A New Fluorescence Band of Anthocyanins as a Simple Oxidation Biomarker of Food Products
Abstract
1. Introduction
2. Results
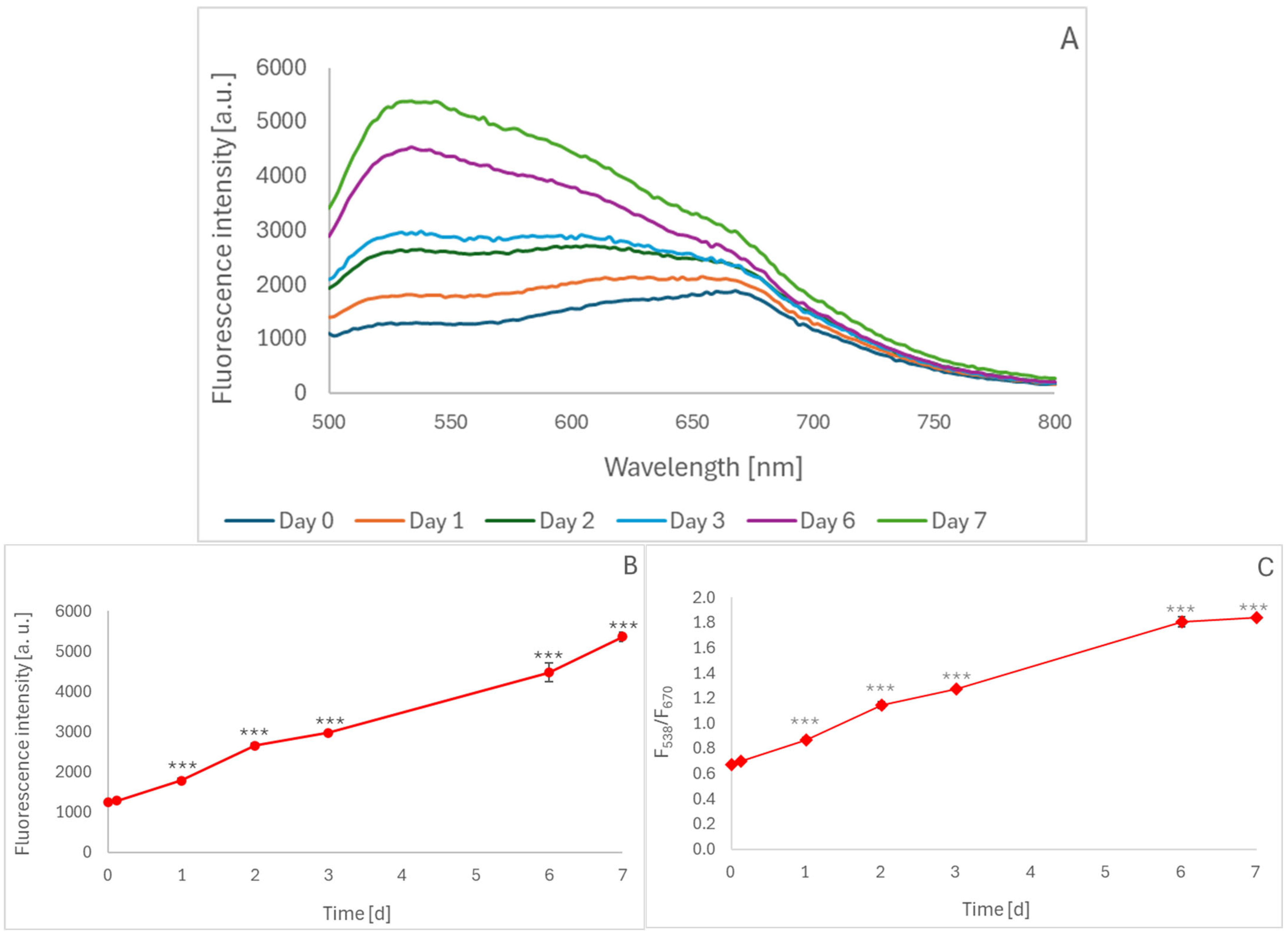
2.1. Oxidation of Blueberry Juice During Aerobic Storage
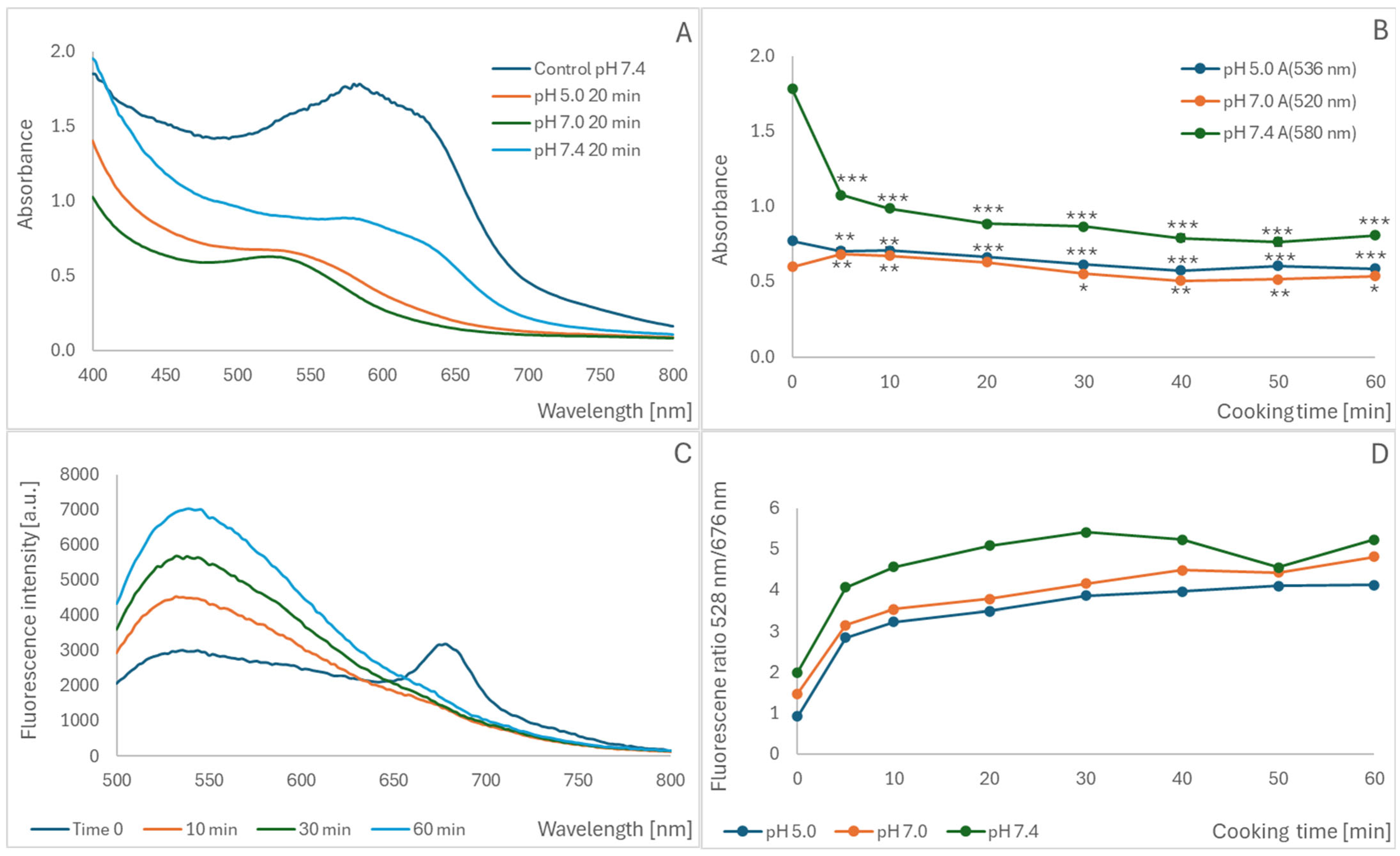
2.2. Oxidation of Blueberry Homogenate During Cooking
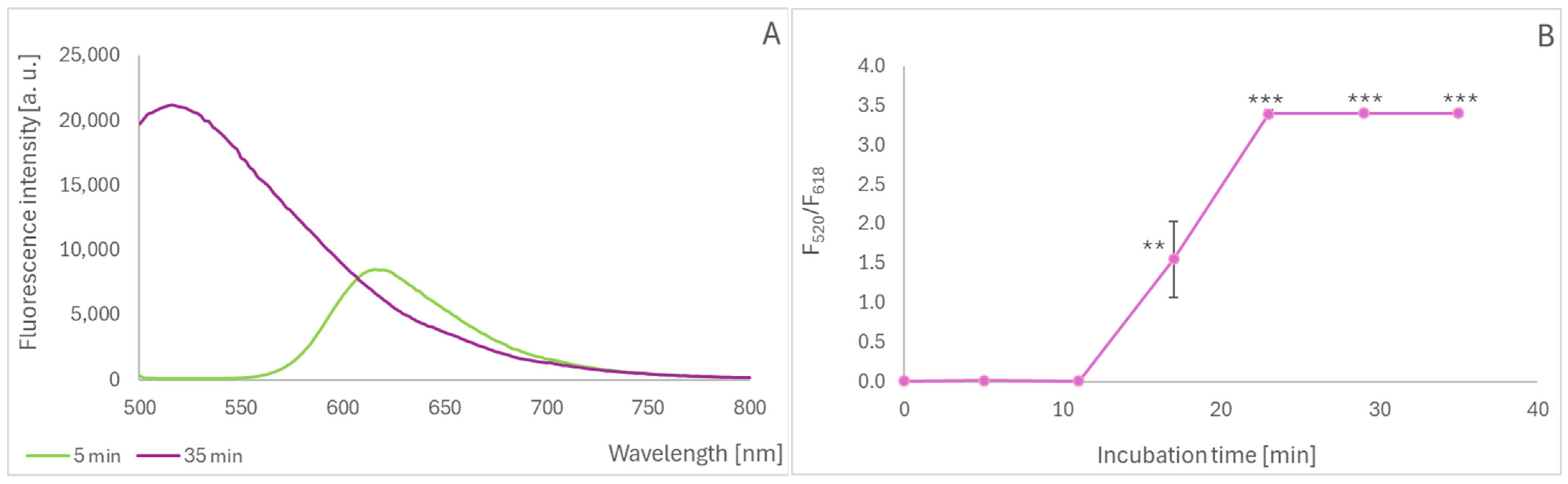
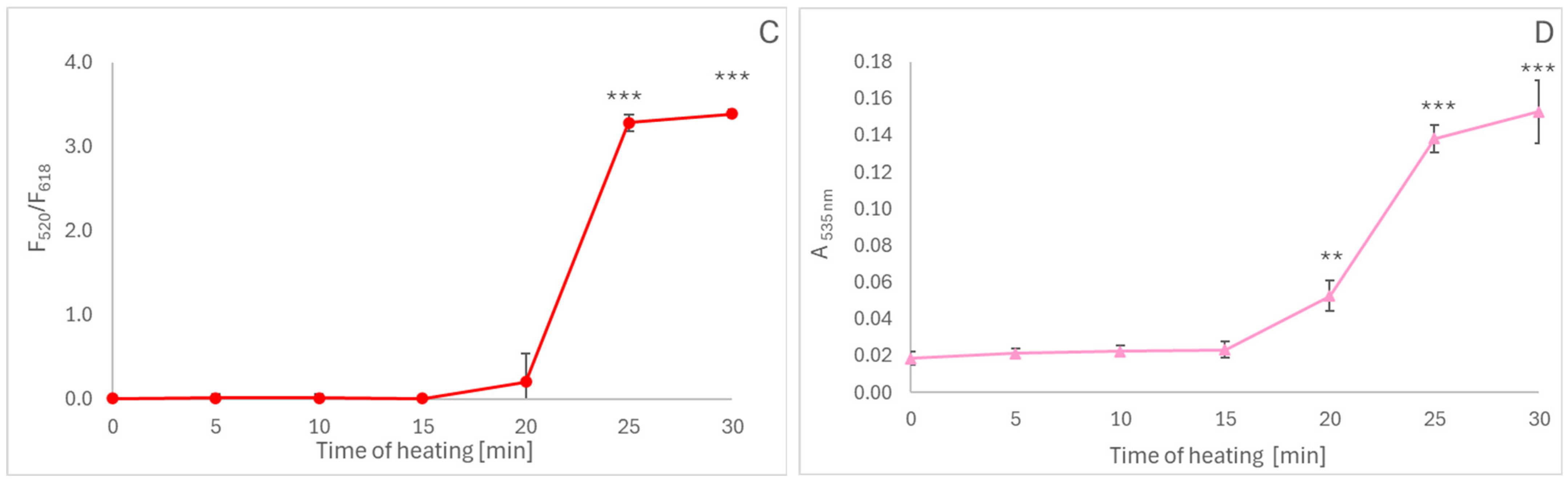
2.3. Oxidation of Black Carrot Anthocyanins During Cooking
2.4. Oil Oxidation During Simulated Frying
3. Discussion
4. Materials and Methods
4.1. Material and Equipment
4.2. Oxidation of Anthocyanin-Rich Juice During Aerobic Incubation
4.3. Cooking of Blueberry Homogenate
4.4. Cooking of Black Carrots
4.5. Oil Oxidation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, T.C.; Giusti, M.M. Anthocyanins—Nature’s bold, beautiful, and health-promoting colors. Foods 2019, 8, 550. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.; Appelhagen, I.; Martin, C. Natural blues: Structure meets function in anthocyanins. Plants 2021, 10, 726. [Google Scholar] [CrossRef]
- Li, Z.; Ahammed, G.J. Plant stress response and adaptation via anthocyanins: A review. Plant Stress 2023, 10, 100230. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.O.; Freitas, A.A.; Maçanita, A.L.; Quina, F.H. Chemistry and photochemistry of natural plant pigments: The anthocyanins. J. Phys. Org. Chem. 2016, 29, 594–599. [Google Scholar] [CrossRef]
- Dangles, O.; Fenger, J.A. The chemical reactivity of anthocyanins and its consequences in food science and nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef]
- Mohammad, S.S.; Santos, R.O.; Barbosa, M.I.; Junior, J.L.B. Anthocyanins: Chemical properties and health benefits: A review. Curr. Nutr. Food Sci. 2021, 17, 662–672. [Google Scholar] [CrossRef]
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and proanthocyanidins: Chemical structures, food sources, bioactivities, and product development. Food Rev. Int. 2023, 39, 4581–4609. [Google Scholar] [CrossRef]
- Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Ağçam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic digestion, bioavailability, therapeutic effects, current pharmaceutical/industrial use, and innovation potential. Antioxidants 2022, 12, 48. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Dudek, A.; Spiegel, M.; Strugała-Danak, P.; Gabrielska, J. Analytical and theoretical studies of antioxidant properties of chosen anthocyanins; a structure-dependent relationships. Int. J. Mol. Sci. 2022, 23, 5432. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant activity of anthocyanins and anthocyanidins: A critical review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef] [PubMed]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and human health—A focus on oxidative stress, inflammation and disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Islam, P.; Subhan, N.; Rahman, M.M.; Khan, F.; Burrows, G.E.; Nahar, L.; Sarker, S.D. Potential health benefits of anthocyanins in oxidative stress related disorders. Phytochem. Rev. 2021, 20, 705–749. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Gui, H.; Sun, L.; Liu, R.; Si, X.; Li, D.; Wang, Y.; Shu, C.; Sun, X.; Jiang, Q.; Qiao, Y.; et al. Current knowledge of anthocyanin metabolism in the digestive tract: Absorption, distribution, degradation, and interconversion. Crit. Rev. Food Sci. Nutr. 2023, 63, 5953–5966. [Google Scholar] [CrossRef]
- Kumkum, R.; Aston-Mourney, K.; McNeill, B.A.; Hernández, D.; Rivera, L.R. Bioavailability of anthocyanins: Whole foods versus extracts. Nutrients 2024, 16, 1403. [Google Scholar] [CrossRef]
- Li, Z.; Tian, J.; Cheng, Z.; Teng, W.; Zhang, W.; Bao, Y.; Wang, Y.; Song, B.; Chen, Y.; Li, B. Hypoglycemic bioactivity of anthocyanins: A review on proposed targets and potential signaling pathways. Crit. Rev. Food Sci. Nutr. 2023, 63, 7878–7895. [Google Scholar] [CrossRef]
