Biodegradable Contact Lenses for Targeted Ocular Drug Delivery: Recent Advances, Clinical Applications, and Translational Perspectives
Abstract
1. Introduction
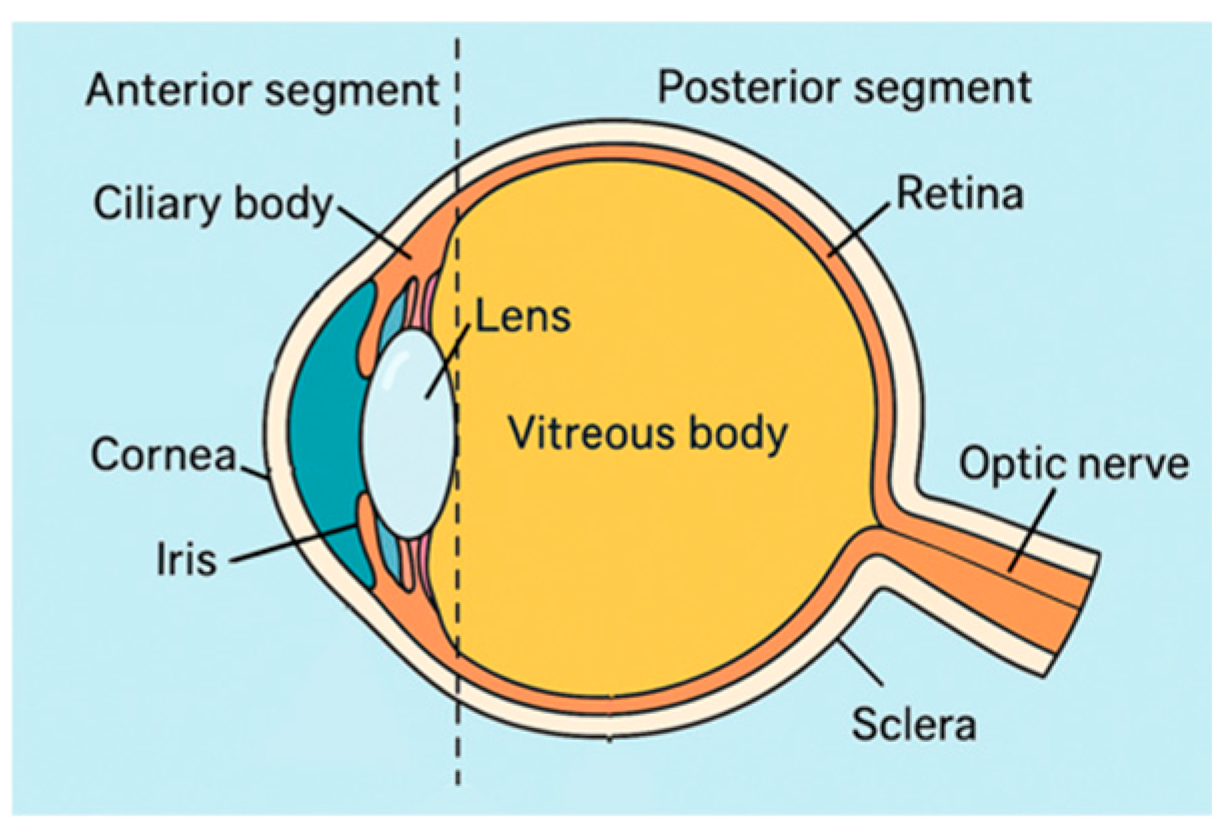
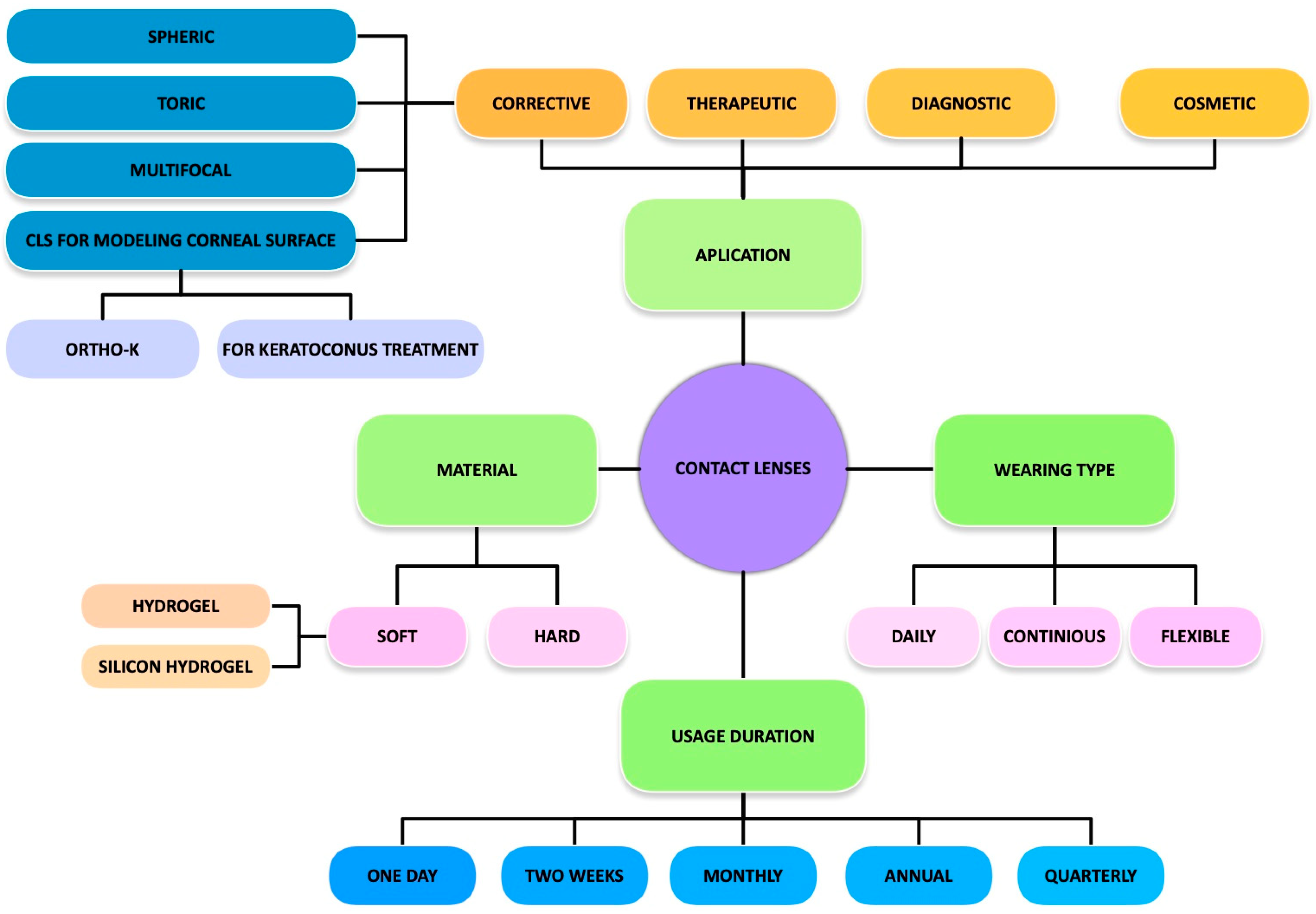
1.1. Modern Contact Lens Classification
1.2. DECL Performance and Design Considerations
1.3. Scope and Structure of This Review
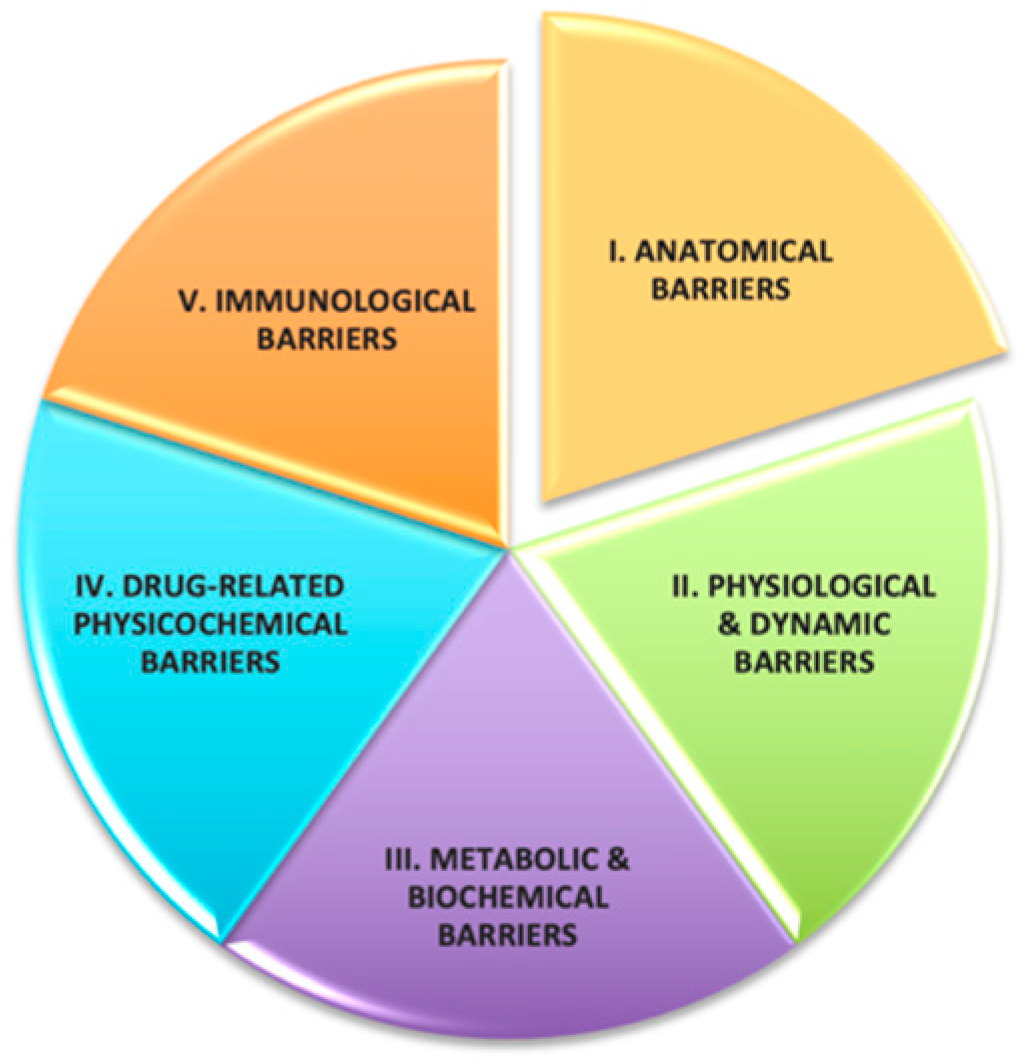
2. Materials and Methods
2.1. Materials Used in DMCLs
2.2. Biopolymers
2.2.1. Synthetic Biodegradable Polymers
2.2.2. Natural Biodegradable Polymers
2.2.3. Emerging Biodegradable Polymers
3. Controlled Drug Release Strategies
4. Drug Delivery Systems Based on Polymeric Materials
5. Recent Advances in Biopolymer-Based Contact Lenses
5.1. Nanowafer-Based Contact Lenses
5.2. Microneedle-Based Contact Lenses
5.3. Personalized and Self-Medication Technologies
6. Biopolymer-Based Contact Lenses for Ocular Drug Delivery
6.1. Corneal Disorders
6.2. Bacterial, Fungal, and Viral Keratitis
6.3. Noninfectious Keratitis and Postoperative Inflammation
6.4. Corneal Wound Healing
6.5. Keratoconus and Myopia
6.6. Glaucoma
6.7. Cataract Surgery and Postoperative Inflammation
6.8. Proliferative Ocular Diseases
6.9. Ocular Cystinosis
6.10. Uveitis
6.11. Color Vision Deficiency (CVD)
7. Advanced Coating and Loading Strategies
7.1. Biopolymer-Based Coatings for Controlled Drug Release and Antimicrobial Protection
7.2. Asymmetric Drug Loading Strategies
8. Biodegradable Polymers Beyond Contact Lenses: Intraocular Drug Delivery Systems
9. Technologically Enhanced Contact Lenses
9.1. Smart Contact Lenses
9.2. Diabetic-Eye Disease Monitoring and Therapy
9.3. Intraocular Pressure Monitoring Lenses
9.4. Intelligent Therapeutic Drug Delivery Platforms
9.5. Digital or Electronic Contact Lenses
9.6. Clinical Application of Continuous IOP Monitoring
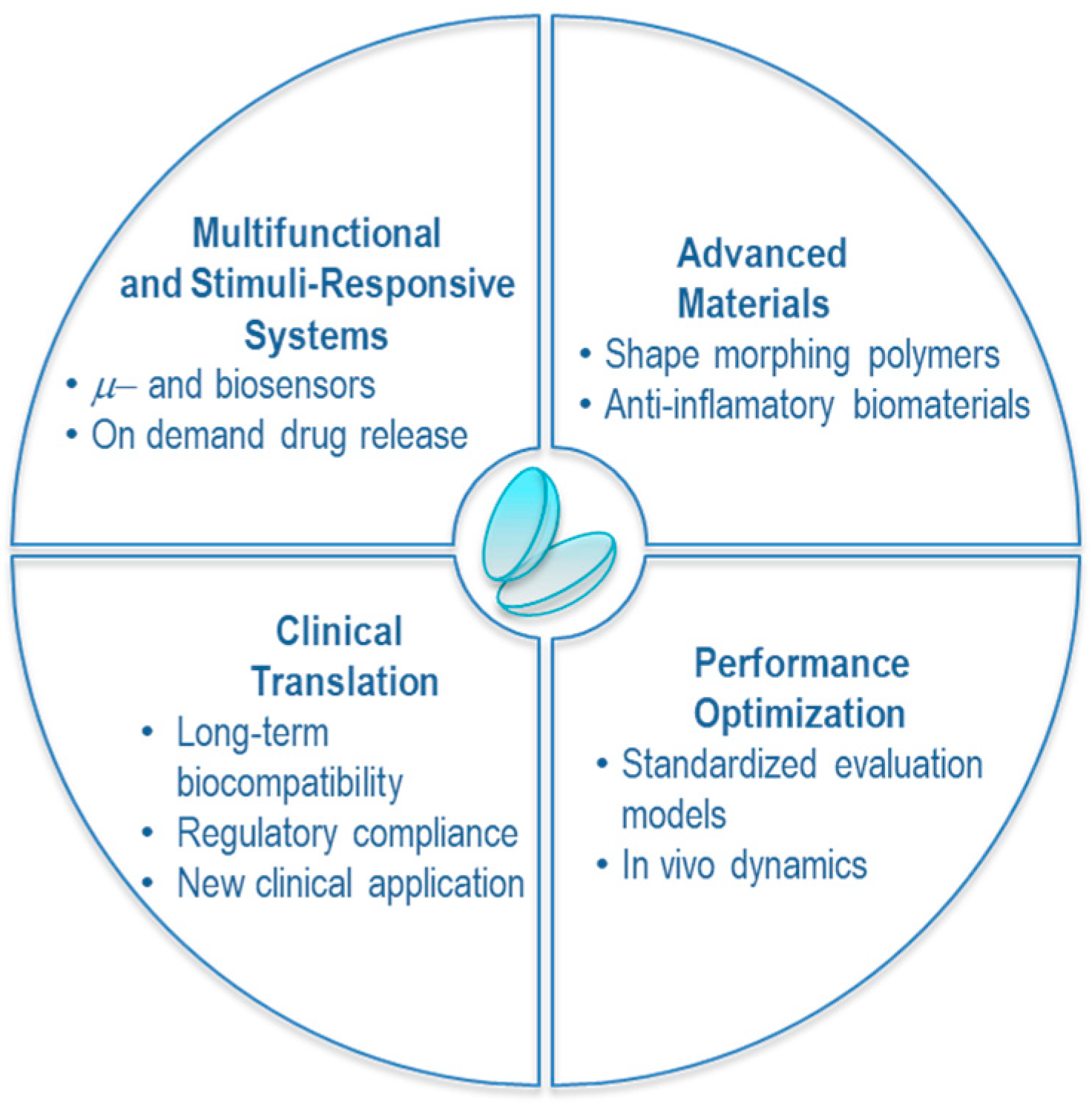
10. Outlook
11. Clinical Translation and Future Implementation
12. Conclusions and Perspectives
- Robust preclinical models that simulate dynamic ocular environments.
