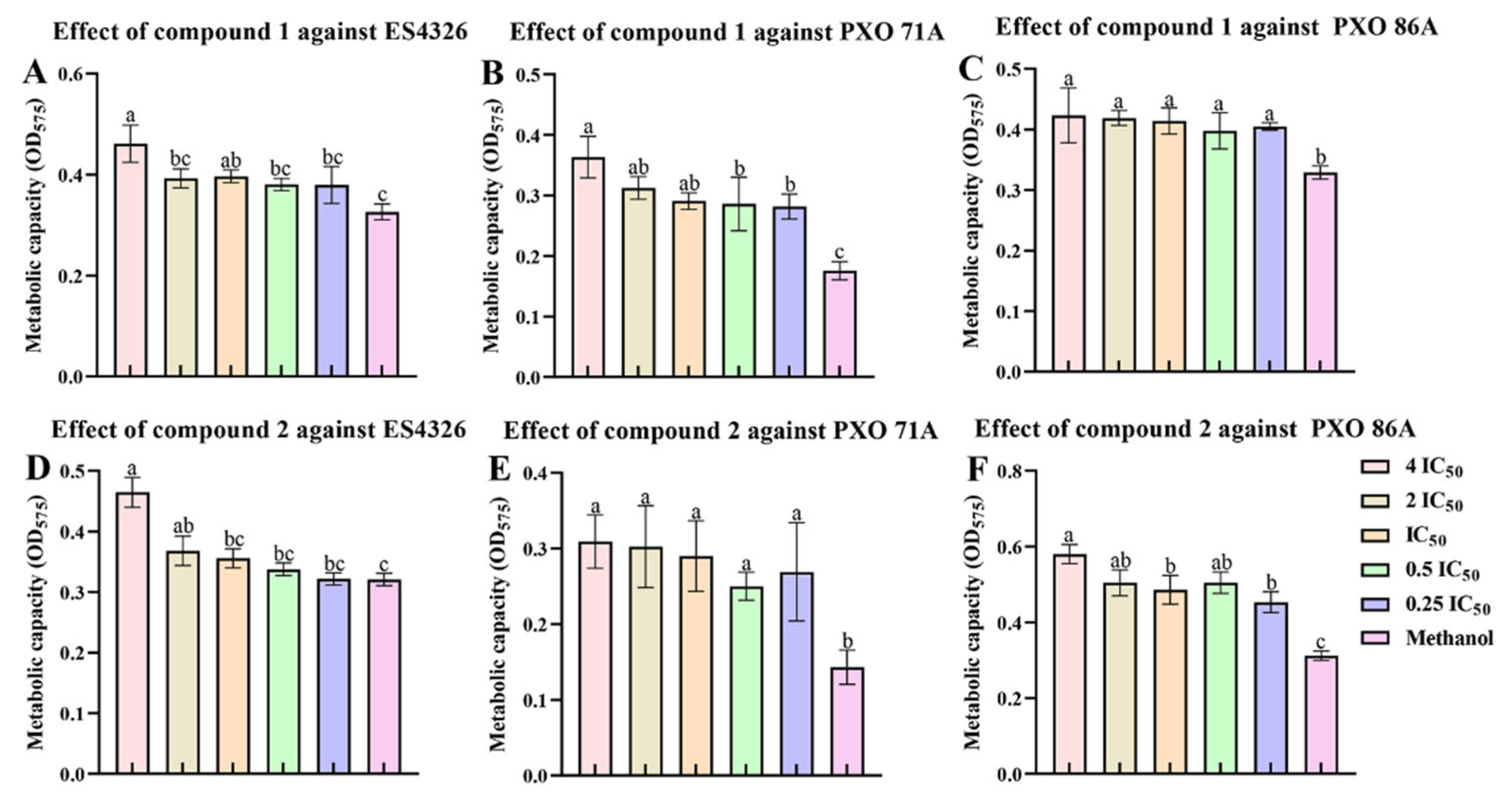
2.5. Ailanstigols A and B Lead to a Reduction in Bacterial Viability
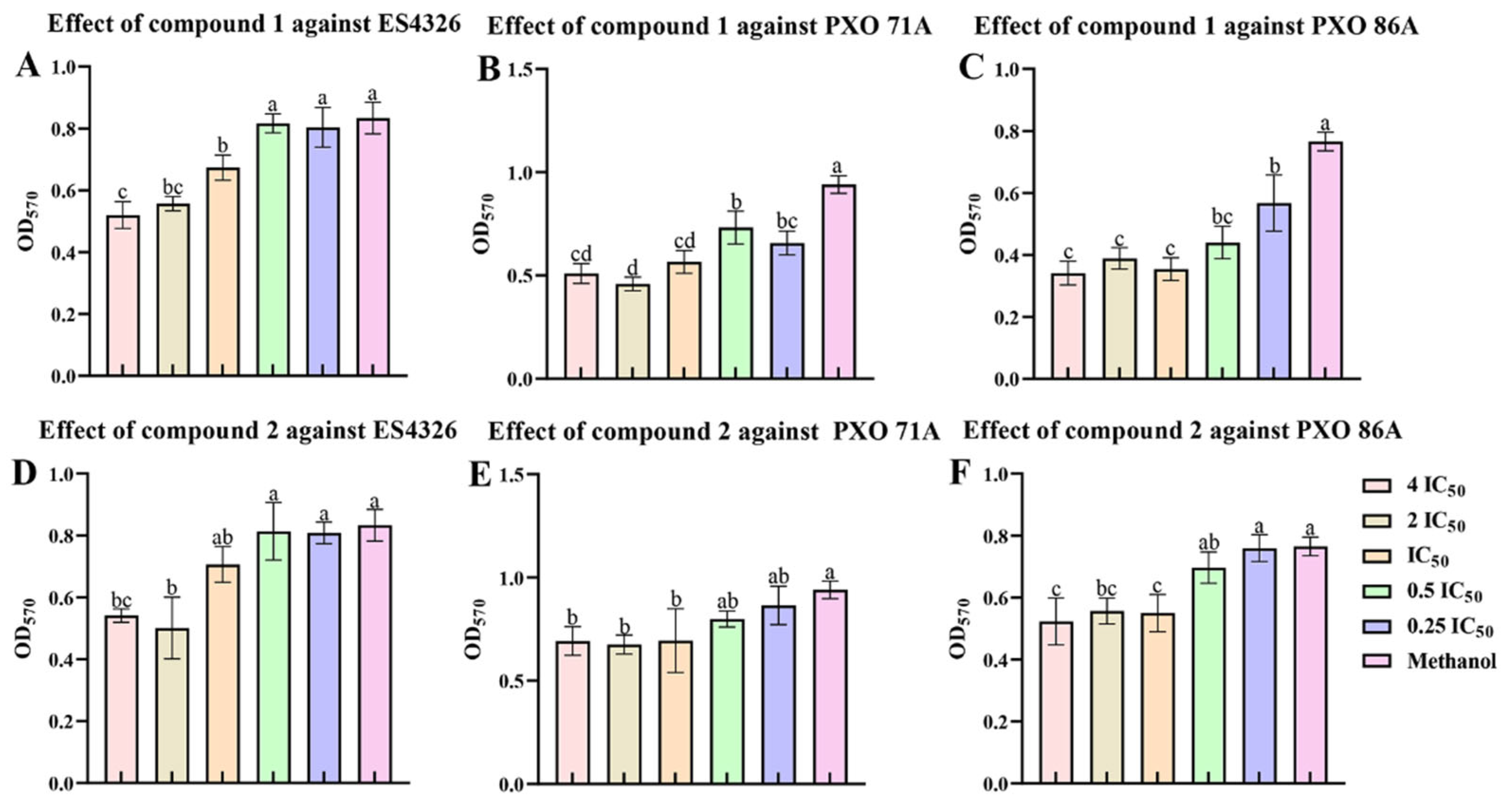
2.6. Ailanstigols A and B Inhibit the Formation of Bacterial Biofilm
3. Materials and Methods
3.1. Collection of Ailanthus altissima Leaves
3.2. Extraction and Isolation of Specialized Metabolites from A. altissima Leaves
3.3. Preparation of NA and KB Culture Media
3.4. ECD Spectra Calculations
3.5. HPLC Analysis
3.6. Activation of Bacteria
3.7. Testing for Antimicrobial Activity in the Isolated Compounds
3.8. Bacterial Protein Leakage Assay
3.9. Bacterial Viability Assay
3.10. Bacterial Biofilm Inhibition Assay
3.11. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.D.; Kang, Z.L.; Hua, J.; Feng, Y.L.; Luo, S.H. Root exudate sesquiterpenoids from the invasive weed Ambrosia trifida regulate rhizospheric Proteobacteria. Sci. Total Environ. 2022, 834, 155263. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.C.; Tran, T.V.; Nguyen, T.T.T.; Nguyen, D.H.; Alhassan, M.; Lee, T. New frontiers of invasive plants for biosynthesis of nanoparticles towards biomedical applications: A review. Sci. Total Environ. 2023, 857, 159278. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, J.Z.; Liu, M.; Yan, Z.Q.; Xu, X.L.; Kuzyakov, Y. Invasive plant competitivity is mediated by nitrogen use strategies and rhizosphere microbiome. Soil Biol. Biochem. 2024, 192, 109361. [Google Scholar] [CrossRef]
- Inoue, Y.; Takikawa, Y. Primers for specific detection and identification of Pseudomonas syringae pv. maculicola and P. cannabina pv. alisalensis. Appl. Microbiol. Biotechnol. 2021, 105, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, J.L.; Yu, J.; Cornish, D.A.; Tanner, D.J.; Windner, R.; Chapman, J.R.; Taylor, R.K.; Mackay, J.F.; Dowlut, S. Identification, virulence, and distribution of two biovars of Pseudomonas syringae pv. actinidiae in New Zealand. Plant Dis. 2013, 97, 708–719. [Google Scholar] [CrossRef]
