Bulky Ligand-Induced Hindrance in Photocatalytic CO2 Reduction over Various Tris(bipyridine)cobalt(II) Chloride Complexes
Abstract
1. Introduction
2. Results and Discussion
2.1. Photocatalytic CO2 Reduction
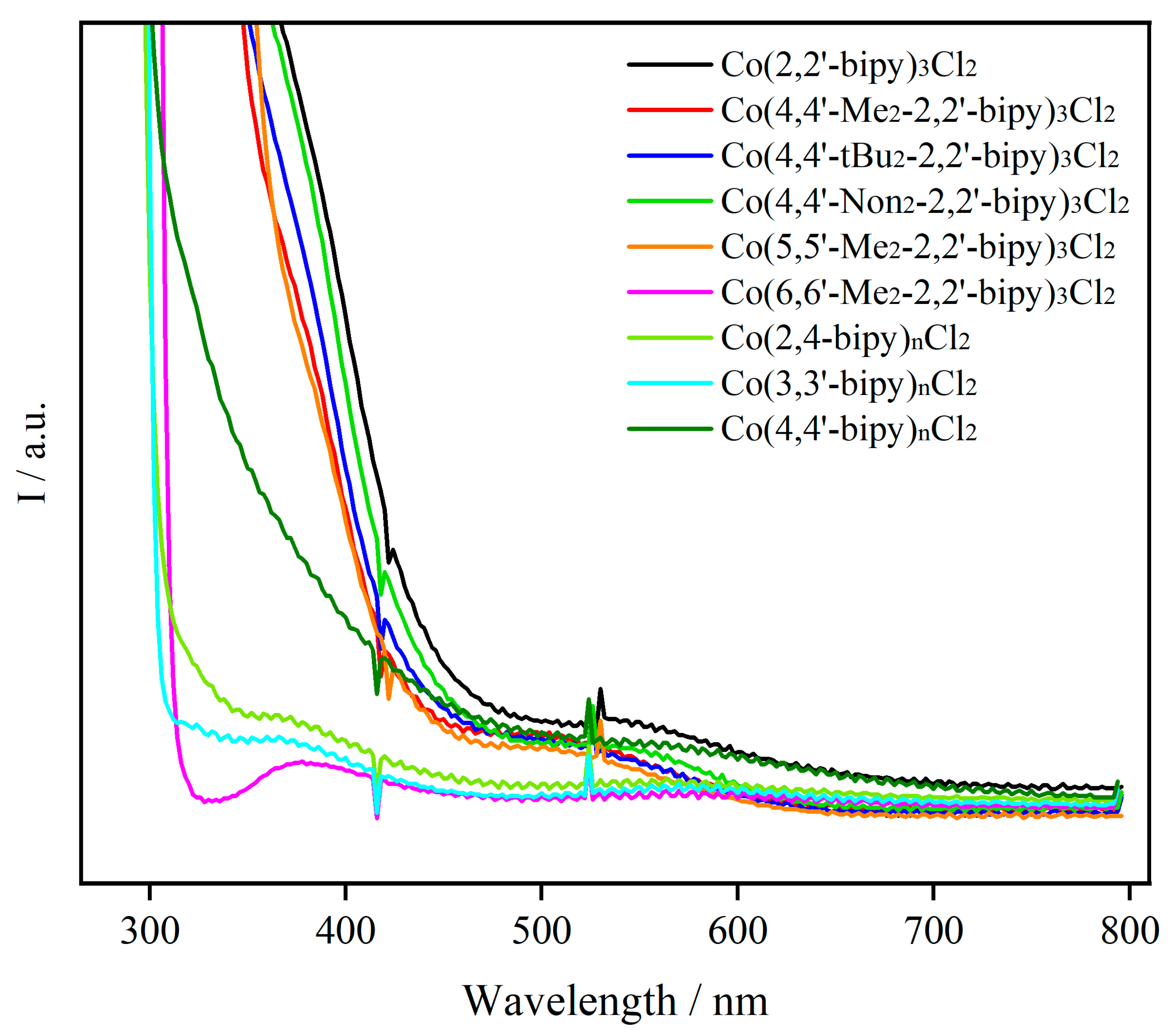
2.2. UV–Vis Absorption
2.3. FT-IR Analysis
2.4. Electronic Observation
2.5. Mechanism Discussion
3. Materials and Methods
3.1. Materials
3.2. Crystallographic Studies
3.3. UV–Vis Test
3.4. FT-IR Inspection
3.5. Electrochemical Measurements
3.6. Photocatalytic CO2 Reduction Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, Z.; Zhu, X.; Gao, X.; An, C.; Wang, Z.; Zuo, C.; Dionysiou, D.; He, H.; Jiang, Z. Enhancing photocatalytic CO2 reduction with TiO2-based materials: Strategies, mechanisms, challenges, and perspectives. Environ. Sci. Ecotechnol. 2024, 20, 100368. [Google Scholar] [CrossRef]
- Behroozi, A.H.; Xu, R. Photocatalytic CO2 reduction: Photocatalysts, membrane reactors, and hybrid processes. Chem. Catal. 2023, 3, 100550. [Google Scholar] [CrossRef]
- Yohannes, A.G.; Lee, C.; Talebi, P.; Mok, D.H.; Karamad, M.; Back, S.; Siahrostami, S. Combined high-throughput DFT and ML screening of transition metal nitrides for electrochemical CO2 reduction. ACS Catal. 2023, 13, 9007–9017. [Google Scholar] [CrossRef]
- Chen, M.; Liu, H.; Wang, Y.; Zhong, Z.Y.; Zeng, Y.; Jin, Y.X.; Ye, D.Q.; Chen, L.M. Cobalt catalyzed ethane dehydrogenation to ethylene with CO2: Relationships between cobalt species and reaction pathways. J. Colloid Interface Sci. 2024, 660, 124–135. [Google Scholar] [CrossRef]
- Su, B.; Kong, Y.H.; Wang, S.B.; Zuo, S.W.; Lin, W.; Fang, Y.X.; Hou, Y.D.; Zhang, G.G.; Zhang, H.B.; Wang, X.C. Hydroxyl-bonded Ru on metallic TiN surface catalyzing CO2 reduction with H2O by infrared light. J. Am. Chem. Soc. 2023, 145, 27415–27423. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Jin, R.; Li, A.; Bi, Y.; Ruan, Q.; Deng, Y.; Zhang, Y.; Yao, S.; Sankar, G.; Ma, D.; et al. Highly selective oxidation of methane to methanol at ambient conditions by titanium dioxide-supported iron species. Nat. Catal. 2018, 1, 889–896. [Google Scholar] [CrossRef]
- Wang, S.B.; Wang, X.C. Multifunctional metal-organic frameworks for photocatalysis. Small 2015, 11, 3097. [Google Scholar] [CrossRef]
- Yan, G.; Sun, X.D.; Zhang, Y.; Li, H.; Huang, H.W.; Jia, B.H.; Su, D.W.; Ma, T.Y. Metal-free 2D/2D van der Waals heterojunction based on covalent organic frameworks for highly efficient solar energy catalysis. Nano-Micro Lett. 2023, 15, 132. [Google Scholar] [CrossRef]
- Fu, Y.H.; Wu, J.Y.; Du, R.F.; Guo, K.; Ma, R.; Zhang, F.M.; Zhu, F.M.; Zhu, W.D.; Fan, M.H. Temperature modulation of defects in NH2-UiO-66(Zr) for photocatalytic CO2 reduction. RSC Adv. 2019, 9, 37733–37738. [Google Scholar] [CrossRef]