- Standardized protocols for evaluating long-term safety, biocompatibility, and degradation.
- Scalable, cost-effective manufacturing workflows compliant with regulatory standards.
- Customizable designs tailored to the patient’s ocular anatomy, pharmacogenomic profile, and therapeutic timeline.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaudana, R.; Jwala, J.; Boddu, S.H.S.; Mitra, A.K. Recent perspectives in ocular drug delivery. Pharm. Res. 2009, 26, 1197–1216. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, X.; Chen, C.; Wang, Q.; Chen, X. Polydopamine-modified therapeutic contact lenses for enhanced ocular drug delivery. Int. J. Mol. Sci. 2023, 24, 12976. [Google Scholar] [CrossRef]
- Kim, J.; Conway, A.; Chauhan, A. Extended delivery of ophthalmic drugs by silicone hydrogel contact lenses. Biomaterials 2008, 29, 2259–2269. [Google Scholar] [CrossRef]
- Efron, N. Rigid Lens Materials. In Contact Lens Practice; Elsevier: Amsterdam, The Netherlands, 2024; pp. 122–130.e1. [Google Scholar] [CrossRef]
- Walline, J.J.; Robboy, M.W.; Hilmantel, G.; Tarver, M.E.; Afshari, N.A.; Dhaliwal, D.K.; Morse, C.L.; Quinn, C.J.; Repka, M.X.; Eydelman, M.B. Food and Drug Administration, American Academy of Ophthalmology, American Academy of Optometry, American Association for Pediatric Ophthalmology and Strabismus, American Optometric Association, American Society of Cataract and Refractive Surgery, and Contact Lens Association of Ophthalmologists Co-Sponsored Workshop: Controlling the Progression of Myopia: Contact Lenses and Future Medical Devices. Eye Contact Lens Sci. Clin. Pract. 2018, 44, 205–211. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, Z.; Mo, F.; Wang, Y. A Review of Functional Hydrogels for Flexible Chemical Sensors. Adv. Sens. Res. 2024, 3, 2300021. [Google Scholar] [CrossRef]
- Sarac, B.; Yücer, S.; Sahin, H.; Unal, M.; Ciftci, F. Wearable and implantable bioelectronic: Biosensing contact lens and applications. Chem. Eng. J. 2024, 491, 152016. [Google Scholar] [CrossRef]
- Dhara, M. Polymer hydrogels: Classification and recent advances. J. Macromol. Sci. Part A 2024, 61, 265–288. [Google Scholar] [CrossRef]
- Dar, U.A.; Tantary, A.A.; Ali, A. Polysaccharide-based hydrogels: History and chronological developments. In Polysaccharides-Based Hydrogels; Elsevier: Amsterdam, The Netherlands, 2024; pp. 21–42. [Google Scholar] [CrossRef]
- Nicolson, P.C.; Vogt, J. Soft contact lens polymers: An evolution. Biomaterials 2001, 22, 3273–3283. [Google Scholar] [CrossRef]
- Baghban, R.; Talebnejad, M.R.; Meshksar, A.; Heydari, M.; Khalili, M.R. Recent advancements in nanomaterial-laden contact lenses for diagnosis and treatment of glaucoma, review and update. J. Nanobiotechnol. 2023, 21, 402. [Google Scholar] [CrossRef]
- Higashide, T.; Udagawa, S.; Nakazawa, K.; Yamashita, Y.; Tsuchiya, S.; Ohkubo, S.; Sugiyama, K. Prediction of glaucoma progression by 24-h contact lens sensor profile in patients with normal-tension glaucoma. Sci. Rep. 2024, 14, 21564. [Google Scholar] [CrossRef] [PubMed]
- Abdulamier, A.A.; Shaker, L.M.; Al-Amiery, A.A. Advancements in the chemistry of contact Lenses: Innovations and applications. Results Chem. 2024, 12, 101872. [Google Scholar] [CrossRef]
- ISO 11980:2012; Ophthalmic Optics—Contact Lenses and Contact Lens Care Products—Guidance for Clinical Investigations. International Organization for Standardization: Geneva, Switzerland, 2012.
- U.S. Food and Drug Administration. Hydrogen Peroxide-Based Contact Lens Care Products: Consumer Labeling Recommendations—Premarket Notification (510(k)) Submissions; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2023.
- U.S. Food and Drug Administration. Soft (Hydrophilic) Daily Wear Contact Lenses—Performance Criteria for Safety and Performance Based Pathway; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2023.
- European Parliament. Council of the European Union Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC (Text with EEA Relevance); European Parliament: Brussels, Belgium, 2017. [Google Scholar]
- Alqurashi, A.; Abouaini, R.; Khashan, K.; Albarqi, Z. Regulatory considerations in the development of drug-eluting contact lenses. Int. J. Pharm. 2022, 622, 121822. [Google Scholar]
- Gulsen, D.; Chauhan, A. Ophthalmic Drug Delivery through Contact Lenses. Investig. Opthalmol. Vis. Sci. 2004, 45, 2342. [Google Scholar] [CrossRef]
- Ciolino, J.B.; Stefanescu, C.F.; Ross, A.E.; Salvador-Culla, B.; Cortez, P.; Ford, E.M.; Wymbs, K.A.; Sprague, S.L.; Mascoop, D.R.; Rudina, S.S.; et al. In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials 2014, 35, 432–439. [Google Scholar] [CrossRef]
- Pereira-da-Mota, A.F.; Phan, C.-M.; Concheiro, A.; Jones, L.; Alvarez-Lorenzo, C. Testing drug release from medicated contact lenses: The missing link to predict in vivo performance. J. Control. Release 2022, 343, 672–702. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Types of Contact Lenses; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2025.
- Lim, L.; Lim, E.W.L. Therapeutic Contact Lenses in the Treatment of Corneal and Ocular Surface Diseases—A Review. Asia-Pac. J. Ophthalmol. 2020, 9, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.B. A new drug delivery system for the eye. IMS Ind. Med. Surg. 1971, 40, 11–13. [Google Scholar]
- Maurice, D. Prolonged release systems and topically applied Drugs. Sight-Sav. Rev. 1972, 42, 42–50. [Google Scholar]
- Maddox, Y.T.; Bernstein, H.N. An evaluation of the bronite hydrophilic contact lens for use in a drug delivery system. Contact Lens J. 1972, 4, 789–790. [Google Scholar]
- Hynes, A. The Promise of Drug-Eluting Contact Lenses. Mod. Optom. 2024, 11–13. [Google Scholar]
- Lanier, O.L.; Manfre, M.G.; Bailey, C.; Liu, Z.; Sparks, Z.; Kulkarni, S.; Chauhan, A. Review of Approaches for Increasing Ophthalmic Bioavailability for Eye Drop Formulations. AAPS PharmSciTech 2021, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; De Marco, I. Contact Lenses as Ophthalmic Drug Delivery Systems: A Review. Polymers 2021, 13, 1102. [Google Scholar] [CrossRef]
- Chaudhari, P.; Ghate, V.M.; Lewis, S.A. Next-generation contact lenses: Towards bioresponsive drug delivery and smart technologies in ocular therapeutics. Eur. J. Pharm. Biopharm. 2021, 161, 80–99. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Wuchte, L.D.; DiPasquale, S.A.; Byrne, M.E. In vivo drug delivery via contact lenses: The current state of the field from origins to present. J. Drug Deliv. Sci. Technol. 2021, 63, 102413. [Google Scholar] [CrossRef]
- Li, Q.; Ma, C.; Ma, Y.; Ma, Y.; Mao, Y.; Meng, Z. Sustained bimatoprost release using gold nanoparticles laden contact lenses. J. Biomater. Sci. Polym. Ed. 2021, 32, 1618–1634. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.S.; Carrasquillo, K.G.; Cottrell, P.D.; Fernández-Velázquez, F.J.; Gil-Cazorla, R.; Jalbert, I.; Pucker, A.D.; Riccobono, K.; Robertson, D.M.; Szczotka-Flynn, L.; et al. BCLA CLEAR—Medical use of contact lenses. Contact Lens Anterior Eye 2021, 44, 289–329. [Google Scholar] [CrossRef]
- Rykowska, I.; Nowak, I.; Nowak, R. Soft contact lenses as drug delivery systems: A review. Molecules 2021, 26, 5577. [Google Scholar] [CrossRef]
- Mofidfar, M.; Abdi, B.; Ahadian, S.; Mostafavi, E.; Desai, T.A.; Abbasi, F.; Sun, Y.; Manche, E.E.; Ta, C.N.; Flowers, C.W. Drug delivery to the anterior segment of the eye: A review of current and future treatment strategies. Int. J. Pharm. 2021, 607, 120924. [Google Scholar] [CrossRef]
- Allyn, M.M.; Luo, R.H.; Hellwarth, E.B.; Swindle-Reilly, K.E. Considerations for Polymers Used in Ocular Drug Delivery. Front. Med. 2022, 8, 787644. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, S.; Li, J.; Falcone, N.; Cui, Q.; Shah, S.; Hartel, M.C.; Yu, N.; Young, P.; de Barros, N.R.; et al. Lab-on-a-Contact Lens: Recent Advances and Future Opportunities in Diagnostics and Therapeutics. Adv. Mater. 2022, 34, 2108389. [Google Scholar] [CrossRef] [PubMed]
- Raina, N.; Pahwa, R.; Bhattacharya, J.; Paul, A.K.; Nissapatorn, V.; de Lourdes Pereira, M.; Oliveira, S.M.R.; Dolma, K.G.; Rahmatullah, M.; Wilairatana, P.; et al. Drug Delivery Strategies and Biomedical Significance of Hydrogels: Translational Considerations. Pharmaceutics 2022, 14, 574. [Google Scholar] [CrossRef] [PubMed]
- Tsung, T.-H.; Chen, Y.-H.; Lu, D.-W. Updates on Biodegradable Formulations for Ocular Drug Delivery. Pharmaceutics 2023, 15, 734. [Google Scholar] [CrossRef]
- Zhao, L.; Song, J.; Du, Y.; Ren, C.; Guo, B.; Bi, H. Therapeutic applications of contact lens-based drug delivery systems in ophthalmic diseases. Drug Deliv. 2023, 30, 2219419. [Google Scholar] [CrossRef]
- Aljaberi, H.A.; Noori, Z.T.M. Contact Lens as Drug Delivery System for Glaucoma Treatment: A Review. Open Ophthalmol. J. 2023, 17, e187494452212300. [Google Scholar] [CrossRef]
- Yuan, W.; Zhao, F.; Liu, X.; Xu, J. Development of corneal contact lens materials and current clinical application of contact lenses: A review. Biointerphases 2023, 18, 050801. [Google Scholar] [CrossRef]
- Sah, R.; Sharma, N.; Priyadarshini, K.; Titiyal, J.S. Contact lenses for the treatment of ocular surface diseases. Indian J. Ophthalmol. 2023, 71, 1135–1141. [Google Scholar] [CrossRef]
- Lal Goyal, J.; Choudhary, A.; Singh, P.; Gupta, S. Contact lenses as ophthalmic drug delivery systems—The future of treatment for ocular infection and injuries—A Review. Afr. J. Biomed. Res. 2024, 27, 4S. [Google Scholar]
- Chang, W.-H.; Liu, P.-Y.; Lin, M.-H.; Lu, C.-J.; Chou, H.-Y.; Nian, C.-Y.; Jiang, Y.-T.; Hsu, Y.-H.H. Applications of Hyaluronic Acid in Ophthalmology and Contact Lenses. Molecules 2021, 26, 2485. [Google Scholar] [CrossRef]
- Wang, Y.; Jacobs, D.S. Role of therapeutic contact lenses in management of corneal disease. Curr. Opin. Ophthalmol. 2022, 33, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yadav, S. Contact lenses as ophthalmic drug delivery system. Int. Res. J. Mod. Eng. Technol. Sci. 2023, 5, 3934–3942. [Google Scholar] [CrossRef]
- Nguyen, D.C.T.; Dowling, J.; Ryan, R.; McLoughlin, P.; Fitzhenry, L. Pharmaceutical-loaded contact lenses as an ocular drug delivery system: A review of critical lens characterization methodologies with reference to ISO standards. Contact Lens Anterior Eye 2021, 44, 101487. [Google Scholar] [CrossRef] [PubMed]
- Ms Rynjah, H.; Kaur, H.; Zaman, I.; Rahman, S.S. S.S. Shaping the future of vision: A comprehensive review of optical innovations. J. Popul. Ther. Clin. Pharmacol. 2025, 32, 46–49. [Google Scholar] [CrossRef]
- Hajirasouliha, E.; Zandi, M.; Hashemi Tabatabaei, M.; Zarrinbakhsh, P. Ocular contact lenses: Smart materials for biomedical applications. Polym. Bull. 2024, 81, 7791–7832. [Google Scholar] [CrossRef]
