Graphene Quantum Dots for Glioblastoma Treatment and Detection–Systematic Review
Abstract
1. Introduction
2. Results and Discussion
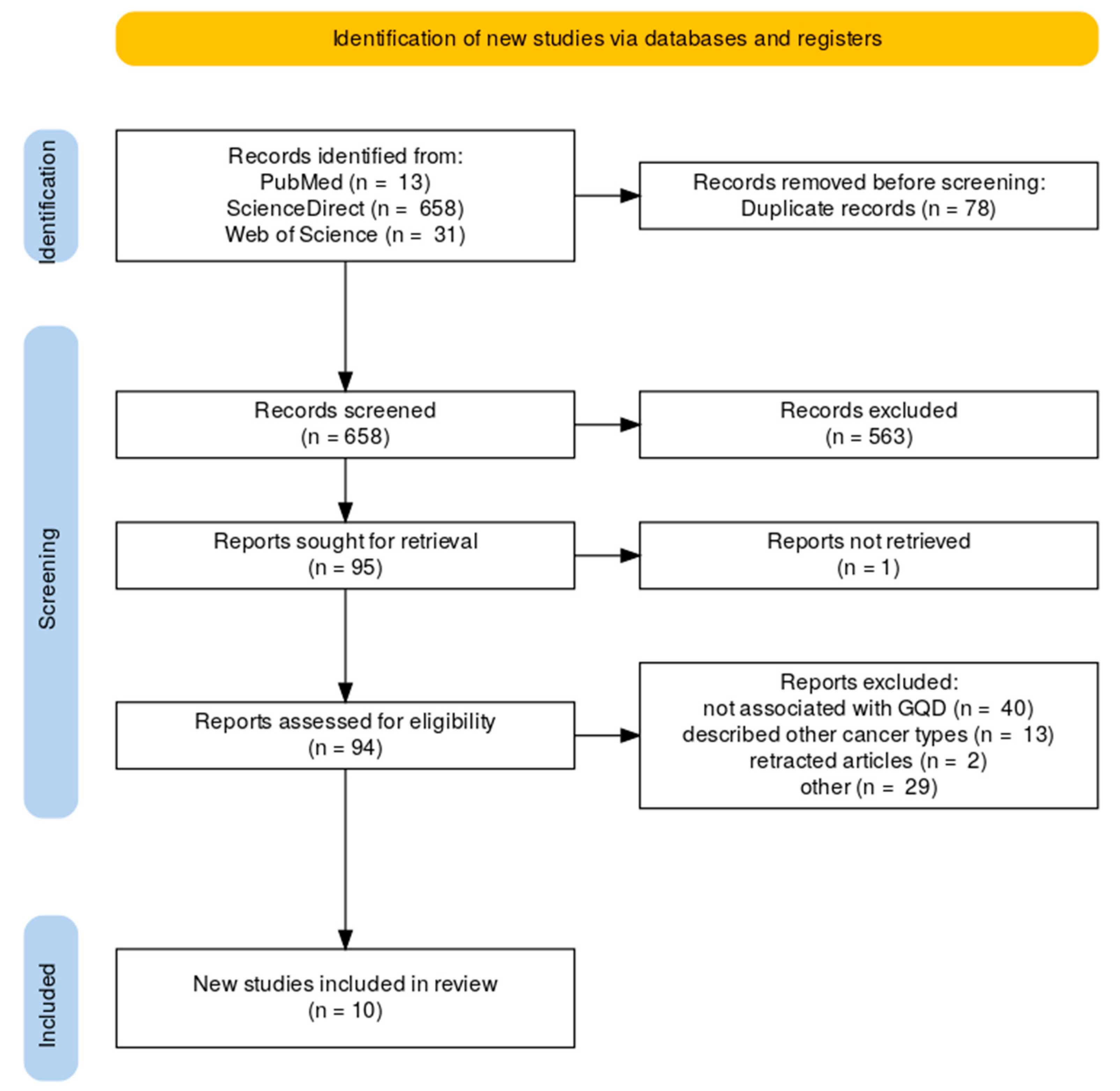
2.1. Literature Review Results
2.2. GQDs as PTT-Inducing Agent
2.3. GQDs as a Pretreatment Agent in Neoadjuvant Glioblastoma Therapy
2.4. GQDs as a Component of Glioblastoma Detecting Biosensors
2.5. GQDs for Drug Delivery in Glioblastoma
3. Methods
3.1. Literature Review
3.2. Data Extraction
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DLS | Digital Light Scattering |
| AFM | Atomic Force Microscopy |
| TEM | Transmission Electron Microscopy |
| TNF-α | Tumor Necrosis Factor alpha |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| BDNF | Brain-Derived Neurotropic Factor |
| N.D. | No Data |
References
- Rubinstein, I.; Weinberg, G.L. Nanomedicines for chronic non-infectious arthritis: The clinician’s perspective. Nanomedicine 2012, 8, S77–S82. [Google Scholar] [CrossRef] [PubMed]
- Kotcherlakota, R.; Das, S.; Patra, C.R. Chapter 16—Therapeutic applications of green-synthesized silver nanoparticles. In Green Synthesis, Characterization and Applications of Nanoparticles; Shukla, A.K., Iravani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 389–428. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, R.; Mishra, J.; Dutta, K.; Ahlawat, P.; Kumar, A.; Dhanasekaran, S.; Gupta, A.K.; Sinha, S.; Bishi, D.K.; et al. Strategies facilitating the permeation of nanoparticles through blood-brain barrier: An insight towards the development of brain-targeted drug delivery system. J. Drug Deliv. Sci. Technol. 2023, 86, 104694. [Google Scholar] [CrossRef]
- Speranza, G. Carbon nanomaterials: Synthesis, functionalization and sensing applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Barati, F.; Avatefi, M.; Moghadam, N.B.; Asghari, S.; Ekrami, E.; Mahmoudifard, M. A review of graphene quantum dots and their potential biomedical applications. J. Biomater. Appl. 2022, 37, 1137–1158. [Google Scholar] [CrossRef]
- Kalluri, A.; Dharmadhikari, B.; Debnath, D.; Patra, P.; Kumar, C.V. Advances in Structural Modifications and Properties of Graphene Quantum Dots for Biomedical Applications. ACS Omega 2023, 8, 21358–21376. [Google Scholar] [CrossRef]
- Chung, S.; Revia, R.A.; Zhang, M. Graphene Quantum Dots and Their Applications in Bioimaging, Biosensing, and Therapy. Adv. Mater. 2021, 33, e1904362. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Chapter 5—Graphene quantum dots, graphene nanoplatelets, and graphene nanoribbons with polymers. In Graphene to Polymer/Graphene Nanocomposites; Kausar, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 91–116. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Huang, P.; Shi, J.J.; Zhang, M.; Jiang, X.H.; Zhong, H.X.; Ding, Y.M.; Cao, X.; Wu, M.; Lu, J. Anomalous Light Emission and Wide Photoluminescence Spectra in Graphene Quantum Dot: Quantum Confinement from Edge Microstructure. J. Phys. Chem. Lett. 2016, 7, 2888–2892. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, H.; Sun, L. Graphene quantum dots: Versatile photoluminescence for energy, biomedical, and environmental applications. J. Mater. Chem. C Mater. 2015, 3, 1157–1165. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, L.; Shi, M.; Wang, Y.; Meng, X.; Chen, Y.; Huang, Q.; Liu, C. A Review of Advances in Graphene Quantum Dots: From Preparation and Modification Methods to Application. C J. Carbon Res. 2024, 10, 7. [Google Scholar] [CrossRef]
