Abstract
This study was the first to analyze the chemical compositions and bioactivities of Serissa japonica leaf oil. The oil, obtained via hydro-distillation with a 0.1% yield, contained 64 compounds, predominantly non-terpenic compounds (39.0%), oxygenated sesquiterpenes (31.4%), and oxygenated monoterpenes (25.6%). Major constituents included 1,8-cineole, (E)-nerolidol, and iso-longifolol. The oil showed good antioxidant activity (IC50 ≈ 62.79 ± 0.77 µg/mL for DPPH and 57.82 ± 1.12 µg/mL for ABTS) and a good anti-tyrosinase effect (IC50 ≈ 195.6 ± 3.82 µg/mL). The trend was similar to anti-inflammatory activity, with an IC50 value of 63.03 ± 3.22, for NO inhibition without cytotoxicity at 100 µg/mL. The bovine serum albumin (BSA) blocking assay demonstrated an IC50 value of 59.31 ± 0.71 µg/mL, indicating a good interaction regarding enzyme inhibition. Moreover, the computational modeling of the possible association between tyrosinase and cyclooxygenase-2 highlighted their antioxidant and anti-inflammatory properties. The results pointed out the usefulness of S. japonica essential oil as a natural candidate for managing oxidative stress and inflammation.
1. Introduction
Essential oils (EOs) are a rich source of plant-derived compounds known for their antioxidant, anti-inflammatory, and anti-glucosidase properties by reducing oxidative stress and modulating immune response [1,2]. The antioxidants in EOs help alleviate oxidative stress by neutralizing harmful radicals, thus safeguarding cells and supporting overall well-being [3]. The secondary metabolites found in EOs, such as phenolic compounds, monoterpenes, and sesquiterpenes, impact the mechanisms of inflammation and the immune response. As a result, they exhibit significant anti-inflammatory activity [4,5]. Chronic inflammation and oxidative stress are interconnected pathological processes that contribute to cancer development and progression. The pathophysiology of several age-related degenerative diseases, including diabetes, cancer, and heart failure, is closely linked to oxidative DNA damage [6]. Oxidative stress, hyperglycemia, and the emergence of diabetic complications are strongly correlated [7]. Inflammation and oxidative stress are both causes and effects of diabetic kidney diseases, so they could be regarded as reciprocal causes of the illness. Moreover, EOs have attracted attention for their potential anti-glucosidase activity, which may aid in managing type 2 diabetes. The regulation of blood glucose levels relies on α-glucosidase, an enzyme that plays an essential role in carbohydrate metabolism [8]. By blocking this enzyme, EOs could assist in slowing carbohydrate breakdown [9].
Plants in the Rubiaceae family have shown potential health benefits. They contain compounds that may help lower blood pressure, reduce cholesterol, manage blood sugar, and provide anti-inflammatory and antioxidant effects [10]. This family is edible and rich in bioactive compounds that affect multiple metabolic pathways. The structural diversity of terpenoids, anthraquinones, iridoids, and indole alkaloids adds to their worth as nutraceuticals, offering a range of health-promoting effects [11]. The phytochemical investigations of the Serissa genus have obtained several metabolites, including triterpenes, steroids, and lignans. The leaves, stems, and roots are commonly processed into powders, brewed as teas, or boiled to create extracts. The S. japonica is a flowering plant species in the Rubiaceae family. This species is native to subtropical forests and moist grasslands in Southeast Asia, ranging from India and China to Japan. The plant grows 2–4 feet tall and slightly wider, with rigid branches covered in small, glossy leaves and numerous white flowers [12]. Recent research has revealed that S. japonica contained a distinct array of lignan compounds, comprising furofuran-, tetrahydrofuran-, and arylnaphthalene-type lignans [13].
Despite the broad range of therapeutic benefits associated with this family and genus, there is a lack of scientific data specifically supporting S. japonica EO. For the first time, chemical compositions and biological activities such as antioxidants (DPPH and ABTS), anti-tyrosinase, anti-inflammatory (inhibition of nitric oxide (NO) production and bovine serum albumin (BSA) denaturation), and anti-α-glucosidase activities were evaluated. Molecular docking analysis was performed to elucidate the interactions between the predominant bioactive compounds and their target enzymes.
