A Review: Using Ionic Liquids for Lignin Extraction from Lignocellulose and High-Value Utilization
Abstract
1. Introduction
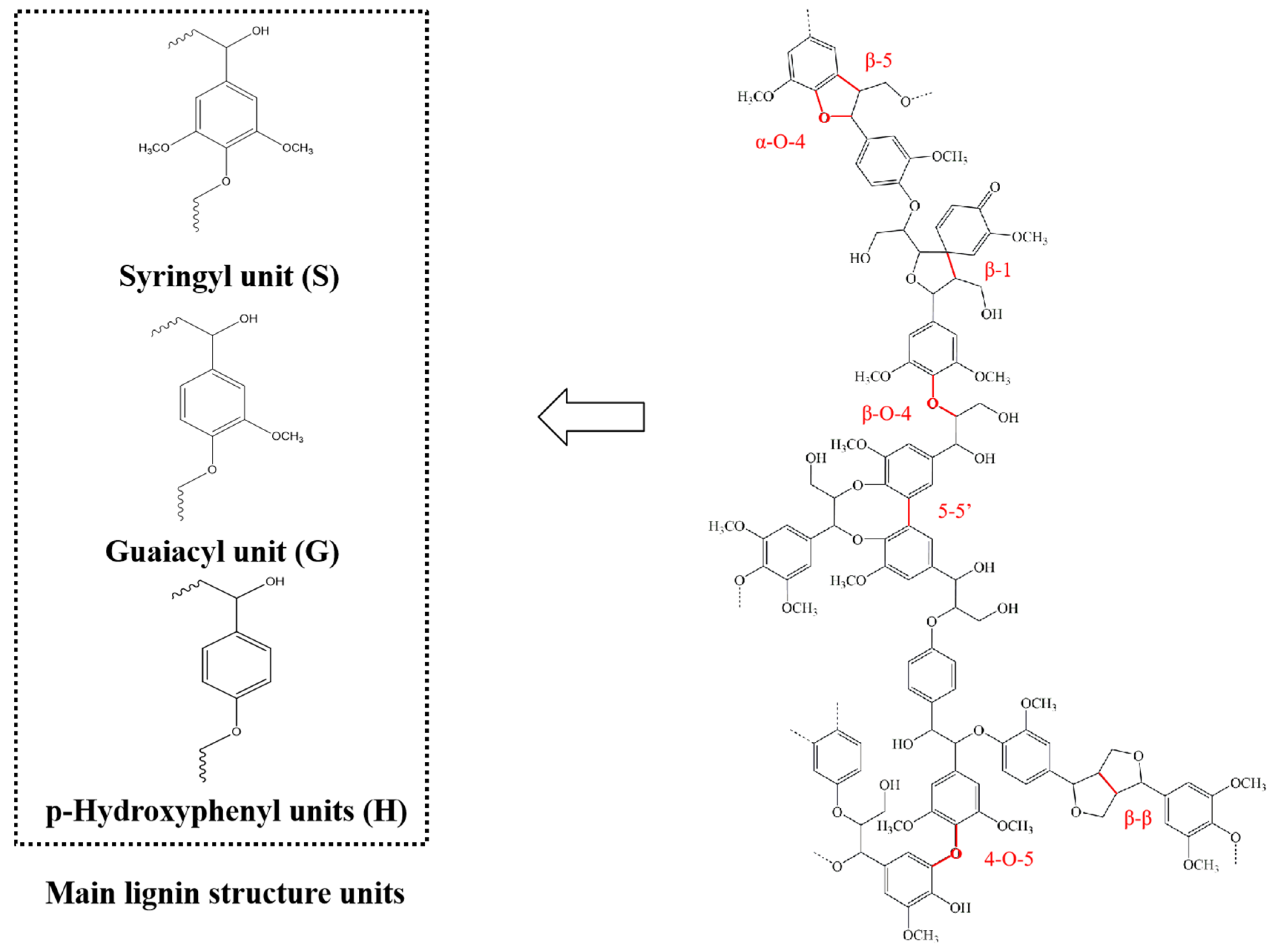
2. Structure of Lignin
3. Extraction of Lignin from Lignocellulose by Ionic Liquids
3.1. Technology of Lignin Extraction
3.2. Process of Lignin Extraction
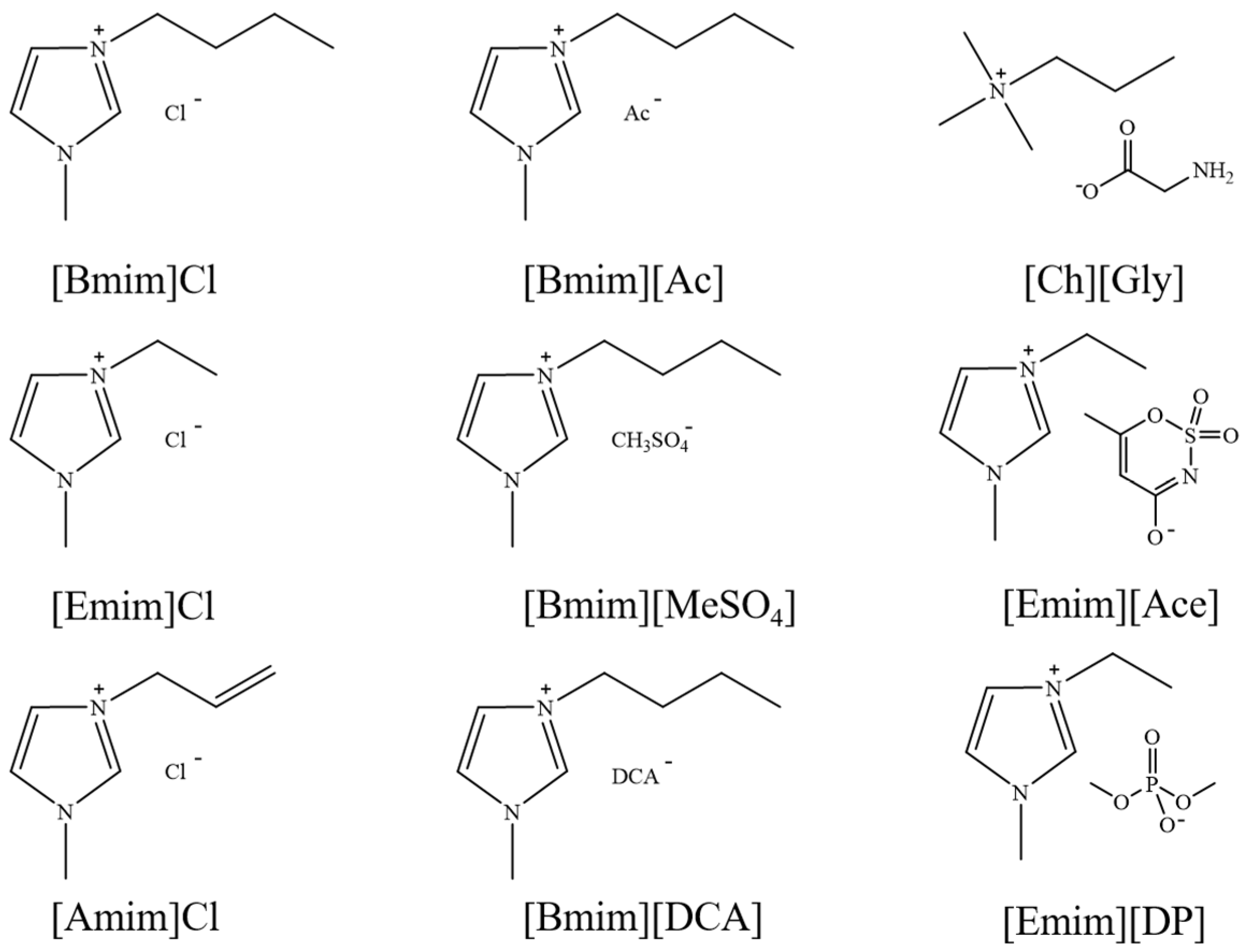
3.3. Aprotic Ionic Liquids (AILs)
- (1)
- Common AILs for lignin extraction
- (2)
- AILs-solvents system
- (3)
- AILs recycle
- (4)
- AILs binary composite system
- (5)
- Summary of this section
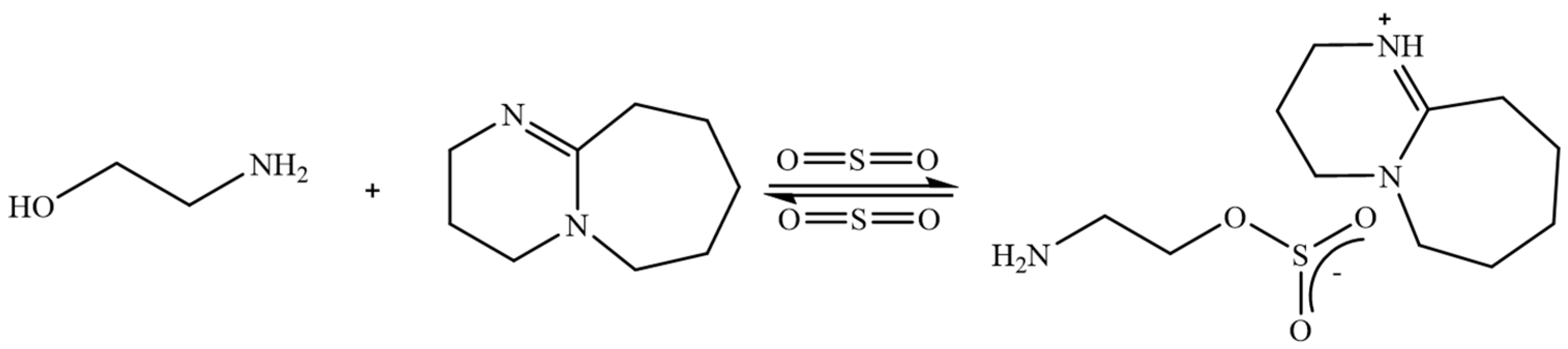
3.4. Protic Ionic Liquids (PILs)
3.5. Combination Process
4. The Mechanism of Lignin Extraction by Ionic Liquid
4.1. Dissolution of Lignin in Ionic Liquids
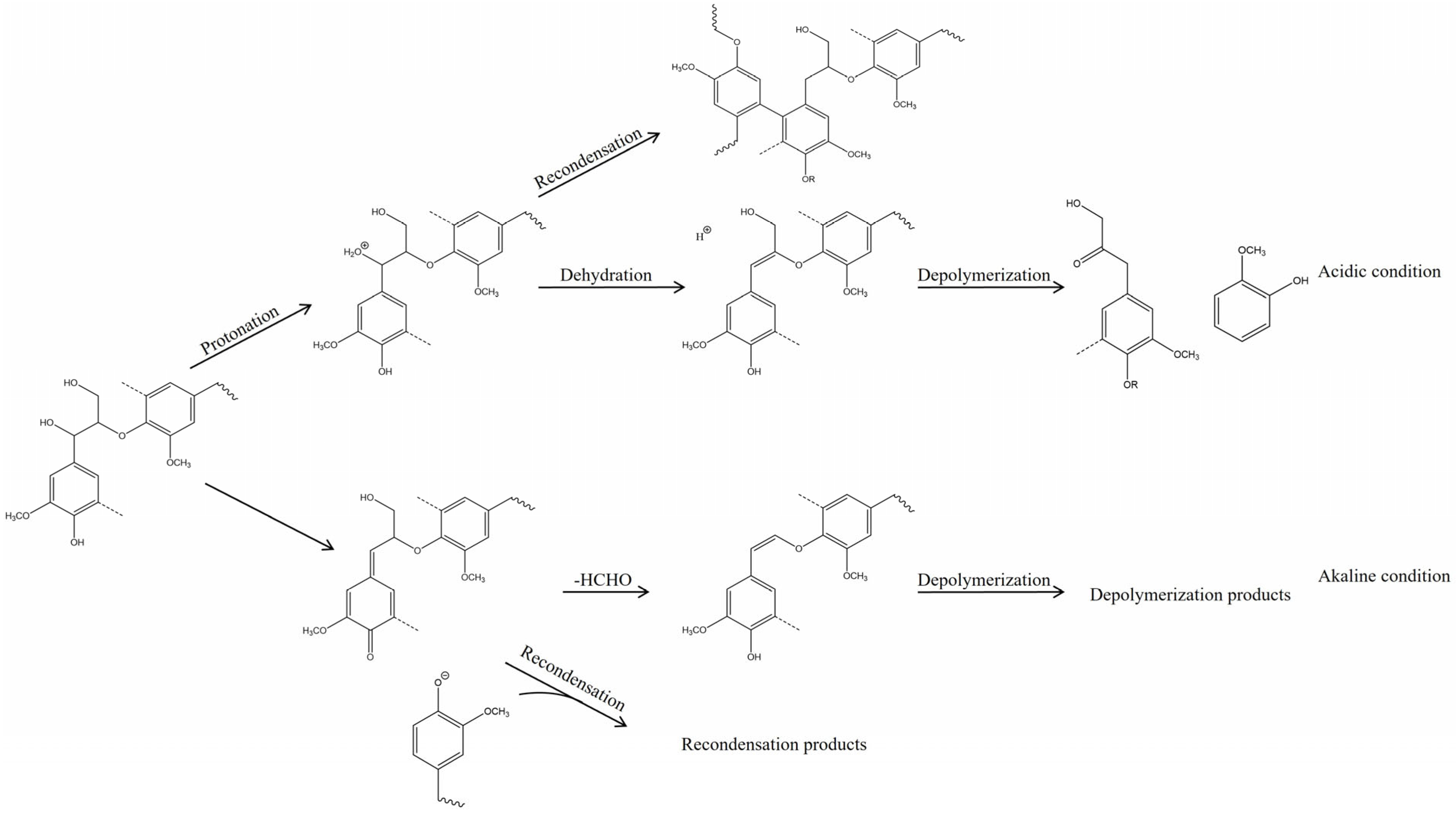
4.2. The Depolymerization Mechanism of Lignin in Ionic Liquids
5. Computational Simulation Method for Screening Ionic Liquids
5.1. Screening ILs by Computational Simulation Method
5.2. Computational Simulation Method in Lignin Valorization
5.3. Challenges
6. The Application of Ionic Liquids in the Value-Added Utilization of Lignin
6.1. Lignin Degradation
6.2. Lignin Modification
- (1)
- Lignin functionalization
- (2)
- Lignin derived materials
7. Conclusions and Future Directions
- (1)
- Ionic liquids have shown great potential in the separation of lignin from lignocellulose. The selective extraction of lignin can be achieved by adjusting the structure of ionic liquid. The lignin-first strategy has become a key focus in the field of biorefining. Current studies place greater emphasis on preserving lignin’s native structure while utilizing cellulose. Adjusting IL structures or incorporating auxiliary agents may help achieve this goal. Future studies should focus on developing IL-based systems in this direction to enable the efficient utilization of lignocellulosic biomass.
- (2)
- Investigating the lignin extraction mechanism by ILs helps us understand IL–lignin interactions and develop more efficient lignin extraction systems. However, the structural complexity and diversity of lignin lead to variations in its degradation mechanisms, and the changes in lignin structure and molecular weight during IL-mediated dissolution still lack detailed and systematic studies. Computational simulations have enhanced our understanding of the mechanisms underlying lignin dissolution and extraction, enabling faster and more effective screening of ILs capable of dissolving lignin. Current computational studies often employ simple lignin models, neglecting the structural complexity of lignocellulose and its impact on simulations. Additionally, few studies have explored simulations of IL extraction systems that preserve lignin’s structural integrity. Future research should refine computational models and expand the application scope of simulation tools.
- (3)
- Compared with organic solvents, the industrial application of ILs is hindered by their higher viscosity and elevated costs. After pretreatment, IL recovery is necessary to reduce cost. Current IL recycling typically involves adding antisolvents to separate ILs from lignocellulose components, but this process incurs non-negligible energy consumption for distillation. Additionally, the relatively high viscosity of ILs limits pretreatment efficiency. In future research, computational simulations could be leveraged to guide the development of IL systems with lower cost, reduced toxicity, and improved viscosity, thereby enhancing their industrial feasibility.
