An Activated Glassy Carbon Electrode for Rapid, Simple, and Sensitive Voltammetric Analysis of Diclofenac in Tablets
Abstract
1. Introduction
2. Results and Discussion
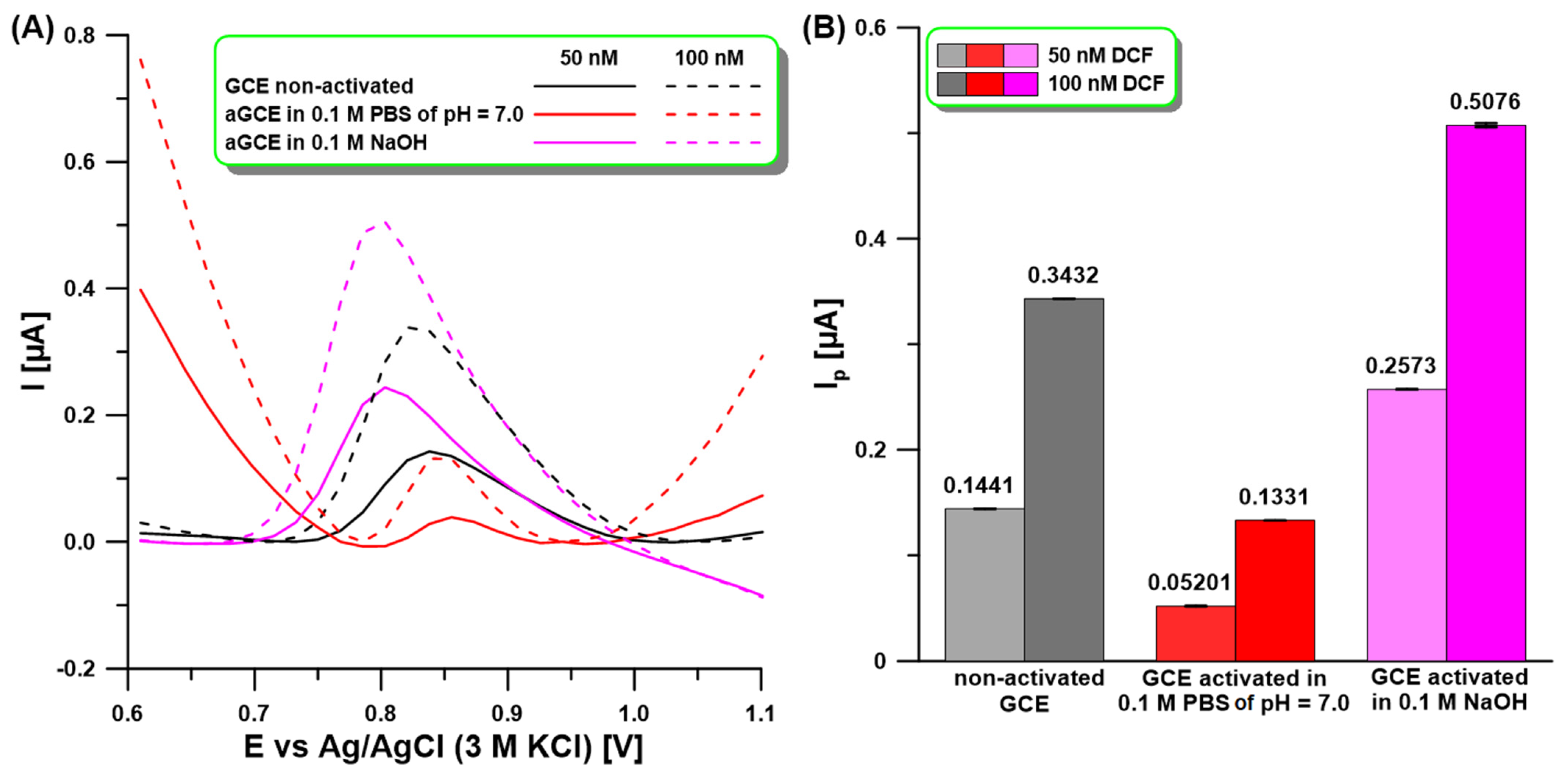
2.1. Activation of the Electrode Surface
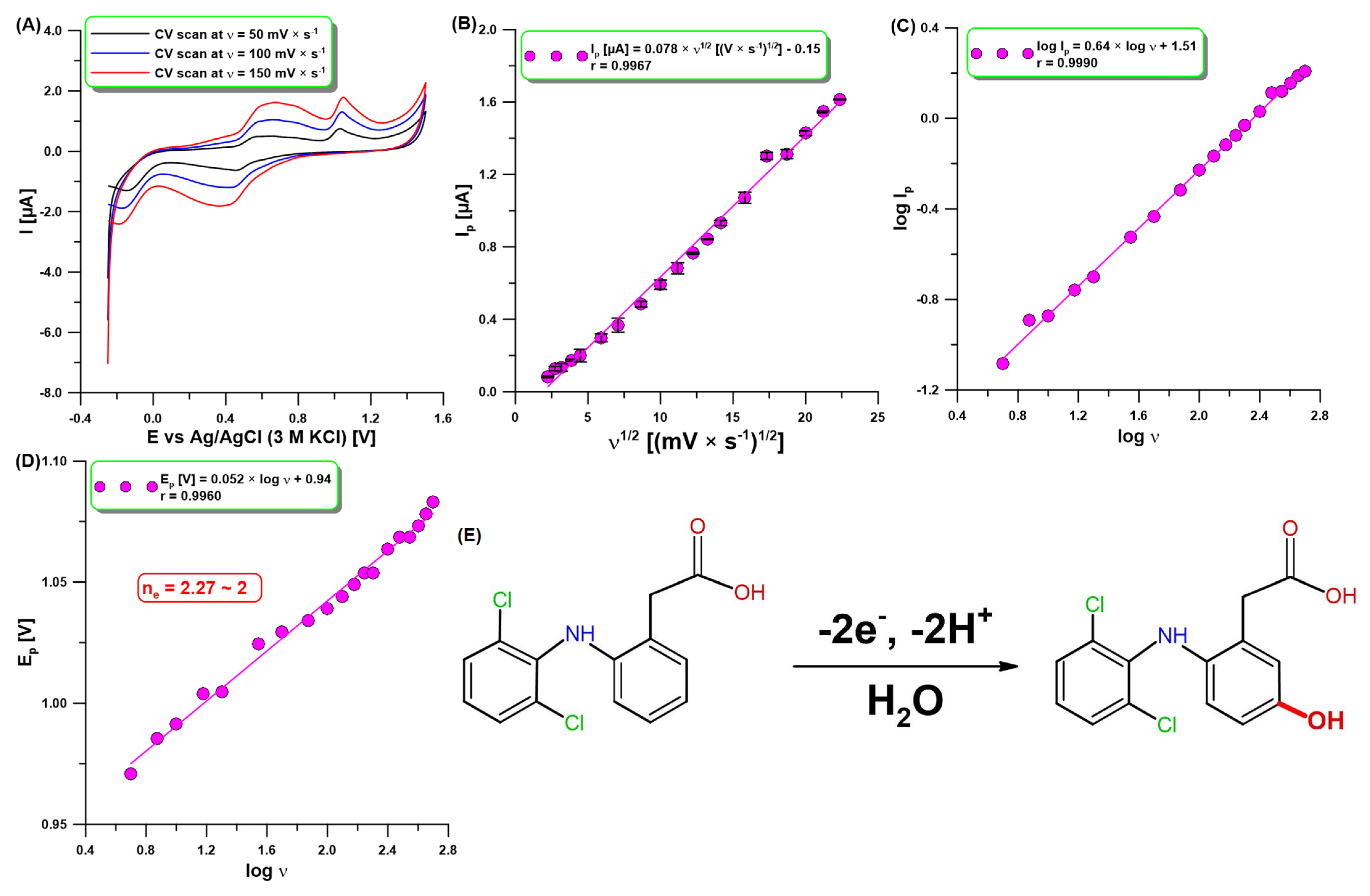
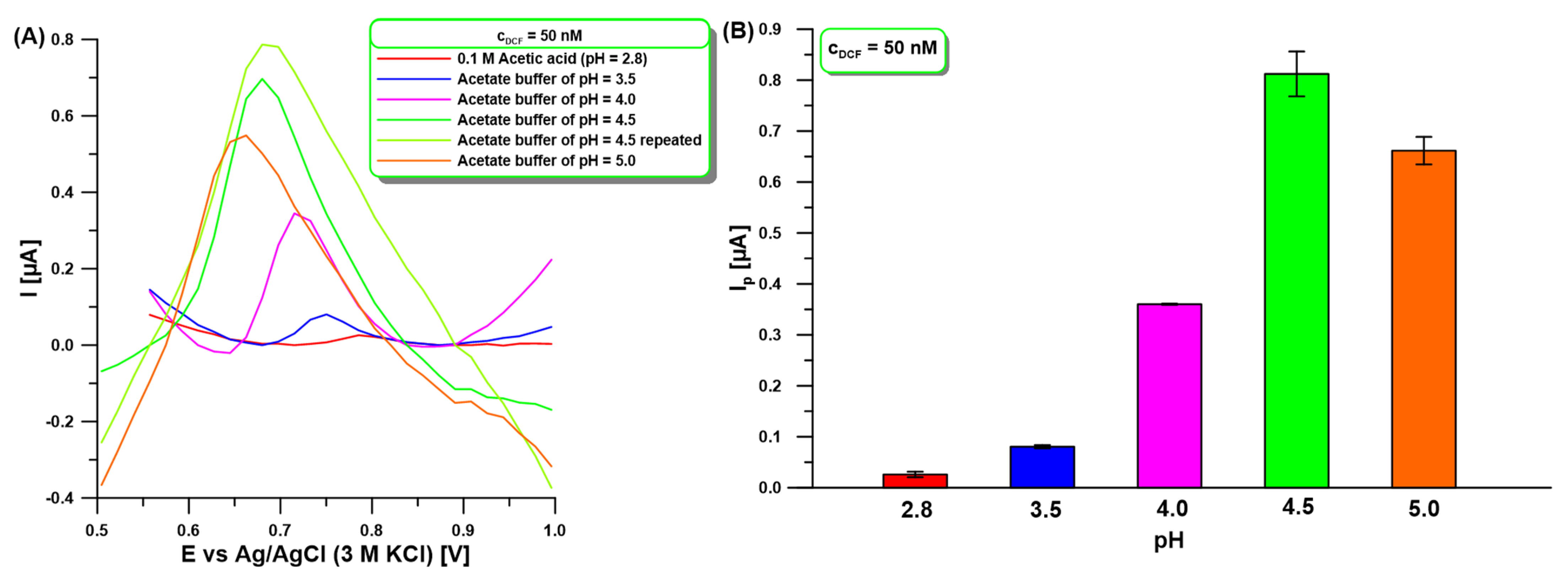
2.2. Voltammetric Behavior of DCF
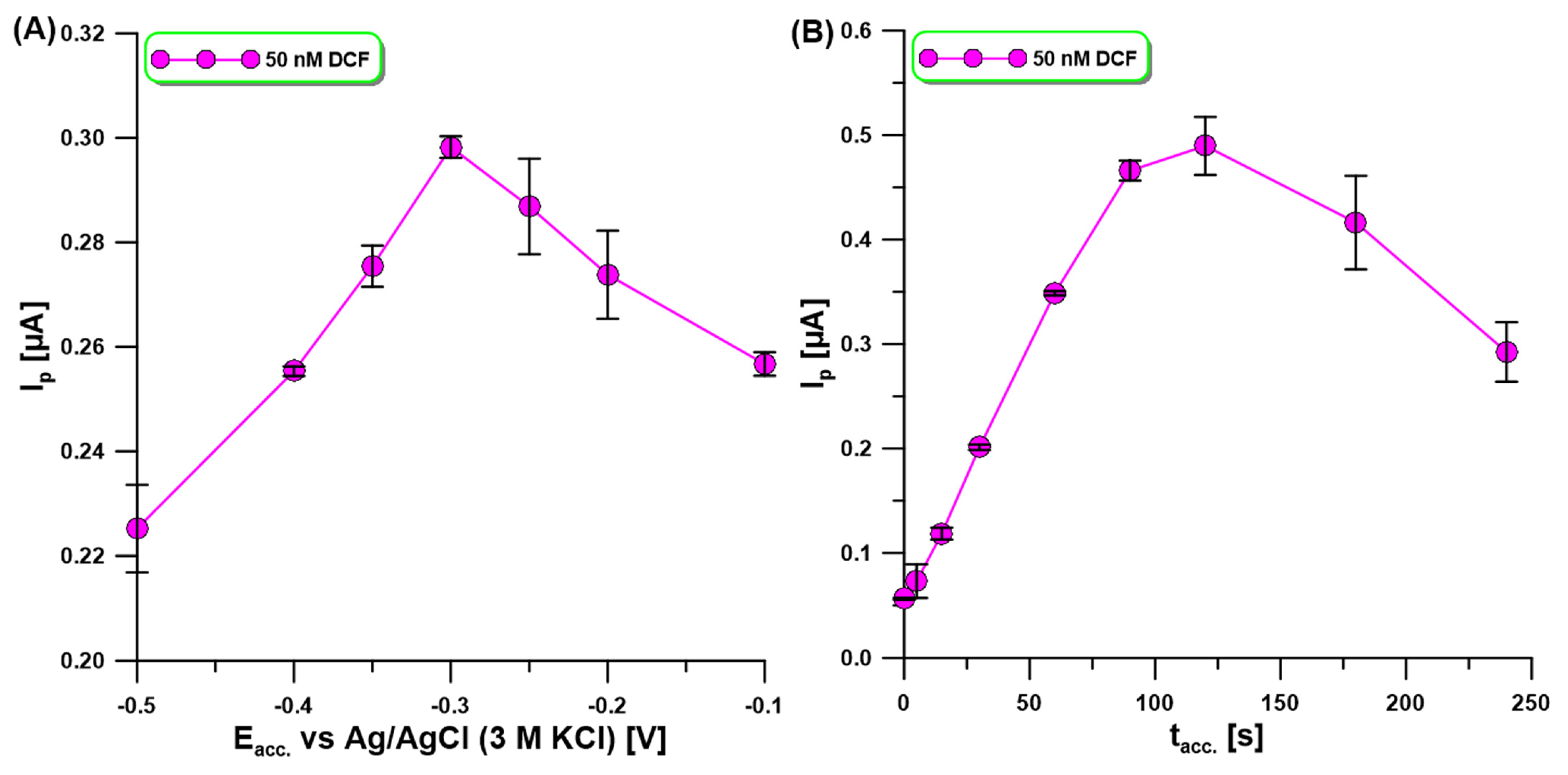
2.3. Step of DCF Accumulation
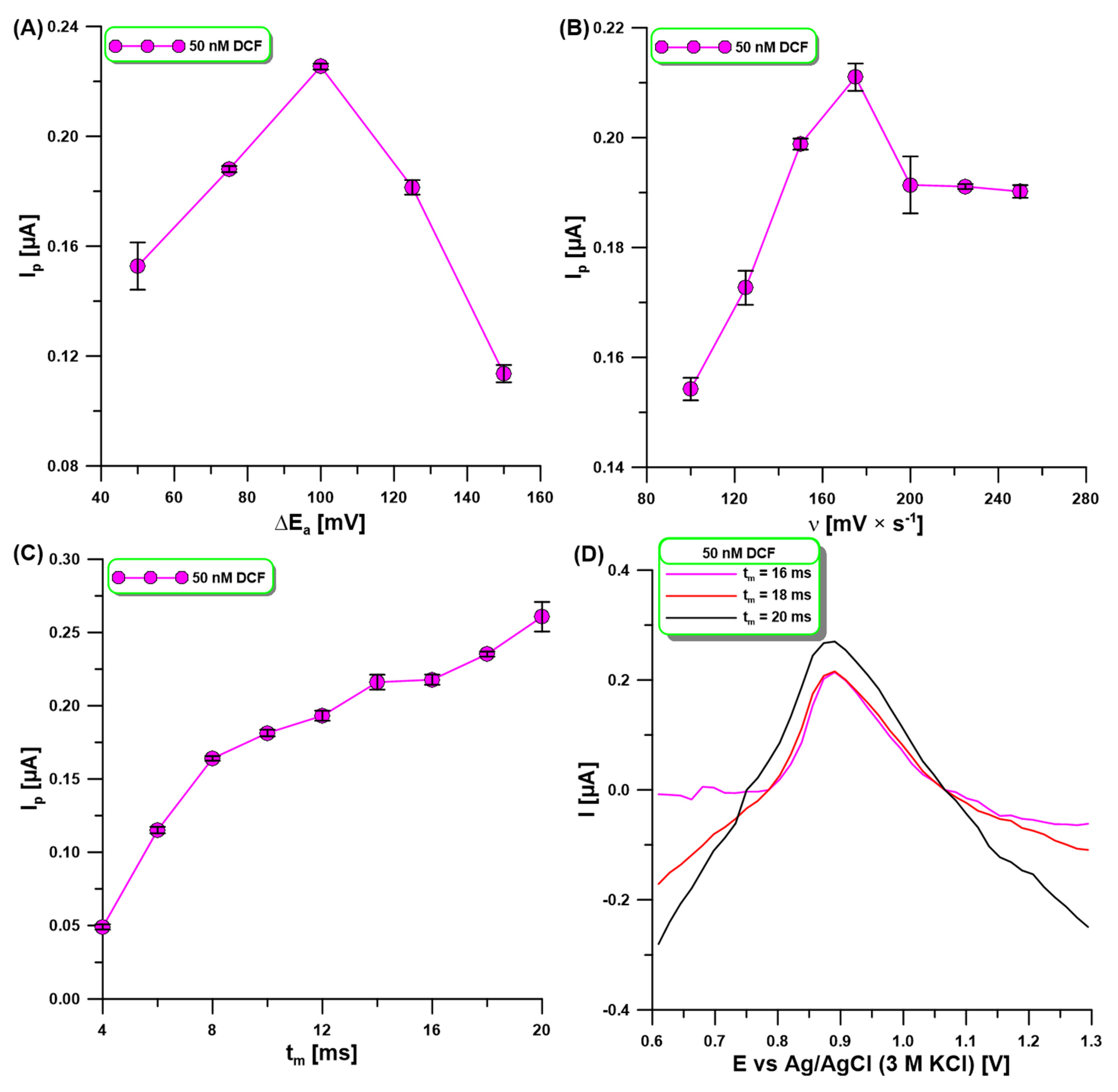
2.4. Signal Registration Technique Parameters
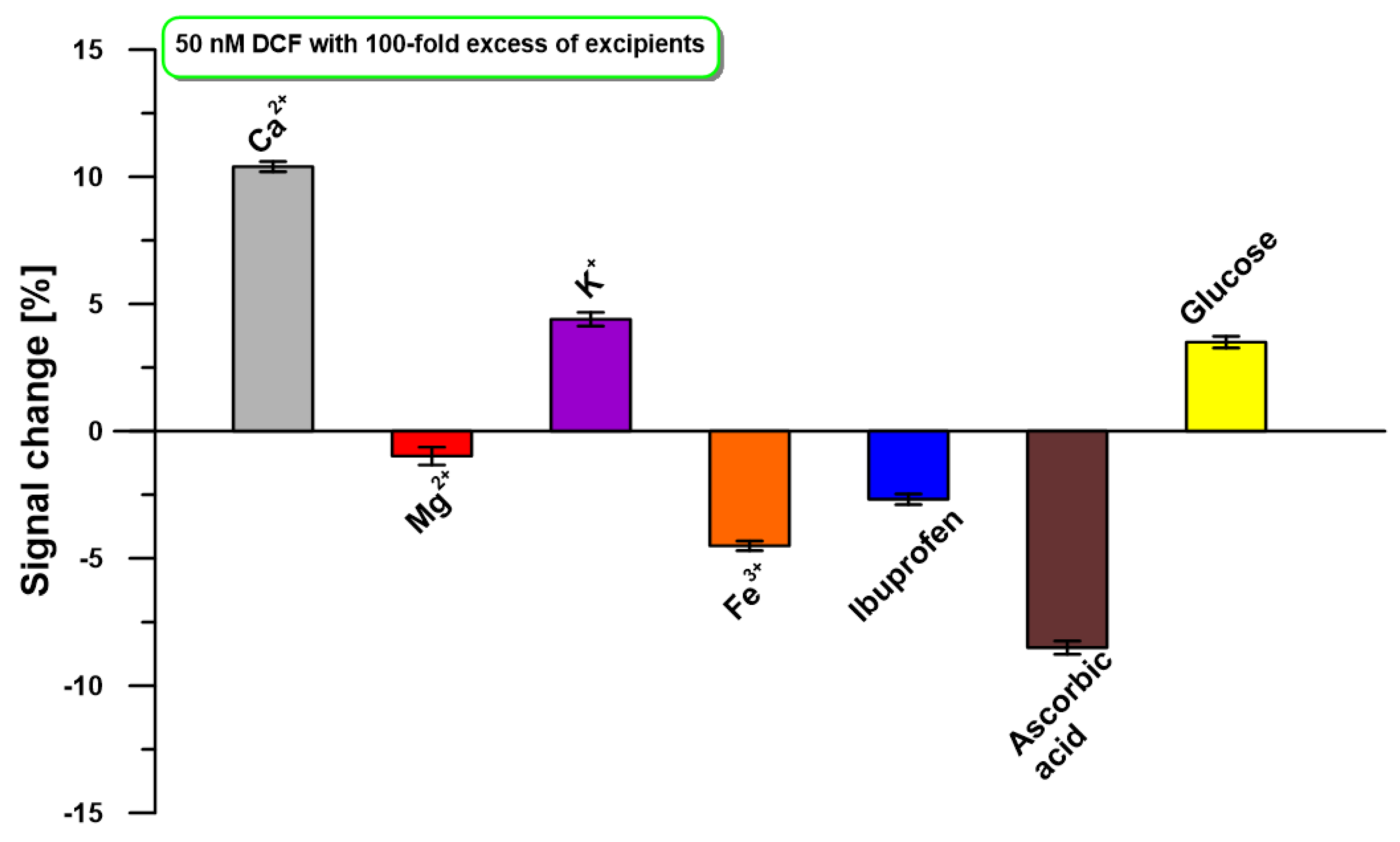
2.5. Selectivity, Repeatability, and Reproducibility
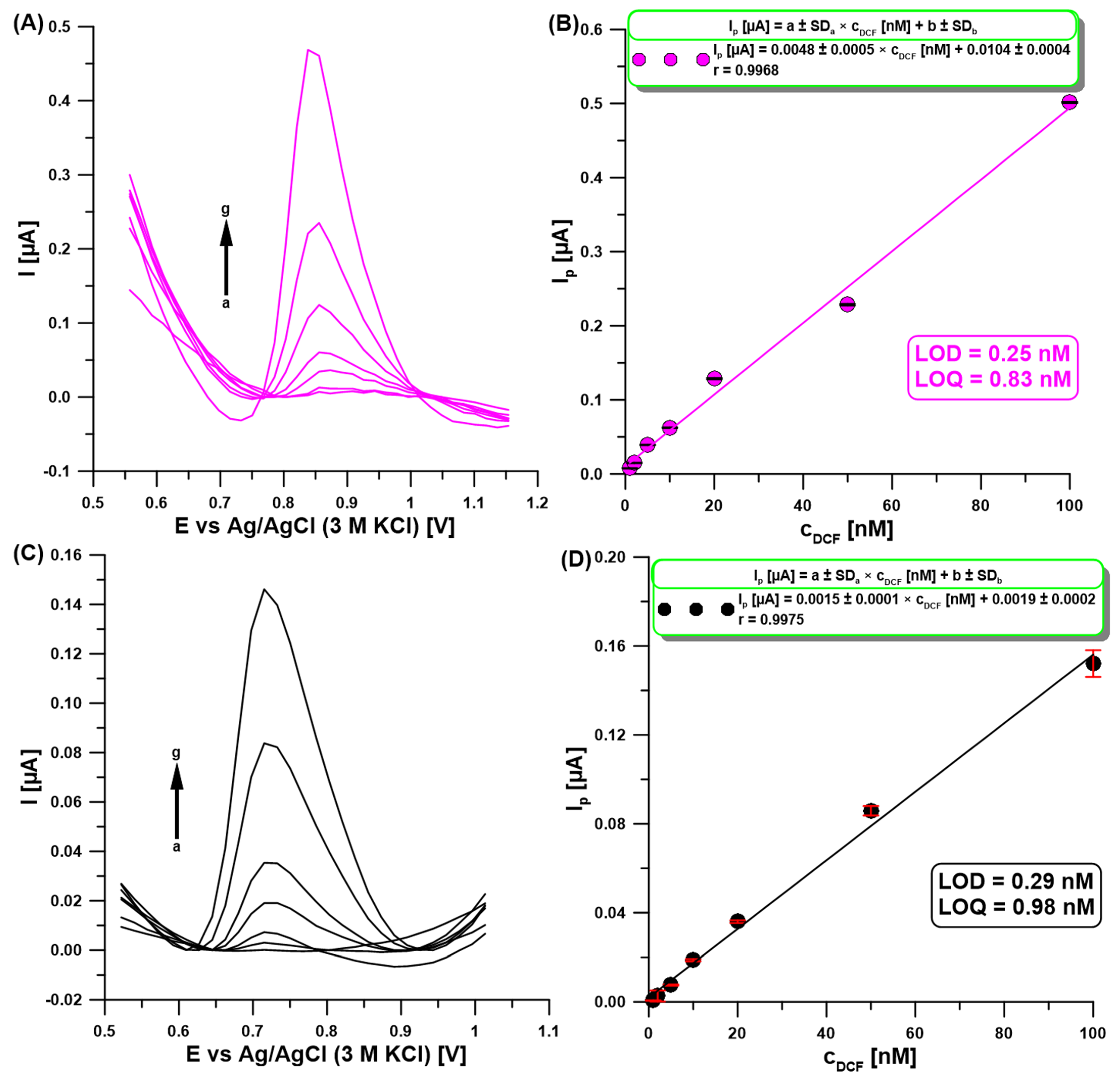
2.6. Analysis of Performance and Practical Application
3. Materials and Methods
3.1. Instrumentation
3.2. Reagents
3.3. aGCE Preparation and DCF Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbas, A.; Atousa, B.; Tayyebeh, M. Gold nanoparticle/multi-walled carbon nanotube modified glassy carbon electrode as a sensitive voltammetric sensor for the determination of diclofenac sodium. Mater. Sci. Eng. C 2016, 59, 168–176. [Google Scholar]
- Vinoda, B.P.; Shweta, J.M.; Suresh, M.T.; Mohammed, A.A.; Nagaraj, P.S. Development of a glycine-MWCNT nanohybrid via electropolymerization for enhanced electrochemical detection of diclofenac. J. Mol. Struct. 2025, 1319, 139535. [Google Scholar]
- Berber-Mendoza, M.S.; Díaz-Flores, P.E.; Jiménez-Peña, A.K.; Medellín-Castillo, N.A.; Flores-Rojas, A.I.; Álvarez-Valero, M.d.C.; Fernández-González, C.; Alexandre-Franco, M. Synthesis of a sustainable material based on pecan nutshell for the elimination of diclofenac in aqueous solution: Characterization and adsorption studies. Sustain. Chem. Environ. 