Study on Mechanism of Surfactant Adsorption at Oil–Water Interface and Wettability Alteration on Oil-Wet Rock Surface
Abstract
1. Introduction
2. Results and Discussion
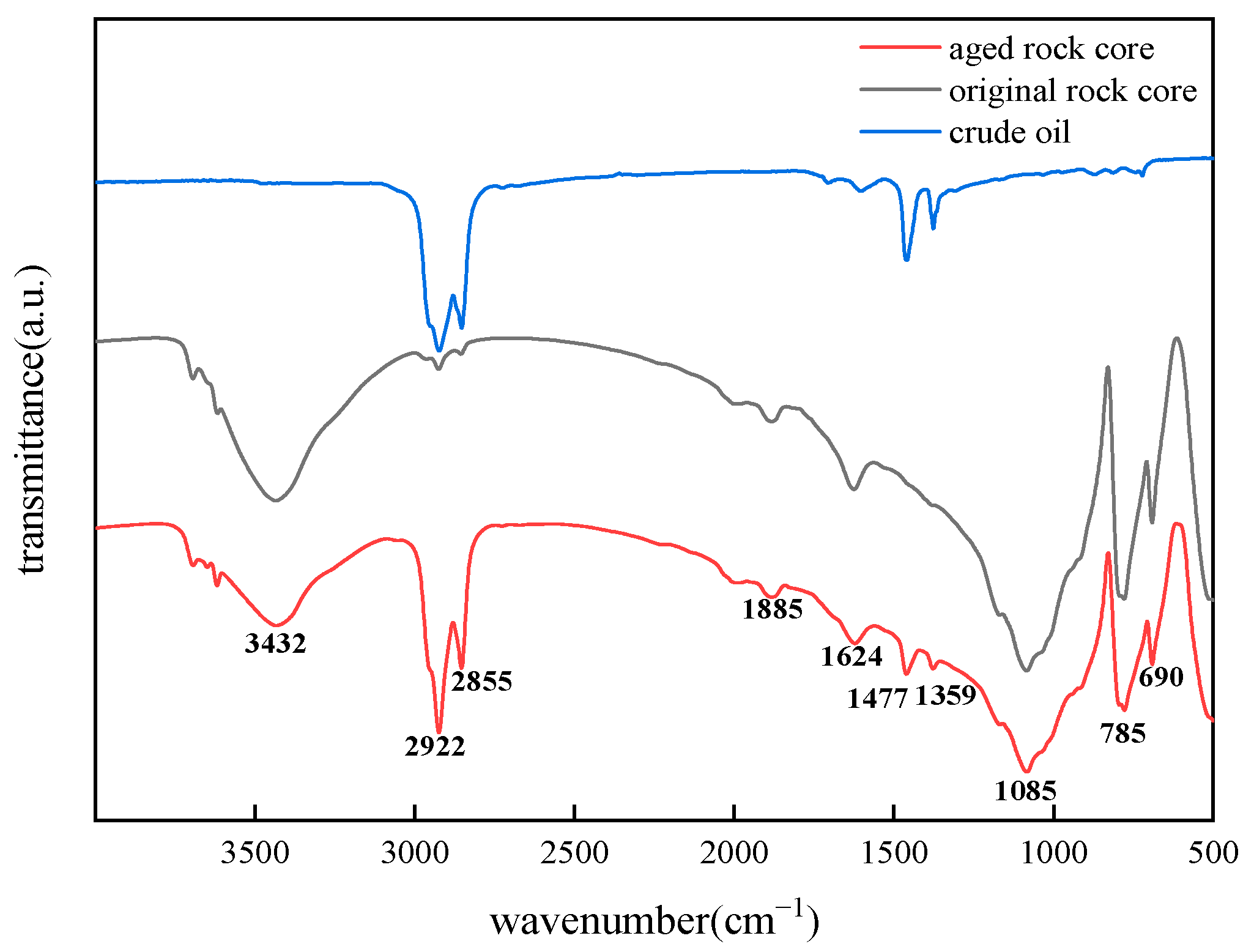
2.1. FT-IR Characterization Results
2.2. Surface (Interface) Properties of Surfactants
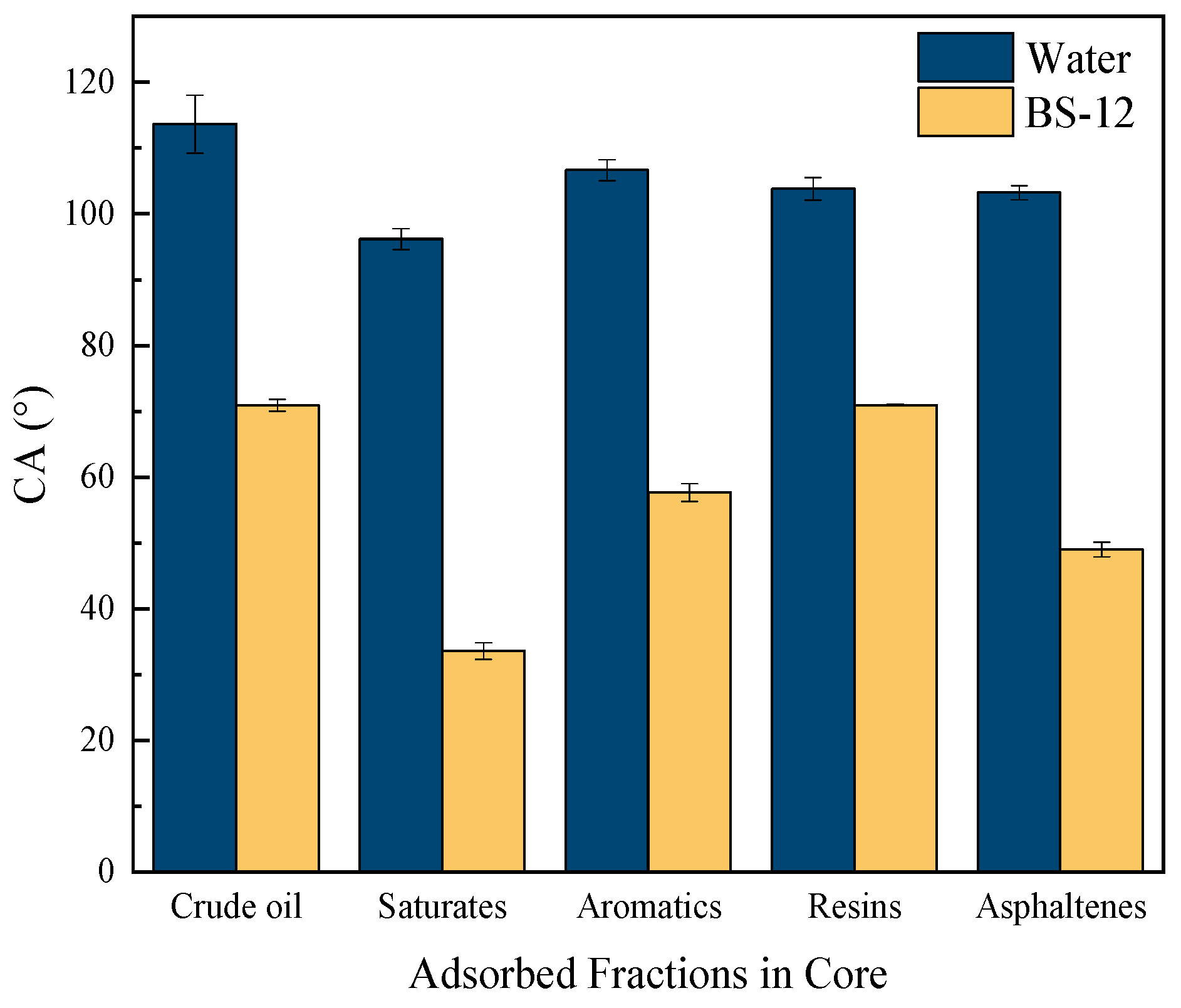
2.3. Wettability Performance of Surfactants
2.4. SEM Images Results
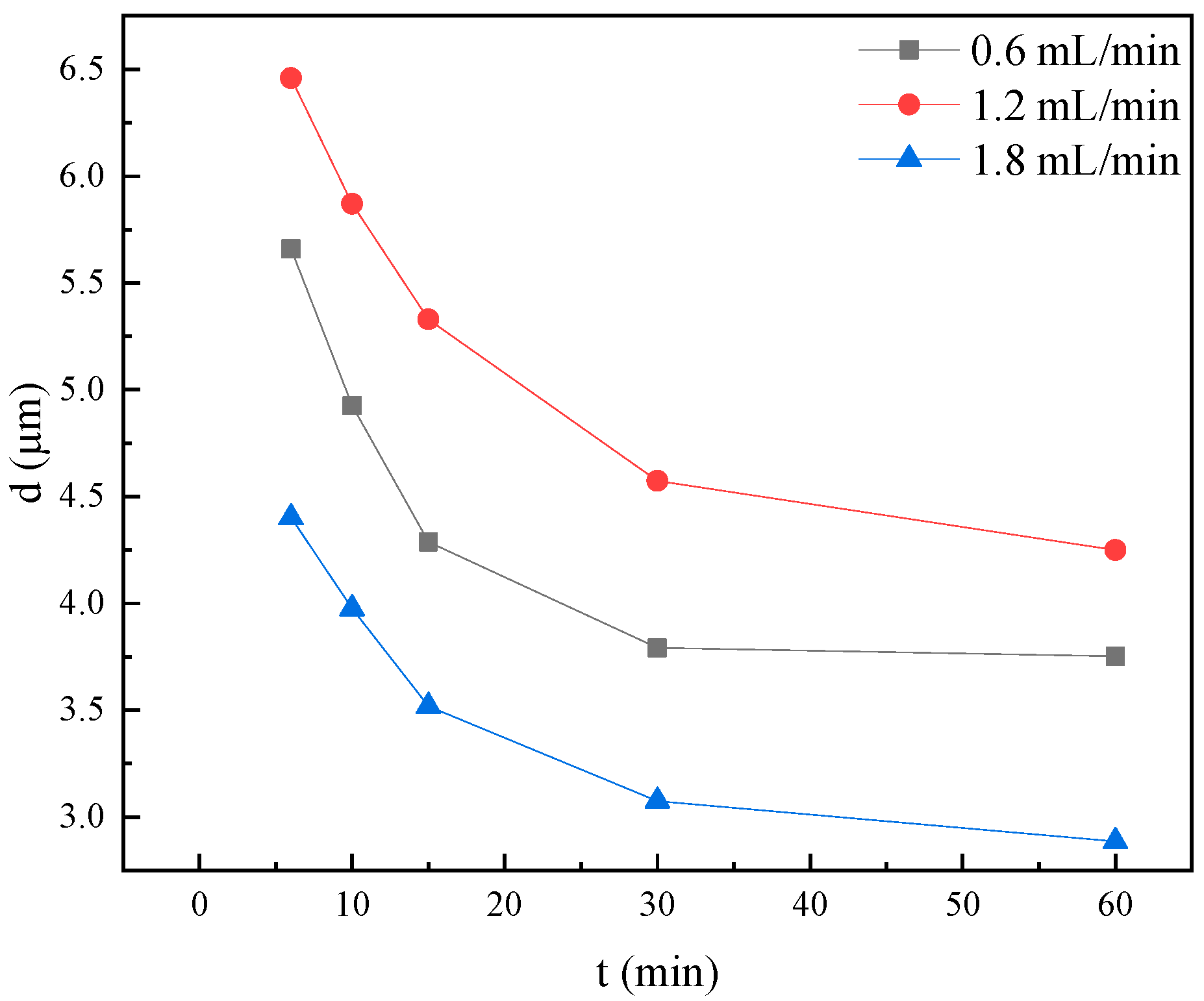
2.5. Core Displacement Experiment Results
3. Experimental Section
3.1. Reagents and Instruments
3.2. Experimental Procedures
3.2.1. Core Surface Preparation
3.2.2. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
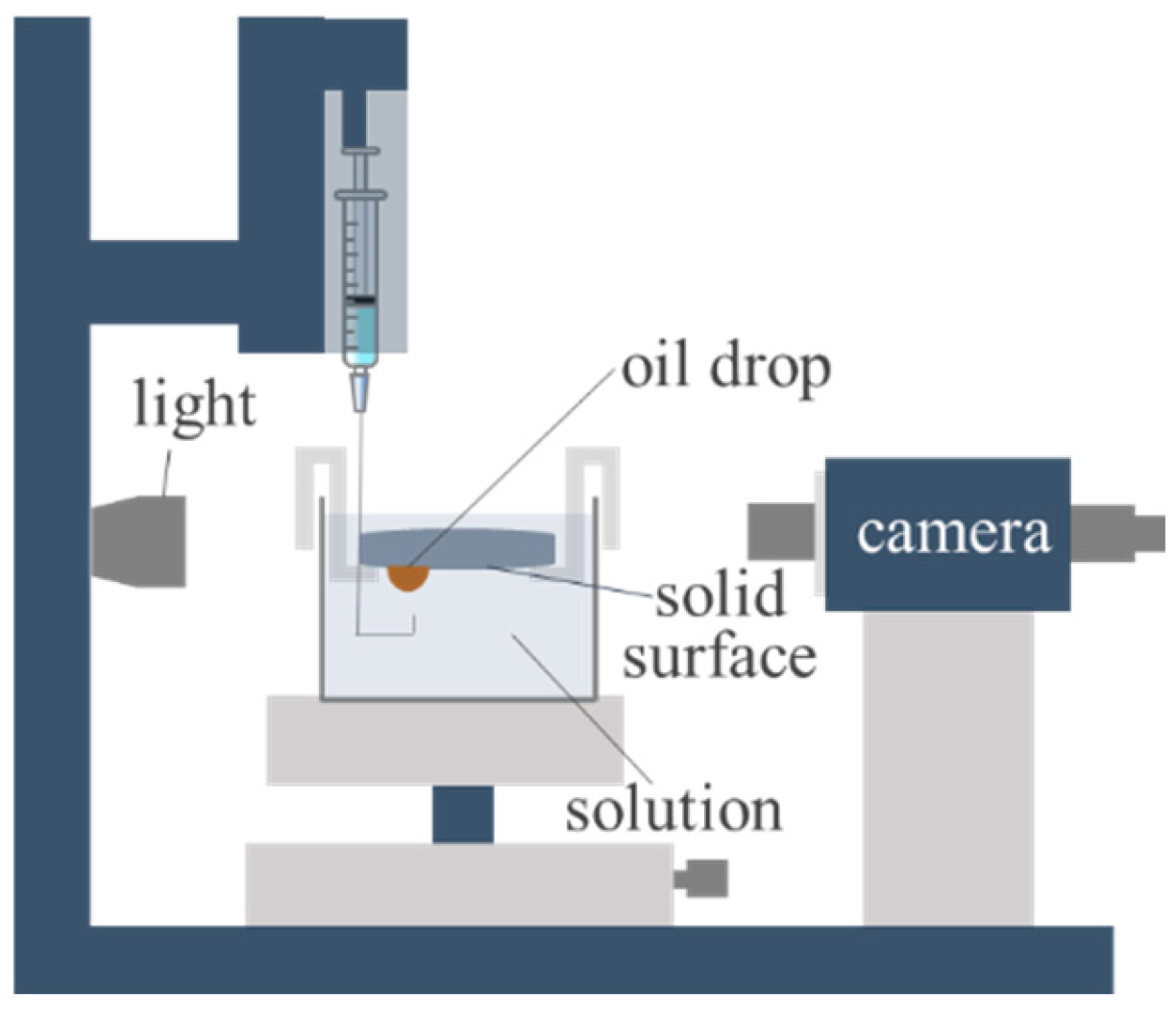
3.2.3. Wettability Measurement
3.2.4. Surface Tension Measurement
3.2.5. Interfacial Tension (IFT) Measurement
3.2.6. Scanning Electron Microscopy (SEM) Analysis
3.2.7. Core Flooding Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Liu, W.; Yu, J.; Liu, C.; Cao, Y.; Sun, M.; Li, M.; Meng, Z.; Yan, X. Technology Progress in High-Frequency Electromagnetic in Situ Thermal Recovery of Heavy Oil and Its Prospects in Low-Carbon Situations. Energies 2024, 17, 4715. [Google Scholar] [CrossRef]
- Dauyltayeva, A.; Mukhtarov, A.; Sagandykova, D.; Shakeel, M.; Pourafshary, P.; Musharova, D. Screening of chemicals to enhance oil recovery in a mature sandstone oilfield in Kazakhstan: Overcoming challenges of high residual oil. Appl. Sci. 2023, 13, 10307. [Google Scholar] [CrossRef]
