Abstract
This study investigated the defluorination of PFOA and PFOS using a vacuum ultraviolet (VUV)/sulfite system, and evaluated its potential application in quantifying individual perfluoroalkyl substances (PFAS). Results showed that 81.9% and 87.5% defluorination of PFOA and PFOS were achieved after 120 min of photoreaction under conditions of pH 12 and 20 mM of sulfite. Higher pH and sulfite dosage facilitated the reaction, while competing ions could suppress the defluorination efficiency. Based on the optimized defluorination conditions for individual PFAS, the potential of fluoride release amount, as an indirect quantification indicator, was further assessed. A strong linearity between the fluoride release and initial PFAS concentration (R2 > 0.999) was observed in the PFAS concentration range of 2–100 μM, and such linearity was also shown in the presence of sediment leachates. This correlation enabled the estimation of individual PFAS concentrations by measuring fluoride release after defluorination treatment. The approach was further demonstrated in an adsorption experiment, where calculated distribution coefficients (Koc) for PFAS–sediment interactions were consistent with previously reported values, supporting the analytical validity of the method under controlled conditions. Overall, this work presents a simple and cost-effective indirect analytical strategy of applying a VUV/sulfite defluorination system for individual PFAS quantitative detection in complex environmental matrices.
1. Introduction
Perfluoroalkyl substances (PFAS) are a class of synthetic organic pollutants with high environmental persistence and they have become a global research focus in environmental science, due to their ubiquitous distribution and ecological risks [1,2]. These chemicals exhibit excellent chemical stability and surfactant properties due to the multiple carbon–fluorine bonds (C-F), and are thus widely used in industrial manufacturing and consumer products [3,4]. During product usage and disposal, PFAS are inevitably released into the environment through multiple pathways, including wastewater discharge and landfill leachate leakage. PFAS have high water solubility due to the polar functional groups at their termini, facilitating cross-media migration through surface runoff and precipitation infiltration [5,6]. The combination of mobility and persistence leads to their widespread accumulation in aquatic systems, with detections even in glacial meltwater and atmospheric deposition [7,8]. In China’s Yangtze River Basin, many studies have reported the occurrence of PFAS in the major tributaries and adjacent lakes [9,10]. Among the various PFAS, perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) are commonly used as representative model compounds due to their frequent detection in various environmental matrices and biota.
The analysis of PFAS involves a range of analytical techniques, each with specific advantages and limitations. Liquid chromatography–tandem mass spectrometry (LC-MS/MS) is regarded as the “gold standard” for its excellent sensitivity and selectivity, enabling trace-level quantification and structural identification of PFAS in complex matrices [4,11]. However, its high operational costs, specialized equipment, and the demand for well-trained personnel limit accessibility in resource-constrained institutions or regions [12]. Simpler techniques such as high-performance liquid chromatography (HPLC) with UV or fluorescence detection are more cost-effective but suffer from low sensitivity due to weak PFAS chromophores, and thus often require derivatization [13]. Total organic fluorine (TOF) analysis methods, such as combustion ion chromatography (CIC), photolysis-coupled ion chromatography analysis, fluorine-19 nuclear magnetic resonance (19F-NMR), and particle-induced gamma-ray emission (PIGE), have been developed to quantify total fluorine content [14,15,16,17]. While these TOF methods offer a view of total fluorine determination in the environmental medium, they still involve high costs or limited availability. Therefore, there is a demand to fill the gap between comprehensive but expensive methods and accessible routine analysis. These issues pose an important challenge in exploring simplified and cost-effective alternatives to facilitate PFAS analysis in the absence of advanced instruments.
Ultraviolet advanced reduction processes (UV-ARPs) are often considered for the reduction of compounds [18,19]. They utilize UV light to activate chemical reductants, and generate highly reactive species in water. In a representative UV/sulfite system, sulfite ions (SO32−), which exhibit strong UV absorption, could generate hydrated electrons (eaq−) and sulfite radicals (SO3•−) via Equation (1) under the irradiation of UV light. The eaq−, with a reduction potential of −2.9 V, is recognized as one of the most powerful natural reductants and it can directly attack the highly electronegative fluorine atoms in PFAS molecules, destabilizing the C-F bonds and initiating sequential defluorination reactions [3,20]. Although eaq− is quickly quenched by dissolved oxygen (k = 1.9 × 1010 M−1 s−1), its lifetime can be prolonged under inert gas conditions, thereby enhancing contaminant removal efficiency [21]. In addition, compared to conventional UV systems operating at 254 nm with a sulfite activation quantum yield of 0.39, vacuum ultraviolet (VUV) irradiation at 185 nm exhibits superior reactivity, achieving a higher quantum yield of 0.85 in sulfite-activated reduction systems [22]. Its higher photon energy enables the simultaneous activation of both sulfite and water molecules (Equation (2)), leading to the acceleration of pollutant degradation [23]. Experimental evidence has confirmed that VUV-driven advanced oxidation/reduction processes (AO/RPs) outperform conventional UV systems in eliminating UV-resistant micro-pollutants [24,25,26].
SO32− + hν → eaq− + SO3•−
H2O + hν (<190 nm) → eaq− + H+ + HO•
Given that fluoride release amount directly correlates with PFAS mineralization, the (V)UV/sulfite system holds potential not only to be used as a treatment approach, but also in conducting simple and economical quantification for individual PFAS through routine fluoride determination. Although UV-ARPs exhibit high treatment ability under ideal conditions, the ubiquitous presence of eaq− quenchers in complex aqueous matrices can substantially compromise degradation efficiency, thereby affecting the defluorination extent. The common cations and anions in natural waters interact with reactive intermediates or modulate solution properties (e.g., pH, ionic strength, and light transmittance), thereby affecting the generation and reactivity of reactive species [24,27,28,29]. These ion-specific interactions may exert either inhibitory or synergistic effects on defluorination kinetics, depending on their chemical nature and concentration. Although the significance of ionic effects on the compound degradation has been widely recognized, understanding of their influences in VUV/sulfite-mediated defluorination remains limited. Investigating the influence of coexisting ions is also helpful for evaluating its applicability in the indirect determination of PFAS concentration.
In this study, the defluorination of two representative PFAS (PFOA and PFOS) was investigated using a VUV-activated sulfite system, with a focus on the influence of key operational parameters and environmentally relevant inorganic ions. The potential of released fluoride as an indirect quantification indicator was further assessed by evaluating its correlation with initial PFAS concentrations in both ultrapure water and sediment leachates. A simulated adsorption experiment was also conducted to demonstrate the practical applicability of this approach. This study provides a simple and cost-effective analysis process for laboratories lacking advanced instruments, enabling the quantification of individual PFAS in complex environmental matrices through fundamental laboratory procedures.
