Abstract
This review examines how plastics break down into dangerous pollutants like microplastics, nanoplastics, and persistent organic pollutants (POPs) that can contaminate the environment, make their way into the human food chain, and provoke toxicological effects in humans. According to the reviewed literature, new biomarkers associated with their exposure should be identified, and new methods for detecting them in the environment and in food should be developed and validated. It would also be interesting to improve research on the interaction between micro- and nanoplastics and human cells, their impact on DNA, and their long-term health effects. Promoting sustainable practices and adherence to the 3R strategies (reduce, reuse, and recycle) to transform hazardous waste into valuable resources is crucial to protecting public health from dangerous contaminants as we wait on the development of new diagnostic methods and more stringent legislation.
1. Introduction
Some plastic materials can contaminate the environment with POPs (such as dioxins and furans) [1] and impact carbon emissions [2]. Improper plastic disposal can damage ecosystems and human health, not only because of the adverse effects linked to their existence [3] but also because plastic waste can accumulate and transport heavy metals, pesticides, and other persistent organic pollutants [4]. In the ocean, plastic debris is fragmented into microplastics (MPs) and nanoplastics (NPs) [5], which can adhere to marine salt or be ingested by marine organisms, subsequently entering the food chain and building up in humans, leading to uncertain consequences [6]. Marine plastic pollution increases atmospheric CO2 levels, diminishing the ocean’s ability to sequester carbon [7]. The significant global crisis due to plastic waste is exacerbated by gas emissions from plastic incineration [8,9]. This paper reviewed papers discussing what happens to plastics when they are disposed of in different ecosystems (terrestrial, marine, air), how their derivatives can affect human health, the methods by which it is possible to determine their derivatives in traces, and sustainable approaches designed to reduce plastic waste.
2. Plastics and Micro- and Nanoplastics
2.1. Plastics Classification
Plastics are polymeric molecules with high molecular weight [10] and with additives such as plasticizers, flame retardants, stabilizers, pigments, and lubricants. The additives do not chemically bond with plastics except for some reactive organic additives that integrate into the polymer chain [11]. “Plastics” refers to substances readily molded into diverse shapes and sizes. It originates from the Greek word “plasticos” [12]. Plastics can be classified based on origin, temperature-dependent behavior, and preservative techniques (Figure 1).
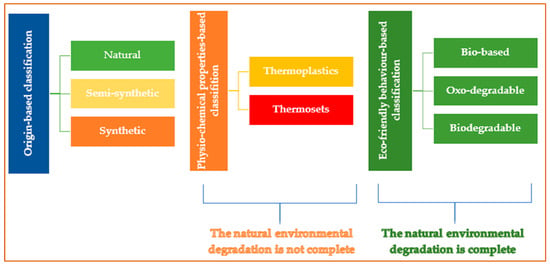
Figure 1.
Plastics classification.
Origin-based classification divides plastics into natural, semi-synthetic, and synthetic subdomains. Natural polymers such as chitin, lignin, and starch are derived from substances like horn, amber, and tortoiseshell, while synthetic polymers, including silicone, polyethylene, polystyrene, and nylon, are produced from petrochemicals [13] (Table 1).

Table 1.
Plastics application overview.
Semi-synthetic plastics are created by altering natural polymer materials like cellulose [28].
Plastics can be classified as thermoplastics based on their physicochemical properties and thermosets. The thermoplastics are suitable for recycling. They can soften upon heating, can melt, and can be reshaped multiple times [29].
Thermosets are materials that cannot be remelted after being formed. They retain their structural integrity when subjected to heat [30]. It is essential to understand that neither thermoplastics nor thermosets undergo complete natural environmental degradation.
On the contrary, bio-based, oxo-degradable, and biodegradable plastics are expected to degrade in the environment, yet their ability to biodegrade is a subject of debate. Biodegradable plastics can produce MPs since their degradation is designed for specific conditions, like composting, and they face challenges in breaking down in natural environments like soil or ocean ecosystems. Various factors, such as temperature, microbial presence, oxygen levels, and environmental conditions, can affect their degradation [31]. Numerous bioplastics produce residues (including POPs), undermining their assertions of being sustainable [32]. Additionally, the biodegradation of bioplastics may present certain disadvantages. As they decompose, they can release nutrients that can lead to eutrophication, and the availability of microorganisms responsible for biodegradation to higher-trophic-level organisms might decrease, potentially disrupting food webs and resulting in the accumulation of toxins [33]. Thus, properly managing biodegradation in complex ecosystems is vital to weigh the benefits against potential risks [34].
Bio-based plastics like bio-polyethylene (bio-PE), polyethylene terephthalate (bio-PET), and bio-polyamides (bio-PA) are derived from renewable sources such as plants, corn cellulose, and starch [31]. Because they come from sustainable materials, they have a reduced carbon footprint and improved circularity. However, scientific evidence concerning the biodegradability and sustainability of these new materials is still lacking [35].
Oxo-degradable plastics comprise polyethylene mixed with TDPA™ (London, UK) to improve polymer chains. They allow much fragmentation but do not bring the compound down to its elementary base [36]. Oxo-degradable plastics are classified into hydro-degradable (which break down from hydrolysis) and photodegradable plastics (which break down from UV radiation) [37].
Bioplastics (e.g., PLA, PHA, polylactide, and polycaprolactone) are polymers that form biogases and biomass when they are subjected to microbial degradation [38].
2.2. Micro- and Nanoplastics
Under ecological stress (ultraviolet rays and digestion) and biotic processes, synthetic plastics can decompose into microplastics ( 5000 µm) and nanoplastics (diameter ≤ 1000 nm) [10]. The fibers and fragments are the most frequently encountered forms in seawater [39]. Elements leading to the presence of MPs and NPs in the environment include urban dust, markings on road surfaces, and beauty products [40].
Microplastics can be divided into primary microplastics (i.e., microbeads in personal care items, plastic pellets used in industry, and synthetic fibers from textiles released during washing), which are released directly into the environment, and secondary microplastics (i.e., plastic bottle fragments, fishing net fibers, and vehicle tire abrasion), which are produced from the degradation of larger plastic items [41]. The primary sources of secondary plastics are laundering textiles, made from mixed synthetic fibers [42], and the wear of car tires [43].
2.3. Degradation of Plastics
Six processes can break down plastic materials: photodegradation, thermal (or oxidative) degradation, ozone degradation, mechanochemical degradation, catalytic degradation, and biodegradation.
Photochemical degradation reactions occur on the polymer surface. Ultraviolet radiation breaks C–C bonds by forming esters, aldehydes, and propyls [44].
Thermal degradation reactions affect the entire polymer, initiating from the weakest bonds. They necessitate the presence of ultraviolet light and elevated temperatures [45]. Ozone degrades polymeric materials by generating reactive oxygen species [46].
The mechanochemical process can reduce the weight of the molecules by ultrasonic irradiation (which generates radical reactions on the chain side) or mechanical stress.
Catalytic degradation process reduces polyolefins into oils and gas [12].
Biodegradation consists of natural and synthetic plastic degradation by bacteria, fungi, and Actinomycetes. The process results in low-molar-mass molecules such as acids, terpenes, aldehydes, water, and gases such as methane, carbon dioxide, and nitrogen [47]. It can happen in aerobic and anaerobic modes. Aerobic biodegradation produces CO2 and H2O. Anaerobic processes generate CO2 and CH4 [48].
Polymeric materials’ biodegradation comprises biodeterioration, biofragmentation, assimilation, and mineralization (Figure 2).
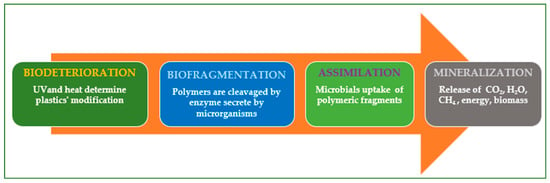
Figure 2.
Polymeric materials’ biodegradation.
Biodeterioration refers to the process in which mechanical, physical, and chemical stresses alter the plastic waste (the decline of the plastic materials can be observed without the aid of instruments). Biofragmentation refers to how microbial enzymes (e.g., lipase, manganese peroxidase, esterase, amidase, and laccase) decrease the polymers’ molecular weight [49]. Assimilation refers to how microorganisms employ polymers as carbon and nitrogen sources during aerobic respiration, anaerobic respiration, and fermentation [47].
Mineralization refers to the process in which organic material transforms into minerals, water, and gases such as methane (CH4), carbon dioxide (CO2), and nitrogen compounds. Mineralization concludes when microorganisms transform all carbon atoms into carbon dioxide [50]. Table 2 presents a side-by-side analysis of various techniques employed in plastic degradation, offering valuable insights into their effectiveness and applications.

Table 2.
Comparative analysis of plastic breakdown processes.
Microplastic type, morphology, dimensions, and coloration provide significant insights to identifying the microplastics’ origin [51]. Fragments with sharp edges are recent entries into marine environments or are derived from the recent disintegration of more extensive plastic materials. Conversely, fragments with smooth edges originate from older materials that have undergone continuous abrasion by other particles or sediments [52].
The standard approach for evaluating the biodegradability of synthetic polymers involves measuring CO2 emissions from soils that contain biodegradable plastics compared to those that do not. Because the breakdown of soil organic matter might lead to overestimating their levels, stable carbon isotopes can be measured to distinguish between CO2 resulting from plastic degradation and soil organic matter’s mineralization [53].
3. Plastics’ Impact on the Ecosystem
Nearly 80% of plastic waste in the past 75 years has ended up in landfills or natural environments, where it can remain for decades or centuries, slowly breaking into smaller pieces. The kind of plastic, how much there is, its additives, and its shape can harm ecosystems differently [54].
3.1. Microplastics’ Impact on the Terrestrial Ecosystem
MPs can influence soil pH, water retention capacity, and conductivity [55,56,57]. They affect plant tissues’ elemental composition and oxidative stress response and decrease plants’ biomass and chlorophyll levels [58,59]. MPs can interfere with soil microbes’ metabolic processes [60] and improve the growth of ones able to metabolize the microplastic, such as Aeromicrobium, Mycobacterium, and Amycolatopsis [61] (Figure 3).
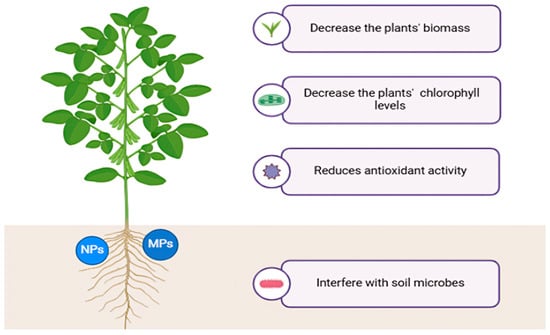
Figure 3.
Impact of plastic derivatives on plants.
Microplastics can carry other pollutants that can adhere to their surfaces using hydrophobic interactions, non-covalent bonding, electrostatic attraction (e.g., van der Waals forces), and microporous mechanisms [62].
3.2. Microplastics’ Impact on the Aquatic Ecosystem
Aquatic ecosystems collect microplastics from wastewater treatment facilities, precipitation, soil erosion, sewer overflow events, and agricultural plastic materials. The plastic collection can be influenced by flow rate, hydraulic gradient, retention time, water volume, and seasonal changes [63]. Freshwater ecosystems are responsible for 80% of the MPs in marine environments. MPs can intensify the melting of Antarctic glaciers by affecting their ability to absorb light [64]. MPs in aquatic ecosystems can end up in the sediment from minor water bodies to larger aquatic ecosystems [65]. MPs can move human-made pollutants, including organic polycyclic aromatic hydrocarbons (PAHs), organochlorine compounds (OCs), and polychlorinated biphenyls (PCBs), and inorganic pollutants like cadmium (Cd), nickel (Ni), chromium (Cr), and lead (Pb) between various environments. Human-made pollutants can be categorized into regulated priority contaminants and Contaminants of Emerging Concern (CECs). CECs are unregulated due to inadequate data demonstrating their environmental hazards. This second category includes chemicals in personal care items, pharmaceuticals, organophosphorus flame retardants, and endocrine-disrupting substances [66].
High temperatures, acidic pH, and salinity can increase pollutants’ adsorption on plastic surfaces [67].
Elevated temperatures amplify microplastics’ negative effect on digestive functions and increase the metabolic demand for aerobic respiration [68].
The pH level can influence electrostatic interactions [69]. When the pH exceeds the MPs’ zero charge point, the MPs’ surfaces gain a negative charge. Conversely, if the pH surpasses the organic pollutants’ acid dissociation constant, the pollutants become deprotonated and anionic, resulting in electrostatic repulsion that hinders their adsorption by MPs [70].
Salinity neutralizes plastics’ surface charges by reducing electrostatic interactions that facilitate the sorption of other contaminants into plastics [71].
The ecological consequences of MPs on algae and marine organisms (e.g., fish, whales, corals, fur seals, and zooplankton) across various trophic levels have been highlighted in numerous studies. MPs can diminish developmental activity in tissues, reduce growth, and alter the gene expression of algal species [72].
In fish, they can determine DNA damage and neurotoxicity, interfering with the immune system, metabolic homeostasis, and oxidative and inflammatory responses [73].
MPs in over 150 fish species from marine and freshwater environments have been reported [74]. The data is critical since these fish can transfer plastic pollutants to higher-level predators, including birds and humans, favoring their bioaccumulation and toxic consequences [75].
3.3. Microplastics’ Impact on the Atmosphere
One significant contributor to air pollution with microplastics comes from textiles, mainly clothing, which can emit more than 1100 fibers for every gram of acrylic fabric. Studies indicate higher levels of airborne microplastics inside buildings than outdoors [76]. Activities that produce airborne MPs are putting clothing on or taking it off, drying garments, breaking down larger plastic items, and producing industrial emissions. Factors like rainfall, pollution, humidity, and particle size influence the distribution and settling of microplastics. The wind can carry MPs over long distances, polluting terrestrial ecosystems and perpetuating a cycle that worsens the issue of microplastic pollution in the environment [77].
Honey bees (Apis mellifera) can be bioindicators of environmental MP pollution since they can collect MPs on their wings and heads [78].
