Search for Antiviral Preparations in Series of New Derivatives of N-Substituted Piperidines
Abstract
1. Introduction
2. Results and Discussion
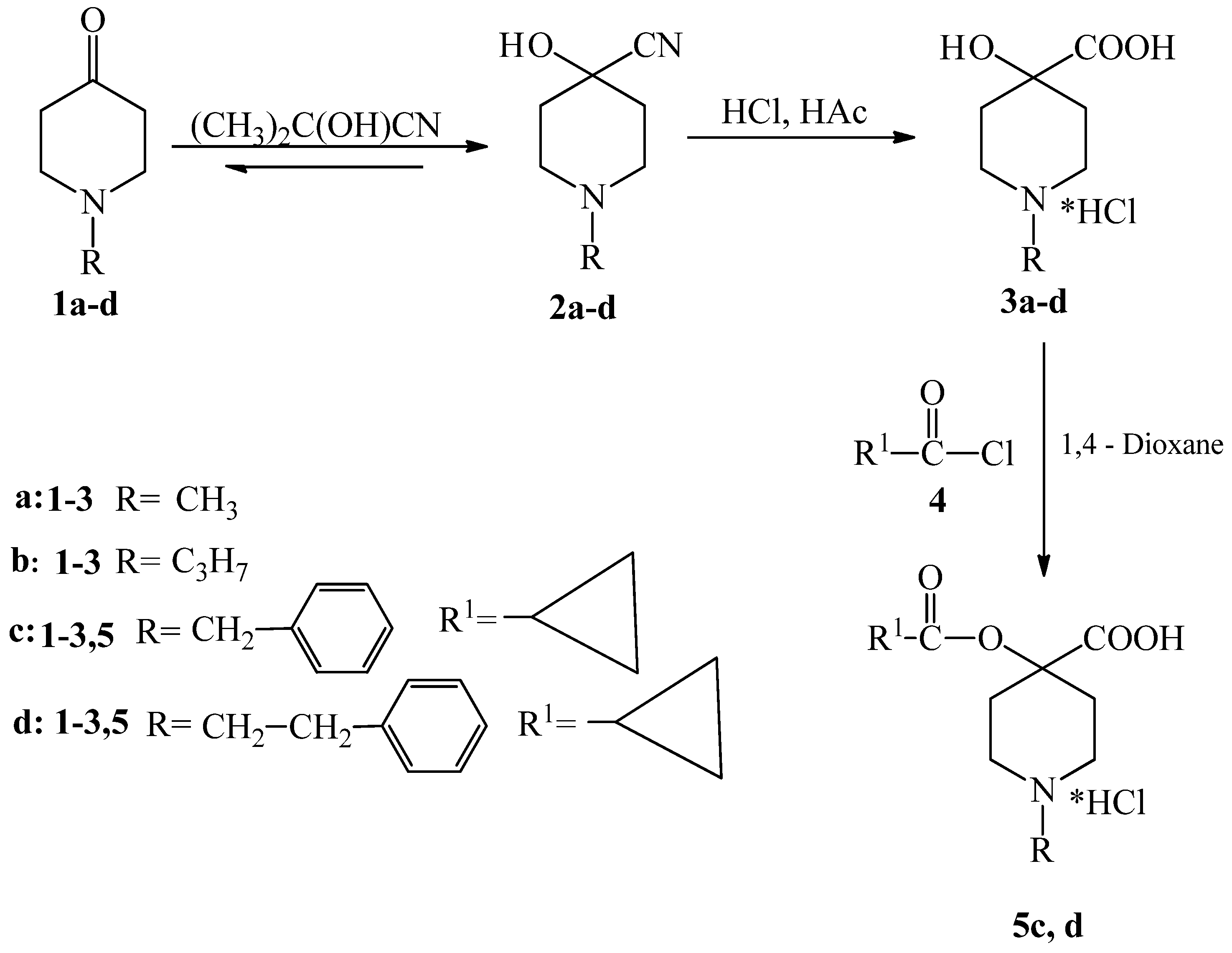
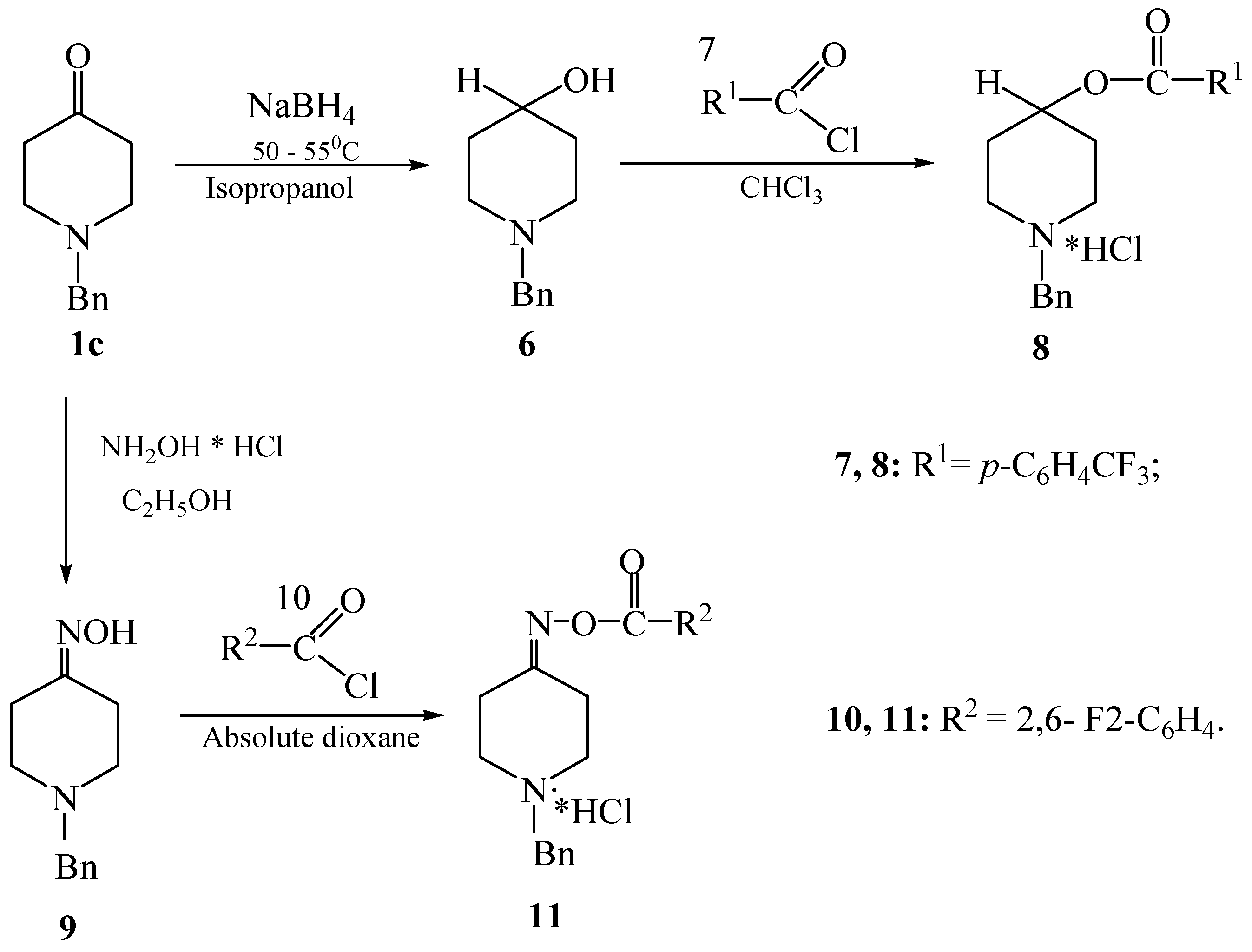
2.1. Synthesis of New Piperidine Derivatives