2. Results and Discussion
2.1. Defluorination of PFAS by VUV/Sulfite
2.1.1. Effect of Irradiation Time on the Defluorination of PFAS
The effect of VUV irradiation time on the defluorination performance of PFOA and PFOS was investigated. As shown in Figure 1, both compounds exhibited progressive fluoride releases, reaching 0.307 mM (81.9%) for PFOA and 0.372 mM (87.5%) for PFOS after 120 min. Although the defluorination efficiencies between PFOA and PFOS were quite similar, there existed slight differences in fluoride release amount, which were attributed to the different numbers of fluorine atoms in the parent molecules. Rapid defluorination occurred within the first 30 min, followed by a slower stage, likely due to sulfite depletion and the increasing stability of residual C–F bonds [30]. As the reaction proceeds, increased resistance to reduction occurs in residual C-F bonds, which are often situated at sterically hindered or electronically stable positions characterized by high bond dissociation energies [26,31]. Although UV-ARPs can achieve complete PFAS degradation with sufficient treatment time, defluorination is often incomplete [30,32]. For example, Bentel et al. [33] reported that while PFOA and PFOS were fully degraded after 8 h and 48 h UV treatment, respectively, their defluorination efficiencies were similar at ~50% over 48 h. This discrepancy arises because degradation can produce short-chain intermediates that retain fluorine, limiting the detectable fluoride release amount [34].
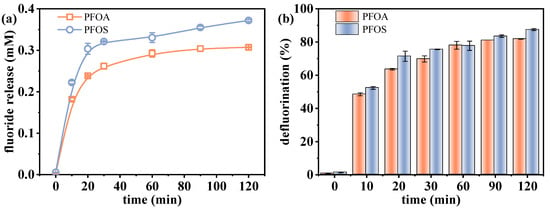
Figure 1.
Changes in defluorination (a) amount and (b) efficiency with reaction time. Initial experimental conditions: [PFOA] = [PFOS] = 25 μM, [sulfite] = 20 mM, pH = 12.
These results confirm the effectiveness of the VUV/sulfite system in facilitating the reductive defluorination of PFOA and PFOS, demonstrating its potential as a promising treatment approach for highly stable fluorinated pollutants. The fluoride release amount at 120 min was slightly higher than that at 60 min. Nevertheless, the time cost, whether for wastewater treatment or sample pretreatment, is an essential factor to consider. In this study, a reaction time of 60 min was selected for subsequent experiments to achieve a balance between defluorination efficiency and time cost.
2.1.2. Effect of Initial pH on the Defluorination
The initial pH of the reaction solution plays a critical role in determining sulfite species, and subsequently influences the defluorination efficiency of perfluorinated compounds in the VUV/sulfite system. The two pKa values of sulfite (1.8 and 7.2) [35,36] result in its predominant existence as a mixture of SO32− and HSO3− in the pH range of 4–9, while SO32− becomes the dominant species at pH values above 9 (Figure 2a). The absorbance of sulfite increases under higher pH conditions, as shown in Figure 2b, making it more susceptible to photoexcitation under irradiation. In a previous work, Luo et al. [36] estimated that SO32− has a higher quantum yield than HSO3− under photolysis at 266 nm (0.06 versus 0.01), with alkaline conditions conducive to enhancing the reaction efficiency.
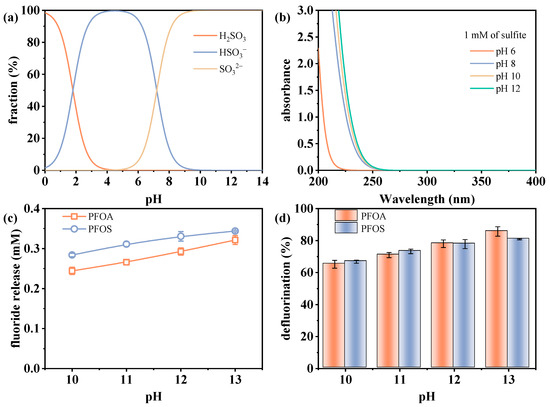
Figure 2.
(a) Species distribution of sulfite, (b) absorbance of sulfite in UV range, and changes in defluorination (c) amount and (d) efficiency under different pH conditions. Initial experimental conditions in (c,d): [PFOA] = [PFOS] = 25 μM, [sulfite] = 20 mM, pH = 10–13, reaction time = 60 min.
Figure 2c,d present the defluorination performance of PFOA and PFOS under varying initial pH values from 10 to 13. The results show that the fluoride release amount of PFOA increased from 0.244 to 0.321 mM, and that of PFOS increased from 0.284 to 0.344 mM as the pH increased from 10 to 13, with corresponding defluorination efficiencies of 65.2–85.7% and 66.8–80.9%, respectively. The defluorination trends in Figure 2c,d confirm that alkaline conditions favor the photoreduction process in the UV/sulfite system. The observed trend is not only related to the pH-dependent photoactivity of sulfite but also to the propensity of eaq− reacting with protons under acidic-to-neutral conditions (Equation (3)), which competitively reduces their availability for PFAS defluorination [37]. In contrast, under alkaline conditions, eaq− is enhanced through reactions with H• and OH− via Equation (4) [32], which increases degradation efficiency.
H+ + eaq− → H•
H• + OH− → eaq− + H2O
2.1.3. Effect of Sulfite Concentration on the Defluorination
As a key source of eaq−, sulfite generates a higher concentration of eaq− at elevated concentrations, thereby enhancing the reductive degradation process. The effect of varying sulfite concentrations (0–25 mM) on the defluorination performance was investigated. As shown in Figure 3, with the increase in sulfite concentration from 0 to 25 mM, the fluoride release amount of PFOA increased from 0.039 to 0.306 mM, and that of PFOS increased from 0.063 to 0.331 mM, corresponding to efficiencies of 10.3–81.7% and 14.8–77.9%, respectively.
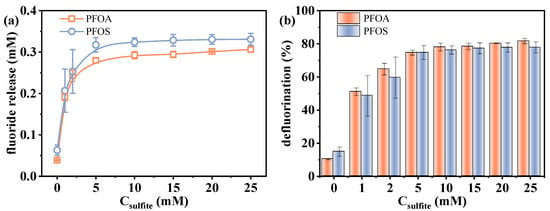
Figure 3.