- White, F.F.; Yang, B. Host and pathogen factors controlling the Rice-Xanthomonas oryzae interaction. Plant Physiol. 2009, 150, 1677–1686. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef]
- Lelano, F.; Scrano, L.; De Franchi, S.; Bonomo, M.G.; Salzano, G.; Milan, S.; Milella, L.; Bufo, S.A. Identification and antimicrobial activity of most representative secondary metabolites from different plant species. Chem. Biol. Technol. Agric. 2018, 5, 13. [Google Scholar]
- Li, S.; Jiang, S.X.; Jia, W.T.; Guo, T.M.; Wang, F.; Li, J.; Yao, Z.L. Natural antimicrobials from plants: Recent advances and future prospects. Food Chem. 2024, 432, 137231. [Google Scholar] [CrossRef]
- Aghraz, A.; Albergamo, A.; Benameur, Q.; Salvo, A.; Larhsini, M.; Markouk, M.; Gervasi, T.; Cicero, N. Polyphenols contents, heavy metals analysis and in vitro antibacterial activity of extracts from Cladanthus arabicus and Bubonium imbricatum of Moroccan Origin. Nat. Prod. Res. 2020, 34, 63–70. [Google Scholar] [CrossRef]
- Kundu, P.; Laskar, S. A brief resume on the genus Ailanthus: Chemical and pharmacological aspects. Phytochem. Rev. 2010, 9, 379–412. [Google Scholar] [CrossRef]
- De Feo, V.; Mancini, E.; Voto, E.; Curini, M.; Digilio, M.C. Bioassay-oriented isolation of an insecticide from Ailanthus altissima. J. Plant Interact. 2009, 4, 119–123. [Google Scholar] [CrossRef]
- Tan, Q.W.; Ni, J.C.; Shi, J.T.; Zhu, J.X.; Chen, Q.J. Two novel quassinoid glycosides with antiviral activity from the samara of Ailanthus altissima. Molecules 2020, 25, 5679. [Google Scholar] [CrossRef]
- Tan, Q.W.; Zhu, J.X.; Ju, Y.Y.; Chi, X.L.; Cao, T.D.; Zheng, L.P.; Chen, Q.J. Antiviral activity of ailanthone from Ailanthus altissima on the rice stripe virus. Viruses 2024, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Boukhibar, H.; Laouani, A.; Touzout, S.N.; Alenazy, R.; Alqasmi, M.; Bokhari, Y.; Saguem, K.; Ben-Attia, M.; El-Bok, S.; Merghni, A. Chemical composition of Ailanthus altissima (Mill.) Swingle methanolic leaf extracts and assessment of their antibacterial activity through oxidative stress induction. Antibiotics 2023, 12, 1253. [Google Scholar] [CrossRef]