- Saini, A.; Sharma, M.; Singh, I.; Swami, R. From Vision Correction to Drug Delivery: Unraveling the Potential of Therapeutic Contact Lens. Curr. Drug Deliv. 2025, 22, 140–159. [Google Scholar] [CrossRef]
- Hatamie, S.; Shih, P.; Chen, B.; Hatami, N. Carbohydrate Polymers, Polymeric Nano Drugs, and Nanoparticles Are Used for Advanced Drug Delivery and Therapeutics in Ocular Diseases. Polym. Adv. Technol. 2025, 36, e70094. [Google Scholar] [CrossRef]
- Borgohain, R.; Patel, P.N. Polymeric Membranes in Contact Lens Technology for Glaucoma Treatment: Breakthroughs, Obstacles, and Emerging Opportunities. Polym. Adv. Technol. 2025, 36, e70135. [Google Scholar] [CrossRef]
- Saxena, A.; Mishra, D.; Singh, K.; George, S.D. Microfluidic contact lens: Fabrication approaches and applications. Microsyst. Nanoeng. 2025, 11, 59. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, H.; Ke, L.; Liu, M.; Wang, C.; Jun Loh, X.; Li, Z.; Wu, Y. Recent Advances in New Copolymer Hydrogel-Formed Contact Lenses for Ophthalmic Drug Delivery. ChemNanoMat 2021, 7, 564–579. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Rykowska, I.; Nowak, I.; Nowak, R. Drug-Eluting Stents and Balloons-Materials, Structure Designs, and Coating Techniques: A Review. Molecules 2020, 25, 4624. [Google Scholar] [CrossRef] [PubMed]
- Shaker, L.M.; Al-Amiery, A.; Isahak, W.N.R.W. Revolutionizing contact lens manufacturing: Exploring cutting-edge techniques and innovations for enhanced vision and comfort. Int. J. Low-Carbon Technol. 2024, 19, 359–385. [Google Scholar] [CrossRef]
- Luo, L.-J.; Nguyen, D.D.; Huang, C.-C.; Lai, J.-Y. Therapeutic hydrogel sheets programmed with multistage drug delivery for effective treatment of corneal abrasion. Chem. Eng. J. 2022, 429, 132409. [Google Scholar] [CrossRef]
- Pillay, R.; Hansraj, R.; Rampersad, N. Historical Development, Applications and Advances in Materials Used in Spectacle Lenses and Contact Lenses. Clin. Optom. 2020, 12, 157–167. [Google Scholar] [CrossRef]
- Sheardown, H.; Hicks, E.A.; Tayab, A.; Muirhead, B. Ophthalmologic Applications. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1135–1152. [Google Scholar] [CrossRef]
- Lohmann, V.; Jones, G.R.; Truong, N.P.; Anastasaki, A. The thermodynamics and kinetics of depolymerization: What makes vinyl monomer regeneration feasible? Chem. Sci. 2024, 15, 832–853. [Google Scholar] [CrossRef]
- Chatterjee, S.; Upadhyay, P.; Mishra, M.; Srividya, M.; Akshara, M.R.; Kamali, N.; Zaidi, Z.S.; Iqbal, S.F.; Misra, S.K. Advances in chemistry and composition of soft materials for drug releasing contact lenses. RSC Adv. 2020, 10, 36751–36777. [Google Scholar] [CrossRef]
- Imperiale, J.C.; Acosta, G.B.; Sosnik, A. Polymer-based carriers for ophthalmic drug delivery. J. Control. Release 2018, 285, 106–141. [Google Scholar] [CrossRef]
- Weiner, A.L. Drug Delivery Systems in Ophthalmic Applications. In Ocular Therapeutics; Elsevier: Amsterdam, The Netherlands, 2008; pp. 7–43. [Google Scholar] [CrossRef]
- Li, Z.; Liu, M.; Ke, L.; Wang, L.-J.; Wu, C.; Li, C.; Li, Z.; Wu, Y.-L. Flexible polymeric nanosized micelles for ophthalmic drug delivery: Research progress in the last three years. Nanoscale Adv. 2021, 3, 5240–5254. [Google Scholar] [CrossRef]
- Kim, H.M.; Woo, S.J. Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics 2021, 13, 108. [Google Scholar] [CrossRef]
- Moiseev, R.V.; Kaldybekov, D.B.; Filippov, S.K.; Radulescu, A.; Khutoryanskiy, V.V. Maleimide-Decorated PEGylated Mucoadhesive Liposomes for Ocular Drug Delivery. Langmuir 2022, 38, 13870–13879. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Caballero-George, C.; Marin, E.; Briceño, M.I. Critical evaluation of biodegradable polymers used in nanodrugs. Int. J. Nanomed. 2013, 8, 3071–3091. [Google Scholar] [CrossRef]
- Baino, F.; Kargozar, S. Regulation of the Ocular Cell/Tissue Response by Implantable Biomaterials and Drug Delivery Systems. Bioengineering 2020, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.R.; Park, S.-K.; Kim, E.J.; Kim, D.-K.; Yoon, Y.C.; Myung, D.; Lee, H.J.; Na, K.-S. Dexamethasone acetate loaded poly(ε-caprolactone) nanofibers for rat corneal chemical burn treatment. Sci. Rep. 2024, 14, 21806. [Google Scholar] [CrossRef]
- Kumar Thota, V.; Dhanraj, S. Synthesis, characterization and solution behaviour of culturally diversified organization. Mater. Today Proc. 2023, 81, 1105–1108. [Google Scholar] [CrossRef]
- Souto, E.B.; Dias-Ferreira, J.; López-Machado, A.; Ettcheto, M.; Cano, A.; Camins Espuny, A.; Espina, M.; Garcia, M.L.; Sánchez-López, E. Advanced Formulation Approaches for Ocular Drug Delivery: State-Of-The-Art and Recent Patents. Pharmaceutics 2019, 11, 460. [Google Scholar] [CrossRef]
- Sovadinova, I.; Palermo, E.F.; Urban, M.; Mpiga, P.; Caputo, G.A.; Kuroda, K. Activity and Mechanism of Antimicrobial Peptide-Mimetic Amphiphilic Polymethacrylate Derivatives. Polymers 2011, 3, 1512–1532. [Google Scholar] [CrossRef]
- Ali, U.; Juhanni, K.; Karim, A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Brannigan, R.P.; Khutoryanskiy, V.V. Synthesis and evaluation of mucoadhesive acryloyl-quaternized PDMAEMA nanogels for ocular drug delivery. Colloids Surf. B Biointerfaces 2017, 155, 538–543. [Google Scholar] [CrossRef]
- Jung, H.J.; Chauhan, A. Temperature sensitive contact lenses for triggered ophthalmic drug delivery. Biomaterials 2012, 33, 2289–2300. [Google Scholar] [CrossRef] [PubMed]
- González-Chomón, C.; Silva, M.; Concheiro, A.; Alvarez-Lorenzo, C. Biomimetic contact lenses eluting olopatadine for allergic conjunctivitis. Acta Biomater. 2016, 41, 302–311. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Lai, J.-Y. Advancing the stimuli response of polymer-based drug delivery systems for ocular disease treatment. Polym. Chem. 2020, 11, 6988–7008. [Google Scholar] [CrossRef]
- Yavuz, B.; Bozdağ Pehlivan, S.; Ünlü, N. Dendrimeric Systems and Their Applications in Ocular Drug Delivery. Sci. World J. 2013, 2013, 732340. [Google Scholar] [CrossRef] [PubMed]
- Lancina, M.G.; Yang, H. Dendrimers for ocular drug delivery. Can. J. Chem. 2017, 95, 897–902. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural Polymers. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–89. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Kathuria, A.; Shamloo, K.; Jhanji, V.; Sharma, A. Categorization of Marketed Artificial Tear Formulations Based on Their Ingredients: A Rational Approach for Their Use. J. Clin. Med. 2021, 10, 1289. [Google Scholar] [CrossRef]
- Dutescu, R.M.; Panfil, C.; Schrage, N. Comparison of the effects of various lubricant eye drops on the in vitro rabbit corneal healing and toxicity. Exp. Toxicol. Pathol. 2017, 69, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, X.; Yang, W.; He, K.; Ye, X. Injectable in situ cross-linking hyaluronic acid/carboxymethyl cellulose based hydrogels for drug release. J. Biomater. Sci. Polym. Ed. 2018, 29, 1643–1655. [Google Scholar] [CrossRef]
- Stefanache, A.; Lungu, I.I.; Anton, N.; Damir, D.; Gutu, C.; Olaru, I.; Plesea Condratovici, A.; Duceac (Covrig), M.; Constantin, M.; Calin, G.; et al. Chitosan Nanoparticle-Based Drug Delivery Systems: Advances, Challenges, and Future Perspectives. Polymers 2025, 17, 1453. [Google Scholar] [CrossRef]
- Han, H.; Li, S.; Xu, M.; Zhong, Y.; Fan, W.; Xu, J.; Zhou, T.; Ji, J.; Ye, J.; Yao, K. Polymer- and lipid-based nanocarriers for ocular drug delivery: Current status and future perspectives. Adv. Drug Deliv. Rev. 2023, 196, 114770. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, R.; Zarembinski, T.I.; Doty, N.; Jiang, C.; Regatieri, C.; Zhang, X.; Young, M.J. The Application of Hyaluronic Acid Hydrogels to Retinal Progenitor Cell Transplantation. Tissue Eng. Part A 2013, 19, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.; Kim, S.; Huh, J.; Koh, M.Y.; Hwang, J.Y.; Kang, B.; Li, X.; Lee, M.S.; Lee, H.; Kim, H.M.; et al. Self-sealing hyaluronic acid-coated 30-gauge intravitreal injection needles for preventing vitreous and drug reflux through needle passage. Sci. Rep. 2021, 11, 16996. [Google Scholar] [CrossRef] [PubMed]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Demirci, A.; Catchmark, J.M. Pullulan: Biosynthesis, production, and applications. Appl. Microbiol. Biotechnol. 2011, 92, 29–44. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Khanda, S.M. Pullulan: An exopolysaccharide and its various applications. Carbohydr. Polym. 2013, 95, 540–549. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Recent insights on applications of pullulan in tissue engineering. Carbohydr. Polym. 2016, 153, 455–462. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef]
- Wong, F.S.Y.; Tsang, K.K.; Chu, A.M.W.; Chan, B.P.; Yao, K.M.; Lo, A.C.Y. Injectable cell-encapsulating composite alginate-collagen platform with inducible termination switch for safer ocular drug delivery. Biomaterials 2019, 201, 53–67. [Google Scholar] [CrossRef]
- Abedin Zadeh, M.; Khoder, M.; Al-Kinani, A.A.; Younes, H.M.; Alany, R.G. Retinal cell regeneration using tissue engineered polymeric scaffolds. Drug Discov. Today 2019, 24, 1669–1678. [Google Scholar] [CrossRef]
- Belin, M.W.; Lim, L.; Rajpal, R.K.; Hafezi, F.; Gomes, J.A.P.; Cochener, B. Corneal Cross-Linking: Current USA Status: Report From the Cornea Society. Cornea 2018, 37, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Batul, R.; Tamanna, T.; Khaliq, A.; Yu, A. Recent progress in the biomedical applications of polydopamine nanostructures. Biomater. Sci. 2017, 5, 1204–1229. [Google Scholar] [CrossRef]
- Buzalewicz, I.; Kaczorowska, A.; Fijałkowski, W.; Pietrowska, A.; Matczuk, A.K.; Podbielska, H.; Wieliczko, A.; Witkiewicz, W.; Jędruchniewicz, N. Quantifying the Dynamics of Bacterial Biofilm Formation on the Surface of Soft Contact Lens Materials Using Digital Holographic Tomography to Advance Biofilm Research. Int. J. Mol. Sci. 2024, 25, 2653. [Google Scholar] [CrossRef]
- Zegans, M.E.; Becker, H.I.; Budzik, J.; O’Toole, G. The Role of Bacterial Biofilms in Ocular Infections. DNA Cell Biol. 2002, 21, 415–420. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, X.; Tang, J.; Han, Y.; Lin, Q. Drug-Eluting Hydrophilic Coating Modification of Intraocular Lens via Facile Dopamine Self-Polymerization for Posterior Capsular Opacification Prevention. ACS Biomater. Sci. Eng. 2021, 7, 1065–1073. [Google Scholar] [CrossRef]
- Jiang, P.; Choi, A.; Swindle-Reilly, K.E. Controlled release of anti-VEGF by redox-responsive polydopamine nanoparticles. Nanoscale 2020, 12, 17298–17311. [Google Scholar] [CrossRef]
- Poinard, B.; Kamaluddin, S.; Tan, A.Q.Q.; Neoh, K.G.; Kah, J.C.Y. Polydopamine Coating Enhances Mucopenetration and Cell Uptake of Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 4777–4789. [Google Scholar] [CrossRef]