Changes in defluorination (a) amount and (b) efficiency with varied sulfite dosages. Initial experimental conditions: [PFOA] = [PFOS] = 25 μM, [sulfite] = 0–25 mM, pH = 12, reaction time = 60 min.
Interestingly, in the absence of sulfite, over 10% defluorination was still observed for both PFOA and PFOS. This indicates that the VUV irradiation alone is capable of exciting water molecules to generate a limited amount of eaq− (Equation (2)), which can also initiate partial PFAS degradation [26]. Direct photolysis may also make a certain contribution, as Xin et al. [38] found that the presence or absence of O2 had no significant influence on the photolysis rate of PFOA at 222 nm, suggesting a non-radical pathway. A sharp increase in defluorination was observed as the sulfite dosage increased from 0 to 5 mM. However, further increasing the sulfite concentration from 5 to 25 mM resulted in a slower rise in defluorination efficiency. Even at the highest sulfite concentration tested, complete defluorination was not achieved. As has been mentioned above, this deceleration is likely related to the increasing difficulty in cleaving the residual C-F bonds. In addition, although more sulfite generates more eaq−, excessive sulfite can also consume these electrons through side reactions (Equation (5)), limiting their availability for PFAS degradation [39]. Zhang et al. [25] also reported a two-stage trend (fast and slow) in the degradation rate constant of monochloroacetic acid in the VUV/sulfite system, demonstrating the side effect of the excessive sulfite. As a result, while sulfite is essential in enhancing defluorination, its overuse can counteract the system’s overall efficiency, emphasizing the necessity for optimal sulfite concentrations.
eaq− + SO32− → Products
2.2. Effect of Ions on the Defluorination of PFAS
In natural aquatic environments, PFAS coexist with a range of ions derived from natural sources or anthropogenic activities. These constituents can affect the physicochemical properties of the aquatic system, compete for reactive species, or interact directly with PFAS, and thereby alter the efficiency of degradation processes [40]. To better understand the VUV/sulfite system under realistic conditions, this section examines the effects of representative anions and cations abundant in surface waters on the defluorination efficiency of PFOA and PFOS.
2.2.1. Effect of Anions
The effects of four kinds of typical anions, including Cl−, SO42−, CO32−, and NO3−, on PFAS defluorination were investigated. Considering that coexisting ions may consume eaq− and reduce defluorination efficiency, a 20 mM sulfite concentration was chosen in the following experiments to minimize these effects and provide a foundation for the quantification analysis using the VUV/sulfite system as a pretreatment approach. The experimental results of defluorination under varying ion concentrations (0–20 mM) are presented in Figure 4.
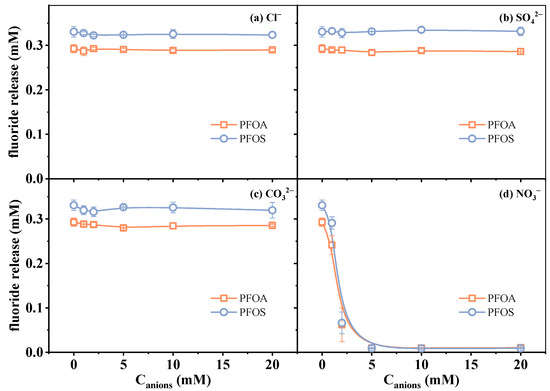
Figure 4.
Changes in fluoride release amount in the presence of (a) Cl−, (b) SO42−, (c) CO32−, and (d) NO3−. Initial experimental conditions: [PFOA] = [PFOS] = 25 μM, [sulfite] = 20 mM, pH = 12, [Cl−] = [SO42−] = [CO32−] = [NO3−] = 0–20 mM, reaction time = 60 min.
The results showed that Cl−, SO42−, and CO32− exhibited a negligible influence on the fluoride release amount of both PFOA and PFOS under the investigated conditions. Previous studies on anions affecting the ARPs have reported distinct mechanistic pathways. It was reported that SO42− can be directly photolyzed into SO4•− and eaq−, and a high concentration of SO42− could thus enhance the reductive reaction [24]. The defluorination efficiency in this work was not significantly improved, likely due to the lower dosage of SO42− compared to Liu et al.’s work [24]. Gu et al. [37] reported a slower reaction rate of PFOS when adding 1 mM of CO32− in the VUV/sulfite system at pH 10, which is considered to be caused by the competitive quenching of eaq−, though near-complete contaminant removal can still be achieved with extended reaction durations by compensating for scavenging effects. Such a negative effect on the reaction rate is more pronounced when using UV (254 nm) as a light source and increasing CO32− concentrations, as reported by Ren et al. [32]. In our work, the combination of elevated sulfite dosage (20 mM) and strongly alkaline conditions (pH 12) synergistically enhanced eaq− yields, effectively masking the slight interference caused by CO32− which was reported under the conditions of lower reductant and lower pH value. The influence of Cl− on pollutant degradation varies with the reaction mechanisms, as it can either scavenge radicals or generate reactive chlorine species [41,42,43]. Under VUV irradiation, Cl− may also produce eaq− (ΦCl−,185 nm = 0.43) or absorb light (εCl−,185 nm = 3500 M−1 cm−1) [24], affecting reductive efficiency. However, in this study, its effect on defluorination was minimal under the tested conditions, possibly due to the combined influence of these factors. In contrast, the presence of NO3− led to an obvious suppression of the defluorination efficiency. This inhibitory effect has been widely observed in many reported reductive systems [23,34,44]. Such an effect is attributed to the strong electron-scavenging ability of nitrate ions via Equation (6). This competitive reaction reduces the availability of eaq− for attacking PFAS molecules, thereby hindering the reductive defluorination process. Moreover, the absorbance of NO3− in the UV range would slightly decrease the photon flux available for sulfite photolysis, though this is likely a secondary effect [25,45].
NO3− + eaq− → (NO3)•2−
2.2.2. Effect of Cations
To assess the potential effect of common cations in an aqueous environment, four kinds of cations, including Na+, K+, Ca2+, and Mg2+, were selected. Experimental results under varying ion concentrations (0–20 mM) are presented in Figure 5. The results showed that Na+ and K+ had negligible effects on the fluoride release amount of both PFOA and PFOS in the investigated concentration range. These two monovalent cations are commonly used as inert counterions in ionic strength influence studies, owing to their high solubility and minimal direct involvement in redox processes [46,47].