- (4)
- Meanwhile, current recycling processes have problems such as inefficient lignin recovery and low lignin purity. In future studies, we should adopt the lignin-first strategy to optimize ILs pretreatment procedures. Lignin fraction should be implemented to maximize its utilization from lignocellulose, thereby enhancing the overall economic value of lignocellulose.
- (5)
- In addition, ionic liquids also show good application value in lignin value-added utilization. The structural tunability of ionic liquids can meet the specific needs of lignin value-added. However, most of the applications are still in the laboratory and fail to carry out large-scale industrial production. In the future, we should further expand its application scope and promote the transformation of lignin from “industrial waste” to “renewable resources” to achieve greater results.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Zheng, X.; Lin, X.; Liu, X.; Han, D.; Zhang, Q. Total component transformation of corn stalk to ethyl levulinate assisted by ionic liquid pretreatment. Cellulose 2024, 31, 3533–3543. [Google Scholar] [CrossRef]
- Zhang, K.; Jiang, Z.; Li, X.; Wang, D.; Hong, J. Enhancing simultaneous saccharification and co-fermentation of corncob by Kluyveromyces marxianus through overexpression of putative transcription regulator. Bioresour. Technol. 2024, 399, 130627. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Li, R.; Xu, P.; Li, T.; Deng, R.; Chen, S.; Zhang, Q. The cornerstone of realizing lignin value-addition: Exploiting the native structure and properties of lignin by extraction methods. Chem. Eng. J. 2020, 402, 126237. [Google Scholar] [CrossRef]
- Zubeltzu, J.; Formoso, E.; Rezabal, E. Lignin solvation by ionic liquids: The role of cation. J. Mol. Liq. 2020, 303, 112588. [Google Scholar] [CrossRef]
- Asim, A.M.; Uroos, M.; Muhammad, N. Extraction of lignin and quantitative sugar release from biomass using efficient and cost-effective pyridinium protic ionic liquids. RSC Adv. 2020, 10, 44003–44014. [Google Scholar] [CrossRef]
- Halder, P.; Kundu, S.; Patel, S.; Setiawan, A.; Atkin, R.; Parthasarthy, R.; Paz-Ferreiro, J.; Surapaneni, A.; Shah, K. Progress on the pre-treatment of lignocellulosic biomass employing ionic liquids. Renew. Sustain. Energy Rev. 2019, 105, 268–292. [Google Scholar] [CrossRef]
- Abu-Omar, M.M.; Barta, K.; Beckham, G.T.; Luterbacher, J.S.; Ralph, J.; Rinaldi, R.; Román-Leshkov, Y.; Samec, J.S.M.; Sels, B.F.; Wang, F. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 2021, 14, 262–292. [Google Scholar] [CrossRef]
- Melro, E.; Alves, L.; Antunes, F.E.; Medronho, B. A brief overview on lignin dissolution. J. Mol. Liq. 2018, 265, 578–584. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef]
- Vásquez-Garay, F.; Carrillo-Varela, I.; Vidal, C.; Reyes-Contreras, P.; Faccini, M.; Teixeira Mendonça, R. A Review on the Lignin Biopolymer and Its Integration in the Elaboration of Sustainable Materials. Sustainability 2021, 13, 2697. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Goswami, S.; Banerjee, D.; Garcia, V.; Zhou, E.; Olmsted, C.N.; Majumder, E.L.W.; Kumar, D.; Awasthi, D.; Mukhopadhyay, A.; et al. Perspective on Lignin Conversion Strategies That Enable Next Generation Biorefineries. ChemSusChem 2024, 17, e202301460. [Google Scholar] [CrossRef] [PubMed]
- Sitotaw, Y.W.; Habtu, N.G.; Gebreyohannes, A.Y.; Nunes, S.P.; Van Gerven, T. Ball milling as an important pretreatment technique in lignocellulose biorefineries: A review. Biomass Convers. Biorefin. 2023, 13, 15593–15616. [Google Scholar] [CrossRef]
- Konan, D.; Koffi, E.; Ndao, A.; Peterson, E.C.; Rodrigue, D.; Adjallé, K. An Overview of Extrusion as a Pretreatment Method of Lignocellulosic Biomass. Energies 2022, 15, 3002. [Google Scholar] [CrossRef]
- Sun, S.-F.; Yang, H.-Y.; Yang, J.; Shi, Z.-J. Structural characterization of poplar lignin based on the microwave-assisted hydrothermal pretreatment. Int. J. Biol. Macromol. 2021, 190, 360–367. [Google Scholar] [CrossRef]
- Mohammadabadi, S.I.; Javanbakht, V. Lignin extraction from barley straw using ultrasound-assisted treatment method for a lignin-based biocomposite preparation with remarkable adsorption capacity for heavy metal. Int. J. Biol. Macromol. 2020, 164, 1133–1148. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Ewulonu, C.M.; Ralph, J.; Xu, F.; Zhang, Q.; Wu, M.; Huang, Y. Mild Alkaline Pretreatment for Isolation of Native-Like Lignin and Lignin-Containing Cellulose Nanofibers (LCNF) from Crop Waste. ACS Sustain. Chem. Eng. 2019, 7, 14135–14142. [Google Scholar] [CrossRef]
- Chin, D.W.K.; Lim, S.; Pang, Y.L.; Lim, C.H.; Shuit, S.H.; Lee, K.M.; Chong, C.T. Effects of Organic Solvents on the Organosolv Pretreatment of Degraded Empty Fruit Bunch for Fractionation and Lignin Removal. Sustainability 2021, 13, 6757. [Google Scholar] [CrossRef]
- Hidayati, S.; Satyajaya, W.; Fudholi, A. Lignin isolation from black liquor from oil palm empty fruit bunch using acid. J. Mater. Res. Technol. 2020, 9, 11382–11391. [Google Scholar] [CrossRef]
- Hasanov, I.; Shanmugam, S.; Kikas, T. Extraction and isolation of lignin from ash tree (Fraxinus exselsior) with protic ionic liquids (PILs). Chemosphere 2022, 290, 133297. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.; González, A.; Ballesteros, I.; Negro, M.J. Production of xylooligosaccharides, bioethanol, and lignin from structural components of barley straw pretreated with a steam explosion. Bioresour. Technol. 2021, 342, 125953. [Google Scholar] [CrossRef]
- Dharmaraja, J.; Shobana, S.; Arvindnarayan, S.; Francis, R.R.; Jeyakumar, R.B.; Saratale, R.G.; Ashokkumar, V.; Bhatia, S.K.; Kumar, V.; Kumar, G. Lignocellulosic biomass conversion via greener pretreatment methods towards biorefinery applications. Bioresour. Technol. 2023, 369, 128328. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Lai, C.; Hua, Q.; Wang, P.; Huang, C.; Ling, Z.; Yong, Q. Lignin-associated factors impede enzymatic hydrolysis of hydrothermally pretreated birch and poplar wood. Ind. Crops Prod. 2025, 226, 120704. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Lei, F.; Jiang, J. Co-production bioethanol and xylooligosaccharides from sugarcane bagasse via autohydrolysis pretreatment. Renew. Energy 2020, 162, 2297–2305. [Google Scholar] [CrossRef]
- Hu, M.; Yuan, L.; Cai, Z.; Zhang, W.; Fu, Q.; Ji, D. Ammonia fiber expansion-assisted deep eutectic solvent treatment for wheat straw fraction separation and bioconversion. Bioresour. Technol. 2023, 367, 128242. [Google Scholar] [CrossRef]