- Chen, F.; Gao, W.; Qiu, X.; Zhang, H.; Liu, L.; Liao, P.; Fu, W.; Luo, Y. Graphene quantum dots in biomedical applications: Recent advances and future challenges. Front. Lab. Med. 2017, 1, 192–199. [Google Scholar] [CrossRef]
- Wang, K.; Dong, J.; Sun, L.; Chen, H.; Wang, Y.; Wang, C.; Dong, L. Effects of elemental doping on the photoluminescence properties of graphene quantum dots. RSC Adv. 2016, 6, 91225–91232. [Google Scholar] [CrossRef]
- Fan, Z.; Zhou, S.; Garcia, C.; Fan, L.; Zhou, J. PH-Responsive fluorescent graphene quantum dots for fluorescence-guided cancer surgery and diagnosis. Nanoscale 2017, 9, 4928–4933. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Khatun, Z.; Huh, K.M.; Park, S.Y.; Lee, D.Y.; Cho, K.J.; Lee, Y.-K. In Vivo Biodistribution and Toxicology of Carboxylated Graphene Quantum Dots. ACS Nano 2013, 7, 6858–6867. [Google Scholar] [CrossRef] [PubMed]
- Perini, G.; Palmieri, V.; Ciasca, G.; De Spirito, M.; Papi, M. Unravelling the potential of graphene quantum dots in biomedicine and neuroscience. Int. J. Mol. Sci. 2020, 21, 3712. [Google Scholar] [CrossRef] [PubMed]
- Sherman, J.H.; Bobak, A.; Arsiwala, T.; Lockman, P.; Aulakh, S. Targeting drug resistance in glioblastoma (Review). Int. J. Oncol. 2024, 65, 80. [Google Scholar] [CrossRef]
- Mondragon-Soto, M.; Rodríguez-Hernández, L.A.; Moreno Jiménez, S.; Gómez Amador, J.L.; Gutierrez-Aceves, A.; Montano-Tello, H.; Reyes-Moreno, I.; Santos-Zambrano, J.; Castro-Martinez, E.; Gonzalez-Aguilar, A. Clinical, Therapeutic, and Prognostic Experience in Patients With Glioblastoma. Cureus 2022, 14, e29856. [Google Scholar] [CrossRef]
- Mehta, M.; Wen, P.; Nishikawa, R.; Reardon, D.; Peters, K. Critical review of the addition of tumor treating fields (TTFields) to the existing standard of care for newly diagnosed glioblastoma patients. Crit. Rev. Oncol. Hematol. 2017, 111, 60–65. [Google Scholar] [CrossRef]
- Osawa, T.; Tosaka, M.; Horiguchi, K.; Sugawara, K.; Yokoo, H.; Yoshimoto, Y. Elderly patients aged over 75 years with glioblastoma: Preoperative status and surgical strategies. Interdiscip. Neurosurg. 2021, 25, 101127. [Google Scholar] [CrossRef]
- Kim, M.; Ladomersky, E.; Mozny, A.; Kocherginsky, M.; O’shea, K.; Reinstein, Z.Z.; Zhai, L.; Bell, A.; Lauing, K.L.; Bollu, L.; et al. Glioblastoma as an age-related neurological disorder in adults. Neurooncol. Adv. 2021, 3, vdab125. [Google Scholar] [CrossRef]
- Noorani, I.; de la Rosa, J. Breaking barriers for glioblastoma with a path to enhanced drug delivery. Nat. Commun. 2023, 14, 5909. [Google Scholar] [CrossRef] [PubMed]
- Male, D.; Gromnicova, R.; McQuaid, C. Chapter Five—Gold Nanoparticles for Imaging and Drug Transport to the CNS. In International Review of Neurobiology; Al-Jamal, K.T., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 130, pp. 155–198. [Google Scholar] [CrossRef]
- Noble, C.O.; Krauze, M.T.; Drummond, D.C.; Forsayeth, J.; Hayes, M.E.; Beyer, J.; Hadaczek, P.; Berger, M.S.; Kirpotin, D.B.; Bankiewicz, K.S.; et al. Pharmacokinetics, Tumor Accumulation and Antitumor Activity of Nanoliposomal Irinotecan Following Systemic Treatment of Intracranial Tumors. Nanomedicine 2014, 9, 2099–2108. [Google Scholar] [CrossRef]