- Ye, X.; Chen, W.; Tu, P.; Jia, R.; Liu, Y.; Tang, Q.; Chen, C.; Yang, C.; Zheng, X.; Chu, Q. Antihyperglycemic effect of an anthocyanin, cyanidin-3-O-glucoside, is achieved by regulating GLUT-1 via the Wnt/β-catenin-WISP1 signaling pathway. Food Funct. 2022, 13, 4612–4623. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, Y.; Liu, X.; Deng, Y.; Wei, B.; Shi, L. Lingonberry anthocyanins inhibit hepatic stellate cell activation and liver fibrosis via TGFβ/Smad/ERK signaling pathway. J. Agric. Food Chem. 2021, 69, 13546–13556. [Google Scholar] [CrossRef]
- Qiu, T.; Sun, Y.; Wang, X.; Zheng, L.; Zhang, H.; Jiang, L.; Zhu, X.; Xiong, H. Drum drying-and extrusion-black rice anthocyanins exert anti-inflammatory effects via suppression of the NF-κB/MAPKs signaling pathways in LPS-induced RAW 264.7 cells. Food Biosci. 2021, 41, 100841. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Liu, S.; Zhang, M.; Wang, C.; Xia, X.; Lou, Y.; Xu, H. The effect of lipid metabolism regulator anthocyanins from Aronia melanocarpa on 3T3-L1 preadipocytes and C57BL/6 mice via activating AMPK signaling and gut microbiota. Food Funct. 2021, 12, 6254–6270. [Google Scholar] [CrossRef] [PubMed]
- Bei, R.; Masuelli, L.; Turriziani, M.; Volti, G.L.; Malaguarnera, M.; Galvano, F. Impaired expression and function of signaling pathway enzymes by anthocyanins: Role on cancer prevention and progression. Curr. Enzyme Inhib. 2009, 5, 184–197. [Google Scholar] [CrossRef]
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, microbiome and health benefits in aging. Molecules 2021, 26, 537. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Shen, G.X. Impact of anthocyanin component and metabolite of Saskatoon berry on gut microbiome and relationship with fecal short chain fatty acids in diet-induced insulin resistant mice. J. Nutr. Biochem. 2023, 111, 109201. [Google Scholar] [CrossRef]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Brncic, S.R.