Profiling of Volatile Organic Compounds, Including Halogenated Substances, in Okinawan Red Alga Portieria hornemannii
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of HS-SPME Temperature on the VOCs of P. hornemannii
2.2. Identification of VOCs Emitted from P. hornemannii Using SLE-GC-MS
3. Materials and Methods
3.1. Materials
3.2. VOCs Extraction Using HS-SPME
3.3. SLE Extraction
3.4. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Jong, E.; Vijge, M.J. From Millennium to Sustainable Development Goals: Evolving discourses and their reflection in policy coherence for development. Earth Syst. Gov. 2021, 7, 100087. [Google Scholar] [CrossRef]
- Fast, G.; Widerberg, O. Governance through goals in action: How multi-stakeholder partnerships translate and connect the SDGs. Earth Syst. Gov. 2025, 23, 100238. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Lopez-Alonso, M.; Hayes, M.; de Nys, R. Assessment of the functional properties of protein extracted from the brown seaweed Himanthalia elongata (Linnaeus) S. F. Gray. Food Res. Int. 2017, 99, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, K.; Jeon, Y. Bio-functionalities of proteins derived from marine algae—A review. Food Res. Int. 2012, 48, 948–960. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Asljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef]
- Sivaraman, L.; Deshmukhe, G.; Xavier, K.A.M.; Hirekudel, S.K.; Desai, A.S.; Balange, A.K. Effect of incorporation of seaweed (Portieria hornemanii) and tilapia fish protein concentrate on the nutritional, physical and sensory attributes of cookies. Int. J. Food Sci. Technol. 2023, 58, 4415–4424. [Google Scholar] [CrossRef]
- Payo, D.A.; Colo, J.; Calumpong, H.; de Clerck, O. Variability of non-polar secondary metabolites in the red alga Portieria. Mar. Drugs 2011, 9, 2438–2468. [Google Scholar] [CrossRef]
- Unnikrishnan, P.S.; Suthindhiran, K.; Jayasri, M.A. Effect marine red algal (Portieria hornemanii) extracts on starch digestion rate and its possible role in diabetes management. Aquac. Int. 2023, 31, 3239–3256. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Y. Marine volatile organic compounds and their impacts on marine aerosol—A review. Sci. Total Environ. 2024, 768, 145054. [Google Scholar] [CrossRef]