- Azadi, A.; Hamidi, M.; Rouini, M.-R. Methotrexate-loaded chitosan nanogels as ‘Trojan Horses’ for drug delivery to brain: Preparation and in vitro/in vivo characterization. Int. J. Biol. Macromol. 2013, 62, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, L.-P.; Dai, W.; Dong, H.; Wen, Y.; Zhang, X. Graphene quantum dots for the inhibition of β amyloid aggregation. Nanoscale 2015, 7, 19060–19065. [Google Scholar] [CrossRef]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef]
- Wang, H.; Mu, Q.; Wang, K.; Revia, R.A.; Yen, C.; Gu, X.; Tian, B.; Liu, J.; Zhang, M. Nitrogen and boron dual-doped graphene quantum dots for near-infrared second window imaging and photothermal therapy. Appl. Mater. Today 2019, 14, 108–117. [Google Scholar] [CrossRef]
- Perini, G.; Palmieri, V.; Ciasca, G.; D’Ascenzo, M.; Primiano, A.; Gervasoni, J.; De Maio, F.; De Spirito, M.; Papi, M. Enhanced chemotherapy for glioblastoma multiforme mediated by functionalized graphene quantum dots. Materials 2020, 13, 4139. [Google Scholar] [CrossRef] [PubMed]
- Perini, G.; Palmieri, V.; Ciasca, G.; D’ascenzo, M.; Gervasoni, J.; Primiano, A.; Rinaldi, M.; Fioretti, D.; Prampolini, C.; Tiberio, F.; et al. Graphene quantum dots’ surface chemistry modulates the sensitivity of glioblastoma cells to chemotherapeutics. Int. J. Mol. Sci. 2020, 21, 6301. [Google Scholar] [CrossRef]
- Wang, M.; Li, B.; Du, Y.; Bu, H.; Tang, Y.; Huang, Q. Fluorescence imaging-guided cancer photothermal therapy using polydopamine and graphene quantum dot-capped Prussian blue nanocubes. RSC Adv. 2021, 11, 8420–8429. [Google Scholar] [CrossRef]
- Perini, G.; Palmieri, V.; Ciasca, G.; Primiano, A.; Gervasoni, J.; De Spirito, M.; Papi, M. Functionalized Graphene Quantum Dots Modulate Malignancy of Glioblastoma Multiforme by Downregulating Neurospheres Formation. C 2021, 7, 4. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Dega, N.K.; Tran, H.L.; Darmonto, W.; Doong, R.A. Application of sulfur-doped graphene quantum dots@gold-carbon nanosphere for electrical pulse-induced impedimetric detection of glioma cells. Biosens. Bioelectron. 2021, 181, 113151. [Google Scholar] [CrossRef] [PubMed]
- Perini, G.; Rosa, E.; Friggeri, G.; Di Pietro, L.; Barba, M.; Parolini, O.; Ciasca, G.; Moriconi, C.; Papi, M.; De Spirito, M.; et al. INSIDIA 2.0 High-Throughput Analysis of 3D Cancer Models: Multiparametric Quantification of Graphene Quantum Dots Photothermal Therapy for Glioblastoma and Pancreatic Cancer. Int. J. Mol. Sci. 2022, 23, 3217. [Google Scholar] [CrossRef]
- Perini, G.; Palmieri, V.; Friggeri, G.; Augello, A.; De Spirito, M.; Papi, M. Carboxylated graphene quantum dots-mediated photothermal therapy enhances drug-membrane permeability, ROS production, and the immune system recruitment on 3D glioblastoma models. Cancer Nanotechnol. 2023, 14, 13. [Google Scholar] [CrossRef]
- Ostovar, S.; Pourmadadi, M.; Zaker, M.A. Co-biopolymer of chitosan/carboxymethyl cellulose hydrogel improved by zinc oxide and graphene quantum dots nanoparticles as pH-sensitive nanocomposite for quercetin delivery to brain cancer treatment. Int. J. Biol. Macromol. 2023, 253, 127091. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Wang, B.; Xie, Y.; Cui, X.; Jiao, J.; Jiao, J.; Zhang, Y. A label-free electrochemical biosensor based on graphene quantum dots-nanoporous gold nanocomposite for highly sensitive detection of glioma cell. Anal. Chim. Acta 2025, 1337, 343555. [Google Scholar] [CrossRef]