- Si, Y.H.; Chen, W.M.; Shang, S.K. g-C3N4/Pt/BiVO4 nanocomposites for highlyefficient visible-light photocatalytic removal of contaminants and hydrogen generation. Nanotechnology 2020, 31, 125706. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, J.; Song, H.; Liu, S.; Huang, Y.F.; Huang, Y.Q.; Waterhouse, G.; Wang, Z.Y.; Mao, Y.; Liang, Z.W.; et al. Synergistically enhancing CO2 adsorption/activation and electron transfer in ZIF-67/Ti3C2Tx MXene for boosting photocatalytic CO2 reduction. Sep. Purif. Technol. 2024, 341, 126817. [Google Scholar] [CrossRef]
- Jian, H.; Lu, M.; Zheng, H.; Yan, S.; Wang, M. Electrochemical Water Oxidation and CO2 Reduction with a Nickel Molecular Catalyst. Molecules 2024, 29, 578. [Google Scholar] [CrossRef] [PubMed]
- Ziessel, R.; Hawecker, J.; Lehn, J.M. Photogeneration of carbon monoxide and of hydrogen via simultaneous photochemical reduction of carbon dioxide and water by visible-light irradiation of organic solutions containing tris(2, 2′-bipyridine)ruthenium(II) and cobalt(II) species as homogeneous catalysts. Helv. Chim. Acta 1986, 69, 1065–1084. [Google Scholar]
- Hawecker, J.; Lehn, J.-M.; Ziessel, R.J. Efficient photochemical reduction of CO2 to CO by visible light irradiation of systems containing Re(bipy)(CO)3X or Ru(bipy)32+-Co2+ combinations as homogeneous catalysts. J. Chem. Soc. Chem. Commun. 1983, 536–538. [Google Scholar] [CrossRef]
- Du, M.; Chen, Y.; Wang, W.; Xu, X.; Li, Y.; Zhang, Y.; Li, Z.; Zou, Z. Construction of new active sites: Cu substitution enabled surface frustrated lewis pairs over calcium hydroxyapatite for CO2 hydrogenation. Adv. Sci. 2021, 8, 2101382. [Google Scholar]
- Kuehnel, M.F.; Orchard, K.L.; Dalle, K.E.; Reisner, E. Selective photocatalytic CO2 reduction in water through anchoring of a molecular Ni catalyst on CdS nanocrystals. J. Am. Chem. Soc. 2017, 139, 7217–7223. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.L.; Cheung, P.L.; Lessio, M.; Carter, E.A.; Kubiak, C.P. Kinetic and Mechanistic Effects of Bipyridine (bpy) Substituent, Labile Ligand, and Brønsted Acid on Electrocatalytic CO2 Reduction by Re(bpy) Complexes. ACS Catal. 2018, 8, 2021–2029. [Google Scholar] [CrossRef]
- Martinez, J.F.; La Porte, N.T.; Wasielewski, M.R. Electron transfer from photoexcited naphthalene-1,4:5,8-bis(dicarboximide) radical anion to Mn(bpy)(CO)3X and Re(bpy)(CO)3X CO2 reduction catalysts linked via a saturated methylene bridge. J. Photochem. Photobiol. A Chem. 2019, 372, 21–28. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wang, X.; Gao, J.L.; Li, D.B. Merging an organic TADF photosensitizer and a simple terpyridine-Fe(III) complex for photocatalytic CO2 reduction. Chem. Commun. 2020, 56, 12170–12173. [Google Scholar] [CrossRef]
- Chen, L.; Chen, G.; Leung, C.F.; Cometto, C.; Robert, M.; Lau, T.C. Molecular quaterpyridine-based metal complexes for small molecule activation: Water splitting and CO2 reduction. Chem. Soc. Rev. 2020, 49, 7271–7283. [Google Scholar] [CrossRef]
