Optimization of Activated Carbon Synthesis from Spent Coffee Grounds for Enhanced Adsorption Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical Properties
2.2. Carbonization
2.3. Activation
2.4. Adsorption Efficiency
3. Results and Discussion
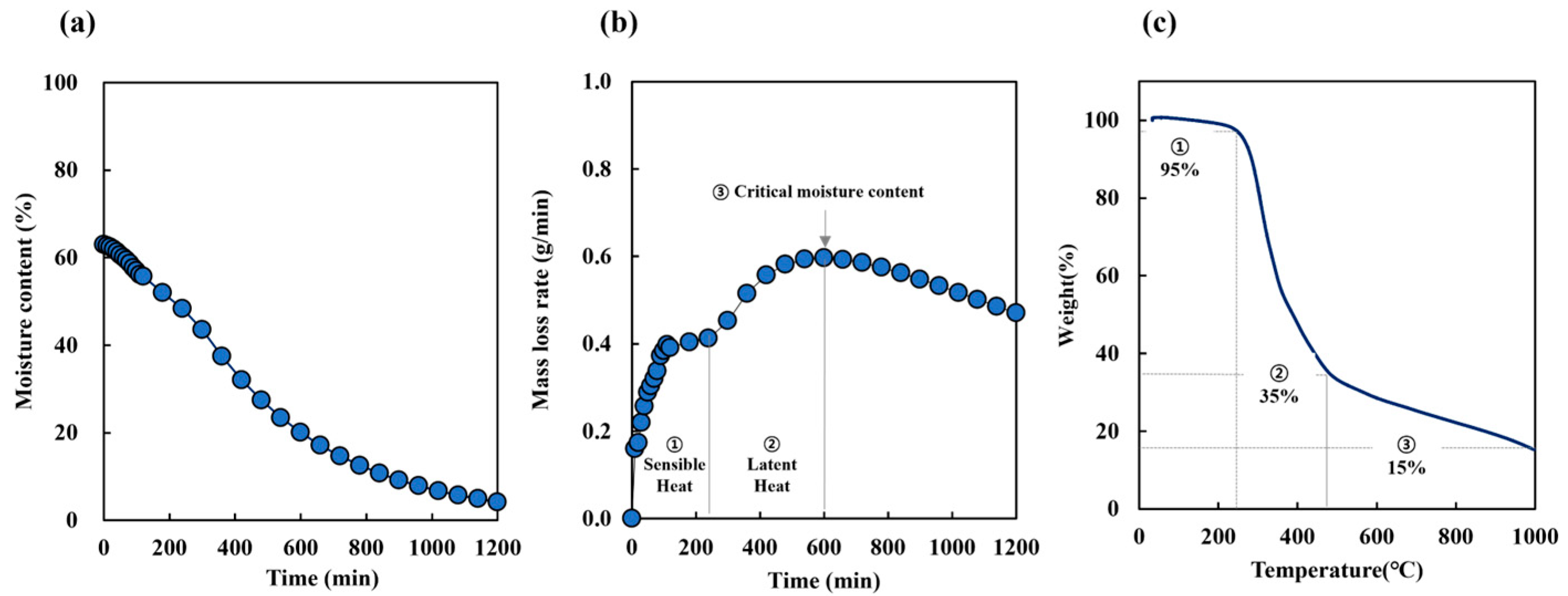
3.1. SCG Drying Conditions
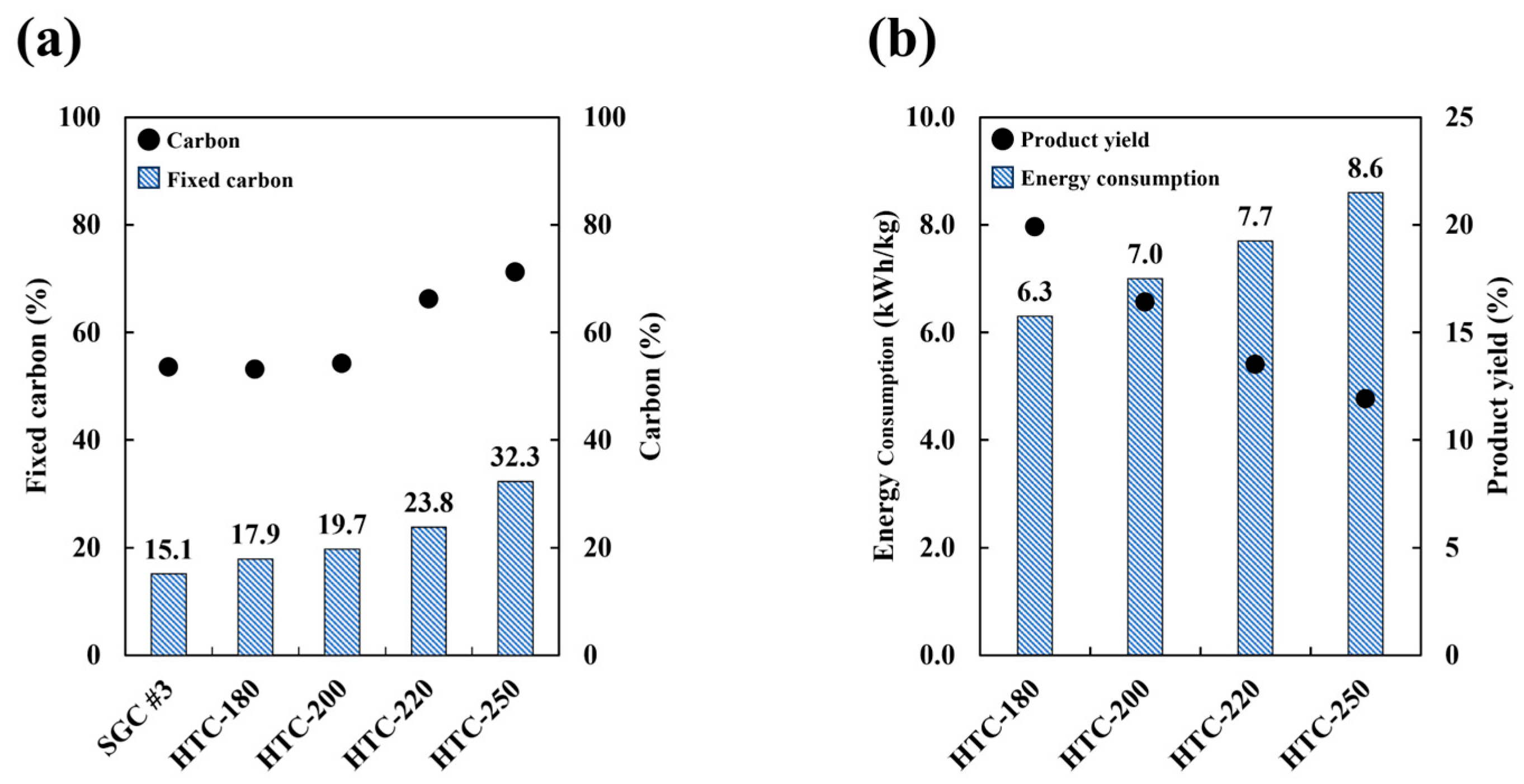
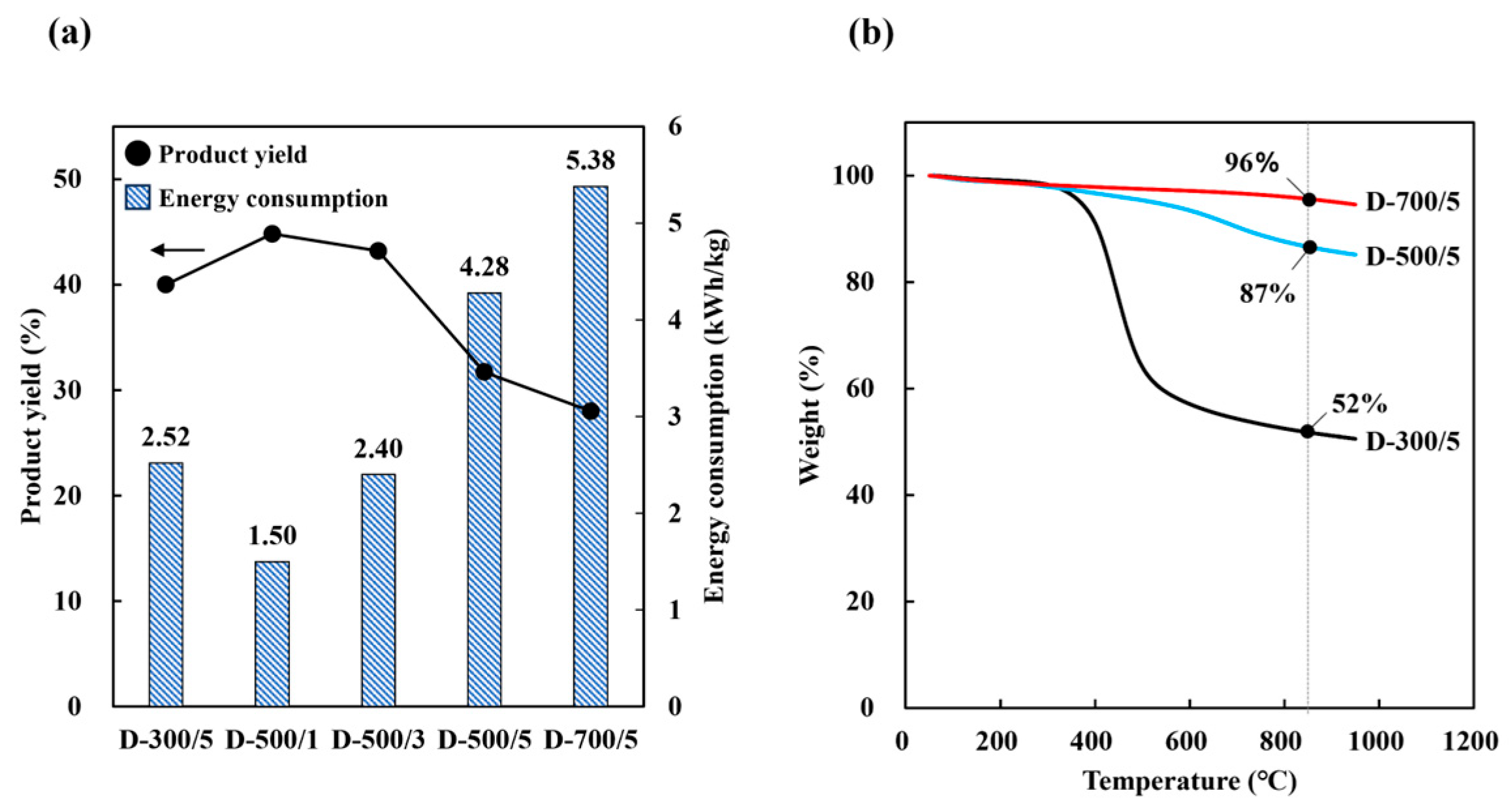
3.2. Carbonization Properties
3.2.1. Hydrothermal Carbonization
3.2.2. Drying Carbonization
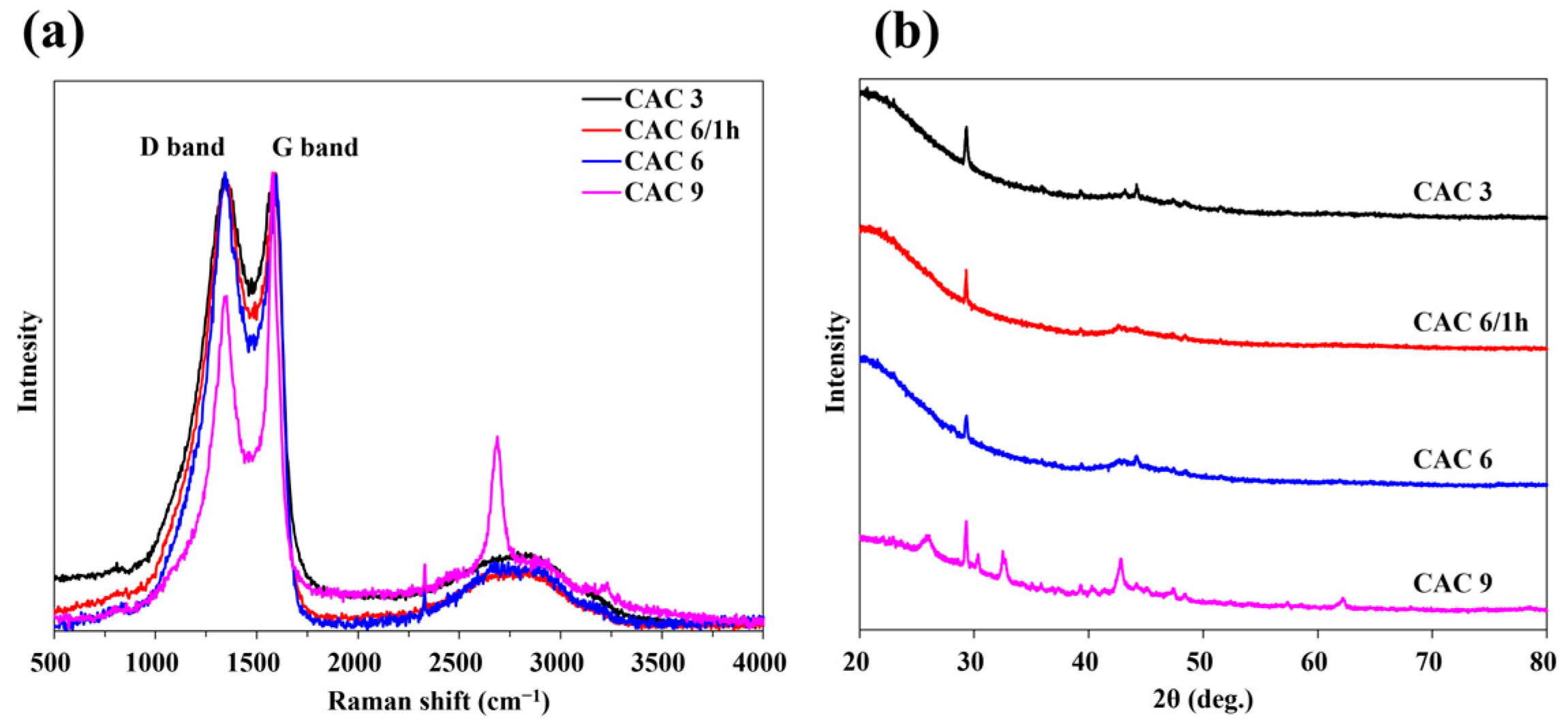
3.3. Properties and Characteristics of Chemically Activated Carbon
3.3.1. Morphology of Chemically Activated Carbon
3.3.2. Microstructural and Textural Properties
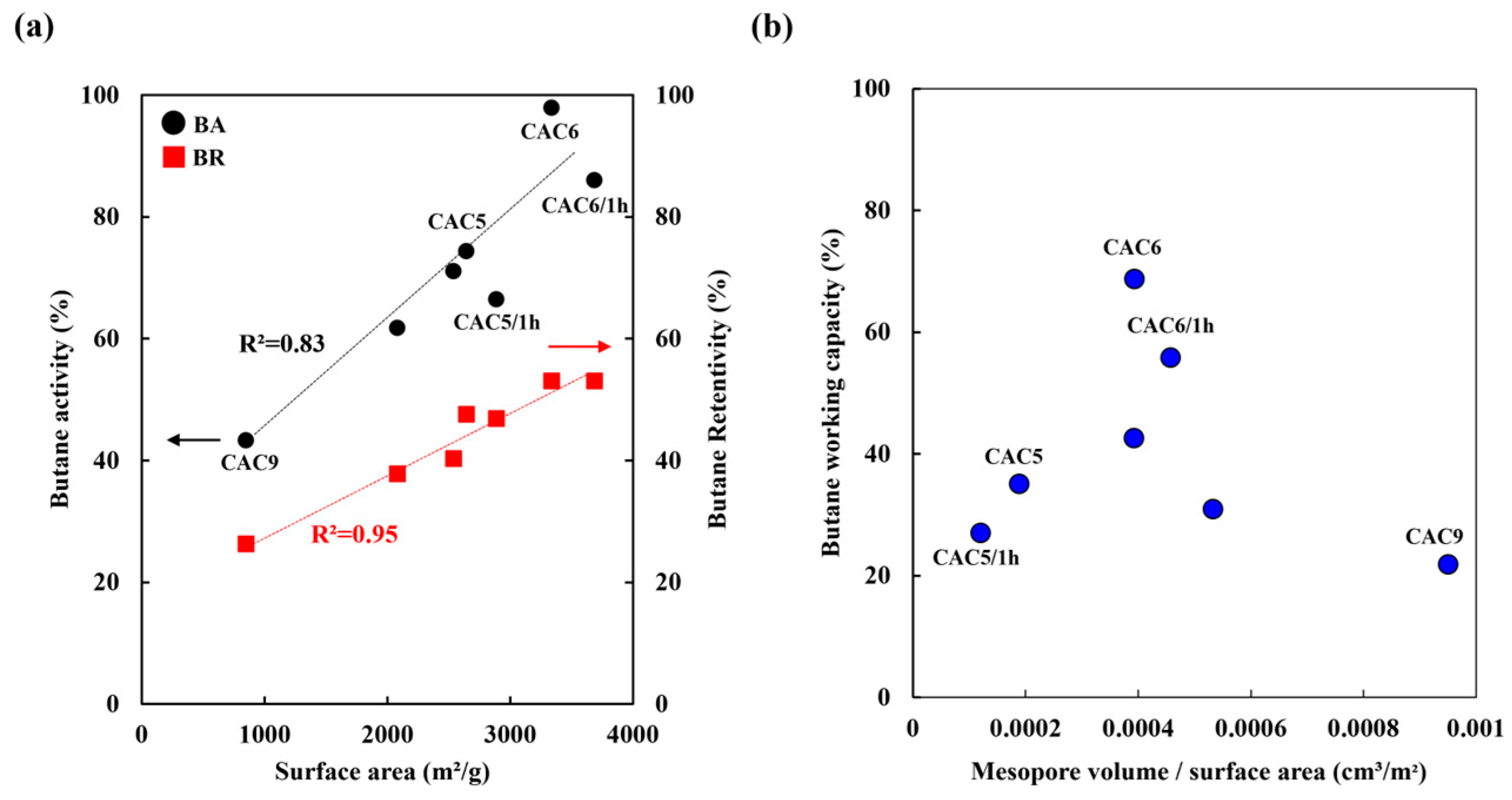
3.4. Butane Working Capacity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McNutt, J.; He, Q. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, X.; Chen, J.; Nilghaz, A.; Yun, X.; Wan, X.; Tian, J. Manufacturing biodegradable lignocellulosic films with tunable properties from spent coffee grounds: A sustainable alternative to plastics. Int. J. Biol. Macromol. 2024, 273, 132918. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Getachew, A.T.; Chun, B.S. Influence of pretreatment and modifiers on subcritical water liquefaction of spent coffee grounds: A green waste valorization approach. J. Clean. Prod. 2017, 142, 3719–3727. [Google Scholar] [CrossRef]