- Self, A.; Farell, M.; Samineni, L.; Kumar, M.; Gomez, E.W. 2D Materials for Combination Therapy to Address Challenges in the Treatment of Cancer. Adv. NanoBiomed Res. 2023, 3, 2300070. [Google Scholar] [CrossRef]
- Kong, C.; Chen, X. Combined Photodynamic and Photothermal Therapy and Immunotherapy for Cancer Treatment: A Review. Int. J. Nanomed. 2022, 17, 6427–6446. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Wang, X.; Guan, X.; Zhang, W.; Ma, J. Recent advances in selective photothermal therapy of tumor. J. Nanobiotechnol. 2021, 19, 335. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, X.; Xiong, J.; Peng, S.; Huang, W.; Joshi, R.; Cai, Y.; Liu, Y.; Li, R.; Yuan, K.; et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci. Rep. 2018, 8, 8720. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y. Advances in nanomaterial-mediated photothermal cancer therapies: Toward clinical applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xia, G.; Yang, N.; Yuan, L.; Li, J.; Wang, Q.; Li, D.; Ding, L.; Fan, Z.; Li, J. Noble Metal Nanoparticle-Based Photothermal Therapy: Development and Application in Effective Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 5632. [Google Scholar] [CrossRef]
- Duan, S.; Hu, Y.; Zhao, Y.; Tang, K.; Zhang, Z.; Liu, Z.; Wang, Y.; Guo, H.; Miao, Y.; Du, H.; et al. Nanomaterials for photothermal cancer therapy. RSC Adv. 2023, 13, 14443–14460. [Google Scholar] [CrossRef]
- Simón, M.; Jørgensen, J.T.; Norregaard, K.; Henriksen, J.R.; Clergeaud, G.; Andresen, T.L.; Hansen, A.E.; Kjaer, A. Neoadjuvant Gold Nanoshell-Based Photothermal Therapy Combined with Liposomal Doxorubicin in a Mouse Model of Colorectal Cancer. Int. J. Nanomed. 2023, 18, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Naser Mohammed, S.; Mishaal Mohammed, A.; Al-Rawi, K.F. Novel combination of multi-walled carbon nanotubes and gold nanocomposite for photothermal therapy in human breast cancer model. Steroids 2022, 186, 109091. [Google Scholar] [CrossRef]
- Su, S.; Wang, J.; Vargas, E.; Wei, J.; Martínez-Zaguilán, R.; Sennoune, S.R.; Pantoya, M.L.; Wang, S.; Chaudhuri, J.; Qiu, J. Porphyrin Immobilized Nanographene Oxide for Enhanced and Targeted Photothermal Therapy of Brain Cancer. ACS Biomater. Sci. Eng. 2016, 2, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, C.; Qian, Y.; Hu, L.; Fang, J.; Tong, W.; Nie, R.; Chen, Q.; Wang, H. Magnetic-induced graphene quantum dots for imaging-guided photothermal therapy in the second near-infrared window. Biomaterials 2020, 232, 119700. [Google Scholar] [CrossRef]
- Carmignani, A.; Battaglini, M.; Marino, A.; Pignatelli, F.; Ciofani, G. Drug-Loaded Polydopamine Nanoparticles for Chemo/Photothermal Therapy against Colorectal Cancer Cells. ACS Appl. Bio Mater. 2024, 7, 2205–2217. [Google Scholar] [CrossRef]
- Hong, H.; Kim, M.K.; Lee, W.; Jeon, M.; Lee, C.; Kim, H.; Im, H.-J.; Piao, Y. Injectable biocompatible nanocomposites of Prussian blue nanoparticles and bacterial cellulose as a safe and effective photothermal cancer therapy. J. Nanobiotechnol. 2023, 21, 365. [Google Scholar] [CrossRef]
- Dacarro, G.; Taglietti, A.; Pallavicini, P. Prussian blue nanoparticles as a versatile photothermal tool. Molecules 2018, 23, 1414. [Google Scholar] [CrossRef]
- West, H.; Jin, J. Neoadjuvant Therapy. JAMA Oncol. 2015, 1, 550. [Google Scholar] [CrossRef] [PubMed]
- NCI. Dictionary of Cancer Terms—NCI n. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms (accessed on 17 February 2025).
