Bio-Based Flame-Retardant Systems for Polymers Obtained via Michael 1,4-Addition
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of Bio-Sourced Components
2.2. Evaluation of the Properties and Structure of Michael Addition Polymers with Flame-Retardant Systems
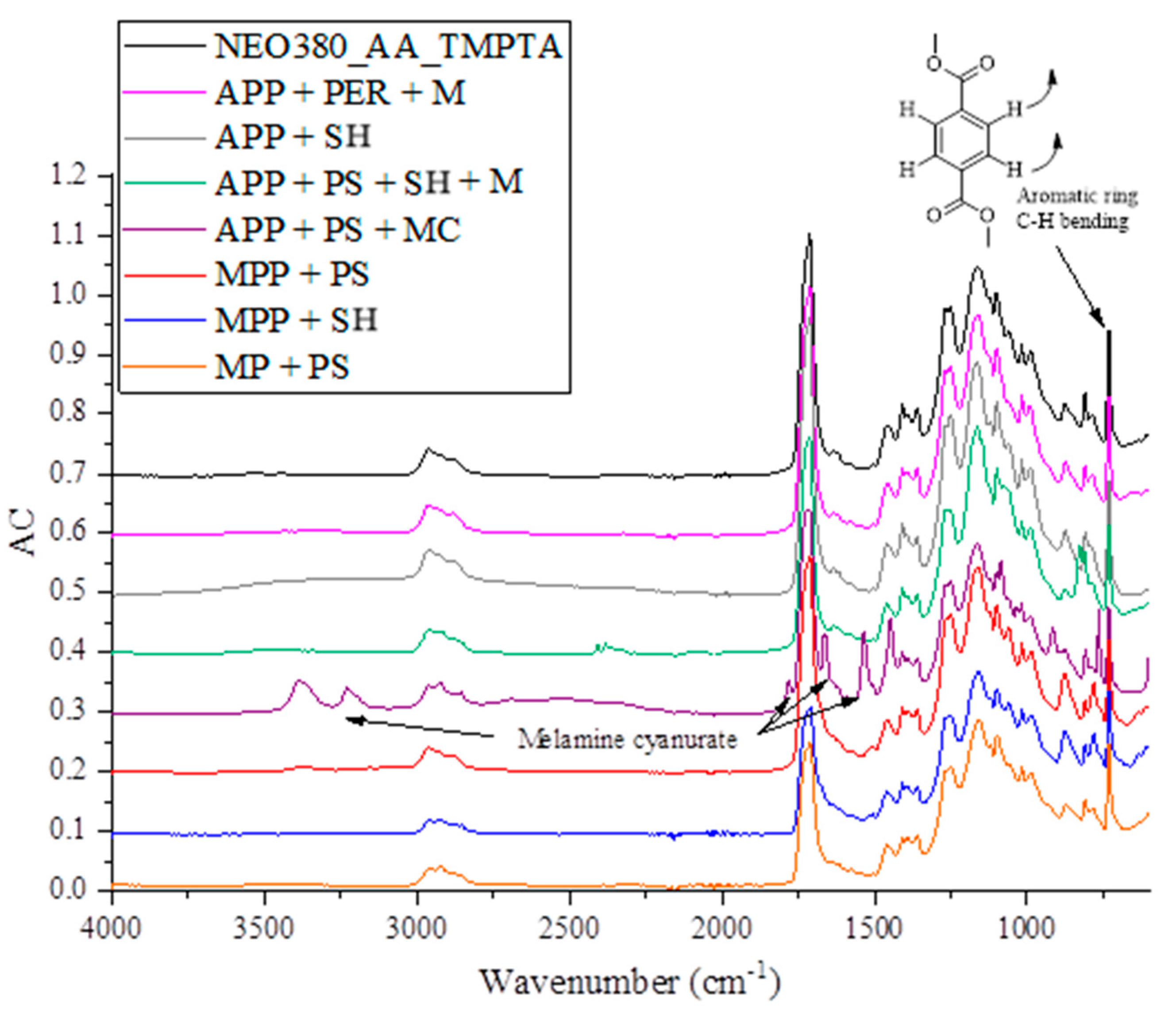
2.2.1. Chemical Structure
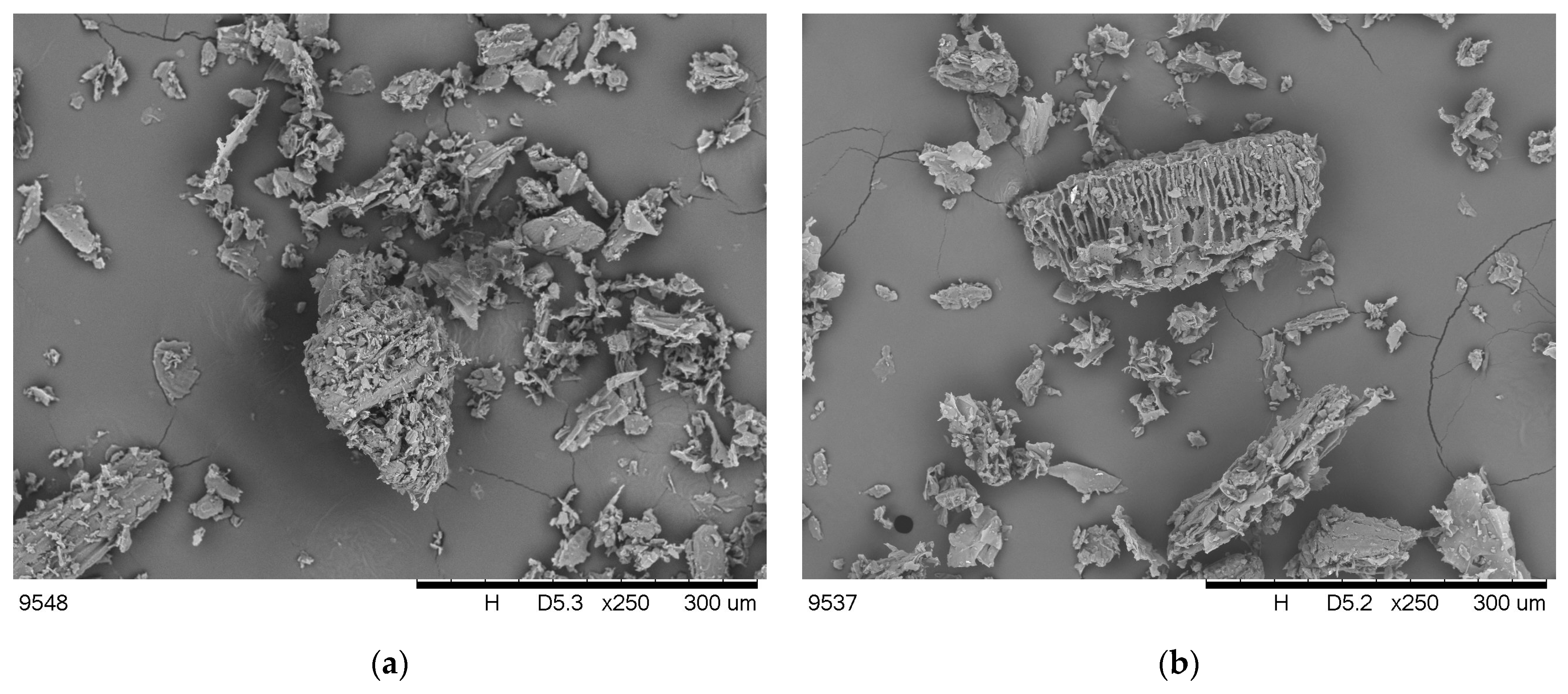
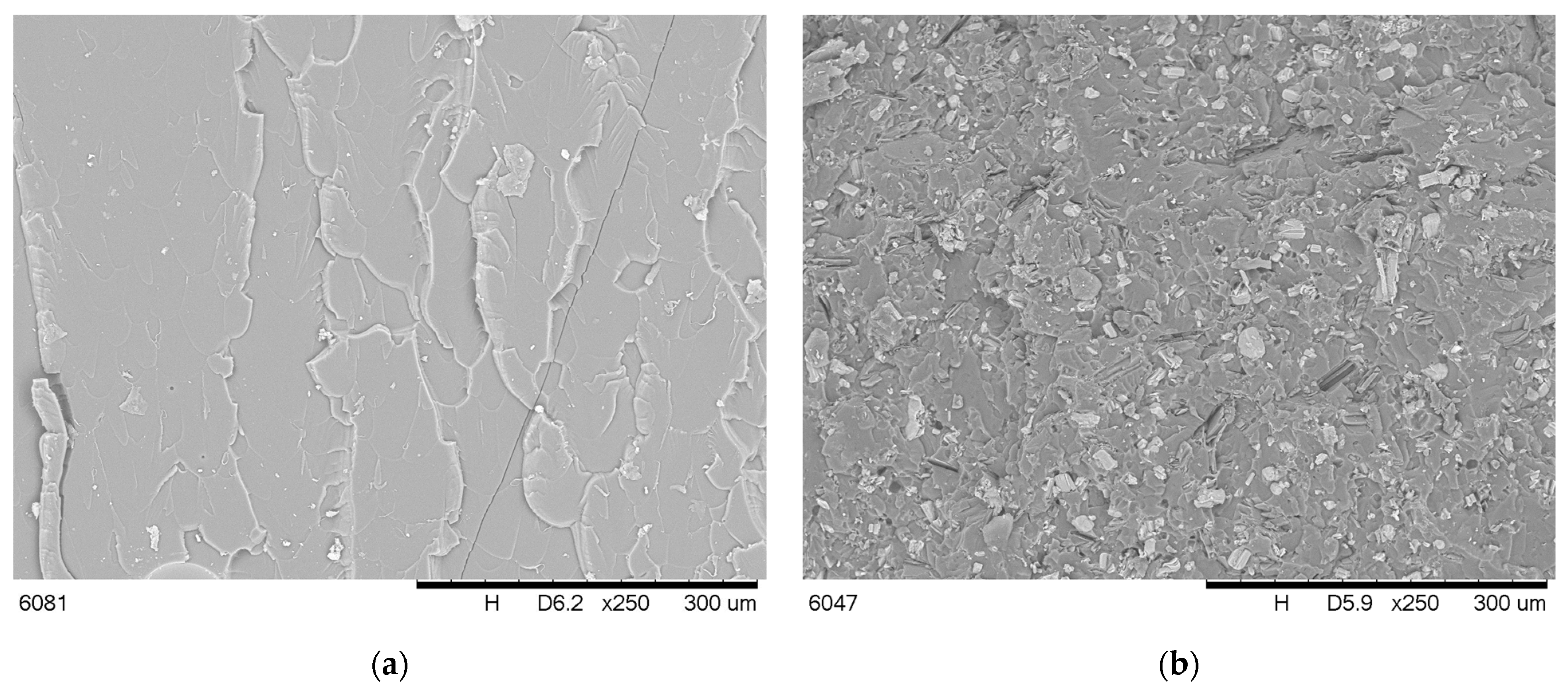
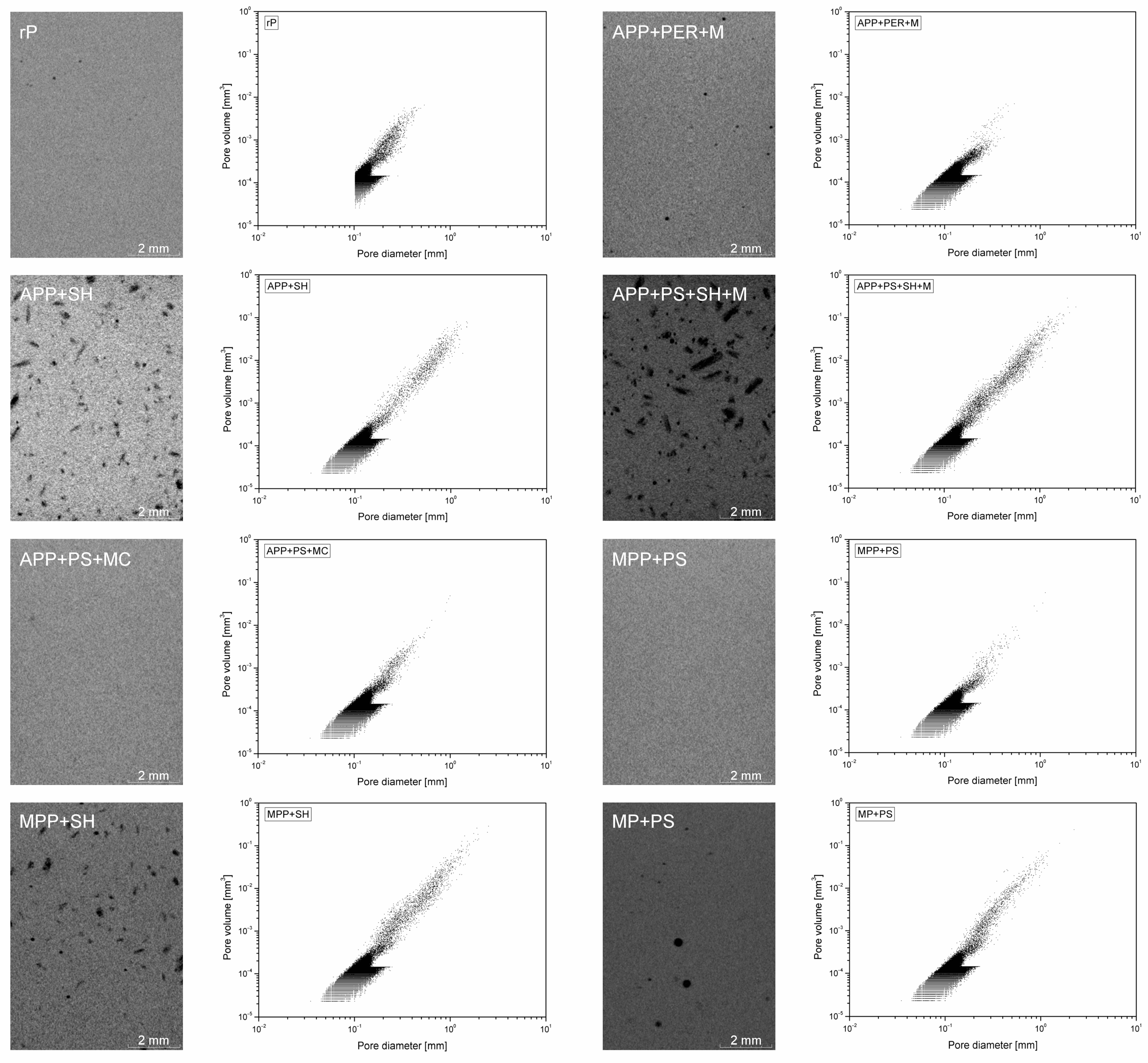
2.2.2. Microstructure and Contact Angle
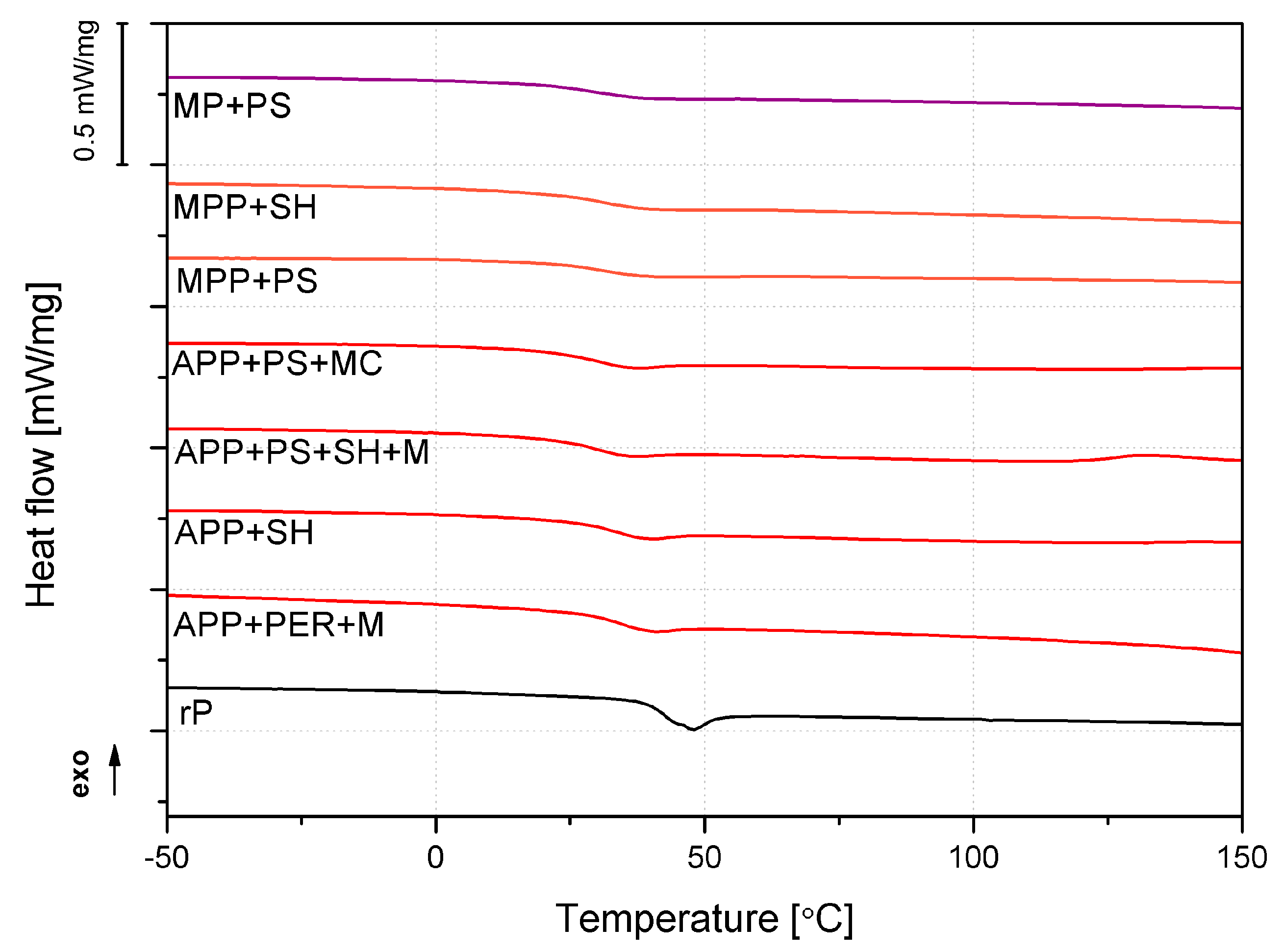
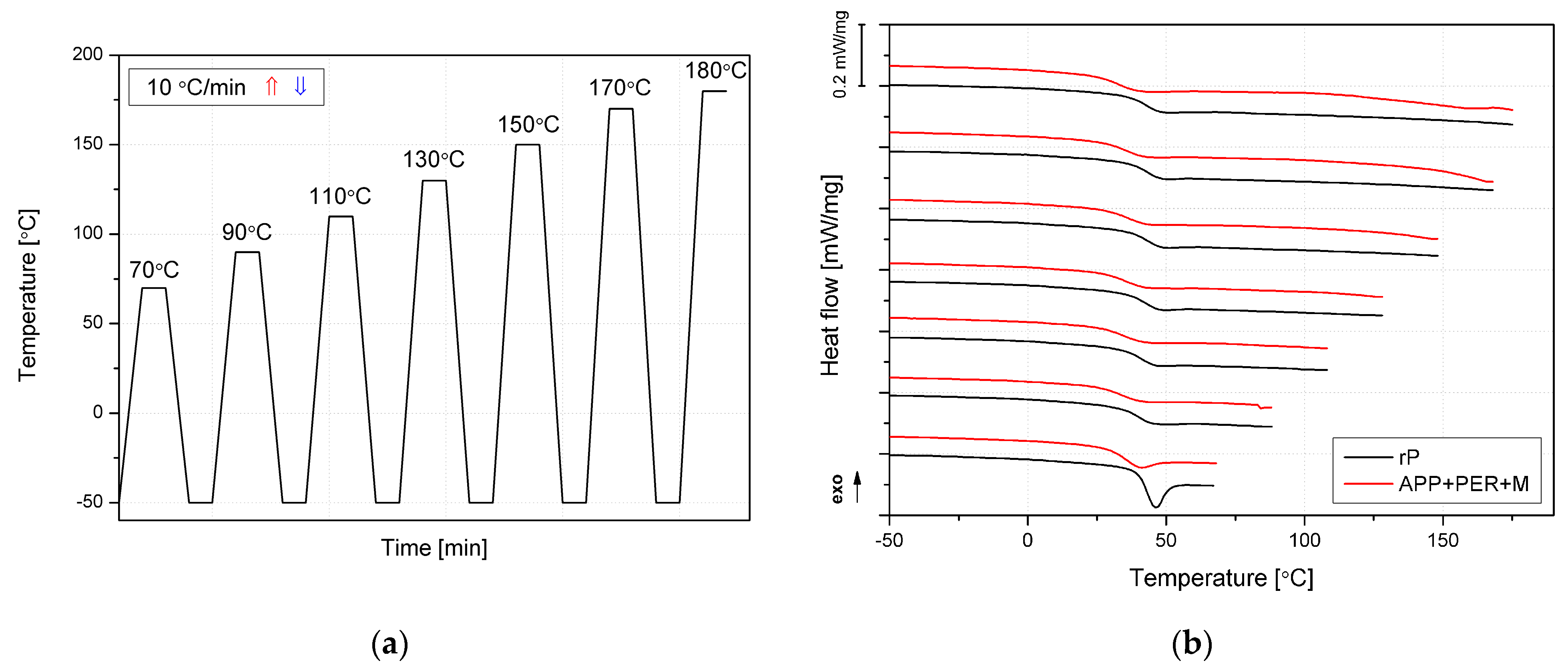