3.4. The Plastisphere
The plastisphere refers to the micro-ecosystem formed by microorganisms that colonize plastic surfaces. Microbes enhance further microbial attachment by producing an extracellular polymeric substance (EPS) [79,80] (Figure 4). The microbial adhesion to plastics is affected by pH, temperature, and salinity [81].
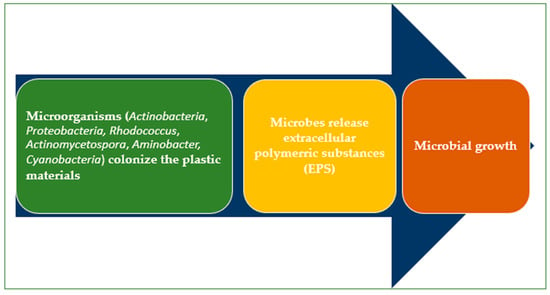
Figure 4.
Biofilm formation on microplastic surfaces.
Cyanobacteria communities and other microorganisms (natural biofilm producers) [81,82] serve as the first microbial colonizers [83]. They produce enzymes with depolymerase activity that help to biodegrade the plastics. The initial attachment of the bacteria is influenced by the MPs’ physicochemical properties (e.g., shape, hydrophobicity, crystallinity, surface roughness, and surface charge) [84], the leaching of additives, and the establishment of an eco-corona [85].
The formation of the plastisphere can happen in terrestrial and aquatic ecosystems.
Diatoms (Mastogloia, Cyclotella, Navicula, Amphora, Sellaphora, Nitzschia, and Pleurosigma), algae (red, brown, and green algae), Rhodococcus, Flavobacterium, Pseudomonas, Saprospiraceae, Planctomycetes, Hyphomonadaceae, and Erythrobacteraceae are microorganisms that colonize the plastisphere in the marine environment along with pioneer colonizers bacterial families like Alpha- and Gamma-proteobacteria [61].
The microbial community in the terrestrial plastisphere closely resembles that of the aquatic plastisphere. Research has identified Actinobacteria, Proteobacteria Rhodococcus, Actinomycetospora, Aminobacter, and Cyanobacteria (Nostoc and Scytonema) in the terrestrial plastisphere [86], along with pioneering bacterial families like Geodermatophilaceae, Beijerinckiaceae, Nocardioides, and Rubrobacteriaceae [87].
4. Microplastics in Food
MPs are recognized as a significant emerging threat in the food sector. Their tiny size allows them to be easily absorbed by various organisms at different trophic levels and with diverse feeding strategies. Microplastic contamination is present throughout the marine food web’s five primary trophic levels. Numerous individual marine species exhibit bioaccumulation of microplastics across four primary consumer trophic levels. A meta-analysis study proved that the effect of environmental exposure to chemical additives on bioaccumulation is more significant than that of chemical additives linked to microplastics. The bioaccumulation of microplastics seems to be more closely related to the feeding strategies of marine species than to their trophic levels [88].
MP rates depend on the materials utilized in processing or storage, the techniques employed during production, and environmental contaminants [89].
MPs are discovered in plentiful amounts in seafood [90], sea salt [91], drinking water [92,93], sugar [94], meat products [95], milk [96], and honey [97,98,99,100].
MPs can contaminate tap and bottled drinking water [93]. Sunlight [99,100], the pressure and heat used during bottling operations [98], and the detergents used in washing machines that clean the bottles before filling them [101] can produce MPs.
Fibers, films, fragments, and spherules from MPs of various origins can taint sugar. This contamination may occur at multiple points in the production cycle, including processing, purification, refinement, and drying, where air currents from dryers can transport MPs [5].
The agricultural and milking processes can produce POPs [102,103]. Multilayer paper packaging can release polypropylene in milk [102].
In some honey samples, styrene, phthalates, and bisphenol A were detected, probably due to the contamination of bees during the pollination process [104].
The MPs in sea salt are attributed to the degradation of plastic materials in the ecosystem, their discharge from industrial processes, and their accumulation linked to the evaporation of seawater. An individual who consumes the daily salt intake (5 g) recommended by the World Health Organization ingests from 37 to 1000 microplastic particles yearly through sea salt [105]. Coagulation or filtration processes can be used to avoid this problem. Coagulation effectively separates solute from solvent, aggregating colloidal particles into larger flocs with coagulants until sedimentation or filtration. Nanofiltration and ultrafiltration use high pressures and large filter surface areas that limit their employment [106].
Consuming fish contaminated with MPs poses low risks for human health when the intestine is discarded [107] since the MPs accumulate in marine organisms’ digestive systems [108].
MP contamination negatively impacts terrestrial fauna. MPs enter the terrestrial food web via primary consumers, such as insects, which transfer them to higher trophic levels through predation. Birds, mammals, soil organisms, and insects can ingest MPs through contaminated food sources like plants and prey. Emerging research suggests that MP exposure may reduce reproductive success and cause developmental abnormalities in terrestrial species, impacting population dynamics and potentially leading to declines in wildlife [109]. Polypropylene and polyethylene (fibers and fragments) were found in South China livestock [110]. Plastic cutting boards and the materials used for packaging can play a role in introducing MPs into meat products [111].
5. Microplastic Emissions in Culinary Environments
MPs from kitchen utensils, food contact materials, packaging, and cooking methods influence the degree of food contamination.
5.1. Kitchen Utensil Contamination
Nonstick cookware, plastic cutting boards, and disposable cutlery are the most significant sources of MPs. Plastics’ disintegration facilitates chemical leaching and promotes the liberation of microplastics [112,113]. Heat deforms the objects and favors their release [114]. Trace elements, such as Fe3+, Cu2+, and Ca2+/HCO3−, can mitigate the emission of MPs [115].
Polyethylene MPs were found in chicken and fish prepared on polyethylene cutting boards [116,117].
Ceramic salt mills release polyethylene terephthalate, kitchen blenders discharge up to 0.78 million polypropylene MPs in 30 s [118], and dish sponges emit 100 and 200 micro-nylon and PET particles in 30 s [119].
Thus, it is recommended that glass or metal utensils be used to reduce the likelihood of MP contamination.
5.2. Food Packaging Contamination
The type of material used in food packaging, along with temperature and duration of contact [119,120], can lead to the release of MPs and stabilizers that can potentially cause cancer and gastrointestinal issues [121,122,123]. Food packaging can discharge harmful metals such as lead and cadmium, which have the potential to cause infertility and cancer [124]. Using biodegradable packaging such as chitosan, milk proteins, seaweed films, and glass containers reduces exposure to MPs [125,126].
The intake of MPs can vary based on factors such as human age, the type of food packaging, the specific food items consumed, cooking techniques, and airborne contaminants present while cooking.
The process of thermal expansion and contraction renders plastics more vulnerable to degradation. Cooler temperatures can cause plastics to become brittle, heightening the chances of MPs being released due to mechanical strain [127]. Hernandez and colleagues found that at 95 °C, a single tea bag can release about 11.6 billion microplastics and 3.1 billion nanoplastics into tea [128].
People who consume take-out meals approximately one to two times per week are exposed to an estimated intake of 170 to 638 MPs [129].
Baking paper and aluminum foil may emit bisphenols when used to cook acidic foods, while a reduced release is observed when they are utilized for fatty foods [130].
Transparent plastic containers release higher levels of phthalate esters than opaque ones, likely due to the need for additional plasticizers [131].
Extruded polystyrene packaging releases MPs in meat and food preparation areas [132].
The vacuum packaging process, high temperature, storage time, the chemical composition of the packaging material, and the type of food influence the contamination rate of the packaged food [133].
Paper bags made from recycled materials may contain elevated levels of phthalate esters whose migration rates are considerably greater than those observed from plastic [134]. The ink used to decorate paper can release poly-fluoroalkyl substances (PFAs) [135].
5.3. Cooking Methods’ Interference with Plastic Contamination
Heat energy has the potential to alter the oxidation processes of plastics. It occurs primarily because the –C–C− bond within the molecular makeup of plastics can rapidly oxidize to form –C double-bond O− and –C–O− bonds under highly thermophilic conditions, thereby increasing degradation rates and improving hydrophobicity [136]. The contamination risk decreases when foods are stored at cooler temperatures. Microwaving alters the packaging material and increases the release of MPs into the foods [137]. Steaming and boiling disperse MPs into the environment through evaporation. Frying accumulates MPs in the cooking medium [137] (Figure 5).
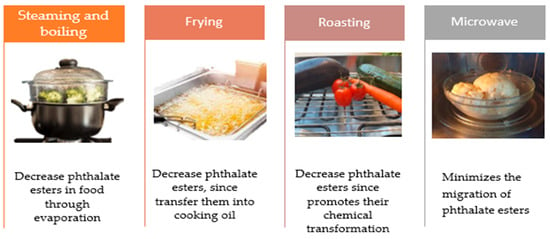
Figure 5.
Potential food contamination with phthalate esters from containers during cooking.
6. Plastic Residues’ Impact on Human Health
Plastic residues can enter the human body, as demonstrated by their being found in feces [138]. The lack of long-term studies on the effects of micro- and nanoplastics on human health constrains our ability to assess their impact.
6.1. Routes of Exposure
MPs can enter our bodies when we consume contaminated foods such as seafood and salt or drink tainted water. They can also be inhaled from the air, especially indoors, where synthetic materials and dust are present, or absorbed through the skin via personal care products [139]. MPs can affect the placenta, liver, gut, lungs, heart system [140,141], thyroid, testes, and ovaries of humans [142].
Textiles, traffic exposure, industrial emissions, cigarette smoking, and construction activities can release MPs into the environment [143]. MPs resist lung degradation and can accumulate [144], causing respiratory distress and triggering cytotoxic and inflammatory responses. Moreover, MPs can contribute to interstitial fibrosis, chronic bronchitis, and asthma-like reactions [145].
Depending on their size, dosage, and individual susceptibility, NPs, bisphenol A, and phthalates can be absorbed through the derma [146]. Dermal contact with NPs can be due to the continuous release of fibers from clothes. Plastic particles that can arise from cosmetic products such as hand washes, face masks, hand cleansers, or toothpaste with a size greater than 100 nm do not get absorbed [147].
Individuals are estimated to swallow between 39,000 and 52,000 MPs annually [148], which can cause inflammatory responses, alterations in gut microbiota composition, and negative impacts on metabolic processes [149].
Following ingestion, MPs enter the enterocytes. Internalization occurs via M cells or other cells in the intestinal mucosa near Peyer’s patches through micropinocytosis, phagocytosis, and receptor-mediated endocytosis. Paracellular transport occurs through the spaces between cells, driven by differences in concentration and the size of particles. This transport is facilitated when the tight junctions are disrupted during cellular renewal and through the temporary gaps due to the epithelial injury or movement of macrophages. Intestinal macrophages and dendritic cells can directly uptake particles from the intestinal lumen [95].
6.2. Harmful Effects on Human Health
Micro- and nanoplastics can deteriorate organs and systems, harming organelles and membranes and affecting gene expression [150].
NPs can interact with mitochondria, the endoplasmic reticulum, and lysosomes, leading to oxidative stress and cytotoxic impacts. In in vivo tests using rats and Eriocheir sinensis, MPs increased the body’s production of ROS [151]. The production of reactive nitrogen species (RNS) and reactive oxygen species (ROS) is influenced by MPs’ dosage, particle size, surface characteristics, and exposure time [152]. The halogenated organic compounds and heavy metal ions adsorbed onto microplastics can exacerbate oxidative stress [153].
In vitro data suggested that polystyrene produced elevated ROS through the P62/Nrf2/Keap1 pathway [154]. MPs and NPs can disrupt the (phosphatidylinositiol3-kinase/protein kinase B) PI3/AKT signaling pathway, which determines cell apoptosis [155].
They initiate ROS-mediated P53 signaling, which activates cellular death pathways [156] and can trigger inflammation [157].
MPs and NPs contribute to metabolic syndrome by stimulating the mitogen-activated protein kinases (MAPKs) [158] and can activate the reactive oxygen species/transforming growth factor-β/Smad (ROS/TGF-β/Smad) pathway, causing fibrotic damage [137].
Finally, they can promote carcinogenesis by activating the nuclear factor erythroid 2-related factor (Nrf2) [135,158].
MPs cross the blood–brain barrier, negatively affecting neurological functions and impairing memory and learning. They increase the levels of harmful substances such as malondialdehyde and reactive oxygen species (ROS) and reduce the levels of glutathione and acetylcholine, which are essential for cognitive performance [159].
Exposure to traffic pollution can determine mild cognitive impairment and can increase the risk of developing Alzheimer’s disease [147].
Additionally, MPs promote microthrombosis and neuronal cell apoptosis, decreasing the expression of connexins within the blood–brain barrier [160].
Prolonged exposure to MPs disrupts the tight junctions between lung cells, compromising lung barrier integrity [161,162].
MPs can absorb heavy metals (e.g., cadmium), which are liver-toxic [163]. The consequent liver fibrosis [164] and steatosis can determine glucose metabolism disorders [165].
Dietary exposure to MPs reduces mucus secretion, promotes epithelial cell apoptosis, and increases intestinal permeability [166]. MPs impact the richness and diversity of the gut microbiome (they increase Staphylococcus and reduce Parabacteroides levels) [167].
The kidney serves as a primary site for the MPs accumulation. MPs can promote the generation of reactive ROS [168], which intensify endoplasmic reticulum stress and induce inflammation and renal damage [169].
MPs can inflame the testes, disturb the testicular blood barrier, and induce inflammatory pathways that attenuate sperm count and motility and increase malformations [170].
In females, they cause ovarian inflammation and lower the extrusion rate of polar bodies and oocyte quality, along with apoptosis of granulosa cells and the fibrosis of the uterus, plausibly resulting in infertility in females [170].
Assessing dose–response relationships for microplastics is challenging, as they may be independent of microplastics themselves due to additives that may leach from them and chemicals they have absorbed. Microplastics found in the environment are usually smaller than 5 mm, yet studies often target a narrow size range of 0.5 to 50 µm, restricting the exploration of their toxicological impacts. The differences in size, composition, and type of microplastics further hinder the ability to determine a threshold for their harmful effects. Additionally, most research has focused on the impact of polystyrene microplastics on higher mammals, resulting in a substantial knowledge gap regarding other types [171].