- Baskin, A.S.; Huppert, L.A.; Kelil, T.; Singer, L.; Mukhtar, R.A. The neoadjuvant approach to treatment of breast cancer: Multidisciplinary management to improve outcomes. Surg. Oncol. Insight 2024, 1, 100059. [Google Scholar] [CrossRef]
- Springfeld, C.; Ferrone, C.R.; Katz, M.H.G.; Philip, P.A.; Hong, T.S.; Hackert, T.; Büchler, M.W.; Neoptolemos, J. Neoadjuvant therapy for pancreatic cancer. Nat. Rev. Clin. Oncol. 2023, 20, 318–337. [Google Scholar] [CrossRef]
- Smith, H.G.; Nilsson, P.J.; Shogan, B.D.; Harji, D.; Gambacorta, M.A.; Romano, A.; Brandl, A.; Qvortrup, C. Neoadjuvant treatment of colorectal cancer: Comprehensive review. BJS Open 2024, 8, zrae038. [Google Scholar] [CrossRef]
- Qu, X.; Zhou, D.; Lu, J.; Qin, D.; Zhou, J.; Liu, H.-J. Cancer nanomedicine in preoperative therapeutics: Nanotechnology-enabled neoadjuvant chemotherapy, radiotherapy, immunotherapy, and phototherapy. Bioact. Mater. 2023, 24, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Saracchini, S.; Foltran, L.; Tuccia, F.; Bassini, A.; Sulfaro, S.; Micheli, E.; Del Conte, A.; Bertola, M.; Gion, M.; Lorenzon, M.; et al. Phase II study of liposome-encapsulated doxorubicin plus cyclophosphamide, followed by sequential trastuzumab plus docetaxel as primary systemic therapy for breast cancer patients withHER2 overexpression or amplification. Breast 2013, 22, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Wang, M.; Wang, Y.; Shi, S.; Hu, X.; Zhang, Q.; Fan, D.; Xu, P. Biomimetic Nanomedicine Coupled with Neoadjuvant Chemotherapy to Suppress Breast Cancer Metastasis via Tumor Microenvironment Remodeling. Adv. Funct. Mater. 2021, 31, 2100262. [Google Scholar] [CrossRef]
- Nabian, N.; Ghalehtaki, R.; Zeinalizadeh, M.; Balaña, C.; Jablonska, P.A. State of the neoadjuvant therapy for glioblastoma multiforme—Where do we stand? Neurooncol. Adv. 2024, 6, vdae028. [Google Scholar] [CrossRef]
- Bihan, C.; Foscolo, S.; Boone, M.; Blonski, M.; Coutte, A.; Darlix, A.; Beauchesne, P.; Lefranc, M.; Lorgis, V.; Taillandier, L.; et al. Upfront bevacizumab and temozolomide or fotemustine before radiotherapy for patients with glioblastoma and severe neurological impairment at Diagnosis. Case Rep. Oncol. 2012, 5, 530–536. [Google Scholar] [CrossRef]
- Kregielewski, K.; Fraczek, W.; Grodzik, M. Graphene Oxide Enhanced Cisplatin Cytotoxic Effect in Glioblastoma and Cervical Cancer. Molecules 2023, 28, 6253. [Google Scholar] [CrossRef]
- Gao, Y.; Dorn, P.; Liu, S.; Deng, H.; Hall, S.R.R.; Peng, R.W.; Schmid, R.A.; Marti, T.M. Cisplatin-resistant A549 non-small cell lung cancer cells can be identified by increased mitochondrial mass and are sensitive to pemetrexed treatment. Cancer Cell Int. 2019, 19, 317. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Luo, C.; Wang, C.; Zhang, F.; Zhang, J.; Guo, S. Graphene quantum dots enhance anticancer activity of cisplatin via increasing its cellular and nuclear uptake. Nanomedicine 2016, 12, 1997–2006. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martinkova, P.; Kostelnik, A.; Valek, T.; Pohanka, M. Main streams in the construction of biosensors and their applications. Int. J. Electrochem. Sci. 2017, 12, 7386–7403. [Google Scholar] [CrossRef]
- Monošík, R.; Streďanský, M.; Šturdík, E. Biosensors—Classification, characterization and new trends. Acta Chim. Slovaca 2012, 5, 109–120. [Google Scholar] [CrossRef]
- Bright, K.; Ioana Voiculescu Anita, N.P.; Alexandrina, U. A Review of Biosensors and Their Applications. ASME Open J. Eng. 2023, 2, 020201. [Google Scholar] [CrossRef]
- Tabish, T.A.; Hayat, H.; Abbas, A.; Narayan, R.J. Graphene quantum dot–based electrochemical biosensing for early cancer detection. Curr. Opin. Electrochem. 2021, 30, 100786. [Google Scholar] [CrossRef]
- Iannazzo, D.; Espro, C.; Celesti, C.; Ferlazzo, A.; Neri, G. Smart biosensors for cancer diagnosis based on graphene quantum dots. Cancers 2021, 13, 3194. [Google Scholar] [CrossRef]
