Therapeutic Potential of Ginsenosides in Anthracycline-Induced Cardiotoxicity
Abstract
1. Introduction
2. Mechanisms and Prevention of Anthracycline Cardiotoxicity
2.1. Classification of the AIC
2.2. Mechanisms Associated with the AIC
2.2.1. Oxidative-Stress-Mediated AIC
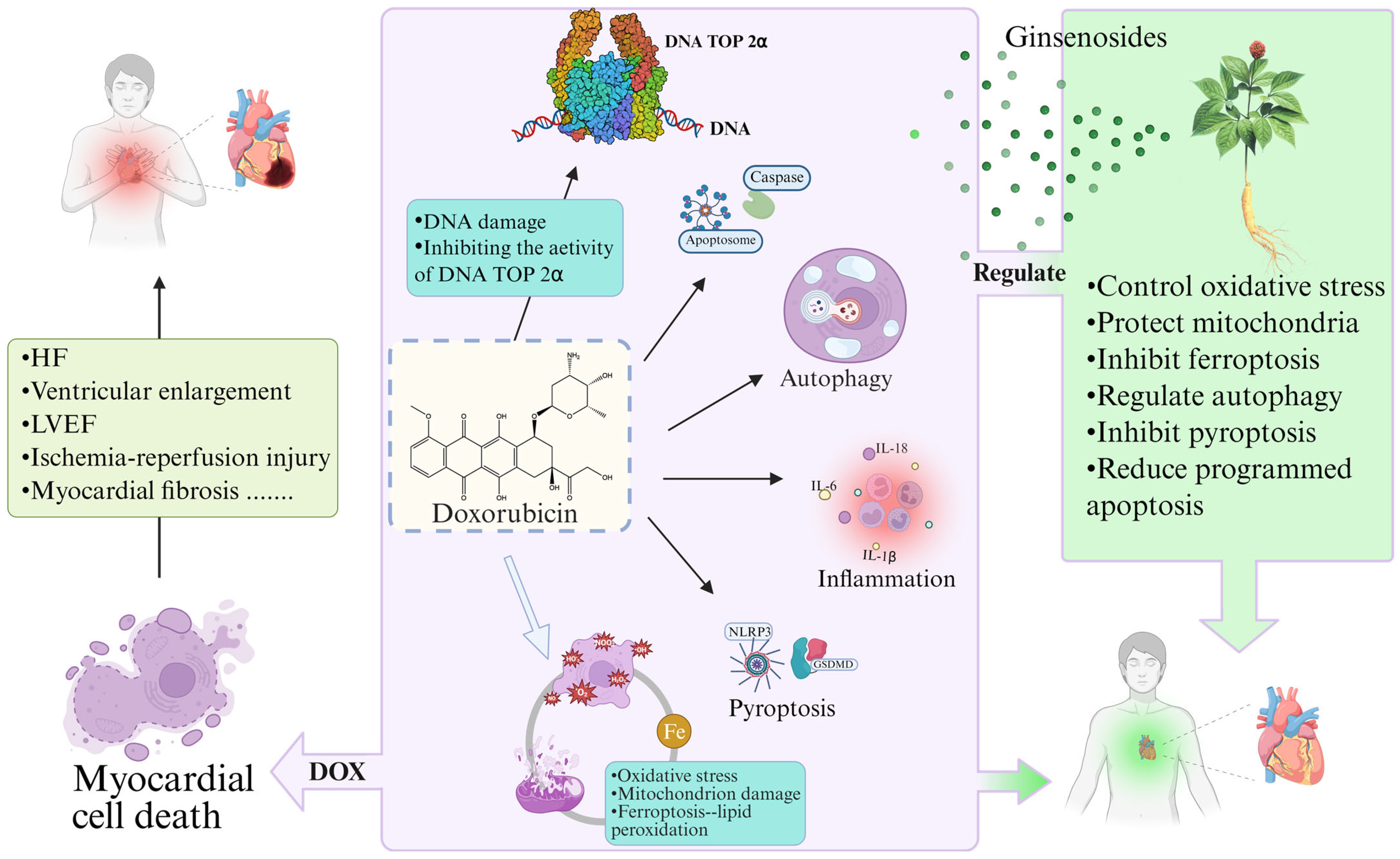
2.2.2. ANTs Induce Cardiomyocyte Death
2.2.3. DNA TOP 2β-Mediated Cardiotoxicity
2.3. Clinical Management of AIC
3. Classification of Ginsenosides and Their Therapeutic Effects on Cardiovascular Diseases
3.1. Classification of Ginsenosides
3.2. Ginsenosides Used to Treat Cardiovascular Diseases
4. Therapeutic Effects and Mechanisms of Ginsenosides in AIC
4.1. Ginsenosides Inhibit Oxidative Stress
| Ginsenosides | Models | Disease | Mechanism | Reference |
|---|---|---|---|---|
| Rg3 | Angiotensin II (Ang II)-induced cardiac hypertrophy in vitro; in vivo transverse aortic constriction constructs a rat model of cardiac hypertrophy. | Hypertensive Cardiac Hypertrophy (HCH); Myocarditis (MC) | ANP, BNP, and β-MHC ↓; myocardial fibrosis-related proteins (MyHc, CollagenI, and TGF-β1) ↓; upregulation of Nrf2/HO-1, SIRT1 pathway; downregulation of NF-κB pathway; Regulation of Nrf2/HO-1, SIRT1/NF-κB pathway inhibits inflammation and oxidative stress. | [98] |
| Rg5 | Ang II induces cardiac inflammation and remodeling. | IL-1β, IL-16, TNF ↓; p-JNK ↓; AP-1 ↓; Inhibition of the JNK/AP-1 pathway blocks inflammation. | [99] | |
| Rb3 | Ang II injury to cardiomyocytes. | NADPH ↓; ROS ↓; NOX-2, NOX-4, p67 ↓; NO, NOS ↑; Reversing NADPH overexpression to resist oxidative damage. | [82] | |
| F1 | A mouse model of atherosclerosis constructed by feeding a high-fat diet. | Atherosclerosis | LOX-1, TLR4 ↓; MPO ↓; G-CSF, ICAM-1, MIP-1δ, IL-1α, IL-15, IL-16 ↓; A20, NF-κB ↓; Mediated A20 inhibition of the NF-κB signaling pathway relieves inflammation. | [100] |
| Ginsenosides | Models | Disease | Mechanism | Reference |
|---|---|---|---|---|
| Rg1 Rd Rk3 Rh1 Rb1 Rd Rc | In vitro cultivation of H9c2 cardiomyocytes subjected to hypoxia/reoxygenation (H/R) injury | MI, HF, Ischemic Cardiomyopathy (ICM) | SOD, GSH-Px, GSH ↑; LDH ↑; Nrf2, HO-1 ↑; TNF-α ↓; Activates Nrf2/HO-1 signaling pathway, inhibits JNK phosphorylation, and regulates Akt and MAPK pathways to prevent oxidative stress | [101,102,103,104,105,106,107] |
| Rg1 | In vivo and in vitro ischemia/reperfusion (I/R) injury models | Protection of cardiomyocytes against hypoxia-induced cellular injury by upregulation of HIF-1α through activation of the PI3K/Akt/mTOR pathway | [108] | |
| Rb1 Re | Rat myocardial I/R model: H2O2-induced oxidative stress | CK, MDA ↓; LDH ↑; SOD, eNOS, NO ↑; increase NO content to inhibit oxidative stress | [109,110] | |
| Rb1 | In vitro H/R model of H9c2 cardiomyocytes | SOD, CAT, GSH-Px ↑; MDA ↓; PARP-1/2 ↑; ERα, ERβ, p-Akt ↑; p-JNK, p-ERK 1/2 ↓; Activation of ER-dependent crosstalk across Akt, JNK, and ERK 1/2 pathways prevents H/R damage | [111] | |
| Rg1 | In vitro H/R model of H9c2 cardiomyocytes | CK, LDH ↓; MMP, ATP ↑; Bax/Bcl-2, ROS ↓; GDH ↓; MFN2 ↑; Regulates GDH and MFN2, maintains mitochondrial dynamics to prevent H/R injury | [112] | |
| Rg5 | Modeling myocardial ischemia | Promotes Akt translocation, increases mitochondrial hexokinase-II (HK-II) binding to mitochondria; inhibits dynamin-related protein 1 (Drp1) recruitment and mitochondrial fission; mPTP ↓; ATP ↑; Regulation of HK-II and Drp1 increases resistance to hypoxia/reoxygenation injury | [113] | |
| Rg2 | Ang II injury to cardiomyocytes | MI | Col-1, Col-3, α-SMA ↓; p-Akt ↑; Masson staining shows Rg2 reduces MI-induced cardiac fibrosis in mice | [114] |
| Re | Constructing a rat MI model | P-AMPKα ↑; TGF-β1 ↓; Smad2/3 ↓; FAK, p-PI3K/p110α, p-Akt ↑; Regulation of AMPK/TGF-β1/Smad2/3 and FAK/PI3K p110α/Akt signaling pathways | [115] |

4.2. Inhibition of Ferroptosis by Ginsenosides
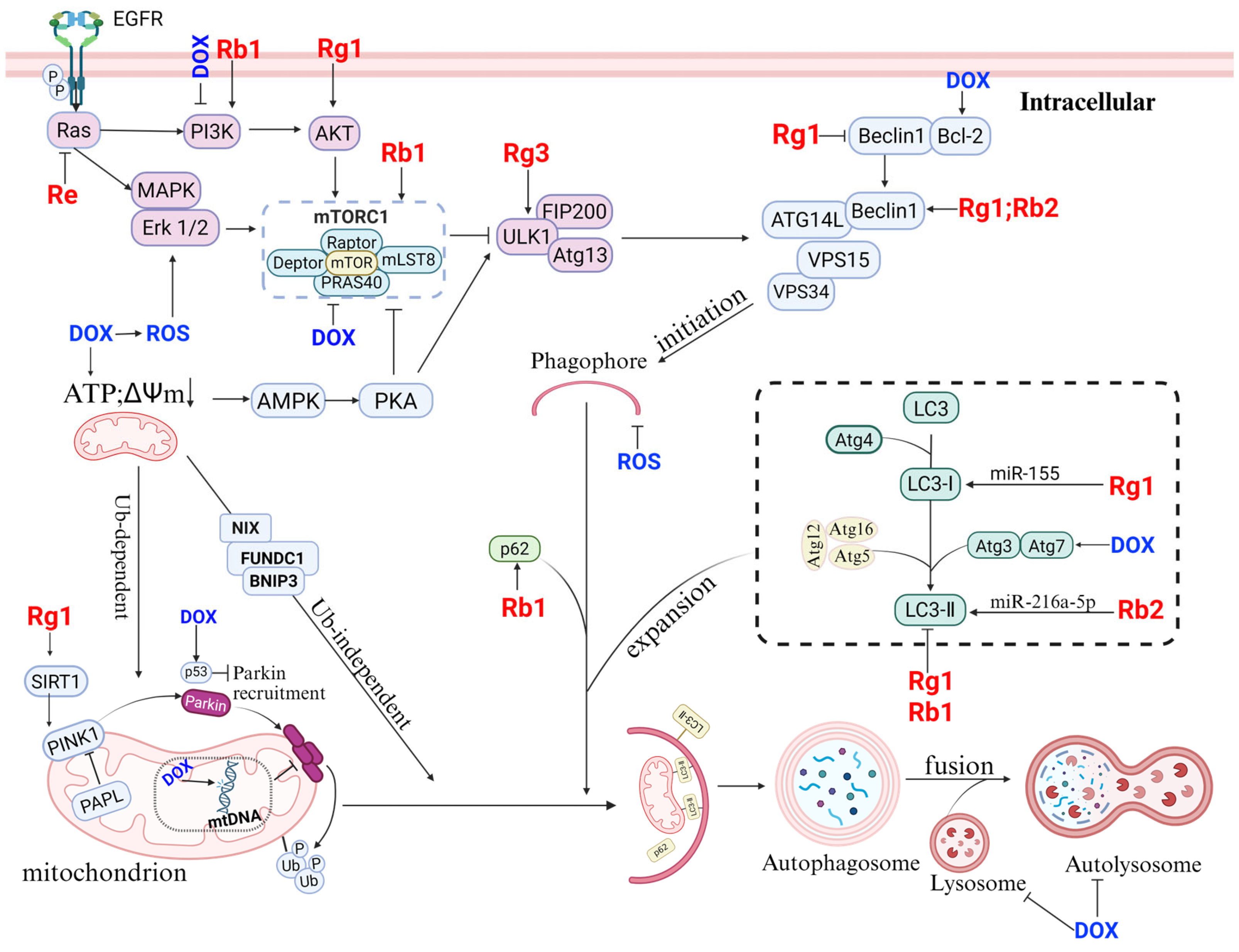
4.3. Regulation of Autophagy by Ginseng Saponins
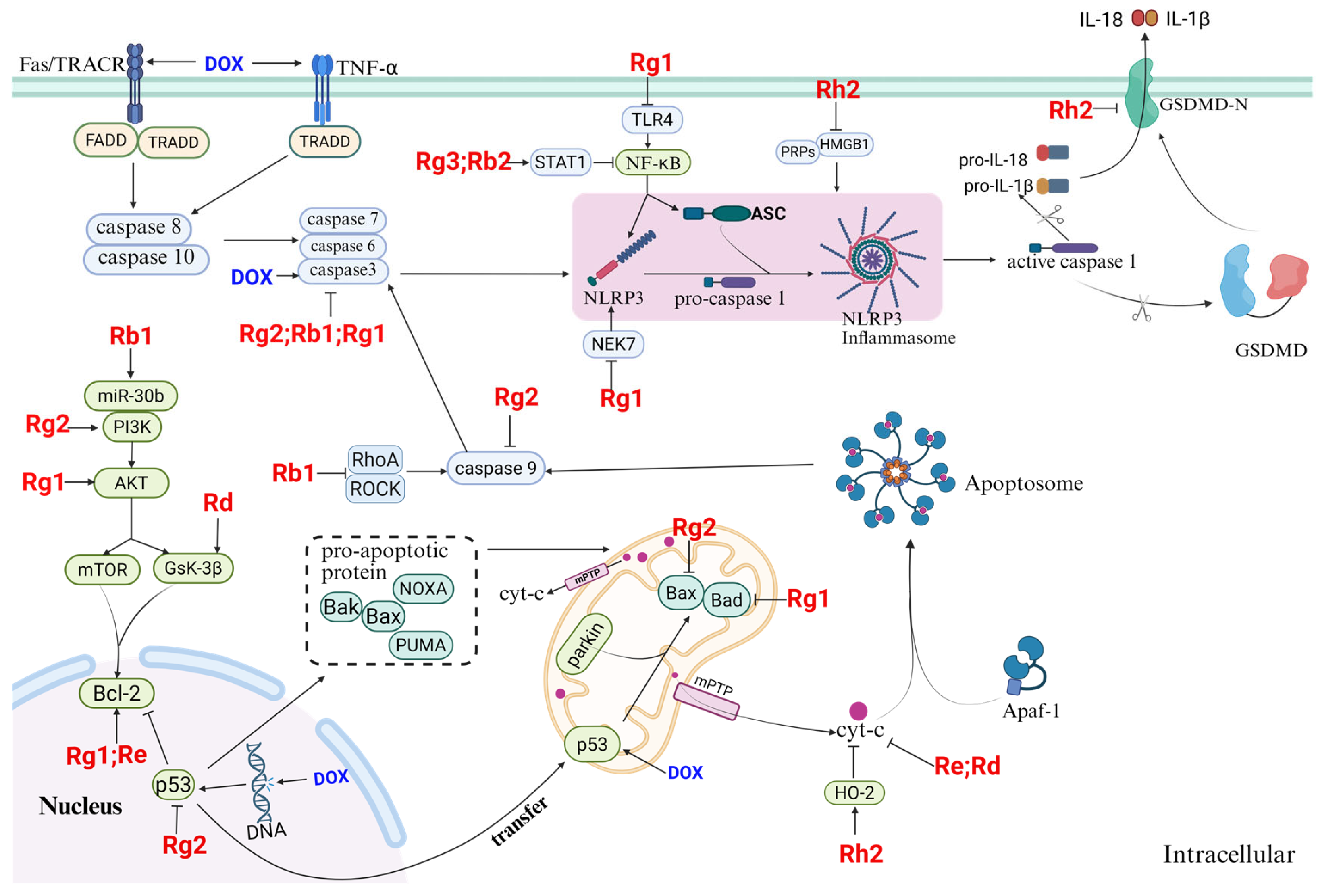
4.4. Inhibitory Effects of Ginsenosides on Pyroptosis
4.5. Inhibition of Apoptosis by Ginsenosides
4.6. Protective Effects of Other Factors on AIC
5. Clinical Applications of Ginseng and Ginsenosides
| Ingredients | Disease | Subjects | Outcome | References |
|---|---|---|---|---|
| Panax ginseng extract (PGE) | The effects of ginseng extract on blood pressure and heart rate | 30 healthy adult subjects. Oral administration of 200 mg of ginseng extract. Monitoring of ECG parameters and blood pressure. | QTc interval prolongation and diastolic blood pressure after 2 h of ingestion. | [181] |
| Powder composed of Radix Ginseng, Radix Notoginseng, and Succinum | Coronary artery angina | 116 patients with coronary artery angina pectoris. Randomized double-blind trial of treatment group and control group (compound Danshen tablets). | The general symptoms, physical strength, ECG parameters, and lipid metabolism in the ginseng treatment group were all better than those in the control group. | [188] |
| Red ginseng extract | ST-elevation acute myocardial infarction (AMI) | 50 patients with AMI. Measurement of coronary flow reserve (CFR) and changes in absolute numbers of circulating angiogenic cells. | During the 8-month follow-up period, the CFR in the red ginseng group was significantly better than that in the placebo group; CD34+, CXCR4+, and CD117+ levels increased and inflammation slowed down. | [189] |
| PGE | The effect of PGE on lipid metabolism—lipid-lowering research | Eight adult male subjects, 6 g/day, 8 weeks. Testing serum MDA, SOD, CAT, serum total cholesterol (TC), triglyceride (TG), LDL, HDL, and other indicators. | MDA, TC, TG, LDL ↓; HDL, SOD, CAT ↑. Lowering blood lipids and antioxidant properties. | [190] |
| Korean red ginseng (KRG) | Endothelial function | 16 healthy participants on four occasions were administered: KRG root (3 g), KRG ginsenosides extract, KRG polysaccharides extract, and cornstarch control. Assessment of flow-mediated vasodilatation. | Maximum vasodilation occurred 180 min after taking KRG ginsenoside extract. Improves endothelial function in healthy individuals. | [191] |
| Ginsenoside Rg3–enriched Korean red ginseng (Rg3-KRG) | Arterial stiffness and peripheral and central BP | 23 healthy subjects. 400 mg Rg3-KRG extract or 400 mg wheat bran control. Measurement of aortic augmentation index and central BP. | Brachial systolic and diastolic BP ↓. Lowers central and peripheral arterial pressures in healthy adults. | [192] |
| Ginseng (Rb1/Rg1) | Blood lipid levels. | Patients with metabolic syndrome, healthy volunteers, postmenopausal women. Meta-analysis. | Total cholesterol, LDL, triglycerides ↓. Regulate blood lipid levels. | [154] |
| Ingredients | Disease | Subjects | Outcome | References |
|---|---|---|---|---|
| Shenmai injection (SMI) | CAD and CHF. Efficacy and safety. | 240 patients with CHF complicated by CAD. CHF standard treatment drugs and SMI (100 mL/day). 1 week. Endpoints: NYHA functional classification, SF-36. Heart survey score, traditional Chinese medicines syndrome score, LVEF, and BNP level. | Each endpoint is superior to the placebo group. SMI can further improve the course of patients with CHF complicated by CAD. | [193] |
| SMI | Coronary heart disease (CHD) | 40 patients with OPCABG. Injecting SMI before performing OPCABG. Indicators: cardiac output (CO), stroke volume (SV), and the ejection fraction (EF) during surgery. | CO, SV, EF ↑. Improving the safety of anesthesia. | [194] |
| Shenmai and compound danshen injection (SM-DS) | Myocardial reperfusion injury after percutaneous coronary intervention (PCI) in patients with acute AMI. | 38 patients with AMI who underwent PCI treatment. SM-DS therapy was used before and after PCI surgery. The integrated left ventricular ejection isometric index (Tei) was determined by echocardiogram. Monitoring MDA, SOD, IL-6, and TNF-α levels. | SOD ↑; MDA, IL-6, TNF-α ↓; The improvement time of the Tei index in the treatment group was earlier than that in the control group. SM-DS could reduce the myocardial reperfusion injury in patients with AMI after PCI. | [195] |