7. Analytical Methods Used to Detect Plastic Residues
The analytical protocols to determine MPs and NPs in foods involve chromatographic methods to eliminate the matrix interferents and microscopy or spectroscopic apparatuses to identify and quantify them (Figure 6) [172]. The lack of agreement regarding the essential information required for safety assessments complicates identifying what should be measured and documented [173]. The lack of standardized methods and consistent measurement units creates significant challenges in assessing the distribution and composition of microplastics (MPs). This inconsistency hampers our ability to accurately analyze their presence and impact, making it challenging to develop effective mitigation and environmental protection strategies [173].
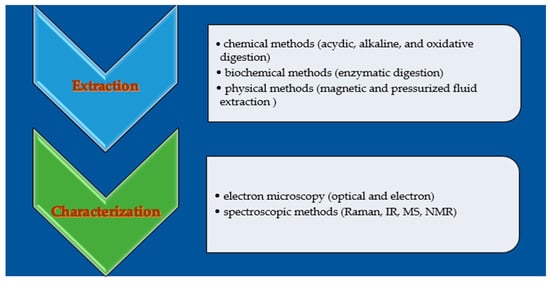
Figure 6.
Analytical methods used to evaluate the plastic residues in foods.
7.1. The Extraction of Microplastics from Biological Specimens
A cleanup procedure must not affect MPs’ integrity and must eliminate the contaminants to allow a precise identification and dosage of MPs.
Chemical (alkaline, acidic, or oxidative digestion), physical (pressurized fluid extraction or magnetic separation), or enzymatic methods can be employed to extract microplastics from biological samples. Chemicals can damage microplastics, so physical or enzymatic procedures are preferable.
FTIR and Raman spectroscopies are employed to evaluate the effects of digestion on the mass, morphology, and surface area of the polymer particles [174].
Alkaline, acidic, and oxidative digestion differ in cost and accessibility. Alkaline digestion is widely accessible due to its simplicity. It is favored for its low cost and efficiency in removing organic material while preserving microplastic integrity. Acidic digestion is moderately accessible but requires careful handling. It may degrade some plastics, affecting cost-effectiveness. Oxidative digestion is the least accessible, needing controlled environments and expertise. It requires specialized equipment, raising costs [175].
Alkaline digestion is a hydrolytic method that employs NaOH or KOH (KOH is the most effective agent) to break down tissues. Alkaline agents can degrade PET (more NaOH than KOH) [172]. The effectiveness of alkaline digestion decreases when applied to water and sediment samples, mainly due to the presence of biogenic organic materials from plant sources, including woody debris, leaves, and algae, along with components from carapaces and shells [176] that contain complex compounds like cellulose, lignin, hemicellulose, humic substances, tannins, and chitin, which are poorly digested [177,178,179,180].
Nitric acid is widely employed for acidic digestion. It can degrade proteins, lipids, and other organic materials of biological tissues [181]. Some of the protocols use a 3:1 mixture of nitric and hydrochloric acids. This mixture has the added advantage of improving the degradation of organic substances and aiding in eliminating some inorganic materials like calcium carbonate that are likely to occur in the samples. In addition, the combination of nitric and perchloric acid in a 1:1 ratio is sometimes employed. This combination is quite effective because perchloric acid is a powerful oxidizer that disassembles intricate organic structures and ensures complete separation of MPs from the biological matrix [182]. Peroxymonosulfuric acid, produced by combining hydrogen peroxide with sulfuric acid, is an oxidizing agent significantly stronger than nitric acid. It is employed to eliminate large quantities of organic contaminants in studies on lake sediment pollution and river sediments [177].
Oxidative digestion employs hydrogen peroxide with a ferric ion (Fe (II)) (Fenton’s reagent) or sodium hypochlorite (NaClO) [174] to break complex compounds into carboxylic acids, carbon dioxide, aldehydes, and water under relatively mild conditions. It is followed by density separation or filtration [183].
Pressurized fluid extraction (PLE) employs solvents under subcritical temperature and pressure conditions to recover semi-volatile organics from solid materials. PLE can be combined with scanning electron microscopy, visual inspection, micro-Fourier-transform infrared spectroscopy, and GC coupled to tandem mass spectrometry to detect MPs [183]. In the initial extraction phase, semi-volatile organics are removed using methanol at 100 °C. The recovery of the MPs from the residual matrix is obtained using dichloromethane at 180 °C [184].
Dynamic PLE requires a specialized high-pressure pump to control the solvent flow rate accurately, along with solvent preheating coils and a back pressure regulator, as opposed to a simple open/close valve commonly found in static systems [185].
Magnetic separation consists of binding sustainable, cost-efficient, and low-toxic magnetic nanoparticles to MPs to allow their separation by applying magnets [186]. The magnetic separation method does not impose a minimum size restriction for the MP separation, as the size dependency can be adjusted by changing the size and type of magnetic nanoparticles used. Hydrophobic and electrostatic interactions are used as driving forces. Hydrophilic magnetic nanoparticles separate MPs using hydrogen bonds. In contrast, hydrophobic nanoparticles employ electrostatic attraction [187]. Magnetic nanoparticles are employed to collect polyethylene, polyethylene terephthalate, polypropylene, polyamide, polystyrene, and polyvinyl chloride [187].
Enzymatic digestion employs enzymes, such as proteinase, cellulase, lipase, or chitinase, to eliminate organic matter. Due to its selective nature, enzymatic hydrolysis reduces the risk of modifying microplastics, which occurs with chemical hydrolysis [188].
7.2. Plastics Characterization
Plastics characterization can be performed by microscopy and spectroscopy (Table 3).
The characterization performed by optical and electron microscopy provides information about MP size, distribution, morphology, thickness, topography, state of degradation, and color. Optical microscopy cannot resolve submicron particles; instead, the electron microscope, whose resolution range is about 1 nm and whose field of view is approximately 1 mm2, succeeds [189].
IR spectroscopy, Raman scattering, and Fourier transform infrared (FT-IR) spectroscopy yield distinctive spectra crucial for identifying and characterizing plastics. Raman spectroscopy exhibits heightened sensitivity to nonpolar symmetric bonds, whereas FT-IR is more adept at identifying polar functional groups [190]. Both techniques exhibit high detection limit values (approximately 20 µm) that can be further reduced (to approximately 1 µm) if imaging is used. Nevertheless, NPs measuring less than 1 µm can be overlooked, resulting in an underestimation of microplastic concentrations in food. Surface additives can also interfere with the estimation of microplastics [191].
Various techniques, including gas chromatography-mass spectrometry (GC-MS), liquid chromatography–tandem mass spectrometry (LC-MS/MS), time-of-flight mass spectrometry (ToF-MS), atmospheric solids analysis probe mass spectrometry (ASAP-MS) [192], isotope ratio mass spectrometry (IR-MS) [193], and single particle–inductively coupled plasma mass spectrometry (SP-ICP-MS), are employed to analyze MPs and NPs in food matrices [194]. Each methodology has varying degrees of accessibility and cost-effectiveness. GC-MS and LC-MS/MS techniques are commonly found in food safety laboratories, making them relatively accessible. Py-GC–MS is considered a technique of choice for analyzing MPs. These methods offer outstanding sensitivity but require expensive equipment and highly trained personnel. ToF-MS and ASAP-MS are not as frequently used due to the specialized nature of the equipment needed. These techniques allow for rapid analyses but can be costly, especially in high resolution. IR-MS and SP-ICP-MS are utilized in environmental and regulatory labs. Their accessibility is not widespread. These methods excel in isotopic and elemental analysis, although the instruments are expensive, and maintenance can be quite high [195].
A key challenge in MPs analysis is their complex composition, often involving multiple polymers that can co-pyrolyze, affecting yield and quantification accuracy. Research on polymer cross-interference during pyrolysis is limited. Diluting reference materials is more manageable for solutions than solids [196].
NMR provides precise 3D structural information based on molecular vibrations while preserving sample integrity. NMR is used with little frequency to characterize higher-molecular-weight compounds due to the complexity of the spectra. Polyvinyl chloride, polyethylene, polystyrene, polyethylene terephthalate, and polyamide were studied using NMR [197].

Table 3.
Comparisons among different detection methods.
Table 3.
Comparisons among different detection methods.
| Method | Detection Limit | Features | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Optical microscopy | 50 μm | Quantification; non-destructive | Rapid and cost-effective identification of particle characteristics such as size, color, shape, and surface structure. | Major inaccuracies may occur, requiring a considerable amount of time; errors in identification. | [189,198] |
| Electron microscopy | 1 nm | Quantification; non-destructive | Electron microscopes can achieve resolutions up to 0.2 nanometers. | Preparing samples for electron microscopy can be time-consuming and may alter the specimen, potentially introducing artifacts. | [189,198] |
| FTIR | 10 μm | Identification; non-destructive | Non-destructive. It has a spectrum database. It can detect several thousand particles with a single measurement. Reliable outcomes, exceptional sensitivity, rapid screening capabilities, and eco-friendliness. | It is costly and requires skilled operators. Pretreatment is essential. It is labor-intensive and can be easily affected by water. Sample preprocessing, organic soil matter, and the age of microplastics may also alter the chemical bonds of the samples. | [190,191,198] |
| Raman | 1 μm | Identification; non-destructive | Non-destructive nature; capable of identifying additives. Excellent spatial resolution, great precision, high sensitivity, exceptional specificity of fingerprint spectrum, and no harm to the sample, with no need for specific sample thickness. It has a spectrum database. | The spectral library is not as extensive as FTIR and may require a significant amount of time. Additives and impurities can lead to interference. Adequate sample preparation is essential. Choosing the appropriate wavelength is vital for reducing sample fluorescence and obtaining a strong signal. | [190,191,198] |
| MS | The lowest detectable limit for PC is 27.7 µg/kg, while for PET is 178.3 µg/kg | Identification and quantification; destructive | It offers an effective detection limit, facilitates high-volume sample input, and operates quickly and automatically. Can measure additives. | Needs pretreatment. | [192,193,194,198] |
| NMR | 0.2~10 µg/mL | Identification and quantification; non-destructive | NMR is infrequently employed for the characterization of higher-molecular-weight compounds because of the intricate nature of the spectra. | [197] |
8. Plastic Waste Management
Plastic waste will reach 265 million tons annually by 2060 [199]. Key plastic challenges arise from their long degradability, treatment expenses, and environmental problems that occur in poor recycling. The basic circular economy principle can be adopted to overcome this challenge. The circular economy promotes repairing, upgrading, and redistributing second-hand goods. This concept is reduced to the 3Rs—reduce, reuse, recycle. With the implementation of this circular economy model, it is anticipated that longevity will be given to waste that can be turned into resources of great value for further use [200]. Gunter Pauli coined the term “upcycling” to refer to waste conversion (by depolymerization, polymerization, and functionalization) into polymers, molecules, and materials [201,202,203]. Upcycling occurs when plastic waste is reused without diminishing its quality or functionality for subsequent applications [201]. Upcycling of plastic waste can be used in the production of high-performance fuels (e.g., hydrogen and liquid alkanes) [204] and carbon materials (e.g., carbon dots [205], nanofibers [206], nanosheets [207], microspheres [208], three-dimensional porous carbon [209], graphite, graphene [210], and carbon nanotubes [211]). For example, polycarbonate (PC) can be made by repolymerizing bis (hydroxyethyl ether) [212], fiberglass-reinforced plastic by the depolymerization of PET [213], and cyclic carbonates and carbamates by the depolymerization of BPA-PC [214]. Erickson et al. examined the economic and environmental advantages of establishing an upcycling infrastructure in the continental United States to produce low-density polyethylene and polypropylene from post-consumer plastic waste. They suggested that this infrastructure could create a market valued at nearly USD 20 billion annually, using a computational framework that combines techno-economic assessment, life-cycle evaluation, and value chain optimization [215].
Primary (closed loop), secondary (mechanical), tertiary (chemical), and incineration methods can be employed to convert plastic waste into reusable materials (Table 4). Only primary recycling produces high-quality plastic; the others give lower-quality plastic, often called “downgrading” or “downcycling”, since high temperatures and mechanical stress degrade the polymers [216].

Table 4.
Comparisons among different recycling and upcycling technologies.
Mechanical recycling is utilized for PET and PE plastics. Recycled plastics have lower quality than virgin plastics [201]. Recycling is not possible for heavily contaminated or multi-material plastics. This process includes sorting and cleaning the plastic waste, producing pellets, and heating it before making new products. Mechanical recycling may release volatile organic compounds into the environment [216].
Thermochemical and catalytic processes (e.g., pyrolysis, gasification, hydrocracking, and depolymerization) transform plastic waste into fuels or monomers. Chemical recycling is more adept at processing mixed or contaminated plastics than physical recycling [217,218,219].
Biodegradation is a nontoxic approach that can transform plastic by using enzymes. Ongoing efforts are directed towards developing strains with improved hydrolytic activity against plastic polymers by altering amino acid sequences at active sites through genetic techniques. Significant areas of investigation involve transcriptional regulators and how to enhance the enzymes’ ability to degrade specific polymers. Only a few studies have examined how the modifications affect the enzymes’ specificity [220,221,222].
9. MPs Legislation
MPs present a significant and persistent threat to human health and the environment. Their long-term effects could negatively impact future generations worldwide. Microplastic regulations vary across regions, reflecting the differences in environmental priorities and regulatory approaches. The European Union (EU) has notably advanced in this domain. The EU aims to accomplish a 30% decrease in MPs emissions by 2030 by implementing bans, improved waste management practices, and stricter regulations [223]. On 25 September 2023, the European Commission approved Regulation (EU) 2023/2055 limiting the intentional incorporation of microplastics in products. This initiative aimed at reducing the release of plastic pellets into the environment, along with its associated Impact Assessment (IA), arises from the Commission’s pledge to address the unintended discharge of microplastics [224].
