Dynamic Migration Characteristics of Potassium During Agricultural Waste Combustion and the Mechanism of Combined Chlorine–Sulfur Action
Abstract
1. Introduction
2. Results and Discussion
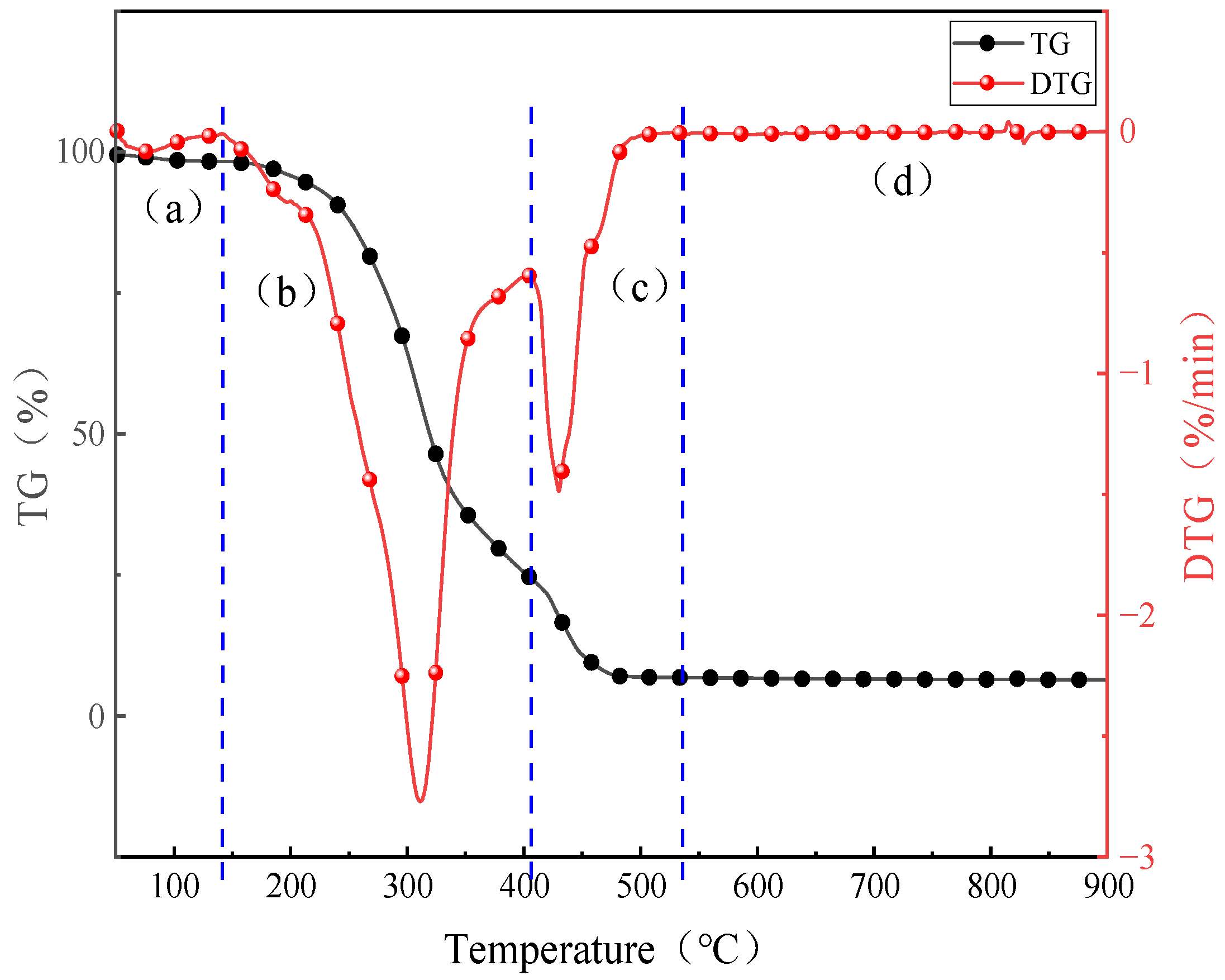
2.1. Combustion Behavior of AW
2.2. Migration Behavior of K
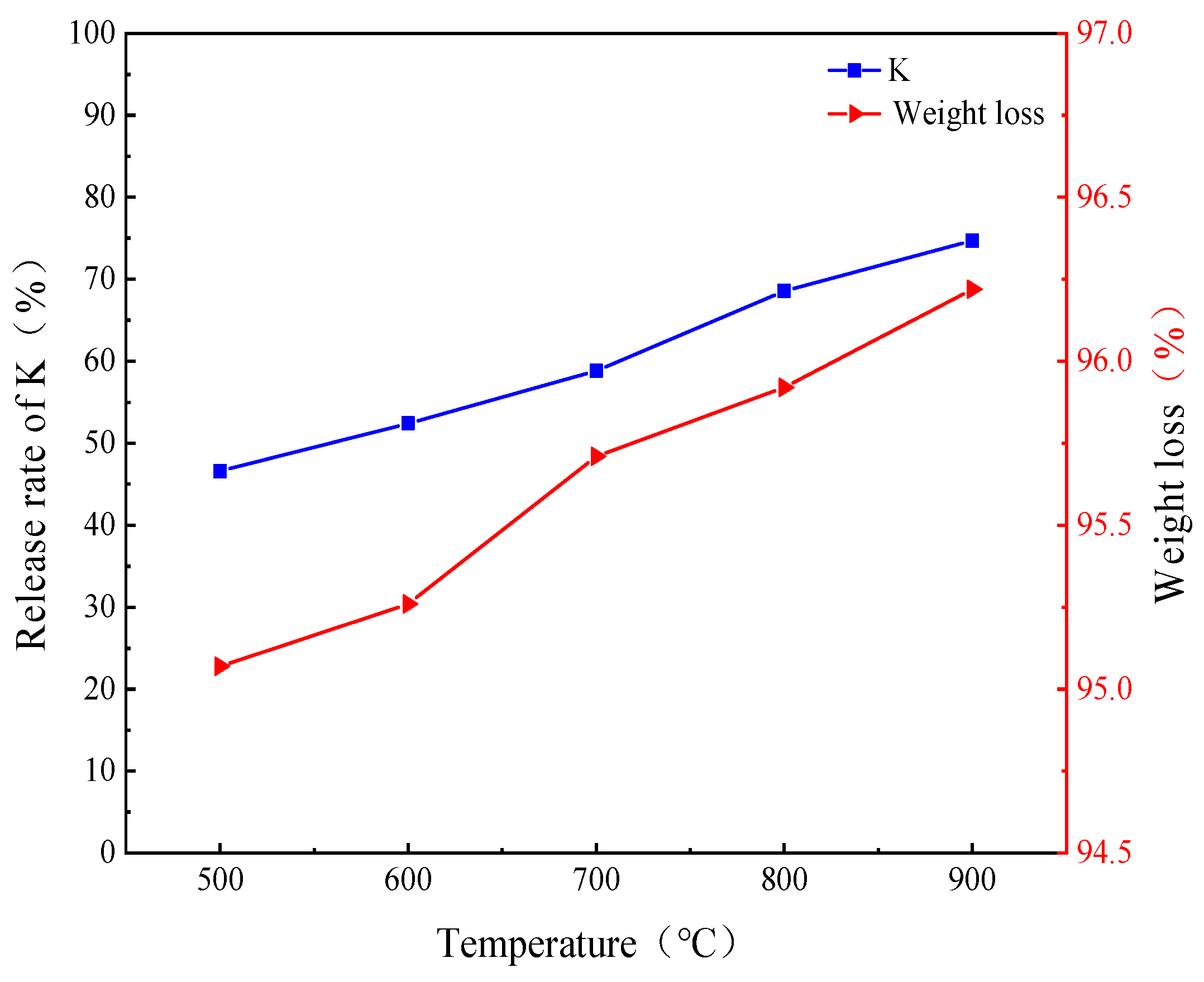
2.2.1. Precipitation Characteristics of K
2.2.2. Migration and Transformation Characteristics of K
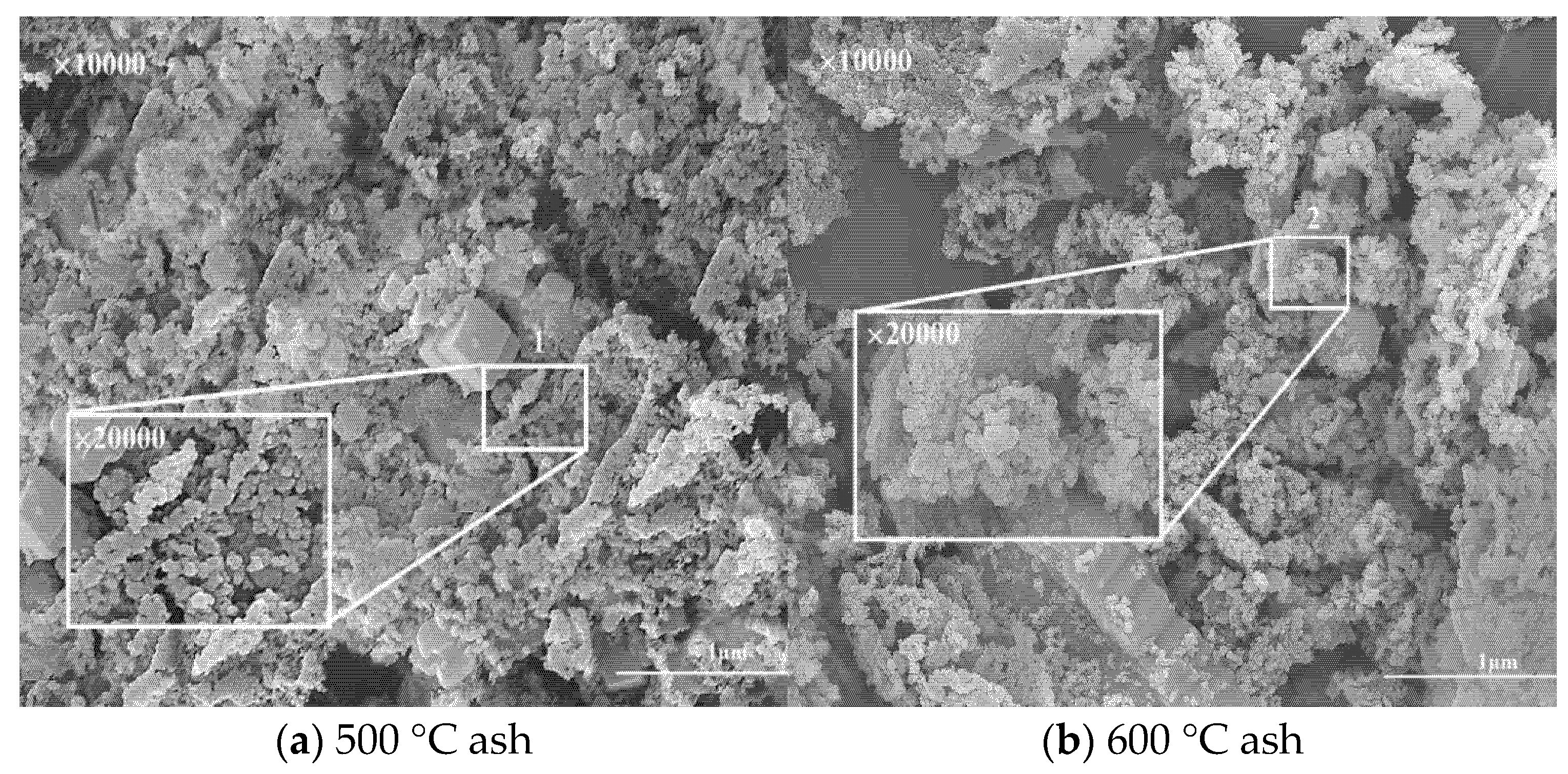
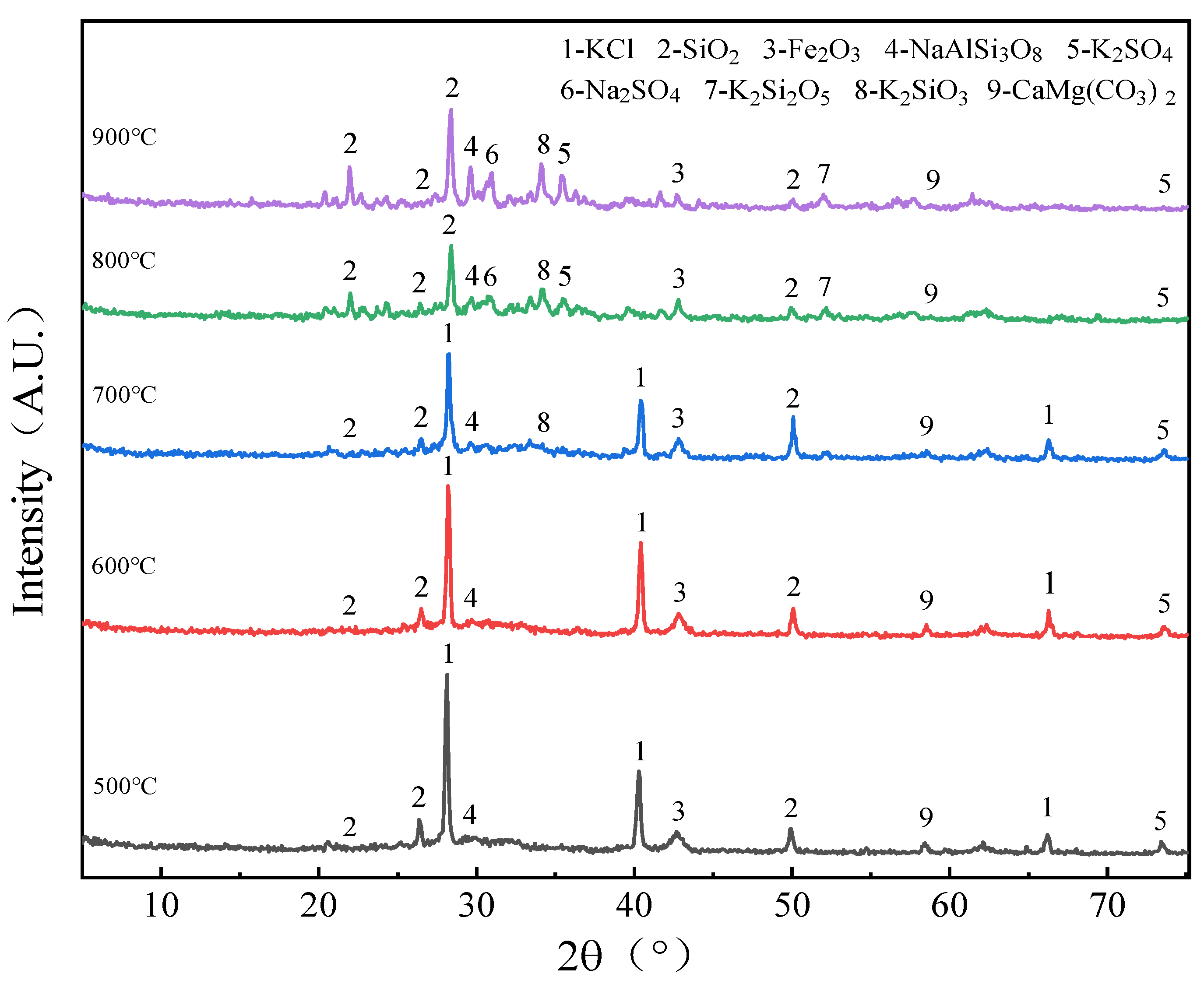
2.2.3. Change Rule of Minerals in Ash with Temperature Rise
2.2.4. Migration and Transformation of K During Combustion
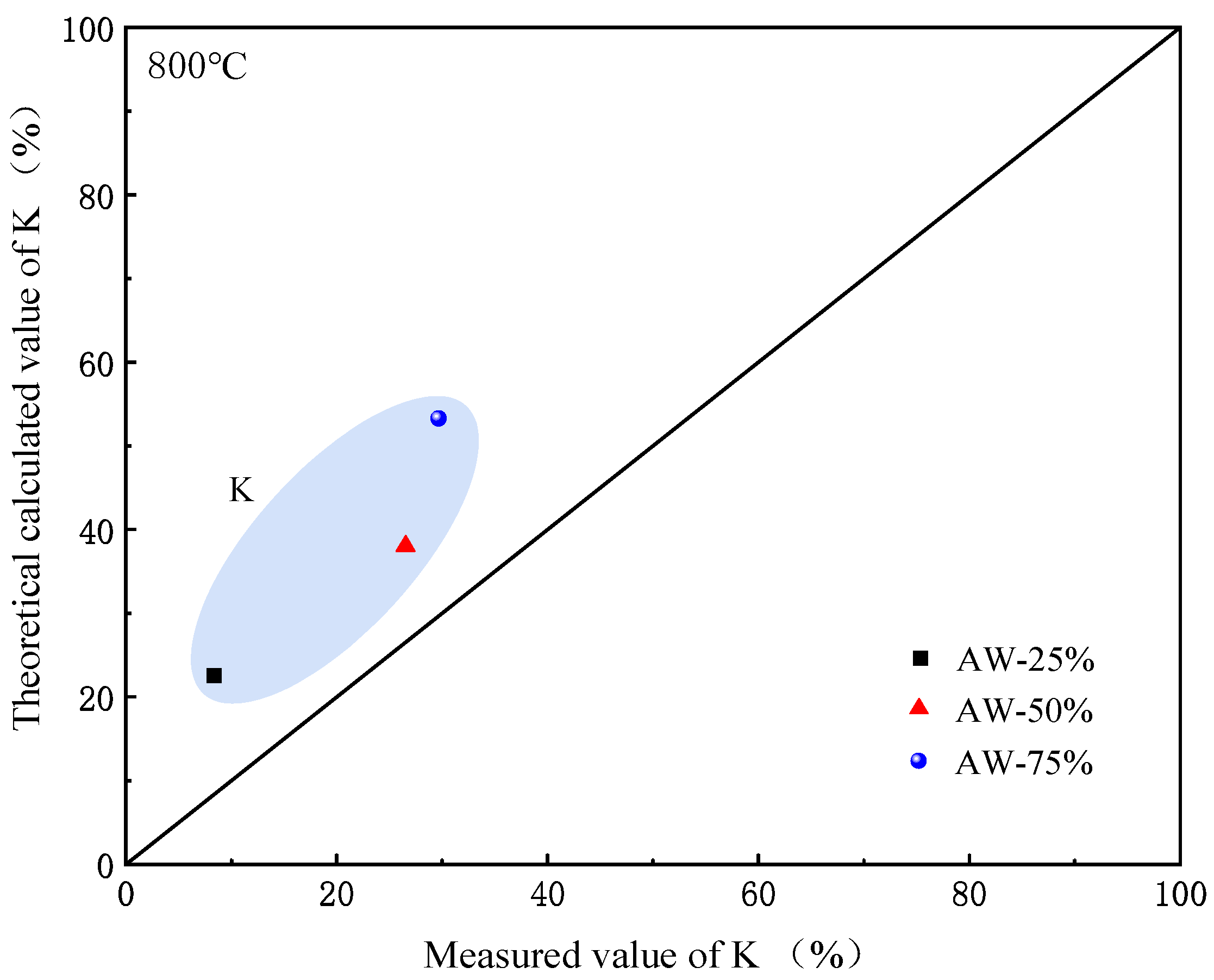
2.3. Research on the Dynamic Migration Law of Potassium Using FactSage
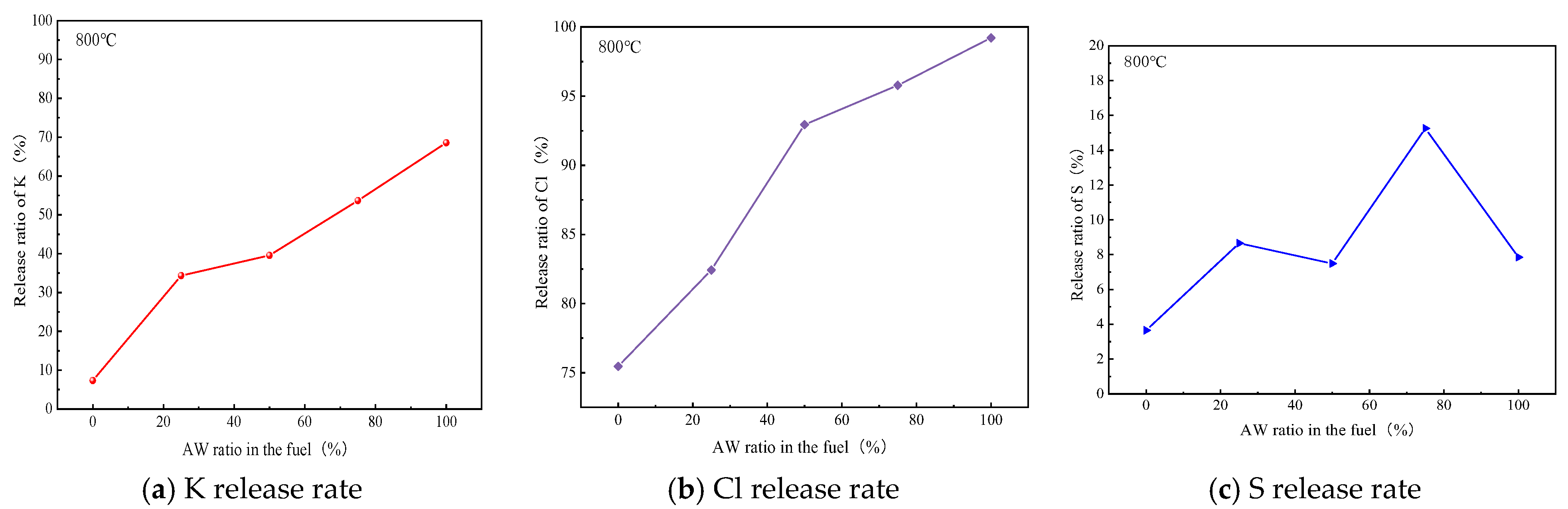
2.4. Influence of Competition Mechanism Between Chlorine and Sulfur on Migration and Transformation of Potassium
2.4.1. Effects of Chlorine on Potassium Release and Transformation
2.4.2. Joint Competition Mechanism of Chlorine, Sulfur, and Potassium
3. Materials and Methods
3.1. Raw Material Preparation
3.2. Ash Sample Preparation and Properties
3.3. Analytical Methods
3.3.1. Chemical Fractionation Analysis
3.3.2. Chlorine Content Analysis
3.3.3. Thermogravimetric Analysis
3.3.4. Scanning Electron Microscope–Energy Dispersive X-Ray Analysis
3.3.5. X-Ray Powder Diffraction Analysis
3.4. Calculation Method
3.4.1. Potassium Content and Release Rate
3.4.2. Chlorine Release Rate
3.4.3. Thermodynamic Equilibrium Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; He, Y.; Wang, Z.; Xia, J.; Wan, K.D.; Whiddon, D.; Cen, K.F. Characteristics of alkali species release from a burning coal/biomass blend. Appl. Energy 2018, 215, 523–531. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, H.; Xu, K.; Xu, K.L.; Li, L. Investigation on the fusion characterization and melting kinetics of ashes from co-firing of anthracite and pine sawdust. Renew. Energy 2020, 145, 835–846. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Y.; Zhang, H.; Zhang, M.; Liu, Q.; Yang, H.R.; Lu, J.F. Improved sequential extraction method for determination of alkali and alkaline earth metals in Zhundong coals. Fuel 2016, 181, 951–957. [Google Scholar] [CrossRef]
- Li, G.; Li, S.; Huang, Q.; Yao, Q. Fine particulate formation and ash deposition during pulverized coal combustion of high-sodium lignite in a down-fired furnace. Fuel 2015, 143, 430–437. [Google Scholar] [CrossRef]
- Zevenhoven-Onderwater, M.; Backman, R.; Skrifvars, B.J.; Hupa, M. The ash chemistry in fluidised bed gasification of biomass fuels. Part I: Predicting the chemistry of melting ashes and ash-bed material interaction. Fuel 2001, 80, 1489–1502. [Google Scholar] [CrossRef]