2.2. The Determination of Cytotoxicity of the Investigational Drugs In Vitro
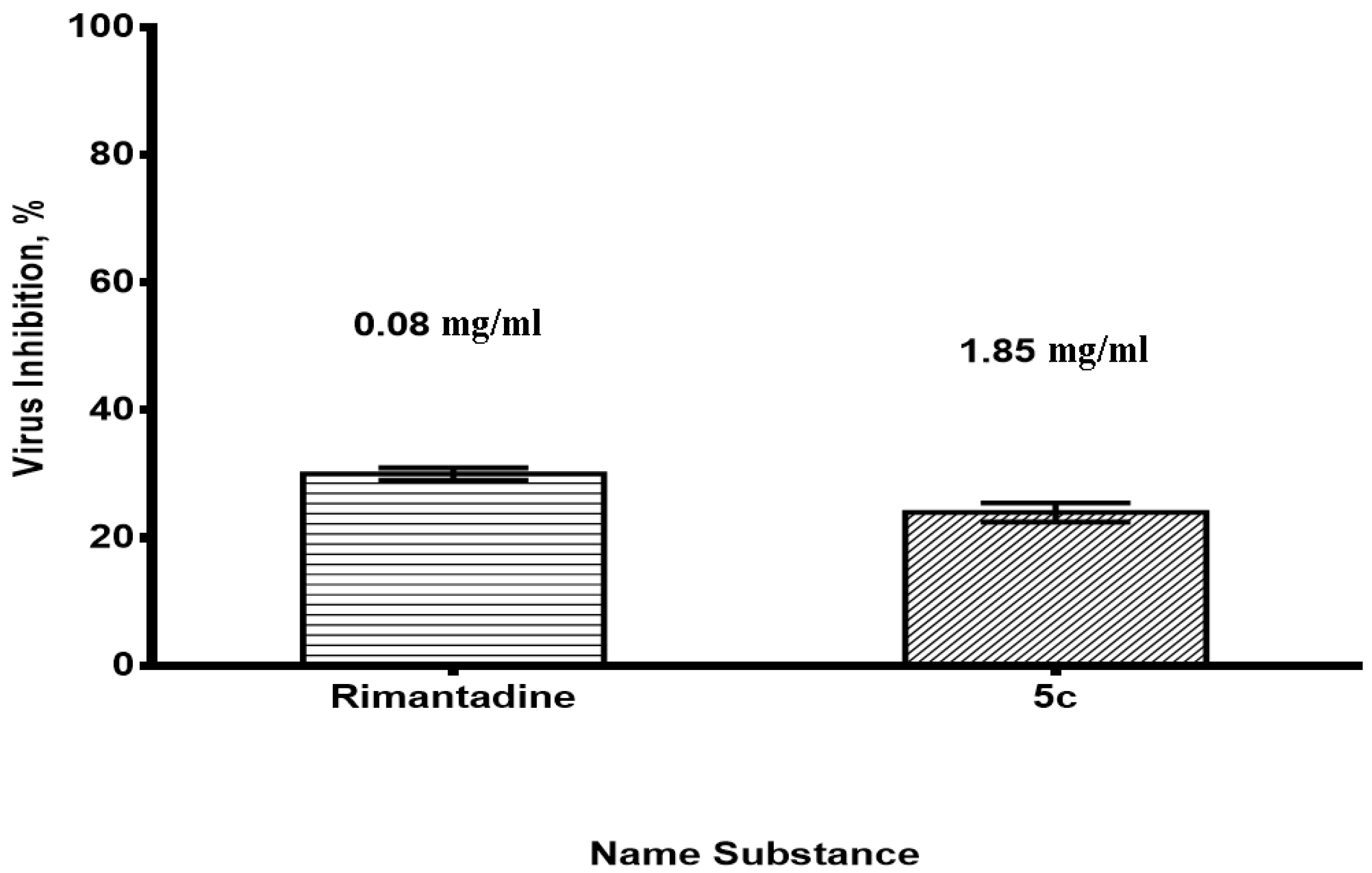
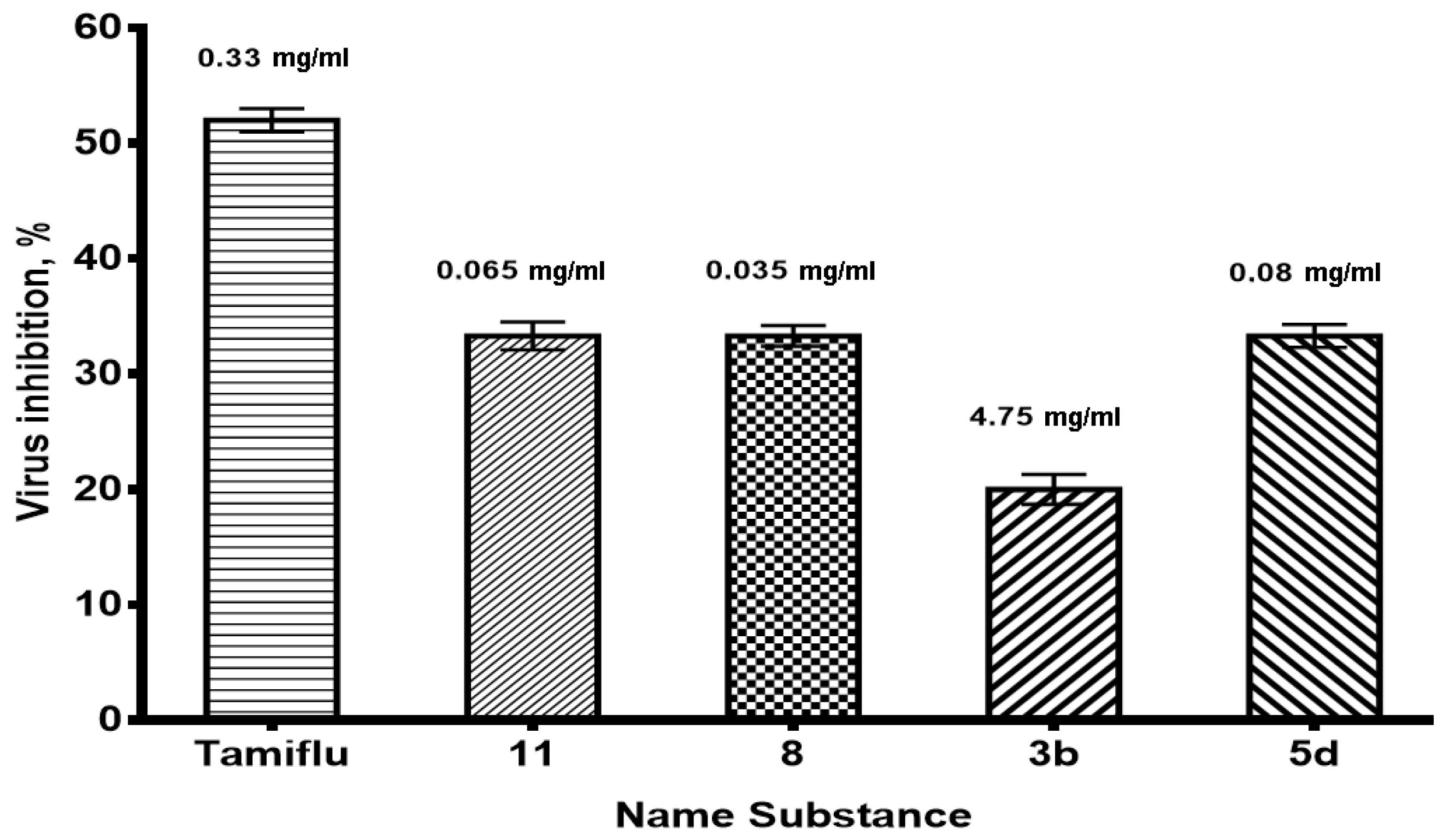
2.3. The Study of the Antiviral Activity of the Investigated Substances in the In Vitro Experiments
3. Materials and Methods
3.1. Chemical Experimental Part
3.1.1. Reagents and Equipment
3.1.2. Synthesis of Piperidine Carboxylic Acids (3a–d)
3.1.3. Synthesis of Piperidine-Containing Esters of Cyclopropanecarboxylic Acid (5c–d)
3.1.4. Synthesis of Piperidine-Containing Esters of Fluorobenzoic Acids (8, 11)
3.2. Biological Experimental Part
- -
- RIMANTADIN-STI, manufactured by Irbit Chemical and Pharmaceutical Plant OJSC, Moscow, Russia.
- -
- Tamiflu, Roche produced by Cenexi S.a.S, Osny, France.