- Serafín, V.; Valverde, A.; Martínez-García, G.; Martínez-Periñán, E.; Comba, F.; Garranzo-Asensio, M.; Barderas, R.; Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Graphene quantum dots-functionalized multi-walled carbon nanotubes as nanocarriers in electrochemical immunosensing. Determination of IL-13 receptor α2 in colorectal cells and tumor tissues with different metastatic potential. Sens. Actuators B Chem. 2019, 284, 711–722. [Google Scholar] [CrossRef]
- Serafín, V.; Valverde, A.; Garranzo-Asensio, M.; Barderas, R.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Simultaneous amperometric immunosensing of the metastasis-related biomarkers IL-13Rα2 and CDH-17 by using grafted screen-printed electrodes and a composite prepared from quantum dots and carbon nanotubes for signal amplification. Microchim. Acta 2019, 186, 411. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, M.; Baghban, H.N.; Shadjou, N.; Mokhtarzadeh, A. Ultrasensitive electrochemical immunosensing of tumor suppressor protein p53 in unprocessed human plasma and cell lysates using a novel nanocomposite based on poly-cysteine/graphene quantum dots/gold nanoparticle. Int. J. Biol. Macromol. 2018, 107, 1348–1363. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A.; Hayat, H.; Abbas, A.; Narayan, R.J. Graphene Quantum Dots-Based Electrochemical Biosensing Platform for Early Detection of Acute Myocardial Infarction. Biosensors 2022, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Au, D.T.; Ying, Z.; Hernández-Ochoa, E.O.; Fondrie, W.E.; Hampton, B.; Migliorini, M.; Galisteo, R.; Schneider, M.F.; Daugherty, A.; Rateri, D.L.; et al. LRP1 (low-density lipoprotein receptor-related protein 1) regulates smooth muscle contractility by modulating Ca2+ signaling and expression of cytoskeleton-related proteins. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2651–2664. [Google Scholar] [CrossRef]
- Parrasia, S.; Szabò, I.; Zoratti, M.; Biasutto, L. Peptides as Pharmacological Carriers to the Brain: Promises, Shortcomings and Challenges. Mol. Pharm. 2022, 19, 3700–3729. [Google Scholar] [CrossRef]
- LN-229—CRL-2611|ATCC. n.d. Available online: https://www.atcc.org/products/crl-2611 (accessed on 19 February 2025).
- Shruthi, N.R.; Behera, M.M.; Naik, S.K.; Das, S.K.; Gopan, S.; Ghosh, A.; Sahu, R.N.; Patra, S.; Purkait, S. Elevated expression of cholesterol transporter LRP-1 is crucially implicated in the pathobiology of glioblastoma. Front. Neurol. 2022, 13, 1003730. [Google Scholar] [CrossRef]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef]
- Prange, C.J.; Hu, X.; Tang, L. Smart chemistry for traceless release of anticancer therapeutics. Biomaterials 2023, 303, 122353. [Google Scholar] [CrossRef]
- Power, E.A.; Rechberger, J.S.; Gupta, S.; Schwartz, J.D.; Daniels, D.J.; Khatua, S. Drug delivery across the blood-brain barrier for the treatment of pediatric brain tumors—An update. Adv. Drug Deliv. Rev. 2022, 185, 114303. [Google Scholar] [CrossRef]
- Nasrollahi, F.; Koh, Y.R.; Chen, P.; Varshosaz, J.; Khodadadi, A.A.; Lim, S. Targeting graphene quantum dots to epidermal growth factor receptor for delivery of cisplatin and cellular imaging. Mater. Sci. Eng. C 2019, 94, 247–257. [Google Scholar] [CrossRef]
- Yu, C.; Long, Z.; Qiu, Q.; Liu, F.; Xu, Y.; Zhang, T.; Guo, R.; Zhong, W.; Huang, S.; Chen, S. Graphene quantum dots-based targeted nanoprobes detecting drug delivery, imaging, and enhanced chemotherapy of nasopharyngeal carcinoma. Bioeng. Transl. Med. 2022, 7, e10270. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Symbol | Full Name | GQD Source | Size (Method) | Use |
|---|---|---|---|---|---|---|
| Wang et al. [29] | 2019 | N-B-GQD | Graphene quantum dots doped with nitrogen and boron | Synthesis by authors | 4.7 nm (TEM) | PTT/imaging |
| Perini et al. [30] | 2020 | NF-GQD | non-functionalized graphene quantum dots | Sigma Aldrich, St. Louis, MO, USA | <10 nm (DLS, AFM) | pretreatment |
| DMF-GQD | dimethylformamide-functionalized graphene quantum dots | ACS Materials, Pasadena, CA, USA | ||||