2. Results
2.1. GC-MS Profiles
The EO from S. japonica leaves was obtained as a yellow liquid with a yield of 0.1% (v/w, fresh weight basis), typical of low-yielding species within the Rubiaceae family. GC-MS analysis identified 64 compounds, accounting for 99.4% of the oil’s composition (Figure 1, Table 1). Non-terpenic compounds were the dominant class (39.0%), followed by oxygenated sesquiterpenes (31.4%), oxygenated monoterpenes (25.6%), oxygenated diterpenes (2.0%), sesquiterpene hydrocarbons (1.1%), and monoterpene hydrocarbons (0.3%). Diterpene hydrocarbons were absent. The major constituents were (E)-nerolidol (16.1%), 1,8-cineole (7.3%), iso-longifolol (5.8%), and isoamyl dodecanoate (4.3%). Other notable compounds (>1.0%) included α-terpineol (3.6%), benzeneacetaldehyde (3.3%), 2-methyl-1,3-cyclohexadiene (3.0%), borneol (3.0%), n-octanol (2.3%), (3Z)-hexenol (2.0%), benzaldehyde (2.0%), camphor (2.0%), cis-phytol (2.0%), n-tetradecanol (1.9%), endo-fenchol (1.8%), n-nonanol (1.8%), cedroxyde (1.6%), linalool (1.5%), methyl decyl ketone (1.4%), geranyl acetone (1.4%), n-nonanal (1.3%), terpinen-4-ol (1.3%), norpatchoulenol (1.3%), bulnesol (1.3%), n-Heptadecane (1.3%), fenchone (1.2%), (3E)-hexenal (1.1%), (2E)-decenal (1.1%), and valerianol (1.0%) (Figure 1).
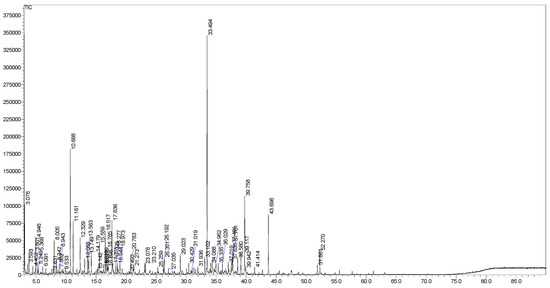
Figure 1.
GC chromatogram of Serissa japonica leaf EO.

Table 1.
Chemical composition of Serissa japonica leaf EO.
2.2. Biological Activities of EO
2.2.1. Antioxidant and Anti-Tyrosinase Detections
The antioxidant activity of the EO from S. japonica leaves was assessed using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) assays. The EO showed good antioxidant activity with an IC50 value of 62.79 ± 0.77 µg/mL for DPPH. A similar trend was observed in the ABTS assay, where the EO demonstrated a good effect with an IC50 value of 57.82 ± 1.12 µg/mL. The positive control exhibited lower IC50 values of 2.15 ± 0.03 µg/mL for DPPH and 1.95 ± 0.05 µg/mL for ABTS (Figure 2).
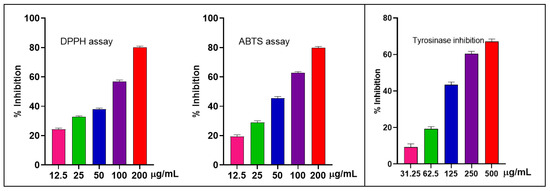
Figure 2.
Free radical scavenging activities (DPP and ABTS) and tyrosinase inhibition from S. japonica leaf EO.
Tyrosinase is crucial for melanin formation and is activated under oxidative conditions; thus, samples that efficiently scavenge free radicals may also modulate tyrosinase activity. As a result, the EO had moderate anti-tyrosinase activity with an IC50 value of 195.6 ± 3.82 µg/mL compared to vitamin C (IC50 ≈ 70.69 ± 1.65 µg/mL) (Figure 2).
2.2.2. The Anti-Inflammatory Effects in LPS-Stimulated RAW 264.7 Macrophages and BSA
The Griess assay was used to determine the inhibitory effects of S. japonica leaf EO on LPS-induced NO production in RAW 264.7 cells. EO showed good NO inhibition with the IC50 value of 63.03 ± 3.22 µg/mL. To ensure that the observed anti-inflammatory effects were not due to cytotoxicity, cell viability was evaluated in parallel using the MTT assay. Only the adherent cells at the bottom of the culture plate were used to assess viability, reflecting the survival activity of cells following treatment. The results indicated that cell viability remained high, with a survival rate of 95.78% at the highest tested concentration (100 µg/mL). It suggested that the EO is safe for use.
Regarding BSA inhibition, the pattern was similar to that of NO inhibition. It showed good properties with the IC50 value of 59.31 ± 0.71 µg/mL compared to the positive control diclofenac (IC50 ≈ 39.19 ± 1.44 µg/mL) (Figure 3).