- Kim, M.S.; Koo, N.; Kim, J.G. Characteristics and recycling of spent coffee grounds. Life Sci. Nat. Resour. Res. 2012, 20, 59–69. [Google Scholar]
- Gottstein, V.; Bernhardt, M.; Dilger, E.; Keller, J.; Bretling-Utzmann, C.M.; Schwarz, S.; Kuballa, T.; Lachenmeier, D.W.; Bunzel, M. Coffee silver skin: Chemical characterization with special consideration of dietary fiber and heat-induced contaminants. Foods 2021, 10, 1705. [Google Scholar] [CrossRef]
- World Bank. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018; Available online: https://datatopics.worldbank.org/what-a-waste/ (accessed on 13 February 2025).
- Hwang, M.J.; Koh, S.C.; Chang, J.S. Characteristics of activated carbons made from coffee ground and their capacities for acid orange 7 removal. KIEAE J. 2016, 6, 38–39. [Google Scholar]
- Johnson, K.; Liu, Y.; Lu, M. A review of recent advances in spent coffee grounds upcycle technologies and practices. Front. Chem. Eng. 2022, 4, 838605. [Google Scholar] [CrossRef]
- Ibitoye, S.E.; Mahamood, R.M.; Jen, T.; Loha, C.; Akinlabi, E.T. An overview of biomass solid fuels: Biomass sources, processing methods, and morphological and microstructural properties. J. Bioresour. Bioprod. 2023, 8, 333–360. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Feng, C.; Hu, Y.; Liu, Y.; Tian, J. Biocomposites Based on Spent Coffee Grounds and Application in Packaging. Interdiscip. Res. Print. Packag. 2022, 896, 455–461. [Google Scholar]