3.3. The Cell Culture and Virus
3.4. Determination of Cytotoxicity of Substances In Vitro
3.5. The Determination of an Antiviral Action of the Substances In Vitro
3.6. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khater, F.; Moorman, P. Complications of influenza. South Med. J. 2003, 96, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Piedra, A. Influenza virus pneumonia: Pathogenesis, treatment, and prevention. Semin. Respir. Infect. 1995, 10, 216–233. [Google Scholar] [PubMed]
- Paules, C.; Subbarao, K. Influenza. Lancet 2019, 393, 1627–1640. [Google Scholar] [CrossRef]
- Widmer, K.; Zhu, Y.; Williams, J.; Griffin, R.; Edwards, K. Influenza complications in children. Clin. Infect. Dis. 2020, 70, 1375–1383. [Google Scholar]
- Monto, A.; Fukuda, K. Lessons from Influenza Pandemics of the 20th Century. Clin. Infect. Dis. 2021, 72, 2006–2012. [Google Scholar]
- Glezen, W.; Schmier, K.; Kuehn, C.; Ryan, K.; Oxford, J. The Burden of Influenza B: A Structured Literature Review. Am. J. Public Health 2020, 103, e43–e51. [Google Scholar] [CrossRef]
- Matrosovich, M.; Tusikov, A.; Bovin, N.; Gambaryan, A.; Klimov, A.; Castrucci, M.R.; Donatelli, I.; Kawaoka, Y. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hem agglutinins after their introduction into mammals. J. Virol. 2000, 74, 8502–8512. [Google Scholar] [CrossRef]
- Moorman, J. Viral characteristics of influenza. South Med. J. 2003, 96, 758–761. [Google Scholar] [CrossRef]
- Stevens, J.; Blixt, O.; Glaser, L.; Taubenberger, J.K.; Palese, P.; Paulson, J.C.; Wilson, I.A. Glycan microarray analysis at the hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. J. Mol. Biol. 2006, 355, 1143–1155. [Google Scholar] [CrossRef]
- Van, K.; Nicoll, A. A human case of swine influenza virus infection in Eurupe—Implications for human health and research. Euro Surveill. 2009, 14, 19124. [Google Scholar]
- Myers, K.; Olsen, W.; Gray, C. Cases at swine influenza in humans: Review at the literature. Clin. Infect. Dis. 2007, 44, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.; McCarthy, T.; Capuano, W.; Setterquist, S.F.; Olsen, C.W.; Alavanja, M.C.; Lynch, C.F. Swine workers and swine influenza virus infections. Emerg. Infect. Dis. 2007, 13, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Oxford, S.; Lambkin, R. Targeting influenza virus neuraminidase—A new strategy for antiviral therapy. Drug Discov. Today 1998, 3, 448–456. [Google Scholar] [CrossRef]