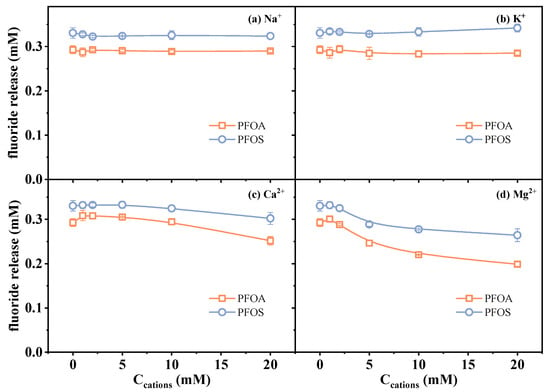
Figure 5.
Changes in fluoride release amount in the presence of (a) Na+, (b) K+, (c) Ca2+, and (d) Mg2+. Initial experimental conditions: [PFOA] = [PFOS] = 25 μM, [sulfite] = 20 mM, pH = 12, [Na+] = [K+] = [Ca2+] = [Mg2+] = 0–20 mM, reaction time = 60 min.
Contrasting with monovalent cations, the presence of Ca2+ and Mg2+ led to a noticeable decrease in the measured fluoride release amount (Figure 5c,d). In order to distinguish whether it is the result of actual degradation inhibition or analytical deviation, fluoride recovery experiments were conducted by preparing standard fluoride solutions (0.3 mM F−) containing varied concentrations of Ca2+ or Mg2+ via an ion-selective electrode in the absence of PFAS and sulfite. The measured signal of F− decreased progressively with increasing concentrations of Ca2+ and Mg2+ (91.2% and 88.4% at 20 mM Ca2+/Mg2+) (Figure 6). This phenomenon is likely attributed to the formation of less soluble fluoride salts. Fovet and Gal [48] demonstrated that Ca2+ and Mg2+ ions can significantly interfere with the potentiometric determination of F− when using a fluoride ion-selective electrode. This interference is caused by the precipitation of fluoride salts (CaF2 and MgF2), which decreases the measurable fluoride ion activity. These results suggest that the observed decrease in defluorination may be related to the analytical deviation, i.e., precipitation leading to the decrease in detectable dissolved F− concentration. This finding highlights the importance of considering ionic speciation and solubility when interpreting defluorination data in systems containing divalent metal ions. In addition, the influence of the two divalent metal cations on reductive reaction efficiency may also be related to factors such as PFAS–cation complex formation [5], as the decrease in defluorination was observed to exceed the signal loss induced by the precipitation of fluoride salts (Figure 5c,d vs. Figure 6).
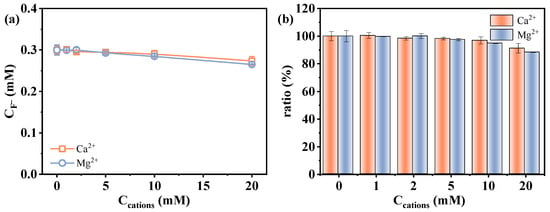
Figure 6.
Effect of Ca2+ and Mg2+ on the determination of CF− using fluoride ion-selective electrode: (a) detected F− concentration and (b) F− recovery. Conditions: [F−] = 0.3 mM, [Ca2+] = [Mg2+] = 0–20 mM.
Overall, these results demonstrate that although monovalent cations have a minimal effect, divalent cations such as Ca2+ and Mg2+ can interfere with the determined amount of defluorination due to the reasons of both the analytical technique and reduction efficiency. This underscores the necessity of incorporating ion-matrix correction strategies into studies of PFAS degradation.
2.3. Application of VUV/Sulfite System in PFAS Quantification Analysis
2.3.1. Establishment of Calibration Curves
The feasibility of applying the VUV/sulfite system for the indirect quantification of individual PFAS via defluorination is investigated. The defluorination performance of PFOA and PFOS was examined under varying initial concentrations (2–100 μM). As shown in Figure 7, a strong linear correlation (R2 > 0.999) was observed between the fluoride release amount and initial PFAS concentrations, though there was a slight decrease in defluorination efficiency with increasing PFAS concentrations due to the fixed generation rate of eaq− under constant VUV/sulfite conditions. This indicates that, under sufficient sulfite dosage, the system is capable of achieving a stable response to various PFAS concentrations up to 100 μM. Such excellent linearity provides a solid foundation for indirect PFAS determination. The limit of detection (LOD) was calculated according to Equation (7):
where SD represents the standard deviation of blank sample measurements. The obtained LODs for these two PFAS were 0.0549 and 0.0492 μM, respectively. In addition, the measurement accuracy was evaluated by repeatedly determining the sample with a PFAS concentration of 25 μM 10 times. As shown in Table 1, the calculated recoveries of PFOA and PFOS were 100.8% and 101.9%, and the relative standard deviations (RSDs) were 2.95% and 3.63%, respectively. These results reflect the robustness of the defluorination efficiency of the VUV/sulfite system in ultrapure water medium, which enables PFAS quantification by determining the fluoride release amount after reductive pretreatment.
LOD = 3 × SD
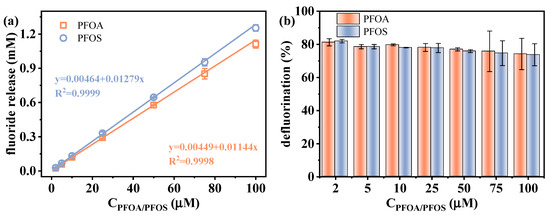
Figure 7.
(a) Relationship between the fluoride release amount and initial PFAS concentrations, and (b) defluorination efficiency of PFOA/PFOS under varied initial PFAS concentrations. Initial experimental conditions: [PFOA] = [PFOS] = 2–100 μM, [sulfite] = 20 mM, pH = 12, reaction time = 60 min.

Table 1.
Detection limit and measurement accuracy of PFOA and PFOS.
Then, the effect of sediments on the correlation between the fluoride release amount and initial PFAS concentrations was assessed using leachates from three sediments (S1, S2, S3) spiked with 2–50 µM of PFOA or PFOS and treated with VUV/sulfite. As shown in Figure 8 and Table 2, both PFOA and PFOS retained excellent linearity, despite an overall decrease in defluorination efficiency relative to ultrapure water. This decrease may be attributed to NO3− and divalent metal ions dissolved from the sediments, as has been discussed above. Additionally, sediment-derived dissolved organic matter (DOM) can quench eaq− and reduce VUV light utilization efficiency through competitive photon absorption [25,49]. PFOA calibration curves remained similar across all sediments, whereas PFOS showed greater variation, likely due to its stronger complexation tendency, which may reduce its availability for eaq−-driven reduction [5,50]. To counteract these effects and improve the response signal, optimization of operational parameters (such as sulfite dosage and treatment duration) is necessary. Using actual environmental matrices to establish calibration curves not only counteracts the matrix-induced effect of defluorination efficiency but also helps eliminate the influence of background fluoride in the sample, since both standards and test samples undergo identical processing. For samples with high PFAS concentrations, dilution can reduce matrix effects while maintaining detectability. Mild pretreatment (e.g., UV pre-irradiation or solid-phase extraction to remove DOM) may also be considered prior to defluorination analysis to reduce their impact.