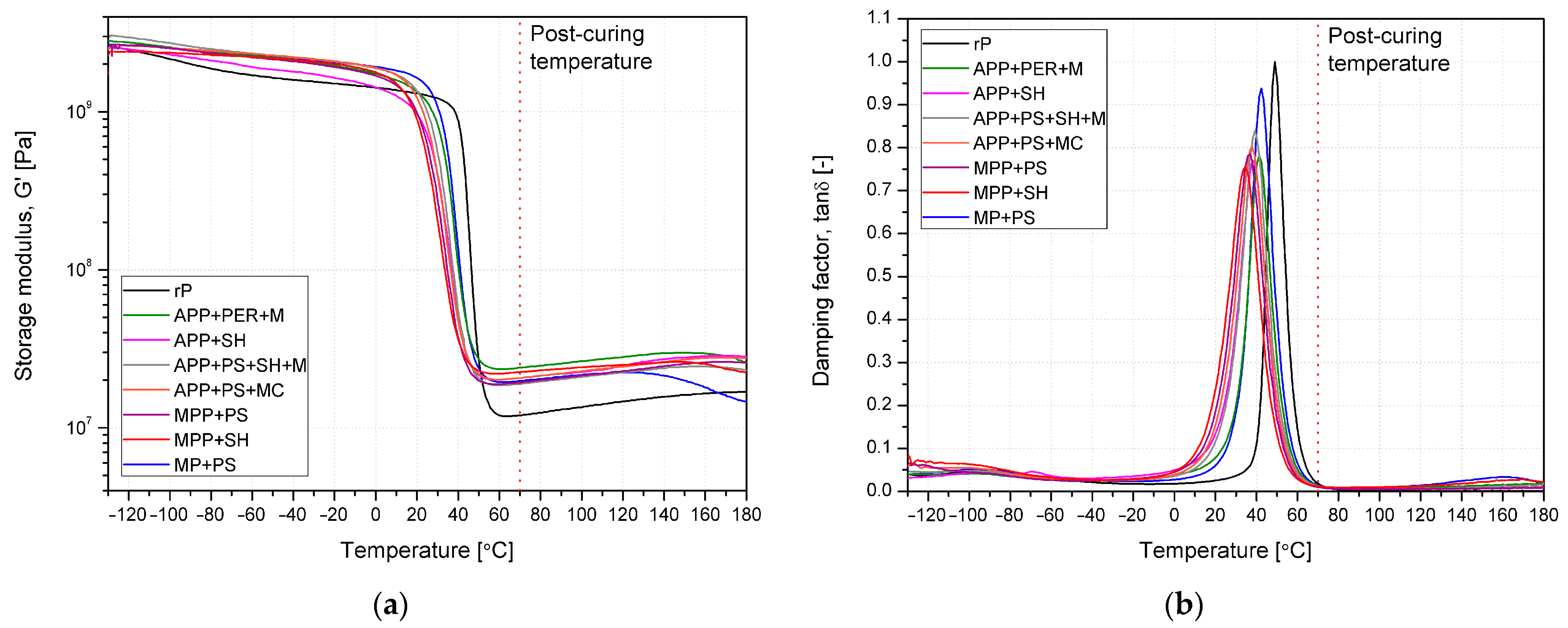
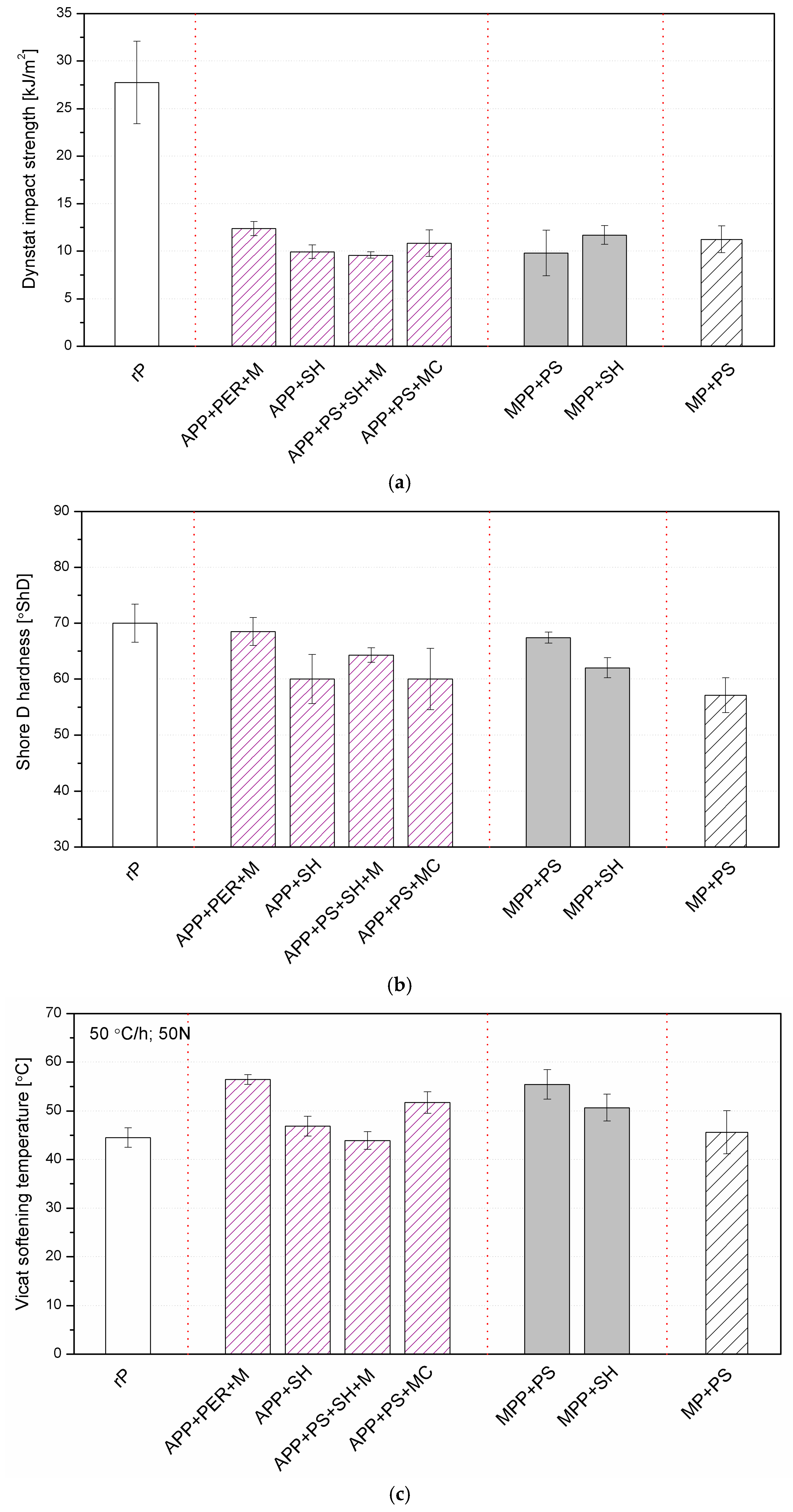
2.2.3. Thermal, Mechanical and Thermomechanical Analyses
2.3. Assessment of the Effectiveness of the Developed Systems on the Michael Reaction Polymer Flammability
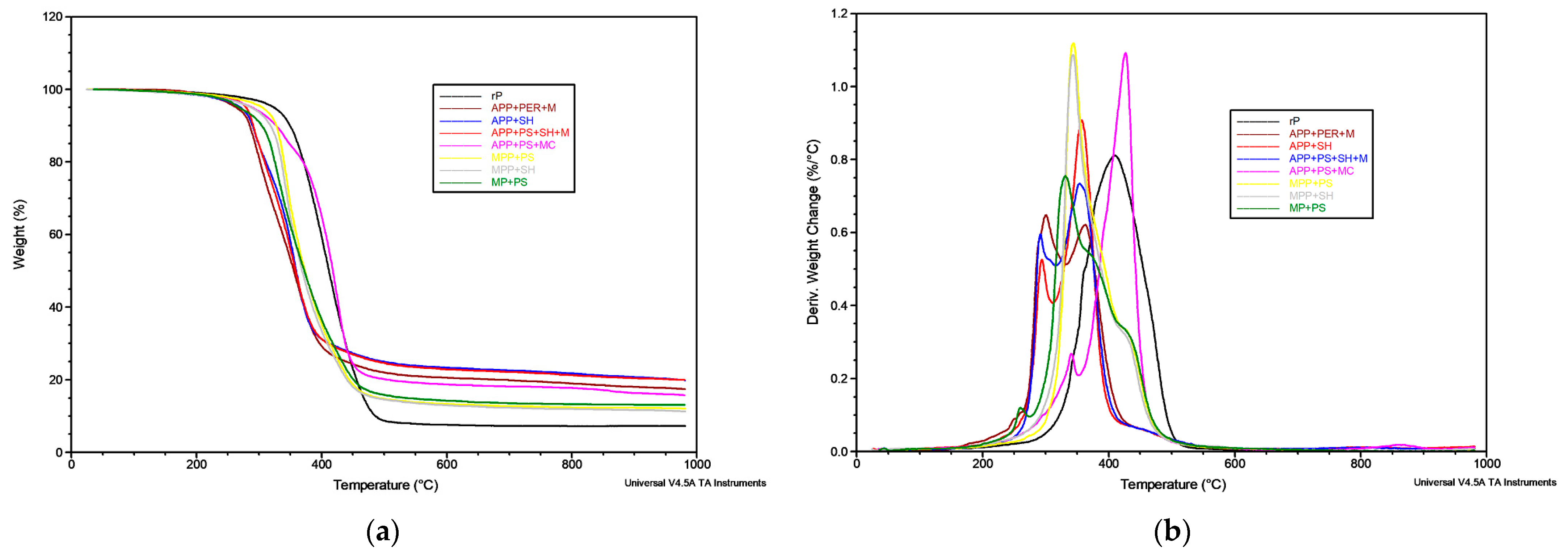