- Sun, B.; Wu, G.; Zhao, H.; Wu, W.; Sun, C. Oil Emulsion and Displacement Mechanism of Surfactants during Water Flooding in Common Heavy Oil Reservoir. Oilfield Chem. 2024, 41, 138–145. [Google Scholar]
- Kumar, S.; Mandal, A. Studies on interfacial behavior and wettability change phenomena by ionic and nonionic surfactants in presence of alkalis and salt for enhanced oil recovery. Appl. Surf. Sci. 2016, 372, 42–51. [Google Scholar] [CrossRef]
- Zhong, X.; Song, J.; Yang, Y.; Cao, L.; Chen, L.; Zhao, H. Molecular insight into the enhanced oil recovery potential of a seawater-based zwitterionic/anionic surfactant compound for heavy oil reservoirs. J. Mol. Liq. 2024, 408, 125315. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Liang, X.; Shi, L.; Chen, M.; Wang, X.; Liu, W.; Ye, Z. New amphiphilic macromolecule as viscosity reducer with both asphaltene dispersion and emulsifying capacity for offshore heavy oil. Energy Fuels 2021, 35, 1143–1151. [Google Scholar] [CrossRef]
- Pei, H.; Shu, Z.; Zhang, G.; Ge, J.; Jiang, P.; Qin, Y.; Cao, X. Experimental study of nanoparticle and surfactant stabilized emulsion flooding to enhance heavy oil recovery. Pet. Sci. Eng. 2018, 163, 476–483. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, M.; Han, Y.; Wang, Y.; Yuan, Y. Comparative Study between Ultra-low Interfacial Tension and Emulsification Oriented Compound Systems on Recovering Heavy Oil. Oilfield Chem. 2020, 37, 715–720. [Google Scholar]
- Belhaj, A.F.; Elraies, K.A.; Mahmood, S.M.; Zulkifli, N.N.; Akbari, S.; Hussien, O.S.E. The effect of surfactant concentration, salinity, temperature, and PH on surfactant adsorption for chemical enhanced oil recovery: A review. Pet. Explor. Prod. Technol. 2020, 10, 125–137. [Google Scholar] [CrossRef]