- Wan, F.-G.; Chen, Y.-L.; Zheng, J.-L.; Jin, W.-Y.; Chen, T.-H.; Zhu, Q.-L.; Zhan, Q.-H.; Jiang, L.-H.; Chen, S.; Song, W.-H.; et al. Exploring eutrophic effects of marine sediments underneath fish cage farms: Insights from changes in eukaryotic and bacterial communities and volatile organic compounds. Sci. Total Environ. 2025, 967, 178820. [Google Scholar] [CrossRef]
- Pozzer, A.C.; Gómez, P.A.; Weiss, J. Volatile organic compounds in aquatic ecosystems—Detection, origin, significance and applications. Sci. Total Environ. 2022, 838, 156155. [Google Scholar] [CrossRef] [PubMed]
- Puspitasari, D.; Punta-Sánchez, I.; Calle, J.L.P.; Setyaningsih, W.; Lideman, L.; Palma, M.; Ningrum, A.; Manikharda. Optimization of HS-SPME combined with GC-MS for key marker volatile organic compound analysis Kappaphycus alvarezii with a chemometric approach. J. Food Meas. Charact. 2024, 18, 3510–3526. [Google Scholar] [CrossRef]
- Garcia-Jimenez, P.; Brito-Romano, O.; Robaina, R.R. Production of volatiles by the red seaweed Gelidium arbuscula (Rhodophyta): Emission of ethylene and dimethyl sulfide. J. Phycol. 2013, 49, 661–669. [Google Scholar] [CrossRef]
- Hutchings, J.L.; Grebneva, Y.; Dilmetz, S.J.; Pincher, D.W.M.; Hoffmann, P. Analytical methods for the analysis of bromoform in red seaweed Asparagopsis armata and Asparagopsis taxiformis—A review. Algal Res. 2024, 79, 103478. [Google Scholar] [CrossRef]
- Ishigami, S.; Fukada, R.; Nagasaka, G.; Tsuruta, T.; Nishikawa, K.; Sasaki, Y.; Nimura, K.; Oshima, I.; Yamagishi, Y.; Morimoto, Y.; et al. Halogenated cyclic monoterpenoids with anti-biofouling activity from the Okinawan red marine algae Portieria hornemanii. Chem. Biodivers. 2024, 21, e202400436. [Google Scholar] [CrossRef]
- Fei, D.; Xie, M.; Guang, Y.; Xu, J.; Wu, N.; Cai, J.; Lai, Y.; Zhou, Y. HS-SPME-GC-MS combined with multivariate analysis assay for volatile organic compounds of commercial eggs and native eggs. Food Anal. Methods 2024, 18, 347–358. [Google Scholar] [CrossRef]
- Munier, M.; Jubeau, S.; Wijaya, A.; Morancais, M.; Dumay, J.; Marchal, L.; Jaouen, P.; Fleurence, J. Physicochemical factors affecting the stability of two pigments: R-phycoerythrin of Grateloupia turuturu and B-phycoerythrin of Porphyridium cruentum. Food Chem. 2014, 150, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Barahona, L.F.; Rorrer, G.L. Isolation of halogenated monoterpenes from bioreactor-cultured microplantlets of the macrophytic red algae Ochtodes secundiramea and Portieria hornemannii. J. Nat. Prod. 2003, 66, 743–751. [Google Scholar] [CrossRef]