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; Pascual-Teresa, S. A Review of factors affecting anthocyanin bioavailability: Possible implications for the inter-individual variability. Foods 2019, 9, 2. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural colorants from plant pigments and their encapsulation: An emerging window for the food industry. LWT 2022, 153, 112527. [Google Scholar] [CrossRef]
- Zeng, S.; Lin, S.; Jiang, R.; Wei, J.; Wang, Y. Biotechnology advances in natural food colorant acylated anthocyanin production. Food Front. 2025, 6, 698–715. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Kossyvaki, D.; Contardi, M.; Athanassiou, A.; Fragouli, D. Colorimetric indicators based on anthocyanin polymer composites: A review. Polymers 2022, 14, 4129. [Google Scholar] [CrossRef]
- Zhao, Y.W.; Wang, C.K.; Huang, X.Y.; Hu, D.G. Anthocyanin stability and degradation in plants. Plant Signal. Behav. 2021, 16, 1987767. [Google Scholar] [CrossRef] [PubMed]
- Oancea, S. A review of the current knowledge of thermal stability of anthocyanins and approaches to their stabilization to heat. Antioxidants 2021, 10, 1337. [Google Scholar] [CrossRef] [PubMed]
- Satake, R.; Yanase, E. Mechanistic studies of hydrogen-peroxide-mediated anthocyanin oxidation. Tetrahedron 2018, 74, 6187–6191. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Neves, D.; Andrade, P.B.; Videira, R.A.; de Freitas, V.; Cruz, L. Berry anthocyanin-based films in smart food packaging: A mini-review. Food Hydrocoll. 2022, 133, 107885. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Y.; Zhao, L.; Wang, Y. Anthocyanin-based pH-sensitive smart packaging films for monitoring food freshness. J. Agric. Food Res. 2022, 9, 100340. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, M.; Shen, D.; Mujumdar, A.S.; Ma, Y. Sensing materials for fresh food quality deterioration measurement: A review of research progress and application in supply chain. Crit. Rev. Food Sci. Nutr. 2024, 64, 8114–8132. [Google Scholar] [CrossRef]