- You, T.; Li, X.; Wang, R.; Zhang, X.; Xu, F. Effects of synergistic fungal pretreatment on structure and thermal properties of lignin from corncob. Bioresour. Technol. 2019, 272, 123–129. [Google Scholar] [CrossRef]
- Zhang, M.; Jia, L.; Li, M.; Yoo, C.G.; Peng, H.; Arvelli, S.; Zhao, J. Challenges and Perspectives in Lignin-Derived Polyurethane Foam Synthesis. Adv. Sustain. Syst. 2025, 9, 2401054. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: Mechanistic insight and advancements. Chemosphere 2021, 264, 128523. [Google Scholar] [CrossRef]
- Hussain, S.M.S.; Adewunmi, A.A.; Alade, O.S.; Murtaza, M.; Mahboob, A.; Khan, H.J.; Mahmoud, M.; Kamal, M.S. A review of ionic liquids: Recent synthetic advances and oilfield applications. J. Taiwan Inst. Chem. Eng. 2023, 153, 105195. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Jiang, N.; Ragauskas, A.J. Ionic Liquid as a Green Solvent for Lignin. J. Wood Chem. Technol. 2007, 27, 23–33. [Google Scholar] [CrossRef]
- Glas, D.; Van Doorslaer, C.; Depuydt, D.; Liebner, F.; Rosenau, T.; Binnemans, K.; De Vos, D.E. Lignin solubility in non-imidazolium ionic liquids. J. Chem. Technol. Biotechnol. 2015, 90, 1821–1826. [Google Scholar] [CrossRef]
- Sun, N.; Rahman, M.; Qin, Y.; Maxim, M.L.; Rodríguez, H.; Rogers, R.D. Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem. 2009, 11, 646–655. [Google Scholar] [CrossRef]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; da Costa Lopes, A.M.; Łukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Q.; He, F.; Yue, W.; Hu, G.; Gan, J.; Xie, H. Pretreatment of Corn Stover by Levulinic Acid-Based Protic Ionic Liquids for Enhanced Enzymatic Hydrolysis. ACS Sustain. Chem. Eng. 2022, 10, 7134–7148. [Google Scholar] [CrossRef]
- Buarque, F.S.; de Souza, C.E.C.; Ferreira, R.M.; Sabino, T.O.; Teixeira, O.M.J.; Bandeira, L.F.M.; Fraga, A.C.; Coelho, M.A.Z.; Ribeiro, B.D. Dissolution and enzymatic hydrolysis of sugarcane bagasse using ionic liquids and deep eutectic solvents. Process Biochem. 2024, 147, 257–267. [Google Scholar] [CrossRef]
- Strehmel, V.; Strunk, D.; Wetzel, H.; Strehmel, N. Investigation of lignin obtained by processing of Betula pendula with ionic liquids. Sustain. Chem. Pharm. 2017, 6, 107–113. [Google Scholar] [CrossRef]
- Muhammad, N.; Man, Z.; Bustam, M.A.; Mutalib, M.I.A.; Rafiq, S. Investigations of novel nitrile-based ionic liquids as pre-treatment solvent for extraction of lignin from bamboo biomass. J. Ind. Eng. Chem. 2013, 19, 207–214. [Google Scholar] [CrossRef]
- Wang, F.-L.; Li, S.; Sun, Y.-X.; Han, H.-Y.; Zhang, B.-X.; Hu, B.-Z.; Gao, Y.-F.; Hu, X.-M. Ionic liquids as efficient pretreatment solvents for lignocellulosic biomass. RSC Adv. 2017, 7, 47990–47998. [Google Scholar] [CrossRef]
- Ma, H.-H.; Zhang, B.-X.; Zhang, P.; Li, S.; Gao, Y.-F.; Hu, X.-M. An efficient process for lignin extraction and enzymatic hydrolysis of corn stalk by pyrrolidonium ionic liquids. Fuel Process. Technol. 2016, 148, 138–145. [Google Scholar] [CrossRef]
- Anugwom, I.; Eta, V.; Virtanen, P.; Mäki-Arvela, P.; Hedenström, M.; Hummel, M.; Sixta, H.; Mikkola, J.-P. Switchable Ionic Liquids as Delignification Solvents for Lignocellulosic Materials. ChemSusChem 2014, 7, 1170–1176. [Google Scholar] [CrossRef]
- Khokarale, S.G.; Le-That, T.; Mikkola, J.-P. Carbohydrate Free Lignin: A Dissolution–Recovery Cycle of Sodium Lignosulfonate in a Switchable Ionic Liquid System. ACS Sustain. Chem. Eng. 2016, 4, 7032–7040. [Google Scholar] [CrossRef]
- Anugwom, I.; Mäki-Arvela, P.; Virtanen, P.; Willför, S.; Damlin, P.; Hedenström, M.; Mikkola, J.-P. Treating birch wood with a switchable 1,8-diazabicyclo-[5.4.0]-undec-7-ene-glycerol carbonate ionic liquid. Holzforschung 2012, 66, 809–815. [Google Scholar]
- Anugwom, I.; Rujana, L.; Wärnå, J.; Hedenström, M.; Mikkola, J.P. In quest for the optimal delignification of lignocellulosic biomass using hydrated, SO2 switched DBU MEASIL switchable ionic liquid. Chem. Eng. J. 2016, 297, 256–264. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Cheng, G. A two-stage pretreatment using dilute sodium hydroxide solution followed by an ionic liquid at low temperatures: Toward construction of lignin-first biomass pretreatment. Bioresour. Technol. Rep. 2019, 7, 100286. [Google Scholar] [CrossRef]
- Kim, J.; Pahari, S.; Ryu, J.; Zhang, M.; Yang, Q.; Yoo, C.G.; Kwon, J.S.-I. Advancing biomass fractionation with real-time prediction of lignin content and MWd: A kMC-based multiscale model for optimized lignin extraction. Chem. Eng. J. 2024, 479, 147226. [Google Scholar] [CrossRef]
- Xu, J.; Dai, L.; Gui, Y.; Yuan, L.; Zhang, C.; Lei, Y. Synergistic benefits from a lignin-first biorefinery of poplar via coupling acesulfamate ionic liquid followed by mild alkaline extraction. Bioresour. Technol. 2020, 303, 122888. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, Y.; Abdeltawab, A.A.; Yakout, S.M.; Chen, X.; Yu, G. Cholinium amino acids-glycerol mixtures: New class of solvents for pretreating wheat straw to facilitate enzymatic hydrolysis. Bioresour. Technol. 2017, 245, 625–632. [Google Scholar] [CrossRef]
- Papa, G.; Feldman, T.; Sale, K.L.; Adani, F.; Singh, S.; Simmons, B.A. Parametric study for the optimization of ionic liquid pretreatment of corn stover. Bioresour. Technol. 2017, 241, 627–637. [Google Scholar] [CrossRef]
- Kim, K.H.; Dutta, T.; Ralph, J.; Mansfield, S.D.; Simmons, B.A.; Singh, S. Impact of lignin polymer backbone esters on ionic liquid pretreatment of poplar. Biotechnol. Biofuels 2017, 10, 101. [Google Scholar] [CrossRef]
- Ren, H.; Zong, M.-H.; Wu, H.; Li, N. Efficient Pretreatment of Wheat Straw Using Novel Renewable Cholinium Ionic Liquids To Improve Enzymatic Saccharification. Ind. Eng. Chem. Res. 2016, 55, 1788–1795. [Google Scholar] [CrossRef]
- da Câmara Rocha, J.; Ribeiro, V.T.; da Costa Filho, J.D.B.; Leitão, A.L.d.S.; Cavalcante, J.D.N.; de Araújo Padilha, C.E.; dos Santos, E.S. The use of ionic liquid pretreatment aims to enhance the enzymatic hydrolysis of green coconut fiber and produce lignin. Biomass Convers. Biorefin. 2025, 15, 2495–2510. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Chai, Y.; Zheng, X.-P.; Du, Y.-P.; Zhang, Y.-C.; Zheng, Y.-Z. Pretreatment of tea stem by biocompatible ionic liquids for enhanced enzymatic hydrolysis of cellulose and formation of UV-blocking chitosan film. Food Chem. 2025, 474, 143172. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, B.; Hu, L.; Wu, Z.; Xu, N.; Dai, B.; He, J. Enzymatic in situ saccharification of lignocellulose in a compatible ionic liquid-cellulase system. Chem. Eng. J. 2015, 267, 163–169. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, L.; Gu, Z.; Hong, Y.; Li, Z.; Li, C. Effects of ionic liquid/water mixture pretreatment on the composition, the structure and the enzymatic hydrolysis of corn stalk. Ind. Crops Prod. 2018, 122, 142–147. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Li, K.; An, Q.; Ma, H.; Yang, L.; Wei, L. Water addition enhanced thermal stability of alkylimidazolium acetate in Ionosolv treatment of lignin. Int. J. Biol. Macromol. 2019, 141, 1055–1064. [Google Scholar] [CrossRef]