- Jafari, D.; Wits, W.W. The utilization of selective laser melting technology on heat transfer devices for thermal energy conversion applications: A review. Renew. Sustain. Energy Rev. 2018, 91, 420–442. [Google Scholar] [CrossRef]
- Su, Z.; Ouyang, T.; Chen, J.; Xu, P.H.; Tan, J.Q.; Chen, N.; Huang, H.Z. Green and efficient configuration of integrated waste heat and cold energy recovery for marine natural gas/diesel dual-fuel engine. Energy Convers. Manag. 2020, 209, 112650. [Google Scholar] [CrossRef]
- Kleinhans, U.; Wieland, C.; Frandsen, F.J.; Spliethoff, H. Ash formation and deposition in coal and biomass fired combustion systems: Progress and challenges in the field of ash particle sticking and rebound behavior. Prog. Energy Combust. Sci. 2018, 68, 65–168. [Google Scholar] [CrossRef]
- Chen, X.; Tang, J.; Tian, X.; Wang, L. Influence of biomass addition on Jincheng coal ash fusion temperatures. Fuel 2015, 160, 614–620. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, Z.; Li, J.; Zhang, B.H.; Zhou, H.D.; Xu, K.L. Experimental investigation of physicochemical and slagging characteristics of inorganic constituents in ash residues from gasification of different herbaceous biomass. Energy 2020, 198, 117367. [Google Scholar] [CrossRef]
- Deng, L.; Jiang, J.; Tie, Y.; Ma, S.H.; Fan, G.F.; Zhu, T.; Che, D.F. Potassium transformation and release during biomass combustion. Can. J. Chem. Eng. 2023, 101, 337–346. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, W.; Rokni, E.; Chen, G.Y.; Sun, R.; Levendis, Y.A. Release of alkalis and chlorine from combustion of waste pinewood in a fixed bed. Energy Fuels 2019, 33, 1256–1266. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Bai, X.S.; Fatehi, H. A multi-step predictive model for the release and transformation of K-Cl-S-containing species from biomass. Combust. Flame 2023, 247, 112512. [Google Scholar] [CrossRef]
- Wang, M.; Xu, D.; Bai, Y.; Yu, G.S.; Zhang, J.X.; Zhang, S.J.; Xu, J.; Zhang, H.; Zhang, S.; Wei, J.T. Dynamic investigation on potassium migration and transformation during biochar combustion and its correlation with combustion reactivity. Fuel 2023, 340, 127540. [Google Scholar] [CrossRef]
- Fatehi, H.; Costa, M.; Bai, X.S. Numerical study on K/S/Cl release during devolatilization of pulverized biomass at high temperature. Proc. Combust. Inst. 2021, 38, 3909–3917. [Google Scholar] [CrossRef]