- Moss, A.K.; Jing, X.D.; Archer, J.S. Laboratory investigation of wettability and hysteresis effects on resistivity index and capillary pressure characteristics. J. Pet. Sci. Eng. 1999, 24, 231–242. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, L.; Liu, K.; Yu, L.; Liu, J. Effects of wettability and pore water occurrence of gas storage space of shale reservoirs. J. Cent. South Univ. (Sci. Technol.) 2022, 53, 3575–3589. [Google Scholar]
- Souayeh, M.; Al-Maamari, R.S.; Aoudia, M. Experimental Investigation of Wettability Alteration of Oil-Wet Carbonates by a Non-ionic Surfactant. Energy Fuels 2018, 32, 11222–11233. [Google Scholar] [CrossRef]
- Jarrahian, K.; Seiedi, O.; Sheykhan, M.; Vafaie Sefti, M.; Ayatollahi, S. Wettability alteration of carbonate rocks by surfactants: A mechanistic study. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 1–10. [Google Scholar] [CrossRef]
- Katende, A.; Sagala, F. A Critical Review of Low Salinity Water Flooding: Mechanism, Laboratory and Field Application. Mol. Liq. 2019, 278, 627–649. [Google Scholar] [CrossRef]
- Buckley, J.S.; Liu, Y.; Monsterleet, S. Mechanisms of Wetting Alteration by Crude Oils. SPE J. 1998, 3, 54–61. [Google Scholar] [CrossRef]
- Standnes, D.C.; Austad, T. Wettability alteration in chalk: 2. Mechanism for wettability alteration from oil-wet to water-wet using surfactants. J. Pet. Sci. Eng. 2000, 28, 123–143. [Google Scholar] [CrossRef]