Three key principles were identified to standardize legislation on MPs and pave the way for a sustainable and environmentally responsible future [225]:
1. Precautionary Principle (PP): This principle, embedded in environmental legislation (European Union, European Treaty, Article 191; REACH CE 1907/2006) [226], advocates for preventive measures in risk management, particularly in the absence of scientific consensus, while also promoting a circular economy.
2. Principle of Proportionality (PrP): It is defined in the European Union’s European Treaty, Article 5 [227], which mandates necessary actions to support the Precautionary Principle in mitigating environmental risks.
3. Polluter Pays Principle (PPP): As outlined in the European Union, European Treaty, Article 191 [228]. This principle holds producers and consumers responsible for the costs associated with reducing MP pollution, thereby fostering environmental accountability and the creation of sustainable products.
REACH regulation is applied to monomers and polymers. However, polymers are currently exempt from the initial registration and evaluation phases, which are essential to REACH. The European Union does not differentiate between new and existing polymers, meaning all are exempt from these requirements until it becomes feasible to identify those that pose potential risks to human health or the environment. Instead, if a polymer contains more than 2% of a monomer and the annual usage exceeds 1 ton, the importer or producer must register the monomer with the European Chemical Agency [229].
Postle et al. [230] and De Toni et al. [231] investigated the economic viability of registering polymers under the REACH regulation for the European Commission.
Postle et al. [230] categorized polymers into two primary groups: those with identical constituents and those with varying constituents. Polymers with identical constituents are further divided into three scenarios: structurally identical polymers, polymers classified as a single substance under the Dangerous Substances Directive, and polymers with incremental and consistent changes. In contrast, polymers with different constituents are characterized by variations in counter-ions and changes involving similar monomers [230]. De Toni et al. assessed when a polymer can be identified as a “Polymer of Low Concern” (PLC), considering factors such as cationic nature, molecular weight, oligomer content, and the availability of data on human and environmental hazard classifications under the EU CLP regulation [231].
Article 5 of the Treaty on European Union states the mandates that measures addressing environmental risks must be necessary and proportionate, preventing both excessive and insufficient responses [225].
Finally, Article 191 of the European Treaty proposes three Extended Producer Responsibility models.
The “Buy-Back Depository Mechanism” incentivizes consumers to return plastic products by offering buy-back prices at collection points like reverse vending machines [232].
The second model forces the producers, importers, and brand owners to contribute to a fund based on the amount of plastic they market.
The third model sets specific recycling targets for producers according to their plastic volumes, supported by a “Plastic Credit” system that validates recycling efforts [233].
The U.S. has focused on promoting biodegradable and recyclable materials; implementing policies to decrease plastic waste; enhancing recycling and disposal systems; strengthening cleanup efforts for plastic pollution; and preventing microplastic contamination in waterways and oceans [234]. The U.S. Geological Survey (USGS) provided data for environmental decisions, building coalitions for collaborative research, and creating standardized MP sampling protocols [235].
Asian nations have adopted strategies shaped by their economic development, environmental priorities, and regulations (ASEAN Regional Action Plan (2021–2025)) [236], improved the regional cooperation (see ASEAN-Norway, which involves collaboration between ASEAN and Norway [237], and ASEAN+3 Marine Plastic Debris, which includes ASEAN Member States and the People’s Republic of China, Japan, and the Republic of Korea [238]), and encouraged responsible waste disposal among residents and tourists by employing public awareness campaigns. In Indonesia, 2012 waste-bank initiative incentivized households to sort waste [239]. Innovative technologies, such as the ocean cleanup project, are being used to eliminate plastics from Southeast Asian waterways [240]. Japan and South Korea enforce strict rules on microplastics in cosmetics and industry [241], while China focuses on managing plastic waste and banning certain single-use plastics [242]. In Southeast Asia, countries like Indonesia and Malaysia, heavily impacted by plastic pollution, are implementing community cleanups and seeking policy reforms [243].
10. Materials and Methods
10.1. Search Strategy
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were used to guarantee the validity and strength of the research outcomes [244]. Google Scholar databases were employed as information sources (Figure 7).
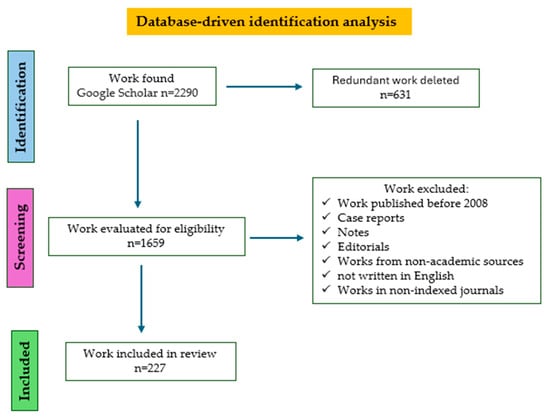
Figure 7.
Flowchart representation of this study using PRISMA.
10.2. Inclusion Criteria
The criteria for eligibility encompassed research articles and reviews on the following topics.
- Food toxicology: Plastic and microplastic residues in foods and a source of contamination of microplastic residues in foods.
- Analytical methods to detect microplastic residues in foods.
- Technological approaches and sustainable strategies to address the challenge of microplastics in foods.
The criteria for exclusion encompassed articles not written in English and publications originating from non-academic sources.
Literature papers published between 2008 and 2024 were discussed in this work.
The search terms utilized included the following: “microplastics in foods”, “cooking methods” microplastics; microplastic “waste recycling”; “nanoplastics toxicity”; “circular economy” microplastic. A total of 244 articles were reviewed.
Papers excluded were works published before 2008, case reports, editorials, notes, works from non-academic sources, works in non-indexed journals, and non-English articles.
11. Conclusions
This review provides a comprehensive overview of the composition, classifications, characteristics, sources, and ecological effects of plastics and their by-products, emphasizing the potential dangers to human health when they infiltrate the food chain. The literature review indicated that humans are exposed to MPs and NPs, and the long-term toxicological impacts remain unknown. Therefore, it is vital to improve public engagement in managing plastic waste to prevent it from entering ecosystems and causing harm until more effective waste recovery methods can be implemented. Further investigation is crucial into the interactions between micro- and nanoplastics and human cells, their effects on DNA, and the long-term health implications. In addition, identifying new biomarkers related to exposure and creating and validating innovative detection techniques for MPs and NPs in environmental and food samples would benefit public authorities and analytical laboratories. Finally, creating advanced technologies that can efficiently decompose microplastics into reusable chemical components and discovering enzymes or microorganisms that can convert microplastics into valuable materials like bioplastics or construction resources could significantly reduce environmental pollution.
Author Contributions
Data curation, conceptualization, writing original draft: I.D.; writing—review and editing: S.S. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bohre, A.; Jadhao, P.R.; Tripathi, K.; Pant, K.K.; Saha, B. Chemical Recycling Processes of Waste Polyethylene Terephthalate Using Solid Catalysts. ChemSusChem 2023, 16, e202300142. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, L.G.; Soares, E.; Oliveira, M. Bio-based thermoplastic composites reinforced with natural fillers for sustainable packaging: A comprehensive review. Polym. Compos. 2025, 35, e6536. [Google Scholar] [CrossRef]
- Wong, S.L.; Nyakuma, B.B.; Wong, K.Y.; Lee, C.T.; Lee, T.H.; Lee, C.H. Microplastics and Nanoplastics in Global Food Webs: A Bibliometric Analysis (2009–2019). Mar. Pollut. Bull. 2020, 158, 111432. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, S.; Preethi, B.; Deena, S.R.; Vijayan, D.S.; Subbaiya, R.; Vickram, S.; Govarthanan, M. Systematic Assessment of Mechanisms, Developments, Innovative Solutions, and Future Perspectives of Microplastics and Ecotoxicity—A Review. Adv. Sust. Sys. 2024, 8, 2400294. [Google Scholar] [CrossRef]
- Makhdoumi, P.; Pirsaheb, M.; Amin, A.A.; Kianpour, S.; Hossini, H. Microplastic pollution in table salt and sugar: Occurrence, qualification and quantification and risk assessment. J. Food Compos. Anal. 2023, 119, 105261. [Google Scholar] [CrossRef]
- Shi, H.; Frias, J.; Sayed, A.; De-la-Torre, G.; Jong, M.; Uddin, S.; Rajaram, R.; Chavanich, S.; Najii, A.; Fernández-Severini, M.D.; et al. Small plastic fragments: A bridge between large plastic debris and micro-&nano-plastics. Trends Analyt. Chem. 2023, 168, 117308. [Google Scholar]
- Shen, M.; Ye, S.; Zeng, G.; Zhang, Y.; Xing, L.; Tang, W.; Wen, X.; Liu, S. Can microplastics pose a threat to ocean carbon sequestration? Mar. Pollut. Bull. 2020, 150, 110712. [Google Scholar] [CrossRef]
- Hamilton, L.A.; Feit, S.; Kelso, M.; Muffett, C.; Rubright, S.M.; Bernhardt, C.; Labbé-Bellas, R. Plastic & Climate. The Hidden Costs of a Plastic Planet. 2019, pp. 1–108. Available online: https://www.ciel.org/wp-content/uploads/2019/05/Plastic-and-Climate-FINAL-2019.pdf (accessed on 18 December 2021).
- Lavers, J.L.; Bond, A.L.; Rolsky, C. Far from a distraction: Plastic pollution and the planetary emergency. Biol. Conserv. 2022, 272, 109655. [Google Scholar] [CrossRef]
- Gilani, I.E.; Sayadi, S.; Zouari, N.; Al-Ghouti, M.A. Plastic waste impact and biotechnology: Exploring polymer degradation, microbial role, and sustainable development implications. Bioresour. Technol. Rep. 2023, 24, 101606. [Google Scholar] [CrossRef]
- Nayanathara Thathsarani Pilapitiya, P.G.C.; Ratnayake, A.S. The World of Plastic Waste: A Review. Clean. Mater. 2024, 11, 100220. [Google Scholar] [CrossRef]
- Elahi, A.; Bukhari, D.A.; Shamim, S.; Rehman, A. Plastics degradation by microbes: A sustainable approach. J. King Saud Univ. Sci. 2021, 33, 101538. [Google Scholar]
- Napper, I.; Thompson, R. Plastic Debris in the Marine Environment: History and Future Challenges. Glob. Chall. 2020, 4, 1900081. [Google Scholar] [CrossRef]
- Wilkes, R.A.; Aristilde, L. Degradation and Metabolism of Synthetic Plastics and Associated Products by Pseudomonas sp.: Capabilities and Challenges. J. Appl. Microbiol. 2017, 123, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Maddah, H.A. Polypropylene as a promising plastic: A review. Amer. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Maafa, I.M. Pyrolysis of Polystyrene Waste: A Review. Polymers 2021, 13, 225. [Google Scholar] [CrossRef]
- Jankauskaite, V.; Macijauskas, G.; Lygaitis, R. Polyethylene terephthalate waste recycling and application possibilities: A review. Mater. Sci. 2008, 14, 119–127. [Google Scholar]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. A New Wave of Industrialization of PHA Biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef]
- Jiao, J.; Zeng, X.; Huang, X. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(butylene succinate) (PBS): Materials, processing, and industrial applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Ghazali, I.; Julmohammad, N.; Huda, N.; Ilyas, R.A. Physical Properties of Thermoplastic Starch Derived from Natural Resources and Its Blends: A Review. Polymers 2021, 13, 1396. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Bhoi, P.R. An overview of non-biodegradable bioplastics. J. Clean. Prod. 2021, 294, 126218. [Google Scholar] [CrossRef]
- Archer, E.; Torretti, M.; Madbouly, S. Biodegradable polycaprolactone (PCL) based polymer and composites. Phys. Sci. Rev. 2021, 8, 255–278. [Google Scholar] [CrossRef]
- Vatanpour, V.; Pasaoglu, M.E.; Barzegar, H.; Teber, O.O.; Kaya, R.; Bastug, M.; Khataee, A.; Koyuncu, I. Cellulose acetate in fabrication of polymeric membranes: A review. Chemosphere 2022, 295, 133914. [Google Scholar] [CrossRef]