| Perini et al. [31] | 2020 | Green GQD | no surface-specific functionalization | Sigma Aldrich, St. Louis, MO, USA | <10 nm (DLS, AFM) | pretreatment |
| COOH-GQD | carboxylated graphene quantum dots | ACS Materials, Pasadena, CA, USA | ||||
| NH2-GQD | aminated graphene quantum dots | ACS Materials, Pasadena, CA, USA | ||||
| Wang et al. [32] | 2021 | PB@PDA@GQD | polydopamine and graphene quantum dot-capped Prussian blue nanocubes | XFNANO Co. Ltd., Nanjing, China | ND | PTT |
| Perini et al. [33] | 2021 | COOH-GQD | carboxylated graphene quantum dots | ACS Materials, Pasadena, CA, USA | <10 nm (TEM) | Treatment |
| NH2-GQD | aminated graphene quantum dots | ACS Materials, Pasadena, CA, USA | ||||
| GQD | no surface-specific functionalization | Sigma Aldrich, St. Louis, MO, USA | ||||
| Ganganboina et al. [34] | 2021 | S-GQDs@@Au-CNS | sulfur-doped graphene quantum dots@gold-carbon nanosphere | Synthesis by authors | 4–11 nm (TEM) | Biosensor |
| Perini et al. [35] | 2022 | GQD | carboxylated graphene quantum dots | ACS Materials, Pasadena, CA, USA | >10 nm (DLS, AFM) | Pretreatment |
| Perini et al. [36] | 2023 | GQD | carboxylated graphene quantum dots | ACS Materials, Pasadena, CA, USA | ~10 nm (DLS, AFM) | PTT/Pretreatment |
| Ostovar et al. [37] | 2023 | CS/CMC/GQD/Zn@QUR | Co-biopolymer of chitosan/carbomethyl cellulose hydrogel improved by zinc oxide and graphene quantum dots nanoparticles | Synthesis by authors | 7.99 nm (DLS) | Drug delivery |
| Wang et al. [38] | 2025 | Ang-2/GQD-NPG/GCE | electrochemical biosensor based on graphene quantum dots-nanoporous gold nanocomposite | Synthesis by authors | ~7 nm (TEM, HR-TEM) | Biosensor |
| Author | Year | Symbol | Use | In Vitro Models (Doses) (Glioblastoma Model Bolded) | In Vivo Models (Doses) | Outcomes |
|---|---|---|---|---|---|---|
| Wang et al. [29] | 2019 | N-B-GQD | PTT/imaging | SF763; 4T1; B16F10 cell lines (12.5; 25; 50; 100; 200; 500 μg/mL) | Nude mice/nude mice with induced C6 glioblastoma (1 mg/mL) | N-B-GQDs were non-toxic at 500 µg/mL over 72 h but reduced glioblastoma cell viability at 100 µg/mL with NIR exposure. In mice, N-B-GQDs combined with NIR effectively inhibited tumor growth and improved visualization of blood vessels and organs, without harming histological or blood parameters. |
| Perini et al. [30] | 2020 | NF-GQD | pretreatment | U87; cortical neurons from E15-18 C57BL/6 mice (50, 100, 200, 250 μg/mL) | - | NF-GQD is biocompatible at 50–250 µg/mL for both glioblastoma and non-cancerous cells. In U87 cells, NF-GQD (200–250 µg/mL) combined with 1 µM Dox significantly reduced viability and enhanced Dox uptake (maximal at 250 µg/mL). |
| DMF-GQD | Conversely, DMF-GQD (200–250 µg/mL) reduced viability in both cell types. Pretreatment with DMF-GQD (100–250 µg/mL) plus 1 µM Dox further decreased viability and increased uptake. Both NF-GQD (200–250 µg/mL) and DMF-GQD (all doses) exhibited synergistic effect with Dox. | |||||
| Perini et al. [31] | 2020 | Green GQD | pretreatment | U87; cortical neurons from E15-18 C57BL/6 mice(50, 100, 200, 250 μg/mL) | - | Green GQD (250 µg/mL) slightly reduces the viability of U87 cells by approximately 20%, but it does not have a significant effect on reactive oxygen species (ROS), DNA fragmentation, or cytokine levels. When pretreated with GQD and then treated with 1 µM doxorubicin, it significantly decreases U87 cell viability and enhances the uptake of Dox, as well as membrane fluidity, without impacting cortical neurons. |
| COOH-GQD | COOH-GQD on its own does not affect cell viability or cytokine levels. However, when administered at concentrations ranging from 200 to 250 µg/mL prior to Dox, it decreases U87 viability while increasing Dox uptake and membrane fluidity, without any effects on cortical neurons. | |||||
| NH2-GQD | NH2-GQD exhibits no influence on viability, ROS, DNA fragmentation, cytokine levels, Dox uptake, or membrane fluidity in either cell type. | |||||