- Cikoš, A.-M.; Flanjak, I.; Bojanić, K.; Babić, S.; Čižmek, L.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Bioprospecting of coralline red alga Amphiroa rigida J.V. Lamouroux: Volatiles, fatty acids and pigments. Molecules 2021, 26, 520–540. [Google Scholar] [CrossRef]
- Vilar, E.G.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. A chemometric approach to characterize the aroma of selected brown and red edible seaweeds/extracts. J. Sci. Food Agric. 2020, 101, 1228–1238. [Google Scholar] [CrossRef]
- Herkenhoff, M.E.; Brodel, O.; Frohme, M. Hops across continents: Exploring how terroir transforms the aromatic profiles of five hop (Humulus lupulus) varieties grown in their countries of origin and in Brazil. Plants 2024, 13, 2675–2693. [Google Scholar] [CrossRef] [PubMed]
- van Gemert, L.J. Flavour Threshold; Oliemans Punter & Partners BV: Zeist, The Netherland, 2011; pp. 1–476. [Google Scholar]
- Egorin, M.J.; Sentz, D.L.; Rosen, D.M.; Ballesteros, M.F.; Kearns, C.M.; Callery, P.S.; Eiseman, J.L. Plasma pharmacokinetics, bioavailability, and tissue distribution in CD2F1 mice of halomon, an antitumor halogenated monoterpene isolated from the red algae Portieria hornemannii. Cancer Chemother. Pharmacol. 1996, 39, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-J.; Huang, T.-Y.; Huang, C.-Y.; Lin, C.-C.; Wang, W.-L.; Huang, H.-C.; Liu, S.-Y.V.; Chao, C.-H.; Sheu, J.-H. Anti-inflammatory halogenated monoterpenes from the red alga Portieria hornemannii. Mar. Drugs 2023, 21, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Coquin, S.; Ormeno, E.; Pasqualini, V.; Monnier, B.; Culioli, G.; Lecareux, C.; Fernandez, C.; Saunier, A. Chemical diversity of mediterranean seagrasses volatilome. Metabolites 2024, 14, 705. [Google Scholar] [CrossRef]
- Bajer, T.; Ligor, M.; Ligor, T.; Buszewski, B. Design of the extraction process for terpenes and other volatiles from allspice by solid-phase microextraction and hydrodistillation. J. Sep. Sci. 2016, 39, 769–775. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, M.; Zhu, K.; Liu, Y.; Wen, H.; Kong, J.; Chen, M.; Cao, L.; Ye, J.; Zhang, H.; et al. Multiomics integrated with sensory evaluations to identify characteristic aromas and key genes in a novel brown navel orange (Citrus sinensis). Food Chem. 2024, 444, 138613. [Google Scholar] [CrossRef]