- Unni, R.; Reshmy, R.; Madhavan, A.; Binod, P.; Pandey, A.; Awasthi, M.K.; Sindhu, R. Cellulose-Based Bioplastics. In Second and Third Generation Bioplastics; CRC Press: Raton, FL, USA, 2024; pp. 57–68. [Google Scholar]
- Bhasney, S.M.; Das, S.R.; Prasad, A.; Mahto, B.; Thangavel, S. Recycling of biopolymersin. In Biodegradable Waste Processing for Sustainable Developments; Arbind, P., Atanu, K.P., Eds.; CRC: Abingdon Oxon, UK, 2024; p. 272. [Google Scholar]
- Khan, A.; Naveed, M.; Rabnawaz, M. Melt-reprocessing of mixed polyurethane thermosets. Green Chem. 2021, 23, 4771–4779. [Google Scholar] [CrossRef]
- Maraveas, C. The Sustainability of Plastic Nets in Agriculture. Sustainability 2020, 12, 3625. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Zhou, J. Ecotoxicity of Biodegradable Microplastics and Bio-Based Microplastics: A Review of in Vitro and in Vivo Studies. Environ. Manag. 2024, 75, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, M.; Daver, F.; Murdoch, B.J.; Ball, A.S.; Ivanova, E.P.; Adhikari, B. Biodegradation of novel bioplastics made of starch, polyhydroxyurethanes and cellulose nanocrystals in soil environment. Sci. Total Environ. 2022, 815, 152684. [Google Scholar] [CrossRef]
- Han, Y.; Teng, Y.; Wang, X.; Ren, W.; Wang, X.; Luo, Y.; Zhang, H.; Christie, P. Soil type driven change in microbial community affects poly (butylene adipate-co-terephthalate) degradation potential. Environ. Sci. Technol. 2021, 55, 4648–4657. [Google Scholar] [CrossRef]
- Malafeev, K.V.; Apicella, A.; Incarnato, L.; Scarfato, P. Understanding the Impact of Biodegradable Microplastics on Living Organisms Entering the Food Chain: A Review. Polymers 2023, 15, 3680. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are Bioplastics and Plant-Based Materials Safer than Conventional Plastics? In Vitro Toxicity and Chemical Composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef] [PubMed]
- Mamin, E.A.; Pantyukhov, P.V.; Olkhov, A.A. Oxo-Additives for Polyolefin Degradation: Kinetics and Mechanism. Macromol 2023, 3, 477–506. [Google Scholar] [CrossRef]
- Goel, V.; Luthra, P.; Kapur, G.S.; Ramakumar, S.S.V. Biodegradable/Bio-plastics: Myths and Realities. J. Polym. Environ. 2021, 29, 3079–3104. [Google Scholar] [CrossRef]
- Efendy, M.; Amir, N.; Mahasin, M.Z.; Wahyu, F.M.; Aroua, M.K.; Gozan, M. Distribution and characteristics of microplastics in seawater, sediment, and crude salt from Madura Island, the largest solar salt production island in Indonesia. Reg. Stud. Mar Sci. 2024, 78, 103803. [Google Scholar] [CrossRef]
- Kabir, S.A.; Bhuiyan, M.A.; Zhang, G.; Pramanik, B.K. Microplastic distribution and ecological risks: Investigating road dust and stormwater runoff across land uses. Environ. Sci. Adv. 2024, 3, 62–75. [Google Scholar] [CrossRef]
- Mathew, J.T.; Inobeme, A.; Adetuyi, B.O.; Adetunji, C.O.; Popoola, O.A.; Olaitan, F.Y.; Akinbo, O.; Shahnawaz, M.; Oyewole, O.A.; Eniola, K.I.T.; et al. General Introduction of Microplastic: Uses, Types, and Generation. In Microplastic Pollution; Springer Nature: Singapore, 2024; pp. 3–21. [Google Scholar]
- Priyadarshini, S.; Jagatee, S.; Das, A.P. Synthetic Fabrics and Microfiber Pollution–An Assessment of Their Global Impact. In Renewable Energy Generation and Value Addition from Environmental Microfiber Pollution Through Advanced Greener Solution; Springer Nature: Cham, Switzerland, 2024; pp. 137–157. [Google Scholar]
- Dagwar, P.P.; Saole, P.; Jeevanasai, A.; Seetha Rama Raju, M.; Sharma, H.; Bahukhandi, K.D. Sources, Types, and Occurrences of Microplastics in Soil, Water, and Air. In Microplastics: Environmental Pollution and Degradation Process; Springer Nature: Singapore, 2024; pp. 23–56. [Google Scholar]
- Xu, Z.; Bai, X.; Ye, Z. Removal and generation of microplastics in wastewater treatment plants: A review. J. Clean. Prod. 2021, 291, 125982. [Google Scholar] [CrossRef]
- Pielichowski, K.; Njuguna, J.; Majka, T.M. Thermal Degradation of Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Oh, S.; Stache, E.E. Recent advances in oxidative degradation of plastics. Chem. Soc. Rev. 2024, 53, 7309–7327. [Google Scholar] [CrossRef]
- Mahmud, A.; Wasif, M.M.; Roy, H.; Mehnaz, F.; Ahmed, T.; Pervez, M.N.; Naddeo, V.; Islam, M.S. Aquatic Microplastic Pollution Control Strategies: Sustainable Degradation Techniques, Resource Recovery, and Recommendations for Bangladesh. Water 2022, 14, 3968. [Google Scholar] [CrossRef]
- Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; de Oliveira, T.V. Biodegradation of Polymers: Stages, Measurement, Standards and Prospects. Macromol 2023, 3, 371–399. [Google Scholar] [CrossRef]
- Kaabel, S.; Therien, J.P.; Dschênes, C.; Dustin, D.D.; Friščić, T.; Auclair, K. Enzymatic depolymerization of highly crystalline polyethylene terephthalate enabled in moist-solid reaction mixtures. Proc. Natl. Acad. Sci. USA 2021, 118, e2026452118. [Google Scholar] [CrossRef] [PubMed]
- Heris, Y.S. Bacterial Biodegradation of Synthetic Plastics: A Review. Bull. Natl. Res. Cent. 2024, 48, 87. [Google Scholar] [CrossRef]
- Rocha-Santos, T.; Duarte, A.C. Characterization and Analysis of Microplastics; Elsevier: Amsterdam, The Netherlands, 2017; Volume 75. [Google Scholar]
- Dimassi, S.N.; Hahladakis, J.N.; Yahia, M.N.D.; Ahmad, M.I.; Sayadi, S.; Al-Ghouti, M.A. Degradation-Fragmentation of Marine Plastic Waste and Their Environmental Implications: A Critical Review. Arab. J. Chem. 2022, 15, 104262. [Google Scholar] [CrossRef]
- Yu, Y.; Lin, S.; Sarkar, B.; Wang, J.; Liu, X.; Wang, D.; Ge, T.; Li, Y.; Zhu, B.; Yao, H. Mineralization and microbial utilization of poly (lactic acid) microplastic in soil. J. Hazard. Mater. 2024, 476, 135080. [Google Scholar] [CrossRef]
- Goudriaan, M.; Morales, V.H.; van der Meer, M.T.J.; Mets, A.; Ndhlovu, R.T.; van Heerwaarden, J.; Simon, S.; Heuer, V.B.; Hinrichs, K.-U.; Niemann, H. A Stable Isotope Assay with C-Labeled Polyethylene to Investigate Plastic Mineralization Mediated by Rhodococcus ruber. Mar. Pollut. Bull. 2023, 186, 114369. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Huang, X.P.; Xiang, L.; Wang, Y.Z.; Li, Y.W.; Li, H.; Cai, Q.Y.; Mo, C.H.; Wong, M.H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Mbachu, O.; Jenkins, G.; Kaparaju, P.; Pratt, C. The rise of artificial soil carbon inputs: Reviewing microplastic pollution effects in the soil environment. Sci. Total Environ. 2021, 780, 146569. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Pant, G.; Pant, K.; Aloo, B.N.; Kumar, G.; Singh, H.B.; Tripathi, V. Microplastic Pollution in Terrestrial Ecosystems and Its Interaction with Other Soil Pollutants: A Potential Threat to Soil Ecosystem Sustainability. Resources 2023, 12, 67. [Google Scholar] [CrossRef]
- Maity, S.; Guchhait, R.; Sarkar, M.B.; Pramanick, K. Occurrence and distribution of micro/nanoplastics in soils and their phytotoxic effects: A review. Plant Cell Environ. 2022, 45, 1011–1028. [Google Scholar] [CrossRef]
- Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Jiangcuo, G.D.; Azeem, K.; Ishfaq, M.; Shakoor, A.; Ayaz, M.; Xu, M.; et al. Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review. Nanomaterials 2021, 11, 2935. [Google Scholar] [CrossRef]
- Rillig, M.C.; Leifheit, E.; Lehmann, J. Microplastic effects on carbon cycling processes in soils. PLoS Biol. 2021, 19, e3001130. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Das, S. Environmental impacts of microplastic and role of plastisphere microbes in the biodegradation and upcycling of microplastic. Chemosphere 2023, 334, 138928. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wei, Y.; Yang, C.; He, Z. Interaction of microplastics and soil pollutants in soil-plant systems. Environ. Pollut. 2022, 315, 120357. [Google Scholar] [CrossRef]
- Upadhyay, S.; Sharma, P.K.; Dogra, K.; Bhattacharya, P.; Kumar, M.; Tripathi, V.; Karmakar, R. Microplastics in freshwater: Unveiling sources, fate, and removal strategies. Groundw. Sustain. Dev. 2024, 26, 101185. [Google Scholar] [CrossRef]
- Zain, N.M.; Fauzi, N.; Subki, N.S.; Ghazali, Z.Z. Occurrence of microplastics in immature aquatic insects of Gua Musang tributaries in Kelantan. IOP Conf. Ser. Earth Environ. Sci. 2022, 1102, 012047. [Google Scholar] [CrossRef]
- Deylami, S.; Cárdenas-Escudero, J.; Ochoa, M.L.; Ayuso-Haro, J.; Galán-Madruga, D.; Ruiz, J.L.U.; Cáceres, J.O. Microplastics in Antarctic air: Revealing current findings. Antarct. Sci. 2025, 37, 104–116. [Google Scholar] [CrossRef]
- Hernández-Mesa, M.; Le Bizec, B.; Dervilly, G. Metabolomics in chemical risk analysis–A review. Anal. Chim. Acta 2021, 1154, 338298. [Google Scholar] [CrossRef]
- Bhaumik, S.; Chakraborty, P. Interactions between microplastics (MPs) and trace/toxic metals in marine environments: Implications and insights—A comprehensive review. Environ. Sci. Pollut. Res. 2024, 31, 59681–59699. [Google Scholar] [CrossRef]
- Trevisan, R.; Ranasinghe, P.; Jayasundara, N.; Di Giulio, R.T. Nanoplastics in Aquatic Environments: Impacts on Aquatic Species and Interactions with Environmental Factors and Pollutants. Toxics 2022, 10, 326. [Google Scholar] [CrossRef]
- Vujić, M.; Vasiljević, S.; Nikić, J.; Kordić, B.; Agbaba, J.; Tubić, A. Sorption Behavior of Organic Pollutants on Biodegradable and Nondegradable Microplastics: pH Effects. Appl. Sci. 2023, 13, 12835. [Google Scholar] [CrossRef]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption behavior of organic pollutants on microplastics. Ecotoxicol. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, L.; Han, X.; Shi, Y.; Huang, J.; Ding, B.; Zhang, Y.; Zhang, Z.; Shi, Y.; Li, F. Effects of ionic strength, cation type and pH on the cotransport of microplastics with PFOA in saturated porous media. Chemosphere 2025, 370, 143942. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, W.R.; Park, E.G.; Lee, D.H.; Kim, J.-m.; Jeong, H.-s.; Roh, H.-Y.; Choi, Y.H.; Srivastava, V.; Mishra, A.; et al. Phenotypic and Gene Expression Alterations in Aquatic Organisms Exposed to Microplastics. Int. J. Mol. Sci. 2025, 26, 1080. [Google Scholar] [CrossRef]
- Eliso, M.C.; Billè, B.; Cappello, T.; Maisano, M. Polystyrene Micro- and Nanoplastics (PS MNPs): A Review of Recent Advances in the Use of -Omics in PS MNP Toxicity Studies on Aquatic Organisms. Fishes 2024, 9, 98. [Google Scholar] [CrossRef]
- Verma, A.; Chand, N.; Upadhyay, P.; Sharma, S.; Prajapati, S.K. Systematic review on microplastics as a threat to terrestrial and aquatic eco-environment. Sustain. Chem. One World 2024, 3, 100013. [Google Scholar] [CrossRef]
- Rakib, M.R.J.; Sarker, A.; Ram, K.; Uddin, M.G.; Walker, T.R.; Chowdhury, T.; Uddin, J.; Khandaker, M.U.; Rahman, M.M.; Idris, A.M. Microplastic Toxicity in Aquatic Organisms and Aquatic Ecosystems: A Review. Water Air Soil Pollut. 2023, 234, 52. [Google Scholar] [CrossRef]
- Jahanzaib, M.; Sharma, S.; Park, D. Microplastics comparison of indoor and outdoor air and ventilation rate effect in outskirts of the Seoul metropolitan city. Emerg. Contam. 2025, 11, 100408. [Google Scholar] [CrossRef]
- Belioka, M.-P.; Achilias, D.S. The Effect of Weathering Conditions in Combination with Natural Phenomena/Disasters on Microplastics’ Transport from Aquatic Environments to Agricultural Soils. Microplastics 2024, 3, 518–538. [Google Scholar] [CrossRef]
- Edo, C.; Fernández-Alba, A.R.; Vejsnæs, F.; van der Steen, J.J.M.; Fernández-Piñas, F.; Rosal, R. Honeybees as Active Samplers for Microplastics. Sci. Total Environ. 2021, 767, 144481. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; de Almeida, M.P.; Neves, C.V.; Neto, J.A.B.; da Fonseca, E.M. The Importance of Biofilms on Microplastic Particles in Their Sinking Behavior and the Transfer of Invasive Organisms between Ecosystems. Micro 2023, 3, 320–337. [Google Scholar] [CrossRef]
- Qin, Y.; Tu, Y.; Chen, C.; Wang, F.; Yang, Y.; Hu, Y. Biofilms on microplastic surfaces and their effect on pollutant adsorption in the aquatic environment. J. Mater. Cycles Waste Manag. 2024, 26, 3303–3323. [Google Scholar] [CrossRef]
- Hou, T.; Sankar Sana, S.; Li, H.; Wang, X.; Wang, Q.; Boya, V.K.N.; Vadde, R.; Kumar, R.; Kumbhakar, D.V.; Zhang, Z.; et al. Development of Plant Protein Derived Tri Angular Shaped Nano Zinc Oxide Particles with Inherent Antibacterial and Neurotoxicity Properties. Pharmaceutics 2022, 14, 2155. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, I.V.; Wichels, A.; Gullans, E.; Krohne, G.; Gerdts, G. The Plastisphere—Uncovering tightly attached plastic “specific” microorganisms. PLoS ONE 2019, 14, e0215859. [Google Scholar] [CrossRef] [PubMed]