- Scalia, G.L.; Saeli, M.; Miglietta, P.P.; Micale, R. Coffee biowaste valorization within circular economy: An evaluation method of spent coffee grounds potentials for mortar production. Int. J. Life Cycle Assess. 2021, 26, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Perestrelo, R.; Câmara, J.S. Valorization of spent coffee grounds as a natural source of bioactive compounds for several industrial applications—A volatilomic approach. Foods 2022, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Rosson, E.; Garbo, F.; Marangoni, G.; Bertani, R. Activated carbon from spent coffee grounds: A good competitor of commercial carbons for water decontamination. Appl. Sci. 2020, 10, 5598. [Google Scholar] [CrossRef]
- Tran, T.K.N.; Ngo, T.C.Q.; Nguyen, Q.V.; Do, T.S.; Hoang, N.B. Chemistry potential and application of activated carbon manufactured from coffee grounds in the treatment of wastewater: A review. Mater. Today Proc. 2022, 60, 1914–1919. [Google Scholar] [CrossRef]
- Malini, K.; Selvakumar, D.; Kumar, N.S. Activated carbon from biomass: Preparation, factors improving basicity and surface properties for enhanced CO2 capture capacity–A review. J. CO2 Util. 2023, 67, 102318. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Wang, S.; Qiao, M.; Zhang, X. Study on Pyrolysis Characteristics and Mechanism of Biomass Waste: Rice Husk, Corn Stalk, and Sawdust. Sustainability 2024, 16, 2889. [Google Scholar]