- Ramesh, C.H.; Koushik, S.; Shunmugaraj, T.; Ramana Murthy, M.V. A red alga Portieria hornemannii (Lyngb.) P. C. Silva 1987 (Gigartinales, Rhizophyllidaceae): A source of fragrance ingredient for perfume industry. Indian J. Geo Mar. Sci. 2020, 49, 898–902. [Google Scholar]
- Tian, M.; Lin, K.; Yang, L.; Jiang, B.; Zhang, B.; Zhu, X.; Ren, D.; Yu, H. Characterization of key aroma compounds in gray sufu fermented using Leuconostoc mesenteroides subsp. Mesenteroides F24 as a starter culture. Food Chem. X 2023, 20, 100881. [Google Scholar] [CrossRef]



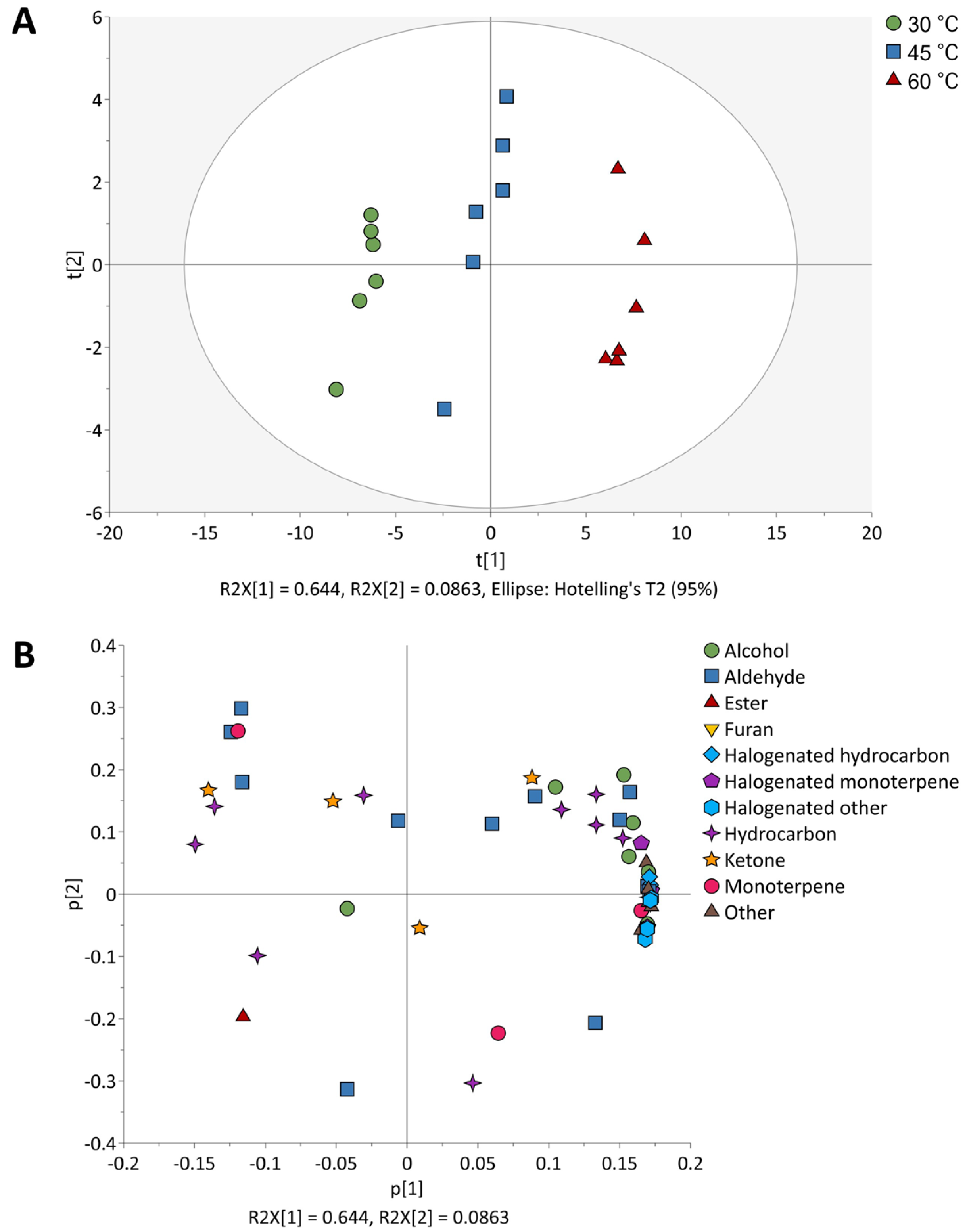
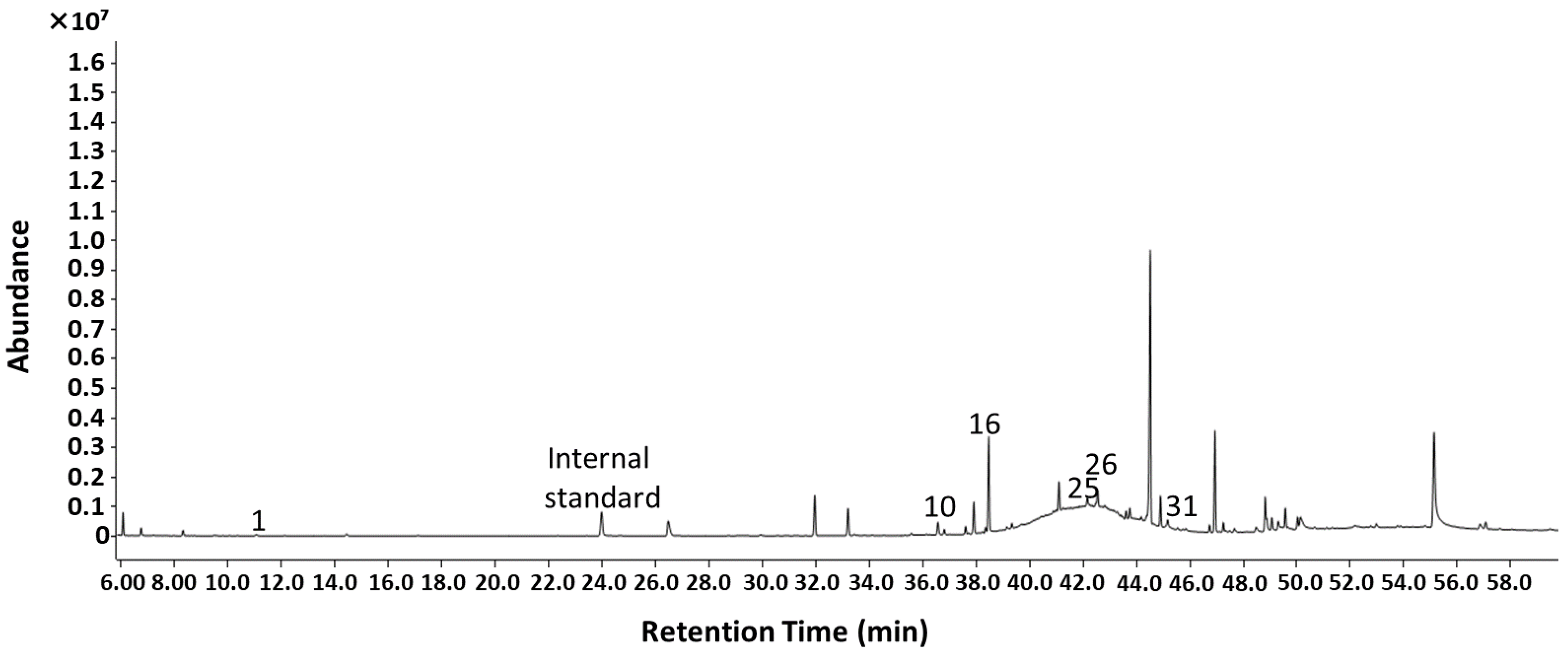
| No. | RI (DB-WAX) 1 | RI (DB-5MS) 2 | Compound (Written in IUPAC Name) | Chemical Group | 30 °C | 45 °C | 60 °C |
|---|---|---|---|---|---|---|---|
| 1 | 711 | - | Ethanal | Aldehyde | nd | nd | 0.43 ± 0.44 |
| 2 | 783 | - | Propanal | Aldehyde | 0.06 ± 0.03 b | 0.10 ± 0.06 b | 0.31 ± 0.22 a |
| 3 | 877 | - | 2-Methyl-2-propenal | Aldehyde | 0.05 ± 0.03 ab | 0.06 ± 0.03 a | 0.01 ± 0.02 b |
| 4 | 884 | 608 | Ethyl acetate | Ester | 0.93 ± 0.21 a | 0.80 ± 0.23 a | 0.54 ± 0.19 b |
| 5 | 949 | - | Octa-1,3-diene | Hydrocarbon | 0.05 ± 0.01 a | 0.05 ± 0.04 a | nd |
| 6 | 976 | 699 | Pentanal | Aldehyde | 0.15 ± 0.04 a | 0.12 ± 0.03 a | 0.07 ± 0.01 b |
| 7 | 1018 | 681 | Pent-1-en-3-one | Ketone | 0.29 ± 0.03 a | 0.21 ± 0.03 ab | 0.16 ± 0.03 b |
| 8 | 1029 | - | (3E,6E)-Octa-1,3,6-triene | Monoterpene | 0.11 ± 0.03 a | 0.11 ± 0.03 a | 0.14 ± 0.03 a |
| 9 | 1051 | 694 | Pentane-2,3-dione | Ketone | 0.04 ± 0.01 a | 0.05 ± 0.01 a | 0.08 ± 0.06 a |
| 10 | 1077 | 800 | Hexanal | Aldehyde | 0.35 ± 0.03 a | 0.33 ± 0.06 a | 0.22 ± 0.05 b |
| 11 | 1100 | - | 1-Ethylcyclohexa-1,4-diene | Hydrocarbon | 0.05 ± 0.01 a | 0.04 ± 0.02 a | nd |
| 12 | 1124 | 747 | (E)-Pent-2-enal | Aldehyde | 0.11 ± 0.02 a | 0.17 ± 0.04 b | 0.24 ± 0.05 c |
| 13 | 1157 | 988 | 7-Methyl-3-methyleneocta-1,6-diene | Monoterpene | 6.29 ± 0.89 a | 6.54 ± 1.52 a | 3.16 ± 0.70 b |
| 14 | 1191 | - | 3-Butyl-4-ethenylcyclopentene | Hydrocarbon | 0.17 ± 0.06 a | 0.11 ± 0.10 a | 0.04 ± 0.06 b |
| 15 | 1213 | 847 | (E)-Hex-2-enal | Aldehyde | 0.07 ± 0.02 a | 0.13 ± 0.06 a | 0.13 ± 0.09 a |