| SMI | CHF | 64 patients with CHF. Basic treatment and SMI. 14 days. Tissue Doppler imaging (TDI) monitoring of eft ventricular diastolic function (LVDF) used. | TDI assessment shows that SMI could effectively improve the LVDF in CHF patients. | [196] |
| SMI | AMI | Meta-analysis of 50 clinical studies of Shenmai for AMI. | The incidence of cardiac failure, the incidence of HF, shock, and reinfarction ↓. No serious adverse drug reactions (ADRs)/adverse events (AEs) were observed, but post-marketing safety evaluation is still required. | [197] |
| Shenfu injection (SFI) | I/RI | 40 patients’ mitral valve replacement (MVR) with cardiopulmonary bypass (CPB). Monitoring systolic SBP, HR, MBP, DBP, and CTn, SOD, and MDA. | MDA, cTnl ↓; SOD ↑. The SBP, MBP, and DBP values and HR were significantly improved in group IV compared with any other groups. | [198] |
| Xinyue capsule | CAD | A randomized, double-blind, controlled clinical trial involving 1054 CAD patients undergoing PCI. Conventional treatment and Xinyue capsule (100 mg/day). 24 weeks. Monitoring ADRs during trials. | Xinyue capsule added on conventional treatment reduced the incidence of cardiac death, nonfatal myocardial infarction, and urgent revascularization. | [199] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TfR | Transferrin Receptor | Akt | protein kinase B |
| FTH1 | Ferritin Heavy Chain 1 | LC3II | light chain 3II |
| IRP1 | Iron Regulatory Protein 1 | Atg | autophagy-related gene |
| Bcl-2 | B cell leukemia-2 | caspase | Cysteine-aspartic protease |
| NOS | Nitric Oxide Synthase | Mdm2 | Mouse double minute 2 homolog |
| GSH | glutathione | CK | creatine kinase |
| SOD | superoxide dismutase | LDH | lactate dehydrogenase |
| ATP | adenosine triphosphate | GPx | glutathione peroxidase |
| eNOS | endothelial nitric oxide synthase | HO-1 | heme oxygenase 1 |
| PI3K | phosphatidylinositol 3-kinase | AST | aspartate transaminase |
| TGF-β | transforming growth factor-β | COL-IV | Collagen Type IV |
| VEGF | Vascular Endothelial Growth Factor | MPO | Myeloperoxidase |
| TNF-α | tumor necrosis factor-α | G-CSF | Granulocyte Colony-Stimulating Factor |
| MMP | Matrix Metalloproteinases | GDH | Glutamate Dehydrogenase |
| α-SMA | α-Smooth Muscle Actin | TLR4 | Toll-like receptor 4 |
| FAK | Focal Adhesion Kinase | SIRT1 | sirtuin 1 |
| p62 | ubiquitin-binding protein p62 | A20 | A20 Zinc Finger Protein |
| GPX4 | Glutathione Peroxidase 4 | SIRT3 | sirtuin 3 |
| ANP | atrial natriuretic peptide | MyHc | myosin heavy chain |
| β-MHC | β-myosin heavy chain | 4-HNE | 4-Hydroxy-2-nonenal |
| GSSG | Glutathione disulfide | Cyt-c | cytochrome c |
| NF-κB | Nuclear Factor-Kappa B | Col | Collagen type |
| PARP | Poly (ADP—Ribose) Polymerase | LC3 | light chain 3 |
| JNK | c-Jun N-terminal Kinase | MFN2 | Mitofusin 2 |
| AP-1 | Activator Protein-1 | Collagen I | Collagen Type I |
| PINK1 | PTEN induced putative kinase 1 | NOX | NADPH oxidase |
| GSK-3β | Glycogen synthase kinase 3β | ER | Estrogen Receptor |
| ARE | Antioxidant Response Element | ERK | Extracellular Signal-Regulated Kinases |
| RhoA | Ras homolog gene family, member A | MIP-1δ | Macrophage Inflammatory Protein-1δ |
| Bax | Bcl-2-associated X apoptosis regulator | H-keap1 | kelch-like ECH-associated protein 1 |
| ICAM-1 | Intercellular Adhesion Molecule-1 | Bad | Bcl-2 associated death promoter |
| VPS | Vacuolar protein sorting 34 | HIF-1α | Hypoxia-Inducible Factor-1α |
| MAPK | Mitogen-Activated Protein Kinase | Smad 2/3 | Mothers against decapentaplegic homolog 2/3 |
| Ras | Rat sarcoma virus oncogene homolog | EHA | European Hematology Association Guidelines |
| NIX | Nip3—like protein X | FUNDC1 | FUN14 Domain—containing 1 |
| SLC7A11 | Solute Carrier Family 7 Member 11 | FIP200 | 200—kDa FAK—family interacting protein |
| COL1A1 | Collagen Type I Alpha 1 Chain | ATG14L | Autophagy—related 14 |
| FADD | Fas—associated death domain protein | SLC7A11 | Solute Carrier Family 7 Member 11 |
| mTOR | mechanistic target of rapamycin | ETC | Electron Transport Chain |
| DMT1 | Divalent Metal Transporter 1 | FPN | Ferroportin |
| PUMA | p53—upregulated modulator of apoptosis | BNIP3 | Bcl—2/adenovirus E1B 19kDa—interacting protein 3 |
| ESC | European Society of Cardiology Guidelines | PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1α |
| TRADD | Tumor Necrosis Factor Receptor 1—associated Death Domain protein | ABCB1 | ATP—Binding Cassette Sub—family B Member 1 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 | AMPK | adenosine monophosphate-activated protein kinase |
| NLRP3 | NOD-like receptor protein-3 GSDME:Gasdermin E | ASC | Apoptosis-associated speck-like protein containing a CARD |
| ROCK | Rho-associated coiled-coil containing protein kinase | NOXA | Novel Oxidation—inducible protein in Astrocytes |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate Hydrogen | CSCO | Chinese Society of Clinical Oncology Guidelines |
| SERCA | Sarcoplasmic/Endoplasmic Reticulum Calcium ATPase | LOX-1 | Lectin-like Oxidized Low-Density Lipoprotein Receptor-1 |
| Apaf1 | Apoptotic Peptidase Activating Factor 1 | ULK1 | unc—51 like autophagy activating kinase 1 |
References
- Qu, P.R.; Jiang, Z.L.; Song, P.P.; Liu, L.C.; Xiang, M.; Wang, J. Saponins and their derivatives: Potential candidates to alleviate anthracycline-induced cardiotoxicity and multidrug resistance. Pharmacol. Res. 2022, 182, 106352. [Google Scholar] [CrossRef] [PubMed]
- Chinese Society of Clinical Oncology (CSCO) Diagnosis and Treatment Guidelines for Colorectal Cancer Working Group. Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for colorectal cancer 2018 (English version). Chin. J. Cancer Res. 2019, 31, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Bou Zerdan, M.; Ghorayeb, T.; Saliba, F.; Allam, S.; Bou Zerdan, M.; Yaghi, M.; Bilani, N.; Jaafar, R.; Nahleh, Z. Triple Negative Breast Cancer: Updates on Classification and Treatment in 2021. Cancers 2022, 14, 1253. [Google Scholar] [CrossRef]
- Marinello, J.; Delcuratolo, M.; Capranico, G. Anthracyclines as Topoisomerase II Poisons: From Early Studies to New Perspectives. Int. J. Mol. Sci. 2018, 19, 3480. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Jiang, P.; Huang, Y. Anthracycline-induced cardiotoxicity: Mechanisms, monitoring, and prevention. Front. Cardiovasc. Med. 2023, 10, 1242596. [Google Scholar] [CrossRef]
- Meattini, I.; Becherini, C.; Martella, F.; Del Bene, M.R.; Saieva, C.; Bacci, C.; Coltelli, L.; Pilato, G.; Visani, L.; Salvestrini, V.; et al. Cardioprotection in patients with anthracycline-treated breast cancer: Final analysis from the 2 × 2 randomized, placebo-controlled, double-blind SAFE trial. ESMO Open 2025, 10, 105116. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Fabiani, I.; Chianca, M.; Cipolla, C.M.; Cardinale, D.M. Anthracycline-induced cardiomyopathy: Risk prediction, prevention and treatment. Nat. Rev. Cardiol. 2025. Online First. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, B.; Wei, Y.; Liu, Y.; Yang, L.; Qiu, Y.; Su, J.; Qiu, M. Bioinspired ginsenoside Rg3 PLGA nanoparticles coated with tumor-derived microvesicles to improve chemotherapy efficacy and alleviate toxicity. Biomater. Sci. 2024, 12, 2672–2688. [Google Scholar] [CrossRef]
- Xie, L.; Liu, H.; Zhang, K.; Pan, Y.; Chen, M.; Xue, X.; Wan, G. Exploring the molecular mechanism of ginseng against anthracycline-induced cardiotoxicity based on network pharmacology, molecular docking and molecular dynamics simulation. Hereditas 2024, 161, 31. [Google Scholar] [CrossRef]
- Tang, M.M.; Zhao, S.T.; Li, R.Q.; Hou, W. Therapeutic mechanisms of ginseng in coronary heart disease. Front. Pharmacol. 2023, 14, 1271029. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, Y.; Zheng, H.; Li, S.; Li, Z.; Yuan, L.; Cheng, X.; He, C.; Sun, J. Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: Pharmacology and mechanisms. Biomed. Pharmacother. 2020, 132, 110915. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, M.; Zhang, Z.; Song, Z.; Xu, J.; Zhang, M.; Gong, M. Overview of Panax ginseng and its active ingredients protective mechanism on cardiovascular diseases. J. Ethnopharmacol. 2024, 334, 118506. [Google Scholar] [CrossRef]