- Zhao, C.C.; Shao, J.H.; Li, X.; Xu, J.; Zhang, P. Antimicrobial constituents from fruits of Ailanthus altissima Swingle. Arch. Pharmacal Res. 2005, 28, 1147–1151. [Google Scholar] [CrossRef]
- DellaGreca, M.; Previtera, L.; Purcaro, R.; Zarrelli, A. Cinnamic acid amides and lignanamides from Aptenia cordifolia. Tetrahedron 2006, 62, 2877–2882. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Tsvetkov, D.E.; Dmitrenok, A.S.; Tsvetkov, Y.E.; Chizhov, A.O.; Nifantiev, N.E. Polyphenolic components of knotwood extracts from Populus tremula (quaking aspen). Russ. Chem. Bull. 2022, 71, 1777–1783. [Google Scholar] [CrossRef]
- Zhang, J.L.; Mohamad, F.H.; Wong, J.H.; Mohamad, H.; Ismail, A.H.; Mohamed Yusoff, A.A.; Osman, H.; Wong, K.T.; Idris, Z.; Abdullah, J.M. The effects of 4-hydroxybenzoic acid identified from Bamboo (Dendrocalamus asper) shoots on Kv1.4 channel. Malays. J. Med. Sci. 2018, 25, 101–113. [Google Scholar] [CrossRef]
- Sarkar, P.; Zohora, F.T.; Jabbar, A.; Tareq, F.S.; Hasan, C.M.; Ahsan, M. Phytochemical studies on the stem bark of Couroupita guianensis Aubl. Asian J. Chem. 2013, 25, 4559–4562. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Wang, Y.M.; Yang, Y.L.; Zeng, Y.; Mei, L.J.; Shi, Y.P.; Tao, Y.D. Antioxidants and α-glucosidase inhibitors from “Liucha” (young leaves and shoots of Sibiraea laevigata). Food Chem. 2017, 230, 117–124. [Google Scholar] [CrossRef]
- Wu, Z.J.; Ouyang, M.A.; Wang, S.B. Two new phenolic water-soluble constituents from branch bark of Davidia involucrata. Nat. Prod. Res. 2008, 22, 483–488. [Google Scholar] [CrossRef]
- Yu, J.H.; Xie, J.C.; Sun, M.; Xiong, S.H.; Xu, C.F.; Zhang, Z.M.; Li, M.J.; Li, C.; Lin, L.M. Plant-derived caffeic acid and its derivatives: An overview of their NMR data and biosynthetic pathways. Molecules 2024, 29, 1625. [Google Scholar] [CrossRef]