2024, 8, 100181. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhu, J.H.; Lv, S.N.; Zhao, J.; Yang, X.S.; Liu, Y.S.; Hu, Z.H.; Cheng, T.; Chen, Y.X.; Wang, L.L. Extraction and determination of diclofenac in food based on sulfonic acid-functionalized covalent organic framework composites by HPLC. Microchem. J. 2025, 212, 113417. [Google Scholar] [CrossRef]
- Roškar, R.; Kmetec, V. Liquid chromatographic determination of diclofenac in human synovial fluid. J. Chromatogr. B 2003, 788, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Ciltas, U. Determination of diclofenac in pharmaceutical preparations by voltammetry and gas chromatography methods. J. Pharm. Anal. 2015, 5, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Thongchai, W.; Liawruangrath, B.; Thongpoon, C.; Machan, T. High performance thin layer chromatographic method for the determination of diclofenac sodium in pharmaceutical formulations. Chiang Mai J. Sci. 2006, 33, 123–128. [Google Scholar]
- Kaale, E.; Nyamweru, B.C.; Manyanga, V.; Chambuso, M.; Layloff, T. The development and validation of a Thin Layer Chromatography densitometry method for the analysis of diclofenac sodium tablets. J. Environ. Anal. Chem. 2013, 4, 73–79. [Google Scholar]
- Mazurek, S.; Szostak, R. Quantitative determination of diclofenac sodium in solid dosage forms by FT-Raman spectroscopy. J. Pharm. Biomed. Anal. 2008, 48, 814–821. [Google Scholar] [CrossRef]
- Arancibia, J.A.; Boldrini, M.A.; Escandar, G.M. Spectrofluorimetric determination of diclofenac in the presence of α-cyclodextrin. Talanta 2000, 52, 261–268. [Google Scholar] [CrossRef]