- Saba, A.; Iqbal, H.M.; Duman, G.; Jablonska, M.; Duman, F. Biomass-Derived Ni-Based Catalysts for Green Hydrogen Production by Glycerol Steam Reforming. Catalysts 2023, 13, 1004. [Google Scholar]
- Zhang, Y.; Huang, Z.; Xie, P.; Lacey, S.D.; Jacob, R.J.; Yao, Y. Flash Joule heating (FJH) is an emerging and profitable technology for converting inexhaustible biomass into flash graphene (FG). Nature 2020, 577, 647–651. [Google Scholar]
- Roychand, R.; Kilmartin-Lynch, S.; Saberian, M.; Li, J.; Zhang, G.; Li, C.Q. Transforming spent coffee grounds into a valuable resource for the enhancement of concrete strength. J. Clean. Prod. 2023, 419, 138205. [Google Scholar] [CrossRef]
- Tay, T.; Ucar, S.; Karagöz, S. Preparation and characterization of activated carbon from waste biomass. J. Hazard. Mater. 2009, 165, 481–485. [Google Scholar] [CrossRef]
- Pal, A.; Thu, K.; Mitra, S.; Ibrahim, I.E.; SaHa, B.B.; Kil, H.S.; Yoon, S.H.; Miyawaki, J. Study on biomass derived activated carbons for adsorptive heat pump application. Int. J. Heat Mass Transf. 2017, 110, 7–19. [Google Scholar] [CrossRef]
- Wickramaarachchi, W.K.P.; Minakshi, M.; Gao, X.; Dabare, R.; Wong, K.W. Hierarchical porous carbon from mango Seed husk for electro-chemcal energy storage. Chem. Eng. J. Adv. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Alwahbi, A.; Khatib, L.A.A.; Dammag, H. Biomass-Based Activated Carbon. In From Biomass to Biobased Products; IntechOpen: London, UK, 2024. [Google Scholar]