- Lu, R.L.; Hu, F.L.; Xia, T. Activity-guided isolation and identification of radical scavenging components in Gao-Cha Tea. J. Food Sci. 2010, 75, H239–H243. [Google Scholar] [CrossRef]
- Lo, S.; Leung, E.; Fedrizzi, B.; Barker, D. Syntheses of mono-acylated luteolin derivatives, evaluation of their antiproliferative and radical scavenging activities and implications on their oral bioavailability. Sci. Rep. 2021, 11, 12595. [Google Scholar] [CrossRef]
- Papan, P.; Kantapan, J.; Sangthong, P.; Meepowpan, P.; Dechsupa, N. Iron (III)-quercetin complex: Synthesis, physicochemical characterization, and MRI cell tracking toward potential applications in regenerative medicine. Contrast Media Mol. Imaging 2020, 2020, 8877862. [Google Scholar] [CrossRef]
- Wu, H.K.; Mao, Y.J.; Sun, S.S.; Xu, Z.Y.; Ma, Y.; Cao, J.X.; Qi, H.; Wu, Z.F.; Li, G.; Yang, W.H. Leojaponic acids A and B, two new homologous terpenoids, isolated from Leonurus japonicus. Chin. J. Nat. Med. 2016, 14, 303–307. [Google Scholar] [CrossRef]
- Zhang, H.J.; Tamez, P.A.; Hoang, V.D.; Tan, G.T.; Van Hung, N.; Xuan, L.T.; Huong, L.M.; Cuong, N.M.; Thao, D.T.; Soejarto, D.D.; et al. Antimalarial compounds from Rhaphidophora decursiva. J. Nat. Prod. 2001, 64, 772–777. [Google Scholar] [CrossRef]
- Lee, J.; Kim, N.H.; Nam, J.W.; Lee, Y.M.; Jang, D.S.; Kim, Y.S.; Nam, S.H.; Seo, E.-K.; Yang, M.S.; Kim, J.S. Scopoletin from the flower buds of Magnolia fargesii inhibits protein glycation, aldose reductase, and cataractogenesis Ex Vivo. Arch. Pharmacal Res. 2010, 33, 1317–1323. [Google Scholar] [CrossRef]
- Qian, Z.H.; Hua, Z.D.; Liu, C.M.; Jia, W. Four types of cannabimimetic indazole and indole derivatives, ADB-BINACA, AB-FUBICA, ADB-FUBICA, and AB-BICA, identified as new psychoactive substances. Forensic Toxicol. 2016, 34, 133–143. [Google Scholar] [CrossRef]