2.3.1. Thermal Stability
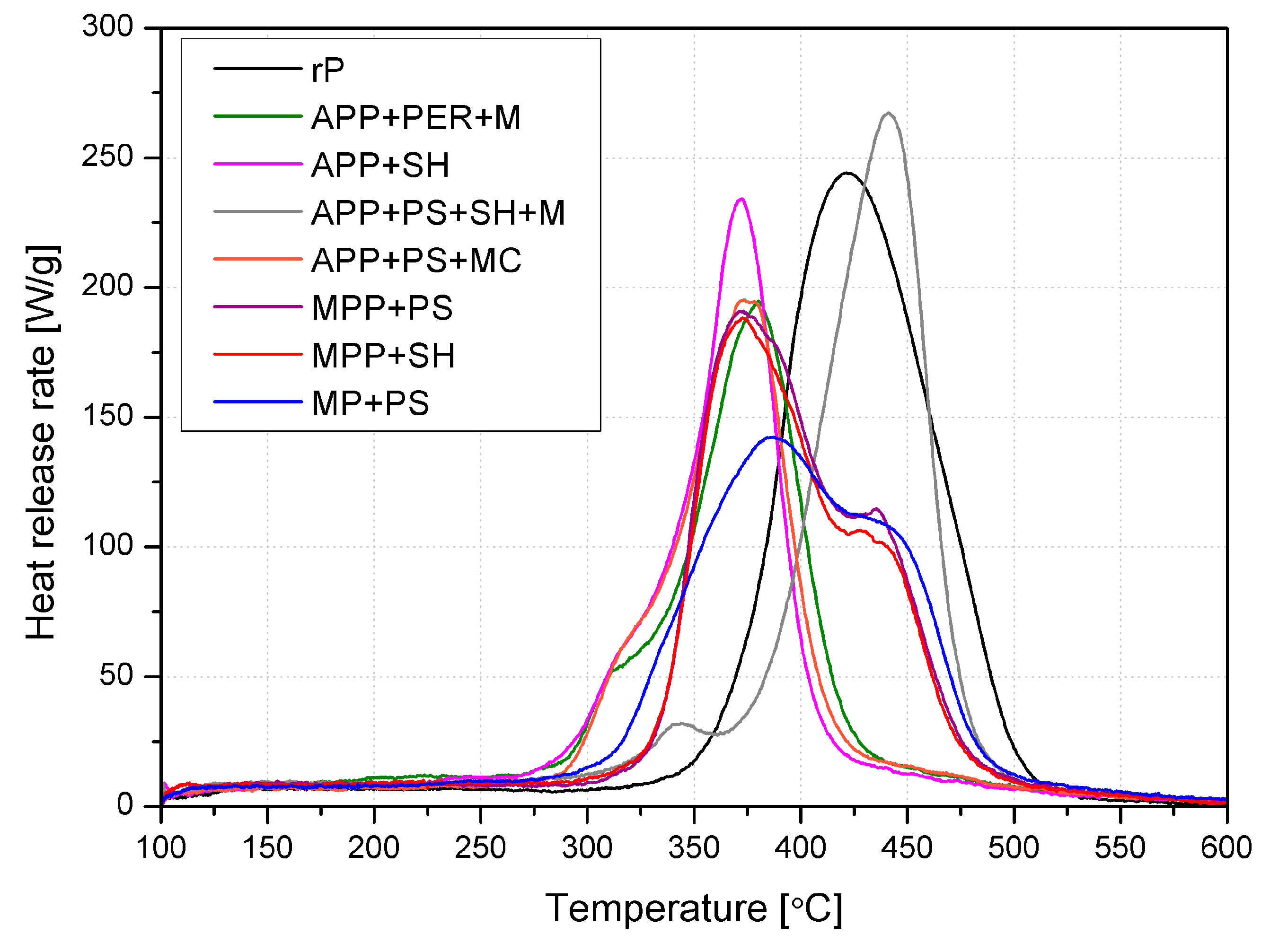
2.3.2. Flammability
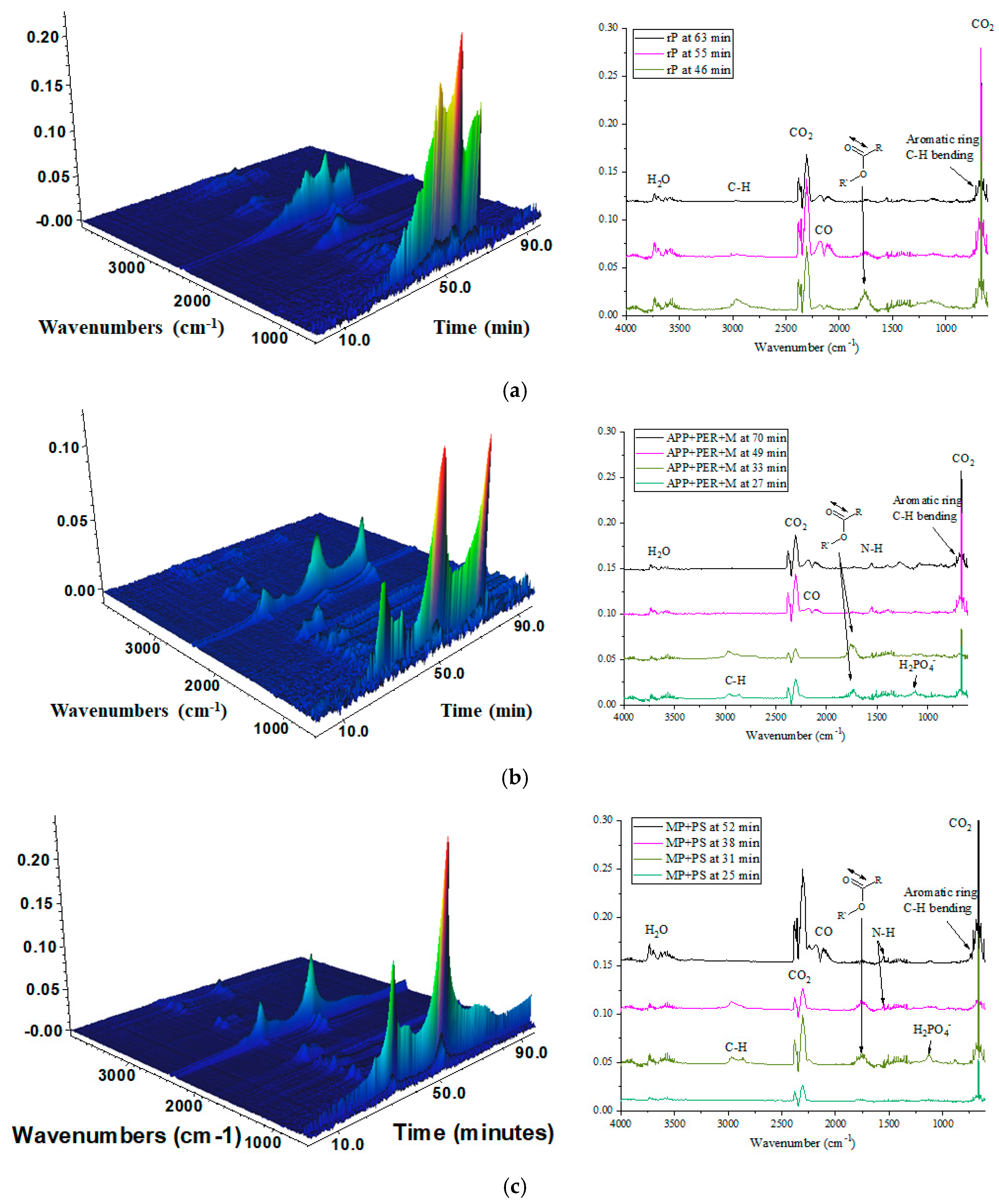
2.3.3. Evolved Gas Analysis
2.3.4. The Mechanism of Flame Retardancy of Developed FR System for Michael Addition Polymer
3. Experimental
3.1. Materials and Preparation
3.2. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Parliament and of the Council. Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency. 2006. Available online: https://eur-lex.europa.eu/eli/reg/2006/1907/oj/eng (accessed on 5 June 2025).