| 16 | 1282 | - | Cyclohexanone | Ketone | 0.12 ± 0.01 a | 0.13 ± 0.05 a | 0.09 ± 0.06 a |
| 17 | 1297 | - | Oct-1-yn-3-ol | Alcohol | 0.14 ± 0.02 b | 0.25 ± 0.08 a | 0.33 ± 0.04 a |
| 18 | 1320 | 762 | (Z)-Pent-2-en-1-ol | Alcohol | 0.08 ± 0.01 b | 0.13 ± 0.05 ab | 0.16 ± 0.05 a |
| 19 | 1349 | 1175 | 6-Butylcyclohepta-1,4-diene | Hydrocarbon | 0.12 ± 0.03 b | 0.16 ± 0.05 ab | 0.22 ± 0.05 a |
| 20 | 1369 | 1156 | 6-[(Z)-But-1-enyl]cyclohepta-1,4-diene | Hydrocarbon | 0.40 ± 0.05 b | 0.49 ± 0.10 ab | 0.62 ± 0.13 a |
| 21 | 1424 | 1055 | (E)-Oct-2-enal | Aldehyde | 0.05 ± 0.03 c | 0.10 ± 0.02 b | 0.22 ± 0.02 a |
| 22 | 1458 | 977 | Oct-1-en-3-ol | Alcohol | 0.09 ± 0.01 c | 0.21 ± 0.05 b | 0.26 ± 0.02 a |
| 23 | 1489 | 970 | (5Z)-Octa-1,5-dien-3-ol | Alcohol | 0.21 ± 0.03 c | 0.58 ± 0.13 b | 0.71 ± 0.05 a |
| 24 | 1500 | - | [(E)-But-1-enyl]benzene | Hydrocarbon | 0.02 ± 0.01 c | 0.09 ± 0.03 b | 0.28 ± 0.04 a |
| 25 | 1514 | 958 | Benzenecarbaldehyde | Aldehyde | 1.32 ± 0.77 b | 2.76 ± 1.61 b | 7.44 ± 2.87 a |
| 26 | 1566 | 1258 | 2-Bromomethyl-6,6-dimethylbicyclo [3.1.1]hept-2-ene | Halogenated monoterpene | 25.20 ± 2.00 c | 47.22 ± 14.86 b | 87.52 ± 17.98 a |
| 27 | 1579 | - | 1-(Methoxymethoxymethyl)-4-prop-1-en-2-ylcyclohexene | Monoterpene | 0.05 ± 0.01 b | 0.12 ± 0.03 b | 0.32 ± 0.10 a |
| 28 | 1583 | 1158 | (2E,6Z)-Nona-2,6-dienal | Aldehyde | 0.09 ± 0.02 b | 0.17 ± 0.04 b | 0.54 ± 0.10 a |
| 29 | 1596 | 1280 | 3-(6-Methyl-2-methylidenehept-5-enylidene)dithiirane | Other | 15.01 ± 0.76 c | 30.64 ± 7.66 b | 57.36 ± 8.55 a |
| 30 | 1602 | 1101 | 2-Methyl-1-benzofuran | Furan | 0.41 ± 0.02 c | 1.85 ± 0.33 b | 7.22 ± 0.82 a |
| 31 | 1618 | 1270 | 2-Bromo-1-methyl-4-propan-2-ylbenzene | Halogenated monoterpene | 0.10 ± 0.01 c | 0.27 ± 0.06 b | 0.88 ± 0.14 a |
| 32 | 1643 | - | 4-Ethenyl-1,2-dimethylbenzene | Hydrocarbon | nd | 0.07 ± 0.06 b | 0.25 ± 0.06 a |
| 33 | 1673 | 1284 | 2-Bromoadamantane | Halogenated hydrocarbon | 0.72 ± 0.04 c | 1.78 ± 0.39 b | 4.09 ± 0.53 a |
| 34 | 1686 | 1067 | (2E)-Octa-2,7-dien-1-ol | Alcohol | 0.14 ± 0.02 c | 0.46 ± 0.10 b | 1.06 ± 0.12 a |
| 35 | 1698 | - | Heptadecane | Hydrocarbon | 0.10 ± 0.03 c | 0.51 ± 0.12 b | 2.12 ± 0.37 a |
| 36 | 1718 | 1356 | 4-Chlorooctahydro-2,4-methano-indene | Halogenated hydrocarbon | 1.50 ± 0.10 c | 4.00 ± 1.09 b | 10.37 ± 1.88 a |