- Wu, S.B.; Ji, Y.P.; Zhu, J.J.; Zhao, Y.; Xia, G.; Hu, Y.H.; Hu, J.F. Steroids from the leaves of Chinese Melia azedarach and their cytotoxic effects on human cancer cell lines. Steroids 2009, 74, 761–765. [Google Scholar] [CrossRef]
- Dao, T.-T.; Tran, T.-L.; Jayeon, K.; Nguyen, P.-H.; Lee, E.-H.; Park, J.; Jang, I.-S.; Oh, W.-K. Terpenylated coumarins as SIRT1 activators isolated from Ailanthus altissima. J. Nat. Prod. 2012, 75, 1332–1338. [Google Scholar] [CrossRef]
- Cocîrlea, M.D.; Simionescu, N.; Petrovici, A.R.; Silion, M.; Biondi, B.; Lastella, L.; Oancea, S. In vitro screening of ecotoxic and cytotoxic activities of Ailanthus altissima leaf extract against target and non-target plant and animal cells. Int. J. Mol. Sci. 2024, 25, 5653. [Google Scholar] [CrossRef]
- Poljuha, D.; Sladonja, B.; Šola, I.; Dudaš, S.; Bilić, J.; Rusak, G.; Motlhatlego, K.E.; Eloff, J.N. Phenolic composition of leaf extracts of Ailanthus altissima (Simaroubaceae) with antibacterial and antifungal activity equivalent to standard antibiotics. Nat. Prod. Commun. 2017, 12, 1609–1612. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Vanara, F.; Fogliatto, S.; Vidotto, F.; Negre, M.; Trotta, F.; Scariot, V. Ailanthone from Ailanthus altissima (Mill.) Swingle as potential natural herbicide. Sci. Hortic. 2019, 257, 108702. [Google Scholar] [CrossRef]
- Yang, D.Q.; Liang, H.; Li, X.X.; Zhang, C.Y.; Lu, Z.W.; Ma, X.Q. Unleashing potential of microbial biosynthesis of monoterpenes via enzyme and metabolic engineering. Biotechnol. Adv. 2025, 79, 108525. [Google Scholar] [CrossRef]
- Chesnut, R.W.; Durham, N.N.; Brown, R.A.; Mawdsley, E.A.; Berlin, K.D. Antibacterial activity of 15-azasteroids alone and in combination with antibiotics. Steroids 1976, 27, 525–541. [Google Scholar] [CrossRef]
- Kamdem, M.H.K.; Ojo, O.; Kemkuignou, B.M.; Talla, R.M.; Fonkui, T.Y.; Silihe, K.K.; Tata, C.M.; Fotsing, M.C.D.; Mmutlane, E.M.; Ndinteh, D.T. Pentacyclic triterpenoids, phytosteroids and fatty acid isolated from the stem-bark of Cola lateritia K. Schum. (Sterculiaceae) of cameroon origin; evaluation of their antibacterial activity. Arabian J. Chem. 2022, 15, 103506. [Google Scholar] [CrossRef]