- Lin, M.; Hu, Y.; An, H.; Guo, T.; Gao, Y.; Peng, K.; Zhao, M.; Zhang, X.; Zhou, H. Silk fibroin-based biomaterials for disc tissue engineering. Biomater. Sci. 2023, 11, 749–776. [Google Scholar] [CrossRef]
- Sodhi, S.; Panitch, A. Glycated Poly(glycerol sebacate) for Ophthalmic Drug Delivery. J. Biomed. Mater. Res. A 2023, 111, 689–699. [Google Scholar]
- Amorim, J.D.P.; Leite, J.L.G.C.; Barros-Timmons, A.; Silva, T.H. Polyhydroxyalkanoates: Promising polymers for regenerative medicine. Int. J. Biol. Macromol. 2022, 209, 1288–1301. [Google Scholar]
- Li, Y.; Yang, X.; Gao, D. Biodegradable poly(trimethylene carbonate) copolymers for ocular implants. Mater. Sci. Eng. C 2021, 126, 112122. [Google Scholar]
- Loh, X.J.; Peh, P.; Liao, S.; Sng, C.; Li, J. Controlled drug delivery from biodegradable poly(ester amide)s. Polym. Chem. 2022, 13, 176–186. [Google Scholar]
- He, Y.; Li, S.; Li, J.; Ren, X.; Yin, J. Zwitterionic hydrogels for extended wear contact lenses. J. Colloid Interface Sci. 2023, 629, 634–643. [Google Scholar]
- Yin, Y.; Zhang, Z.; Yang, M. Methacrylated hyaluronic acid hydrogels for ophthalmic applications. Carbohydr. Polym. 2022, 275, 118706. [Google Scholar] [CrossRef]
- Du, Z.; Wu, Y.; Chen, H.; Li, X. Nanogels in ocular drug delivery: From preclinical to clinical investigation. Adv. Drug Deliv. Rev. 2024, 200, 114034. [Google Scholar]
- Jiang, Y.; Zhang, H.; Wang, S. Polydopamine-functionalized drug carriers for ocular disease therapy. Molecules 2021, 26, 3289. [Google Scholar]
- Mundargi, R.; Babu, V.; Rangaswamy, V.; Patel, P.; Aminabhavi, T. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J. Control. Release 2008, 125, 193–209. [Google Scholar] [CrossRef]
- Sultana, N.; Akhter, S. Biodegradable polymers for ocular drug delivery. J. Drug Deliv. Sci. Technol. 2013, 23, 211–217. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Kapoor, Y.; Thomas, J.C.; Tan, G.; John, V.T.; Chauhan, A. Surfactant-laden soft contact lenses for extended delivery of ophthalmic drugs. Biomaterials 2009, 30, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gan, L.; Qin, Z.; Zhu, J.; Zhu, Q.; Zhang, X.; Gan, Y. Alginate-based ocular drug delivery systems: From preclinical development to clinical application. Drug Discov. Today 2022, 27, 2172–2183. [Google Scholar] [CrossRef]
- Shen, J.; Burgess, D.J. Accelerated in-vitro release testing of implantable PLGA microsphere/PVA hydrogel composite coatings. Int. J. Pharm. 2012, 422, 341–348. [Google Scholar] [CrossRef]
- Rwei, A.Y.; Wang, W.; Kohane, D.S. Photoresponsive nanoparticles for drug delivery. Nano Today 2015, 10, 451–467. [Google Scholar] [CrossRef]
- Refojo, M.F.; Leong, F.L. Poly(hydroxyethyl methacrylate) hydrogels for drug delivery applications. J. Biomater. Appl. 1985, 1, 433–447. [Google Scholar]
- Ai, H.; Jones, S.A.; de Villiers, M.M. Layer-by-layer assembly for drug delivery and related applications. Ther. Deliv. 2013, 4, 881–898. [Google Scholar] [CrossRef][Green Version]
- Hoshino, Y.; Kodama, T.; Okahata, Y.; Shea, K.J. Peptide-imprinted polymer nanoparticles: A plastic antibody. J. Am. Chem. Soc. 2008, 130, 15242–15243. [Google Scholar] [CrossRef]
- Yu, B.; Li, X.-R.; Zhang, X.-M. Mesenchymal stem cell-derived extracellular vesicles as a new therapeutic strategy for ocular diseases. World J. Stem Cells 2020, 12, 178–187. [Google Scholar] [CrossRef]
- Holland, E.J.; Darvish, M.; Nichols, K.K.; Jones, L.; Karpecki, P.M. Efficacy of topical ophthalmic drugs in the treatment of dry eye disease: A systematic literature review. Ocul. Surf. 2019, 17, 412–423. [Google Scholar] [CrossRef]
- Bajracharya, R.; Song, J.G.; Back, S.Y.; Han, H.-K. Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery. Comput. Struct. Biotechnol. J. 2019, 17, 1290–1308. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Pal, D.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Mitra, A.K. Ocular delivery of proteins and peptides: Challenges and novel formulation approaches. Adv. Drug Deliv. Rev. 2018, 126, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Shin, C.S.; Wang, C.; Pflugfelder, S.C.; Acharya, G.; De Paiva, C.S. Dexamethasone Drug Eluting Nanowafers Control Inflammation in Alkali-Burned Corneas Associated with Dry Eye. Investig. Opthalmol. Vis. Sci. 2016, 57, 3222. [Google Scholar] [CrossRef]
- Coursey, T.G.; Henriksson, J.T.; Marcano, D.C.; Shin, C.S.; Isenhart, L.C.; Ahmed, F.; De Paiva, C.S.; Pflugfelder, S.C.; Acharya, G. Dexamethasone nanowafer as an effective therapy for dry eye disease. J. Control. Release 2015, 213, 168–174. [Google Scholar] [CrossRef]
- Yuan, X.; Marcano, D.C.; Shin, C.S.; Hua, X.; Isenhart, L.C.; Pflugfelder, S.C.; Acharya, G. Ocular Drug Delivery Nanowafer with Enhanced Therapeutic Efficacy. ACS Nano 2015, 9, 1749–1758. [Google Scholar] [CrossRef]
- Marcano, D.C.; Shin, C.S.; Lee, B.; Isenhart, L.C.; Liu, X.; Li, F.; Jester, J.V.; Pflugfelder, S.C.; Simpson, J.; Acharya, G. Synergistic Cysteamine Delivery Nanowafer as an Efficacious Treatment Modality for Corneal Cystinosis. Mol. Pharm. 2016, 13, 3468–3477. [Google Scholar] [CrossRef]
- Tummala, G.K.; Rojas, R.; Mihranyan, A. Poly(vinyl alcohol) Hydrogels Reinforced with Nanocellulose for Ophthalmic Applications: General Characteristics and Optical Properties. J. Phys. Chem. B 2016, 120, 13094–13101. [Google Scholar] [CrossRef] [PubMed]
- Than, A.; Liu, C.; Chang, H.; Duong, P.K.; Cheung, C.M.G.; Xu, C.; Wang, X.; Chen, P. Self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drug delivery. Nat. Commun. 2018, 9, 4433. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Saju, A.; Cheerla, K.D.; Gade, S.K.; Garg, P.; Venuganti, V.V.K. Corneal delivery of besifloxacin using rapidly dissolving polymeric microneedles. Drug Deliv. Transl. Res. 2018, 8, 473–483. [Google Scholar] [CrossRef]
- Roy, G.; Galigama, R.D.; Thorat, V.S.; Garg, P.; Venuganti, V.V.K. Microneedle ocular patch: Fabrication, characterization, and ex-vivo evaluation using pilocarpine as model drug. Drug Dev. Ind. Pharm. 2020, 46, 1114–1122. [Google Scholar] [CrossRef]
- Thakur, R.R.S.; Tekko, I.A.; Al-Shammari, F.; Ali, A.A.; McCarthy, H.; Donnelly, R.F. Rapidly dissolving polymeric microneedles for minimally invasive intraocular drug delivery. Drug Deliv. Transl. Res. 2016, 6, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.; Chen, R.K. Self-Adhesive Microneedles with Interlocking Features for Sustained Ocular Drug Delivery. Macromol. Biosci. 2020, 20, 2000089. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhao, G.; Wang, A.; Sun, W.; Mi, S. Pressure-Triggered Microfluidic Contact Lens for Ocular Drug Delivery. ACS Appl. Polym. Mater. 2022, 4, 7290–7299. [Google Scholar] [CrossRef]
- Yang, X.; Yao, H.; Zhao, G.; Ameer, G.A.; Sun, W.; Yang, J.; Mi, S. Flexible, wearable microfluidic contact lens with capillary networks for tear diagnostics. J. Mater. Sci. 2020, 55, 9551–9561. [Google Scholar] [CrossRef]
- Reimondez-Troitiño, S.; Csaba, N.; Alonso, M.J.; de la Fuente, M. Nanotherapies for the treatment of ocular diseases. Eur. J. Pharm. Biopharm. 2015, 95, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. Neuronal death in glaucoma. Prog. Retin. Eye Res. 1999, 18, 39–57. [Google Scholar] [CrossRef]
- Janoria, K.G.; Gunda, S.; Boddu, S.H.; Mitra, A.K. Novel approaches to retinal drug delivery. Expert. Opin. Drug Deliv. 2007, 4, 371–388. [Google Scholar] [CrossRef]
- Ul Hassan, M.; Ahmed, S.; Nasir, H.; Hassan, M.; Ullah, M. Sustained release ocular drug delivery: Recent advances and developments. J. Drug Deliv. Sci. Technol. 2022, 67, 102933. [Google Scholar] [CrossRef]
- Xiang, Y.; Chen, H.; Yu, D.; Xie, H.; Luo, Y. PLGA-based drug delivery systems for sustained release of therapeutic agents in ocular disease treatment. Int. J. Pharm. 2023, 631, 122517. [Google Scholar]
- Yao, C.; Li, C.; Sun, W.; Zhang, H.; Wang, J. Biopolymer-based drug delivery systems for ocular applications: Recent advances and future perspectives. Carbohydr. Polym. 2023, 312, 120791. [Google Scholar] [CrossRef]
- Tummala, H.; Natarajan, J.V.; Yim, D.; Kim, B.; Kim, J.; Wong, T.T.; Choy, K.W.; Venkatraman, S.S. Drug-eluting contact lenses for extended delivery of therapeutic agents. J. Control. Release 2021, 330, 126–136. [Google Scholar] [CrossRef]
- Vedadghavami, A.; Zhang, C.; Bajpayee, A.G. Overcoming negatively charged tissue barriers: Drug delivery using cationic peptides and proteins. Nano Today 2020, 34, 100898. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-Y.; Luo, L.-J.; Nguyen, D.D. Multifunctional glutathione-dependent hydrogel eye drops with enhanced drug bioavailability for glaucoma therapy. Chem. Eng. J. 2020, 402, 126190. [Google Scholar] [CrossRef]
- Chouhan, G.; Moakes, R.J.A.; Esmaeili, M.; Hill, L.J.; deCogan, F.; Hardwicke, J.; Rauz, S.; Logan, A.; Grover, L.M. A self-healing hydrogel eye drop for the sustained delivery of decorin to prevent corneal scarring. Biomaterials 2019, 210, 41–50. [Google Scholar] [CrossRef]
- Ross, A.E.; Bengani, L.C.; Tulsan, R.; Maidana, D.E.; Salvador-Culla, B.; Kobashi, H.; Kolovou, P.E.; Zhai, H.; Taghizadeh, K.; Kuang, L.; et al. Topical sustained drug delivery to the retina with a drug-eluting contact lens. Biomaterials 2019, 217, 119285. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, T.; Wei, Q.; Zeng, Z.; Gong, Y.; Xu, X.; Chen, M.; Zhao, C. “NIR-triggered ROS storage” photodynamic intraocular implant for high-efficient and safe posterior capsular opacification prevention. Asian J. Pharm. Sci. 2022, 17, 838–854. [Google Scholar] [CrossRef]
- Boia, R.; Dias, P.A.N.; Martins, J.M.; Galindo-Romero, C.; Aires, I.D.; Vidal-Sanz, M.; Agudo-Barriuso, M.; de Sousa, H.C.; Ambrósio, A.F.; Braga, M.E.M.; et al. Porous poly(ε-caprolactone) implants: A novel strategy for efficient intraocular drug delivery. J. Control. Release 2019, 316, 331–348. [Google Scholar] [CrossRef]
- Zhao, J.; Xiong, J.; Ning, Y.; Zhao, J.; Wang, Z.; Long, L.; He, H.; Gou, J.; Yin, T.; Tang, X.; et al. A triple crosslinked micelle-hydrogel lacrimal implant for localized and prolonged therapy of glaucoma. Eur. J. Pharm. Biopharm. 2023, 185, 44–54. [Google Scholar] [CrossRef]
- Fan, X.; Torres-Luna, C.; Azadi, M.; Domszy, R.; Hu, N.; Yang, A.; David, A.E. Evaluation of commercial soft contact lenses for ocular drug delivery: A review. Acta Biomater. 2020, 115, 60–74. [Google Scholar] [CrossRef]
- Machida, Y.; Kuroki, S.; Kanekiyo, M.; Kobayashi, M.; Ando, I.; Amiya, S. A structural study of water in a poly(vinyl alcohol) gel by 17O NMR spectroscopy. J. Mol. Struct. 2000, 554, 81–90. [Google Scholar] [CrossRef]