- Yan, B.; Li, K.; Wei, L.; Ma, Y.; Shao, G.; Zhao, D.; Wan, W.; Song, L. Understanding lignin treatment in dialkylimidazolium-based ionic liquid–water mixtures. Bioresour. Technol. 2015, 196, 509–517. [Google Scholar] [CrossRef]
- Akiba, T.; Tsurumaki, A.; Ohno, H. Induction of lignin solubility for a series of polar ionic liquids by the addition of a small amount of water. Green Chem. 2017, 19, 2260–2265. [Google Scholar] [CrossRef]
- Brandt, A.; Ray, M.J.; To, T.Q.; Leak, D.J.; Murphy, R.J.; Welton, T. Ionic liquid pretreatment of lignocellulosic biomass with ionic liquid–water mixtures. Green Chem. 2011, 13, 2489–2499. [Google Scholar] [CrossRef]
- Belesov, A.V.; Ladesov, A.V.; Pikovskoi, I.I.; Faleva, A.V.; Kosyakov, D.S. Characterization of Ionic Liquid Lignins Isolated from Spruce Wood with 1-Butyl-3-methylimidazolium Acetate and Methyl Sulfate and Their Binary Mixtures with DMSO. Molecules 2020, 25, 2479. [Google Scholar] [CrossRef]
- Jin, L.; Yu, X.; Peng, C.; Guo, Y.; Zhang, L.; Xu, Q.; Zhao, Z.K.; Liu, Y.; Xie, H. Fast dissolution pretreatment of the corn stover in gamma-valerolactone promoted by ionic liquids: Selective delignification and enhanced enzymatic saccharification. Bioresour. Technol. 2018, 270, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Kuntapa, M.; Sudaprasert, K.; Tachaapaikoon, C. Effects on delignification of reusing 1-ethyl-3-methylimidazolium acetate and dimethyl sulfoxide in the pretreatment of corn cob for the enhancement of enzymatic hydrolysis in ethanol production. Biomass Convers. Biorefin. 2023, 13, 13037–13050. [Google Scholar] [CrossRef]
- Yang, J.; Lu, X.; Zhou, Q.; Xu, J.; Xin, J.; Zhang, S. Efficient Biomass Pretreatment Process Based on the Simple Reuse of a Low-Viscosity Ionic-Liquid Solvent System. ACS Sustain. Chem. Eng. 2022, 10, 12738–12750. [Google Scholar] [CrossRef]
- Wei, H.-L.; Bu, J.; Zhou, S.-S.; Deng, M.-C.; Zhu, M.-J. A facile ionic liquid and p-toluenesulfonic acid pretreatment of herb residues: Enzymatic hydrolysis and lignin valorization. Chem. Eng. J. 2021, 419, 129616. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, Q.; Wei, L.; Sun, J.; Li, K.; Zhang, J.; Zhai, S.; An, Q. Characterization of lignin streams during ionic liquid/hydrochloric acid/formaldehyde pretreatment of corn stalk. Bioresour. Technol. 2021, 331, 125064. [Google Scholar] [CrossRef]
- Chang, K.-L.; Chen, X.-M.; Wang, X.-Q.; Han, Y.-J.; Potprommanee, L.; Liu, J.-y.; Liao, Y.-L.; Ning, X.-a.; Sun, S.-y.; Huang, Q. Impact of surfactant type for ionic liquid pretreatment on enhancing delignification of rice straw. Bioresour. Technol. 2017, 227, 388–392. [Google Scholar] [CrossRef]
- Pang, Z.; Lyu, W.; Dong, C.; Li, H.; Yang, G. High selective delignification using oxidative ionic liquid pretreatment at mild conditions for efficient enzymatic hydrolysis of lignocellulose. Bioresour. Technol. 2016, 214, 96–101. [Google Scholar] [CrossRef]
- Ziaei-Rad, Z.; Fooladi, J.; Pazouki, M.; Gummadi, S.N. Lignocellulosic biomass pre-treatment using low-cost ionic liquid for bioethanol production: An economically viable method for wheat straw fractionation. Biomass Bioenergy 2021, 151, 106140. [Google Scholar] [CrossRef]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef]
- Achinivu, E.C.; Blankenship, B.W.; Baral, N.R.; Choudhary, H.; Kakumanu, R.; Mohan, M.; Baidoo, E.E.K.; Scown, C.D.; George, A.; Simmons, B.A.; et al. Biomass pretreatment with distillable ionic liquids for an effective recycling and recovery approach. Chem. Eng. J. 2024, 479, 147824. [Google Scholar] [CrossRef]
- Hossain, M.M.; Rawal, A.; Aldous, L. Aprotic vs Protic Ionic Liquids for Lignocellulosic Biomass Pretreatment: Anion Effects, Enzymatic Hydrolysis, Solid-State NMR, Distillation, and Recycle. ACS Sustain. Chem. Eng. 2019, 7, 11928–11936. [Google Scholar] [CrossRef]
- De Gregorio, G.F.; Weber, C.C.; Gräsvik, J.; Welton, T.; Brandt, A.; Hallett, J.P. Mechanistic insights into lignin depolymerisation in acidic ionic liquids. Green Chem. 2016, 18, 5456–5465. [Google Scholar] [CrossRef]
- Lu, J.; Xu, C.; Li, J.; Wang, S.; Xiang, Q.; Chen, X.; Hua, Y.; Li, Y. Effect of nicotinic acid additives on the electrodeposition of Al-Mn alloy from AlCl3-based ionic liquids. Ionics 2022, 28, 3525–3536. [Google Scholar] [CrossRef]
- Muazzam, R.; Asim, A.M.; Uroos, M.; Muhammad, N.; Hallett, J.P. Evaluating the potential of a novel hardwood biomass using a superbase ionic liquid. RSC Adv. 2021, 11, 19095–19105. [Google Scholar] [CrossRef] [PubMed]
- Anuchi, S.O.; Campbell, K.L.S.; Hallett, J.P. Effective pretreatment of lignin-rich coconut wastes using a low-cost ionic liquid. Sci. Rep. 2022, 12, 6108. [Google Scholar] [CrossRef]
- Tolesa, L.D.; Gupta, B.S.; Lee, M.-J. Treatment of Coffee Husk with Ammonium-Based Ionic Liquids: Lignin Extraction, Degradation, and Characterization. ACS Omega 2018, 3, 10866–10876. [Google Scholar] [CrossRef]
- Lin, X.; Jiang, K.; Liu, X.; Bi, H.; Li, T.; Han, D.; Zhang, Q. Effective conversion of corn stalk into ethyl levulinate and crude lignin catalyzed by ionic liquids. Biomass Bioenergy 2023, 175, 106894. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Idris, A.; Chandrasekaram, K.; Alias, Y. Efficiency of bronsted acidic ionic liquids in the dissolution and depolymerization of lignin from rice husk into high value-added products. Ind. Crops Prod. 2020, 157, 112885. [Google Scholar] [CrossRef]
- Achinivu, E.C. Protic Ionic Liquids for Lignin Extraction—A Lignin Characterization Study. Int. J. Mol. Sci. 2018, 19, 428. [Google Scholar] [CrossRef]
- Leal Silva, J.F.; Nakasu, P.Y.S.; da Costa, A.C.; Maciel Filho, R.; Rabelo, S.C. Techno-economic analysis of the production of 2G ethanol and technical lignin via a protic ionic liquid pretreatment of sugarcane bagasse. Ind. Crops Prod. 2022, 189, 115788. [Google Scholar] [CrossRef]