- Moini Jazani, A.; Arabzadeh, A.; Haghi-Aminjan, H.; Nasimi Doost Azgomi, R. The role of ginseng derivatives against chemotherapy-induced cardiotoxicity: A systematic review of non-clinical studies. Front. Cardiovasc. Med. 2023, 10, 1022360. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef]
- Sarhene, M.; Ni, J.Y.; Duncan, E.S.; Liu, Z.; Li, S.; Zhang, J.; Guo, R.; Gao, S.; Gao, X.; Fan, G. Ginsenosides for cardiovascular diseases; update on pre-clinical and clinical evidence, pharmacological effects and the mechanisms of action. Pharmacol. Res. 2021, 166, 105481. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, M.X.; Zhang, L.; Zhang, D.; Li, C.; Li, Y.L. Autophagy, Pyroptosis, and Ferroptosis: New Regulatory Mechanisms for Atherosclerosis. Front. Cell Dev. Biol. 2021, 9, 809955. [Google Scholar] [CrossRef]
- Saleh, Y.; Abdelkarim, O.; Herzallah, K.; Abela, G.S. Anthracycline-induced cardiotoxicity: Mechanisms of action, incidence, risk factors, prevention, and treatment. Heart Fail. Rev. 2021, 26, 1159–1173. [Google Scholar] [CrossRef]
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef]
- Mudd, T.W., Jr.; Khalid, M.; Guddati, A.K. Cardiotoxicity of chemotherapy and targeted agents. Am. J. Cancer Res. 2021, 11, 1132–1147. [Google Scholar]
- Kong, M.; Pan, Q.; Cheng, X.; Li, J.; Gao, Y.; Tian, X. Anthracycline-induced delayed-onset cardiac toxicity: A case report and literature review. Exp. Ther. Med. 2023, 26, 505. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Ameri, P.; Cadeddu, C.; Ghigo, A.; Madonna, R.; Marone, G.; Mercurio, V.; Monte, I.; Novo, G.; Parrella, P.; et al. Antineoplastic Drug-Induced Cardiotoxicity: A Redox Perspective. Front. Physiol. 2018, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS induced lipid peroxidation and their role in ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef]
- Pamplona, R.; Costantini, D. Molecular and structural antioxidant defenses against oxidative stress in animals. American journal of physiology. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R843–R863. [Google Scholar] [CrossRef]
- Stěrba, M.; Popelová, O.; Vávrová, A.; Jirkovský, E.; Kovaříková, P.; Geršl, V.; Simůnek, T. Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxid. Redox Signal. 2013, 18, 899–929. [Google Scholar] [CrossRef]
- Vitale, R.; Marzocco, S.; Popolo, A. Role of Oxidative Stress and Inflammation in Doxorubicin-Induced Cardiotoxicity: A Brief Account. Int. J. Mol. Sci. 2024, 25, 7477. [Google Scholar] [CrossRef]
- Mitry, M.A.; Edwards, J.G. Doxorubicin induced heart failure: Phenotype and molecular mechanisms. Int. J. Cardiol. Heart Vasc. 2016, 10, 17–24. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Schlame, M.; Rua, D.; Greenberg, M.L. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 2000, 39, 257–288. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Sun, Y.; Zhao, X.; Xiao, Y.; Zhou, F.; Lin, L.; Wang, W.; Lin, B.; Wang, Z.; Fang, Z.; et al. An update of the molecular mechanisms underlying anthracycline induced cardiotoxicity. Front. Pharmacol. 2024, 15, 1406247. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Takemura, G.; Kanamori, H.; Takeyama, T.; Watanabe, T.; Morishita, K.; Ogino, A.; Tsujimoto, A.; Goto, K.; Maruyama, R.; et al. Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovasc. Res. 2012, 96, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, S.; Deng, W. Mitophagy in Doxorubicin-Induced Cardiotoxicity: Insights into Molecular Biology and Novel Therapeutic Strategies. Biomolecules 2024, 14, 1614. [Google Scholar] [CrossRef]
- Tan, N.; Luo, H.; Li, W.; Ling, G.; Wei, Y.; Wang, W.; Wang, Y. The dual function of autophagy in doxorubicin-induced cardiotoxicity: Mechanism and natural products. Semin. Cancer Biol. 2025, 109, 83–90. [Google Scholar] [CrossRef]
- Li, Y.; Yan, J.; Yang, P. The mechanism and therapeutic strategies in doxorubicin-induced cardiotoxicity: Role of programmed cell death. Cell Stress Chaperones 2024, 29, 666–680. [Google Scholar] [CrossRef]
- Koleini, N.; Kardami, E. Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity. Oncotarget 2017, 8, 46663–46680. [Google Scholar] [CrossRef]
- Dimitrakis, P.; Romay-Ogando, M.I.; Timolati, F.; Suter, T.M.; Zuppinger, C. Effects of doxorubicin cancer therapy on autophagy and the ubiquitin-proteasome system in long-term cultured adult rat cardiomyocytes. Cell Tissue Res. 2012, 350, 361–372. [Google Scholar] [CrossRef]
- Sishi, B.J.; Bester, D.J.; Wergeland, A.; Loos, B.; Jonassen, A.K.; van Rooyen, J.; Engelbrecht, A.M. Daunorubicin therapy is associated with upregulation of E3 ubiquitin ligases in the heart. Exp. Biol. Med. 2012, 237, 219–226. [Google Scholar] [CrossRef]
- Li, M.; Russo, M.; Pirozzi, F.; Tocchetti, C.G.; Ghigo, A. Autophagy and cancer therapy cardiotoxicity: From molecular mechanisms to therapeutic opportunities. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2020, 1867, 118493. [Google Scholar] [CrossRef] [PubMed]
- Pessah, I.N.; Kim, K.H.; Feng, W. Redox sensing properties of the ryanodine receptor complex. Front. Biosci. A J. Virtual Libr. 2002, 7, a72–a79. [Google Scholar] [CrossRef]
- Wallace, K.B. Adriamycin-induced interference with cardiac mitochondrial calcium homeostasis. Cardiovasc. Toxicol. 2007, 7, 101–107. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, X.; Tan, B.; Zhang, Q.; Zhao, X.; Dong, D. Potential role of endoplasmic reticulum stress in doxorubicin-induced cardiotoxicity-an update. Front. Pharmacol. 2024, 15, 1415108. [Google Scholar] [CrossRef]
- Meng, L.; Lin, H.; Zhang, J.; Lin, N.; Sun, Z.; Gao, F.; Luo, H.; Ni, T.; Luo, W.; Chi, J.; et al. Doxorubicin induces cardiomyocyte pyroptosis via the TINCR-mediated posttranscriptional stabilization of NLR family pyrin domain containing 3. J. Mol. Cell. Cardiol. 2019, 136, 15–26. [Google Scholar] [CrossRef]
- Zheng, X.; Zhong, T.; Ma, Y.; Wan, X.; Qin, A.; Yao, B.; Zou, H.; Song, Y.; Yin, D. Bnip3 mediates doxorubicin-induced cardiomyocyte pyroptosis via caspase-3/GSDME. Life Sci. 2020, 242, 117186. [Google Scholar] [CrossRef]
- Keizer, H.G.; Pinedo, H.M.; Schuurhuis, G.J.; Joenje, H. Doxorubicin (adriamycin): A critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacol. Ther. 1990, 47, 219–231. [Google Scholar] [CrossRef]
- Miranda, C.J.; Makui, H.; Soares, R.J.; Bilodeau, M.; Mui, J.; Vali, H.; Bertrand, R.; Andrews, N.C.; Santos, M.M. Hfe deficiency increases susceptibility to cardiotoxicity and exacerbates changes in iron metabolism induced by doxorubicin. Blood 2003, 102, 2574–2580. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, H.; Wu, Y.; Lu, D.; Li, C.; Yang, X.; Chen, Z.; Qian, J.; Ge, J. COX5A Alleviates Doxorubicin-Induced Cardiotoxicity by Suppressing Oxidative Stress, Mitochondrial Dysfunction and Cardiomyocyte Apoptosis. Int. J. Mol. Sci. 2023, 24, 10400. [Google Scholar] [CrossRef]
- Shi, J.; Abdelwahid, E.; Wei, L. Apoptosis in Anthracycline Cardiomyopathy. Curr. Pediatr. Rev. 2011, 7, 329–336. [Google Scholar] [CrossRef]
- Men, H.; Cai, H.; Cheng, Q.; Zhou, W.; Wang, X.; Huang, S.; Zheng, Y.; Cai, L. The regulatory roles of p53 in cardiovascular health and disease. Cell. Mol. Life Sci. 2021, 78, 2001–2018. [Google Scholar] [CrossRef] [PubMed]