- Campos, G.A.F.; Perez, J.P.H.; Block, I.; Sagu, S.T.; Celis, P.S.; Taubert, A.; Rawel, H. Preparation of activated carbons from spent coffee grounds and coffee parchment and assessment of their adsorbent efficiency. Processes 2021, 9, 1396. [Google Scholar] [CrossRef]
- Ani, J.U.; Akpmie, K.G.; Okoro, U.C.; Onukwuli, O.D.; Ujam, O.T. Potentials of activated carbon produced from biomass materials for sequestration of dyes, heavy metals, and crude oil components from aqueous environment. Appl. Water Sci. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Zięzio, M.; Charmas, B.; Jedynak, K.; Hawryluk, M. Preparation and characterization of activated carbons obtained from the waste materials impregnated with phosphoric acid (V). Appl. Nanosci. 2020, 10, 4703–4716. [Google Scholar] [CrossRef]
- ASTM D1762-84; Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 2021.
- Aller, D.; Bakshi, S.; Laird, D.A. Modified method for proximate analysis of biochars. J. Anal. Appl. Pyrolysis 2017, 124, 335–342. [Google Scholar] [CrossRef]
- Park, J.E.; Lee, G.B.; Jeong, C.J.; Kim, H.; Kim, C.G. Determination of Relationship Between Higher Heating Value and Atomic Ratio of Hydrogen to Carbon in Spent Coffee Grounds by Hydrothermal Carbonization. Energies 2021, 14, 6551. [Google Scholar] [CrossRef]
- ASTM D5228-16; Standard Test Method for Determination of Butane Working Capacity of Activated Carbon. ASTM International: West Conshohocken, PA, USA, 2016.