- Convention on Persistent Organic Pollutants. 2004. Available online: https://www.pops.int/ (accessed on 5 June 2025).
- Restriction of Hazardous Substances’ in Electrical and Electronic Equipment. 2015. Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/rohs-directive_en (accessed on 5 June 2025).
- Levchik, S.V. Introduction to flame retardancy and polymer flammability. In Flame Retardant Polymer Nanocomposites; Wiley: Hoboken, NJ, USA, 2007; pp. 1–29. [Google Scholar]
- Battig, A.; Sanchez-Olivares, G.; Rockel, D.; Maldonado-Santoyo, M.; Schartel, B. Waste not, want not: The use of leather waste in flame retarded EVA. Mater. Des. 2021, 210, 110100. [Google Scholar] [CrossRef]
- Alongi, J.; Han, Z.; Bourbigot, S. Intumescence: Tradition versus novelty. A comprehensive review. Prog. Polym. Sci. 2015, 51, 28–73. [Google Scholar] [CrossRef]
- Suardana, N.P.G.; Ku, M.S.; Lim, J.K. Effects of diammonium phosphate on the flammability and mechanical properties of bio-composites. Mater. Des. 2011, 32, 1990–1999. [Google Scholar] [CrossRef]
- Shukor, F.; Hassan, A.; Saiful Islam, M.; Mokhtar, M.; Hasan, M. Effect of ammonium polyphosphate on flame retardancy, thermal stability and mechanical properties of alkali treated kenaf fiber filled PLA biocomposites. Mater. Des. 2014, 54, 425–429. [Google Scholar] [CrossRef]
- Yu, Y.; Fu, S.; Song, P.; Luo, X.; Jin, Y.; Lu, F.; Wu, Q.; Ye, J. Functionalized lignin by grafting phosphorus-nitrogen improves the thermal stability and flame retardancy of polypropylene. Polym. Degrad. Stab. 2012, 97, 541–546. [Google Scholar] [CrossRef]
- Mandlekar, N.; Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Malucelli, G.; Guan, J.-P. An overview on the use of lignin and its derivatives in fire retardant polymer systems. In Lignin—Trends and Applications; InTech: Tokyo, Japan, 2018. [Google Scholar]
- Salasinska, K.; Barczewski, M.; Borucka, M.; Górny, R.L.; Kozikowski, P.; Celiński, M.; Gajek, A. Thermal Stability, Fire and Smoke Behaviour of Epoxy Composites Modified with Plant Waste Fillers. Polymers 2019, 11, 1234. [Google Scholar] [CrossRef]
- Salasinska, K.; Celiński, M.; Mizera, K.; Kozikowski, P.; Leszczyński, M.K.; Gajek, A. Synergistic effect between histidine phosphate complex and hazelnut shell for flammability reduction of low-smoke emission epoxy resin. Polym. Degrad. Stab. 2020, 181, 109292. [Google Scholar] [CrossRef]
- Abolins, A.; Eihe, D.; Pomilovskis, R.; Fridrihsone, A.; Kirpluks, M. Rapeseed oil as feedstock for the polymeric materials via Michael addition reaction. Ind. Crops Prod. 2023, 204, 117367. [Google Scholar] [CrossRef]
- Pomilovskis, R.; Mierina, I.; Fridrihsone, A.; Kirpluks, M. Bio-Based Polymer Developments from Tall Oil Fatty Acids by Exploiting Michael Addition. Polymers 2022, 14, 4068. [Google Scholar] [CrossRef]
- Pomilovskis, R.; Kaulina, E.; Mierina, I.; Abolins, A.; Kockova, O.; Fridrihsone, A.; Kirpluks, M. Wood pulp industry by-product valorization for acrylate synthesis and bio-based polymer development via Michael addition reaction. J. Bioresour. Bioprod. 2023, 8, 265–279. [Google Scholar] [CrossRef]
- Pomilovskis, R.; Mierina, I.; Beneš, H.; Trhlíková, O.; Abolins, A.; Fridrihsone, A.; Kirpluks, M. The Synthesis of Bio-Based Michael Donors from Tall Oil Fatty Acids for Polymer Development. Polymers 2022, 14, 4107. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.U. Developing plant fibre composites for structural applications by optimising composite parameters: A critical review. J. Mater. Sci. 2013, 48, 6083–6107. [Google Scholar] [CrossRef]
- Kuciel, S.; Rydarowski, H. Biokompozyty z Surowców Odnawialnych; Kracow University of Technology: Kraków, Poland, 2012. [Google Scholar]
- Bledzki, A.K.; Mamun, A.A.; Volk, J. Physical, chemical and surface properties of wheat husk, rye husk and soft wood and their polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 480–488. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.-P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Pirayesh, H.; Khazaeian, A. Using almond (Prunus amygdalus L.) shell as a bio-waste resource in wood based composite. Compos. Part B Eng. 2012, 43, 1475–1479. [Google Scholar] [CrossRef]
- Guler, C.; Copur, Y.; Tascioglu, C. The manufacture of particleboards using mixture of peanut hull (Arachis hypoqaea L.) and European Black pine (Pinus nigra Arnold) wood chips. Bioresour. Technol. 2008, 99, 2893–2897. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Bonnia, N.N.; Ahmad, S. Basic properties of grain by-products and their viability in polypropylene composites. Ind. Crops Prod. 2012, 37, 427–434. [Google Scholar] [CrossRef]
- Niska, O.K.; Sain, M. Wood-Polymer Composites, 1st ed.; Woodhead Publishing: Sawston, UK, 2008; pp. 1–82. [Google Scholar]
- Endres, H.J. Energetic utilization: Biopolymers as a source of energy. Kunststoffe Int. 2010, 100, 83–85. [Google Scholar]
- Salasinska, K.; Ryszkowska, J. The effect of filler chemical constitution and morphological properties on the mechanical properties of natural fiber composites. Compos. Interfaces 2015, 22, 39–50. [Google Scholar] [CrossRef]
- Omer, R.A.; Hughes, A.; Hama, J.R.; Wang, W.; Tai, H. Hydrogels from dextran and soybean oil by UV photo-polymerization. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Salasinska, K.; Mizera, K.; Barczewski, M.; Borucka, M.; Gloc, M.; Celiński, M.; Gajek, A. The influence of degree of fragmentation of Pinus sibirica on flammability, thermal and thermomechanical behavior of the epoxy-composites. Polym. Test. 2019, 79, 106036. [Google Scholar] [CrossRef]
- Barczewski, M.; Sałasińska, K.; Szulc, J. Application of sunflower husk, hazelnut shell and walnut shell as waste agricultural fillers for epoxy-based composites: A study into mechanical behavior related to structural and rheological properties. Polym. Test. 2019, 75, 1–11. [Google Scholar] [CrossRef]
- Speer, D.; Mahon, W.; Behtash, S.; Ayinuola, K.; Wang, X. Isocyanate-Free Foam Using Carbon Michael Addition Chemistry. U.S. Patent Application No. US11161954B2, 14 February 2018. [Google Scholar]