- Niu, Y.; Tan, H. Ash-related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Dai, Y.; Yin, H.; Zhao, J.; Zhu, P.C.; Suo, Z.L. Preparation of Biochar from Straw in Northeast China to Assist in Carbon Neutrality: Data Visualization and Comprehensive Evaluation. Water Air Soil Pollut. 2025, 236, 324. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Cheng, S.; Wang, K.; Cheng, L.; Zhu, J.X.; Zheng, H.S.; Duan, W.J. Emission characteristics and reactivity of volatile organic compounds from typical high-energy-consuming industries in North China. Sci. Total Environ. 2022, 809, 151134. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, S.; Li, Y.; Zhang, Z.Y.; Guo, P.; Yang, H.Y.; Zhang, S.W.; Pan, R.K. The occurrence state of moisture in coal and its influence model on pore seepage. RSC Adv. 2018, 8, 5420–5432. [Google Scholar] [CrossRef]
- Li, R.; Kai, X.; Yang, T.; Sun, Y.; He, Y.Z.; Shen, S.Q. Release and transformation of alkali metals during co-combustion of coal and sulfur-rich wheat straw. Energy Convers. Manag. 2014, 83, 197–202. [Google Scholar] [CrossRef]
- Jones, J.M.; Darvell, L.I.; Bridgeman, T.G.; Pourkashanian, M.; Williams, A. An investigation of the thermal and catalytic behaviour of potassium in biomass combustion. Proc. Combust. Inst. 2007, 31, 1955–1963. [Google Scholar] [CrossRef]
- Fatehi, H.; He, Y.; Wang, Z.; Li, Z.S.; Bai, X.S.; Aldén, M.; Cen, K.F. LIBS measurements and numerical studies of potassium release during biomass gasification. Proc. Combust. Inst. 2015, 35, 2389–2396. [Google Scholar] [CrossRef]
- Van Lith, S.C.; Alonso-Ramírez, V.; Jensen, P.A.; Glarborg, P. Release to the gas phase of inorganic elements during wood combustion. Part 1: Development and evaluation of quantification methods. Energy Fuels 2008, 22, 1598–1609. [Google Scholar] [CrossRef]
- Sams, D.A.; Talverdian, T.; Shadman, F. Kinetics of catalyst loss during potassium-catalysed CO2 gasification of carbon. Fuel 1985, 64, 1208–1214. [Google Scholar] [CrossRef]
- Delannay, F.; Tysoe, W.T.; Heinemann, H. The role of KOH in the steam gasification of graphite: Identification of the reaction steps. Carbon 1984, 22, 401–407. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Lv, Y.; Wan, K.D.; He, Y.; Xia, J.; Cen, K.F. Inhibition of sodium release from Zhundong coal via the addition of mineral additives: A combination of online multi-point LIBS and offline experimental measurements. Fuel 2018, 212, 498–505. [Google Scholar] [CrossRef]
- Glarborg, P.; Marshall, P. Mechanism and modeling of the formation of gaseous alkali sulfates. Combust. Flame 2005, 141, 22–39. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.; Lin, L.; Zhang, X.L. Release of potassium in association with structural evolution during biomass combustion. Fuel 2021, 287, 119524. [Google Scholar] [CrossRef]
- Zhang, S.; Min, G.; Wang, J.; Zhu, S.Z.; Zhang, H.L.; Liu, X.Z. Release characteristics of potassium and chlorine for torrefied wheat straw during a combined pyrolysis-combustion system. Bioresour. Technol. 2020, 312, 123591. [Google Scholar] [CrossRef]