- Oxford, S. Influenza A pandemics at the 20th century with special reference to 1918: Virology, pathology and epidemiology. Rev. Med. Virol. 2000, 10, 119–133. [Google Scholar] [CrossRef]
- Gani, R.; Hughes, H.; Fleming, O.; Griffin, T.; Medlock, J.; Leach, S. Potential impact of antiviral drug use during influenza pandemic. Emerg. Infect. Dis. 2005, 11, 1355–1362. [Google Scholar] [CrossRef]
- Weintraub, M.; Sabol, S.; Kane, M.; Borcherding, R. Recent advances in the synthesis of piperidones and piperidines. Tetrahedron 2003, 59, 2953–2989. [Google Scholar] [CrossRef]
- Pichkhadze, M.; Smagulova, S.; Kadyrova, M.; Praliyev, K.; Yu, V. Local Anesthetic Activity of a Promising Piperidine Derivative (LAS-54) in Combination with Epinephrine. Pharm. Chem. J. 2016, 50, 600–602. [Google Scholar] [CrossRef]
- Sadyrbayeva, F.; Akhmetova, G.; Praliyev, K.; Osman, H.; Korotetskaya, N. New Anti-Infective Preparations of Naphthyloxypropargylpiperidine Series. Eurasian Chem.-Technol. J. 2017, 19, 183–190. [Google Scholar] [CrossRef]
- Zolotareva, D.; Zazybin, A.; Dauletbakov, A.; Belyankova, Y.; Giner, B.; Tursynbek, S.; Seilkhanov, T.; Kairullinova, A. Morpholine, Piperazine, and Piperidine Derivatives as Antidiabetic Agents. Molecules 2024, 29, 3043. [Google Scholar] [CrossRef]
- Frolov, N.A.; Vereshchagin, A. Piperidine Derivatives: Recent Advances in Synthesis and Pharmacological Applications. Int. J. Mol. Sci. 2023, 24, 2937. [Google Scholar] [CrossRef]
- Abdelshaheed, M.; Fawzy, M.; El-Subbagh, I.; Youssef, M. Piperidine Nucleus in the Field of Drug Discovery. Future J. Pharm. Sci. 2021, 7, 188. [Google Scholar] [CrossRef]
- Dawood, S.; Dayl, A. Synthesis, Identification and Molecular Docking Studies of N-Functionalized Piperidine Derivatives Linked to 1,2,3-Triazole Ring. Synth. Commun. 2020, 50, 2422–2431. [Google Scholar] [CrossRef]
- Nairoukh, Z.; Strieth-Kalthoff, F.; Bergander, K.; Glorius, F. Understanding the Conformational Behavior of Fluorinated Piperidines: The Origin of the Axial-F Preference. Chem. Eur. J. 2020, 26, 6141–6146. [Google Scholar] [CrossRef] [PubMed]
- Wagener, T.; Heusler, A.; Nairoukh, Z.; Bergander, K.; Daniliuc, C.; Glorius, F. Accessing (Multi)Fluorinated Piperidines Using Heterogeneous Hydrogenation. ACS Catal. 2020, 10, 12052–12057. [Google Scholar] [CrossRef]
- Le Roch, M.; Renault, J.; Argouarch, G.; Lenci, E.; Trabocchi, A.; Roisnel, T.; Gouault, N.; Lalli, C. Synthesis and Chemoinformatic Analysis of Fluorinated Piperidines as 3D Fragments for Fragment-Based Drug Discovery. J. Org. Chem. 2024, 89, 4932–4946. [Google Scholar] [CrossRef]