- Manshad, A.K.; Rezaei, M.; Moradi, S.; Nowrouzi, I.; Mohammadi, A.H. Wettability alteration and interfacial tension (IFT) reduction in enhanced oil recovery (EOR) process by ionic liquid flooding. J. Mol. Liquids. 2017, 248, 153–162. [Google Scholar] [CrossRef]
- Ma, H.; Ranimol, S.; Cameron, A. Synergetic System of Zwitterionic/Anionic Surfactants with Ultralow Interfacial Tension and High Salt Resistance for Enhancing Heavy Oil Recovery. Energy Fuels 2022, 36, 8216–8223. [Google Scholar] [CrossRef]
- Ahmadi, M.; Hou, Q.; Wang, Y.; Chen, Z. Interfacial and molecular interactions between fractions of heavy oil and surfactants in porous media: Comprehensive review. Adv. Colloid Interface Sci. 2020, 283, 102242. [Google Scholar] [CrossRef]
- Kokal, S.L.; Mohammed, A.-D. Case Studies of Emulsion Behavior at Reservoir Conditions. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 11–14 March 2007. [Google Scholar]
- Chen, Z.; Dong, M.; Husein, M.; Bryant, S. Effects of Oil Viscosity on the Plugging Performance of Oil-in-Water Emulsion in Porous Media. Ind. Eng. Chem. Res. 2018, 57, 7301–7309. [Google Scholar] [CrossRef]
- Lu, J.; Weerasooriya, U.P.; Pope, G.A. Investigation of gravity-stable surfactant floods. Fuel 2014, 124, 76–84. [Google Scholar] [CrossRef]
- Su, H.; Zhou, F.; Liu, Y.; Gao, Y.; Cheng, B.; Dong, R.; Liang, T.; Li, J. Pore-scale investigation on occurrence characteristics and conformance control mechanisms of emulsion in porous media. Pet. Explor. Dev. 2021, 48, 1241–1249. [Google Scholar] [CrossRef]