- Deng, L.; Ye, J.; Jin, X.; Zhu, T.; Che, D.F. Release and transformation of potassium during combustion of biomass. Energy Procedia 2017, 142, 401–406. [Google Scholar] [CrossRef]
- Wei, J.; Wang, M.; Xu, D.; Shi, L.; Li, B.; Bai, Y.H.; Yu, G.S.; Bao, W.N.; Xu, J.; Zhang, H.; et al. Migration and transformation of alkali/alkaline earth metal species during biomass and coal co-gasification: A review. Fuel Process. Technol. 2022, 235, 107376. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Zhong, W.; YU, Z.W. Migration and transformation law of potassium in the combustion of biomass blended coal. J. Fuel Chem. Technol. 2020, 48, 929–936. [Google Scholar] [CrossRef]
- He, J.; Li, J.; Huang, Q.; Yan, J.H. Release characteristics of potassium and sodium during pellet combustion of typical MSW fractions using the FES method. Combust. Flame 2022, 244, 112233. [Google Scholar] [CrossRef]
- Zhao, H.; Song, Q.; Wu, X.; Yao, Q. Study on the transformation of inherent potassium during the fast-pyrolysis process of rice straw. Energy Fuels 2015, 29, 6404–6411. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, H.; Xu, K.; Chen, S.K.; Ge, J.; Xu, Q.W. Systematic study on ash transformation behaviour and thermal kinetic characteristics during co-firing of biomass with high ratios of bituminous coal. Renew. Energy 2020, 147, 1453–1468. [Google Scholar] [CrossRef]
- Okuno, T.; Sonoyama, N.; Hayashi, J.; Li, C.Z.; Sathe, C.; Chiba, T. Primary release of alkali and alkaline earth metallic species during the pyrolysis of pulverized biomass. Energy Fuels 2005, 19, 2164–2171. [Google Scholar] [CrossRef]
- Van Eyk, P.J.; Ashman, P.J.; Nathan, G.J. Mechanism and kinetics of sodium release from brown coal char particles during combustion. Combust. Flame 2011, 158, 2512–2523. [Google Scholar] [CrossRef]
- Knudsen, J.N.; Jensen, P.A.; Dam-Johansen, K. Transformation and release to the gas phase of Cl, K, and S during combustion of annual biomass. Energy Fuels 2004, 18, 1385–1399. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Xia, J.; Vervisch, L.; Wan, K.D.; He, Y.; Whiddon, R.; Bahai, H.; Cen, K.F. Measurement and kinetics of elemental and atomic potassium release from a burning biomass pellet. Proc. Combust. Inst. 2019, 37, 2681–2688. [Google Scholar] [CrossRef]
- Blaesing, M.; Mueller, M. Co-gasification of waste wood with coal: Release of condensable and non-condensable inorganic gas species. Int. J. Hydrogen Energy 2018, 43, 435–441. [Google Scholar] [CrossRef]
- Hervy, M.; Olcese, R.; Bettahar, M.M.; Martine, M.; Aurélien, R.; Libeth, M.; Damien, R.; Guillain, M.; Anthony, D. Evolution of dolomite composition and reactivity during biomass gasification. Appl. Catal. A Gen. 2019, 572, 97–106. [Google Scholar] [CrossRef]