- Fahelelbom, K.M.S.; Saleh, A.; Mansour, R.; Sayed, S. First derivative ATR-FTIR spectroscopic method as a green tool for the quantitative determination of diclofenac sodium tablets. F1000Res 2020, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Sena, M.M.; Chaudhry, Z.F.; Collins, C.H.; Poppi, R.J. Direct determination of diclofenac in pharmaceutical formulations containing B vitamins by using UV spectrophotometry and partial least squares regression. J. Pharm. Biomed. Anal. 2004, 36, 743–749. [Google Scholar] [CrossRef] [PubMed]
- El-Malla, S.F.; Hamza, A.A.; Elagamy, S.H. Effective spectrophotometric methods for resolving the superimposed spectra of Diclofenac Potassium and Methocarbamol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 296, 122662. [Google Scholar] [CrossRef]
- Kormosh, Z.A.; Hunka, I.P.; Bazel, Y.R. A potentiometric sensor for the determination of diclofenac. J. Anal. Chem. 2009, 64, 853–858. [Google Scholar] [CrossRef]
- Das, S.; Chakravorty, A.; Raj, A.; Luktuke, S.; Mini, A.A.; Awasthi, S.; Sana, S.S.; Pandey, S.K.; Raghavan, V. Graphene/MWCNT/copper-nanoparticle fabricated printed electrode for diclofenac detection in milk and drinking water: Electrochemical and in-silico analysis. J. Mol. Liq. 2024, 411, 125750. [Google Scholar] [CrossRef]
- Mokhtari, A.; Karimi-Maleh, H.; Ensafi, A.A.; Beitollahi, H. Application of modified multiwall carbon nanotubes paste electrode for simultaneous voltammetric determination of morphine and diclofenac in biological and pharmaceutical samples. Sens. Actuators B Chem. 2012, 169, 96–105. [Google Scholar] [CrossRef]
- Goodarzian, M.; Khalilzade, M.A.; Karimi, F.; Gupta, V.K.; Keyvanfard, M.; Bagheri, H.; Fouladgar, M. Square wave voltammetric determination of diclofenac in liquid phase using a novel ionic liquid multiwall carbon nanotubes paste electrode. J. Mol. Liq. 2014, 197, 114–119. [Google Scholar] [CrossRef]