- Salasinska, K.; Celiński, M.; Barczewski, M.; Leszczyński, M.K.; Borucka, M.; Kozikowski, P. Fire behavior of flame retarded unsaturated polyester resin with high nitrogen content additives. Polym. Test. 2020, 84, 106379. [Google Scholar] [CrossRef]
- Genest, A.; Portinha, D.; Fleury, E.; Ganachaud, F. The aza-Michael reaction as an alternative strategy to generate advanced silicon-based (macro)molecules and materials. Prog. Polym. Sci. 2017, 72, 61–110. [Google Scholar] [CrossRef]
- Riahipour, R.; Nemati, M.S.; Zadehmohamad, M.; Abadyan, M.r.; Tehrani, M.; Baniassadi, M. Mechanical properties of an epoxy-based coating reinforced with silica aerogel and ammonium polyphosphate additives. Polym. Polym. Compos. 2022, 30, 096739112110690. [Google Scholar] [CrossRef]
- You, G.; Cheng, Z.; Peng, H.; He, H. The synthesis and characterization of a novel phosphorus–nitrogen containing flame retardant and its application in epoxy resins. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Theocaris, P.S.; Papanicolaou, G.C.; Papadopoulos, G.A. The Effect of Filler-Volume Fraction on Crack-Propagation Behavior of Particulate Composites. J. Compos. Mater. 1981, 15, 41–54. [Google Scholar] [CrossRef]
- Sousa, S.P.B.; Ribeiro, M.C.S.; Nóvoa, P.R.O.; Pereira, C.M.; Ferreira, A.J.M. Mechanical behaviour assessment of unsaturated polyester polymer mortars filled with nano-sized Al2O3 and ZrO2 particles. Ciênc. Tecnol. Mater. 2017, 29, e167–e171. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Yang, H.; Yu, B.; Kandola, B.; Deli, D. Comparative study on the synergistic effect of POSS and graphene with melamine phosphate on the flame retardance of poly(butylene succinate). Thermochim. Acta 2012, 543, 156–164. [Google Scholar] [CrossRef]
- Markwart, J.C.; Battig, A.; Zimmermann, L.; Wagner, M.; Fischer, J.; Schartel, B.; Wurm, F.R. Systematically Controlled Decomposition Mechanism in Phosphorus Flame Retardants by Precise Molecular Architecture: P-O vs. P-N. ACS Appl. Polym. Mater. 2019, 1, 1118–1128. [Google Scholar] [CrossRef]
- Ramgobin, A.; Fontaine, G.; Penverne, C.; Bourbigot, S. Thermal Stability and Fire Properties of Salen and Metallosalens as Fire Retardants in Thermoplastic Polyurethane (TPU). Materials 2017, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Schinazi, G.; Price, E.J.; Schiraldi, D.A. Fire testing methods of bio-based flame-retardant polymeric materials. In Bio-Based Flame-retardant Technology for Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 61–95. [Google Scholar]
- Hamciuc, C.; Vlad-Bubulac, T.; Serbezeanu, D.; Macsim, A.-M.; Lisa, G.; Anghel, I.; Şofran, I.-E. Effects of Phosphorus and Boron Compounds on Thermal Stability and Flame Retardancy Properties of Epoxy Composites. Polymers 2022, 14, 4005. [Google Scholar] [CrossRef] [PubMed]
- Levchik, S.V.; Costa, L.; Camino, G. Effect of the fire-retardant, ammonium polyphosphate, on the thermal decomposition of aliphatic polyamides: Part II—Polyamide 6. Polym. Degrad. Stab. 1992, 36, 229–237. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Lyons, J.W. Mechanisms of Fire Retardation with Phosphorus Compound. J. Fire Flammabl. 1970, 1, 302–311. [Google Scholar]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chemie Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef]
- Battig, A.; Markwart, J.C.; Wurm, F.R.; Schartel, B. Hyperbranched phosphorus flame retardants: Multifunctional additives for epoxy resins. Polym. Chem. 2019, 10, 4346–4358. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Warmiński, K.; Krzyżaniak, M.; Tyśkiewicz, K.; Olba-Zięty, E.; Graban, Ł.; Lajszner, W.; Załuski, D.; Wiejak, R.; Kamiński, P.; et al. How does extraction of biologically active substances with supercritical carbon dioxide affect lignocellulosic biomass properties? Wood Sci. Technol. 2020, 54, 519–546. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Olba-Zięty, E. Biomass yield and quality of perennial herbaceous crops in a double harvest in a continental environment. Ind. Crops Prod. 2022, 180, 114752. [Google Scholar] [CrossRef]
- PN-EN ISO 16472; Animal Feeding Stuffs—Determination of Amylase-Treated Neutral Detergent Fibre Content (aNDF). Polish Committee for Standardization: Warsaw, Poland, 2007.
- PN-EN ISO 13906; Animal Feeding Stuffs—Determination of Acid Detergent Fibre (adf) and Acid Detergent Lignin (adl) Contents. Polish Committee for Standardization: Warsaw, Poland, 2009.
- PN-EN ISO 18134-1; Solid Biofuels—Determination of Moisture Content—Oven Method—Part 3: Moisture in Sample for Bulk Analysis. Polish Committee for Standardization: Warsaw, Poland, 2015.
- PN-EN ISO 734; Oilseed Meals—Determination of Oil Content—Extraction Method with Hexane (or Light Petroleum). Polish Committee for Standardization: Warsaw, Poland, 2016.
- PN-EN ISO 16948; Solid Biofuels —Determination of Total Carbon, Hydrogen and Nitrogen Content. Polish Committee for Standardization: Warsaw, Polan, 2015.
- PN-EN ISO 16994; Solid Biofuels—Determination of Total Content of Sulfur and Chlorine. Polish Committee for Standardization: Warsaw, Poland, 2016.
- PN-ISO 587; Solid Fuels. Determination of Chlorine Content Using Eschka Mixture. Polish Committee for Standardization: Warsaw, Poland, 2000.
- DIN 53435; Testing of Plastics—Bending Test and Impact Test on Dynstat Test Specimens. German Institute for Standardisation: Berlin, Germany, 2024.
- ISO 868; Determination of Hardness by the Indentation Method Using a Hardness Tester (Shore Hardness). Polish Committee for Standardization: Warsaw, Poland, 2005.
- ISO 306; Plastics|Thermoplastics|Determination of the Vicat Softening Temperature (VST). Polish Committee for Standardization: Warsaw, Poland, 2006.
- ASTM D7309; Standard Test Method for Determining Flammability Characteristics of Plastics and Other Solid Materials Using Microscale Combustion Calorimetry. ASTM International: West Conshohocken, PA, USA, 2021.