- Turley, H.; Comley, M.; Houlbrook, S.; Nozaki, N.; Kikuchi, A.; Hickson, I.D.; Gatter, K.; Harris, A.L. The distribution and expression of the two isoforms of DNA topoisomerase II in normal and neoplastic human tissues. Br. J. Cancer 1997, 75, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.I.; Hochhauser, D.; Ayton, P.; Camplejohn, R.C.; Whitehouse, R.; Turley, H.; Gatter, K.; Hickson, I.D.; Harris, A.L. Differential expression of the topoisomerase II alpha and beta genes in human breast cancers. Br. J. Cancer 1996, 73, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Pantazi, D.; Tselepis, A.D. Cardiovascular toxic effects of antitumor agents: Pathogenetic mechanisms. Thromb. Res. 2022, 213 (Suppl. S1), S95–S102. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Moreno-Arciniegas, A.; Cádiz, L.; Galán-Arriola, C.; Clemente-Moragón, A.; Ibáñez, B. Cardioprotection strategies for anthracycline cardiotoxicity. Basic Res. Cardiol. 2025, 120, 71–90. [Google Scholar] [CrossRef]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.B.; Ewer, M.; et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- Ferruzzi, G.J.; Campanile, A.; Visco, V.; Loria, F.; Mone, P.; Masarone, D.; Dattilo, G.; Agnelli, G.; Moncada, A.; Falco, L.; et al. Subclinical left ventricular dysfunction assessed by global longitudinal strain correlates with mild cognitive impairment in hypertensive patients. Hypertens. Res. 2025, 48, 1768–1778. [Google Scholar] [CrossRef]
- Gripp, E.A.; Oliveira, G.E.; Feijó, L.A.; Garcia, M.I.; Xavier, S.S.; Sousa, A.S. Global Longitudinal Strain Accuracy for Cardiotoxicity Prediction in a Cohort of Breast Cancer Patients During Anthracycline and/or Trastuzumab Treatment. Arq. Bras. Cardiol. 2018, 110, 140–150. [Google Scholar] [CrossRef]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef]
- Balough, E.; Ariza, A.; Asnani, A.; Hoeger, C.W. Cardiotoxicity of Anthracyclines. Cardiol. Clin. 2025, 43, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, A.A.; Pituskin, E.; Thompson, R.B.; Mackey, J.R.; Koshman, S.L.; Jassal, D.; Pitz, M.; Haykowsky, M.J.; Pagano, J.J.; Chow, K.; et al. Cardiac and cardiometabolic phenotyping of trastuzumab-mediated cardiotoxicity: A secondary analysis of the MANTICORE trial. European heart journal. Cardiovasc. Pharmacother. 2022, 8, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Pudil, R.; Mueller, C.; Čelutkienė, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef]
- Gong, H.; Wang, X.; Shi, Y.J.; Shang, W.J.; Ling, Y.I.; Pan, L.J.; Shi, H.M. Correlation between brain natriuretic peptide levels and the prognosis of patients with left ventricular diastolic dysfunction. Exp. Ther. Med. 2016, 11, 2583–2589. [Google Scholar] [CrossRef][Green Version]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef]
- Ky, B.; Putt, M.; Sawaya, H.; French, B.; Januzzi, J.L., Jr.; Sebag, I.A.; Plana, J.C.; Cohen, V.; Banchs, J.; Carver, J.R.; et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J. Am. Coll. Cardiol. 2014, 63, 809–816. [Google Scholar] [CrossRef]
- Finkelman, B.S.; Putt, M.; Wang, T.; Wang, L.; Narayan, H.; Domchek, S.; DeMichele, A.; Fox, K.; Matro, J.; Shah, P.; et al. Arginine-Nitric Oxide Metabolites and Cardiac Dysfunction in Patients with Breast Cancer. J. Am. Coll. Cardiol. 2017, 70, 152–162. [Google Scholar] [CrossRef]
- Demissei, B.G.; Freedman, G.; Feigenberg, S.J.; Plastaras, J.P.; Maity, A.; Smith, A.M.; McDonald, C.; Sheline, K.; Simone, C.B., 2nd; Lin, L.L.; et al. Early Changes in Cardiovascular Biomarkers with Contemporary Thoracic Radiation Therapy for Breast Cancer, Lung Cancer, and Lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 851–860. [Google Scholar] [CrossRef]
- Jirkovská, A.; Karabanovich, G.; Kubeš, J.; Skalická, V.; Melnikova, I.; Korábečný, J.; Kučera, T.; Jirkovský, E.; Nováková, L.; Bavlovič Piskáčková, H.; et al. Structure-Activity Relationship Study of Dexrazoxane Analogues Reveals ICRF-193 as the Most Potent Bisdioxopiperazine against Anthracycline Toxicity to Cardiomyocytes Due to Its Strong Topoisomerase IIβ Interactions. J. Med. Chem. 2021, 64, 3997–4019. [Google Scholar] [CrossRef]
- Bosch, X.; Rovira, M.; Sitges, M.; Domènech, A.; Ortiz-Pérez, J.T.; de Caralt, T.M.; Morales-Ruiz, M.; Perea, R.J.; Monzó, M.; Esteve, J. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: The OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J. Am. Coll. Cardiol. 2013, 61, 2355–2362. [Google Scholar] [CrossRef]
- Avila, M.S.; Ayub-Ferreira, S.M.; de Barros Wanderley, M.R., Jr.; das Dores Cruz, F.; Gonçalves Brandão, S.M.; Rigaud, V.O.C.; Higuchi-Dos-Santos, M.H.; Hajjar, L.A.; Kalil Filho, R.; Hoff, P.M.; et al. Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity: The CECCY Trial. J. Am. Coll. Cardiol. 2018, 71, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Quinaglia, T.; Onoue, T.; Mahmood, S.S.; Drobni, Z.D.; Gilman, H.K.; Smith, A.; Heemelaar, J.C.; Brahmbhatt, P.; Ho, J.S.; et al. Atorvastatin for Anthracycline-Associated Cardiac Dysfunction: The STOP-CA Randomized Clinical Trial. JAMA 2023, 330, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.S.; Siqueira, S.R.R.; Waldeck, L.; Ayub-Ferreira, S.M.; Takx, R.; Bittencourt, M.S.; Bocchi, E.A. Renin-angiotensin System Antagonists and Beta-blockers in Prevention of Anthracycline Cardiotoxicity: A Systematic Review and Meta-analysis. Arq. Bras. Cardiol. 2023, 120, e20220298. [Google Scholar] [CrossRef]
- de Baat, E.C.; Mulder, R.L.; Armenian, S.; Feijen, E.A.; Grotenhuis, H.; Hudson, M.M.; Mavinkurve-Groothuis, A.M.; Kremer, L.C.; van Dalen, E.C. Dexrazoxane for preventing or reducing cardiotoxicity in adults and children with cancer receiving anthracyclines. Cochrane Database Syst. Rev. 2022, 9, Cd014638. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef]
- Lü, J.M.; Yao, Q.; Chen, C. Ginseng compounds: An update on their molecular mechanisms and medical applications. Curr. Vasc. Pharmacol. 2009, 7, 293–302. [Google Scholar] [CrossRef]
- Geng, X.; Wang, J.; Liu, Y.; Liu, L.; Liu, X.; Zhao, Y.; Wang, C.; Liu, J. Research progress on chemical diversity of saponins in Panax ginseng. Chin. Herb. Med. 2024, 16, 529–547. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Yang, J.; Wang, W.; Zhang, J.; Zhang, R.; Meng, Q. Discovery, semisynthesis, biological activities, and metabolism of ocotillol-type saponins. J. Ginseng Res. 2017, 41, 373–378. [Google Scholar] [CrossRef]
- Chu, L.L.; Huy, N.Q.; Tung, N.H. Microorganisms for Ginsenosides Biosynthesis: Recent Progress, Challenges, and Perspectives. Molecules 2023, 28, 1437. [Google Scholar] [CrossRef]
- Sohn, S.H.; Kim, S.K.; Kim, Y.O.; Kim, H.D.; Shin, Y.S.; Yang, S.O.; Kim, S.Y.; Lee, S.W. A comparison of antioxidant activity of Korean White and Red Ginsengs on H2O2-induced oxidative stress in HepG2 hepatoma cells. J. Ginseng Res. 2013, 37, 442–450. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Hong, Y.H.; Park, S.H.; Lee, J.O.; Lee, J.; Cho, J.Y. Pharmacological potential of ginseng and its major component ginsenosides. J. Ginseng Res. 2021, 45, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, J.; Liu, P.; Lau, C.W.; Gao, Z.; Zhou, D.; Tang, J.; Ng, C.F.; Huang, Y. Ginsenoside Rb3 attenuates oxidative stress and preserves endothelial function in renal arteries from hypertensive rats. Br. J. Pharmacol. 2014, 171, 3171–3181. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, H.; Xie, Z.; Yang, S.; Xu, W.; Hou, J.; Yu, B. Ginsenoside Rb3 protects cardiomyocytes against ischemia-reperfusion injury via the inhibition of JNK-mediated NF-κB pathway: A mouse cardiomyocyte model. PLoS ONE 2014, 9, e103628. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; He, N.; Wang, Z.; Fu, X.; Aung, L.H.H.; Liu, Y.; Li, M.; Cho, J.Y.; Yang, Y.; Yu, T. Functional roles and mechanisms of ginsenosides from Panax ginseng in atherosclerosis. J. Ginseng Res. 2021, 45, 22–31. [Google Scholar] [CrossRef]
- Hou, L.; Zou, Z.; Wang, Y.; Pi, H.; Yuan, Z.; He, Q.; Kuang, Y.; Zhao, G. Exploring the anti-atherosclerosis mechanism of ginsenoside Rb1 by integrating network pharmacology and experimental verification. Aging 2024, 16, 6745–6756. [Google Scholar] [CrossRef]
- Han, A.Y.; Ha, S.M.; Shin, Y.K.; Seol, G.H. Ginsenoside Rg-1 prevents elevated cytosolic Ca(2+) via store-operated Ca(2+) entry in high-glucose-stimulated vascular endothelial and smooth muscle cells. BMC Complement. Med. Ther. 2022, 22, 166. [Google Scholar] [CrossRef]
- Shi, L.; Luo, J.; Wei, X.; Xu, X.; Tu, L. The protective role of ginsenoside Rg3 in heart diseases and mental disorders. Front. Pharmacol. 2024, 15, 1327033. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Hedayati-Goudarzi, M.T.; Alizadeh, A.; Sadeghian-Nodoushan, F.; Soltaninejad, H. A review of cardioprotective effect of ginsenosides in chemotherapy-induced cardiotoxicity. Biomed. Eng. Online 2024, 23, 128. [Google Scholar] [CrossRef]