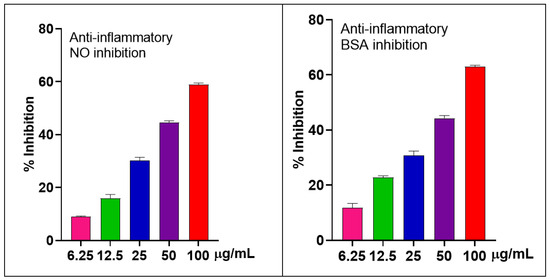
Figure 3.
Inhibitions of NO production (%) in LPS-stimulated RAW 264.7 cells and BSA of the S. japonica leaf EO.
2.2.3. Anti-α-Glucosidase Activity
Shown in Figure 4 is a dose-dependent response to the inhibition of α-glucosidase by S. japonica leaf EO. A notable inhibition of 52.51 ± 0.91% was observed at 500 µg/mL for the EO, whereas at 250 µg/mL, the inhibition was noticeably decreased at 34.82 ± 1.09%. Although there is some inhibitory capability in S. japonica leaf EO, the data suggested that it might not be the best choice for diabetes management.
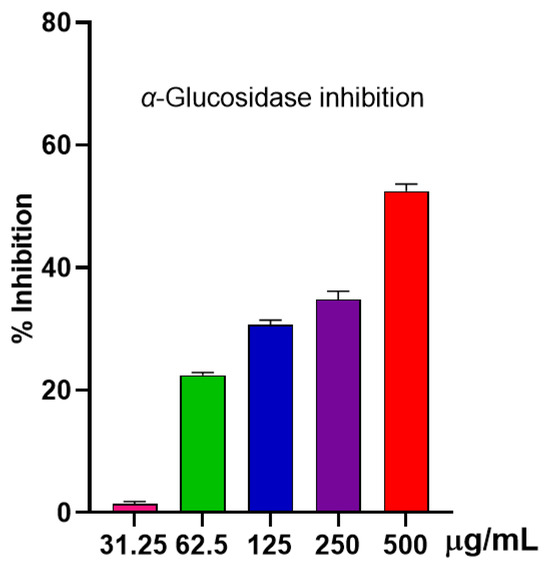
Figure 4.
α-glucosidase inhibition of the EO.
2.3. Molecular Docking Analysis
The EO showed good antioxidant and significant anti-inflammatory effects. Therefore, the main components such as (E)-nerolidol (16.1%) (a), 1,8-cineole (7.3%) (b), and iso-longifolol (5.8%) (c) in this EO were evaluated in silico for anti-tyrosinase and anti-inflammatory effects. Tyrosinase is an enzyme involved in melanin production, which can lead to pigmentation issues when overactive. Antioxidants can reduce oxidative stress, inhibit tyrosinase effects, and contribute to anti-inflammatory responses. Regarding the tyrosinase enzyme, a high binding energy indicates a stronger interaction between a–c and the protein. Three compounds, characterized by a lipophilic carbon framework, showed good energy ranging from −36.3 to −55.7 kcal/mol. Due to their specific chemical structures, the interactions between these three docked compounds and the protein binding site were primarily driven by hydrophobic interactions. Similarly, on the tyrosinase protein, compounds with an OH group (a and c) showed more favorable interactions with the binding site compared to b (Figure 5 and Table 2).
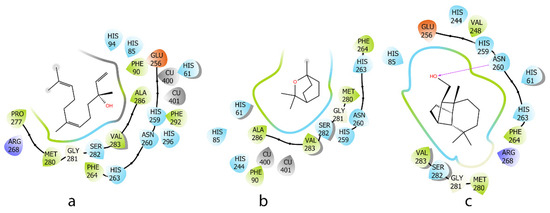
Figure 5.
Two-dimensional interactions of ligands (E)-nerolidol (a), 1,8-cineole (b), and iso-longifolol (c) with tyrosinase protein (2Y9X).

Table 2.
MM_GBSA binding free energy and interaction between ligands and tyrosinase protein (2Y9X).
On the COX-2 protein, compounds a and c, containing an OH group, demonstrated better MM-GBSA energy compared to b, which lacked this functional group. Compound c exhibited the strongest binding energy, with its OH group forming two hydrogen bonds with Arg 2120 and Tyr 2355. In contrast, compound b engaged only in hydrophobic interactions with the binding site, leading to weaker binding (Figure 6 and Table 3).
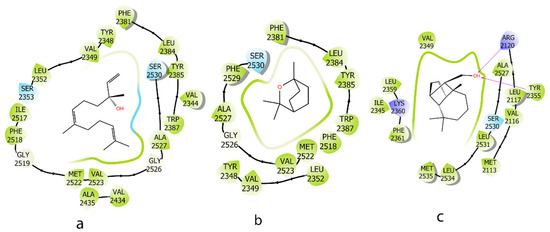
Figure 6.