- Saka, T.; San-Pedro, L.; Abubakar, A.M.; Sylvain, T. Evaluation of the physical properties of various biomass materials for the production of activated carbon. J. Chem. Environ. 2023, 1, 30–39. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, Z.; Chen, B. H/C atomic ratio as a smart linkage between pyrolytic temperatures, aromatic clusters and sorption properties of biochars derived from diverse precursory materials. Sci. Rep. 2016, 6, 22644. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Luo, L.; Deng, S.; Shi, G.; Zhang, S.; Zhang, Y.; Deng, O.; Wang, L.; Zhang, J.; Wei, L. Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Bioresour. Technol. 2018, 267, 431–437. [Google Scholar] [CrossRef]
- Scardaci, V.; Compagnini, G. Raman Spectroscopy Investigation of Graphene Oxide Reduction by Laser Scribing. C 2021, 7, 48. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Konarov, A.; Gosselink, D.; Chen, P.; Adair, K.R.; Sun, Q.; Li, R.; Sun, X. High-Performance Potassium-Ion Batteries with Ultrafine MoO Nanoparticles Embedded in Carbon Nanosheets Anode. J. Power Sources 2020, 476, 229076. [Google Scholar]
- Helmi, M.; Moazami, F.; Hemmati, A.; Ghaemi, A. Synthesis and Characterization of KOH Graphene Oxide-Fe3O4 as a Magnetic Composite Adsorbent for CO2 Capture. J. Phys. Chem. Solids 2023, 178, 111338. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Chen, X. Modeling of experimental adsorption isotherm data. Information 2015, 6, 14–22. [Google Scholar] [CrossRef]
- Furmaniak, S.; Gauden, P.A.; Lezanska, M.; Miskiewicz, R.; Blajet-Kosicka, A.; Kowalczyk, P. The finite pore volume GAB adsorption isotherm model as a simple tool to estimate a diameter of cylindrical nanopores. Molecules 2021, 26, 1509. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.; An, K.; Park, S.; Kim, B. Facile preparation of activated carbon with optimal pore range for high butane working capacity. Carbon Lett. 2020, 30, 297–305. [Google Scholar] [CrossRef]
- Lee, B.-H.; Lee, H.-M.; Chung, D.C.; Kim, B.-J. Effect of Mesopore Development on Butane Working Capacity of Biomass-derived Activated Carbon for Automobile Canister. Nanomaterials 2021, 11, 673. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Li, W.; Peng, J.; Xia, H.; Zhang, L.; Guo, S.; Chen, G. Optimization of Mesoporous Activated Carbon from Coconut Shells by Chemical Activation with Phosphoric Acid. BioResources 2013, 8, 6184–6195. [Google Scholar] [CrossRef]