- Meng, F.H.; Yang, T.H.; Sun, Y.; He, Y.G.; Kai, X.P. Research progress migration and transformation of alkali metals during biomass combustion. Kezaisheng Nengyuan/Renew. Energy Resour. 2010, 28, 111–114. [Google Scholar]
- Niu, Y.; Tan, H.; Wang, X.; Liu, Z.N.; Liu, Y.; Xu, T.M. Study on deposits on the surface, upstream, and downstream of bag filters in a 12 MW biomass-fired boiler. Energy Fuels 2010, 24, 2127–2132. [Google Scholar] [CrossRef]
- Frandsen, F. Ash Formation, Deposition and Corrosion When Utilizing Straw for Heat and Power Production; DTU Chemical Engineering: Lyngby, Denmark, 2010. [Google Scholar]
- Van Lith, S.C.; Jensen, P.A.; Frandsen, F.J.; Glarborg, P. Release to the gas phase of inorganic elements during wood combustion. Part 2: Influence of fuel composition. Energy Fuels 2008, 22, 1598–1609. [Google Scholar] [CrossRef]
- Wang, S.; Duan, W.; Cao, C.; Yang, Z.Y.; Lv, H.M.; Qiu, Z.J. Determination of total sulphur and chloride in solid biomass fuel by tube furnace combustion-ion chromatography. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 440, p. 042104. [Google Scholar]
- Wu, D.; Wang, Y.; Wang, Y.; Li, S.; Wei, X.L. Release of alkali metals during co-firing biomass and coal. Renew. Energy 2016, 96, 91–97. [Google Scholar] [CrossRef]
- Peng, B.; Li, X.; Luo, J.; Yu, X. Fate of chlorine in rice straw under different pyrolysis temperatures. Energy Fuels 2019, 33, 9272–9279. [Google Scholar] [CrossRef]
- Müller, M.; Wolf, K.J.; Smeda, A.; Hilpert, K. Release of K, Cl, and S species during co-combustion of coal and straw. Energy Fuels 2006, 20, 1444–1449. [Google Scholar] [CrossRef]
- Dayton, D.C.; Belle-Oudry, D.; Nordin, A. Effect of coal minerals on chlorine and alkali metals released during biomass/coal cofiring. Energy Fuels 1999, 13, 1203–1211. [Google Scholar] [CrossRef]
- Lind, T.; Kauppinen, E.I.; Hokkinen, J.; Jorma, K.J.; Markku, O.; Minna, A.; Risto, H. Effect of chlorine and sulfur on fine particle formation in pilot-scale CFBC of biomass. Energy Fuels 2006, 20, 61–68. [Google Scholar] [CrossRef]
- Xue, Z.; Zhong, Z.; Zhang, B.; Zhang, J.; Xie, X.W. Potassium transfer characteristics during co-combustion of rice straw and coal. Appl. Therm. Eng. 2017, 124, 1418–1424. [Google Scholar] [CrossRef]
- Broström, M.; Kassman, H.; Helgesson, A.; Magnus, B.; Christer, A.; Rainer, B.; Anders, N. Sulfation of corrosive alkali chlorides by ammonium sulfate in a biomass fired CFB boiler. Fuel Process. Technol. 2007, 88, 1171–1177. [Google Scholar] [CrossRef]
- GB/T 28730–2012; Method for Preparation of Solid Biofuels Sample. China Environmental Science Press: Beijing, China, 2012.