- Bartosz, G.; Grzesik-Pietrasiewicz, M.; Sadowska-Bartosz, I. Fluorescent products of anthocyanidin and anthocyanin oxidation. J. Agric. Food Chem. 2020, 68, 12019–12027. [Google Scholar] [CrossRef]
- Figueiredo, P.; Pina, F. Fluorescence spectra and decays of malvidin 3,5-diglucoside in aqueous solutions. J. Photochem. Photobiol. A Chem. 1990, 52, 411–424. [Google Scholar] [CrossRef]
- Pina, F.; Melo, M.J.; Santos, H.; Lima, J.C.; Abreu, I.; Ballardini, R.; Maestri, M. Excited state proton transfer in synthetic flavylium salts: 4-methyl-7-hydroxyflavylium and 4′,7-dihydroxyflavylium. Example of a four-level molecular device to invert the population of the excited state. New J. Chem. 1998, 22, 1093–1098. [Google Scholar] [CrossRef]
- Collings, D.A. Anthocyanin in the vacuole of red onion epidermal cells quenches other fluorescent molecules. Plants 2019, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- Chanoca, A.; Burkel, B.; Kovinich, N.; Grotewold, E.; Eliceiri, K.W.; Otegui, M.S. Using fluorescence lifetime microscopy to study the subcellular localization of anthocyanins. Plant J. 2016, 88, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Mackon, E.; Ma, Y.; Jeazet Dongho Epse Mackon, G.C.; Li, Q.; Zhou, Q.; Liu, P. Subcellular localization and vesicular structures of anthocyanin pigmentation by fluorescence imaging of black rice (Oryza sativa L.) stigma protoplast. Plants 2021, 10, 685. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Matteini, P.; Oliveira, J.; de Freitas, V.; Mateus, N. Fluorescence approach for measuring anthocyanins and derived pigments in red wine. J. Agric. Food Chem. 2013, 61, 10156–10162. [Google Scholar] [CrossRef]
- Chai, Z.; Herrera-Balandrano, D.D.; Yu, H.; Beta, T.; Zeng, Q.; Zhang, X.; Tian, L.; Niu, L.; Huang, W. A comparative analysis on the anthocyanin composition of 74 blueberry cultivars from China. J. Food Compos. Anal. 2021, 102, 104051. [Google Scholar] [CrossRef]