- Lin, J.L.; Qin, B.; Zhao, G.L. Effect of solvents on photocatalytic reduction of CO2 mediated by cobalt complex. J. Photochem. Photobiol. A Chem. 2018, 354, 181–186. [Google Scholar] [CrossRef]
- Lin, J.L.; Qin, B.; Fang, Z.X. Nickel bipyridine (Ni(bpy)3Cl2) complex used as molecular catalyst for photocatalytic CO2 reduction. Catal. Lett. 2019, 149, 25–33. [Google Scholar] [CrossRef]
- Rojek, T.; Goldeman, W.; Ślepokura, K.; Matczak-Jon, E. Co(II) coordination polymers derived from α,α-disubstituted analogues of zoledronic acid and 4,4′-bipyridine: Synthesis, structures and characterization. Polyhedron 2020, 185, 114594. [Google Scholar] [CrossRef]
- Sivaraj, K.; Elango, K.P. Synthesis, spectral characterization and solvent effects on the electro-reduction of mixed ligand cobalt(III) complexes. J. Solut. Chem. 2010, 39, 1681–1697. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Mahadevi, P.; Sumathi, S.; Metha, A.; Singh, J. Synthesis, spectral, antioxidant, in vitro cytotoxicity activity and thermal analysis of Schiff base metal complexes with 2,2′-bipyridine-4,4′-dicarboxylic acid as co-ligand. J. Mol. Struct. 2022, 1268, 133669. [Google Scholar] [CrossRef]
- Gerasimova, T.P.; Katsyuba, S.A. Bipyridine and phenanthroline IR-spectral bands as indicators of metal spin state in hexacoordinated complexes of Fe(II), Ni(II) and Co(II). Dalton Trans. 2013, 42, 1787. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, J.T.A.; Gaston, N. Design of superatomic systems: Exploiting favourable conditions for the delocalisation of d-electron density in transition metal doped clusters. Phys. Chem. Chem. Phys. 2020, 22, 18585–18594. [Google Scholar] [CrossRef]
- Wong, K.N.; Colson, S.D. The FT-IR spectra of pyridine and pyridine-d5. J. Mol. Spectrosc. 1984, 104, 129–151. [Google Scholar] [CrossRef]
- Jing, H.W.; Zhao, L.; Song, G.Y.; Li, J.Y.; Wang, Z.Y.; Han, Y.; Wang, Z.X. Application of a mixed-ligand metal-organic framework in photocatalytic CO2 reduction, antibacterial activity and dye adsorption. Molecules 2023, 28, 5204. [Google Scholar] [CrossRef]
- Fillon, H.; Gall, E.L.; Gosmini, C.; Périchon, J. Pure acetonitrile as solvent for the efficient electrochemical conversion of aryl bromides in organozinc species and their coupling reaction with acetyl chloride. Tetrahedron Lett. 2002, 43, 5941–5944. [Google Scholar] [CrossRef]
- Budnikova, Y.G.; Kafiyatullina, A.; Kargin, Y.M.; Sinyashin, O. Electrochemical reduction of cobalt and nickel complexes with ligands stabilizing metal in low oxidation state. Russ. Chem. Bull. 2003, 52, 1504–1511. [Google Scholar] [CrossRef]
- Marinescu, S.C.; Winkler, J.R.; Gray, H.B. Molecular mechanisms of cobalt-catalyzed hydrogen evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 15127–15131. [Google Scholar] [CrossRef] [PubMed]