- Arvand, M.; Gholizadeh, T.M.; Zanjanchi, M.A. MWCNTs/Cu(OH)2 nanoparticles/IL nanocomposite modified glassy carbon electrode as a voltammetric sensor for determination of the non-steroidal anti-inflammatory drug diclofenac. Mater. Sci. Eng. C 2012, 32, 1682–1689. [Google Scholar] [CrossRef]
- Postolović, K.; Stanić, Z. Chitosan/TiO2 nanoparticles modified carbon paste electrode as a sensitive voltammetric sensor for the determination of diclofenac sodium as an anti-inflammatory drug. Mater. Today Commun. 2023, 37, 107416. [Google Scholar] [CrossRef]
- Lochab, A.; Baweja, S.; Jindal, K.; Chowdhuri, A.; Tomar, M.; Saxena, R. Experimental and computational insights on the electrochemical detection of diclofenac in the presence of paracetamol using Asparagine-Modified MWCNTs. Microchem. J. 2025, 208, 112430. [Google Scholar] [CrossRef]
- El-Zahry, M.R. Electrochemical sensor based on molybdenum-doped graphene oxide nanorods anchored carbon spheres/vanadium pentoxide nanocomposites for simultaneous determination of diclofenac sodium and posaconazole. Microchem. J. 2024, 206, 111593. [Google Scholar] [CrossRef]
- Gashu, M.; Aragaw, B.A.; Tefera, M.; Abebe, A. Cobalt (II) bis-(1, 10-phenanthroline) complex electropolymerized glassy carbon electrode and its electrocatalytic sensing of diclofenac in pharmaceuticals and biological samples. Colloids Surf. A Physicochem. Eng. Asp. 2024, 693, 133974. [Google Scholar] [CrossRef]
- Killedar, L.; Ilager, D.; Shetti, N.P.; Aminabhavi, T.M.; Reddy, K.R. Synthesis of ruthenium doped titanium dioxide nanoparticles for the electrochemical detection of diclofenac sodium. J. Mol. Liq. 2021, 340, 116891. [Google Scholar] [CrossRef]