- GB/T 28731-2012; Proximate Analysis of Solid Biofuels. China Environmental Science Press: Beijing, China, 2012.
- GB/T 476-2001; Elemental Analysis Methods for Coal. China Environmental Science Press: Beijing, China, 2001.
- Liu, Y.; Cheng, L.; Zhao, Y.; Ji, J.Q.; Wang, Q.H.; Luo, Z.Y.; Bai, Y. Transformation behavior of alkali metals in high-alkali coals. Fuel Process. Technol. 2018, 169, 288–294. [Google Scholar] [CrossRef]
- Deng, L.; Ye, J.; Jin, X.; Che, D.F. Transformation and release of potassium during fixed-bed pyrolysis of biomass. J. Energy Inst. 2018, 91, 630–637. [Google Scholar] [CrossRef]
- Reza, S.K.; Baruah, U.; Chattopadhyay, T.; Sarkar, D. Distribution of forms of potassium in relation to different agroecological regions of North-Eastern India. Arch. Agron. Soil Sci. 2014, 60, 507–518. [Google Scholar] [CrossRef]


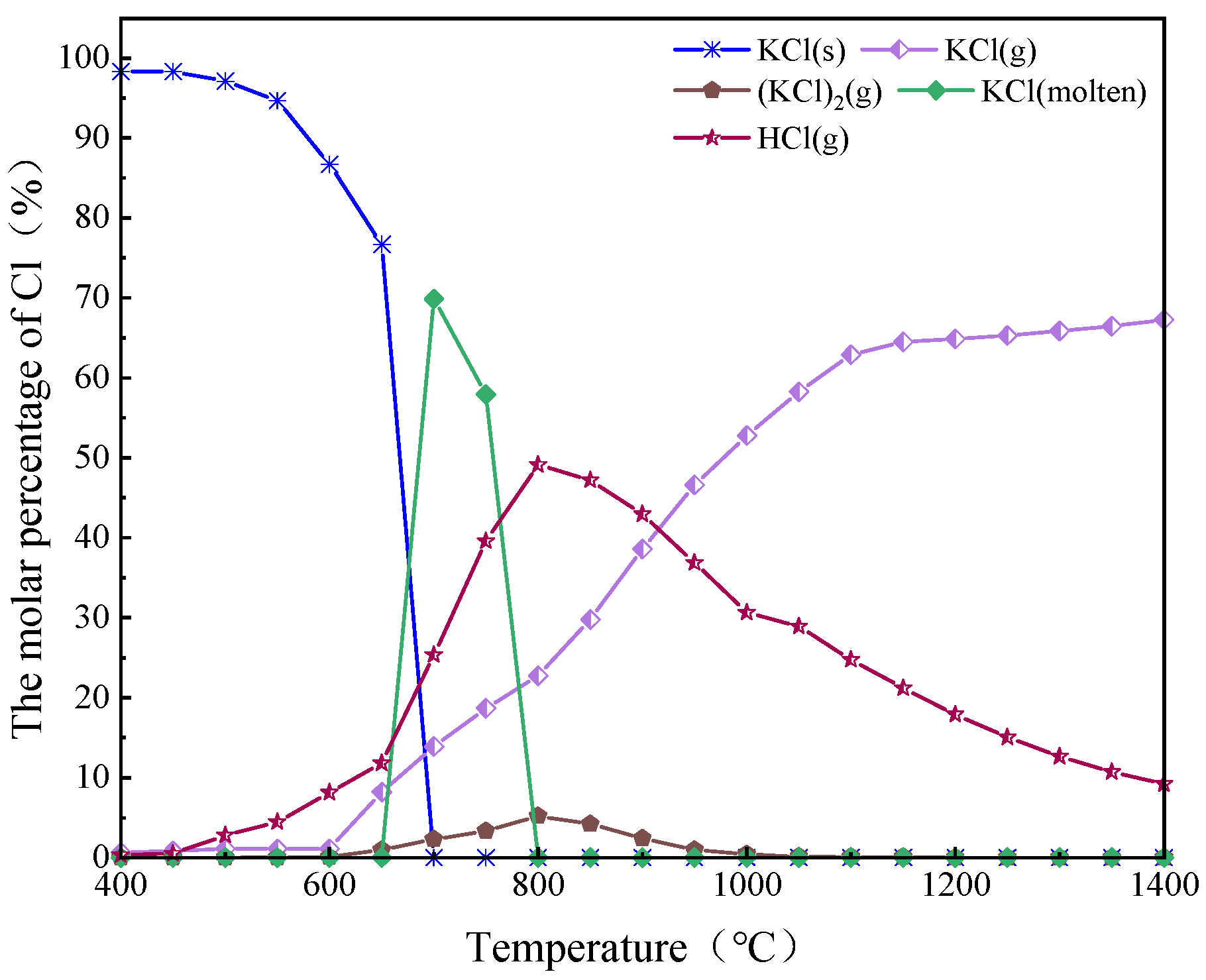
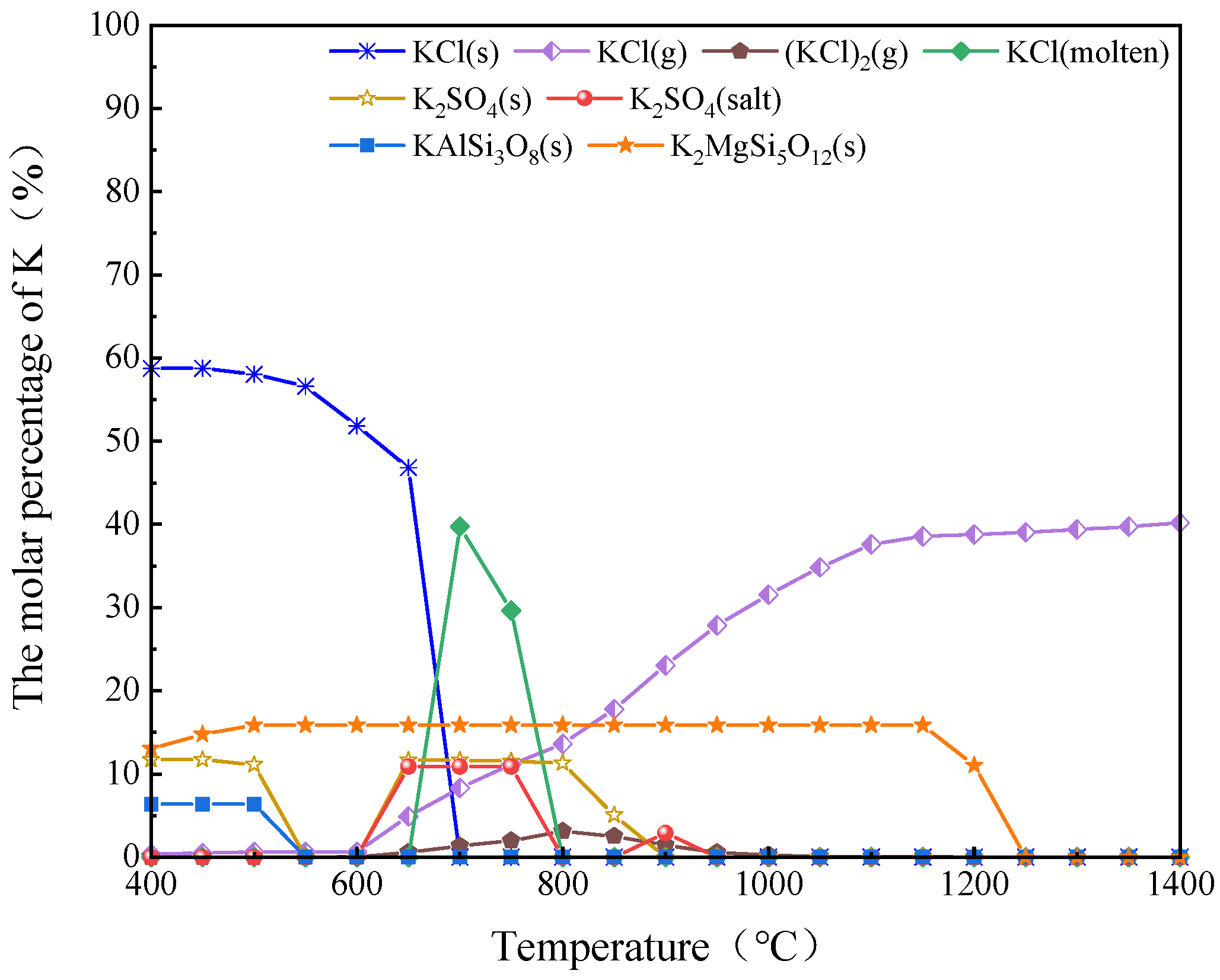
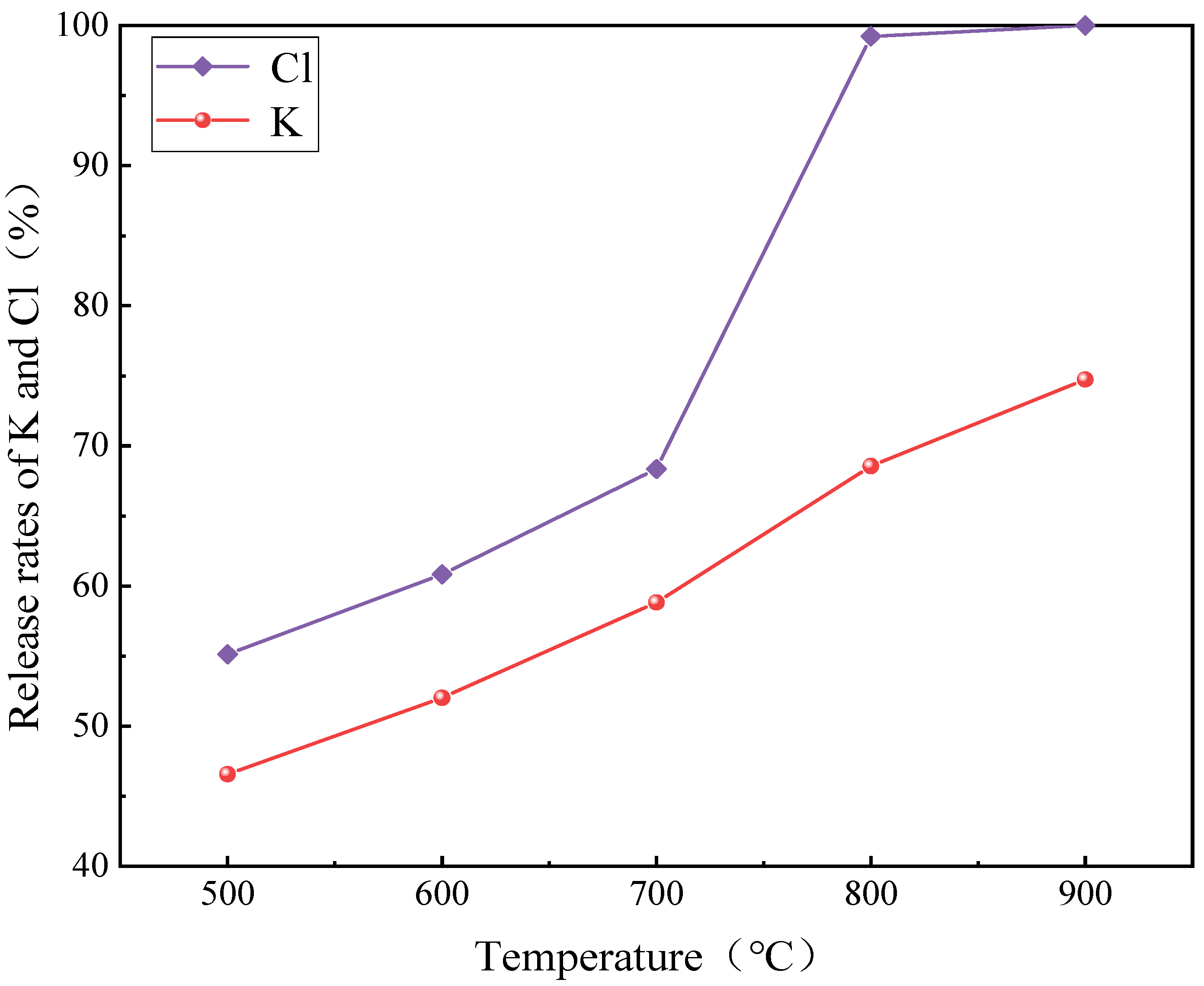
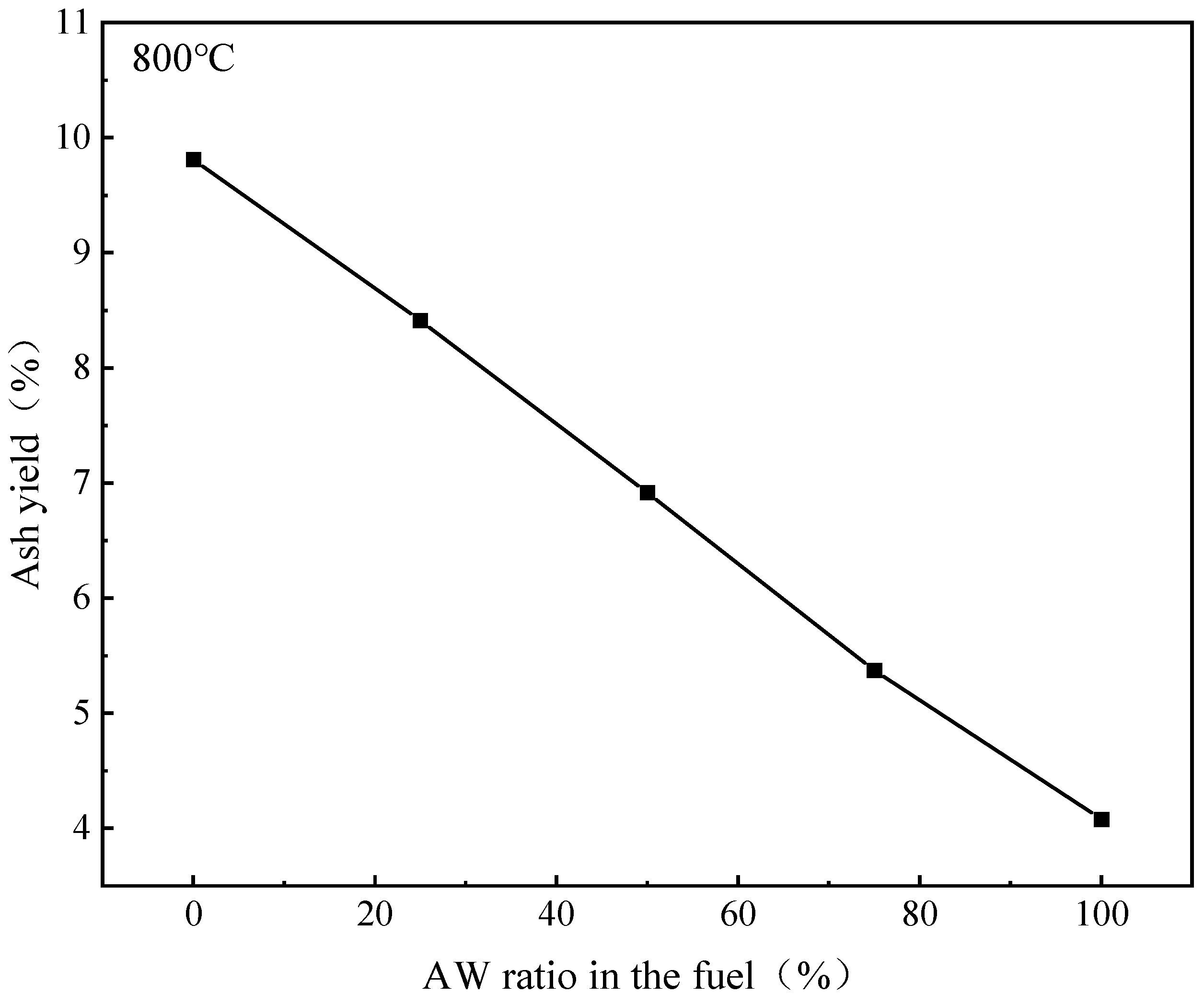


| K content of AW in Different Forms (mg/g) | AW | 500 °C | 600 °C | 700 °C | 800 °C | 900 °C |
|---|---|---|---|---|---|---|
| Water solubility | 19.32 | 10.04 | 8.84 | 6.84 | 4.34 | 3.02 |
| NH4Ac solubility | 0.96 | 0.64 | 0.48 | 0.41 | 0.21 | 0.12 |
| HCl solubility | 0.34 | 0.27 | 0.32 | 0.43 | 0.93 | 0.65 |
| Insolubility | 0.99 | 0.92 | 1.01 | 1.47 | 1.50 | 1.81 |
| Sum total | 22.20 | 11.86 | 10.65 | 9.14 | 6.98 | 5.61 |
| Element | Surface Element Content of Combustion Ash Sample (wt.%) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| C | 1.42 | 0.53 | 0.22 | 0.14 | 0 |