- Hou, B.; Wang, Y.; Huang, Y. Mechanistic study of wettability alteration of oil-wet sandstone surface using different surfactants. Appl. Surf. Sci. 2015, 330, 56–64. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, Z.; Xiao, C.; Gao, M.; Han, L.; Liu, Q.; Zhang, L.; Zhang, Q.; Zhang, L. The synergism of improving interfacial properties between betaine and crude oil for enhanced oil recovery. J. Mol. Liq. 2023, 383, 122046. [Google Scholar] [CrossRef]
- Cui, X.; Pan, Y.; Hu, F.; Han, L.; Zhu, X.; Zhang, L.; Zhou, Z.; Li, G.; Ma, G.; Zhang, L. Dynamic Interfacial Tensions of Surfactant and Polymer Solutions Related to High-Temperature and High-Salinity Reservoir. Molecules 2023, 28, 1279. [Google Scholar] [CrossRef]
- Bian, S.; Liu, P.; Mao, Z.; Huang, W.; Zhu, Y.; Zhang, L.; Hou, Y.; Zhang, L. Studying the factors determining the ultralow interfacial tensions of betaine solutions against crude oil. Colloids Surf. A Physicochem. Eng. Asp. 2024, 686, 133453. [Google Scholar] [CrossRef]
- Yao, Y.; Wei, M.; Kang, W. A review of wettability alteration using surfactants in carbonate reservoirs. Adv. Colloid Interface Sci. 2021, 294, 102477. [Google Scholar] [CrossRef]
- Shi, X.; Xu, Y.; Wu, Y.; Wu, B.; Xi, L. Wettability Changing Mechanism of Oil-wet Sandstone Surface by Anionic Surfactant. Oilfield Chem. 2024, 41, 680–685, 694. [Google Scholar]
- Hou, B.; Wang, Y.; Huang, Y. Mechanism and Influencing Factors of Wettability Alteration of Water-Wet Sandstone Surface by CTAB. J. Dispers. Sci. Technol. 2015, 36, 1587–1594. [Google Scholar] [CrossRef]