Two-dimensional interactions of ligands (E)-nerolidol (a), 1,8-cineole (b), and iso-Longifolol (c) with COX-2 protein (1CVU).

Table 3.
MM_GBSA binding free energy and interaction between ligands and COX-2 protein (1CVU).
3. Discussion
Chemical Profile of EO
The EO of S. japonica leaves, non-terpenic compounds predominated at 39.0%, followed by oxygenated sesquiterpenes (31.4%), oxygenated monoterpenes (25.6%), oxygenated diterpenes (2.0%), sesquiterpene hydrocarbons (1.1%), and monoterpene hydrocarbons (0.3%), with diterpene hydrocarbons being absent. Major constituents included (E)-nerolidol (16.1%), 1,8-cineole (7.3%), and iso-longifolol (5.8%), alongside several compounds exceeding 1.0% abundance. Significant chemotypic divergence is revealed by comparative research within the Serissa genus, particularly regarding S. serissoides, which shows notable seasonal changes. δ-9(10)-tetrahydrocostunolide-1-keto (35.51%), 2-methoxy-4-vinylphenol (10.87%), and 1b,5,5,6a-tetramethyl-octahydro-1-oxa-cyclopropa[a]inden-6-one (7.32%) constitute the majority of the 43 chemicals (78.91% of the total oil) found in S. serissoides oil in fall. Germacrene D (12.311%), 5-propionyl-2-chlorobenzeneacetic acid methyl ester (8.541%), and 2-methoxy-4-vinylphenol (6.513%) are the main ingredients of the 72 chemicals that make up the oil in spring (79.88% of the total oil). Interestingly, despite their taxonomic proximity, S. japonica and S. serissoides do not share any dominating chemicals. In contrast to S. serissoides’ mixed terpenic, non-terpenic, and sesquiterpene-rich fall profile, S. japonica has a higher predominance of non-terpenic compounds (39.0%), suggesting significant genetic or environmental influence on biosynthesis pathways. Adaptive metabolic plasticity is suggested by seasonal shifts in S. serissoides [14].
S. japonica is unique among the Rubiaceae family in that it has a higher (E)-nerolidol concentration (16.1%) than other species. For example, in Geophila repens, β-caryophyllene (23.3%), and β-elemene (8.0%) predominate, while (E)-nerolidol makes up 3.3% [15]. According to earlier research, S. japonica’s high concentration of (E)-nerolidol indicates increased sesquiterpene production, which may be connected to ecological functions such as herbivore deterrence [15]. Broader comparisons within the Rubiaceae family reveal significant chemical diversity. Spathulenol (10.4%) and thujopsan-2-α-ol (9.5%) are found in Psychotria laui oil, whereas (E)-citral (20.6%) and 10-epi-γ-eudesmol (15.9%) are found in Psychotria asiatica oil [16]. Germacrene D (27.70%) is abundant in the flowers of Galium verum, while 2-methylbenzaldehyde (26.27%) dominates the leaves. Cruciata laevipes is dominated by cis-3-hexen-1-ol (9.69%) and β-caryophyllene (19.90%) [17]. S. japonica’s unique chemotypic profile within the family is further supported by the absence of substantial aldehydes or phenylpropanoids. In summary, S. japonica differs from comparable taxa because it has a distinct chemotype within the Rubiaceae family, with a higher (E)-nerolidol and a more significant proportion of non-terpenic compounds.
The Rubiaceae family displays antioxidative, anti-inflammatory, and anti-glucosidase effects owing to their prebiotic activity, free radical neutralizing ability, and immune system-modulating characteristics. The biological activity of the EO derived from S. japonica leaves was assessed. No published studies have reported the antioxidant, anti-inflammatory, and anti-glucosidase activities of EOs from the Serissa genus, rendering this observation a novel addition to the scientific record. Within the Rubiaceae family, comparative insights can be drawn from other species. For example, the EO from Psychotria asiatica leaves exhibited anti-inflammatory properties by reducing nitric oxide production, while showing only mild antioxidant activity [16]. This suggests that S. japonica EO has a relatively more robust antioxidant profile than P. asiatica within the same family. Additionally, the EO from Coffea arabica L. husks, another Rubiaceae species, displayed significant in vivo anti-glucosidase and antioxidant effects in a dose-dependent manner in the DPPH assay [18], further illustrating the variability of antioxidant potential within the family. Moreover, the extract of Psychotria malayana demonstrated significant potential in managing diabetes and inflammatory responses [19].