- Xie, J.T.; Shao, Z.H.; Vanden Hoek, T.L.; Chang, W.T.; Li, J.; Mehendale, S.; Wang, C.Z.; Hsu, C.W.; Becker, L.B.; Yin, J.J.; et al. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur. J. Pharmacol. 2006, 532, 201–207. [Google Scholar] [CrossRef]
- Wang, H.; Yu, P.; Gou, H.; Zhang, J.; Zhu, M.; Wang, Z.H.; Tian, J.W.; Jiang, Y.T.; Fu, F.H. Cardioprotective Effects of 20(S)-Ginsenoside Rh2 against Doxorubicin-Induced Cardiotoxicity In Vitro and In Vivo. Evid.-Based Complement. Altern. Med. 2012, 2012, 506214. [Google Scholar] [CrossRef]
- Li, L.; Ni, J.; Li, M.; Chen, J.; Han, L.; Zhu, Y.; Kong, D.; Mao, J.; Wang, Y.; Zhang, B.; et al. Ginsenoside Rg3 micelles mitigate doxorubicin-induced cardiotoxicity and enhance its anticancer efficacy. Drug Deliv. 2017, 24, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Wang, T.; Jiang, X.; Zhang, H.; Li, P.; Lv, B.; Gao, X. Ginsenoside Rg3 antagonizes adriamycin-induced cardiotoxicity by improving endothelial dysfunction from oxidative stress via upregulating the Nrf2-ARE pathway through the activation of akt. Phytomedicine 2015, 22, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, J.; Liu, S.; Chen, C.; Li, Q.; Qin, M.; Ren, L. Ginsenoside F1 attenuates pirarubicin-induced cardiotoxicity by modulating Nrf2 and AKT/Bcl-2 signaling pathways. J. Ginseng Res. 2023, 47, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, J.; Wang, X.; Wang, L.; Pugliese, M.; Passantino, A.; Li, J. Cardioprotection of Sheng Mai Yin a classic formula on adriamycin induced myocardial injury in Wistar rats. Phytomedicine 2018, 38, 1–11. [Google Scholar] [CrossRef]
- Hou, J.; Yun, Y.; Cui, C.; Kim, S. Ginsenoside Rh2 mitigates doxorubicin-induced cardiotoxicity by inhibiting apoptotic and inflammatory damage and weakening pathological remodelling in breast cancer-bearing mice. Cell Prolif. 2022, 55, e13246. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Sun, B.; Xu, J.; Jiang, J.; Luo, M. Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion injury via Akt/endothelial nitric oxide synthase signaling and the B-cell lymphoma/B-cell lymphoma-associated X protein pathway. Mol. Med. Rep. 2015, 11, 4518–4524. [Google Scholar] [CrossRef]
- Jia, Y.; Gong, M.; Ke, Z. The Pharmacological Mechanisms Underlying the Protective Effect of Ginsenoside Rg3 against Heart Failure. Cardiol. Res. Pract. 2024, 2024, 3373410. [Google Scholar] [CrossRef]
- Ren, B.; Feng, J.; Yang, N.; Guo, Y.; Chen, C.; Qin, Q. Ginsenoside Rg3 attenuates angiotensin II-induced myocardial hypertrophy through repressing NLRP3 inflammasome and oxidative stress via modulating SIRT1/NF-κB pathway. Int. Immunopharmacol. 2021, 98, 107841. [Google Scholar] [CrossRef]
- Yu, T.; Xu, X.; Wei, J.; Xu, J.; Luo, W.; Li, A.; Liang, G.; Wang, M. Ginsenoside Rg5 alleviates Ang II-induced cardiac inflammation and remodeling by inhibiting the JNK/AP-1 pathway. Int. Immunopharmacol. 2023, 120, 110408. [Google Scholar] [CrossRef]
- Qin, M.; Luo, Y.; Lu, S.; Sun, J.; Yang, K.; Sun, G.; Sun, X. Ginsenoside F1 Ameliorates Endothelial Cell Inflammatory Injury and Prevents Atherosclerosis in Mice through A20-Mediated Suppression of NF-kB Signaling. Front. Pharmacol. 2017, 8, 953. [Google Scholar] [CrossRef]
- Li, Q.; Xiang, Y.; Chen, Y.; Tang, Y.; Zhang, Y. Ginsenoside Rg1 Protects Cardiomyocytes Against Hypoxia/Reoxygenation Injury via Activation of Nrf2/HO-1 Signaling and Inhibition of JNK. Cell. Physiol. Biochem. 2017, 44, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, J.; Li, Z. Ginsenoside Rd mitigates myocardial ischemia-reperfusion injury via Nrf2/HO-1 signaling pathway. Int. J. Clin. Exp. Med. 2015, 8, 14497–14504. [Google Scholar] [PubMed]
- Sun, J.; Sun, G.; Meng, X.; Wang, H.; Wang, M.; Qin, M.; Ma, B.; Luo, Y.; Yu, Y.; Chen, R.; et al. Ginsenoside RK3 Prevents Hypoxia-Reoxygenation Induced Apoptosis in H9c2 Cardiomyocytes via AKT and MAPK Pathway. Evid.-Based Complement. Altern. Med. 2013, 2013, 690190. [Google Scholar] [CrossRef]
- Gai, Y.; Ma, Z.; Yu, X.; Qu, S.; Sui, D. Effect of ginsenoside Rh1 on myocardial injury and heart function in isoproterenol-induced cardiotoxicity in rats. Toxicol. Mech. Methods 2012, 22, 584–591. [Google Scholar] [CrossRef]
- Fan, H.J.; Tan, Z.B.; Wu, Y.T.; Feng, X.R.; Bi, Y.M.; Xie, L.P.; Zhang, W.T.; Ming, Z.; Liu, B.; Zhou, Y.C. The role of ginsenoside Rb1, a potential natural glutathione reductase agonist, in preventing oxidative stress-induced apoptosis of H9C2 cells. J. Ginseng Res. 2020, 44, 258–266. [Google Scholar] [CrossRef]
- Zhang, N.; An, X.; Lang, P.; Wang, F.; Xie, Y. Ginsenoside Rd contributes the attenuation of cardiac hypertrophy in vivo and in vitro. Biomed. Pharmacother. 2019, 109, 1016–1023. [Google Scholar] [CrossRef]
- Shi, L.; Fu, W.; Xu, H.; Li, S.; Yang, X.; Yang, W.; Sui, D.; Wang, Q. Ginsenoside Rc attenuates myocardial ischaemic injury through antioxidative and anti-inflammatory effects. Pharm. Biol. 2022, 60, 1038–1046. [Google Scholar] [CrossRef]
- Qin, L.; Fan, S.; Jia, R.; Liu, Y. Ginsenoside Rg1 protects cardiomyocytes from hypoxia-induced injury through the PI3K/AKT/mTOR pathway. Die Pharm. 2018, 73, 349–355. [Google Scholar] [CrossRef]
- Xia, R.; Zhao, B.; Wu, Y.; Hou, J.B.; Zhang, L.; Xu, J.J.; Xia, Z.Y. Ginsenoside Rb1 preconditioning enhances eNOS expression and attenuates myocardial ischemia/reperfusion injury in diabetic rats. J. Biomed. Biotechnol. 2011, 2011, 767930. [Google Scholar] [CrossRef]
- Huang, G.D.; Zhong, X.F.; Deng, Z.Y.; Zeng, R. Proteomic analysis of ginsenoside Re attenuates hydrogen peroxide-induced oxidative stress in human umbilical vein endothelial cells. Food Funct. 2016, 7, 2451–2461. [Google Scholar] [CrossRef]
- Cui, Y.C.; Pan, C.S.; Yan, L.; Li, L.; Hu, B.H.; Chang, X.; Liu, Y.Y.; Fan, J.Y.; Sun, K.; Li, Q.; et al. Ginsenoside Rb1 protects against ischemia/reperfusion-induced myocardial injury via energy metabolism regulation mediated by RhoA signaling pathway. Sci. Rep. 2017, 7, 44579. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Chen, T.; Ren, X.; Zhang, Z.; Huang, W.; Liu, L.; Luo, P.; Zhou, H. Rg1 prevents myocardial hypoxia/reoxygenation injury by regulating mitochondrial dynamics imbalance via modulation of glutamate dehydrogenase and mitofusin 2. Mitochondrion 2016, 26, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Li, J.; Liu, K.; Zhang, L.; Liu, Q.; Liu, B.; Qi, L.W. Ginsenoside Rg5 increases cardiomyocyte resistance to ischemic injury through regulation of mitochondrial hexokinase-II and dynamin-related protein 1. Cell Death Dis. 2017, 8, e2625. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiang, N.; Wang, Z. Ginsenoside Rg2 attenuates myocardial fibrosis and improves cardiac function after myocardial infarction via AKT signaling pathway. Biosci. Biotechnol. Biochem. 2020, 84, 2199–2206. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, J.; Liu, J.; Wang, P.; Wang, C. Ginsenoside Re Preserves Cardiac Function and Ameliorates Left Ventricular Remodeling in a Rat Model of Myocardial Infarction. J. Cardiovasc. Pharmacol. 2020, 75, 91–97. [Google Scholar] [CrossRef]
- Zhai, Y.; Bai, J.; Peng, Y.; Cao, J.; Fang, G.; Dong, Y.; Wang, Z.; Lu, Y.; Wang, M.; Liu, M.; et al. Ginsenoside Rb1 attenuates doxorubicin induced cardiotoxicity by suppressing autophagy and ferroptosis. Biochem. Biophys. Res. Commun. 2024, 710, 149910. [Google Scholar] [CrossRef]
- Zhong, G.; Chen, J.; Li, Y.; Han, Y.; Wang, M.; Nie, Q.; Xu, M.; Zhu, Q.; Chang, X.; Wang, L. Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion-induced ferroptosis via the keap1/Nrf2/GPX4 signaling pathway. BMC Complement. Med. Ther. 2024, 24, 247. [Google Scholar] [CrossRef]
- Ye, J.; Lyu, T.J.; Li, L.Y.; Liu, Y.; Zhang, H.; Wang, X.; Xi, X.; Liu, Z.J.; Gao, J.Q. Ginsenoside Re attenuates myocardial ischemia/reperfusion induced ferroptosis via miR-144-3p/SLC7A11. Phytomedicine 2023, 113, 154681. [Google Scholar] [CrossRef]