- Gillis, P.; Eastman, J.; Hill, D.; Donnelly, J.; Meanwell, A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Kihakh, S.; Melnykov, K.; Bilenko, V.; Trofymchuk, S.; Liashuk, O.; Grygorenko, O. Gem-Difluoro-3-azabicyclo [3.n.1]alkanes and Their Derivatives—Bicyclic Fluorinated Piperidine Isosteres for Drug Discovery. Eur. J. Org. Chem. 2023, 27, e202300937. [Google Scholar] [CrossRef]
- Rizzo, C.; Amata, S.; Pibiri, I.; Pace, A.; Buscemi, S.; Palumbo Piccionello, A. FDA-Approved Fluorinated Heterocyclic Drugs from 2016 to 2022. Molecules 2023, 28, 3248. [Google Scholar] [CrossRef]
- Aiming, S.; David, L.; Kenneth, H.; James, P. 3-Fluoropiperidines and N-Methyl-3-fluoropiperidinium Salts: The Persistence of Axial Fluorine. Chem. Eur. J. 2005, 11, 1579–1591. [Google Scholar]
- Maksatova, A.; Akhmetova, G.; Datkhayev, U.; Omyrzakov, M.; Praliyev, K.; Ross, S.; Seilkhanov, O. Biological active fluorobenzoates of piperidine range. Res. J. Pharm. Technol. 2020, 13, 2261–2265. [Google Scholar] [CrossRef]
- Boshkayeva, A.; Sayakova, G.; Kiyekbayeva, L.; Akhmetova, G.; Bekbayeva, L.; Dyusenova, N.; Mamurova, A.; Akhmetova, A. An approach of quantum chemical methods for the development and substantiation of the structure of new piperidine compounds. Egypt. J. Chem. 2021, 64, 5143–5151. [Google Scholar] [CrossRef]
- Issayeva, U.; Akhmetova, G.; Datkhayev, U.; Omyrzakov, M.; Praliyev, K.; Ross, S. The search for biologically active compounds in the series of N-ethoxyethylpiperidine derivatives. Eurasian Chem.-Technol. J. 2019, 21, 125–133. [Google Scholar] [CrossRef]
- Reed, L.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Spalatin, J.; Hanson, P.; Beard, D. The haemagglutination-elution pattern as a marker in characterizing Newcastle disease virus. Avian Dis. 1970, 14, 542–549. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55. [Google Scholar] [CrossRef]
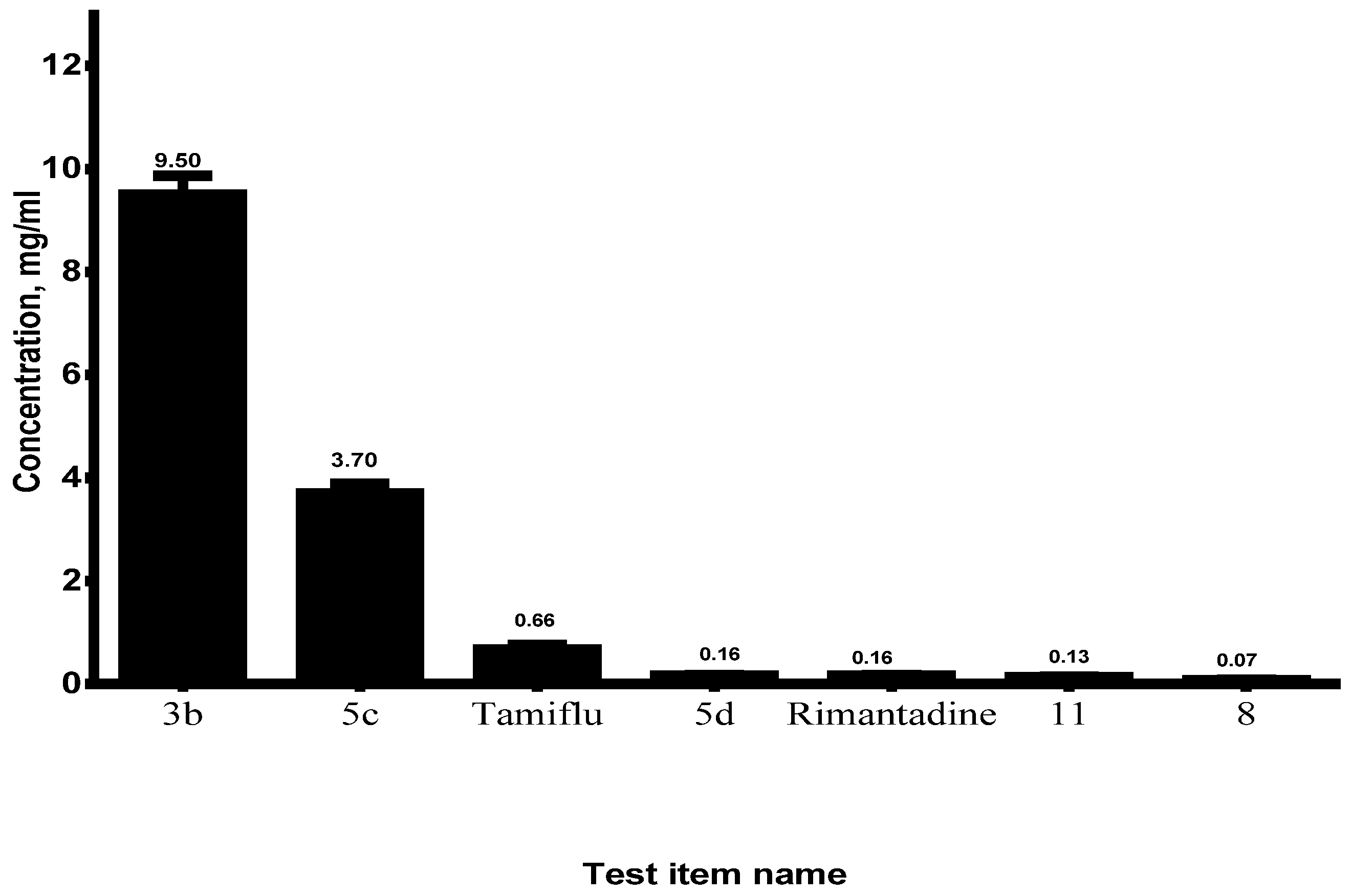
| Name of Substance | CC50 ± SD, mg/mL |
|---|---|
| 3b | 9.50 ± 0.364 |
| 5c | 3.70 ± 0.189 |
| 5d | 0.16 ± 0.006 |
| 8 | 0.07 ± 0.005 |
| 11 | 0.13 ± 0.002 |
| Rimantadine | 0.16 ± 0.011 |
| Tamiflu | 0.66 ± 0.091 |
| Name | Concentration, mg/mL | Titre in HAU * | Titre in HAU for PC 1 |
|---|---|---|---|
| 5c | 1.850 | 3.8 ± 1.1 | 5.0 ± 0 |
| 0.925 | 4.4 ± 0.6 | ||
| 0.463 | 5.0 ± 0 | ||
| 0.231 | 5.0 ± 0 | ||
| 0.116 | 5.0 ± 0 | ||
| 0.058 | 5.0 ± 0 | ||