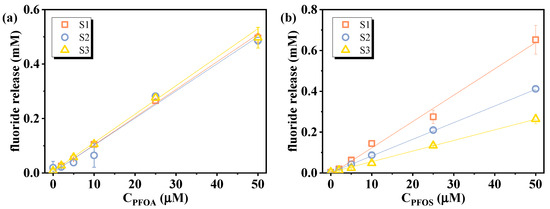
Figure 8.
Calibration curves of (a) PFOA and (b) PFOS under the influence of sediment leachates. Initial experimental conditions: [PFOA] = [PFOS] = 2–50 μM, [sulfite] = 20 mM, pH = 12, reaction time = 60 min.

Table 2.
Equations of calibration curves under the influence of sediment leachates.
Although this method was demonstrated using PFOA and PFOS, it is conceptually applicable to a broader range of PFAS. Previous studies have shown that eaq−-based systems have the ability to degrade various organofluorines, including short-chain PFAS, perfluoro-carboxylates and perfluoro-sulfonate, though defluorination efficiency may vary with molecular structure, particularly for ultra-short-chain or more stable compounds [24,30,33]. Similar to TOF-based approaches, this method measures total fluoride release amount and cannot distinguish the contributions of each PFAS in a mixture, which limits its applicability for mixed PFAS analysis. Nevertheless, these results demonstrate that, under appropriate conditions, fluoride release remains a reliable indirect measurement for individual PFAS quantification in complex medium.
2.3.2. Application in Adsorption Experiment
Based on the established calibration curves in Figure 8, the practical applicability of the VUV/sulfite defluorination system in PFAS quantification was explored through adsorption experiments. The equilibrium concentration of PFAS in the aqueous phase was determined by applying the VUV/sulfite system. Then, the adsorption isotherms of PFOA and PFOS onto three different sediment samples were evaluated, as shown in Figure 9. Both compounds exhibited measurable adsorption to all three sediments, confirming their affinity for natural solid phases. PFOA exhibited similar adsorption behaviors onto the three sediments, while PFOS showed more obvious variability, which may be attributed to its higher susceptibility to the constitution of sediments due to its longer chain [51].
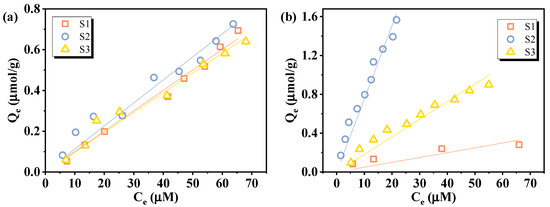
Figure 9.
Adsorption isotherms of (a) PFOA and (b) PFOS onto sediments.
Linear fitting was applied to the sorption isotherm data to calculate the partitioning coefficient (Kd, L/kg) and organic carbon-normalized distribution coefficient (Koc) according to Equations (8) and (9):
where Qe (μmol/g) is the concentration of PFAS in the sediment, Ce (μmol/mL) is the equilibrium concentration of PFAS in water, and foc is the fraction of organic carbon in the sediment (given in Table 3), respectively. Koc enables a standardized comparison of sorption capacities across sediments with varying organic carbon contents. The calculated logKoc values for PFOA ranged from 2.25 to 2.29, and those for PFOS ranged from 1.98 to 3.07, both within the range of values previously reported in the literature [51,52,53]. These results validate the feasibility of employing the VUV/sulfite system as a quantitative tool for analyzing perfluorinated compounds in complex environmental matrices, which offers a promising approach for assessing PFAS behavior in natural sorption processes.
Kd = Qe/Ce
Koc = Kd/foc ×100

Table 3.
Calculated water–sediment partitioning parameters for PFOA and PFOS.
3. Materials and Methods
3.1. Chemicals and Sediments
PFOA (96%) and PFOS (98%) were purchased from Macklin Biochemical (Shanghai, China). Sodium sulfite (Na2SO3), sodium hydroxide (NaOH) and other reagents were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Ultrapure water with 18.2 MΩ cm resistivity, used in all experiments, was obtained from a water purification system (Ming-Che 24UV, Millipore, France). The sediments used in this work were sampled from the river channels in Wuhan City, China. The collected sediment samples (S1, S2 and S3) were dried at room temperature and protected from light. Then, the dried solids were ground and sieved using a 100-mesh sieve.
3.2. Photochemical Reaction
The structure of the reactor used in this work is shown in Figure 10. A VUV disinfection lamp containing light at 185 nm was used as the irradiation light source. A total of 8 quartz tubes were used as the reaction tubes, as quartz allows the penetration of the VUV light. A circular reaction rack was placed on the rotating table, and the VUV lamp was placed in the center of the reaction rack, with 8 quartz tubes evenly placed around the VUV lamp. In the light reaction process, the rotating table was turned on so that the quartz tubes rotated around the ultraviolet lamp to avoid reaction differences due to the light intensity. Each quartz tube was equipped with a PTFE plug to isolate the circulation of air inside and outside of the reaction tube.
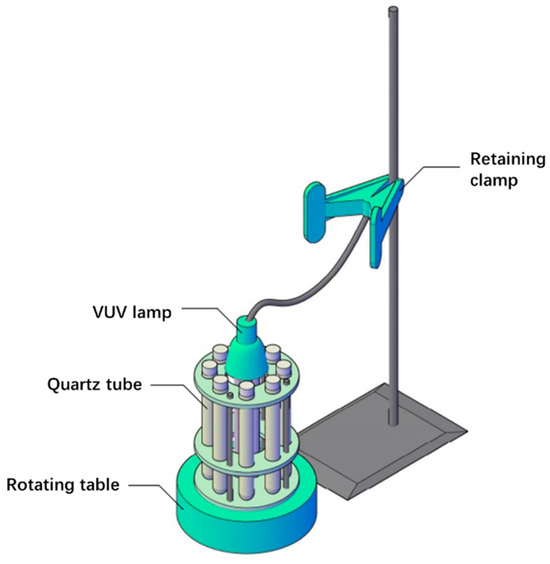
Figure 10.
Photoreactor structure diagram.