- Ung, L.; Pattamatta, U.; Carnt, N.; Wilkinson-Berka, J.L.; Liew, G.; White, A.J.R. Oxidative stress and reactive oxygen species: A review of their role in ocular disease. Clin. Sci. 2017, 131, 2865–2883. [Google Scholar] [CrossRef] [PubMed]
- Mirzajani, H.; Mirlou, F.; Istif, E.; Singh, R.; Beker, L. Powering smart contact lenses for continuous health monitoring: Recent advancements and future challenges. Biosens. Bioelectron. 2022, 197, 113761. [Google Scholar] [CrossRef]
- Luo, H.; Lu, Y.; Wu, T.; Zhang, M.; Zhang, Y.; Jin, Y. Construction of tissue-engineered cornea composed of amniotic epithelial cells and acellular porcine cornea for treating corneal alkali burn. Biomaterials 2013, 34, 6748–6759. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, J.; Chen, X.; Deng, Y.; Gou, J.; Yin, T.; He, H.; Tang, X.; Ni, X.; Yang, L.; et al. An adaptive drug-releasing contact lens for personalized treatment of ocular infections and injuries. J. Control. Release 2024, 369, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Ye, A.; Mei, H.; Zhang, Z.; Song, F.; Jiang, L.; Huang, T.; Li, P.; Du, S.; Feng, Y.; Jiang, T.; et al. Corneal first aid lens: Collagen-based hydrogels loading aFGF as contact lens for treating corneal injuries. J. Control. Release 2025, 379, 251–265. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, S.; Wang, T.; He, X.; Yang, M.; Tao, J.; Zhu, S.; Zhao, H. “Bioactive” Therapeutic Contact Lens Triggered by Biomimetic Chiral Helical Nanoarchitectonics for Rapid Corneal Repair. ACS Nano 2025, 19, 9250–9264. [Google Scholar] [CrossRef]
- Wang, R.; Lu, D.; Wang, H.; Zou, H.; Bai, T.; Feng, C.; Lin, Q. “Kill-release” antibacterial polysaccharides multilayer coating based therapeutic contact lens for effective bacterial keratitis treatment. RSC Adv. 2021, 11, 26160–26167. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Liu, Y.; Qi, X.; Guo, C.; Wu, X. Kaempferol Incorporated Bovine Serum Albumin Fibrous Films for Ocular Drug Delivery. Macromol. Biosci. 2021, 21, 2100269. [Google Scholar] [CrossRef]
- Saleem, W.; Akhtar, S.; Jahan, F.; Ashraf, S.; Shahnaz, G.; Rahdar, A.; Pandey, S. Development of Contact Lenses Loaded Thiolated Nanoparticles for Ocular Delivery of Tobramycin. Bionanoscience 2024, 14, 2665–2677. [Google Scholar] [CrossRef]
- Sadeghi, M.; Tajzadeh, P.; Zarei-Ghanavati, S.; Arefnezhad, M. Chitosan-coated contact lens-based ophthalmic drug delivery system to manage Acanthamoeba keratitis: A preliminary hypothesis. Med. Hypothesis Discov. Innov. Optom. 2022, 2, 114–118. [Google Scholar] [CrossRef]
- Khan, S.A.; Shahid, S.; Mahmood, T.; Lee, C.-S. Contact lenses coated with hybrid multifunctional ternary nanocoatings (Phytomolecule-coated ZnO nanoparticles: Gallic Acid: Tobramycin) for the treatment of bacterial and fungal keratitis. Acta Biomater. 2021, 128, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Chen, M.; Xu, Z. Recent Advances in Biodegradable Polymers for Ocular Drug Delivery. Polymers 2023, 15, 1122. [Google Scholar] [CrossRef]
- Varela-Garcia, A.; Gomez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C. Imprinted Contact Lenses for Ocular Administration of Antiviral Drugs. Polymers 2020, 12, 2026. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, J.; Ding, J. Biodegradable polymeric systems for ocular drug delivery. Acta Biomater. 2021, 123, 101–117. [Google Scholar] [CrossRef]
- Carreira, A.S.; Ferreira, P.; Ribeiro, M.P.; Correia, T.R.; Coutinho, P.; Correia, I.J.; Gil, M.H. New drug-eluting lenses to be applied as bandages after keratoprosthesis implantation. Int. J. Pharm. 2014, 477, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Jeencham, R.; Sutheerawattananonda, M.; Rungchang, S.; Tiyaboonchai, W. Novel daily disposable therapeutic contact lenses based on chitosan and regenerated silk fibroin for the ophthalmic delivery of diclofenac sodium. Drug Deliv. 2020, 27, 782–790. [Google Scholar] [CrossRef]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Delfino, A.; Riva, F.; Icaro Cornaglia, A.; Marrubini, G.; Musitelli, G.; Del Fante, C.; Perotti, C.; et al. Platelet lysate and chondroitin sulfate loaded contact lenses to heal corneal lesions. Int. J. Pharm. 2016, 509, 188–196. [Google Scholar] [CrossRef]
- Dhillon, H.; Bahadur, H.; Raj, A. A comparative study of tarsorrhaphy and amniotic membrane transplantation in the healing of persistent corneal epithelial defects. Indian J. Ophthalmol. 2020, 68, 29. [Google Scholar] [CrossRef]
- Zhao, L.; Qi, X.; Cai, T.; Fan, Z.; Wang, H.; Du, X. Gelatin hydrogel/contact lens composites as rutin delivery systems for promoting corneal wound healing. Drug Deliv. 2021, 28, 1951–1961. [Google Scholar] [CrossRef]
- Yin, C.; Qi, X.; Wu, J.; Guo, C.; Wu, X. Therapeutic contact lenses fabricated by hyaluronic acid and silver incorporated bovine serum albumin porous films for the treatment of alkali-burned corneal wound. Int. J. Biol. Macromol. 2021, 184, 713–720. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, C.; Chen, W.; Chen, K.; Chen, H.; Liu, F.; Liu, D.; Lin, H.; Xie, X.; Chen, W. Wireless-Powered Electrical Bandage Contact Lens for Facilitating Corneal Wound Healing. Adv. Sci. 2022, 9, 2202506. [Google Scholar] [CrossRef] [PubMed]
- Saad, S.; Saad, R.; Jouve, L.; Kallel, S.; Trinh, L.; Goemaere, I.; Borderie, V.; Bouheraoua, N. Corneal crosslinking in keratoconus management. J. Français Ophtalmol. 2020, 43, 1078–1095. [Google Scholar] [CrossRef]
- Shetty, R.; D’Souza, S.; Khamar, P.; Ghosh, A.; Nuijts, R.M.M.A.; Sethu, S. Biochemical Markers and Alterations in Keratoconus. Asia-Pac. J. Ophthalmol. 2020, 9, 533–540. [Google Scholar] [CrossRef]
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An updated review. Contact Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.; Kim, T.Y.; Myung, D.; Hahn, S.K. Smart contact lens containing hyaluronate–rose bengal conjugate for biophotonic myopia vision correction. Biomater. Sci. 2022, 10, 4997–5005. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Wang, Z.; Wang, X.; Zhang, J.; Bin, F.; Chen, W.; Li, H.; Huo, D.; Xiao, D. Fish Fin-Derived Non-Invasive Flexible Bioinspired Contact Lens for Continuous Ophthalmic Drug Delivery. Adv. Sci. 2025, 12, 2412630. [Google Scholar] [CrossRef]
- Pinto, J.; Rao Addoor, K.; Guru, R.B. An Intraocular Lens-based Biodegradable Drug Delivery System for the Treatment of Post-cataract Inflammation. Trends Biomater. Artif. Organs 2021, 35, 415–423. [Google Scholar]
- Subhash, N.E.-; Nair, S.; Srinivas, S.P.; Theruveethi, N.; Bhandary, S.V.-; Guru, B. Development of a biodegradable polymer-based implant to release dual drugs for post-operative management of cataract surgery. Drug Deliv. Transl. Res. 2025, 15, 508–522. [Google Scholar] [CrossRef]
- Wu, C.; Or, P.W.; Chong, J.I.T.; Pathirage Don, I.K.K.; Lee, C.H.C.; Wu, K.; Yu, M.; Lam, D.C.C.; Yang, Y. Extended Delivery of Pirfenidone with Novel, Soft Contact Lenses In Vitro and In Vivo. J. Ocul. Pharmacol. Ther. 2021, 37, 75–83. [Google Scholar] [CrossRef]
- Wu, C.; Or, P.W.; Chong, J.I.T.; Don, I.K.K.P.; Lee, C.H.C.; Wu, K.; Yu, M.; Lam, D.C.C.; Yang, Y. Controllable release of pirfenidone by polyvinyl alcohol film embedded soft contact lenses in vitro and in vivo. Drug Deliv. 2021, 28, 634–641. [Google Scholar] [CrossRef]
- Dixon, P.; Chauhan, A. Carbon Black Tinted Contact Lenses for Reduction of Photophobia in Cystinosis Patients. Curr. Eye Res. 2019, 44, 497–504. [Google Scholar] [CrossRef]
- Xu, X.; Awwad, S.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Brocchini, S.; Gaisford, S.; Goyanes, A.; Basit, A.W. 3D Printed Punctal Plugs for Controlled Ocular Drug Delivery. Pharmaceutics 2021, 13, 1421. [Google Scholar] [CrossRef]
- Liu, Z.; Kompella, U.B.; Chauhan, A. Gold nanoparticle synthesis in contact lenses for drug-less ocular cystinosis treatment. Eur. J. Pharm. Biopharm. 2021, 165, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Bengani, L.C.; Kobashi, H.; Ross, A.E.; Zhai, H.; Salvador-Culla, B.; Tulsan, R.; Kolovou, P.E.; Mittal, S.K.; Chauhan, S.K.; Kohane, D.S.; et al. Steroid-eluting contact lenses for corneal and intraocular inflammation. Acta Biomater. 2020, 116, 149–161. [Google Scholar] [CrossRef]
- DiPasquale, S.A.; Wuchte, L.D.; Mosley, R.J.; Demarest, R.M.; Voyles, M.L.; Byrne, M.E. One Week Sustained In Vivo Therapeutic Release and Safety of Novel Extended-Wear Silicone Hydrogel Contact Lenses. Adv. Healthc. Mater. 2022, 11, 2101263. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.E.; Elsherif, M.; Alam, F.; Yetisen, A.K.; Butt, H. Gold Nanocomposite Contact Lenses for Color Blindness Management. ACS Nano 2021, 15, 4870–4880. [Google Scholar] [CrossRef] [PubMed]
- Roostaei, N.; Hamidi, S.M. Two-dimensional biocompatible plasmonic contact lenses for color blindness correction. Sci. Rep. 2022, 12, 2037. [Google Scholar] [CrossRef] [PubMed]
- Karepov, S.; Ellenbogen, T. Metasurface-based contact lenses for color vision deficiency. Opt. Lett. 2020, 45, 1379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Feng, C.; Song, F.; Du, X. Preparation and Evaluation of Starch Hydrogel/Contact Lens Composites as Epigallocatechin Gallate Delivery Systems for Inhibition of Bacterial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 759303. [Google Scholar] [CrossRef]
- Silva, D.; de Sousa, H.C.; Gil, M.H.; Santos, L.F.; Moutinho, G.M.; Salema-Oom, M.; Alvarez-Lorenzo, C.; Serro, A.P.; Saramago, B. Diclofenac sustained release from sterilised soft contact lens materials using an optimised layer-by-layer coating. Int. J. Pharm. 2020, 585, 119506. [Google Scholar] [CrossRef]
- Shikamura, Y.; Yamazaki, Y.; Matsunaga, T.; Sato, T.; Ohtori, A.; Tojo, K. Hydrogel Ring for Topical Drug Delivery to the Ocular Posterior Segment. Curr. Eye Res. 2016, 41, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; de Sousa, H.C.; Gil, M.H.; Santos, L.F.; Oom, M.S.; Alvarez-Lorenzo, C.; Saramago, B.; Serro, A.P. Moxifloxacin-imprinted silicone-based hydrogels as contact lens materials for extended drug release. Eur. J. Pharm. Sci. 2021, 156, 105591. [Google Scholar] [CrossRef] [PubMed]
- Pereira-da-Mota, A.F.; Vivero-Lopez, M.; Topete, A.; Serro, A.P.; Concheiro, A.; Alvarez-Lorenzo, C. Atorvastatin-Eluting Contact Lenses: Effects of Molecular Imprinting and Sterilization on Drug Loading and Release. Pharmaceutics 2021, 13, 606. [Google Scholar] [CrossRef]
- Pereira-da-Mota, A.F.; Vivero-Lopez, M.; Serramito, M.; Diaz-Gomez, L.; Serro, A.P.; Carracedo, G.; Huete-Toral, F.; Concheiro, A.; Alvarez-Lorenzo, C. Contact lenses for pravastatin delivery to eye segments: Design and in vitro-in vivo correlations. J. Control. Release 2022, 348, 431–443. [Google Scholar] [CrossRef]