In this study, the EO was primarily noted for its antioxidant and anti-inflammatory activities, with the chemical composition consisting mainly of three key compounds: (E)-nerolidol (16.1%) (a), 1,8-cineole (or eucalyptol) (7.3%) (b), and iso-longifolol (5.8%) (c), along with several minor constituents. The high relative abundance of these compounds, as determined by GC-MS analysis, suggests that they are the dominant bioactive constituents in the EO. There is supporting evidence from the literature that these specific constituents have been previously reported to exhibit antioxidant and anti-inflammatory effects [20,21,22,23]. Therefore, we conducted an in silico analysis using the crystallographic structure of the COX-2 and tyrosinase enzymes. The presence of the hydroxyl group significantly improves the binding affinity of a and c by facilitating hydrogen bond formation with critical amino acids in the COX-2 and tyrosinase binding site [22,24]. Compound c, with its ability to form two hydrogen bonds with COX2, demonstrated the most potent binding, followed by one hydrogen bond with tyrosinase protein. Compound a accounted for the highest ratio (16.1%). It relied solely on hydrophobic interactions and showed the second-strongest binding affinity, underscoring the importance of functional groups in achieving strong protein–ligand interactions. This is consistent with prior research identifying (E)-nerolidol as a molecule with strong antioxidant and anti-inflammatory effects [20,21]. Similarly, 1,8-cineole, despite having a slightly lower binding affinity, showed considerable interaction with the COX-2 active site, possibly explaining its contribution to the EO’s anti-inflammatory properties [23]. Regarding the inhibition of α-glucosidase, there is currently no proof that the primary components of EO have any noticeable inhibitory effects. Our findings indicate that the EO exhibited only weak anti-glucosidase activity, reinforcing that these specific compounds may not play a crucial role in α-glucosidase inhibition. This suggests that the EO is unlikely to contribute significantly to regulating blood glucose levels.
4. Materials and Methods
4.1. General Procedures
The bioactivity measurements were conducted using a Thermo Fisher Scientific UV-Vis spectrophotometer for cuvette (U.S.) and SH-1200 microplate reader (Corona electric Co., Ltd., Tokyo, Japan) for 96-wells. The positive controls and reagents were obtained from Sigma Aldrich (St. Louis, MO, USA). Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) were sourced from Waco (Pure Chemical Industrial, Ltd., Osaka, Japan). The RAW 264.7 cells were supplied by the Riken Cell Bank (Tsukuba, Japan). Pure Chemical Industries, Ltd.: Osaka, Japan
4.2. Plant Materials
Fresh leaves of Serissa japonica (1.2 kg) were collected in February 2025 from Thua Thien Hue province, Vietnam (Latitude: 16°48′34.8″ N and Longitude: 107°58′88.6″ E). Identification was verified by Thao Xuan Hoang (Faculty of Biology, University of Education, Hue University), and a voucher specimen (SJL-202502) was deposited at the Faculty of Chemistry, University of Education, Hue University.
4.3. Hydro-Distillation of EO
The leaves were thoroughly washed, chopped into small fragments, and subjected to hydro-distillation for 4.5 h using a Clevenger-type apparatus, following Vietnamese Pharmacopeia guidelines [25]. The hydro-distillation of 1.2 kg of fresh leaves yielded 1.2 g of EO (0.1% w/w, fresh weight). The resulting EO was dried over anhydrous Na2SO4 and stored at 5 °C until analysis.
4.4. The GC-MS Analysis
GC-MS analysis was performed on a Shimadzu GCMS-QP2010 Plus system (Shimadzu, Kyoto, Japan) equipped with an Equity-5 fused silica capillary column (30 m × 0.25 mm, 0.25 μm film thickness; Supelco, PA, USA) [26,27]. Helium was used as the carrier gas at a flow rate of 1.2 mL/min. Injector and interface temperatures were maintained at 280 °C. The oven was set to 60 °C (held for 2 min), increased to 240 °C at 3 °C/min (held for 10 min), then raised to 280 °C at 5 °C/min (held for 35 min). Samples (1.0 µL) were injected in split mode (30:1) at an inlet pressure of 93.2 kPa. Mass spectrometry conditions included an ionization voltage of 70 eV, a detector voltage of 0.80 kV, and a scan range of 40–500 amu at 0.5 scan/s. Retention indices (RIs) were calculated relative to a C7–C40 n-alkane series co-injected with the sample. Compounds were identified by comparing RI values with the literature data and mass spectra with NIST 11 and WILEY 7 libraries [28].
4.5. Biological Activities
DPPH, ABTS radical scavenging, and tyrosinase inhibition activities: The antioxidant capacity of S. japonica leaf EO to neutralize free radicals produced from DPPH and ABTS, as well as its anti-tyrosinase effect, was assessed using the specified methodology, with requisite adaptations to accommodate the laboratory. The detailed protocols for these assessments have been previously outlined in our work [1,29].