- Yang, W.; Guo, Y.; Liu, M.; Chen, X.; Xiao, X.; Wang, S.; Gong, P.; Chen, F. Structure and function of blueberry anthocyanins: A review of recent advances. J. Funct. Foods 2022, 88, 104864. [Google Scholar] [CrossRef]
- Liu, S.; Ma, C.; Zhang, Y.; Wang, Y.; Tian, J.; Li, B.; Zhao, J. Different processing methods on anthocyanin composition and antioxidant capacity in blueberry juice: Based on metabolomics and DFT analysis. EFood 2024, 5, e131. [Google Scholar] [CrossRef]
- Wang, S.; Wang, B.; Dong, K.; Li, J.; Li, Y.; Sun, H. Identification and quantification of anthocyanins of 62 blueberry cultivars via UPLC-MS. Biotechnol. Biotechnol. Equip. 2022, 36, 587–597. [Google Scholar] [CrossRef]
- Algarra, M.; Fernandes, A.; Mateus, N.; de Freitas, V.; da Silva, J.C.E.; Casado, J. Anthocyanin profile and antioxidant capacity of black carrots (Daucus carota L. ssp. sativus var. atrorubens Alef.) from Cuevas Bajas, Spain. J. Food Compos. Anal. 2014, 33, 71–76. [Google Scholar] [CrossRef]
- Blando, F.; Marchello, S.; Maiorano, G.; Durante, M.; Signore, A.; Laus, M.N.; Soccio, M.; Mita, G. Bioactive compounds and antioxidant capacity in anthocyanin-rich carrots: A comparison between the black carrot and the Apulian landrace “Polignano” carrot. Plants 2021, 10, 564. [Google Scholar] [CrossRef]
- Kammerer, D.; Carle, R.; Schieber, A. Quantification of anthocyanins in black carrot extracts (Daucus carota ssp. sativus var. atrorubens Alef.) and evaluation of their color properties. Eur. Food Res. Technol. 2004, 219, 479–486. [Google Scholar] [CrossRef]
- Özkan, M.; Yemenicioğlu, A.; Cemeroğlu, B. Degradation of various fruit juice anthocyanins by hydrogen peroxide. Food Res. Int. 2005, 38, 1015–1021. [Google Scholar] [CrossRef]