- Abdi, B.; Mofidfar, M.; Hassanpour, F.; Kirbas Cilingir, E.; Kalajahi, S.K.; Milani, P.H.; Ghanbarzadeh, M.; Fadel, D.; Barnett, M.; Ta, C.N.; et al. Therapeutic contact lenses for the treatment of corneal and ocular surface diseases: Advances in extended and targeted drug delivery. Int. J. Pharm. 2023, 638, 122740. [Google Scholar] [CrossRef]
- Liu, Z.; Overton, M.; Chauhan, A. Transport of Vitamin E from Ethanol/Water Solution into Contact Lenses and Impact on Drug Transport. J. Ocul. Pharmacol. Ther. 2022, 38, 396–403. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Fan, X.; Domszy, R.; Hu, N.; Wang, N.S.; Yang, A. Hydrogel-based ocular drug delivery systems for hydrophobic drugs. Eur. J. Pharm. Sci. 2020, 154, 105503. [Google Scholar] [CrossRef] [PubMed]
- Holgado, M.A.; Anguiano-Domínguez, A.; Martín-Banderas, L. Contact lenses as drug-delivery systems: A promising therapeutic tool. Arch. Soc. Esp. Oftalmol. (Engl. Ed.) 2020, 95, 24–33. [Google Scholar] [CrossRef]
- Sarmout, M.; Xiao, Y.; Hu, X.; Rakhmetova, A.; Koole, L.H. A novel approach to achieve semi-sustained drug delivery to the eye through asymmetric loading of soft contact lenses. Heliyon 2023, 9, e16916. [Google Scholar] [CrossRef]
- Karamitsos, A.; Lamprogiannis, L.; Karagkiozaki, V.; Laskarakis, A.; Papadopoulou, L.; Fatouros, D.; Ziakas, N.; Logothetidis, S.; Tsinopoulos, I. Design, characterisation and drug release study of polymeric, drug-eluting single layer thin films on the surface of intraocular lenses. IET Nanobiotechnol 2020, 14, 501–507. [Google Scholar] [CrossRef]
- Karamitsos, A.; Lamprogiannis, L.; Karagkiozaki, V.; Koutsogianni, A.; Chakim, Z.; Ziakas, N.G.; Tsinopoulos, I.; Logothetidis, S. Morphological Characteristics of Drug-Eluting Biodegradable Polymeric Thin Films Developed on the Surface of Intraocular Lenses by Three Techniques: A Comparative Study. Cureus 2021, 13, e19674. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, K.; Wang, H.; Ma, L.; Yu, L.; Nie, Y. Stable Fabrication of Zwitterionic Coating Based on Copper-Phenolic Networks on Contact Lens with Improved Surface Wettability and Broad-Spectrum Antimicrobial Activity. ACS Appl. Mater. Interfaces 2020, 12, 16125–16136. [Google Scholar] [CrossRef] [PubMed]
- Yuan Tian, J.J.; Liu, L.; Ross, M.; Sheardown, H. Hyaluronic Acid based Therapeutic Bandage Contact Lenses for Corneal Wound Healing. Investig. Ophthalmol. Vis. Sci. 2022, 63, 3222-A0257. [Google Scholar]
- Gandara-Loe, J.; Souza, B.E.; Missyul, A.; Giraldo, G.; Tan, J.-C.; Silvestre-Albero, J. MOF-Based Polymeric Nanocomposite Films as Potential Materials for Drug Delivery Devices in Ocular Therapeutics. ACS Appl. Mater. Interfaces 2020, 12, 30189–30197. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Das, P.J.; Dey, S.; Nayak, A.K.; Roy, P.K.; Chakrabarti, S.; Marbaniang, D.; Das, S.K.; Ray, S.; Chattopadhyay, P.; et al. Development and optimization of besifloxacin hydrochloride loaded liposomal gel prepared by thin film hydration method using 32 full factorial design. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124071. [Google Scholar] [CrossRef]
- Daza, J.H.U.; Righetto, G.M.; Chaud, M.V.; da Conceição Amaro Martins, V.; Lopes Baratella da Cunha Camargo, I.; Maria de Guzzi Plepis, A. PVA/anionic collagen membranes as drug carriers of ciprofloxacin hydrochloride with sustained antibacterial activity and potential use in the treatment of ulcerative keratitis. J. Biomater. Appl. 2020, 35, 301–312. [Google Scholar] [CrossRef]
- Alvarez-Rivera, F.; Rey-Rico, A.; Venkatesan, J.K.; Diaz-Gomez, L.; Cucchiarini, M.; Concheiro, A.; Alvarez-Lorenzo, C. Controlled Release of rAAV Vectors from APMA-Functionalized Contact Lenses for Corneal Gene Therapy. Pharmaceutics 2020, 12, 335. [Google Scholar] [CrossRef]
- Li, R.; Guan, X.; Lin, X.; Guan, P.; Zhang, X.; Rao, Z.; Du, L.; Zhao, J.; Rong, J.; Zhao, J. Poly(2-hydroxyethyl methacrylate)/β-cyclodextrin-hyaluronan contact lens with tear protein adsorption resistance and sustained drug delivery for ophthalmic diseases. Acta Biomater. 2020, 110, 105–118. [Google Scholar] [CrossRef]
- Lasowski, F.; Rambarran, T.; Rahmani, V.; Brook, M.A.; Sheardown, H. PEG-containing siloxane materials by metal-free click-chemistry for ocular drug delivery applications. J. Biomater. Sci. Polym. Ed. 2021, 32, 581–594. [Google Scholar] [CrossRef]
- Morgan, S.R.; Pilia, N.; Hewitt, M.; Moses, R.L.; Moseley, R.; Lewis, P.N.; Morrison, P.W.J.; Kelly, S.L.; Parker, J.E.; Whitaker, D.; et al. Controlled in vitro delivery of voriconazole and diclofenac to the cornea using contact lenses for the treatment of Acanthamoeba keratitis. Int. J. Pharm. 2020, 579, 119102. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Hu, N.; Fan, X.; Domszy, R.; Yang, J.; Briber, R.M.; Yang, A. Extended delivery of cationic drugs from contact lenses loaded with unsaturated fatty acids. Eur. J. Pharm. Biopharm. 2020, 155, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ongkasin, K.; Masmoudi, Y.; Wertheimer, C.M.; Hillenmayer, A.; Eibl-Lindner, K.H.; Badens, E. Supercritical fluid technology for the development of innovative ophthalmic medical devices: Drug loaded intraocular lenses to mitigate posterior capsule opacification. Eur. J. Pharm. Biopharm. 2020, 149, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.R.; Maulvi, F.A.; Desai, D.M.; Shukla, M.R.; Ranch, K.M.; Vyas, B.A.; Shah, S.A.; Sandeman, S.; Shah, D.O. Multiple drug delivery from the drug-implants-laden silicone contact lens: Addressing the issue of burst drug release. Mater. Sci. Eng. C 2020, 112, 110885. [Google Scholar] [CrossRef]
- Wei, N.; Dang, H.; Huang, C.; Sheng, Y. Timolol loaded microemulsion laden silicone contact lens to manage glaucoma: In vitro and in vivo studies. J. Dispers. Sci. Technol. 2021, 42, 742–750. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shin, K.-S.; Kim, J.-W.; Kang, J.-Y.; Kim, J.-K. Stimulus-Responsive Contact Lens for IOP Measurement or Temperature-Triggered Drug Release. Transl. Vis. Sci. Technol. 2020, 9, 1. [Google Scholar] [CrossRef]
- Pillai, S.K.R.; Reghu, S.; Vikhe, Y.; Zheng, H.; Koh, C.H.; Chan-Park, M.B. Novel Antimicrobial Coating on Silicone Contact Lens Using Glycidyl Methacrylate and Polyethyleneimine Based Polymers. Macromol. Rapid Commun. 2020, 41, 2000175. [Google Scholar] [CrossRef]
- Elsherif, M.; Moreddu, R.; Alam, F.; Salih, A.E.; Ahmed, I.; Butt, H. Wearable Smart Contact Lenses for Continual Glucose Monitoring: A Review. Front. Med. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Chung, W.G.; Kwon, Y.W.; Kim, S.; Hong, Y.-M.; Park, W.; Kim, E.; Lee, J.; Lee, S.; Kim, M.; et al. Smart Contact Lenses as Wearable Ophthalmic Devices for Disease Monitoring and Health Management. Chem. Rev. 2023, 123, 11488–11558. [Google Scholar] [CrossRef]
- Seewoodhary, M. An overview of diabetic retinopathy and other ocular complications of diabetes mellitus. Nurs. Stand. 2021, 36, 71–76. [Google Scholar] [CrossRef]
- Elsherif, M.; Alam, F.; Salih, A.E.; AlQattan, B.; Yetisen, A.K.; Butt, H. Wearable Bifocal Contact Lens for Continual Glucose Monitoring Integrated with Smartphone Readers. Small 2021, 17, 2102876. [Google Scholar] [CrossRef]
- Alvarez-Rivera, F.; Concheiro, A.; Alvarez-Lorenzo, C. Epalrestat-loaded silicone hydrogels as contact lenses to address diabetic-eye complications. Eur. J. Pharm. Biopharm. 2018, 122, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Dennyson Savariraj, A.; Salih, A.; Alam, F.; Elsherif, M.; AlQattan, B.; Khan, A.A.; Yetisen, A.K.; Butt, H. Ophthalmic Sensors and Drug Delivery. ACS Sens. 2021, 6, 2046–2076. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yan, X.; Gu, Z.; Xiu, G.; Xiao, X. Electrochemical Sensing in Contact Lenses. Electroanalysis 2022, 34, 227–236. [Google Scholar] [CrossRef]
- Wuchte, L.; DiPasquale, S.; Masterson, A.; Vance, A.; Goff, J.; Arkles, B.; Sulaiman, S.; Byrne, M. Characterization and analysis of extended-wear silicone hydrogel contact lenses utilizing novel silicone macromers. J. Biomed. Mater. Res. 2022, 110, 1512–1523. [Google Scholar] [CrossRef]
- Kim, T.Y.; Lee, G.-H.; Mun, J.; Cheong, S.; Choi, I.; Kim, H.; Hahn, S.K. Smart contact lens systems for ocular drug delivery and therapy. Adv. Drug Deliv. Rev. 2023, 196, 114817. [Google Scholar] [CrossRef]
- Xiong, J.; Hsiang, E.-L.; He, Z.; Zhan, T.; Wu, S.-T. Augmented reality and virtual reality displays: Emerging technologies and future perspectives. Light. Sci. Appl. 2021, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Aydındoğan, G.; Kavaklı, K.; Şahin, A.; Artal, P.; Ürey, H. Applications of augmented reality in ophthalmology [Invited]. Biomed. Opt. Express 2021, 12, 511. [Google Scholar] [CrossRef]
- Yin, K.; Hsiang, E.-L.; Zou, J.; Li, Y.; Yang, Z.; Yang, Q.; Lai, P.-C.; Lin, C.-L.; Wu, S.-T. Advanced liquid crystal devices for augmented reality and virtual reality displays: Principles and applications. Light. Sci. Appl. 2022, 11, 161. [Google Scholar] [CrossRef]
- Zhan, T.; Yin, K.; Xiong, J.; He, Z.; Wu, S.-T. Augmented Reality and Virtual Reality Displays: Perspectives and Challenges. iScience 2020, 23, 101397. [Google Scholar] [CrossRef]
- Beck, A.; Uhrig, M.; Schuster, A.; Korb, C.; Pfeiffer, N.; Lorenz, K. Comparison of SENSIMED Triggerfish® (TF) 24-Hour Monitoring in Open-Angle Glaucoma Patients Before and After Trabeculectomy. J. Clin. Med. 2025, 14, 2112. [Google Scholar] [CrossRef]


| Criteria | FDA (USA) | EMA/MDR (EU) |
|---|---|---|
| Regulatory status | Medical device—Class II or III (21 CFR 886.5925). | Medical device—Class IIa (per MDR 2017/745) |
| Classification basis | Clinical use, wearing time, material composition. | Clinical use, wearing time, mechanism of action |
| Contact lens categories | • Corrective; • Therapeutic; • Cosmetic; • Orthokeratology. | • Corrective; • Therapeutic; • Cosmetic; • Orthokeratology. |
| Drug-eluting therapeutic lenses | Combination product (drug–device) requires pharmaceutical and device-specific data. Regulated jointly by CDRH and CDER. | Combination products, must comply with MDR and relevant medicinal product legislation (e.g., Directive 2001/83/EC) |
| Pre-market requirements | Evaluation of safety, efficacy, PK/PD, biocompatibility, GMP compliance. | Clinical evaluation, CE documentation, ISO compliance, notified body assessment |
| Wearing time classification | Daily wear (DW)—<24 h Extended wear (EW)—>24 | Based on duration and intended use; >24 h requires additional evaluation under MDR |
| Material compliance | Biocompatibility per ISO 10993 and FDA guidance documents. | Biocompatibility per ISO 10993 and MDR Annex I (General Safety and Performance Requirements) |
| Title | Keywords/Aims | Ref. |
|---|---|---|
| Recent Advancements in Nanomaterial-Laden Contact Lenses for Diagnosis and Treatment of Glaucoma, Review and Update | Glaucoma; Contact Lenses; Drug Delivery System; Diagnosis; Treatment | [12] |