| O | 0.39 | 0.52 | 0.48 | 0.40 | 0.97 |
| Si | 0.02 | 0.08 | 0.06 | 0.16 | 0.45 |
| K | 0.14 | 0.10 | 0.11 | 0.04 | 0.17 |
| Cl | 0.12 | 0.06 | 0.07 | 0 | 0 |
| Mg | 0.04 | 0.12 | 0.11 | 0.13 | 0.23 |
| Ca | 0.02 | 0.07 | 0.07 | 0.19 | 0.13 |
| Al | 0 | 0 | 0 | 0 | 0.02 |
| Other elements | 97.85 | 98.52 | 98.88 | 98.94 | 98.03 |
| Samples | Industrial Analysis (wt.%) | Element Content(wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | V | FC | C | H | O | N | S | K | Cl | |
| Fujian bituminous coal | 10.01 | 32.78 | 57.21 | 71.60 | 4.83 | 11.63 | 1.08 | 0.85 | 0.93 | 0.25 |
| AW25 wt.% | 9.02 | 42.92 | 48.06 | 67.11 | 5.11 | 16.62 | 1.39 | 0.75 | 1.34 | 0.47 |
| AW50 wt.% | 6.54 | 55.68 | 37.78 | 60.78 | 6.17 | 24.45 | 1.42 | 0.64 | 1.59 | 0.71 |
| AW75 wt.% | 4.48 | 66.77 | 28.75 | 54.26 | 6.65 | 32.71 | 1.47 | 0.43 | 1.92 | 0.96 |
| AW100 wt.% | 4.07 | 76.39 | 19.72 | 45.56 | 6.79 | 42.11 | 1.12 | 0.35 | 2.22 | 1.21 |
| Samples | Proximate Analysis (wt.%) | Ultimate Analysis (wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Ad | Vd | FCd | Cd | Hd | Od | Nd | Sd | |
| AW | 4.07 | 76.39 | 19.72 | 45.56 | 6.79 | 42.11 | 1.12 | 0.35 |
| Ash | Chemical Composition (wt.%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K2O | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | CaO | TiO2 | MnO | Fe2O3 | Cl | |
| 500 °C | 22.54 | 0.17 | 18.40 | 1.24 | 31.83 | 2.60 | 1.54 | 7.61 | 0.07 | 0.30 | 4.64 | 10.17 |
| 600 °C | 21.69 | 0.18 | 19.56 | 1.64 | 30.62 | 2.86 | 1.30 | 8.00 | 0.07 | 0.27 | 4.90 | 9.27 |