| 37 | 1757 | 1198 | 4-(1-Methylethyl)benzaldehyde | Aldehyde | 3.06 ± 0.26 c | 8.58 ± 1.85 b | 21.31 ± 4.30 a |
| 38 | 1777 | 1239 | 5,6-Dimethyl-1H-benzimidazole | Other | 8.85 ± 0.35 c | 31.53 ± 6.42 b | 129.95 ± 18.26 a |
| 39 | 1811 | 1352 | 4-(4-Methylpent-3-enyl)-3,6-dihydrodithiine | Other | 0.61 ± 0.08 c | 1.63 ± 0.38 b | 5.51 ± 0.62 a |
| 40 | 1881 | - | 2,7-Dimethylocta-2,6-dien-1-ol | Alcohol | 0.52 ± 0.04 c | 2.40 ± 0.21 b | 9.83 ± 0.73 a |
| 41 | 1903 | 1227 | 5,7-Dimethyl-1H-indazole | Other | 0.23 ± 0.04 b | 2.08 ± 1.06 a | 10.50 ± 3.71 a |
| 42 | 1915 | - | Pentamethylbenzenesulphonamide | Other | 0.40 ± 0.18 b | 2.23 ± 1.08 a | 15.99 ± 4.32 a |
| 43 | 1926 | - | [(E)-Pent-2-en-2-yl]benzene | Hydrocarbon | 0.19 ± 0.25 b | 0.69 ± 0.83 b | 5.81 ± 3.10 a |
| 44 | 1927 | - | 1-Methyl-1,3-dihydroinden-2-one | Ketone | 0.06 ± 0.09 a | 0.08 ± 0.21 a | 2.28 ± 3.54 a |
| 45 | 1935 | - | 2,2-Dimethyl-1,3-dihydroindene | Hydrocarbon | 0.47 ± 0.12 b | 1.09 ± 0.83 a | 3.55 ± 2.85 a |
| 46 | 1943 | - | (E)-3-(4-Methylphenyl)prop-2-enal | Aldehyde | 0.37 ± 0.05 a | 0.79 ± 0.63 a | 3.89 ± 5.04 a |
| 47 | 1953 | 1476 | 1-Acetyl-3-chloro-adamantane | Halogenated other | 0.67 ± 0.08 b | 2.45 ± 0.78 b | 13.51 ± 2.22 a |
| 48 | 1975 | - | 2-Buta-1,3-dien-2-ylphenol | Alcohol | 0.14 ± 0.18 a | 0.31 ± 0.41 a | 0.42 ± 1.03 a |
| 49 | 2005 | - | 1,3-Dibromoadamantane | Halogenated monoterpene | 2.02 ± 0.18 b | 5.33 ± 1.13 b | 30.38 ± 5.17 a |
| 50 | 2037 | 1375 | 2,4,6-Trimethylbenzoyl chloride | Halogenated other | 0.43 ± 0.05 c | 1.66 ± 0.36 b | 6.68 ± 0.63 a |
| 51 | 2046 | 1541 | N-(2-Bromophenyl)hex-5-enamide | Halogenated other | 14.39 ± 2.24 c | 36.36 ± 4.44 b | 150.94 ± 20.41 a |
| 52 | 2054 | - | 6,6-Dimethylbicyclo [3.1.1]hept-2-ene-2-carbonyl bromide | Halogenated other | 0.13 ± 0.04 c | 0.50 ± 0.11 b | 2.93 ± 0.20 a |
| No | RI (DB-Wax) | Compound (Written in IUPAC Name) | Chemical Group | SLE (%) | SPME 30 °C (%) |
|---|---|---|---|---|---|
| 1 | 1160 | 7-Methyl-3-methyleneocta-1,6-diene | Monoterpene | 0.09 ± 0.04 b | 7.20 ± 0.85 a |
| 2 | 1233 | Acetic anhydride | Other | 0.15 ± 0.04 | nd |
| 3 | 1448 | Acetic acid | Carboxylic acid | 1.60 ± 0.97 | nd |
| 4 | 1513 | Benzenecarbaldehyde | Aldehyde | 0.09 ± 0.05 b | 1.51 ± 0.88 a |
| 5 | 1563 | 2-Bromomethyl-6,6-dimethylbicyclo [3.1.1]hept-2-ene | Halogenated monoterpene | 2.94 ± 1.11 b | 28.88 ± 1.58 a |