| Review Article Testing Drug Release from Medicated Contact Lenses: The Missing Link to Predict In Vivo Performance | Drug-Eluting; Contact Lens; In Vitro Release Tests; In Vivo Release Rate Specifications; Therapeutic Response; In Vitro–In Vivo Correlations | [22] |
| Advancements in the chemistry of contact lenses: innovations and applications | Contact Lens Chemistry; Polymer Materials; Hydrogels and Silicone Hydrogels; Smart Contact Lenses; Antimicrobial Coatings | [14] |
| The Promise of Drug-Eluting Contact Lenses | An Overview of Drug-Eluting Contact Lens Technologies and Lenses That Are in Preparation | [28] |
| Review of Approaches for Increasing Ophthalmic Bioavailability for Eye Drop Formulations | Bioavailability; Drug Delivery; Eye Drops; Nanoparticles; Permeability Enhancers | [29] |
| Contact Lenses as Ophthalmic Drug Delivery Systems: A Review | Contact Lenses; Ophthalmic Drug; Polymeric Support; Ocular Drug Delivery | [30] |
| Next-Generation Contact Lenses: Towards Bioresponsive Drug Delivery and Smart Technologies in Ocular Therapeutics | Contact Lens; Drug-Eluting; Ocular Surface; Biosensing; Ocular Therapeutics; Drug Delivery | [31] |
| Controlled Drug Delivery Systems: Current Status and Future Directions | Controlled Release Dosage Forms; Pharmacokinetics; Nano-Drug Delivery; Smart and Stimuli-Responsive Delivery; Intelligent Biomaterials | [32] |
| In Vivo Drug Delivery via Contact Lenses: The Current State of the Field from Origins to Present | Contact Lens; Drug Release; In Vivo; Ophthalmic Therapy; Therapeutic Contact Lens | [33] |
| Sustained Bimatoprost Release using Gold Nanoparticles Laden Contact Lenses | Contact Lens; Animal Studies; Bimatoprost; Gold Nanoparticles; Sustained Drug Delivery | [34] |
| BCLA CLEAR—Medical Use of Contact Lenses | Therapeutic Contact Lens; Bandage Lens; Scleral Lens; Irregular Astigmatism; Aphakia; Ocular Surface Disease | [35] |
| Soft Contact Lenses as Drug Delivery Systems: A Review | Contact Lenses; Drug Delivery; Drug-Controlled Release; Drug Delivery Systems Based on Contact Lenses in Ophthalmic Therapies | [36] |
| Drug Delivery to the Anterior Segment of the Eye: A Review of Current and Future Treatment Strategies | Optimizing Ophthalmic Drug Delivery by Achieving High Drug Concentrations with a Prolonged Duration of Action that is Convenient for Patient Administration | [37] |
| Considerations for Polymers Used in Ocular Drug Delivery | Controlled Release; Drug Delivery; Hydrogel; Ocular Biomaterials; Ocular Implants; Ophthalmic Delivery; Polymer | [38] |
| Lab-on-a-Contact Lens: Recent Advances and Future Opportunities in Diagnostics and Therapeutics | Bioelectronics; Biosensors; Contact Lens; Diagnostics; Integrated Systems; Personalized Healthcare; Therapeutics; Wearable Electronics | [39] |
| Drug Delivery Strategies and Biomedical Significance of Hydrogels: Translational Considerations | Hydrogels; Drug Delivery; Therapeutic Interventions; Clinical Trials; Translation; Biomedical Perspectives; Contact Lenses; Wound Management; Tissue Engineering | [40] |
| Updates on Biodegradable Formulations for Ocular Drug Delivery | Biodegradable Drug Delivery; Ocular Drug Delivery; Biodegradable Polymers; Nanoparticle Drug Delivery; Polymeric Micelles; Liposomes; Hydrogels; Biodegradable Implants | [41] |
| Therapeutic Applications of Contact Lens-Based Drug Delivery Systems in Ophthalmic Diseases | Drug Delivery; Contact Lens; Ophthalmic Diseases; Polymer Material | [42] |
| Contact Lens as Drug Delivery System for Glaucoma Treatment: A Review | Glaucoma; Intraocular Pressure; Gold Nanoparticles (GNPs); Timolol; Drug Delivery; Bioavailability | [43] |
| Development of Corneal Contact Lens Materials and Current Clinical Application of Contact Lenses: A Review | Drug-Eluting Contact Lenses: Progress, Challenges, And Prospects | [44] |
| Contact Lenses for the Treatment of Ocular Surface Diseases | Bandage Contact Lens; Dry Eye; Ocular Surface Disease; Prosthetic Contact Lens; Rigid Gas Permeable; Scleral Contact Lens; Stevens–Johnson Syndrome; Therapeutic Contact Lens | [45] |
| Contact Lenses as Ophthalmic Drug Delivery Systems—The Future of Treatment for Ocular Infection and Injuries—A Review | Therapeutic Contact Lens; Antibiotic-Releasing Contact Lens; Contact Lens Application | [46] |
| Review Applications of Hyaluronic Acid in Ophthalmology and Contact Lenses | Hyaluronic Acid; Contact Lenses; Ophthalmology | [47] |
| Role of Therapeutic Contact Lenses in the Management of Corneal Disease | Keratoconus; Ocular Surface Disease; Scleral Lens; Therapeutic Contact Lens | [48] |
| Contact Lenses as an Ophthalmic Drug Delivery System | Contact Lenses; Ophthalmic Drug; Polymeric Support; Ocular Drug Deliver | [49] |
| Pharmaceutical-Loaded Contact Lenses as an Ocular Drug Delivery System: A Review of Critical Lens Characterization Methodologies Regarding ISO Standards | Ocular Drug Delivery; Therapeutic Contact Lens; Characterization Techniques; Physical Properties; Chemical Properties; ISO Standards | [50] |
| Ocular contact lenses: smart materials for biomedical applications | Contact Lenses; Silicone Acrylate-Based Polymers; Optical Disorders; Therapeutic Lens; Biomaterials | [51,52] |
| From Vision Correction to Drug Delivery: Unraveling the Potential of Therapeutic Contact Lens | Therapeutic Contact Lens; Contact Lenses; Drug Release; Drug Stability; Ocular Surface Disorders; Vision Correction | [53] |
| Carbohydrate Polymers, Polymeric Nano Drugs, and Nanoparticles Are Used for Advanced Drug Delivery and Therapeutics in Ocular Diseases | Carbohydrate Polymers; Polymeric Nano-Drugs; Nanoparticles; Contact Lenses | [54] |
| Polymeric Membranes in Contact Lens Technology for Glaucoma Treatment: Breakthroughs, Obstacles, and Emerging Opportunities | Contact Lenses; Drug Delivery; Glaucoma; Hydrogel, Nanoparticle; Polymers | [55] |
| Microfluidic contact lens: fabrication approaches and applications | Microfluidic Contact Lens | [56] |
| Recent Advances in New Copolymer Hydrogel-Formed Contact Lenses for Ophthalmic Drug Delivery | The Use of HEMA, MAA, DMA, NYP, EGDMA, TRIS, and PDMS in Therapeutic Contact Lenses; The Advantages and Disadvantages of Each Material in Tailoring the Drug Release Rate for Different Encapsulated Payloads, With Particular Emphasis on Their Impact on Therapeutic Efficacy | [57] |
| Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management | Hydrogel; Stimuli-Responsive; Polymeric Hydrogel Nanoparticles; Drug Delivery Systems; Wound Dressing Materials; Tissue Engineering Scaffolds; Modified Contact Lens | [58] |
| Drug-Modified Contact Lenses—Properties, Release Kinetics, and Stability of Active Substances with Particular Emphasis on Cyclosporine A: A Review | Therapeutic Contact Lenses; Polymer Matrix; Drug Stability; Mechanic Parameters; Cyclosporine Stability; Drug Delivery Systems | [59] |
| Monomer | Polymer Properties, Advantages/Limitations | Ref. |
|---|---|---|
| Polyethylene glycols | ||
| Ethylene Glycol (EG) | PEG (poly(ethylene glycol)): Water-soluble and highly biocompatible; exhibits faster degradation than other synthetic polymers; commonly used for surface modification and drug conjugation | [68,69,70] |
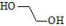 | ||
| Polyvinyl alcohols | ||
| Vinyl Alcohol (VA) | PVA (poly(vinyl alcohol)): Characterized by slow degradation under physiological conditions; typically synthesized using harsh organic solvents | [71] |
 | ||
| Polyesters | ||
| Glycolic Acid (GA) | PGA (poly(glycolic acid)): Exhibits rapid hydrolytic degradation; limited mechanical strength; rarely used alone because of brittleness | [2,72,73] |
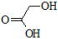 | ||
| Lactic Acid (LA) | PLA (poly(lactic acid)): Synthesized from renewable natural resources; good mechanical strength and processability; limited impact resistance; slow and incomplete biodegradation under physiological conditions | |
 | ||
| GA + LA | PLGA (poly(lactic-co-glycolic acid)) Biocompatible and FDA-approved copolymer; controlled and tunable degradation rate; most applied polymer in ocular drug delivery platforms | |
| ε-Caprolactone (CL) | PCL (poly(caprolactone)): Biodegradable, hydrophobic, excellent biocompatible, semi-crystalline polyester, mechanical flexibility, slow degradation profile, easy to modify, inexpensive, widely explored in ophthalmic drug delivery, not specifically FDA-approved for ophthalmic use | [41,74,75] |
 | ||
| Ortho ester (OE) | POE (poly(ortho ester)): Undergo surface erosion during degradation; limited data available on their application in ocular drug delivery systems | [76] |
 | ||
| Polymethacrylates | ||
| Methyl methacrylate (MMA) | PMMA (poly(methyl methacrylate)): Well-established ophthalmic polymer; cost-effective and resistant to UV radiation and environmental exposure; non-biodegradable; limited chemical and thermal resistance; low oxygen permeability | [77,78] |
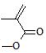 | ||
| 2-Hydroxyethyl Methacrylate (HEMA) | pHEMA (poly(2-hydroxyethyl methacrylate)): Hydrophilic and water-absorbing material; biocompatible but non-biodegradable; rigid when dry and soft, flexible when hydrated; exhibits poor mechanical strength; capable of hydrolysis, ionization, and hydrogen bonding; suitable for modulating slow drug release | [79,80,81] |
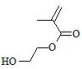 | ||
| 2-(Dimethylamino)ethyl Methacrylate (DMAEM) | PDMAEM (poly-2-(dimethylamino))ethyl methacrylate: Methacrylate-based polymer used in ocular hydrogels, nanoparticle carriers, micelles, and implants; chemically stable but incompatible with strong acids, bases, and oxidizers; prone to auto-polymerization and degradation upon exposure to air, moisture, or light | |
 | ||
| Polyolefins | ||
| Acrylic Acid (AA) | PAA (poly(acrylic acid)): Highly water-soluble and mucoadhesive polymer; biodegradable, yielding acidic degradation products; widely explored for controlled ocular drug delivery | [82] |
 | ||
| Dendrimers | ||
| Ethylenediamine | PAMAM (poly(amidoamine)): Highly branched dendrimer with numerous reactive surface groups; enables facile chemical functionalization; not currently FDA-approved for ophthalmic applications | [83,84] |
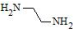 |
| Polymer | Structure | Characteristics | Ref |
|---|---|---|---|
| Polysaccharide biopolymers | |||
| Dextran | 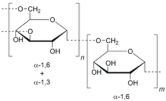 | DEX: Biocompatible, biodegradable, and hydrophilic biopolymer; capable of forming hydrogels; FDA-approved and commonly used in ophthalmic eye drop formulations. | [86,87] |