- Das, S.; Chakravorty, A.; Luktuke, S.; Raj, A.; Mini, A.A.; Ramesh, K.; Grace, A.N.; Raghavan, V. Graphene/gadolinium oxide composite modified screen-printed electrochemical sensor for detection of diclofenac sodium. Results Chem. 2023, 6, 101189. [Google Scholar] [CrossRef]
- Berto, S.; Cagno, E.; Prenesti, E.; Aragona, G.; Bertinetti, S.; Giacomino, A.; Inaudi, P.; Malandrino, M.; Terranova, E.; Abollino, O. Voltammetric Study for the Determination of Diclofenac in Aqueous Solutions Using Electro-Activated Carbon Electrodes. Appl. Sci. 2022, 12, 7983. [Google Scholar] [CrossRef]
- Abd-Elsabour, M.; Abou-Krisha, M.M.; Kenawy, S.H.; Yousef, T.A. A Novel Electrochemical Sensor Based on an Environmentally Friendly Synthesis of Magnetic Chitosan Nanocomposite Carbon Paste Electrode for the Determination of Diclofenac to Control Inflammation. Nanomaterials 2023, 13, 1079. [Google Scholar] [CrossRef] [PubMed]
- Sasal, A.; Tyszczuk-Rotko, K.; Wójciak, M.; Sowa, I. First Electrochemical Sensor (Screen-Printed Carbon Electrode Modified with Carboxyl Functionalized Multiwalled Carbon Nanotubes) for Ultratrace Determination of Diclofenac. Materials 2020, 13, 781. [Google Scholar] [CrossRef]
- Wallans, T.P.; Dos Santos, W.T.P.; Amin, H.M.A.; Compton, R.G. A nano-carbon electrode optimized for adsorptive stripping voltammetry: Application to detection of the stimulant selegiline in authentic saliva. Sensors Actuators B Chem. 2019, 279, 433–439. [Google Scholar]