- Wirth, M.; Joachim, J.; Tooze, S.A. Autophagosome formation—The role of ULK1 and Beclin1-PI3KC3 complexes in setting the stage. Semin. Cancer Biol. 2013, 23, 301–309. [Google Scholar] [CrossRef]
- Iriondo, M.N.; Etxaniz, A.; Varela, Y.R.; Ballesteros, U.; Lázaro, M.; Valle, M.; Fracchiolla, D.; Martens, S.; Montes, L.R.; Goñi, F.M.; et al. Effect of ATG12-ATG5-ATG16L1 autophagy E3-like complex on the ability of LC3/GABARAP proteins to induce vesicle tethering and fusion. Cell. Mol. Life Sci. 2023, 80, 56. [Google Scholar] [CrossRef]
- Wang, H.; Sun, P.; Yuan, X.; Xu, Z.; Jiang, X.; Xiao, M.; Yao, X.; Shi, Y. Autophagy in tumor immune escape and immunotherapy. Mol. Cancer 2025, 24, 85. [Google Scholar] [CrossRef] [PubMed]
- Li, L.F.; Ma, Z.C.; Wang, Y.G.; Tang, X.L.; Tan, H.L.; Xiao, C.R.; Gao, Y. Protective effect of ginsenoside Rb₁ on doxorubicin-induced myocardial autophagy. China J. Chin. Mater. Med. 2017, 42, 1365–1369. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Fan, Y.; Liu, M.L. Ginsenoside Rg1 inhibits autophagy in H9c2 cardiomyocytes exposed to hypoxia/reoxygenation. Mol. Cell. Biochem. 2012, 365, 243–250. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, K.; Liang, F.; Ma, W.; Jiang, X.; Wang, H.; Zhan, H.; Sonkoly, E.; Hu, H.; Zhao, Z. Inhibition of the Ras/ERK1/2 pathway contributes to the protective effect of ginsenoside Re against intimal hyperplasia. Food Funct. 2021, 12, 6755–6765. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, X.; Liu, M.; Liu, X.; Dong, M.; Cheng, J.; Zhang, X.; Zhai, C.; Song, Y.; Lu, H.; et al. Ginsenoside Rb1 Enhances Atherosclerotic Plaque Stability by Improving Autophagy and Lipid Metabolism in Macrophage Foam Cells. Front. Pharmacol. 2017, 8, 727. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.; Fu, F.; Lai, Q.; Zhang, L.; Liu, T.; Yu, B.; Kou, J.; Li, F. Cardioprotective effect of ginsenoside Rb1 via regulating metabolomics profiling and AMP-activated protein kinase-dependent mitophagy. J. Ginseng Res. 2022, 46, 255–265. [Google Scholar] [CrossRef]
- Guan, S.; Xin, Y.; Ding, Y.; Zhang, Q.; Han, W. Ginsenoside Rg1 Protects against Cardiac Remodeling in Heart Failure via SIRT1/PINK1/Parkin-Mediated Mitophagy. Chem. Biodivers. 2023, 20, e202200730. [Google Scholar] [CrossRef]
- Wang, X.; Ling, G.; Wei, Y.; Li, W.; Zhang, Y.; Tan, N.; Li, W.; Li, H.; Qiu, Q.; Wang, W.; et al. Activation of ULK1 to trigger FUNDC1-mediated mitophagy in heart failure: Effect of Ginsenoside Rg3 intervention. Phytomedicine 2023, 120, 155042. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Xin, G.J.; Chen, Y.Y.; Cao, C.; Cui, X.S.; Gao, J.M.; Guo, H.; Fu, J.H. Mechanism of ginsenoside Rg_1 in regulating autophagy through miR-155/Notch1/Hes1 pathway to attenuate hypoxia/reoxygenation injury in HL-1 cells. China J. Chin. Mater. Med. 2024, 49, 6450–6458. [Google Scholar] [CrossRef]
- Peng, Y.; Liao, B.; Zhou, Y.; Zeng, W. Ginsenoside Rb2 improves heart failure by down-regulating miR-216a-5p to promote autophagy and inhibit apoptosis and oxidative stress. J. Appl. Biomed. 2023, 21, 180–192. [Google Scholar] [CrossRef]
- Li, D.; Wang, J.; Hou, J.; Fu, J.; Chang, D.; Bensoussan, A.; Liu, J. Ginsenoside Rg1 protects starving H9c2 cells by dissociation of Bcl-2-Beclin1 complex. BMC Complement. Altern. Med. 2016, 16, 146. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.L.; Chen, H.L.; Wu, D.; Chen, J.X.; Wang, X.X.; Li, R.L.; He, J.H.; Mo, L.; Cen, X.; et al. Ghrelin inhibits doxorubicin cardiotoxicity by inhibiting excessive autophagy through AMPK and p38-MAPK. Biochem. Pharmacol. 2014, 88, 334–350. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, Y.; Liu, H.; Mu, H.; Lu, Y.; Zhang, J.; Huang, J. Oral administration of Ginsenoside Rg1 prevents cardiac toxicity induced by doxorubicin in mice through anti-apoptosis. Oncotarget 2017, 8, 83792–83801. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.W.; Lu, P.; Peng, L.; Jiang, W. Ginsenoside Rb1 Inhibits Cardiomyocyte Autophagy via PI3K/Akt/mTOR Signaling Pathway and Reduces Myocardial Ischemia/Reperfusion Injury. Am. J. Chin. Med. 2021, 49, 1913–1927. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.M.; Li, C.B.; Liu, Q.L.; Li, P.; Yang, H. Ginsenoside Rg1 Prevents Doxorubicin-Induced Cardiotoxicity through the Inhibition of Autophagy and Endoplasmic Reticulum Stress in Mice. Int. J. Mol. Sci. 2018, 19, 3658. [Google Scholar] [CrossRef]
- Zou, G.; Tang, Y.; Yang, J.; Fu, S.; Li, Y.; Ren, X.; Zhou, N.; Zhao, W.; Gao, J.; Ruan, Z.; et al. Signal-induced NLRP3 phase separation initiates inflammasome activation. Cell Res. 2025, 35, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Mou, S.; Zhang, C.; Zhu, T.; Hu, X.; Li, M. Ginsenoside Rh2 Ameliorates Myocardial Infarction by Regulating Cardiomyocyte Pyroptosis Based on Network Pharmacology, Molecular Docking, and Experimental Verification. Am. J. Chin. Med. 2025, 53, 475–499. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Cai, Y.; Yang, D. Ginsenoside Rb2 Inhibits the Pyroptosis in Myocardial Ischemia Progression Through Regulating the SIRT1 Mediated Deacetylation of ASC. Biochem. Genet. 2024, 62, 2595–2611. [Google Scholar] [CrossRef]
- Qi, Z.; Yan, Z.; Wang, Y.; Ji, N.; Yang, X.; Zhang, A.; Li, M.; Xu, F.; Zhang, J. Integrative applications of network pharmacology and molecular docking: An herbal formula ameliorates H9c2 cells injury through pyroptosis. J. Ginseng Res. 2023, 47, 228–236. [Google Scholar] [CrossRef]
- Luo, M.; Yan, D.; Sun, Q.; Tao, J.; Xu, L.; Sun, H.; Zhao, H. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-kB/NLRP3 pathway. J. Cell. Biochem. 2020, 121, 2994–3004. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, H.; Zheng, M.; Xu, W.; Yang, Y.; Shi, F. Ginsenoside Rg3 suppresses the NLRP3 inflammasome activation through inhibition of its assembly. FASEB J. 2020, 34, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Hussaniy, H.A.; Al-Gareeb, A.I.; Negm, W.A.; El-Kadem, A.H.; Batiha, G.E.; Welson, N.N.; Mostafa-Hedeab, G.; Qasem, A.H.; Conte-Junior, C.A. Combination of Panax ginseng C. A. Mey and Febuxostat Boasted Cardioprotective Effects Against Doxorubicin-Induced Acute Cardiotoxicity in Rats. Front. Pharmacol. 2022, 13, 905828. [Google Scholar] [CrossRef]
- Peng, L.; Li, S.; Cai, H.; Chen, X.; Tang, Y. Ginsenoside Rg1 treats chronic heart failure by downregulating ERK1/2 protein phosphorylation. In vitro cellular & developmental biology. Animal 2024, 60, 1085–1098. [Google Scholar] [CrossRef]
- Qiu, B.; Mao, M.; Ma, Z.; Deng, B.; Shen, L.; Zhou, D.; Zheng, W.; Wei, Y. Ginsenoside Rg2 Attenuates Doxorubicin-induced Cardiomyocyte Apoptosis via the PI3K/Akt Pathway. Rev. Bras. Farmacogn. 2022, 32, 433–439. [Google Scholar] [CrossRef]
- Pi, Y.; Chen, X.; Zhang, X.; Cai, H. Ginsenoside Rb1 alleviates ADR-induced H9C2 cell injury by regulating miR-130b. Acta Pol. Pharm. 2022, 78, 825–834. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Ma, Z.; Liang, Q.; Tang, X.; Tan, H.; Xiao, C.; Gao, Y. Ginsenoside Rb1 Inhibits Doxorubicin-Triggered H9C2 Cell Apoptosis via Aryl Hydrocarbon Receptor. Biomol. Ther. 2017, 25, 202–212. [Google Scholar] [CrossRef]
- Wei, H.; Chen, J.; Wang, S.; Fu, F.; Zhu, X.; Wu, C.; Liu, Z.; Zhong, G.; Lin, J. A Nanodrug Consisting of Doxorubicin and Exosome Derived from Mesenchymal Stem Cells for Osteosarcoma Treatment In Vitro. Int. J. Nanomed. 2019, 14, 8603–8610. [Google Scholar] [CrossRef]
- Alyane, M.; Barratt, G.; Lahouel, M. Remote loading of doxorubicin into liposomes by transmembrane pH gradient to reduce toxicity toward H9c2 cells. Saudi Pharm. J. 2016, 24, 165–175. [Google Scholar] [CrossRef]
- Li, C.; Gou, X.; Gao, H. Doxorubicin nanomedicine based on ginsenoside Rg1 with alleviated cardiotoxicity and enhanced antitumor activity. Nanomedicine 2021, 16, 2587–2604. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Fu, W.; Yu, X.; Sui, D. Combination of the ginsenosides Rb3 and Rb2 exerts protective effects against myocardial ischemia reperfusion injury in rats. Int. J. Mol. Med. 2020, 45, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Wang, X.; Lau, W.; Wang, Y.; Xing, Y.; Zhang, X.; Ma, X.; Gao, F. Ginsenoside Rd attenuates myocardial ischemia/reperfusion injury via Akt/GSK-3β signaling and inhibition of the mitochondria-dependent apoptotic pathway. PLoS ONE 2013, 8, e70956. [Google Scholar] [CrossRef] [PubMed]