- Huang, K.; Mohan, M.; George, A.; Simmons, B.A.; Xu, Y.; Gladden, J.M. Integration of acetic acid catalysis with one-pot protic ionic liquid configuration to achieve high-efficient biorefinery of poplar biomass. Green Chem. 2021, 23, 6036–6049. [Google Scholar] [CrossRef]
- Khan, S.; Rauber, D.; Shanmugam, S.; Kay, C.W.M.; Konist, A.; Kikas, T. Efficient Lignin Fractionation from Scots Pine (Pinus sylvestris) Using Ammonium-Based Protic Ionic Liquid: Process Optimization and Characterization of Recovered Lignin. Polymers 2022, 14, 4637. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Malaret, F.; Firth, A.E.J.; Verdía, P.; Abouelela, A.R.; Chen, Y.; Hallett, J.P. Design of a combined ionosolv-organosolv biomass fractionation process for biofuel production and high value-added lignin valorisation. Green Chem. 2020, 22, 5161–5178. [Google Scholar] [CrossRef]
- Chambon, C.L.; Fitriyanti, V.; Verdía, P.; Yang, S.M.; Hérou, S.; Titirici, M.-M.; Brandt-Talbot, A.; Fennell, P.S.; Hallett, J.P. Fractionation by Sequential Antisolvent Precipitation of Grass, Softwood, and Hardwood Lignins Isolated Using Low-Cost Ionic Liquids and Water. ACS Sustain. Chem. Eng. 2020, 8, 3751–3761. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Perspective on Technical Lignin Fractionation. ACS Sustain. Chem. Eng. 2020, 8, 8086–8101. [Google Scholar] [CrossRef]
- Guiao, K.S.; Tzoganakis, C.; Mekonnen, T.H. Green mechano-chemical processing of lignocellulosic biomass for lignin recovery. Chemosphere 2022, 293, 133647. [Google Scholar] [CrossRef]
- Penín, L.; Lange, H.; Santos, V.; Crestini, C.; Parajó, J.C. Characterization of Eucalyptus nitens Lignins Obtained by Biorefinery Methods Based on Ionic Liquids. Molecules 2020, 25, 425. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, D.; Jiang, Y.; Mei, X.; Ren, W.; Sun, M.; Su, C.; Cao, H.; Zhang, C.; Qin, P. Rapid fractionation of corn stover by microwave-assisted protic ionic liquid [TEA][HSO4] for fermentative acetone–butanol–ethanol production. Biotechnol. Biofuels Bioprod. 2024, 17, 62. [Google Scholar] [CrossRef]
- Sun, W.; Greaves, T.L.; Othman, M.Z. Electro-Assisted Pretreatment of Lignocellulosic Materials in Ionic Liquid-Promoted Organic Solvents. ACS Sustain. Chem. Eng. 2020, 8, 18177–18186. [Google Scholar] [CrossRef]
- Sun, Y.-C.; Liu, X.-N.; Wang, T.-T.; Xue, B.-L.; Sun, R.-C. Green Process for Extraction of Lignin by the Microwave-Assisted Ionic Liquid Approach: Toward Biomass Biorefinery and Lignin Characterization. ACS Sustain. Chem. Eng. 2019, 7, 13062–13072. [Google Scholar] [CrossRef]
- Sorn, V.; Chang, K.-L.; Phitsuwan, P.; Ratanakhanokchai, K.; Dong, C.-D. Effect of microwave-assisted ionic liquid/acidic ionic liquid pretreatment on the morphology, structure, and enhanced delignification of rice straw. Bioresour. Technol. 2019, 293, 121929. [Google Scholar] [CrossRef] [PubMed]
- Merino, O.; Fundora-Galano, G.; Luque, R.; Martínez-Palou, R. Understanding Microwave-Assisted Lignin Solubilization in Protic Ionic Liquids with Multiaromatic Imidazolium Cations. ACS Sustain. Chem. Eng. 2018, 6, 4122–4129. [Google Scholar] [CrossRef]
- Abejón, R.; Rabadán, J.; Lanza, S.; Abejón, A.; Garea, A.; Irabien, A. Supported Ionic Liquid Membranes for Separation of Lignin Aqueous Solutions. Processes 2018, 6, 143. [Google Scholar] [CrossRef]
- Zhang, Y.; He, H.; Dong, K.; Fan, M.; Zhang, S. A DFT study on lignin dissolution in imidazolium-based ionic liquids. RSC Adv. 2017, 7, 12670–12681. [Google Scholar] [CrossRef]
- Manna, B.; Datta, S.; Ghosh, A. Understanding the dissolution of softwood lignin in ionic liquid and water mixed solvents. Int. J. Biol. Macromol. 2021, 182, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Choudhary, H.; George, A.; Simmons, B.A.; Sale, K.; Gladden, J.M. Towards understanding of delignification of grassy and woody biomass in cholinium-based ionic liquids. Green Chem. 2021, 23, 6020–6035. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Y.; Wang, Y.; Li, Y.; Li, H.; Li, C. The molecular design and experimental study on the catalytic cleavage of linkages in lignin with binuclear ionic liquid. J. Mol. Liq. 2020, 308, 113128. [Google Scholar] [CrossRef]
- Ge, M.; Fang, T.; Zhou, G.; Li, C.; Li, Y.; Liu, X. Insight into the dual effect of water on lignin dissolution in ionic liquids. Int. J. Biol. Macromol. 2022, 205, 178–184. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Manna, B.; Ghosh, A. Molecular insights into dissolution of lignin bunch in ionic liquid-water mixture for enhanced biomass conversion. Renew. Energy 2023, 206, 47–59. [Google Scholar] [CrossRef]
- Dutta, T.; Isern, N.G.; Sun, J.; Wang, E.; Hull, S.; Cort, J.R.; Simmons, B.A.; Singh, S. Survey of Lignin-Structure Changes and Depolymerization during Ionic Liquid Pretreatment. ACS Sustain. Chem. Eng. 2017, 5, 10116–10127. [Google Scholar] [CrossRef]
- Abouelela, A.R.; Al Ghatta, A.; Verdía, P.; Shan Koo, M.; Lemus, J.; Hallett, J.P. Evaluating the Role of Water as a Cosolvent and an Antisolvent in [HSO4]-Based Protic Ionic Liquid Pretreatment. ACS Sustain. Chem. Eng. 2021, 9, 10524–10536. [Google Scholar] [CrossRef]
- Wen, J.-L.; Yuan, T.-Q.; Sun, S.-L.; Xu, F.; Sun, R.-C. Understanding the chemical transformations of lignin during ionic liquid pretreatment. Green Chem. 2014, 16, 181–190. [Google Scholar] [CrossRef]
- Halder, P.; Kundu, S.; Patel, S.; Parthasarathy, R.; Pramanik, B.; Paz-Ferreiro, J.; Shah, K. TGA-FTIR study on the slow pyrolysis of lignin and cellulose-rich fractions derived from imidazolium-based ionic liquid pre-treatment of sugarcane straw. Energy Convers. Manag. 2019, 200, 112067. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Romero, R.A.; Redondo, A.; Gnanakaran, S. Theoretical Study of the Remarkably Diverse Linkages in Lignin. J. Phys. Chem. Lett. 2011, 2, 2660–2666. [Google Scholar] [CrossRef]
- Dias, R.M.; Vilas-Boas, S.M.; da Costa, M.C. Unveiling the ability of protic and aprotic ionic liquids to dissolve and modify Kraft lignin. Sep. Purif. Technol. 2024, 350, 127977. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Hou, Y. Behavior of oxygen-containing groups in grass lignin during dissolution in basic ionic liquids. Cellulose 2019, 26, 737–749. [Google Scholar] [CrossRef]
- Hasanov, I.; Raud, M.; Kikas, T. The Role of Ionic Liquids in the Lignin Separation from Lignocellulosic Biomass. Energies 2020, 13, 4864. [Google Scholar] [CrossRef]
- Zhang, Y.; He, H.; Liu, Y.; Wang, Y.; Huo, F.; Fan, M.; Adidharma, H.; Li, X.; Zhang, S. Recent progress in theoretical and computational studies on the utilization of lignocellulosic materials. Green Chem. 2019, 21, 9–35. [Google Scholar] [CrossRef]