- Atta, N.F.; Ahmed, R.A.; Amin, H.M.A.; Galal, A. Monodispersed Gold Nanoparticles Decorated Carbon Nanotubes as an Enhanced Sensing Platform for Nanomolar Detection of Tramadol. Electroanalysis 2012, 24, 2135–2146. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.M.; Hassan, H.H.; Badr, I.H.A. Activated Glassy Carbon Electrode as an Electrochemical Sensing Platform for the Determination of 4-Nitrophenol and Dopamine in Real Samples. ACS Omega 2022, 7, 34127–34135. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.M.; Hassan, H.H.; Badr, I.H.A. Glassy carbon electrode electromodification in the presence of organic monomers: Electropolymerization versus activation. Anal. Chem. 2020, 92, 7947–7954. [Google Scholar] [CrossRef] [PubMed]
- Tyszczuk-Rotko, K.; Staniec, K.; Hanaka, A. Green and cost-effective voltammetric assay based on activated glassy carbon electrode for determination of the plant growth regulator methyl jasmonate. Biosens. Bioelectron. 2025, 274, 117217. [Google Scholar] [CrossRef] [PubMed]
- Tyszczuk-Rotko, K.; Keller, A. Simultaneous Measurements of Nanotrace Amounts of Lead and Cadmium Using an Environmentally Friendly Sensor (An Activated Glassy Carbon Electrode Modified with a Bismuth Film). Molecules 2025, 30, 1308. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, F.; Hu, S. Enhanced oxidation of diclofenac sodium at a nano-structured electrochemical sensing film constructed by multi-wall carbon nanotubes–surfactant composite. Mater. Sci. Eng. C 2008, 28, 188–194. [Google Scholar] [CrossRef]
- Goyal, R.N.; Chatterjee, S.; Agrawal, B. Electrochemical investigations of diclofenac at edge plane pyrolytic graphite electrode and its determination in human urine. Sens. Actuators B Chem. 2010, 145, 743–748. [Google Scholar] [CrossRef]