- Xin, G.J.; Chen, Y.Y.; Liu, Z.X.; Xu, S.J.; Zhang, H.Y.; Guo, F.; Peng, H.; Li, L.; Han, X.; Liu, J.X.; et al. Ginsenoside Re regulates mitochondrial biogenesis through Nrf2/HO-1/PGC-1α pathway to reduce hypoxia/reoxygenation injury in H9c2 cells. China J. Chin. Mater. Med. 2024, 49, 1064–1072. [Google Scholar] [CrossRef]
- Hernández-García, D.; Granado-Serrano, A.B.; Martín-Gari, M.; Naudí, A.; Serrano, J.C. Efficacy of Panax ginseng supplementation on blood lipid profile. A meta-analysis and systematic review of clinical randomized trials. J. Ethnopharmacol. 2019, 243, 112090. [Google Scholar] [CrossRef]
- Jia, Y.; Zuo, D.; Li, Z.; Liu, H.; Dai, Z.; Cai, J.; Pang, L.; Wu, Y. Astragaloside IV inhibits doxorubicin-induced cardiomyocyte apoptosis mediated by mitochondrial apoptotic pathway via activating the PI3K/Akt pathway. Chem. Pharm. Bull. 2014, 62, 45–53. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wu, S.S.; Chen, X.M.; Pi, J.K.; Cheng, Y.F.; Zhang, Y.; Wang, X.J.; Luo, D.; Zhou, J.H.; Xu, J.Y.; et al. Saikosaponin D Alleviates DOX-induced Cardiac Injury In Vivo and In Vitro. J. Cardiovasc. Pharmacol. 2022, 79, 558–567. [Google Scholar] [CrossRef]
- Liu, J.; Xin, Y.; Qiu, Z.; Zhang, Q.; He, T.; Qiu, Y.; Wang, W. Cordyceps sinensis-mediated biotransformation of notoginsenoside R1 into 25-OH-20(S/R)-R2 with elevated cardioprotective effect against DOX-induced cell injury. RSC Adv. 2022, 12, 12938–12946. [Google Scholar] [CrossRef]
- Petran, E.M.; Periferakis, A.; Troumpata, L.; Periferakis, A.T.; Scheau, A.E.; Badarau, I.A.; Periferakis, K.; Caruntu, A.; Savulescu-Fiedler, I.; Sima, R.M.; et al. Capsaicin: Emerging Pharmacological and Therapeutic Insights. Curr. Issues Mol. Biol. 2024, 46, 7895–7943. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, A.T.; Troumpata, L.; Periferakis, K.; Georgatos-Garcia, S.; Touriki, G.; Dragosloveanu, C.D.M.; Caruntu, A.; Savulescu-Fiedler, I.; Dragosloveanu, S.; et al. Pinosylvin: A Multifunctional Stilbenoid with Antimicrobial, Antioxidant, and Anti-Inflammatory Potential. Curr. Issues Mol. Biol. 2025, 47, 204. [Google Scholar] [CrossRef]
- Hu, L.F.; Lan, H.R.; Li, X.M.; Jin, K.T. A Systematic Review of the Potential Chemoprotective Effects of Resveratrol on Doxorubicin-Induced Cardiotoxicity: Focus on the Antioxidant, Antiapoptotic, and Anti-Inflammatory Activities. Oxidative Med. Cell. Longev. 2021, 2021, 2951697. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Li, S.; Zha, Z.; Chen, Y.; Wang, Q.; Zhou, S.; Huang, X.; Xu, M. Capsaicin alleviates doxorubicin-induced acute myocardial injury by regulating iron homeostasis and PI3K-Akt signaling pathway. Aging 2023, 15, 11845–11859. [Google Scholar] [CrossRef] [PubMed]
- Katona, M.; Boros, K.; Sántha, P.; Ferdinandy, P.; Dux, M.; Jancsó, G. Selective sensory denervation by capsaicin aggravates adriamycin-induced cardiomyopathy in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 370, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef]
- Shackebaei, D.; Yari, K.; Rahimi, N.; Gorgani, S.; Yarmohammadi, F. Targeting the NLRP3 by Natural Compounds: Therapeutic Strategies to Mitigate Doxorubicin-Induced Cardiotoxicity. Cell Biochem. Biophys. 2025, 83, 1–13. [Google Scholar] [CrossRef]
- Shishtar, E.; Jovanovski, E.; Jenkins, A.; Vuksan, V. Effects of Korean White Ginseng (Panax Ginseng C.A. Meyer) on Vascular and Glycemic Health in Type 2 Diabetes: Results of a Randomized, Double Blind, Placebo-controlled, Multiple-crossover, Acute Dose Escalation Trial. Clin. Nutr. Res. 2014, 3, 89–97. [Google Scholar] [CrossRef]
- Stavro, P.M.; Woo, M.; Heim, T.F.; Leiter, L.A.; Vuksan, V. North American ginseng exerts a neutral effect on blood pressure in individuals with hypertension. Hypertension 2005, 46, 406–411. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Wen, A.; Yang, J.; Yan, Y.; Song, Y.; Liu, X.; Ren, H.; Wu, Y.; Li, Z.; et al. Ginsenoside-Rd improves outcome of acute ischaemic stroke—A randomized, double-blind, placebo-controlled, multicenter trial. Eur. J. Neurol. 2012, 19, 855–863. [Google Scholar] [CrossRef]
- Xia, Z.Y.; Liu, X.Y.; Zhan, L.Y.; He, Y.H.; Luo, T.; Xia, Z. Ginsenosides compound (shen-fu) attenuates gastrointestinal injury and inhibits inflammatory response after cardiopulmonary bypass in patients with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2005, 130, 258–264. [Google Scholar] [CrossRef]
- Chung, T.H.; Kim, J.H.; Seol, S.Y.; Kim, Y.J.; Lee, Y.J. The Effects of Korean Red Ginseng on Biological Aging and Antioxidant Capacity in Postmenopausal Women: A Double-Blind Randomized Controlled Study. Nutrients 2021, 13, 3090. [Google Scholar] [CrossRef]
- Zeng, D.; Wang, J.; Kong, P.; Chang, C.; Li, J.; Li, J. Ginsenoside Rg3 inhibits HIF-1α and VEGF expression in patient with acute leukemia via inhibiting the activation of PI3K/Akt and ERK1/2 pathways. Int. J. Clin. Exp. Pathol. 2014, 7, 2172–2178. [Google Scholar] [PubMed]
- Xu, X.; Lu, Q.; Wu, J.; Li, Y.; Sun, J. Impact of extended ginsenoside Rb1 on early chronic kidney disease: A randomized, placebo-controlled study. Inflammopharmacology 2017, 25, 33–40. [Google Scholar] [CrossRef]
- Zhang, R.; Liao, Y.; Gao, Y.; Tian, H.; Wu, S.; Zeng, Q.; He, Q.; Zhang, R.; Wei, C.; Liu, J. Evaluation of the Efficacy, Safety, and Clinical Outcomes of Ginsenosides as Adjuvant Therapy in Hepatocellular Carcinoma: A Meta-Analysis and Systematic Review. Integr. Cancer Ther. 2024, 23, 15347354241293790. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, Z.; Su, H.; Xu, P.; Qi, H.; Zhao, D.; Li, X. Panax ginseng clinical trials: Current status and future perspectives. Biomed. Pharmacother. 2020, 132, 110832. [Google Scholar] [CrossRef]
- Hamidian, M.; Foroughinia, F.; Haghighat, S.; Attar, A.; Haem, E. Protective effects of Panax ginseng against doxorubicin-induced cardiac toxicity in patients with non-metastatic breast cancer: A randomized, double-blind, placebo-controlled clinical trial. J. Oncol. Pharm. Pract. 2023, 29, 1306–1316. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Yu, L.; Mo, T.; Wu, Q.; Zhou, Z. Aidi injection plus platinum-based chemotherapy for stage IIIB/IV non-small cell lung cancer: A meta-analysis of 42 RCTs following the PRISMA guidelines. J. Ethnopharmacol. 2018, 221, 137–150. [Google Scholar] [CrossRef]
- Wang, C.Q.; Zheng, X.T.; Chen, X.F.; Jiang, H.; Huang, J.; Jiang, Y.; Hu, S.S.; Huang, X.R.; Liu, S.Y.; Gong, Q.H.; et al. The Optimal Adjuvant Strategy of Aidi Injection with Gemcitabine and Cisplatin in Advanced Non-small Cell Lung Cancer: A Meta-analysis of 70 Randomized Controlled Trials. Front. Pharmacol. 2021, 12, 582447. [Google Scholar] [CrossRef]
- Xiao, Z.; Jiang, Y.; Wang, C.Q.; Hu, S.S.; Huang, X.R.; Chen, X.F.; Huang, J.; Shan, L.J.; Tang, Y.H.; Wang, Y.H.; et al. Clinical efficacy and safety of aidi injection combination with vinorelbine and cisplatin for advanced non-small-cell lung carcinoma: A systematic review and meta-analysis of 54 randomized controlled trials. Pharmacol. Res. 2020, 153, 104637. [Google Scholar] [CrossRef]