- MacLean, J.; Mayanna, S.; Benning, L.G.; Horn, F.; Bartholomäus, A.; Wiesner, Y.; Wagner, D.; Liebner, S. The Terrestrial Plastisphere: Diversity and Polymer-Colonizing Potential of Plastic-Associated Microbial Communities in Soil. Microorganisms 2021, 9, 1876. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.D. Terrestrial and Aquatic Plastisphere: Formation, Characteristics, and Influencing Factors. Sustainability 2024, 16, 2163. [Google Scholar] [CrossRef]
- Pfeiffer, T.Ž.; Maronić, D.Š.; Stević, F.; Balkić, A.G.; Bek, N.; Martinović, A.; Mandir, T.; Nikolašević, R.; Janjić, D. Plastisphere development in relation to the surrounding biotic communities. Environ. Pollut. 2022, 306, 119380. [Google Scholar] [CrossRef]
- Roncero-Ramos, B.; Muñoz-Martín, M.; Cantón, Y.; Chamizo, S.; Rodríguez-Caballero, E.; Mateo, P. Land degradation effects on composition of pioneering soil communities: An alternative successional sequence for dryland cyanobacterial biocrusts. Soil Biol. Biochem. 2020, 146, 107824. [Google Scholar] [CrossRef]
- Miralles, I.; Lázaro, R.; Sánchez-Marañón, M.; Soriano, M.; Ortega, R. Biocrust cover and successional stages influence soil bacterial composition and diversity in semiarid ecosystems. Sci. Total Environ. 2020, 709, 134654. [Google Scholar] [CrossRef]
- Brachner, A.; Fragouli, D.; Duarte, I.F.; Farias, P.M.A.; Dembski, S.; Ghosh, M.; Barisic, I.; Zdzieblo, D.; Vanoirbeek, J.; Schwabl, P.; et al. Assessment of human health risks posed by nano-and microplastics is currently not feasible. Int. J. Environ. Res. Public Health 2020, 17, 8832. [Google Scholar] [CrossRef]
- Santonicola, S.; Volgare, M.; Cocca, M.; Dorigato, G.; Giaccone, V.; Colavita, G. Impact of Fibrous Microplastic Pollution on Commercial Seafood and Consumer Health: A Review. Animals 2023, 13, 1736. [Google Scholar] [CrossRef]
- Miller, M.E.; Hamann, M.; Kroon, F.J. Bioaccumulation and Biomagnification of Microplastics in Marine Organisms: A Review and Meta-Analysis of Current Data. PLoS ONE 2020, 15, e0240792. [Google Scholar] [CrossRef] [PubMed]
- Parvin, F.; Nath, J.; Hannan, T.; Tareq, S.M. Proliferation of microplastics in commercial sea salts from the world longest sea beach of Bangladesh. Environ. Adv. 2022, 7, 100173. [Google Scholar] [CrossRef]
- Nirmala, K.; Rangasamy, G.; Ramya, M.; Shankar, V.U.; Rajesh, G. A critical review on recent research progress on microplastic pollutants in drinking water. Environ. Res. 2023, 222, 115312. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Laneri, S.; Di Lorenzo, R.; Neri, I.; Dini, I.; Ciampaglia, R.; Grumetto, L. Monitoring of Pollutants Content in Bottled and Tap Drinking Water in Italy. Molecules 2022, 27, 3990. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Hu, Z.; Li, H.; Zheng, H.; Yang, S.; Yu, W.; Tang, B.; Yang, H.; He, R.; Guo, W.; et al. Recent advances in microplastic removal from drinking water by coagulation: Removal mechanisms and influencing factors. Environ. Pollut. 2024, 349, 123863. [Google Scholar] [CrossRef]
- Prata, J.C.; Dias-Pereira, P. Microplastics in Terrestrial Domestic Animals and Human Health: Implications for Food Security and Food Safety and Their Role as Sentinels. Animals 2023, 13, 661. [Google Scholar] [CrossRef]
- Adjama, I.; Dave, H.; Balarabe, B.Y.; Masiyambiri, V.; Marycleopha, M. Microplastics in dairy products and human breast milk: Contamination status and greenness analysis of available analytical methods. J. Hazard. Mater. Lett. 2024, 5, 100120. [Google Scholar] [CrossRef]
- Alma, A.M.; de Groot, G.S.; Buteler, M. Microplastics Incorporated by Honeybees from Food Are Transferred to Honey, Wax and Larvae. Environ. Pollut. 2023, 320, 121078. [Google Scholar] [CrossRef]
- Schiano, M.E.; D’Auria, L.J.; D’Auria, R.; Seccia, S.; Rofrano, G.; Signorelli, D.; Sansone, D.; Caprio, E.; Albrizio, S.; Cocca, M. Microplastic contamination in the agri-food chain: The case of honeybees and beehive products. Sci. Total Environ. 2024, 948, 174698. [Google Scholar] [CrossRef]
- Zuccarello, P.; Ferrante, M.; Cristaldi, A.; Copat, C.; Grasso, A.; Sangregorio, D.; Fiore, M.; Oliveri Conti, G. Exposure to microplastics (<10 μm) associated to plastics bottles mineral water consumption: The first quantitative study. Water Res. 2019, 157, 365–371. [Google Scholar]
- Samandra, S.; Mescall, O.J.; Plaisted, K.; Symons, B.; Xie, S.; Ellis, A.V.; Clarke, B.O. Assessing exposure of the Australian population to microplastics through bottled water consumption. Sci. Total Environ. 2022, 837, 155329. [Google Scholar] [CrossRef]
- Weisser, J.; Beer, I.; Hufnagl, B.; Hofmann, T.; Lohninger, H.; Ivleva, N.P.; Glas, K. From the Well to the Bottle: Identifying Sources of Microplastics in Mineral Water. Water 2023, 12, 841. [Google Scholar] [CrossRef]
- Di Fiore, C.; Carriera, F.; Russo, M.V.; Avino, P. Are Microplastics a Macro Issue? A Review on the Sources of Contamination, Analytical Challenges and Impact on Human Health of Microplastics in Food. Foods 2023, 12, 3915. [Google Scholar] [CrossRef]
- Kannan, K.; Vimalkumar, K. A Review of Human Exposure to Microplastics and Insights into Microplastics as Obesogens. Front. Endocrinol. 2021, 12, 724989. [Google Scholar] [CrossRef] [PubMed]
- Katsara, K.; Viskadourakis, Z.; Alissandrakis, E.; Kountourakis, N.; Kenanakis, G.; Papadakis, V.M. Microplastics’ Detection in Honey: Development of Protocols in a Simulation. Appl. Sci. 2024, 14, 4720. [Google Scholar] [CrossRef]
- WHO. Issues New Guidance on Dietary Salt and Potassium. Available online: https://www.who.int/news/item/31-01-2013-who-issues-new-guidance-on-dietary-salt-and-potassium (accessed on 31 August 2021).
- Lee, P.S.; Jung, S.M. Quantitative Analysis of Microplastics Coagulation-Removal Process for Clean Sea Salt Production. Int. J. Environ. Sci. Technol. 2021, 19, 5205–5216. [Google Scholar] [CrossRef]
- Rainieri, S.; Barranco, A. Microplastics, a food safety issue? Trends Food Sci. Technol. 2019, 84, 55–57. [Google Scholar] [CrossRef]
- Ciaralli, L.; Valente, T.; Monfardini, E.; Libralato, G.; Manfra, L.; Berto, D.; Rampazzo, F.; Gioacchini, G.; Chemello, G.; Piermarini, R.; et al. Rose or Red, but Still under Threat: Comparing Microplastics Ingestion between Two Sympatric Marine Crustacean Species (Aristaeomorpha foliacea and Parapenaeus longirostris). Animals 2024, 14, 2212. [Google Scholar] [CrossRef]
- Jeong, E.; Lee, J.-Y.; Redwan, M. Animal Exposure to Microplastics and Health Effects: A Review. Emerg. Contam. 2024, 10, 100369. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef]
- Habib, R.Z.; Kindi, R.A.; Salem, F.A.; Kittaneh, W.F.; Poulose, V.; Iftikhar, S.H.; Mourad, A.-H.I.; Thiemann, T. Microplastic Contamination of Chicken Meat and Fish through Plastic Cutting Boards. Int. J. Environ. Res. Public Health 2022, 19, 13442. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined Effects of UV Exposure Duration and Mechanical Abrasion on Microplastic Fragmentation by Polymer Type. Environ. Sci. Tech. 2017, 51, 4368–4376. [Google Scholar] [CrossRef] [PubMed]
- Enfrin, M.; Lee, J.; Gibert, Y.; Basheer, F.; Kong, L.; Dum’ee, L.F. Release of hazardous nanoplastic contaminants due to microplastics fragmentation under shear stress forces. J. Hazard. Mater. 2020, 384, 121393. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Qi, F.; Gibson, C.T.; Lei, Y.; Fang, C. Investigating kitchen sponge-derived microplastics and nanoplastics with Raman imaging and multivariate analysis. Sci. Total Environ. 2022, 824, 153963. [Google Scholar] [CrossRef]
- Yadav, H.; Khan, M.R.H.; Quadir, M.; Rusch, K.A.; Mondal, P.P.; Orr, M.; Xu, E.G.; Iskander, S.M. Cutting Boards: An Overlooked Source of Microplastics in Human Food? Environ. Sci. Tech. 2023, 57, 8225–8235. [Google Scholar] [CrossRef]
- Shi, Y.; Li, D.; Xiao, L.; Sheerin, E.D.; Mullarkey, D.; Yang, L.; Bai, X.; Shvets, I.V.; Boland, J.J.; Wang, J.J. The influence of drinking water constituents on the level of microplastic release from plastic kettles. J. Hazard. Mater. 2022, 425, 127997. [Google Scholar] [CrossRef]
- Muhib, M.I.; Rahman, M.M. Microplastics contamination in fish feeds: Characterization and potential exposure risk assessment for cultivated fish of Bangladesh. Heliyon 2023, 9, e19789. [Google Scholar] [CrossRef]
- Luo, Y.; Awoyemi, O.S.; Naidu, R.; Fang, C. Detection of microplastics and nanoplastics released from a kitchen blender using Raman imaging. J. Hazard. Mater. 2023, 453, 131403. [Google Scholar] [CrossRef]
- Blanco-Zubiaguirre, L.; Zabaleta, I.; Prieto, A.; Olivares, M.; Zuloaga, O.; Elizalde, M.P. Migration of photoinitiators, phthalates and plasticizers from paper and cardboard materials into different simulants and foodstuffs. Food Chem. 2021, 344, 128597. [Google Scholar] [CrossRef]
- Zheng, X.; Feng, Q.; Chen, J.; Yan, J.; Li, X.; Guo, L. Quantification analysis of microplastics released from disposable polystyrene tableware with fluorescent polymer staining. Sci. Total Environ. 2023, 864, 161155. [Google Scholar] [CrossRef]
- Schiano, M.E.; Sodano, F.; Magli, E.; Corvino, A.; Fiorino, F.; Rimoli, M.G.; Seccia, S.; Albrizio, S. Quantitative Determination of BPA, BPB, BPF and BPS Levels in Canned Legumes from Italian Market. Food Chem. 2023, 416, 135642. [Google Scholar] [CrossRef] [PubMed]
- Thornton Hampton, L.M.; Bouwmeester, H.; Brander, S.M.; Coffin, S.; Cole, M.; Hermabessiere, L.; Mehinto, A.C.; Miller, E.; Rochman, C.M.; Weisberg, S.B. Research recommendations to better understand the potential health impacts of microplastics to humans and aquatic ecosystems. Microplastics Nanoplastics 2022, 2, 18. [Google Scholar] [CrossRef]
- Schiano, M.E.; Sodano, F.; Cassiano, C.; Fiorino, F.; Seccia, S.; Rimoli, M.G.; Albrizio, S. Quantitative Determination of Bisphenol A and Its Congeners in Plant-Based Beverages by Liquid Chromatography Coupled to Tandem Mass Spectrometry. Foods 2022, 11, 3853. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Dini, I. The Potential of Algae in the Nutricosmetic Sector. Molecules 2023, 28, 4032. [Google Scholar] [CrossRef]
- Dini, I. “Edible Beauty”: The Evolution of Environmentally Friendly Cosmetics and Packaging. Antioxidants 2024, 13, 742. [Google Scholar] [CrossRef]
- Oliveri Conti, G.; Rapisarda, P.; Ferrante, M. Relationship between climate change and environmental microplastics: A one health vision for the platysphere health. One Health Adv. 2024, 2, 17. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Ren, J. Analysis of Microplastics in Takeaway Food Containers in China Using FPA-FTIR Whole Filter Analysis. Molecules 2022, 27, 2646. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Sonego, E.; Di Filippo, P.; Riccardi, C.; Pomata, D.; Bannò, A.; Simonetti, G.; Buiarelli, F. Occurrence and migration study of chemicals from baking paper and aluminium foil. Food Chem. 2023, 409, 135260. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Yang, A.; Wang, Y.; Hou, S.; Zheng, N.; Liang, D.; Hua, X.; Dong, D. Health risks of population exposure to phthalic acid esters through the use of plastic containers for takeaway food in China. Sci. Total Environ. 2021, 785, 147347. [Google Scholar] [CrossRef]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic contamination of packaged meat: Occurrence and associated risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Alak, G.; Köktürk, M.; Atamanalp, M. Evaluation of phthalate migration potential in vacuum-packed. Sci. Rep. 2024, 14, 7944. [Google Scholar] [CrossRef]
- Carwile, J.L.; Seshasayee, S.M.; Ahrens, K.A.; Hauser, R.; Chavarro, J.E.; Fleisch, A.F. Dietary correlates of urinary phthalate metabolite concentrations in 6–19 Year old children and adolescents. Environ. Res. 2022, 204, 112083. [Google Scholar] [CrossRef] [PubMed]
- Alimba, C.G.; Faggio, C.; Sivanesan, S.; Ogunkanmi, A.L.; Krishnamurthi, K. Micro (nano)-plastics in the environment and risk of carcinogenesis: Insight into possible mechanisms. J. Hazard. Mater. 2021, 416, 126143. [Google Scholar] [CrossRef]
- Lin, Z.; Jin, T.; Zou, T.; Xu, L.; Xi, B.; Xu, D.; He, J.; Xiong, L.; Tang, C.; Peng, J.; et al. Current Progress on Plastic/Microplastic Degradation: Fact Influences and Mechanism. Environ. Pollut. 2022, 304, 119159. [Google Scholar] [CrossRef]
- Wang, J.; Weng, X.; Liu, S.; Zhang, H.; Zhu, Q.; Fu, Q.; Wang, T.; Liao, C.; Jiang, G. Occurrence, Fate, and Reduction Measures of Phthalates in the Cooking Process: A Review. Environ. Health 2023, 1, 300–314. [Google Scholar] [CrossRef]