- Bartosz, G.; Rajzer, K.; Grzesik-Pietrasiewicz, M.; Sadowska-Bartosz, I. Hydrogen peroxide is formed upon cooking of vegetables. Acta Biochim. Pol. 2022, 69, 471–474. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Hydrogen peroxide: A ubiquitous component of beverages and food. Int. J. Mol. Sci. 2025, 26, 3397. [Google Scholar] [CrossRef]
- Bartosz, G.; Baran, S.; Grzesik-Pietrasiewicz, M.; Sadowska-Bartosz, I. Antioxidant capacity and hydrogen peroxide formation by black and orange carrots. Agric. Food Sci. 2022, 31, 71–77. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, J.M. Monitoring of used frying oils and frying times for frying chicken nuggets using peroxide value and acid value. Korean J. Food Sci. Anim. Resour. 2016, 36, 612. [Google Scholar] [CrossRef]
- Machado, M.; Rodriguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Vegetable oils oxidation: Mechanisms, consequences and protective strategies. Food Rev. Int. 2023, 39, 4180–4197. [Google Scholar] [CrossRef]
- Erickson, M.D.; Yevtushenko, D.P.; Lu, Z.X. Oxidation and thermal degradation of oil during frying: A review of natural antioxidant use. Food Rev. Int. 2023, 39, 4665–4696. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rak, M.; Bartosz, G.; Sadowska-Bartosz, I. A New Fluorescence Band of Anthocyanins as a Simple Oxidation Biomarker of Food Products. Molecules 2025, 30, 2510. https://doi.org/10.3390/molecules30122510
Rak M, Bartosz G, Sadowska-Bartosz I. A New Fluorescence Band of Anthocyanins as a Simple Oxidation Biomarker of Food Products. Molecules. 2025; 30(12):2510. https://doi.org/10.3390/molecules30122510
Chicago/Turabian StyleRak, Małgorzata, Grzegorz Bartosz, and Izabela Sadowska-Bartosz. 2025. "A New Fluorescence Band of Anthocyanins as a Simple Oxidation Biomarker of Food Products" Molecules 30, no. 12: 2510. https://doi.org/10.3390/molecules30122510
APA StyleRak, M., Bartosz, G., & Sadowska-Bartosz, I. (2025). A New Fluorescence Band of Anthocyanins as a Simple Oxidation Biomarker of Food Products. Molecules, 30(12), 2510. https://doi.org/10.3390/molecules30122510