- Tyszczuk-Rotko, K.; Pietrzak, K.; Sasal, A. Adsorptive stripping voltammetric method for the determination of caffeine at an integrated three-electrode screen-printed sensor with carbon/carbon nanofibers working electrode. Adsorption 2019, 25, 913–921. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusion less electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Richard, A.; Camara, F.A.; Ramézani, H.; Mathieu, N.; Delpeux, S.; Bhatia, S.K. Structure of diclofenac in an aqueous medium and its adsorption onto carbons: Molecular insights through simulation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 686, 133373. [Google Scholar] [CrossRef]
- Amin, H.M.A.; El-Kady, M.F.; Atta, N.F.; Galal, A. Gold Nanoparticles Decorated Graphene as a High Performance Sensor for Determination of Trace Hydrazine Levels in Water. Electroanalysis 2018, 30, 1757–1766. [Google Scholar] [CrossRef]
| Electrode | Technique | Linear Range [nM] | LOD [nM] | Application | Reference |
|---|---|---|---|---|---|
| GCE/MWCNT/AuNP | SWAdSV | 30–200,000 | 20.0 | Tablet and human urine | [1] |
| CPE/MWCNT/poly(Gly) | DPV | 500–1000 | 79.6 | Tablet and urine | [2] |
| GRP/MWCNT/CuNP@OA | DPV | 17,410–206,450 | 57.7 | Drinking water and milk | [15] |
| CPE/VF/MWCNT | SWV | 500–600,000 | 200 | Tablet and urine | [16] |
| CNTPE/IL | SWV | 30–750,000 | 90 | Tablet and urine | [17] |
| GCE/MWCNT/Cu(OH)2 | DPV | 180–119,000 | 40.0 | Tablet, human and fish blood serum, and seawater | [18] |
| CPE/chitosan/TiO2NP | DPAdSV | 20–10,000 10,000–100,000 | 13.0 | Tablet and synthetic urine | [19] |
| ITO/IL/Asp-MWCNT | CV | 40,000–160,000 | 9.40 | Water samples | [20] |
| Mo@GO/CS-V2O5 | SWV | 10–200 | 10.2 | Human plasma and urine | [21] |