- Barcelo, D.; Pico, Y.; Alfarhan, A.H. Microplastics: Detection in human samples, cell line studies, and health impacts. Environ. Tox. Pharm. 2023, 101, 104204. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, M.; Feng, Z.; Wang, Z.; Lv, M.; Chang, J.; Chen, L.; Wang, C. Human Microplastics Exposure and Potential Health Risks to Target Organs by Different Routes: A Review. Curr. Pollut. Rep. 2023, 9, 468–485. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, Y.; Zhong, Q.; Ma, F.; Zhang, Y. The Impact of Microplastic Particles on Population Dynamics of Predator and Prey: Implication of the Lotka-Volterra Model. Sci. Rep. 2020, 10, 4500. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Ahmad, S.; Guo, X.L.; Ullah, S.; Nabi, G.; Wanghe, K. A review of the endocrine disrupting effects of micro and nano plastic and their associated chemicals in mammals. Front. Endocrinol. 2023, 13, 1084236. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Li, X.; Wang, S.; Tu, C.; Qiu, L.; Zhang, H.; Zhong, C.; Li, S.; Liu, Y.; Liu, J.; et al. New Evidence of Microplastics in the Lower Respiratory Tract: Inhalation through Smoking. Environ. Sci. Technol. 2023, 57, 8496–8505. [Google Scholar] [CrossRef]
- Vattanasit, U.; Kongpran, J.; Ikeda, A. Airborne microplastics: A narrative review of potential effects on the human respiratory system. Sci. Total Environ. 2023, 904, 166745. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Heidari, S.; Sepahvand, P.; Afkhami, H.; Kheradjoo, H. Microplastics and environmental effects: Investigating the effects of microplastics on aquatic habitats and their impact on human health. Front. Public Health 2024, 12, 1411389. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, B.; Gu, C.; Shen, C.; Yin, S.; Aamir, M.; Li, F. Are we underestimating the sources of microplastic pollution in terrestrial environment? J. Hazard. Mater. 2020, 400, 123228. [Google Scholar] [CrossRef]
- Borriello, L.; Scivicco, M.; Cacciola, N.A.; Esposito, F.; Severino, L.; Cirillo, T. Microplastics, a Global Issue: Human Exposure through Environmental and Dietary Sources. Foods 2023, 12, 3396. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, W.; Chan, H.; Peng, J.; Zhu, P.; Li, J.; Jiang, X.; Zhang, Z.; Wang, Y.; Tan, Z.; et al. Polystyrene Microplastics Induce Size-Dependent Multi-Organ Damage in Mice: Insights into Gut Microbiota and Fecal Metabolites. J. Hazard. Mater. 2024, 461, 132503. [Google Scholar] [CrossRef]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Ośko, J.; Knez, E.; Grembecka, M. Microplastics and Oxidative Stress—Current Problems and Prospects. Antioxidants 2024, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Palić, D. Micro- and Nano-Plastics Activation of Oxidative and Inflammatory Adverse Outcome Pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, M.; Xiong, F.; Xu, K.; Huang, J.; Liu, J.; Wang, D.; Pu, Y. Polystyrene micro-and nanoplastics induce gastric toxicity through ROS mediated oxidative stress and P62/Keap1/Nrf2 pathway. Sci. Total Environ. 2024, 912, 169228. [Google Scholar] [CrossRef]
- Zhou, N.; Wang, Z.; Yang, L.; Zhou, W.; Qin, Z.; Zhang, H. Size-Dependent Toxicological Effects of Polystyrene Microplastics in the Shrimp Litopenaeus Vannamei Using a Histomorphology, Microbiome, and Metabolic Approach. Environ. Pollut. 2023, 316, 120635. [Google Scholar] [CrossRef]
- Das, A. The emerging role of microplastics in systemic toxicity: Involvement of reactive oxygen species (ROS). Sci. Total Environ. 2023, 895, 165076. [Google Scholar] [CrossRef]
- Zhong, G.; Rao, G.; Tang, L.; Wu, S.; Tang, Z.; Huang, R.; Ruan, Z.; Hu, L. Combined effect of arsenic and polystyrene-nanoplastics at environmentally relevant concentrations in mice liver: Activation of apoptosis, pyroptosis and excessive autophagy. Chemosphere 2022, 300, 134566. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Fan, X.; Xu, T.; He, Y.; Chi, Q.; Li, Z.; Li, S. Polystyrene Nanoplastics Exacerbated Lipopolysaccharide-Induced Necroptosis and Inflammation via the ROS/MAPK Pathway in Mice Spleen. Environ. Toxicol. 2022, 37, 2552–2565. [Google Scholar] [CrossRef]
- Hayden, M.R. Overview and New Insights into the Metabolic Syndrome: Risk Factors and Emerging Variables in the Development of Type 2 Diabetes and Cerebrocardiovascular Disease. Medicina 2023, 59, 561. [Google Scholar] [CrossRef]
- Wang, S.; Han, Q.; Wei, Z.; Wang, Y.; Xie, J.; Chen, M. Polystyrene Microplastics Affect Learning and Memory in Mice by Inducing Oxidative Stress and Decreasing the Level of Acetylcholine. Food Chem. Toxicol. 2022, 162, 278–6915. [Google Scholar] [CrossRef]
- Yin, K.; Wang, D.; Zhao, H.; Wang, Y.; Zhang, Y.; Liu, Y.; Li, B.; Xing, M. Polystyrene Microplastics Up-Regulates Liver Glutamine and Glutamate Synthesis and Promotes Autophagy-Dependent Ferroptosis and Apoptosis in the Cerebellum through the Liver-Brain Axis. Environ. Pollut. 2022, 307, 119449. [Google Scholar] [CrossRef]
- Abad López, A.P.; Trilleras, J.; Arana, V.A.; García-Alzate, L.S.; Grande-Tovar, C.D. Atmospheric microplastics: Exposure, toxicity, and detrimental health effects. RSC Adv. 2023, 13, 7468–7489. [Google Scholar] [CrossRef] [PubMed]
- Olesiejuk, K.; Chałubiński, M. How does particulate air pollution affect barrier functions and inflammatory activity of lung vascular endothelium? Allergy 2023, 78, 629–638. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Bi, L.; Jin, L.; Peng, R. Understanding the Mechanistic Roles of Microplastics Combined with Heavy Metals in Regulating Ferroptosis: Adding New Paradigms Regarding the Links with Diseases. Environ. Res. 2024, 242, 117732. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Yang, K.; Cheng, X.; Guo, C.; Xing, X.; Sun, H.; Liu, D.; Liu, X.; Wang, D. Accumulation of Polystyrene Microplastics Induces Liver Fibrosis by Activating CGAS/STING Pathway. Environ. Pollut. 2022, 300, 118986. [Google Scholar] [CrossRef]
- Zhao, Y.; Bao, Z.; Wan, Z.; Fu, Z.; Jin, Y. Polystyrene microplastic exposure disturbs hepatic glycolipid metabolism at the physiological, biochemical, and transcriptomic levels in adult zebrafish. Sci. Total Environ. 2020, 710, 136279. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.R.; Wright, S.J.; Gant, T.W. A critical review of microplastics toxicity and potential adverse outcome pathway in human gastrointestinal tract following oral exposure. Toxicol. Lett. 2023, 385, 51–60. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, J.; Chen, Q.; Xu, L.; Yue, X.; Qiao, D. Chronic exposure to polyvinyl chloride microplastics induces liver injury and gut microbiota dysbiosis based on the integration of liver transcriptome profiles and full-length 16S rRNA sequencing data. Sci. Total Environ. 2022, 839, 155984. [Google Scholar] [CrossRef]
- Ma, S.; Xiao, Y.; Zhang, X.; Xu, Y.; Zhu, K.; Zhang, K.; Li, X.; Zhou, H.; Chen, G.; Guo, X. Dietary exposure to polystyrene microplastics exacerbates liver damage in fulminant hepatic failure via ROS production and neutrophil extracellular trap formation. Sci. Total Environ. 2024, 907, 167403. [Google Scholar] [CrossRef]
- Zou, H.; Chen, Y.; Qu, H.; Sun, J.; Wang, T.; Ma, Y.; Yuan, Y.; Bian, J.; Liu, Z. Microplastics Exacerbate Cadmium-Induced Kidney Injury by Enhancing Oxidative Stress, Autophagy, Apoptosis, and Fibrosis. Int. J. Mol. Sci. 2022, 23, 14411. [Google Scholar] [CrossRef]
- Marcelino, R.C.; Cardoso, R.M.; Domingues, E.L.B.C.; Gonçalves, R.V.; Lima, G.D.A.; Novaes, R.D. The emerging risk of microplastics and nanoplastics on the microstructure and function of reproductive organs in mammals: A systematic review of preclinical evidence. Life Sci. 2022, 295, 120404. [Google Scholar] [CrossRef]
- Rubio-Armendáriz, C.; Alejandro-Vega, S.; Paz-Montelongo, S.; Gutiérrez-Fernández, Á.J.; Carrascosa-Iruzubieta, C.J.; Hardisson-de la Torre, A. Microplastics as Emerging Food Contaminants: A Challenge for Food Safety. Int. J. Environ. Res. Public Health 2022, 19, 1174. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.D.; Li, R.; Li, Z.; Wang, D. Health Risk of Human Exposure to Microplastics: A Review. Environ. Chem. Lett. 2024, 22, 1155–1183. [Google Scholar] [CrossRef]
- Duncan, T.V.; Khan, S.A.; Patri, A.K.; Wiggins, S. Regulatory Science Perspective on the Analysis of Microplastics and Nanoplastics in Human Food. Anal. Chem. 2024, 96, 4343–4358. [Google Scholar] [CrossRef]
- Lao, W.; Dial, S.; Salmon, M.; Wong, C.S. Development and validation of an acid/alkaline digestion method for efficient microplastic extraction from wastewater treatment plant effluents: Sulfuric acid concentration and contact time do matter. Sci. Total Environ. 2024, 917, 170528. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, F.; Fischer, E.K. Various Digestion Protocols Within Microplastic Sample Processing—Evaluating the Resistance of Different Synthetic Polymers and the Efficiency of Biogenic Organic Matter Destruction. Front. Environ. Sci. 2020, 8, 572424. [Google Scholar] [CrossRef]
- Rani, M.; Ducoli, S.; Depero, L.E.; Prica, M.; Tubić, A.; Ademovic, Z.; Morrison, L.; Federici, S. A Complete Guide to Extraction Methods of Microplastics from Complex Environmental Matrices. Molecules 2023, 28, 5710. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Han, J.; Zhou, H.; Lau, Y.L.; An, W.; Wei, P.; Cheung, S.G.; Yang, Y.; Tam, N.F. Development of a Digestion Method for Determining Microplastic Pollution in Vegetal-Rich Clayey Mangrove Sediments. Sci. Total Environ. 2020, 707, 136030. [Google Scholar] [CrossRef]
- Schrank, I.; Möller, J.N.; Imhof, H.K.; Hauenstein, O.; Zielke, F.; Agarwal, S.; Löder, M.G.J.; Greiner, A.; Laforsch, C. Microplastic Sample Purification Methods-Assessing Detrimental Effects of Purification Procedures on Specific Plastic Types. Sci. Total Environ. 2022, 833, 154824. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Garrido-Amador, P.; Martínez, I.; Samper, M.D.; López-Martínez, J.; Gómez, M.; Packard, T.T. Novel methodology to isolate microplastics from vegetal-rich samples. Mar. Pollut. Bull. 2018, 129, 61–69. [Google Scholar] [CrossRef]
- Möller, J.N.; Löder, M.G.J.; Laforsch, C. Finding Microplastics in Soils: A Review of Analytical Methods. Environ. Sci. Technol. 2020, 54, 2078–2090. [Google Scholar] [CrossRef]
- Gulizia, A.M.; Brodie, E.; Daumuller, R.; Bloom, S.B.; Corbett, T.; Santana, M.M.F.; Motti, C.A.; Vamvounis, G.; Gulizia, A.M.; Brodie, E.; et al. Evaluating the Effect of Chemical Digestion Treatments on Polystyrene Microplastics: Recommended Updates to Chemical Digestion Protocols. Macromol. Chem. Phys. 2022, 223, 2100485. [Google Scholar] [CrossRef]
- Perez, M.; Parra, S.; Ferrada, C.; Bravo, M.; Perez, P.A.; Quiroz, W. Development of a new methodology for the determination of PET microplastics in sediment, based on microwave-assisted acid digestion. PLoS ONE 2024, 19, e0314520. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pettinato, M.; Campardelli, R.; De Marco, I.; Perego, P. High-Pressure Technologies for the Recovery of Bioactive Molecules from Agro-Industrial Waste. Appl. Sci. 2022, 12, 3642. [Google Scholar] [CrossRef]
- Tan, M.; Sun, Y.; Gui, J.; Wang, J.; Chen, X.; Wu, D. The efficiency of different digestion and separation methods for extracting microplastics in typical organic solid waste. Int. J. Environ. Res. 2022, 16, 16. [Google Scholar] [CrossRef]
- Özkaymak, G.; Aygün, A. Microplastic Detection and Analytical Methods in Wastewater Treatment Plants. In Pollutants and Recent Trends in Wastewater Treatment; Springer Nature: Cham, Switzerland, 2024; pp. 129–144. [Google Scholar]
- Barp, L.; Višnjevec, A.M.; Moret, S. Pressurized Liquid Extraction: A Powerful Tool to Implement Extraction and Purification of Food Contaminants. Foods 2023, 12, 2017. [Google Scholar] [CrossRef]
- Grbic, J.; Nguyen, B.; Guo, E.; You, J.B.; Sinton, D.; Rochman, C.M. Magnetic Extraction of Microplastics from Environmental Samples. Environ. Sci. Technol. Lett. 2019, 6, 68–72. [Google Scholar] [CrossRef]
- Zandieh, M.; Liu, J.W. Removal and Degradation of Microplastics Using the Magnetic and Nanozyme Activities of Bare Iron Oxide Nanoaggregates. Angew. Chem. Int. Ed. 2022, 61, e202212013. [Google Scholar] [CrossRef]
- Solanki, P.; Putatunda, C.; Kumar, A.; Bhatia, R.; Walia, A. Microbial proteases: Ubiquitous enzymes with innumerable uses. 3 Biotech 2021, 11, 428. [Google Scholar] [CrossRef]