| 700 °C | 17.50 | 0.21 | 22.94 | 2.08 | 32.14 | 3.38 | 1.44 | 9.22 | 0.08 | 0.30 | 4.79 | 5.94 |
| 800 °C | 12.80 | 0.28 | 25.99 | 2.31 | 35.5 | 4.01 | 1.81 | 10.44 | 0.09 | 0.33 | 4.74 | 0.67 |
| 900 °C | 11.32 | 0.24 | 26.86 | 2.19 | 32.96 | 3.70 | 2.33 | 10.21 | 0.10 | 0.34 | 4.91 | 0.09 |
| Samples | Element Content (kmol/t) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | Si | K | Na | Al | Ca | Mg | Fe | Cl | P | |
| AW | 37.97 | 67.90 | 26.31 | 0.80 | 0.11 | 0.76 | 0.57 | 0.09 | 0.04 | 0.14 | 0.10 | 0.03 | 0.34 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhou, Y.; Zhao, G.; Yuan, Q. Dynamic Migration Characteristics of Potassium During Agricultural Waste Combustion and the Mechanism of Combined Chlorine–Sulfur Action. Molecules 2025, 30, 2495. https://doi.org/10.3390/molecules30122495
Li J, Zhou Y, Zhao G, Yuan Q. Dynamic Migration Characteristics of Potassium During Agricultural Waste Combustion and the Mechanism of Combined Chlorine–Sulfur Action. Molecules. 2025; 30(12):2495. https://doi.org/10.3390/molecules30122495
Chicago/Turabian StyleLi, Jian, Yunlong Zhou, Guochao Zhao, and Qixin Yuan. 2025. "Dynamic Migration Characteristics of Potassium During Agricultural Waste Combustion and the Mechanism of Combined Chlorine–Sulfur Action" Molecules 30, no. 12: 2495. https://doi.org/10.3390/molecules30122495
APA StyleLi, J., Zhou, Y., Zhao, G., & Yuan, Q. (2025). Dynamic Migration Characteristics of Potassium During Agricultural Waste Combustion and the Mechanism of Combined Chlorine–Sulfur Action. Molecules, 30(12), 2495. https://doi.org/10.3390/molecules30122495