In order to investigate defluorination efficiency of PFOA/PFOS in the VUV/sulfite system under different conditions, solutions containing the desired amount of several reagents were prepared, including PFOA/PFOS as the reaction substrate, NaOH which provided the alkaline conditions, and anions or cations when needed. After each reagent was added into the quartz tube, ultrapure water was added to make up the total reaction volume to 10 mL. Then, a certain amount of sodium sulfite powder was added into the tube. The mixed reaction solution was aerated under argon (99.999%) for 30 min to remove the dissolved oxygen. After aeration, the quartz tube was quickly plugged with a PTFE plug to isolate the solution from contact with air. The prepared quartz tubes were then placed on the reaction rack, and the VUV lamp was turned on to start the reaction. When the desired reaction time was reached, we turned off the VUV lamp to stop the reaction. All experiments were conducted at least in duplicate.
3.3. Simulated Adsorption Experiments
Batch adsorption experiments were conducted in 15 mL centrifuge tubes under controlled conditions (25 °C) for 24 h. A weight of 0.5 g of sediment was equilibrated with 10 mL of PFAS solution under varied initial concentrations. After 24 h of adsorption, 5 mL of the filtered reaction solution was transferred into a quartz tube and subjected to photoreaction treatment according to the procedure described in Section 3.2. The fluoride concentration in the solution was then measured after the treatment using the VUV/sulfite system. In order to establish a fluoride–PFAS calibration curve under the influence of the sediment leachates, ultrapure water was used to prepare a control mixture with the same solid-to-liquid ratio as that in the adsorption experiment. This mixture was shaken for 24 h to extract soluble components from the sediment. The extract was then transferred into a quartz tube, spiked with a known concentration of PFAS, and subjected to the same photoreaction process as described in Section 3.2.
3.4. Analytical Methods
The released fluoride ion (F−) was measured by an ion-selective electrode (PXSJ-216F, Shanghai Yidian Analysis Instrument Co., Ltd., Shanghai, China) according to the Chinese standard of GB 7484-87 [54] (water quality–determination of fluoride–ion-selective electrode method). TISAB III (total ionic strength adjustment buffer, containing (CH2)6N4, KNO3, and C6H4Na2S2·H2O) was used during the analysis to avoid further pH adjustment. The property of sediments was analyzed according to the national standard methods. The spectrum of sulfite under varied pH conditions was recorded using a UV-Vis spectrophotometer (UV-3600, Shimadzu, Japan) using a 1 cm quartz cuvette at room temperature.
4. Conclusions
In this study, the defluorination of PFOA and PFOS using a VUV/sulfite system was investigated, with an exploration of its potential application in PFAS quantification. High defluorination efficiency of both PFOA and PFOS was achieved within 60 min of reductive reaction under the conditions of 20 mM of sulfite and pH 12. Operational parameters, including irradiation time, initial pH, and sulfite concentration, affected the defluorination efficiency: a higher pH value, higher dosage of sulfite and longer reaction time all enhanced the defluorination efficiency. The effects of common environmental ions occurring in surface waters were also evaluated. NO3− and divalent cations (Ca2+ and Mg2+) showed inhibitory effects, likely due to electron scavenging and precipitation, while other ions such as Cl−, SO42−, CO32−, Na+, and K+ exhibited negligible effects under the experimental conditions. Calibration curves established under varying PFAS concentrations exhibited excellent linearity (R2 > 0.999), and maintained robustness even in the presence of sediment leachates, despite a reduction in absolute fluoride release amount. Furthermore, the VUV/sulfite defluorination system was successfully applied to quantify the PFAS concentration in a sediment adsorption experiment. Both PFOA and PFOS showed measurable adsorption behaviors, and the calculated logKoc values were consistent with those reported in the literature. Overall, this work highlights the dual function of the VUV/sulfite system, offering an effective approach for PFAS degradation, and a rapid, cost-effective and reliable indirect analytical strategy for quantifying individual PFAS species in complex environmental matrices.
Author Contributions
Conceptualization, J.X. and F.W.; methodology, Y.C. and L.J.; validation, S.T., X.M. and Y.C.; formal analysis, S.T., X.M. and Y.C.; investigation, S.T., X.M. and Y.C.; writing—original draft preparation, S.T.; writing—review and editing, J.X. and F.W.; visualization, S.T., X.M. and L.J.; supervision, J.X. and F.W.; project administration, J.X.; funding acquisition, J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hubei Provincial Natural Science Foundation of China (2023AFD204) and the National Natural Science Foundation of China (No. 42077350).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.
Acknowledgments
Comments from the anonymous reviewers are appreciated.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cousins, I.T.; Johansson, J.H.; Salter, M.E.; Sha, B.; Scheringer, M. Outside the safe operating space of a new planetary boundary for per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Technol. 2022, 56, 11172–11179. [Google Scholar] [CrossRef]
- Milinovic, J.; Lacorte, S.; Vidal, M.; Rigol, A. Sorption behaviour of perfluoroalkyl substances in soils. Sci. Total Environ. 2015, 511, 63–71. [Google Scholar] [CrossRef]
- Li, F.; Duan, J.; Tian, S.; Ji, H.; Zhu, Y.; Wei, Z.; Zhao, D. Short-chain per- and polyfluoroalkyl substances in aquatic systems: Occurrence, impacts and treatment. Chem. Eng. J. 2020, 380, 122506. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Tang, S.; Qiu, J.; Luo, Y.; Yang, C.; Lai, X.; Wang, Q.; Cao, H. Per- and polyfluoroalkyl substances (PFAS) in consumer products: An overview of the occurrence, migration, and exposure assessment. Molecules 2025, 30, 994. [Google Scholar] [CrossRef]