- Dong, K.; Liu, X.; Dong, H.; Zhang, X.; Zhang, S. Multiscale Studies on Ionic Liquids. Chem. Rev. 2017, 117, 6636–6695. [Google Scholar] [CrossRef]
- Yu, K.; Ding, W.-L.; Lu, Y.; Wang, Y.; Liu, Y.; Liu, G.; Huo, F.; He, H. Ionic liquids screening for lignin dissolution: COSMO-RS simulations and experimental characterization. J. Mol. Liq. 2022, 348, 118007. [Google Scholar] [CrossRef]
- Mohan, M.; Simmons, B.A.; Sale, K.L.; Singh, S. Multiscale molecular simulations for the solvation of lignin in ionic liquids. Sci. Rep. 2023, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Baran, K.; Barczak, B.; Kloskowski, A. Modeling lignin extraction with ionic liquids using machine learning approach. Sci. Total Environ. 2024, 935, 173234. [Google Scholar] [CrossRef]
- Sun, Q.; Fu, X.; Wang, P.; Li, K.; Wei, L.; Zhai, S.; An, Q. Selective production of p-hydroxybenzaldehyde in the oxidative depolymerization of alkali lignin catalyzed by copper-containing imidazolium-based ionic liquids. J. Mol. Liq. 2023, 385, 122378. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, C.; Cui, Y.; Ye, J.; He, B.; Liu, X.; Sun, J. Efficient demethylation of lignin for polyphenol production enabled by low-cost bifunctional protic ionic liquid under mild and halogen-free conditions. Chem. Eng. J. 2022, 443, 136486. [Google Scholar] [CrossRef]
- Janesko, B.G. Acid-catalyzed hydrolysis of lignin β-O-4 linkages in ionic liquid solvents: A computational mechanistic study. Phys. Chem. Chem. Phys. 2014, 16, 5423–5433. [Google Scholar] [CrossRef]
- Taylor, B.R.; Kumar, N.; Mishra, D.K.; Simmons, B.A.; Choudhary, H.; Sale, K.L. Computational Advances in Ionic Liquid Applications for Green Chemistry: A Critical Review of Lignin Processing and Machine Learning Approaches. Molecules 2024, 29, 5073. [Google Scholar] [CrossRef]
- Wufuer, A.; Wang, Y.; Dai, L. Enhanced lignin degradation by a two-step acidic protic bio-based ionic liquid pretreatment method. Biomass Convers. Biorefin. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Peng, M.; Nakabayashi, M.; Kim, K.; Kamiya, K.; Qian, E.W. Lignin depolymerization with alkaline ionic liquids and ethylene glycol in a continuous flow reactor. Fuel 2023, 335, 126960. [Google Scholar] [CrossRef]
- Tolesa, L.D.; Gupta, B.S.; Tiwikrama, A.H.; Wu, Y.-C.; Lee, M.-J. Alkali lignin degradation with aqueous ammonium-based ionic liquid solutions. J. Clean. Prod. 2020, 258, 120724. [Google Scholar] [CrossRef]
- Kang, S.; Bu, D.; Xue, L.; Wang, Q.; Jiang, M. Degradation of lignin catalyzed by nanochannels solid acids in ionic liquid. Polym. Degrad. Stab. 2019, 166, 1–7. [Google Scholar] [CrossRef]
- De Gregorio, G.F.; Prado, R.; Vriamont, C.; Erdocia, X.; Labidi, J.; Hallett, J.P.; Welton, T. Oxidative Depolymerization of Lignin Using a Novel Polyoxometalate-Protic Ionic Liquid System. ACS Sustain. Chem. Eng. 2016, 4, 6031–6036. [Google Scholar] [CrossRef]
- Kang, Y.; Lu, X.; Zhang, G.; Yao, X.; Xin, J.; Yang, S.; Yang, Y.; Xu, J.; Feng, M.; Zhang, S. Metal-Free Photochemical Degradation of Lignin-Derived Aryl Ethers and Lignin by Autologous Radicals through Ionic Liquid Induction. ChemSusChem 2019, 12, 4005–4013. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Li, D.; Jiang, J.; Li, K.; Zhang, K.; An, Q.; Zhai, S.; Wei, L. 1-Ethyl-3-methylimidazolium acetate ionic liquid as simple and efficient catalytic system for the oxidative depolymerization of alkali lignin. Int. J. Biol. Macromol. 2021, 183, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, J.; Liu, M.; Wang, S.; Li, W.; An, Q.; Li, K.; Wei, L.; Han, C.; Zhai, S. Enhanced oxidative depolymerization of lignin in cooperative imidazolium-based ionic liquid binary mixtures. Bioresour. Technol. 2022, 357, 127333. [Google Scholar] [CrossRef]
- Singh, K.; Mehra, S.; Kumar, A. Metal-based ionic liquids: Effective catalysts in aqueous media for the selective production of vanillin from alkali lignin at room temperature. Green Chem. 2022, 24, 9629–9642. [Google Scholar] [CrossRef]
- Mehta, M.J.; Kulshrestha, A.; Sharma, S.; Kumar, A. Room temperature depolymerization of lignin using a protic and metal based ionic liquid system: An efficient method of catalytic conversion and value addition. Green Chem. 2021, 23, 1240–1247. [Google Scholar] [CrossRef]
- Libretti, C.; Santos Correa, L.; Meier, M.A.R. From waste to resource: Advancements in sustainable lignin modification. Green Chem. 2024, 26, 4358–4386. [Google Scholar] [CrossRef]
- Suzuki, S.; Kurachi, S.; Wada, N.; Takahashi, K. Selective Modification of Aliphatic Hydroxy Groups in Lignin Using Ionic Liquid. Catalysts 2021, 11, 120. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Wang, H.; Deuss, P.J. Tuning lignin properties by mild ionic-liquid-mediated selective alcohol incorporation. Chem Catal. 2022, 2, 1407–1427. [Google Scholar] [CrossRef]
- Kurc, B.; Gross, X.; Pigłowska, M.; Kołodziejczak-Radzimska, A.; Klapiszewski, Ł. Lignin activation with selected ionic liquids based on kinetic and thermodynamic analyses. Int. J. Biol. Macromol. 2025, 305, 141144. [Google Scholar] [CrossRef]
- Pin, T.C.; Rabelo, S.C.; Pu, Y.; Ragauskas, A.J.; Costa, A.C. Effect of Protic Ionic Liquids in Sugar Cane Bagasse Pretreatment for Lignin Valorization and Ethanol Production. ACS Sustain. Chem. Eng. 2021, 9, 16965–16976. [Google Scholar] [CrossRef]
- Majira, A.; Godon, B.; Foulon, L.; van der Putten, J.C.; Cézard, L.; Thierry, M.; Pion, F.; Bado-Nilles, A.; Pandard, P.; Jayabalan, T.; et al. Enhancing the Antioxidant Activity of Technical Lignins by Combining Solvent Fractionation and Ionic-Liquid Treatment. ChemSusChem 2019, 12, 4799–4809. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, J.; Dzieniszewska, A.; Sabura, E.; Rzepa, G.; Muszyński, M.; Kyzioł–Komosińska, J. Modified kraft lignin with imidazolium ionic liquids: Towards novel lignin-Ils biomaterials. Mater. Chem. Phys. 2025, 339, 130605. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H.; Pizzi, A. Ionic liquid- modified lignin as a bio- coupling agent for natural fiber- recycled polypropylene composites. Compos. Part B Eng. 2020, 181, 107587. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Liang, J.; Lu, L.; Xie, Y.; Qin, C.; Liang, C.; Huang, C.; Yao, S. Optimizing lignin demethylation using a novel proton- based ionic liquid: 1, 2-propanediamine/glycolic acid catalyst. Int. J. Biol. Macromol. 2024, 279, 135172. [Google Scholar] [CrossRef]