- Lyu, Y.; Chai, D.; Li, X.; Zhang, F.; Liu, L. Enlightenment of interfacial behavior and adhesion for study on wall sticking mechanism of condensate oil. Oil Gas Storage Transp. 2021, 40, 1338–13481. [Google Scholar]
- Ge, Q.; Ma, T.; Lun, Z. Effect of Interfacial Characteristics on Oil Removal Efficiency of Different Surfactants. Contemp. Chem. Industry 2024, 53, 1267–1271. [Google Scholar]
- Zhao, X.; Feng, Y.; Liao, G.; Liu, W. Visualizing in-situ emulsification in porous media during surfactant flooding: A microfluidic study. J. Colloid Interface Sci. 2020, 578, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, D.; Wang, Z.; Cao, R. The formation and viscoelasticity of pore-throat scaleemulsion in porous media. Petrol. Explor. Develop. 2017, 44, 111–118. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, G.; Bai, Y.; Wang, P.; Liu, X.; Wang, X. Probing the generation mechanism of emulsion based on shearing actions between multiphase fluids in porous media. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129997. [Google Scholar] [CrossRef]
- Wang, H.; Wei, B.; Hou, J.; Sun, Z.; Du, Q.; Zhou, K. Investigation of microflow mechanisms and emulsion size distribution in porous media. Phys. Fluids 2023, 35, 102003. [Google Scholar] [CrossRef]
- Wydro, P.; Paluch, M. Surface properties of cationic–nonionic mixed surfactant systems. Colloids Surf. A Physicochem. Eng. Asp. 2004, 245, 75–79. [Google Scholar] [CrossRef]
- Chen, L.; Hu, Z.Y.; Zhu, H.L. Synthesis of Cleavable Aryl Sulfonate anionic surfactants and a study of their surface activity. J. Surfactants Deterg. 2008, 11, 97–102. [Google Scholar] [CrossRef]