- Zhao, L.; Bian, J.; Zhang, Y.; Zhu, L.; Liu, Z. Comparison of the sorption behaviors and mechanisms of perfluorosulfonates and perfluorocarboxylic acids on three kinds of clay minerals. Chemosphere 2014, 114, 51–58. [Google Scholar] [CrossRef]
- Sharma, B.M.; Bharat, G.K.; Tayal, S.; Larssen, T.; Bečanová, J.; Karásková, P.; Whitehead, P.G.; Futter, M.N.; Butterfield, D.; Nizzetto, L. Perfluoroalkyl substances (PFAS) in river and ground/drinking water of the Ganges River basin: Emissions and implications for human exposure. Environ. Pollut. 2016, 208, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Kirchgeorg, T.; Dreyer, A.; Gabrielli, P.; Gabrieli, J.; Thompson, L.G.; Barbante, C.; Ebinghaus, R. Seasonal accumulation of persistent organic pollutants on a high altitude glacier in the Eastern Alps. Environ. Pollut. 2016, 218, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kannan, K. Perfluorinated acids in air, rain, snow, surface runoff, and lakes: Relative importance of pathways to contamination of urban lakes. Environ. Sci. Technol. 2007, 41, 8328–8334. [Google Scholar] [CrossRef]
- Tang, A.; Zhang, X.; Li, R.; Tu, W.; Guo, H.; Zhang, Y.; Li, Z.; Liu, Y.; Mai, B. Spatiotemporal distribution, partitioning behavior and flux of per- and polyfluoroalkyl substances in surface water and sediment from Poyang Lake, China. Chemosphere 2022, 295, 133855. [Google Scholar] [CrossRef]
- Liu, T.; Qian, X.; Wang, S.; Wang, H.; Wei, S.; Chen, H. Occurrence and transport of perfluoroalkyl acids (PFAAs) in a Yangtze River water diversion project during water diversion and flooding. Water Res. 2021, 205, 117662. [Google Scholar] [CrossRef]
- Rehman, A.U.; Crimi, M.; Andreescu, S. Current and emerging analytical techniques for the determination of PFAS in environmental samples. Trends Environ. Anal. Chem. 2023, 37, e00198. [Google Scholar] [CrossRef]
- Aborode, A.T.; Adesola, R.O.; Idris, I.; Sakariyau Adio, W.; Olapade, S.; Oluwafisayo, G.; Onifade, I.A.; Fakorede, S.; Bakare-Abidola, T.; Olaoye, J.; et al. Challenges associated with PFAS detection method in Africa. Environ. Health Insights 2025, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, Y.; Yang, R.; Jiang, Y.; Meng, M.; Liu, Z.; Ni, L.; Wu, W.; Liu, H. Adsorption for perfluorooctanoic acid with graphitic-phase carbon nitride and its HPLC fluorescence determination. Can. J. Chem. Eng. 2020, 98, 394–403. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Pan, H.; Wei, Y.; Yang, Y.; Xu, N.; Chen, B.; Mohseni, M.; Esfahani, E.B. Total organic fluorine (TOF) analysis by completely converting TOF into fluoride with vacuum ultraviolet. J. Hazard. Mater. 2022, 429, 128389. [Google Scholar] [CrossRef]
- Whitehead, H.D.; Venier, M.; Wu, Y.; Eastman, E.; Urbanik, S.; Diamond, M.L.; Shalin, A.; Schwartz-Narbonne, H.; Bruton, T.A.; Blum, A.; et al. Fluorinated compounds in North American cosmetics. Environ. Sci. Technol. Lett. 2021, 8, 538–544. [Google Scholar] [CrossRef]
- Gauthier, J.R.; Mabury, S.A. Experimentally determined aqueous diffusion coefficients of PFAS using 19F NMR diffusion-ordered spectroscopy. ACS ES&T Water 2024, 4, 4615–4624. [Google Scholar] [CrossRef]
- D’Agostino, L.A.; Mabury, S.A. Identification of novel fluorinated surfactants in aqueous film forming foams and commercial surfactant concentrates. Environ. Sci. Technol. 2014, 48, 121–129. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, T.; Yu, S.; Yi, P.; Li, L. Influence of UV lamp, sulfur(IV) concentration, and pH on bromate degradation in UV/sulfite systems: Mechanisms and applications. Water Res. 2017, 111, 288–296. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Shan, C.; Zhang, W.; Pan, B. A novel combined process for efficient removal of Se(VI) from sulfate-rich water: Sulfite/UV/Fe(III) coagulation. Chemosphere 2018, 211, 867–874. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, C.; Li, F.; Chen, J.; Zhou, Q. Photo-reductive defluorination of perfluorooctanoic acid in water. Water Res. 2010, 44, 2939–2947. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Banayan Esfahani, E.; Mohseni, M. Fluence-based photo-reductive decomposition of PFAS using vacuum UV (VUV) irradiation: Effects of key parameters and decomposition mechanism. J. Environ. Chem. Eng. 2022, 10, 107050. [Google Scholar] [CrossRef]
- Wu, S.; Shen, L.; Lin, Y.; Yin, K.; Yang, C. Sulfite-based advanced oxidation and reduction processes for water treatment. Chem. Eng. J. 2021, 414, 128872. [Google Scholar] [CrossRef]
- Liu, S.; Chen, G.; Shi, Q.; Gan, J.; Jin, B.; Men, Y.; Liu, H. Promotive effects of chloride and sulfate on the near-complete destruction of perfluorocarboxylates (PFCAs) in brine via hydrogen-tuned 185-nm UV photolysis: Mechanisms and kinetics. Environ. Sci. Technol. 2024, 58, 10347–10356. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Liu, X.; Cui, F.; Zhao, Z. Efficient reductive and oxidative decomposition of haloacetic acids by the vacuum-ultraviolet/sulfite system. Water Res. 2022, 210, 117974. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, T.; Kim, J.; Kim, M.K.; Eom, S.; Choi, Y.; Zoh, K.D. Reductive degradation mechanism of perfluorooctanoic acid (PFOA) during vacuum ultraviolet (VUV) reactions combining with sulfite and iodide. Chemosphere 2024, 348, 140759. [Google Scholar] [CrossRef]
- Li, S.; Xu, J.; Chen, W.; Yu, Y.; Liu, Z.; Li, J.; Wu, F. Multiple transformation pathways of p-arsanilic acid to inorganic arsenic species in water during UV disinfection. J. Environ. Sci. 2016, 47, 39–48. [Google Scholar] [CrossRef]
- Luo, T.; Le Crom, S.; Luong, N.T.; Hanna, K.; Boily, J.F. Goethite-bound copper controls the fate of antibiotics in aquatic environments. ACS ES&T Water 2024, 4, 638–647. [Google Scholar] [CrossRef]