| Rimantadine | 0.080 | 4.2 ± 0.4 | 6.0 ± 0 |
| 0.040 | 4.2 ± 0.4 | ||
| 0.020 | 4.2 ± 0.4 | ||
| 0.010 | 4.2 ± 0.4 | ||
| 0.005 | 4.2 ± 0.4 | ||
| 0.003 | 4.2 ± 0.4 |
| Name | Concentration, mg/mL | Titre in HAU * | Titre in HAU for PC 1 |
|---|---|---|---|
| 11 | 0.0650 | 4.0 ± 0 | 6.0 ± 0 |
| 0.0325 | 4.0 ± 0 | ||
| 0.0163 | 6.0 ± 0 | ||
| 0.0081 | 6.0 ± 0 | ||
| 0.0041 | 6.0 ± 0 | ||
| 0.0020 | 6.0 ± 0 | ||
| 8 | 0.0350 | 4.0 ± 0 | 6.0 ± 0 |
| 0.0175 | 5.0 ± 0 | ||
| 0.0088 | 5.0 ± 0 | ||
| 0.0044 | 6.0 ± 0 | ||
| 0.0022 | 6.0 ± 0 | ||
| 0.0011 | 6.0 ± 0 | ||
| 3b | 4.75 | 4.0 ± 0 | 5.0 ± 0 |
| 2.38 | 4.4 ± 0.6 | ||
| 1.19 | 5.0 ± 0 | ||
| 0.59 | 5.0 ± 0 | ||
| 0.30 | 5.0 ± 0 | ||
| 0.15 | 5.0 ± 0 | ||
| 5d | 0.0800 | 4.0 ± 0 | 6.0 ± 0 |
| 0.0400 | 4.8 ± 0.5 | ||
| 0.0200 | 6.0 ± 0 | ||
| 0.0100 | 6.0 ± 0 | ||
| 0.0050 | 6.0 ± 0 | ||
| 0.0025 | 6.0 ± 0 | ||
| Tamiflu | 0.33 | 2.4 ± 0.5 | 5.0 ± 0 |
| 0.17 | 2.4 ± 0.5 | ||
| 0.08 | 3.0 ± 0 | ||
| 0.04 | 3.0 ± 0 | ||
| 0.02 | 4.0 ± 0 | ||
| 0.01 | 4.0 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmetova, G.S.; Issayeva, U.B.; Praliyev, K.D.; Korotetskiy, I.S.; Seilkhanov, T.M.; Ross, S.A.; Omyrzakov, M.T.; Datkhayev, U.M.; Tassibekov, K.S.; Ivanova, L.N.; et al. Search for Antiviral Preparations in Series of New Derivatives of N-Substituted Piperidines. Molecules 2025, 30, 2540. https://doi.org/10.3390/molecules30122540
Akhmetova GS, Issayeva UB, Praliyev KD, Korotetskiy IS, Seilkhanov TM, Ross SA, Omyrzakov MT, Datkhayev UM, Tassibekov KS, Ivanova LN, et al. Search for Antiviral Preparations in Series of New Derivatives of N-Substituted Piperidines. Molecules. 2025; 30(12):2540. https://doi.org/10.3390/molecules30122540
Chicago/Turabian StyleAkhmetova, Gulmira S., Ulzhalgas B. Issayeva, Kaldybay D. Praliyev, Ilya S. Korotetskiy, Tulegen M. Seilkhanov, Samir A. Ross, Manas T. Omyrzakov, Ubaidilla M. Datkhayev, Khaidar S. Tassibekov, Lyudmila N. Ivanova, and et al. 2025. "Search for Antiviral Preparations in Series of New Derivatives of N-Substituted Piperidines" Molecules 30, no. 12: 2540. https://doi.org/10.3390/molecules30122540
APA StyleAkhmetova, G. S., Issayeva, U. B., Praliyev, K. D., Korotetskiy, I. S., Seilkhanov, T. M., Ross, S. A., Omyrzakov, M. T., Datkhayev, U. M., Tassibekov, K. S., Ivanova, L. N., & Zubenko, N. V. (2025). Search for Antiviral Preparations in Series of New Derivatives of N-Substituted Piperidines. Molecules, 30(12), 2540. https://doi.org/10.3390/molecules30122540