| Cellulose | 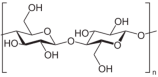 | CEL: Biocompatible and biodegradable via enzymatic degradation and hydrolysis; chemically reactive and amenable to conjugation; FDA-approved for ophthalmic applications. | [85] |
| Carboxymethylcellulose |  | CMC: Biocompatible and hydrophilic linear polymer; an effective matrix for experimental biopolymer-based hydrogels and thin films enabling sustained local drug release. | [85,88,89] |
| Chitosan |  | CHI: Mucoadhesive, biocompatible, exhibits antimicrobial and anti-inflammatory properties, enhances drug retention on the ocular surface. Poor solubility at neutral and alkaline pH; batch-to-batch variability in molecular weight and degree of deacetylation affects stability and reproducibility. | [90,91] |
| Hyaluronic acid |  | HA: Naturally occurring, biocompatible, and biodegradable polysaccharide with high water retention capacity; exhibits viscoelastic and mucoadhesive properties; widely used in ocular formulations to promote wound healing and lubrication. | [92,93,94] |
| Pullulan | 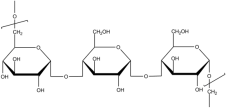 | PUL: Biocompatible, nonionic, and biodegradable polysaccharide; water-soluble and stable across a wide range of temperatures and pH; insoluble in most organic solvents; oxygen-impermeable, viscosity-enhancing, and easily processed for ocular formulations. | [95,96,97] |
| Guar gum |  | GG (Galactomannan): Biocompatible, water-soluble, and mucoadhesive polysaccharide; nonionic and hydrolytically degradable; exhibits strong swelling capacity and increases viscosity; FDA-approved for ophthalmic use. Limited solubility in alcohols and organic solvents; unstable in solution over time. | [98] |
| Protein biopolymers | |||
| Collagen |  | COL: Biocompatible and enzymatically degradable structural protein. The primary sequence motif repeats Gly–X–Y, where Gly = glycine (every third residue), X = usually proline, and Y = usually hydroxyproline or hydroxylysine. Relatively easy to process and widely available from animal sources (e.g., bovine, porcine), recombinant collagen offers a safer and more sustainable alternative via plant and yeast expression systems. | [99,100,101] |
| Gelatin | 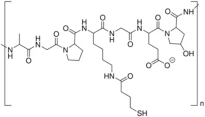 | GEL: Biocompatible, biodegradable, and water-soluble protein derived from collagen; forms gel and increases viscosity; cost-effective and widely available; exhibits lower gelation temperature and higher aqueous solubility than native collagen. | |
| Other biopolymers | |||
| Poly(dopamine) |  | PDA: Biocompatible and low-toxicity polymer formed via oxidative polymerization of dopamine; extensively explored in drug delivery for its strong adhesion to diverse surfaces; widely used in developing biofunctional coatings and nanostructures. | [102] |
| Polymer | Application in Ophthalmology | Research Status | Ref. |
|---|---|---|---|
| Silk fibroin | SF: Used in transparent corneal scaffolds and drug-loaded therapeutic contact lenses | Emerging clinical interest | [110] |
| Poly(hydroxy alkanoates) | PHA/P3HB: Controlled drug release in ocular implants and corneal patches | Preclinical and translational studies | [112] |
| Poly(glycerol sebacate) | PGS: Flexible biodegradable substrate for ocular implants and hydrogels | Experimental phase | [111] |
| Poly(trimethylene carbonate) | PTMC: Tested as a coating for intraocular lens systems | Investigational | [113] |
| Poly(ester amide) | PEA: Ocular drug carriers with tunable degradation for retinal delivery | Advanced preclinical development | [114] |
| Zwitterionic hydrogels | Z-HYD: Biofilm-resistant hydrogels for drug-eluting contact lenses | Proof-of-concept studies | [115] |
| Methacrylated hyaluronic acid | MeHA: Enhanced HA hydrogels for sustained drug release in CLs | In vitro/in vivo validation | [116] |
| Polysaccharide nanogels | PSNG: Nanogels for anterior and posterior segment drug delivery | Exploratory nanomedicine studies | [117] |
| Polydopamine derivatives | PDA-PEG, PDA-HA: ROS/pH-sensitive coatings for targeted ocular drug release | High potential, under development | [118] |
| Condition | Lens Type/Carrier | Active Agent | Mechanism of Action | Validation |
|---|---|---|---|---|
| Corneal wound healing | Gelatin hydrogel, HA/Pluronic®, BSA/Ag/HA, electric lenses | Rutin, HA, silver, e-stimulation | Anti-inflammatory, ROS scavenging, epithelial repair | In vivo (rabbit, mouse) |
| Keratoconus and myopia | HA-RB conjugate lens (photoactivated) | Rose Bengal | Collagen photo-crosslinking without epithelial disruption | In vivo (preclinical) |
| Glaucoma | Microstructured lenses, DEX-ring, bromfenac-loaded hydrogel | Dexamethasone, bromfenac | Sustained/pressure-responsive delivery | In vivo (rabbit) |
| Cataract (postop) | PLGA-coated IOLs, dual-drug implants (DEX + MOX) | Dexamethasone, moxifloxacin | Anti-inflammatory + antibacterial postop protection | In vivo (rabbit) |
| Proliferative ocular diseases | Silicone-PVA layered lenses | Pirfenidone | Antifibrotic, extended tear residence | In vivo (rabbit) |
| Ocular cystinosis | Cysteamine + carbon black or gold NP lenses | Cysteamine, vitamin E, gold NP | Prolonged release, UV protection, cystine binding | In vivo, in vitro |
| Uveitis | DEX-ring, bromfenac hydrogel lenses | Dexamethasone, bromfenac | Sustained anti-inflammatory release | In vivo (rabbit) |
| Color vision deficiency (CVD) | Nanocomposite/metasurface/plasmonic lenses | Optical modulation | Wavelength filtering for enhanced color vision | In silico, prototyping |
| Ophthalmic Diseases | Techniques | Active Principle | Findings/Results | Ref. |
|---|---|---|---|---|
| Bacterial keratitis | Coating | Copper ions | Endows CLs with the ability to effectively inhibit biofilm formation | [212] |
| Bacterial keratitis | FRP free radical polymerization | EGCG epigallocatechin gallate | Sustained drug release over 14 days; significantly inhibits P. aeruginosa adhesion | [199] |
| Fungal keratitis | Nanocoatings | Gallic acid, tobramycin | Significant antimycotic, biofilm inhibition, and antifouling properties | [171] |
| Viral keratitis | Hydrogels based on HEMA, EGDMA, MAA, AIBN; molecular imprinting | Acyclovir, valacyclovir | Releases the drug in a sustained manner for 10 h | [173] |
| Noninfectious keratitis | DPF drug–polymer film | Vancomycin | Sustainably released for more than 8 h | [176] |
| Corneal wound healing | FRP free radical polymerization | Rutin | Sustained rutin release over 14 days; facilitates corneal wound healing | [179] |
| FRP free radical polymerization | HA hyaluronic acid | Reduces ocular inflammation; supports corneal healing in preclinical models | [213] | |
| DPF drug–polymer film | HA, silver | Prolonged hyaluronic acid retention accelerates corneal healing | [180] | |
| Glaucoma | Polyurethane film produced by solvent casting; soaking | Brimonidine tartrate | Prolonged drug release up to 14 days | [214] |
| Conjunctivitis | Lipid-based film—drug-loaded liposomes by hydration method; soaking | Besifloxacin hydrochloride | Biphasic release: initial burst + sustained (80% released in 10 h) | [215] |
| Ulcerative keratitis | Soaking | Ciprofloxacin hydrochloride, tobramycin | Antibacterial activity for 48 h | [216] |
| Corneal gene therapy | HEMA hydrogels; soaking | rAAV | Efficacy in transduction/triggering cell proliferation | [217] |
| Conjunctivitis | HEMA/CD hyaluronan; soaking | Diclofenac sodium | Therapeutic effect for conjunctivitis | [218] |
| Retinoblastoma | PEG-modified silicone; soaking | Roscovitine | Prolonged drug release | [219] |
| Acanthamoeba keratitis | Commercial hydrogel-based CLs; soaking | Voriconazole, diclofenac sodium | Sustained release, cell proliferation | [220] |
| Commercial CLs based on silicone or HEMA hydrogels; soaking | Tetracaine, bupivacaine, ketotifen, diclofenac, flurbiprofen; loading of fatty acids (i.e., oleic acid, linoleic, linolenic acid) | Initial burst release: 30–90% (dependent on drug–lens system) followed by sustained release phase | [221] | |
| Endophthalmitis after cataract surgery | Commercial foldable acrylic CLs; supercritical impregnation | Gatifloxacin | Improvement in impregnation yield | [222] |
| Posterior capsule opacification after cataract surgery | Commercial foldable acrylic CLs; supercritical impregnation | Methotrexate | Prolonged drug release for more than 100 days, inhibition of fibrosis | [222] |
| Ocular hypertension, glaucoma | Silicone CLs, implants based on Irgacure, EGDMA, DMA, NVP, siloxane, and HEMA, then embedded into silicone CLs; soaking | Bimatoprost, hyaluronic acid, timolol | High burst effect in drug release profiles | [223] |
| Glaucoma | Sil-DMA-HEMA | Timolol | [224] | |
| Glaucoma | HEMA-DMA/GMA/Sil | Timolol | [225] | |
| Proliferative ocular diseases | Drug–polymer film | Pirfenidone | Increased duration of pirfenidone | [189,190] |
| Ocular cystinosis | Nanoparticles | Gold NPs | Cystine binding for ocular cystinosis management | [193] |
| Uveitis | Drug–polymer film | Dexamethasone | Provides 7-day corneal anti-inflammatory activity and 5-day anterior uveitis suppression | [194] |
| Uveitis | Molecular imprinting | Bromfenac | Eight-day sustained release of bromfenac in vivo | [195] |
| Color vision deficiency | Metasurfaces | Metasurfaces | Spectral correction of misperceived pigments for color vision enhancement | [198] |
| Bacterial keratitis | Coating | Glycidyl methacrylate | Against MRSA with killing efficacy > 99.99% | [226] |
| Ocular inflammation-related disorders | PLGA: thin film | Dexamethasone | Provides >30-day sustained dexamethasone release with demonstrated efficacy | [187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rykowska, I.; Nowak, I.; Nowak, R.; Michałkiewicz, O. Biodegradable Contact Lenses for Targeted Ocular Drug Delivery: Recent Advances, Clinical Applications, and Translational Perspectives. Molecules 2025, 30, 2542. https://doi.org/10.3390/molecules30122542
Rykowska I, Nowak I, Nowak R, Michałkiewicz O. Biodegradable Contact Lenses for Targeted Ocular Drug Delivery: Recent Advances, Clinical Applications, and Translational Perspectives. Molecules. 2025; 30(12):2542. https://doi.org/10.3390/molecules30122542
Chicago/Turabian StyleRykowska, Iwona, Iwona Nowak, Rafał Nowak, and Ola Michałkiewicz. 2025. "Biodegradable Contact Lenses for Targeted Ocular Drug Delivery: Recent Advances, Clinical Applications, and Translational Perspectives" Molecules 30, no. 12: 2542. https://doi.org/10.3390/molecules30122542
APA StyleRykowska, I., Nowak, I., Nowak, R., & Michałkiewicz, O. (2025). Biodegradable Contact Lenses for Targeted Ocular Drug Delivery: Recent Advances, Clinical Applications, and Translational Perspectives. Molecules, 30(12), 2542. https://doi.org/10.3390/molecules30122542