- Girão, A.V. SEM/EDS and Optical Microscopy Analysis of Microplastics. In Handbook of Microplastics in the Environment; Rocha-Santos, T., Costa, M., Mouneyrac, C., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–22. [Google Scholar]
- Andoh, C.N.; Attiogbe, F.; Ackerson, N.O.B.; Antwi, M.; Adu-Boahen, K. Fourier Transform Infrared Spectroscopy: An analytical technique for microplastic identification and quantification. Infrared Phys. Technol. 2023, 136, 105070. [Google Scholar] [CrossRef]
- Wu, P.; Wu, X.; Huang, Q.; Yu, Q.; Jin, H.; Zhu, M. Mass spectrometry-based multimodal approaches for the identification and quantification analysis of microplastics in food matrix. Front. Nutr. 2023, 10, 1163823. [Google Scholar] [CrossRef]
- Vitali, C.; Janssen, H.-G.; Ruggeri, F.S.; Nielen, M.W.F. Rapid single particle atmospheric solids analysis probe-mass spectrometry for multimodal analysis of microplastics. Anal. Chem. 2022, 95, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Birch, Q.T.; Potter, P.M.; Pinto, P.X.; Dionysiou, D.D.; Al-Abed, S.R. Isotope Ratio Mass Spectrometry and Spectroscopic Techniques for Microplastics Characterization. Talanta 2021, 224, 121743. [Google Scholar] [CrossRef] [PubMed]
- Laborda, F.; Trujillo, C.; Lobinski, R. Analysis of Microplastics in Consumer Products by Single Particle-Inductively Coupled Plasma Mass Spectrometry Using the Carbon-13 Isotope. Talanta 2021, 221, 121486. [Google Scholar] [CrossRef]
- Meyers, N.; Kopke, K.; Buhhalko, N.; Mattsson, K.; Janssen, C.R.; Everaert, G.; De Witte, B. Value for money: A cost-effectiveness analysis of microplastic analytics in seawater. Microplast. Nanoplast. 2024, 4, 4. [Google Scholar] [CrossRef]
- Matsueda, M.; Mattonai, M.; Iwai, I.; Watanabe, A.; Teramae, N.; Robberson, W.; Ohtani, H.; Kim, Y.M.; Watanabe, C. Preparation and Test of a Reference Mixture of Eleven Polymers with Deactivated Inorganic Diluent for Microplastics Analysis by Pyrolysis-GC–MS. J. Anal. Appl. Pyrolysis 2021, 154, 104993. [Google Scholar] [CrossRef]
- Adelugba, A.; Emenike, C. Comparative Review of Instrumental Techniques and Methods for the Analysis of Microplastics in Agricultural Matrices. Microplastics 2024, 3, 1–21. [Google Scholar] [CrossRef]
- Dong, H.; Wang, X.; Niu, X.; Zeng, J.; Zhou, Y.; Suona, Z.; Yuan, Y.; Chen, X. Overview of analytical methods for the determination of microplastics: Current status and trends. TrAC Trends Anal. Chem. 2023, 167, 117261. [Google Scholar] [CrossRef]
- Murti, Z.; Soedjati, D.; Barkah, A.; Rahardjo, P. Review of the circular economy of plastic waste in various countries and potential applications in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2022, 1098, 012014. [Google Scholar] [CrossRef]
- Bernat, K. Post-Consumer Plastic Waste Management: From Collection and Sortation to Mechanical Recycling. Energies 2023, 16, 3504. [Google Scholar] [CrossRef]
- Pauli, G. UpCycling: Wirtschaften nach dem Vorbild der Natur für Mehr Arbeitsplätze und eine Saubere Umwelt; Carl Bertelsmann: Gütersloh, Germany, 1999. [Google Scholar]
- Jehanno, C.; Alty, J.W.; Roosen, M.; De Meester, S.; Dove, A.P.; Chen, E.Y.-X.; Leibfarth, F.A.; Sardon, H. Critical advances and future opportunities in upcycling commodity polymers. Nature 2022, 603, 803–814. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chu, H.Y.; Wang, C.C. Converting waste PET plastics to high value-added MOFs-based functional materials: A state of the art review. Coord. Chem. Rev. 2024, 518, 216106. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Qian, H.; Nie, Y.; Bai, X.; Xia, T.; Rehman, M.L.U.; Li, Q.; Ju, M. Upcycling and catalytic degradation of plastic wastes. Cell Rep. Phys. Sci. 2021, 2, 100514. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumari, M.; Chauhan, P.; Ram Chaudhary, G. Upcycling of plastic waste into fluorescent carbon dots: An environmentally viable transformation to biocompatible C-dots with potential prospective in analytical applications. Waste Manag. 2021, 120, 675–686. [Google Scholar] [CrossRef]
- Kumari, M.; Chaudhary, G.R.; Chaudhary, S.; Umar, A. Transformation of solid plastic waste to activated carbon fibres for wastewater treatment. Chemosphere 2022, 294, 133692. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.; Wen, X.; Hu, G.; Liu, H.; Gong, Z.; Bi, S.; Wei, Q.; Niu, R.; Gong, J. Sustainable upcycling of waste polyethylene terephthalate into hierarchically porous carbon nanosheet for interfacial solar steam and hydroelectricity generation. Sustain. Mater. Technol. 2024, 41, e01022. [Google Scholar] [CrossRef]
- Ko, S.; Kwon, Y.J.; Lee, J.U.; Jeon, Y.-P. Preparation of synthetic graphite from waste PET plastic. J. Ind. Eng. Chem. 2020, 83, 449–458. [Google Scholar] [CrossRef]
- Min, J.; Wen, X.; Tang, T.; Chen, X.; Huo, K.; Gong, J.; Azadmanjiri, J.; He, C.; Mijowska, E. A general approach towards carbonization of plastic waste into a well-designed 3D porous carbon framework for super lithium-ion batteries. Chem. Commun. 2020, 56, 9142–9145. [Google Scholar] [CrossRef]
- Gharpure, A.; Kowalik, M.; Vander Wal, R.L.; van Duin, A.C. Upcycling Plastic Waste into Graphite Using Graphenic Additives for Energy Storage: Yield, Graphitic Quality, and Interaction Mechanisms via Experimentation and Molecular Dynamics. ACS Sustain. Chem. Eng. 2024, 12, 4565–4575. [Google Scholar] [CrossRef]
- Balu, R.; Dutta, N.K.; Roy Choudhury, N. Plastic Waste Upcycling: A Sustainable Solution for Waste Management, Product Development, and Circular Economy. Polymers 2022, 14, 4788. [Google Scholar] [CrossRef]
- Kim, J.G. Chemical recycling of poly(bisphenol A carbonate). Polym. Chem. 2020, 11, 4830–4849. [Google Scholar] [CrossRef]
- Rorrer, N.A.; Nicholson, S.; Carpenter, A.; Biddy, M.J.; Grundl, N.J.; Beckham, G.T. Combining reclaimed PET with bio-based monomers enables plastics upcycling. Joule 2019, 3, 1006–1027. [Google Scholar] [CrossRef]
- Williamson, J.B.; Na, C.G.; Johnson, R.R.; Daniel, W.F.M.; Alexanian, E.J.; Leibfarth, F.A. Chemo- and regioselective functionalization of isotactic polypropylene: A mechanistic and structure–property study. J. Am. Chem. Soc. 2019, 141, 12815–12823. [Google Scholar] [CrossRef] [PubMed]
- Erickson, E.D.; Tominac, P.A.; Ma, J.; Aguirre-Villegas, H.; Zavala, V.M. Evaluating the economic and environmental benefits of deploying a national-scale, thermo-chemical plastic waste upcycling infrastructure in the United States. Comput. Chem. Eng. 2024, 189, 108800. [Google Scholar] [CrossRef]
- Zhaoa, X.; Korey, M.; Li, K.; Copenhaver, K.; Tekinalp, H.; Celik, S.; Kalaitzidou, K.; Ruan, R.; Ragauskas, A.J.; Ozcan, S. Plastic waste upcycling toward a circular economy. Chem. Eng. J. 2021, 428, 131928. [Google Scholar] [CrossRef]
- Ding, Q.; Zhu, H. The Key to Solving Plastic Packaging Wastes: Design for Recycling and Recycling Technology. Polymers 2023, 15, 1485. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kwon, E.E.; Lam, S.S.; Chen, W.H.; Rinklebe, J.; Park, Y.K. Chemical recycling of plastic waste via thermocatalytic routes. J. Clean. Prod. 2021, 321, 128989. [Google Scholar] [CrossRef]
- Liu, X.H.; Xu, S.M.; Zhang, F.; Wang, X.L.; Wang, Y.Z. Chemical upcycling of polymeric materials. Acta Polym. Sin. 2022, 53, 1005–1022. [Google Scholar]
- Awoyera, P.O.; Adesina, A. Plastic wastes to construction products: Status, limitations and future perspective. Case Stud. Constr. Mater. 2020, 12, e00330. [Google Scholar] [CrossRef]
- Dogu, O.; Pelucchi, M.; Van de Vijver, R.; Van Steenberge, P.H.M.; D’hooge, D.R.; Cuoci, A.; Mehl, M.; Frassoldati, A.; Faravelli, T.; Van Geem, K.M. The chemistry of chemical recycling of solid plastic waste via pyrolysis and gasification: State-of-the-art, challenges, and future directions. Prog. Energy Combust. Sci. 2021, 84, 100901. [Google Scholar] [CrossRef]
- Martín-González, D.; de la Fuente Tagarro, C.; De Lucas, A.; Bordel, S.; Santos-Beneit, F. Genetic Modifications in Bacteria for the Degradation of Synthetic Polymers: A Review. Int. J. Mol. Sci. 2024, 25, 5536. [Google Scholar] [CrossRef]
- Plastics Strategy. Available online: https://environment.ec.europa.eu/strategy/plastics-strategy_en#:~:text=The%20plastics%20strategy%20aims%20to,and%20production%20patterns%20for%20plastics (accessed on 10 March 2025).
- Regulation (EU) 2023/2055 of 25 September 2023 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Synthetic Polymer Microparticles. Official Journal of the European Union. 27 September 2023, p. L 238/67. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=oj:JOL_2023_238 (accessed on 27 September 2023).
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- REACH CE 1907/2006. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/PDF/?uri=CELEX:32006R1907R(01) (accessed on 30 December 2016).
- Consolidated Version of the Treaty on European Union. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:2bf140bf-a3f8-4ab2-b506-fd71826e6da6.0023.02/DOC_1&format=PDF (accessed on 26 October 2012).
- Precautionary Principle. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:12016E191 (accessed on 7 June 2016).
- ECHA. Available online: http://echa.europa.eu/regulations/reach/registration (accessed on 26 February 2016).
- Postle, M.; Holmes, P.; Camboni, M.; Footitt, A.; Tuffnell, N.; Blainey, M.; Stevens, G.; Pye, A. Review of REACH with Regard to the Registration Requirements on Polymers; Final Report. Part A: Polymers. Prepared for the European Commission, DG Environment; Risk & Policy Analysis Limited: Loddon, UK, 2012. [Google Scholar]
- Toni, A.D.; Saïdi, N.; Santos, L.R.; Mudgal, S. Technical Assistance Related to the Review of REACH with Regard to the Registration Requirements on Polymers; Final Report Prepared for the European Commission (DG ENV), in Collaboration with PIEP; European Commission–DG ENV: Brussels, Belgium, 2015. [Google Scholar]
- Ministry of Housing & Urban Affairs (MOHUA). Plastic Waste Management Issues, Solutions & Case Studies. Available online: https://sbmurban.org/storage/app/media/pdf/SBM%20Plastic%20Waste%20Book.pdf (accessed on 15 January 2021).
- Niaounakis, M. 10—Legislation and Regulatory Framework. In Recycling of Flexible Plastic Packaging; Niaounakis, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2020; pp. 369–393. [Google Scholar]
- National Strategy to Prevent Plastic Pollution. Available online: https://www.epa.gov/circulareconomy/national-strategy-prevent-plastic-pollution (accessed on 1 November 2024).
- New Strategy for Current and Future USGS Microplastics Research. Available online: https://www.usgs.gov/news/featured-story/small-particles-big-problems-a-strategy-addressing-microplastics-science-gaps (accessed on 18 June 2024).
- ASEAN Regional Action Plan for Combating Marine Debris in the ASEAN Member States (2021–2025). Available online: https://asean.org/book/asean-regional-action-plan-for-combating-marine-debris-in-the-asean-member-states-2021-2025-2/ (accessed on 18 June 2024).
- ASEAN-Norway Sectoral Dialogue Partnership: Practical Cooperation Areas (2021–2025). Available online: https://policycommons.net/artifacts/1550631/asean-norway-sectoral-dialogue-partnership/2240456/ (accessed on 24 March 2021).
- ASEAN Plus Three Statement on Active Ageing. 2016. Available online: https://asean.Org/asean-plus-three-statement-on-active-ageing/ (accessed on 11 May 2020).
- Ng, C.H.; Mistoh, M.A.; Teo, S.H.; Galassi, A.; Ibrahim, A.; Sipaut, C.S.; Foo, J.; Seay, J.; Taufiq-Yap, Y.H.; Janaun, J. Plastic waste and microplastic issues in Southeast Asia. Front. Environ. Sci. 2023, 11, 1–15. [Google Scholar] [CrossRef]
- Omeyer, L.; Duncan, E.; Aisembom, K. Priorities to inform research on marine plastic pollution in Southeast Asia. Sci. Total Environ. 2022, 841, A1656704. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, J.P.; Mouneyrac, C.; Costa, M.; Duarte, A.C.; Rocha-Santos, T. The Role of Legislation, Regulatory Initiatives and Guidelines on the Control of Plastic Pollution. Front. Environ. Sci. 2020, 8, 104. [Google Scholar] [CrossRef]
- Liu, C.; Liu, C. Exploring Plastic-Management Policy in China: Status, Challenges and Policy Insights. Sustainability 2023, 15, 9087. [Google Scholar] [CrossRef]
- Kuan, S.H.; Low, F.S.; Chieng, S. Towards regional cooperation on sustainable plastic recycling: Comparative analysis of plastic waste recycling policies and legislations in Japan and Malaysia. Clean Technol. Environ. Policy 2022, 24, 761–777. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).