| 6 | 1600 | 2-Methyl-1-benzofuran | Furan | 0.21 ± 0.04 b | 0.47 ± 0.02 a |
| 7 | 1622 | 2-(2-Ethoxyethoxy)ethanol | Alcohol | 0.08 ± 0.06 | nd |
| 8 | 1635 | 2-Hydroxyethyl acetate | Ester | 0.04 ± 0.02 | nd |
| 9 | 1668 | 2-Bromoadamantane | Halogenated hydrocarbon | 0.19 ± 0.10 b | 0.83 ± 0.03 a |
| 10 | 1686 | (2E)-Octa-2,7-dien-1-ol | Alcohol | 0.13 ± 0.08 a | 0.16 ± 0.03 a |
| 11 | 1700 | Heptadecane | Hydrocarbon | 1.61 ± 0.36 a | 0.11 ± 0.03 b |
| 12 | 1709 | (1E,4E,8E)-2,6,6,9-Tetramethylcycloundeca-1,4,8-triene | Hydrocarbon | 0.51 ± 0.09 b | 0.71 ± 0.09 a |
| 13 | 1743 | 2,6-Diethylbromobenzene | Halogenated hydrocarbon | 0.16 ± 0.07 | nd |
| 14 | 1750 | 4-(1-Methylethyl)benzaldehyde | Aldehyde | 2.92 ± 0.26 b | 3.51 ± 0.32 a |
| 15 | 1766 | 5,6-Dimethyl-1H-benzimidazole | Other | 0.79 ± 0.12 | nd |
| 16 | 1771 | 2-Ethyl-1-benzofuran | Furan | 10.65 ± 0.87 | nd |
| 17 | 1797 | 2-(2-Butoxyethoxy)ethanol | Alcohol | 1.63 ± 0.75 | nd |
| 18 | 1851 | 2-Methyl-2,3-dihydroinden-1-one | Ketone | 4.99 ± 2.67 | nd |
| 19 | 1853 | 2,3-Dimethyl-1-benzofuran | Furan | 1.12 ± 0.68 | nd |
| 20 | 1857 | 2,3-Dihydro-3-methyl-1H-inden-1-one | Ketone | 2.92 ± 0.83 | nd |
| 21 | 1894 | 1-Methyl-1,3-dihydroinden-2-one | Ketone | 1.28 ± 1.04 | nd |
| 22 | 1896 | 2-Ethenyl-1,3,5-trimethylbenzene | Hydrocarbon | 2.62 ± 1.28 | nd |
| 23 | 1915 | Ethyl-2-benzofuran | Furan | 2.46 ± 1.58 | nd |
| 24 | 1925 | 7,11,15-Trimethyl-3-methylidenehexadec-1-ene | Hydrocarbon | 8.93 ± 1.70 | nd |
| 25 | 1943 | 1-[4-(2-Chloroethyl)phenyl]ethanone | Halogenated other | 9.54 ± 2.05 | nd |
| 26 | 1955 | [(2E,7R,11R)-3,7,11,15-Tetramethylhexadec-2-enyl]acetate | Ester | 5.92 ± 1.14 | nd |
| 27 | 1976 | 6-Methoxy-3-methylbenzofuran | Furan | 3.76 ± 1.87 | nd |
| 28 | 1992 | 2-tert-Butylphenol mesylate | Other | 4.07 ± 0.40 | nd |
| 29 | 2054 | N-(2-Bromophenyl)hex-5-enamide | Halogenated other | 4.30 ± 0.50 b | 16.54 ± 2.80 a |
| 30 | 2067 | 5,7-Dimethyl-1H-indazole | Other | 1.57 ± 2.44 a | 0.27 ± 0.04 a |
| 31 | 2086 | 1-Bromo-2,2-dimethyl-1-(1-hexynyl)-cyclopropane | Halogenated hydrocarbon | 1.12 ± 0.51 | nd |
| 32 | 2096 | 2,4,7,9-Tetramethyl-5-decyne-4,7-diol | Alcohol | 0.83 ± 0.28 | nd |
| 33 | 2147 | 2,4,6-Trimethylbenzoyl chloride | Halogenated other | 0.77 ± 0.12 a | 0.49 ± 0.05 b |
| 34 | 2186 | 1-(3-Chloro-2-propenyl)-4-methoxy-benzene | Halogenated hydrocarbon | 0.27 ± 0.06 | nd |