Anti-inflammatory evaluation: The inhibitory effect of S. japonica leaf EO on LPS-induced NO generation in RAW 264.7 cells was assessed. The nitrite concentration, was evaluated utilizing the Griess reaction. The cytotoxicity assay was evaluated by MTT assay. Our previous studies have delineated comprehensive procedures for these assays [29].
Bovine serum albumin (BSA) assay: The ability of the EO to prevent the denaturation of BSA was assessed using a modified version described by Sakat et al. (2010) [30]. 10 mg/mL of EO was dissolved in DMSO, and then further diluted in phosphate-buffered saline (pH 6.3) to achieve final concentrations ranging from 6.25 to 100 µg/mL. 500 µL of the sample was mixed with 180 µL of a 5% BSA solution and buffer to make 2 mL. The mixtures were thoroughly mixed and incubated at 37 °C for 10 min, followed by heating at 100 °C for 3 min. After cooling, the absorbance was measured at 660 nm using a UV-Visible spectrophotometer. Diclofenac was used as a positive control [29].
Anti-α-glucosidase assay: The α-glucosidase inhibition experiment was conducted using previously established conditions with minor adjustments. The α-glucosidase enzyme was diluted with 0.1 M sodium phosphate buffer (pH 6.8) to achieve a concentration of 1 U/mL. Samples (500, 250, 125, 62.5, and 31.125 µg/mL) were combined with α-glucosidase solution (20 μL) in a 96-well plate. Then, p-NPG (0.53 mM; 380 μL), the substrate, was introduced, and the mixture was incubated in dry baths at 37 °C for 40 min. The reaction was halted by adding 500 μL of 0.1 M Na2CO3 solution. The absorbance of the generated p-nitrophenol was quantified by assessing the reduction in absorbance at 400 nm [31].
4.6. In Silico Analysis
The COX-2 protein (PDB: 1CVU) [32] and the Tyrosinase protein (PDB: 2Y9X) [33] utilized in this investigation were sourced from the Protein Data Bank and processed via the Protein Preparation Workflow [34]. The ligands were prepared using LigPrep. Docking was later performed with Induced Fit Docking with Enhanced Precision [35]. The protein–ligand complex was subjected to energy computation by MM-GBSA to assess the binding affinity and stability of the interactions [36].
Statistical Analysis: The data of bioassays were analyzed using GraphPad Prism, version 9.3.0, GraphPad Software, San Diego, California, with mean ± standard deviation (SD), n = 3.
5. Conclusions
For the first time, we reported on both the chemical constituents and biological effects of EO obtained from S. japonica leaves. We detected 64 chemical components using GC-MS, with 1,8-cineole, (E)-nerolidol, and iso-longifolol accounting for 7.3%, 16.1%, and 5.8% of the oil, respectively. EO inhibited good antioxidants, as evidenced by ABTS and DPPH assays, and exhibited anti-inflammatory activities without cytotoxicity through NO inhibition and BSA denaturation assays. However, it exhibited weak α-glucosidase inhibition. In silico analysis of the main components of tyrosinase and COX-2 enzymes was conducted to support these activities. Taken together, the S. japonica leaves EO was not a strong candidate for diabetes treatment, but its anti-inflammatory without cytotoxicity and antioxidant properties suggested the potential for medicinal applications.