- Hoai Thi, N.; Duc Viet, H.; Hung Quoc, V.; Anh Tuan, L.; Hien Minh, N.; Kodama, T.; Ito, T.; Morita, H.; Raal, A. Antibacterial activities of chemical constituents from the aerial parts of Hedyotis pilulifera. Pharm. Biol. 2017, 55, 787–791. [Google Scholar]
- Merlani, M.; Nadaraia, N.; Amiranashvili, L.; Petrou, A.; Geronikaki, A.; Ciric, A.; Glamoclija, J.; Carevic, T.; Sokovic, M. Antimicrobial activity of some steroidal hydrazones. Molecules 2023, 28, 1167. [Google Scholar] [CrossRef]
- Savage, P.B.; Li, C.H.; Taotafa, U.; Ding, B.W.; Guan, Q.Y. Antibacterial properties of cationic steroid antibiotics. FEMS Microbiol. Lett. 2002, 217, 1–7. [Google Scholar] [CrossRef]
- Han, Q.Q.; Feng, L.L.; Zhang, Y.; Zhang, R.G.; Wang, G.L.; Zhang, Y.L. Effect of juglone against Pseudomonas syringae pv actinidiae planktonic growth and biofilm formation. Molecules 2021, 26, 7580. [Google Scholar] [CrossRef]
- Tao, S.Q.; Zhang, J.K.; Zhu, D.H.; Wu, Y.Y.; Zheng, X.K.; Feng, W.S. Flavonoids and phenols from the stems of Ephedra equisetina. Phytochemistry 2024, 220, 114003. [Google Scholar] [CrossRef]
- Cui, J.P.; Bahrami, A.K.; Pringle, E.G.; Hernandez-Guzman, G.; Bender, C.L.; Pierce, N.E.; Ausubel, F.M. Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc. Natl. Acad. Sci. USA 2005, 102, 1791–1796. [Google Scholar] [CrossRef]
- Malapaka, V.R.R.; Barrese, A.A.; Tripp, B.C.; Tripp, B.C. High-throughput screening for antimicrobial compounds using a 96-well format bacterial motility absorbance assay. J. Biomol. Screen. 2007, 12, 849–854. [Google Scholar] [CrossRef]
- Mao, C.Y.; Xiang, Y.M.; Liu, X.M.; Zheng, Y.F.; Yeung, K.W.K.; Cui, Z.D.; Yang, X.J.; Li, Z.Y.; Liang, Y.Q.; Zhu, S.L.; et al. Local photothermal/photodynamic synergistic therapy by disrupting bacterial membrane to accelerate reactive oxygen species permeation andprotein leakage. ACS Appl. Mater. Interfaces 2019, 11, 17902–17914. [Google Scholar] [CrossRef]