- Tory, J.; Setterfield-Price, B.; Dryfe, R.A.W.; Hartl, F. [M(CO)4(2,2′-bipyridine)] (M=Cr, Mo, W) complexes as efficient catalysts for electrochemical reduction of CO2 at a gold electrode. ChemElectroChem 2015, 2, 213–217. [Google Scholar] [CrossRef]
- Ikeda, A.; Hunge, Y.M.; Teshima, K.; Uetsuka, H.; Terashima, C. Enhancing CO2 reduction: Insights from in-liquid microwave plasma chemical vapor deposition. Energy Fuels 2024, 38, 11918–11926. [Google Scholar] [CrossRef]
- Takagi, K.; Suzuki, N.; Hunge, Y.M.; Kuriyama, H.; Hayakawa, T.; Serizawa, I.; Terashima, C. Synergistic effect of Ag decorated in-liquid plasma treated titanium dioxide catalyst for efficient electrocatalytic CO2 reduction application. Sci. Total Environ. 2023, 902, 166018. [Google Scholar] [CrossRef]
- Qiu, Y.; Ahmad, S.F.; Song, R.R. Multi-facet investigation of integrating a multigeneration system and landfill gas-based combustion process using an environmentally friendly thermal design arrangement. J. Environ. Chem. Eng. 2025, 13, 115241. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]


 | |||||
| Entry | Ligands | CO/μmol | H2/μmol | CO + H2/μmol | Sel./% [b] |
| 1 | CdS | 1.2 | 5.6 | 6.8 | 17.6 |
| 2 | Co(II)+2,2′-bipy | / [c] | 0.1 | / | / |
| 3 | CdS+2,2′-bipy | 0.1 | 6.5 | 6.6 | 1.5 |
| 4 | Ru(bipy)3Cl2 | 49.8 | 10.1 | 59.9 | 83.1 |
| 5 | Dark | / | / | / | / |
| 6 | 2,2′-bipy | 40.8 | 8.6 | 49.4 | 82.6 |
| 7 | 4,4′-Me2-2,2′-bipy | 35.4 | 7.5 | 42.9 | 82.5 |
| 8 | 4,4′-tBu2-2,2′-bipy | 28.7 | 6.2 | 34.9 | 82.2 |
| 9 | 4,4′-Non2-2,2′-bipy | 0.8 | 1.5 | 2.3 | 0.34 |
| 10 | 5,5′-Me2-2,2′-bipy | 11.8 | 3.6 | 15.4 | 76.6 |
| 11 | 6,6′-Me2-2,2′-bipy | / | 3.2 | 3.2 | / |
| 12 | 2,4-bipy | / | 2.8 | 2.8 | / |
| 13 | 3,3′-bipy | / | 0.5 | 0.5 | / |
| 14 | 4,4′-bipy | 6.3 | 4.5 | 10.9 | 57.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Liao, R.; Li, L.; Yao, S.; Li, S.; Zheng, Y.; Fei, F. Bulky Ligand-Induced Hindrance in Photocatalytic CO2 Reduction over Various Tris(bipyridine)cobalt(II) Chloride Complexes. Molecules 2025, 30, 2573. https://doi.org/10.3390/molecules30122573
Lin J, Liao R, Li L, Yao S, Li S, Zheng Y, Fei F. Bulky Ligand-Induced Hindrance in Photocatalytic CO2 Reduction over Various Tris(bipyridine)cobalt(II) Chloride Complexes. Molecules. 2025; 30(12):2573. https://doi.org/10.3390/molecules30122573
Chicago/Turabian StyleLin, Jinliang, Rongying Liao, Li Li, Shuli Yao, Shengkai Li, Yun Zheng, and Fei Fei. 2025. "Bulky Ligand-Induced Hindrance in Photocatalytic CO2 Reduction over Various Tris(bipyridine)cobalt(II) Chloride Complexes" Molecules 30, no. 12: 2573. https://doi.org/10.3390/molecules30122573
APA StyleLin, J., Liao, R., Li, L., Yao, S., Li, S., Zheng, Y., & Fei, F. (2025). Bulky Ligand-Induced Hindrance in Photocatalytic CO2 Reduction over Various Tris(bipyridine)cobalt(II) Chloride Complexes. Molecules, 30(12), 2573. https://doi.org/10.3390/molecules30122573