- Oliveira, C.; Lima, D.L.D.; Silva, C.P.; Calisto, V.; Otero, M.; Esteves, V.I. Photodegradation of sulfamethoxazole in environmental samples: The role of pH, organic matter and salinity. Sci. Total Environ. 2019, 648, 1403–1410. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Gao, J.; Yu, Y.; Men, Y.; Gu, C.; Liu, J. Accelerated degradation of perfluorosulfonates and perfluorocarboxylates by UV/sulfite + iodide: Reaction mechanisms and system efficiencies. Environ. Sci. Technol. 2022, 56, 3699–3709. [Google Scholar] [CrossRef]
- Lorpaiboon, W.; Ho, J. High-level quantum chemical prediction of C-F bond dissociation energies of perfluoroalkyl substances. J. Phys. Chem. A 2023, 127, 7943–7953. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Bergmann, U.; Leiviskä, T. Reductive degradation of perfluorooctanoic acid in complex water matrices by using the UV/sulfite process. Water Res. 2021, 205, 117676. [Google Scholar] [CrossRef] [PubMed]
- Bentel, M.J.; Yu, Y.; Xu, L.; Li, Z.; Wong, B.M.; Men, Y.; Liu, J. Defluorination of per- and polyfluoroalkyl substances (PFASs) with hydrated electrons: Structural dependence and implications to PFAS remediation and management. Environ. Sci. Technol. 2019, 53, 3718–3728. [Google Scholar] [CrossRef]
- Song, Z.; Tang, H.; Wang, N.; Zhu, L. Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system. J. Hazard. Mater. 2013, 262, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Luo, T.; Xu, J.; Li, J.; Wu, F.; Brigante, M.; Mailhot, G. Enhanced oxidation of aniline using Fe(III)-S(IV) system: Role of different oxysulfur radicals. Chem. Eng. J. 2019, 362, 183–189. [Google Scholar] [CrossRef]
- Luo, T.; Wang, Z.; Wang, Y.; Liu, Z.; Pozdnyakov, I.P. Different role of bisulfite/sulfite in UVC-S(IV)-O2 system for arsenite oxidation in water. Molecules 2019, 24, 2307. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, T.; Wang, H.; Han, H.; Dong, W. Hydrated electron based decomposition of perfluorooctane sulfonate (PFOS) in the VUV/sulfite system. Sci. Total Environ. 2017, 607–608, 541–548. [Google Scholar] [CrossRef]
- Xin, X.; Kim, J.; Ashley, D.C.; Huang, C.H. Degradation and defluorination of per- and polyfluoroalkyl substances by direct photolysis at 222 nm. ACS ES&T Water 2023, 3, 2776–2785. [Google Scholar] [CrossRef]
- Maza, W.A.; Breslin, V.M.; Plymale, N.T.; Desario, P.A.; Epshteyn, A.; Owrutsky, J.C.; Pate, B.B. Nanosecond transient absorption studies of the pH-dependent hydrated electron quenching by HSO3−. Photochem. Photobiol. Sci. 2019, 18, 1526–1532. [Google Scholar] [CrossRef]
- Olawade, D.B.; Ijiwade, J.O.; Fapohunda, O.; Ige, A.O.; Olajoyetan, D.O.; Wada, O.Z. Predictive modeling of PFAS behavior and degradation in novel treatment scenarios: A review. Process Saf. Environ. Prot. 2025, 196, 106869. [Google Scholar] [CrossRef]
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of water matrix on the removal of micropollutants by advanced oxidation technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef]
- Huang, Y.; Sheng, B.; Wang, Z.; Liu, Q.; Yuan, R.; Xiao, D.; Liu, J. Deciphering the degradation/chlorination mechanisms of maleic acid in the Fe(II)/peroxymonosulfate process: An often overlooked effect of chloride. Water Res. 2018, 145, 453–463. [Google Scholar] [CrossRef]
- Li, X.; Shen, J.; Sun, Z.; Zhang, W.; Ma, F.; Gu, Q. Insights into the impacts of chloride ions on the oxidation of 2,4-dinitrotoluene using ferrous activated persulfate: Removal efficiency, reaction mechanism, transformation pathway, and toxicity assessment. Chemosphere 2023, 317, 137887. [Google Scholar] [CrossRef] [PubMed]
- Milh, H.; Yu, X.; Cabooter, D.; Dewil, R. Degradation of ciprofloxacin using UV-based advanced removal processes: Comparison of persulfate-based advanced oxidation and sulfite-based advanced reduction processes. Sci. Total Environ. 2021, 764, 144510. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, H.; Luo, T.; Liu, Z.; Xia, J.; Zhang, X. Phototransformation of p-arsanilic acid in aqueous media containing nitrogen species. Chemosphere 2018, 212, 777–783. [Google Scholar] [CrossRef]
- Xu, J.; Marsac, R.; Wei, C.; Wu, F.; Boily, J.F.; Hanna, K. Cobinding of pharmaceutical compounds at mineral surfaces: Mechanistic modeling of binding and cobinding of nalidixic acid and niflumic acid at goethite surfaces. Environ. Sci. Technol. 2017, 51, 11617–11624. [Google Scholar] [CrossRef]
- Cheng, W.; Zhou, L.; Marsac, R.; Boily, J.F.; Hanna, K. Effects of organic matter–goethite interactions on reactive transport of nalidixic acid: Column study and modeling. Environ. Res. 2020, 191, 110187. [Google Scholar] [CrossRef] [PubMed]
- Fovet, Y.; Gal, J.Y. Formation constants β2 of calcium and magnesium fluorides at 25 °C. Talanta 2000, 53, 617–626. [Google Scholar] [CrossRef]
- Fennell, B.D.; Odorisio, A.; Mckay, G. Quantifying hydrated electron transformation kinetics in UV-advanced reduction processes using the Re-,UV method. Environ. Sci. Technol. 2022, 56, 10329–10338. [Google Scholar] [CrossRef]
- Tipplook, M.; Hisama, K.; Koyama, M.; Fujisawa, K.; Hayashi, F.; Sudare, T.; Teshima, K. Cation-doped nanocarbons for enhanced perfluoroalkyl substance removal: Exotic bottom-up solution plasma synthesis and characterization. ACS Appl. Mater. Interfaces 2024, 16, 61832–61845. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, L.; Yang, L.; Liu, Z.; Zhang, Y. Distribution and desorption of perfluorinated compounds in fractionated sediments. Chemosphere 2012, 88, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, Y.; Zhao, X.; Du, P.; Liu, S.; Lv, J.; Xu, F.; Meng, W.; Xu, J. Distribution, source characterization and inventory of perfluoroalkyl substances in Taihu Lake, China. Chemosphere 2015, 127, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Fagbayigbo, B.O.; Opeolu, B.O.; Fatoki, O.S.; Olatunji, O.S.; Akharame, M.O.; Human, I.S. Sorption and partitioning of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) onto sediments of Diep and Plankenburg river systems Western Cape, South Africa. Environ. Technol. Innov. 2022, 25, 102110. [Google Scholar] [CrossRef]
- Chinese Standard of GB 7484-87. Available online: https://www.codeofchina.com/standard/GBT7484-1987.html (accessed on 28 April 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).