- Chen, J.L.; Steele, T.W.J.; Stuckey, D.C. Metabolic reduction of resazurin; location within the cell for cytotoxicity assays. Biotechnol. Bioeng. 2018, 115, 351–358. [Google Scholar] [CrossRef]
- Dieltjens, L.; Appermans, K.; Lissens, M.; Lories, B.; Kim, W.; Van der Eycken, E.V.; Foster, K.R.; Steenackers, H.P. Inhibiting bacterial cooperation is an evolutionarily robust anti-biofilm strategy. Nat. Commun. 2020, 11, 107. [Google Scholar] [CrossRef]
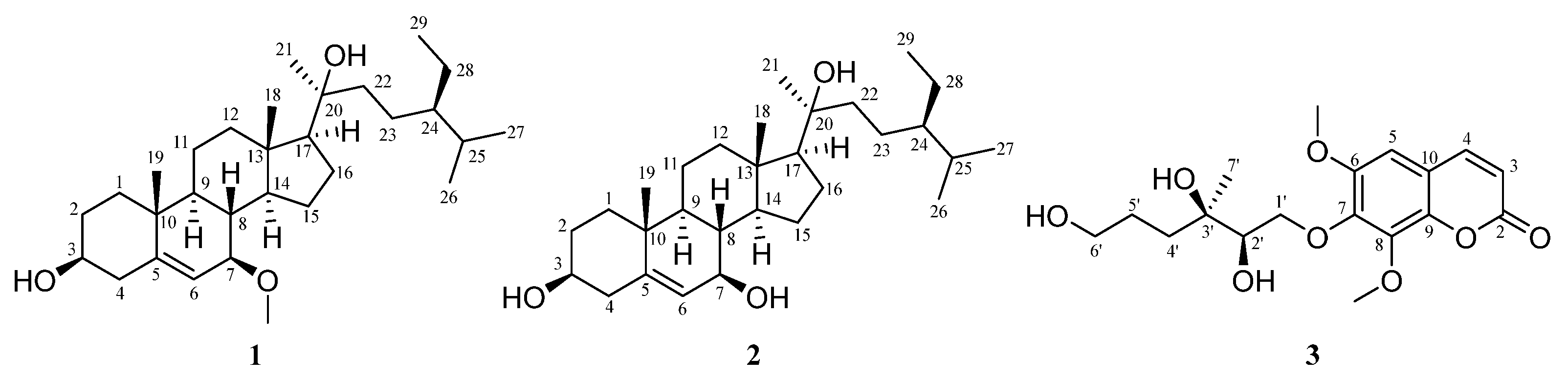
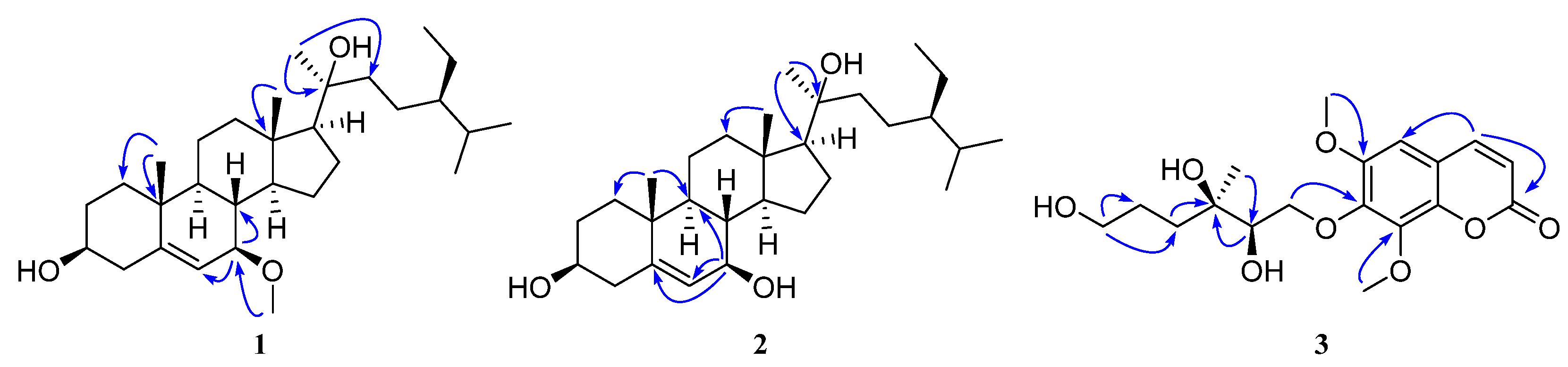
| Position | Compound 1 | Compound 2 | ||
|---|---|---|---|---|
| δH (J[Hz]) | δC | δH (J[Hz]) | δC | |
| 1 | 1.10, m 1.84, m | 38.1, t | 1.04, m 1.86, m | 38.2, t |
| 2 | 1.48, m 1.88, m | 32.1, t | 1.48, m 1.78, m | 32.3, t |
| 3 | 3.45, m | 72.2, d | 3.41, m | 72.1, d |
| 4 | 2.29, m | 43.1, t | 2.24, m | 42.5, t |
| 5 | - | 148.0, s | - | 144.1, s |
| 6 | 5.77, d (4.1) | 121.5, d | 5.27, br s | 127.4, d |
| 7 | 3.34, br s | 75.2, d | 3.72, br d (8.2) | 73.7, d |
| 8 | 1.50, m | 38.0, d | 1.45, m | 40.7, d |
| 9 | 1.28, m | 44.1, d | 1.02, m | 50.0, d |
| 10 | - | 38.6, s | - | 37.6, s |
| 11 | 1.53, m | 21.9, t | 1.54, m | 22.2, t |
| 12 | 1.17, m 2.05, m | 41.1, t | 1.20, m 2.09, m | 41.5, t |
| 13 | - | 43.5, s | - | 44.3, s |
| 14 | 1.49, m | 50.6, d | 1.13, m | 58.0, d |
| 15 | 1.15, m 1.33, m | 24.8, t | 1.24, m 1.44, m | 27.0, t |
| 16 | 1.16, m | 25.5, t | 1.13, m | 25.4, t |
| 17 | 1.48, m | 59.1, d | 1.44, m | 58.7, d |
| 18 | 0.85, s | 13.7, q | 0.87, s | 14.1, q |
| 19 | 0.99, s | 18.8, q | 1.06, s | 19.4, q |
| 20 | - | 76.2, s | - | 76.2, s |
| 21 | 1.24, s | 26.2, q | 1.24, s | 26.2, q |
| 22 | 1.52, m | 43.4, t | 1.28, m 1.51, m | 43.4, t |
| 23 | 1.67, m | 23.4, t | 1.65, m 1.79, m | 23.8, t |
| 24 | 0.94, m | 47.7, d | 0.93, m | 47.7, d |
| 25 | 1.69, m | 30.4, d | 1.69, m | 30.4, d |
| 26 | 0.87, d (6.5) | 20.1, q | 0.87, d (6.6) | 20.0, q |