- Peng, Z.; Wu, W.W.; Yi, P. The Efficacy of Ginsenoside Rg3 Combined with First-line Chemotherapy in the Treatment of Advanced Non-Small Cell Lung Cancer in China: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Pharmacol. 2020, 11, 630825. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, T.; Cao, H.; Sun, H.; Liu, G. Ginsenoside Rg3 for Chemotherapy-Induced Myelosuppression: A Meta-Analysis and Systematic Review. Front. Pharmacol. 2020, 11, 649. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Cheng, Y.; Chen, Q.; Tan, H.; Son, D.; Chang, D.; Bian, Z.; Fang, H.; Xu, H. Safety and antifatigue effect of Korean Red Ginseng: A randomized, double-blind, and placebo-controlled clinical trial. J. Ginseng Res. 2019, 43, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Song, S.W.; Kim, H.N.; Shim, J.Y.; Yoo, B.Y.; Kim, D.H.; Lee, S.H.; Park, J.S.; Kim, M.J.; Yoo, J.H.; Cho, B.; et al. Safety and tolerability of Korean Red Ginseng in healthy adults: A multicenter, double-blind, randomized, placebo-controlled trial. J. Ginseng Res. 2018, 42, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.H.; Yoo, S.R.; Kim, H.G.; Cho, J.H.; Son, C.G. Safety and tolerability of Panax ginseng root extract: A randomized, placebo-controlled, clinical trial in healthy Korean volunteers. J. Altern. Complement. Med. 2012, 18, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Bilgi, N.; Bell, K.; Ananthakrishnan, A.N.; Atallah, E. Imatinib and Panax ginseng: A potential interaction resulting in liver toxicity. Ann. Pharmacother. 2010, 44, 926–928. [Google Scholar] [CrossRef]
- Abebe, W. Herbal medication: Potential for adverse interactions with analgesic drugs. J. Clin. Pharm. Ther. 2002, 27, 391–401. [Google Scholar] [CrossRef]
- Yu, S.E.; Mwesige, B.; Yi, Y.S.; Yoo, B.C. Ginsenosides: The need to move forward from bench to clinical trials. J. Ginseng Res. 2019, 43, 361–367. [Google Scholar] [CrossRef]
- Hu, Q.R.; Hong, H.; Zhang, Z.H.; Feng, H.; Luo, T.; Li, J.; Deng, Z.Y.; Chen, F. Methods on improvements of the poor oral bioavailability of ginsenosides: Pre-processing, structural modification, drug combination, and micro- or nano- delivery system. J. Ginseng Res. 2023, 47, 694–705. [Google Scholar] [CrossRef]
- Yuan, J.; Guo, W.; Yang, B.; Liu, P.; Wang, Q.; Yuan, H. 116 cases of coronary angina pectoris treated with powder composed of radix ginseng, radix notoginseng and succinum. J. Tradit. Chin. Med. Chung I Tsa Chih Ying Wen Pan 1997, 17, 14–17. [Google Scholar]
- Ahn, C.M.; Hong, S.J.; Choi, S.C.; Park, J.H.; Kim, J.S.; Lim, D.S. Red ginseng extract improves coronary flow reserve and increases absolute numbers of various circulating angiogenic cells in patients with first ST-segment elevation acute myocardial infarction. Phytother. Res. 2011, 25, 239–249. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, K.S. Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacol. Res. 2003, 48, 511–513. [Google Scholar] [CrossRef]
- Jovanovski, E.; Peeva, V.; Sievenpiper, J.L.; Jenkins, A.L.; Desouza, L.; Rahelic, D.; Sung, M.K.; Vuksan, V. Modulation of endothelial function by Korean red ginseng (Panax ginseng C.A. Meyer) and its components in healthy individuals: A randomized controlled trial. Cardiovasc. Ther. 2014, 32, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Jovanovski, E.; Bateman, E.A.; Bhardwaj, J.; Fairgrieve, C.; Mucalo, I.; Jenkins, A.L.; Vuksan, V. Effect of Rg3-enriched Korean red ginseng (Panax ginseng) on arterial stiffness and blood pressure in healthy individuals: A randomized controlled trial. J. Am. Soc. Hypertens. 2014, 8, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Xian, S.; Yang, Z.; Lee, J.; Jiang, Z.; Ye, X.; Luo, L.; Jin, L.; Yang, T.; Ye, S.; Lu, D. A randomized, double-blind, multicenter, placebo-controlled clinical study on the efficacy and safety of Shenmai injection in patients with chronic heart failure. J. Ethnopharmacol. 2016, 186, 136–142. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, H.; Wang, J.; Li, X.M. Effects of Shenmai injection on the values of CO, SV, and EF in patients undergoing off-pump coronary artery bypass graft: A randomized, clinical trial. Medicine 2018, 97, e0085. [Google Scholar] [CrossRef]
- Geng, Q.X.; Zhu, X.L.; Zhang, X.H. Effect of combined therapy of shenmai and compound danshen injection on myocardial reperfusion injury after percutaneous coronary intervention in patients with acute myocardial infarction. Chin. J. Integr. Tradit. West. Med. 2004, 24, 496–499. [Google Scholar]
- Ma, R.G.; Wang, C.X.; Shen, Y.H.; Wang, Z.Q.; Ma, J.H.; Huang, L.S. Effect of Shenmai Injection on ventricular diastolic function in patients with chronic heart failure: An assessment by tissue Doppler imaging. Chin. J. Integr. Med. 2010, 16, 173–175. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, W.; Xie, Y.M.; Wang, L.X.; Nie, X.L.; Zhang, Y.L. Meta-analysis of Shenmai injection treatment for acute myocardial infarction. China J. Chin. Mater. Med. 2012, 37, 2760–2767. [Google Scholar]
- Zheng, C.D.; Min, S. Cardioprotection of Shenfu Injection against myocardial ischemia/reperfusion injury in open heart surgery. Chin. J. Integr. Med. 2008, 14, 10–16. [Google Scholar] [CrossRef]
- Guo, M.; Wang, P.; Du, J.; Fu, C.; Yang, Q.; Gao, Z.; Zhu, M.; Lv, S.; Deng, Y.; Li, T.; et al. Xinyue Capsule in patients with stable coronary artery disease after percutaneous coronary intervention: A multicenter, randomized, placebo-controlled trial. Pharmacol. Res. 2020, 158, 104883. [Google Scholar] [CrossRef]
- Hyun, S.H.; Bhilare, K.D.; In, G.; Park, C.K.; Kim, J.H. Effects of Panax ginseng and ginsenosides on oxidative stress and cardiovascular diseases: Pharmacological and therapeutic roles. J. Ginseng Res. 2022, 46, 33–38. [Google Scholar] [CrossRef]
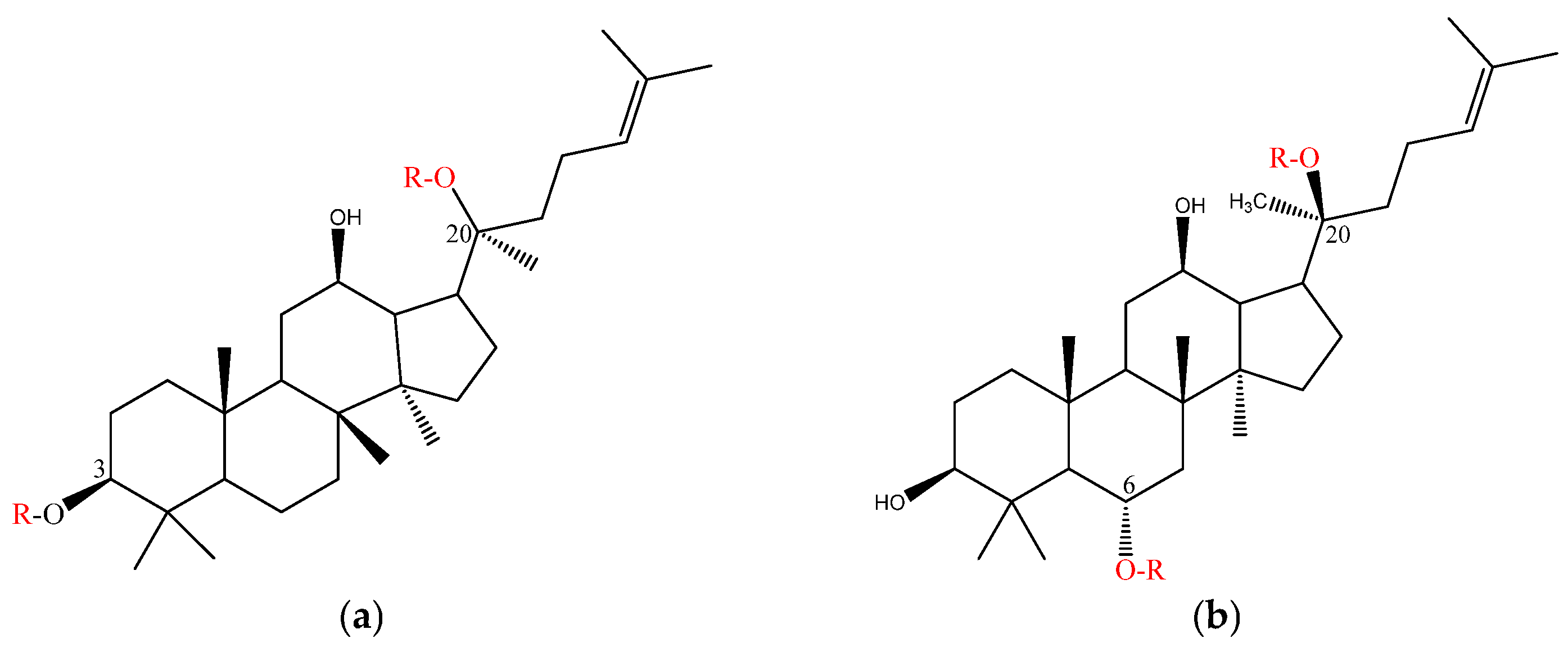

| Class | -OR of Points | Representative Ginsenosides | |
|---|---|---|---|
| Dammarane type | Protopanaxadiol (PPD) | C3; C20 | C-K, Rd, Rg3, Rb, Ra1, Ra2, Ra3, Rb, Rc, Rd, Rg3, Rh1, Rh2, Rh3, Rh4, F2 |
| Protopanaxatriol (PPT) | C6; C20 | Re, Rf, F1, F3, F4, F5, Rg1, Rg2, Rh1, notoginsenoside R1 | |
| Ocotillol type | C24; C20 | ginsenoside R2, notoginsenoside R1, pseudoginsenoside F11 Makonoside-Rs | |
| Oleanolic acid type | Ro, Ri | ||
| Animal Model | Treatment Protocol | Autophagy Marker Change | Effects of Autophagy Targeting | Reference |
|---|---|---|---|---|
| Male C57BL/6 mice. 8–10 weeks old. DOX 20 mg/kg i.p. weekly. | Gavage administration of Rb1 (40 mg/kg/day). | LC3-I, p62 ↓. | Inhibiting autophagy | [116,122] |
| H/R treatment, H9c2 cardiomyocytes | Rg1 (100 μmol/L), 24 h. | LC3-II, Beclin-1, p62 ↓, inhibiting AMPK pathway. | Inhibiting autophagy | [123] |
| Male rats weighing 280–320 g. Balloon-injury. | Gavage administration of Re 12.5/25 mg/kg, 2 weeks. | ERK1/2, LC3-I, p62 ↓ | Inhibiting autophagy | [124] |
| Mouse primary peritoneal macrophages. ox-LDL (100 μg/mL)–24 h. | Ox-LDL and 10/20/40/80 μM Rb1(24 h). | AMPK, LC3-II ↑; SQSTM1/p62 degradation. | Rb1 rescues autophagy flux, inducing autophagy. | [125] |
| ICR male mice. Coronary artery ligation (CAL). | Rb1 6 mg/kg i.p. after 20 min of CAL. | PINK1,Parkin,LC3-II/LC3-I ↑, p62 ↓ to increase degradation. | Rb1 exerts cardioprotective functions through activation of mitophagy via AMPKα pathway. | [126] |
| C57BL/6 male mice (9–11 weeks old). Left anterior descending coronary artery (LAD). 28 days. | Gavage administration of Rg1 (20 mg/kg). | LC3-II ↑, p62 ↓ GRg1 significantly increases SIRT1 expression and activates the PINK1/Parkin signaling pathway. | Rg1 enhances mitochondrial autophagy and alleviates HF. | [127] |
| Male Sprague Dawley (SD) rats. The left anterior descending branch-ligated HF rat model. OGD/R H9c2 cell model. | Gavage administration of Rb2 (10 mg/kg, 20 mg/kg, daily for 3 days) | miR-216a-5p ↓, LC3B II/I, Beclin1 ↑. | Rb2 promotes autophagy treatment for HF. | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, R.; Zhao, Z.; Han, X.; Shang, M.; Liu, G.; Xu, F.; Cai, S. Therapeutic Potential of Ginsenosides in Anthracycline-Induced Cardiotoxicity. Molecules 2025, 30, 2527. https://doi.org/10.3390/molecules30122527
Bai R, Zhao Z, Han X, Shang M, Liu G, Xu F, Cai S. Therapeutic Potential of Ginsenosides in Anthracycline-Induced Cardiotoxicity. Molecules. 2025; 30(12):2527. https://doi.org/10.3390/molecules30122527
Chicago/Turabian StyleBai, Rongrong, Zhigao Zhao, Xing Han, Mingying Shang, Guangxue Liu, Feng Xu, and Shaoqing Cai. 2025. "Therapeutic Potential of Ginsenosides in Anthracycline-Induced Cardiotoxicity" Molecules 30, no. 12: 2527. https://doi.org/10.3390/molecules30122527
APA StyleBai, R., Zhao, Z., Han, X., Shang, M., Liu, G., Xu, F., & Cai, S. (2025). Therapeutic Potential of Ginsenosides in Anthracycline-Induced Cardiotoxicity. Molecules, 30(12), 2527. https://doi.org/10.3390/molecules30122527