- Rosen, M.J.; Joy, T.K. Surfactants and Interfacial Phenomena, 4th ed.; Wiley: New York, NY, USA, 2012. [Google Scholar]










| Surfactant | M/g·mol−1 | CMC/mol·dm−3 | γCMC/mN·m−1 | Γmax/μmol·m−2 | Amin/nm2 | pC20 |
|---|---|---|---|---|---|---|
| SDBS | 348.5 | 1.92 × 10−3 | 30.50 | 2.23 | 0.74 | 3.55 |
| CTAB | 364.5 | 9.71 × 10−4 | 31.56 | 1.33 | 1.26 | 4.36 |
| BS-12 | 335.5 | 6.26 × 10−4 | 30.15 | 1.42 | 1.17 | 4.54 |
| OP-10 | 646 | 3.17 × 10−4 | 29.63 | 2.76 | 0.60 | 4.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Tong, Y.; Zhang, Y.; Yang, P.; Wang, C.; Liu, J. Study on Mechanism of Surfactant Adsorption at Oil–Water Interface and Wettability Alteration on Oil-Wet Rock Surface. Molecules 2025, 30, 2541. https://doi.org/10.3390/molecules30122541
Tang X, Tong Y, Zhang Y, Yang P, Wang C, Liu J. Study on Mechanism of Surfactant Adsorption at Oil–Water Interface and Wettability Alteration on Oil-Wet Rock Surface. Molecules. 2025; 30(12):2541. https://doi.org/10.3390/molecules30122541
Chicago/Turabian StyleTang, Xinyu, Yaoyao Tong, Yuhui Zhang, Pujiang Yang, Chuangye Wang, and Jinhe Liu. 2025. "Study on Mechanism of Surfactant Adsorption at Oil–Water Interface and Wettability Alteration on Oil-Wet Rock Surface" Molecules 30, no. 12: 2541. https://doi.org/10.3390/molecules30122541
APA StyleTang, X., Tong, Y., Zhang, Y., Yang, P., Wang, C., & Liu, J. (2025). Study on Mechanism of Surfactant Adsorption at Oil–Water Interface and Wettability Alteration on Oil-Wet Rock Surface. Molecules, 30(12), 2541. https://doi.org/10.3390/molecules30122541