| 27 | 0.86, d (6.5) | 19.6, q | 0.86, d (6.6) | 19.6, q |
| 28 | 1.66, m | 24.1, t | 1.33, m | 24.1, t |
| 29 | 0.89, t (7.3) | 12.4, q | 0.88, t (7.3) | 12.4, q |
| 7-OMe | 3.35, s | 56.9, q | ||
| Position | Compound 3 | |
|---|---|---|
| δH (J[Hz]) | δC | |
| 2 | - | 160.4, s |
| 3 | 6.34, d (9.5) | 115.7, d |
| 4 | 7.92, d (9.5) | 144.7, d |
| 5 | 7.10, s | 105.6, d |
| 6 | - | 150.9, s |
| 7 | - | 145.9, s |
| 8 | - | 141.8, s |
| 9 | - | 143.7, s |
| 10 | - | 115.6, s |
| 1′ | 4.08, dd (10.3, 8.3); 4.54, dd (10.3, 2.6) | 77.0, t |
| 2′ | 3.80, dd (8.3, 2.6) | 76.1, d |
| 3′ | - | 73.3, s |
| 4′ | 1.55, m; 1.68, m | 36.6, t |
| 5′ | 1.65, m | 27.3, t |
| 6′ | 3.53, m | 63.2, t |
| 7′ | 1.13, s | 22.8, q |
| 6-OMe | 3.90, s | 56.7, q |
| 8-OMe | 4.00, s | 62.0, q |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wu, Y.; Gao, Z.; Liu, Z.; Hua, J.; Luo, S. New Steroids Obtained from Ailanthus altissima Leaves Inhibit the Invasive Bacteria Xanthomonas oryzae pv. oryzae and Pseudomonas syringae pv. maculicola. Molecules 2025, 30, 2576. https://doi.org/10.3390/molecules30122576
Yang Y, Wu Y, Gao Z, Liu Z, Hua J, Luo S. New Steroids Obtained from Ailanthus altissima Leaves Inhibit the Invasive Bacteria Xanthomonas oryzae pv. oryzae and Pseudomonas syringae pv. maculicola. Molecules. 2025; 30(12):2576. https://doi.org/10.3390/molecules30122576
Chicago/Turabian StyleYang, Yuhong, Yue Wu, Zhengyi Gao, Zhixiang Liu, Juan Hua, and Shihong Luo. 2025. "New Steroids Obtained from Ailanthus altissima Leaves Inhibit the Invasive Bacteria Xanthomonas oryzae pv. oryzae and Pseudomonas syringae pv. maculicola" Molecules 30, no. 12: 2576. https://doi.org/10.3390/molecules30122576
APA StyleYang, Y., Wu, Y., Gao, Z., Liu, Z., Hua, J., & Luo, S. (2025). New Steroids Obtained from Ailanthus altissima Leaves Inhibit the Invasive Bacteria Xanthomonas oryzae pv. oryzae and Pseudomonas syringae pv. maculicola. Molecules, 30(12), 2576. https://doi.org/10.3390/molecules30122576