Author Contributions
Conceptualization, T.V.P.; methodology, T.V.P., T.-Y.V. and H.M.N.; formal analysis T.V.P., T.-Y.V. and H.M.N.; investigation, T.V.P., T.-Y.V. and H.M.N.; resources, T.V.P.; data curation, H.M.N. and T.V.P.; writing—original draft preparation, T.V.P.; writing—review and editing, H.M.N.; visualization, T.V.P.; supervision, H.M.N.; project administration, H.M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nguyen, H.M.; Pham, T.V.; Vo, H.Q.; Nguyen, H.T.; Nguyen, L.T.K.; Nguyen, B.C.; Chung, K.L.; Ho, D.V. Essential oil from Vietnamese Peperomia leptostachya Hook. & Arn.(Piperaceae): Chemical composition, antioxidant, anti-inflammatory, cytotoxic activities, and in silico analysis. Molecules 2024, 29, 2808. [Google Scholar] [CrossRef]
- John, R.; Manilal, A.; Varghese, L.S.; Govindarajan, S. Chemical composition, antimicrobial and antioxidant activities of Vateria indica L. oleo-gum resin (white dammar) essential oil. J. Essent. Oil Bear. Plants 2024, 27, 1516–1523. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative stress: The role of antioxidant phytochemicals in the prevention and treatment of diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Moraes, F.d.S.R.; Moraes, L.H.R.; Macedo, A.B.; Valduga, A.H.; Mizobuti, D.S.; Rocha, G.L.d.; Silva, H.N.M.d.; Salvador, M.J.; Minatel, E. Anti-inflammatory and antioxidant activities of Citrus aurantifolia (Christm.) Swingle essential oil in dystrophic muscle cells: Implication of the PGC-1α pathway. J. Essent. Oil Bear. Plants 2024, 27, 34–46. [Google Scholar] [CrossRef]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative stress in ageing and chronic degenerative pathologies: Molecular mechanisms involved in counteracting oxidative stress and chronic inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef]
- Ceriello, A. Oxidative stress and diabetes-associated complications. Endocr. Pract. 2006, 12, 60–62. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and oxidative stress: An integral, updated and critical overview of their metabolic interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Lin, H.-R.; Yang, C.-S.; Liaw, C.-C.; Sung, P.-J.; Kuo, Y.-H.; Cheng, M.-J.; Chen, J.-J. Antioxidant and anti-α-glucosidase activities of various solvent extracts and major bioactive components from the fruits of Crataegus pinnatifida. Antioxidants 2022, 11, 320. [Google Scholar] [CrossRef]
- González-Castelazo, F.; Soria-Jasso, L.E.; Torre-Villalvazo, I.; Cariño-Cortés, R.; Muñoz-Pérez, V.M.; Ortiz, M.I.; Fernández-Martínez, E. Plants of the Rubiaceae family with effect on metabolic syndrome: Constituents, pharmacology, and molecular targets. Plants 2023, 12, 3583. [Google Scholar] [CrossRef]
- Mamun-or-Rashid, A.; Hossain, M.S.; Hassan, N.; Dash, B.K.; Sapon, M.A.; Sen, M.K. A review on medicinal plants with antidiabetic activity. J. Pharmacogn. Phytochem. 2014, 3, 149–159. [Google Scholar] [CrossRef]
- Thunberg, C.P. Nova Genera Plantarum; Upsaliæ: London, UK, 1798; Volume 9, pp. 1743–1828. [Google Scholar]
- Zhang, D.Y.; Wang, X.X.; Wang, M.; Su, B.L.; Wang, Y.X.; Li, J.Y.; Yi, J.; Chen, X.Y.; Zhuang, P.Y.; Liu, H. Chemotaxonomic significance of lignans from Serissa japonica (Thunb.) Thunb. Biochem. Syst. Ecol. 2021, 99, 104350. [Google Scholar] [CrossRef]
- Ni, S.F.; Fu, C.X.; Pan, Y.J.; Lu, Y.B.; Wu, P.; Chan, Y.S.G. Contrastive analysis of volatile oil from Serissa serissoides in different seasons. China J. Chin. Mater. Med. 2004, 28, 54–58. [Google Scholar]
- Rao, H.; Lai, P.; Gao, Y. Chemical composition, antibacterial activity, and synergistic effects with conventional antibiotics and nitric oxide production inhibitory activity of essential oil from Geophila repens (L.) IM Johnst. Molecules 2017, 22, 1561. [Google Scholar] [CrossRef]
- Tran, T.D.; Le, A.T.; Le, T.Q.; Pham, T.V. Major sesquiterpenoids in Psychotria laui leaf essential oil exhibit anti-inflammatory and cytotoxic properties. Nat. Prod. Commun. 2024, 19, 1934578X241293925. [Google Scholar] [CrossRef]
- Tava, A.; Biazzi, E.; Ronga, D.; Avato, P. Identification of the volatile components of Galium verum L. and Cruciata leavipes opiz from the western Italian Alps. Molecules 2020, 25, 2333. [Google Scholar] [CrossRef]