- Zhao, W.; Luo, L.; Wang, H.; Fan, M. Synthesis of Bamboo-Based Activated Carbons with Super-High Specific Surface Area for Hydrogen Storage. BioResources 2017, 12, 1246–1262. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.; Park, S.; An, K.; Kim, B. Pitch-derived activated carbon fibers for emission control of low-concentration hydrocarbon. Nanomaterials 2019, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
| Name | H-180 | H-200 | H-220 | H-250 | D-300/5 | D-500/1 | D-500/3 | D-500/5 | D-700/5 |
|---|---|---|---|---|---|---|---|---|---|
| Carbonization method | Hydrothermal | Drying | |||||||
| Temperature (°C) | 180 | 200 | 220 | 250 | 300 | 500 | 500 | 500 | 700 |
| Time (h) | 1 | 1 | 1 | 1 | 5 | 1 | 3 | 5 | 5 |
| Name | CAC 1 | CAC 2 | CAC 3 | CAC 4 | CAC5/1 h | CAC5/2 h | CAC 5 | CAC6/1 h | CAC6/2 h | CAC 6 | CAC 7 | CAC 8 | CAC 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 750 | 750 | 750 | 850 | 850 | 850 | 850 | 850 | 850 | 850 | 950 | 950 | 950 |
| Time (h) | 3 | 3 | 3 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 3 | 3 | 3 |
| Agent ratio (w/w) | 1 | 2 | 3 | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 1 | 2 | 3 |
| Sample Number | #1 | #2 | #3 | #4 | #5 | Average |
|---|---|---|---|---|---|---|
| Moisture contents (%) | 57.0 | 56.0 | 64.6 | 43.0 | 65.0 | 57.1 ± 8.9 |
| Sample Number | #1 | #2 | #3 | #4 | #5 | Average | |
|---|---|---|---|---|---|---|---|
| Proximate Analysis (wt%) | Volatiles | 88.4 | 82.6 | 87.5 | 80.4 | 84.5 | 84.7 ± 3.3 |
| Fixed-C | 11.0 | 16.4 | 17.6 | 17.5 | 13.4 | 15.2 ± 2.9 | |
| Ash | 0.6 | 1.0 | 0.9 | 1.9 | 2.1 | 1.3 ± 0.7 | |
| Elemental analysis (wt%) | C | 50.2 | 51.8 | 54.1 | 55.2 | 53.5 | 53.0 ± 2.0 |
| H | 7.2 | 7.2 | 6.7 | 7.1 | 7.1 | 7.1 ± 0.2 | |
| O | 39.1 | 37.7 | 32.7 | 32.9 | 33.9 | 35.3 ± 2.9 | |
| N | 2.3 | 2.2 | 2.6 | 2.4 | 2.3 | 2.4 ± 0.2 | |
| S | 0 | 0 | 0 | 0 | 0 | 0 | |
| H/C (-) | 0.14 | 0.14 | 0.12 | 0.13 | 0.13 | 0.13 | |
| Samples | D-300/5 | D-500/1 | D-500/3 | D-500/5 | D-700/5 | |
|---|---|---|---|---|---|---|
| Proximate analysis (wt%) | Volatiles (VR) | 59.2 (▼32%) | 12.9 (▼85%) | 12.8 (▼85%) | 11.7 (▼87%) | 7.4 (▼92%) |
| Fixed-C (CR) | 21.9 (▲24%) | 84.8 (▲382%) | 84.2 (▲378%) | 83.7 (▲376%) | 84.5 (▲380%) | |
| Elemental analysis (wt%) | C | 71.9 | 83.3 | 82.6 | 85.2 | 88.9 |
| H | 6.9 | 2.8 | 2.8 | 2.7 | 1.2 | |
| H/C (-) | 0.09 | 0.03 | 0.03 | 0.03 | 0.01 | |
| Yield (%) | 40.0 | 44.8 | 43.2 | 31.7 | 28.0 | |
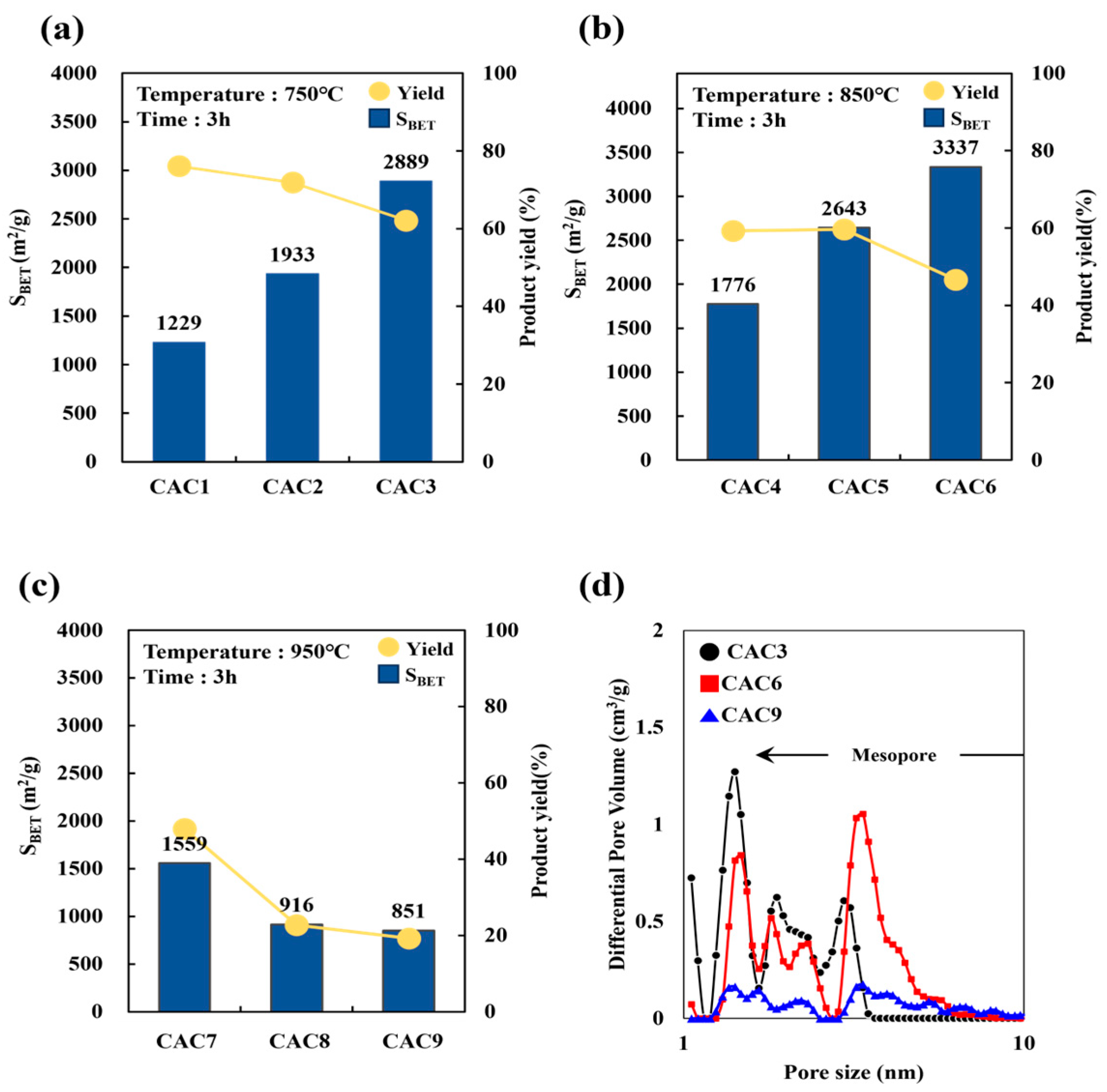
| Sample | CAC 1 | CAC 2 | CAC 3 | CAC 4 | CAC 5 | CAC 6 | CAC 7 | CAC 8 | CAC 9 |