| GCE/poly(Co(Phen)2) | SWAdSV | 1000–250,000 | 56.0 | Pharmaceutical, milk, serum, and urine | [22] |
| CPE/Ru-TiO2 | SWV | 100–2000 | 1.48 | Urine sample | [23] |
| SPCPE/GE/Gd2O3 | DPV | 5890–66,700 | 28.0 | - | [24] |
| aGCE * | DPV | 10–50 | 5.03 | - | [25] |
| aSPCE | DPV | 67–490 | 22.0 | - | [25] |
| CPE/M-ChsNC | DPV | 25–4000 | 7.00 | Tablet and human serum | [26] |
| SPCE/MWCNTs-COOH | DPAdSV | 0.1–10 | 0.028 | River water | [27] |
| aGCE | DPAdSV | 1–100 | 0.25 | Tablet | This work |
| DCF Content [mg/tablet] ± SD (n = 3) | |||
|---|---|---|---|
| Sample (Content) | Found DPAdSV | Coefficient of Variation * [%] | Recovery ** [%] |
| A (25) | 24.4 ± 0.77 | 3.2 | 97.6 |
| B (25) | 25.5 ± 1.45 | 5.7 | 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyszczuk-Rotko, K.; Keller, A.; Liwak, A. An Activated Glassy Carbon Electrode for Rapid, Simple, and Sensitive Voltammetric Analysis of Diclofenac in Tablets. Molecules 2025, 30, 2530. https://doi.org/10.3390/molecules30122530
Tyszczuk-Rotko K, Keller A, Liwak A. An Activated Glassy Carbon Electrode for Rapid, Simple, and Sensitive Voltammetric Analysis of Diclofenac in Tablets. Molecules. 2025; 30(12):2530. https://doi.org/10.3390/molecules30122530
Chicago/Turabian StyleTyszczuk-Rotko, Katarzyna, Aleksy Keller, and Aleksandra Liwak. 2025. "An Activated Glassy Carbon Electrode for Rapid, Simple, and Sensitive Voltammetric Analysis of Diclofenac in Tablets" Molecules 30, no. 12: 2530. https://doi.org/10.3390/molecules30122530
APA StyleTyszczuk-Rotko, K., Keller, A., & Liwak, A. (2025). An Activated Glassy Carbon Electrode for Rapid, Simple, and Sensitive Voltammetric Analysis of Diclofenac in Tablets. Molecules, 30(12), 2530. https://doi.org/10.3390/molecules30122530