- Lozada-Ramírez, J.D.; Guerrero-Moras, M.C.; González-Peña, M.A.; Silva-Pereira, T.S.; Anaya de Parrodi, C.; Ortega-Regules, A.E. Stabilization of anthocyanins from coffee (Coffea arabica L.) husks and in vivo evaluation of their antioxidant activity. Molecules 2023, 28, 1353. [Google Scholar] [CrossRef]
- Syed Mohamad, S.N.A.; Khatib, A.; So’ad, S.Z.M.; Ahmed, Q.U.; Ibrahim, Z.; Nipun, T.S.; Humaryanto, H.; AlAjmi, M.F.; Khalifa, S.A.; El-Seedi, H.R. In vitro anti-diabetic, anti-inflammatory, antioxidant activities and toxicological study of optimized Psychotria malayana Jack leaves extract. Pharmaceuticals 2023, 16, 1692. [Google Scholar] [CrossRef]
- Chan, W.-K.; Tan, L.T.-H.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Nerolidol: A sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- Fonsêca, D.V.; Salgado, P.R.; de Carvalho, F.L.; Salvadori, M.G.S.; Penha, A.R.S.; Leite, F.C.; Borges, C.J.S.; Piuvezam, M.R.; Pordeus, L.C.d.M.; Sousa, D.P. Nerolidol exhibits antinociceptive and anti-inflammatory activity: Involvement of the GABA ergic system and proinflammatory cytokines. Fundam. Clin. Pharmacol. 2016, 30, 14–22. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Fang, H.; Yang, H.; Wu, T.; Shi, X.; Pang, C. Isolongifolene alleviates liver ischemia/reperfusion injury by regulating AMPK-PGC1α signaling pathway-mediated inflammation, apoptosis, and oxidative stress. Int. Immunopharmacol. 2022, 113, 109185. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.O.B.P.B.; Rodrigues, L.B.; Cesário, F.R.A.S.; de Oliveira, M.R.C.; Tintino, C.D.M.; e Castro, F.F.; Alcantara, I.S.; Fernandes, M.N.M.; de Albuquerque, T.R.; da Silva, M.S.A. Anti-edematogenic and anti-inflammatory activity of the essential oil from Croton rhamnifolioides leaves and its major constituent 1,8-cineole (eucalyptol). Biomed. Pharmacother. 2017, 96, 384–395. [Google Scholar] [CrossRef]
- Barone, M.; Pannuzzo, G.; Santagati, A.; Catalfo, A.; De Guidi, G.; Cardile, V. Molecular docking and fluorescence characterization of benzothieno [3,2-d] pyrimidin-4-one sulphonamide thio-derivatives, a novel class of selective cyclooxygenase-2 Inhibitors. Molecules 2014, 19, 6106–6122. [Google Scholar] [CrossRef]
- Ministry of Health. Vietnamese Pharmacopoeia; Medical Publishing House: Hanoi, Vietnam, 2017; p. 89. [Google Scholar]
- Pham, T.V.; Le, A.T.; Nguyen, N.H.; Nguyen, H.H.; Tran, G.-B.; Do, B.H. Phytochemical analysis and investigation of the anti-inflammatory and anticancerous activity of Walsura robusta leaf volatile compounds. J. Essent. Oil Bear. Plants 2025, 28, 83–94. [Google Scholar] [CrossRef]
- Pham, T.V.; Hoang, T.X.; Do, B.H.; Nguyen, K.Q.T.; Nguyen, N.H.; Tran, G.B. Chemical compositions, molecular docking, anti-inflammatory, and anti-cancer effects of the leaf essential oils isolated from three species of the Rutaceae family in Vietnam. J Chem. Biodiver. 2025, 22, e202401466. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Texensis Publishing: Gruver, TX, USA, 2007; pp. 46–52. [Google Scholar]
- Nguyen, H.M.; Nguyen, K.P.; Le, A.T.P.; Nguyen, N.H.T.; Vu-Huynh, L.K.; Le, C.K.T.; Delpe Acharige, A.; Hull, K.; Romo, D. Antioxidant, anti-tyrosinase, hepatoprotective, and anti-inflammatory potential in flowers and seeds of Ochna integerrima (Lour.) Merr. J Nat. Prod. Res. 2024, 17, 1–11. [Google Scholar] [CrossRef]
- Sakat, S.; Juvekar, A.R.; Gambhire, M.N. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. J Int. Pharm. Pharm. Sci. 2010, 2, 146–155. [Google Scholar]
- Shulgau, Z.; Palamarchuk, I.V.; Sergazy, S.; Urazbayeva, A.; Ramankulov, Y.; Kulakov, I.V. Synthesis, computational study, and in vitro α-Glucosidase inhibitory action of 1, 3, 4-Thiadiazole derivatives of 3-Aminopyridin-2 (1 H)-ones. Pharmaceuticals 2024, 17, 377. [Google Scholar] [CrossRef]
- Kiefer, J.R.; Pawlitz, J.L.; Moreland, K.T.; Stegeman, R.A.; Hood, W.F.; Gierse, J.K.; Stevens, A.M.; Goodwin, D.C.; Rowlinson, S.W.; Marnett, L.J. Structural insights into the stereochemistry of the cyclooxygenase reaction. Nature 2000, 405, 97–101. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef]
- Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.; Honig, B.; Shaw, D.E.; Friesner, R.A. A hierarchical approach to all-atom protein loop prediction. Proteins Struct. Funct. Bioinf. 2004, 55, 351–367. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).