|---|---|---|---|---|---|---|---|---|---|
| SBET (m2/g) | 1230 | 1933 | 2889 | 1776 | 2643 | 3337 | 1559 | 916 | 851 |
| VT (cm3/g) | N.A. | N.A. | 1.29 | N.A. | 1.28 | 1.95 | N.A. | N.A. | 0.84 |
| VMES (cm3/g) | N.A. | N.A. | 0.35 | N.A. | 0.50 | 1.53 | N.A. | N.A. | 0.80 |
| VMIC (cm3/g) | N.A. | N.A. | 0.94 | N.A. | 0.78 | 0.42 | N.A. | N.A. | 0.04 |
| Yield (wt%) | 76.1 | 71.9 | 62.1 | 59.3 | 59.6 | 46.7 | 47.9 | 22.7 | 19.2 |
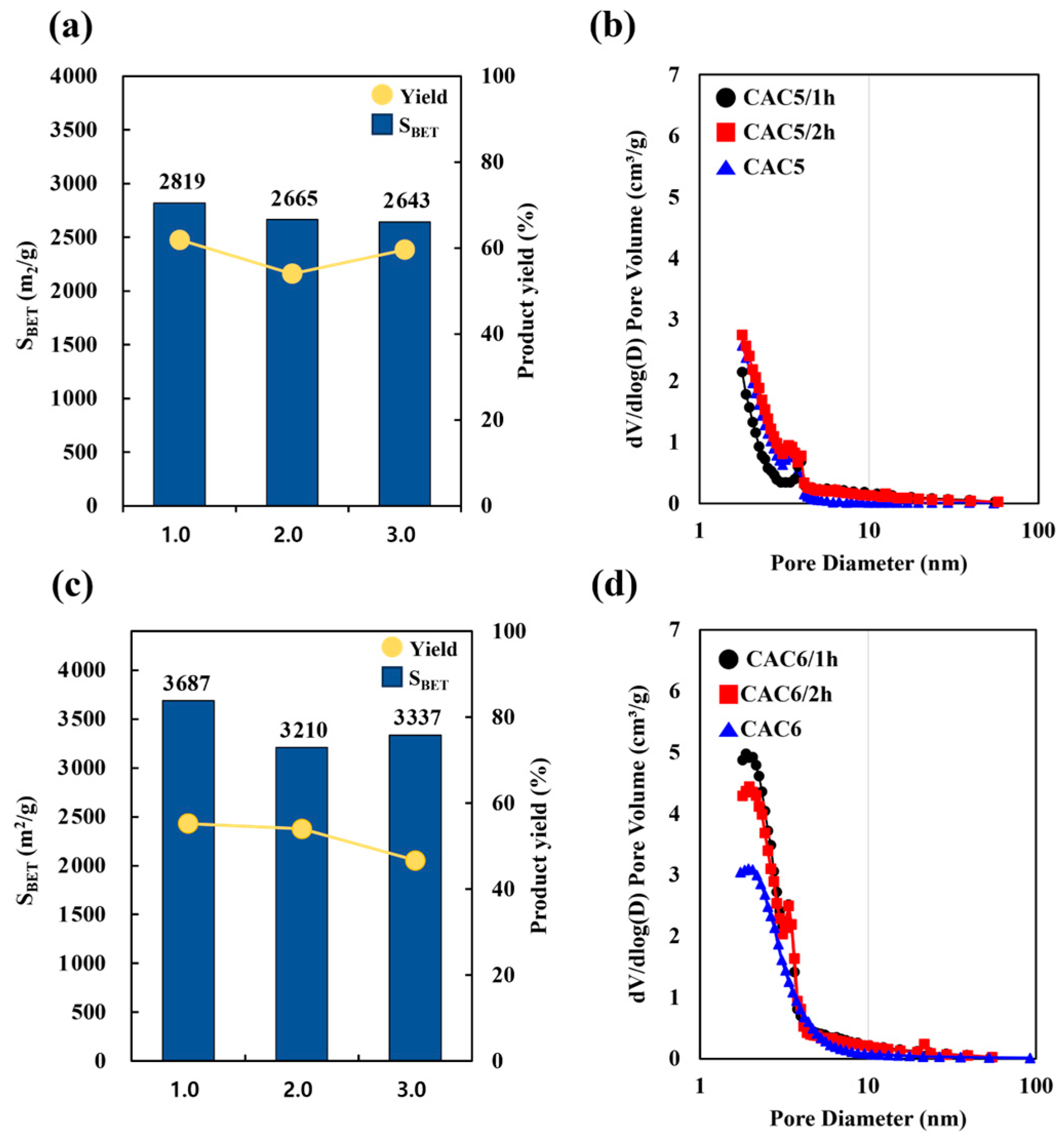
| Sample | CAC 5/1 h | CAC 5/2 h | CAC 5 | CAC 6/1 h | CAC 6/2 h | CAC 6 |
|---|---|---|---|---|---|---|
| SBET (m2/g) | 2819 | 2665 | 2643 | 3687 | 3210 | 3337 |
| VT (cm3/g) | 1.40 | 1.41 | 1.28 | 2.11 | 1.87 | 1.95 |
| VMES (cm3/g) | 0.49 | 0.69 | 0.50 | 1.45 | 1.33 | 1.53 |
| VMIC (cm3/g) | 0.91 | 0.73 | 0.78 | 0.66 | 0.54 | 0.42 |
| Yield (wt%) | 61.9 | 60.0 | 59.6 | 55.2 | 54.0 | 46.7 |
| Raw Material | Activator | SBET (m2/g) | VT (cm3/g) | VMES (cm3/g) | Ref. |
|---|---|---|---|---|---|
| Spent coffee grounds | KOH | 3687 | 2.11 | 1.45 | This study |
| Waste palm trunk | KOH | 2927 | 2.51 | 0.10 | [27] |
| Mango seed | KOH | 1944 | 0.39 | - | [23] |
| Coconut shell | H3PO4 | 891 | 0.72 | 0.46 | [43] |
| Bamboo | KOH | 3208 | 1.01 | - | [44] |
| Spent coffee grounds | H3PO4 | 720 | 0.46 | 0.051 | [23] |
| Spent coffee grounds | KOH | 2746 | 2.32 | 1.34 | [8] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyeon, G.-W.; Lee, G.B.; Kang, D.J.; Lee, S.E.; Seong, K.M.; Park, J.-E. Optimization of Activated Carbon Synthesis from Spent Coffee Grounds for Enhanced Adsorption Performance. Molecules 2025, 30, 2557. https://doi.org/10.3390/molecules30122557
Hyeon G-W, Lee GB, Kang DJ, Lee SE, Seong KM, Park J-E. Optimization of Activated Carbon Synthesis from Spent Coffee Grounds for Enhanced Adsorption Performance. Molecules. 2025; 30(12):2557. https://doi.org/10.3390/molecules30122557
Chicago/Turabian StyleHyeon, Geon-Woong, Gi Bbum Lee, Da Jung Kang, Sang Eun Lee, Kwang Mo Seong, and Jung-Eun Park. 2025. "Optimization of Activated Carbon Synthesis from Spent Coffee Grounds for Enhanced Adsorption Performance" Molecules 30, no. 12: 2557. https://doi.org/10.3390/molecules30122557
APA StyleHyeon, G.-W., Lee, G. B., Kang, D. J., Lee, S. E., Seong, K. M., & Park, J.-E. (2025). Optimization of Activated Carbon Synthesis from Spent Coffee Grounds for Enhanced Adsorption Performance. Molecules, 30(12), 2557. https://doi.org/10.3390/molecules30122557