- Luo, Q.; Li, C.; Zhao, W.; Ding, W.; Liu, Y.; Xiao, W.; Liu, H.; Pang, X.; Sun, J. Lignin Demethylated by Protic Ionic Liquid as a Novel and Sustainable Chrome-Free Tanning Agent for Eco-Leather Production. ACS Sustain. Chem. Eng. 2024, 12, 9682–9694. [Google Scholar] [CrossRef]
- Anuchi, S.O.; Campbell, K.L.S.; Hallett, J.P. Effects of the Ionic Liquid Structure on Porosity of Lignin-Derived Carbon Materials. ACS Sustain. Chem. Eng. 2023, 11, 15228–15241. [Google Scholar] [CrossRef]
- Al Aiti, M.; Das, A.; Kanerva, M.; Järventausta, M.; Johansson, P.; Scheffler, C.; Göbel, M.; Jehnichen, D.; Brünig, H.; Wulff, L.; et al. Dry-Jet Wet Spinning of Thermally Stable Lignin-Textile Grade Polyacrylonitrile Fibers Regenerated from Chloride-Based Ionic Liquids Compounds. Materials 2020, 13, 3687. [Google Scholar] [CrossRef]
- Yang, S.M.; Shaffer, M.S.P.; Brandt-Talbot, A. High Lignin Content Carbon Fiber Precursors Wet-Spun from Low-Cost Ionic Liquid Water Mixtures. ACS Sustain. Chem. Eng. 2023, 11, 8800–8811. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, B.; Zhang, Y.; Cao, Q.; Yang, C.; Li, Y.; Zhou, J. Biomimetic lignin/poly(ionic liquids) composite hydrogel dressing with excellent mechanical strength, self-healing properties, and reusability. Chem. Eng. J. 2020, 400, 125984. [Google Scholar] [CrossRef]
- Hu, F.; Dong, B.; Zhao, R.; Li, Z.; Zhang, Y.; Zhang, F.; Liu, W.; Yu, D. Lignosulfonate sodium and ionic liquid synergistically promote tough hydrogels for intelligent wearable human-machine interaction. Int. J. Biol. Macromol. 2024, 254, 127958. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Zhao, Y.; Chen, Z.A.; Wang, S.; Ren, Y.; Wang, Q.; Shao, J.; Wang, W.; Dong, X. Thermoresponsive Lignin-Reinforced Poly(Ionic Liquid) Hydrogel Wireless Strain Sensor. Research 2021, 2021, 9845482. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hou, Y.; Li, Y.; Xiao, H. Heteroatom-doped porous carbon microspheres derived from ionic liquid-lignin solution for high performance supercapacitors. J. Colloid Interface Sci. 2022, 614, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Cao, M.; Liu, R.; Tian, B.; Liu, S.; Li, J.; Li, S.; Strehmel, B.; James, T.D.; Chen, Z. Photocured room temperature phosphorescent materials from lignosulfonate. Nat. Commun. 2024, 15, 1590. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Nimir, W.; Al-Othman, A.; Ka’ki, A. High proton conduction in zirconium silicate/lignin/ionic liquids based- membranes for high temperature PEM fuel cells. Process Saf. Environ. Prot. 2024, 190, 779–791. [Google Scholar] [CrossRef]
- Nisha, S.S.; Nikzad, M.; Al Kobaisi, M.; Truong, V.K.; Sbarski, I. The role of ionic-liquid extracted lignin micro/nanoparticles for functionalisation of an epoxy-based composite matrix. Compos. Sci. Technol. 2019, 174, 11–19. [Google Scholar] [CrossRef]
- Rashid, T.; Sher, F.; Khan, A.S.; Khalid, U.; Rasheed, T.; Iqbal, H.M.N.; Murugesan, T. Effect of protic ionic liquid treatment on the pyrolysis products of lignin extracted from oil palm biomass. Fuel 2021, 291, 120133. [Google Scholar] [CrossRef]
- Monaci, S.; Mezzetta, A.; Guazzelli, L.; Mecerreyes, D.; Forsyth, M.; Somers, A. Lignin-Based Bio Ionic Liquids for Enhanced Corrosion Protection of Metals. ACS Sustain. Chem. Eng. 2025, 13, 2023–2037. [Google Scholar] [CrossRef]
- Yu, Q.; Yang, Z.; Huang, Q.; Lv, H.; Zhou, K.; Yan, X.; Wang, X.; Yang, W.; Zhou, C.; Yu, B.; et al. Lignin composite ionic liquid lubricating material as a water-based lubricating fluid additive with excellent lubricating, anti-wear and anti-corrosion properties. Tribol. Int. 2022, 174, 107742. [Google Scholar] [CrossRef]

| Major Pretreatment Methods | Conventional Type | Characteristic |
|---|---|---|
| Milling treatment | Physical pretreatment |
|
| Microwave treatment | Physical pretreatment |
|
| Acid treatment | Chemical pretreatment |
|
| Alkakine treatment | Chemical pretreatment |
|
| Organic treatment | Chemical pretreatment |
|
| Ionic liquid treatment | Chemical pretreatment |
|
| Hot water treatment | Physicochemical pretreatment |
|
| Steam explosion treatment | Physicochemical pretreatment |
|
| Fungi treatment (Brown Fungi, White Fungi) | Biological pretreatment |
|
| Abbreviation | Name | Structure | Reference | Lignin Yield |
|---|---|---|---|---|
| [TMG][HSO4] | 1,1,3,3-tetramethylguanidinium hydrogen sulfate |  | [75] | 81% |
| [DMBA][HSO4] | N,N,N-dimethyl butylammonium hydrogen sulfate |  | [76] | 73% |
| [TEA][HSO4] | Triethylammonium hydrogen sulfate | 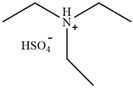 | [70] | 80% |
| [DIPEA][Ac] | N,N-Diisopropylethylammonium acetate | 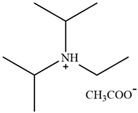 | [77] | 71.2% |
| [DIPEA][P] | N,N-Diisopropylethylammonium propanoate | 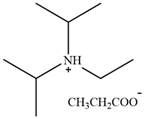 | [77] | 63.7% |
| [DIPEA][O] | N,N-Diisopropylethylammonium octanoate | 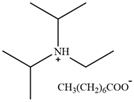 | [77] | 54.2% |
| [C3H6SO3Hmim][HSO4] | 1-(3-sulfopropyl)-3-methylimidazolium bisulfate | 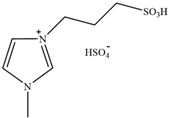 | [78] | 79.94% |
| [C3SO3HMIM][Cl] | 1-methyl-3-(3-sulfopropyl)-imidazolium chloride |  | [79] | 78% |
| [Py][Ac] | Pyridinium acetate | 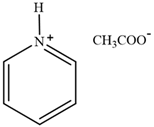 | [80] | 76% |
| [Py][For] | Pyridinium formate | 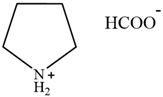 | [21] | Not reported |
| [MEA][Ac] | 2-hydroxyethylammonium acetate | 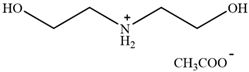 | [81] | Not reported |
| HAc-[EOA][OAc] | Acetic acid-Ethanolamine acetate | 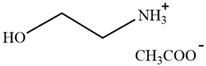 | [82] | 46% |
| [N11H(2OH)][LAC] | 2-hydroxyethyl ammonium lactate |  | [83] | 56% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yang, J.; He, W.; Zhao, S.; Fang, Z.; Guo, K.; Li, Y. A Review: Using Ionic Liquids for Lignin Extraction from Lignocellulose and High-Value Utilization. Molecules 2025, 30, 2514. https://doi.org/10.3390/molecules30122514
Li X, Yang J, He W, Zhao S, Fang Z, Guo K, Li Y. A Review: Using Ionic Liquids for Lignin Extraction from Lignocellulose and High-Value Utilization. Molecules. 2025; 30(12):2514. https://doi.org/10.3390/molecules30122514
Chicago/Turabian StyleLi, Xinyu, Jiming Yang, Wei He, Shuangfei Zhao, Zheng Fang, Kai Guo, and Yuguang Li. 2025. "A Review: Using Ionic Liquids for Lignin Extraction from Lignocellulose and High-Value Utilization" Molecules 30, no. 12: 2514. https://doi.org/10.3390/molecules30122514
APA StyleLi, X., Yang, J., He, W., Zhao, S., Fang, Z., Guo, K., & Li, Y. (2025). A Review: Using Ionic Liquids for Lignin Extraction from Lignocellulose and High-Value Utilization. Molecules, 30(12), 2514. https://doi.org/10.3390/molecules30122514