| Wang et al. [32] | 2021 | PB@PDA@GQD | PTT | C6, BV2 (12,5; 25; 50; 75; 100; 200 μg/mL) | Balb/c mice with induced C6 glioblastoma (6 mg/kg) | PB@PDA@GQD is non-toxic to BV2 cells and maintains over 80% C6 cell viability across 12.5–200 µg/mL, but 200 µg/mL PB@PDA@GQD with NIR decreases C6 viability to 8%. In Balb/c mice with C6 tumors, PB@PDA@GQD with NIR significantly inhibits tumor growth. |
| Perini et al. [33] | 2021 | COOH-GQD | Treatment | U87MG (50, 100, 200 μg/mL) | - | COOH-GQD at concentrations of 50, 100, and 200 µg/mL did not result in significant changes in U87 cell viability over a period of 14 days. However, COOH-GQD at 200 µg/mL inhibited neurosphere growth, affecting the average size and density, and also led to a decrease in membrane fluidity while impacting clusterization and connection formation. |
| NH2-GQD | NH2-GQD at the same concentrations did not affect U87 cell viability after 14 days, did not inhibit neurosphere growth, showed no significant change in membrane fluidity, and did not influence clusterization or connection formation. | |||||
| GQD | GQD, similarly to COOH-GQD at concentrations of 50, 100, and 200 µg/mL also showed no significant effect on U87 cell viability. GQD 200 µg/mL inhibited neurosphere growth and caused a decrease in membrane fluidity, while also inhibiting clusterization and connection formation. | |||||
| Ganganboina et al. [34] | 2021 | S-GQDs@@Au-CNS | Biosensor | Glioma cells (not specified) | - | A manufactured biosensor allows for the detection of low concentrations of glioblastoma cells, starting with 40 cells per mL. |
| Perini et al. [35] | 2022 | GQD | Pretreatment | U87MG (200 μg/mL) | - | At 200 µg/mL, GQD + 1 µM Dox reduces U87 spheroid viability by 30%, while adding NIR irradiation further decreases spheroid volume by 70% over 14 days. However, NIR irradiation does not enhance viability reduction beyond GQD + Dox treatment alone. |
| Perini et al. [36] | 2023 | GQD | PTT/Pretreatment | U87, human fibroblasts (50; 100; 200; 250 μg/mL) | - | GQD (50–250 µg/mL) is non-cytotoxic and enhances membrane fluidity at 250 µg/mL in tested cells. When combined with 1 µM Dox, it increases Dox uptake and reduces viability at ≥200 µg/mL in U87 cells. A similar effect occurs with GQD at 200–250 µg/mL alongside 100 µM TMZ. In 3D U87 spheroids, GQD alone or with drugs does not affect viability but increases membrane permeability at 200 µg/mL. The combination of GQD and PTT reduces spheroid growth, with PTT plus chemotherapy being more effective than the drugs alone. This approach also improves drug penetration, boosts immune cell recruitment, and elevates ROS production in treated spheroids. |
| Ostovar et al. [37] | 2023 | CS/CMC/GQD/Zn@QUR | Drug delivery | U87, L929 | - | The composite CS/CMC/GQD/Zn@QUR has an improved controlled release profile and shows greater inhibitory effects on U87 cells compared to pure QUR, while keeping toxicity levels acceptable in non-cancerous cells. |
| Wang et al. [38] | 2025 | Ang-2/GQD-NPG/GCE | Biosensor | U251, 3T3, SCC7, 4T1, B16–F10, HeLa, HepG2, LN229, PC-9, LL/2, hCMEC/D3 and LO2 | - | Ang-2/GQD-NPG/GCE enables selective glioblastoma cell detection in serum and culture, with a detection limit of 1 cell/mL and stability for 21 days. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kregielewski, K.; Fraczek, W.; Grodzik, M. Graphene Quantum Dots for Glioblastoma Treatment and Detection–Systematic Review. Molecules 2025, 30, 2483. https://doi.org/10.3390/molecules30122483
Kregielewski K, Fraczek W, Grodzik M. Graphene Quantum Dots for Glioblastoma Treatment and Detection–Systematic Review. Molecules. 2025; 30(12):2483. https://doi.org/10.3390/molecules30122483
Chicago/Turabian StyleKregielewski, Kacper, Wiktoria Fraczek, and Marta Grodzik. 2025. "Graphene Quantum Dots for Glioblastoma Treatment and Detection–Systematic Review" Molecules 30, no. 12: 2483. https://doi.org/10.3390/molecules30122483
APA StyleKregielewski, K., Fraczek, W., & Grodzik, M. (2025). Graphene Quantum Dots for Glioblastoma Treatment and Detection–Systematic Review. Molecules, 30(12), 2483. https://doi.org/10.3390/molecules30122483