| 35 | 2221 | Methyl 14-methylpentadecanoate | Ester | 0.12 ± 0.07 | nd |
| 36 | 2225 | 1-Acetyl-5-chloro-adamantane | Halogenated other | 0.01 ± 0.01 | nd |
| 37 | 2258 | 7,7-Bis(bromomethyl)-1-methylbicyclo [2.2.1]heptan-2-one | Halogenated other | 3.92 ± 0.77 | nd |
| 38 | 2271 | 9,10-Dichloro-tricyclo [3.3.2.0(1,5)]decan-2-one | Halogenated other | 1.91 ± 0.28 | nd |
| 39 | 2285 | (Z)-1-Bromo-8-methyl-hydrindan-2-on | Halogenated other | 2.01 ± 0.57 | nd |
| 40 | 2311 | 2,4-di-tert-Butylphenol | Alcohol | 0.44 ± 0.38 | nd |
| 41 | 2361 | Docosahexaenoic acid | Carboxylic acid | 0.83 ± 0.20 | nd |
| 42 | 2388 | Methyl docosahexaenoate | Ester | 0.81 ± 0.30 | nd |
| 43 | 2449 | (Z)-9-Octadecenoic acid | Carboxylic acid | 1.01 ± 0.70 | nd |
| 44 | 2485 | Dodecanoic acid | Carboxylic acid | 1.09 ± 0.44 | nd |
| 45 | 2541 | 4-Benzyloxytricyclo [4.3.1.0(3,8)]decan-10-ol | Alcohol | 0.89 ± 0.36 | nd |
| 46 | 2548 | Bis-(2-methylpropyl)ester-1,2-benzenedicarboxylic acid | Ester | 0.82 ± 0.42 | nd |
| 47 | 2593 | 2-Octadecyloxyethanol | Alcohol | 0.18 ± 0.18 | nd |
| 48 | 2599 | Docosane | Hydrocarbon | 1.03 ± 0.68 | nd |
| 49 | 2666 | Hexadecyl-2-ethylhexanoate | Ester | 0.38 ± 0.61 | nd |
| 50 | 2719 | Octan-2-yl palmitate | Ester | 0.24 ± 0.24 | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tani, K.; Sasaki, Y.; Ishii, T.; Asikin, Y. Profiling of Volatile Organic Compounds, Including Halogenated Substances, in Okinawan Red Alga Portieria hornemannii. Molecules 2025, 30, 2534. https://doi.org/10.3390/molecules30122534
Tani K, Sasaki Y, Ishii T, Asikin Y. Profiling of Volatile Organic Compounds, Including Halogenated Substances, in Okinawan Red Alga Portieria hornemannii. Molecules. 2025; 30(12):2534. https://doi.org/10.3390/molecules30122534
Chicago/Turabian StyleTani, Kazuki, Yu Sasaki, Takahiro Ishii, and Yonathan Asikin. 2025. "Profiling of Volatile Organic Compounds, Including Halogenated Substances, in Okinawan Red Alga Portieria hornemannii" Molecules 30, no. 12: 2534. https://doi.org/10.3390/molecules30122534
APA StyleTani, K., Sasaki, Y., Ishii, T., & Asikin, Y. (2025). Profiling of Volatile Organic Compounds, Including Halogenated Substances, in Okinawan Red Alga Portieria hornemannii. Molecules, 30(12), 2534. https://doi.org/10.3390/molecules30122534