| Bio-Sourced Component | Celulose [%] | Hemicellulose [%] | Lignin [%] | Fat [%] | Humidity [%] | C [%] | N [%] | H [%] | S [%] | Cl [%] | Heat of Combustion [J/g] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SH | 30.7 ± 0.2 | 21.6 ± 0.2 | 23.1 ± 0.1 | 2.7 ± 0.1 | 9.8 ± 0.3 | 47.7 ± 0.1 | 0.9 ± 0.0 | 5.6 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 20,081 ± 46 |
| PS | 33.4 ± 0.4 | 14.3 ± 0.3 | 30.2 ± 0.3 | 1.4 ± 0.0 | 6.1 ± 0.1 | 47.4 ± 0.2 | 1.0 ± 0.0 | 6.0 ± 0.1 | 0.1 ± 0.0 | 0.3 ± 0.0 | 19,570 ± 30 |
| Material | Porosity [%] |
|---|---|
| rP | 3.48 |
| APP + PER + M | 3.99 |
| APP + SH | 7.78 |
| APP + PS + SH + M | 8.05 |
| APP + PS + MC | 3.44 |
| MPP + PS | 2.89 |
| MPP + SH | 6.91 |
| MP + PS | 4.67 |
| Material | Tg DSC | Tg DMA | tanδ at Tg DMA | G’ at −20 °C | G’ at 20 °C | G’ at 80 °C |
|---|---|---|---|---|---|---|
| [°C] | [-] | [Pa] | ||||
| rP | 40.9 | 49.0 | 1.003 | 1.51 × 109 | 1.31 × 109 | 1.27 × 107 |
| APP + PER + M | 31.6 | 42.4 | 0.938 | 2.05 × 109 | 1.63 × 109 | 2.04 × 107 |
| APP + SH | 32.0 | 41.3 | 0.782 | 1.96 × 109 | 1.34 × 109 | 2.47 × 107 |
| APP + PS + SH + M | 29.7 | 37.9 | 0.769 | 1.64 × 109 | 9.61 × 108 | 2.13 × 107 |
| APP + PS + MC | 28.7 | 39.1 | 0.839 | 2.08 × 109 | 1.34 × 109 | 1.96 × 107 |
| MPP + PS | 29.4 | 38.0 | 0.801 | 2.09 × 109 | 1.22 × 109 | 2.13 × 107 |
| MPP + SH | 28.1 | 37.0 | 0.785 | 1.92 × 109 | 9.79 × 108 | 2.00 × 107 |
| MP + PS | 26.7 | 34.7 | 0.755 | 2.01 × 109 | 8.79 × 108 | 2.31 × 107 |
| Material | T5% [°C] | DTG1 [°C; %/min] | DTG2 [°C; %/min] | DTG3 [°C; %/min] | DTG4 [°C; %/min] | Residue at 1000 °C in Inert Atmosphere | Residue at 1000 °C in Air |
|---|---|---|---|---|---|---|---|
| rP | 324 | - | - | 363; 0.50 | 410; 0.81 | 7.32 | 0.00 |
| APP + PER + M | 263 | 251; 0.09 | 300; 0.65 | 363; 0.62 | 453; 0.06 | 17.42 | 1.73 |
| APP + SH | 270 | - | 294; 0.52 | 358; 0.90 | 440; 0.07 | 19.85 | 2.15 |
| APP + PS + SH + M | 289 | 242; 0.03 | 295; 0.01 | 342; 0.27 | 426; 1.09 | 15.71 | 8.78 |
| APP + PS + MC | 279 | 241; 0.03 | 291; 0.59 | 354; 0.73 | 452; 0.06 | 19.88 | 1.91 |
| MPP + PS | 305 | 273; 0.04 | - | 343; 1.12 | 425; 0.34 | 12.09 | 3.71 |
| MPP + SH | 287 | 242; 0.03 | - | 342; 1.02 | 423; 0.33 | 11.28 | 0.99 |
| MP + PS | 268 | 260; 0.12 | - | 332; 0.75 363; 0.55 | 423; 0.34 | 13.03 | 2.73 |
| Material | pHRR [W/g] | TpHRR [°C] | THR [kJ/g] | HRC [J/g·K] |
|---|---|---|---|---|
| rP | 252 ± 16 | 417 ± 3 | 22.6 ± 0.3 | 277 ± 23 |
| APP + PER + M | 192 ± 10 | 377 ± 3 | 16.1 ± 0.5 | 211 ± 9 |
| APP + SH | 236 ± 4 | 370 ± 2 | 16.3 ± 0.2 | 260 ± 7 |
| APP + PS + SH + M | 268 ± 18 | 442 ± 2 | 19.9 ± 0.5 | 294 ± 19 |
| APP + PS + MC | 193 ± 11 | 372 ± 2 | 15.5 ± 0.5 | 210 ± 12 |
| MPP + PS | 191 ± 6 | 372 ± 3 | 19.5 ± 0.5 | 209 ± 6 |
| MPP + SH | 186 ± 7 | 373 ± 3 | 19.5 ± 0.4 | 204 ± 10 |
| MP + PS | 138 ± 6 | 384 ± 4 | 18.2 ± 0.8 | 152 ± 5 |
| Ammonium Polyphosphate (APP) | Dipentaerythritol (PER) | Melamine (M) | Melamine Cyanurate (MC) | Melamine Polyphosphate (MPP) | Melamine Phosphate (MP) | |
|---|---|---|---|---|---|---|
| Trade name and supplier | Addforce FR APP201, Walter Thieme Handel GmbH (Stade, Germany) | Addforce® FR Penta D40, Walter Thieme Handel GmbH (Stade, Germany) | Addforce FR FA405, Walter Thieme Handel GmbH (Stade, Germany) | Exflam MCA25, Grolman Sp. z o.o. (Neuss, Germany) | Exflam MPP, Grolman Sp. z o.o. (Neuss, Germany) | Exflam MP, Grolman Sp. z o.o. (Neuss, Germany) |
| Particle size | D98 < 15 μm | D98 = 40 μm | D98 = 40 μm | D98 < 25 μm | D98 < 8 μm | D50 < 10 μm |
| P and N content or another main component [%] | P min. 31% N min. 14% | C10H22O7 85% (grupy hydroksylowe 38–40%) | C3H6N6 99.8% | C6H9N9O3 min. 99.5% | P 42–44% N 12–14% | P min. 12% N 36–38% |
| Designation | APP | PER | M | MC | MPP | MP | SH | PS |
|---|---|---|---|---|---|---|---|---|
| rP | ||||||||
| APP + PER + M | 3 | 1 | 1 | |||||
| APP + SH | 3 | 2 | ||||||
| APP + PS + SH + M | 3 | 1 | 0.5 | 0.5 | ||||
| APP + PS + MC | 3 | 1 | 1 | |||||
| MPP + PS | 3 | 2 | ||||||
| MPP + SH | 3 | 2 | ||||||
| MP + PS | 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salasinska, K.; Barczewski, M.; Kirpluks, M.; Pomilovskis, R.; Sulima, P.; Michałowski, S.; Mietliński, P.; Przyborowski, J.A.; Boczkowska, A. Bio-Based Flame-Retardant Systems for Polymers Obtained via Michael 1,4-Addition. Molecules 2025, 30, 2556. https://doi.org/10.3390/molecules30122556
Salasinska K, Barczewski M, Kirpluks M, Pomilovskis R, Sulima P, Michałowski S, Mietliński P, Przyborowski JA, Boczkowska A. Bio-Based Flame-Retardant Systems for Polymers Obtained via Michael 1,4-Addition. Molecules. 2025; 30(12):2556. https://doi.org/10.3390/molecules30122556
Chicago/Turabian StyleSalasinska, Kamila, Mateusz Barczewski, Mikelis Kirpluks, Ralfs Pomilovskis, Paweł Sulima, Sławomir Michałowski, Patryk Mietliński, Jerzy Andrzej Przyborowski, and Anna Boczkowska. 2025. "Bio-Based Flame-Retardant Systems for Polymers Obtained via Michael 1,4-Addition" Molecules 30, no. 12: 2556. https://doi.org/10.3390/molecules30122556
APA StyleSalasinska, K., Barczewski, M., Kirpluks, M., Pomilovskis, R., Sulima, P., Michałowski, S., Mietliński, P., Przyborowski, J. A., & Boczkowska, A. (2025). Bio-Based Flame-Retardant Systems for Polymers Obtained via Michael 1,4-Addition. Molecules, 30(12), 2556. https://doi.org/10.3390/molecules30122556











