Finely Designing Dicarboxylic Acid-Based Protic Ionic Liquids System for Tailoring Lignin Structure via Demethylation Strategy
Abstract
1. Introduction
2. Results and Discussions
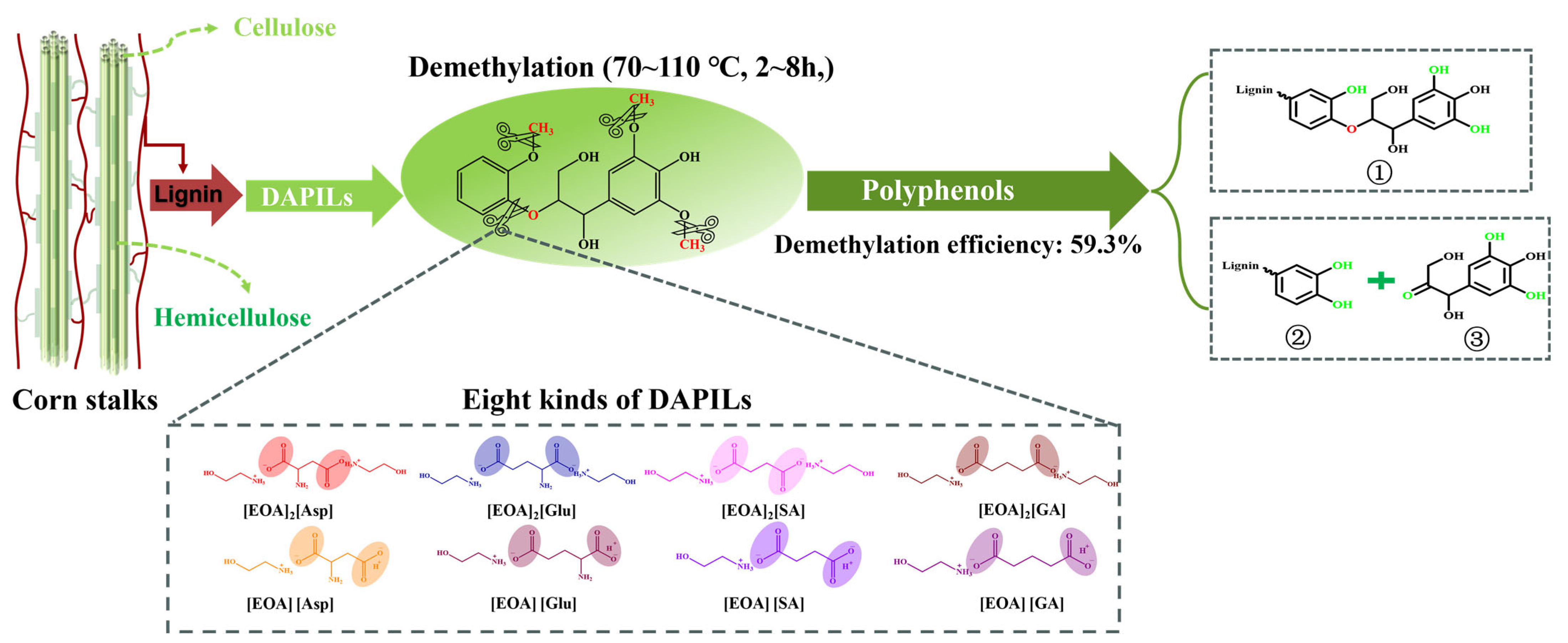
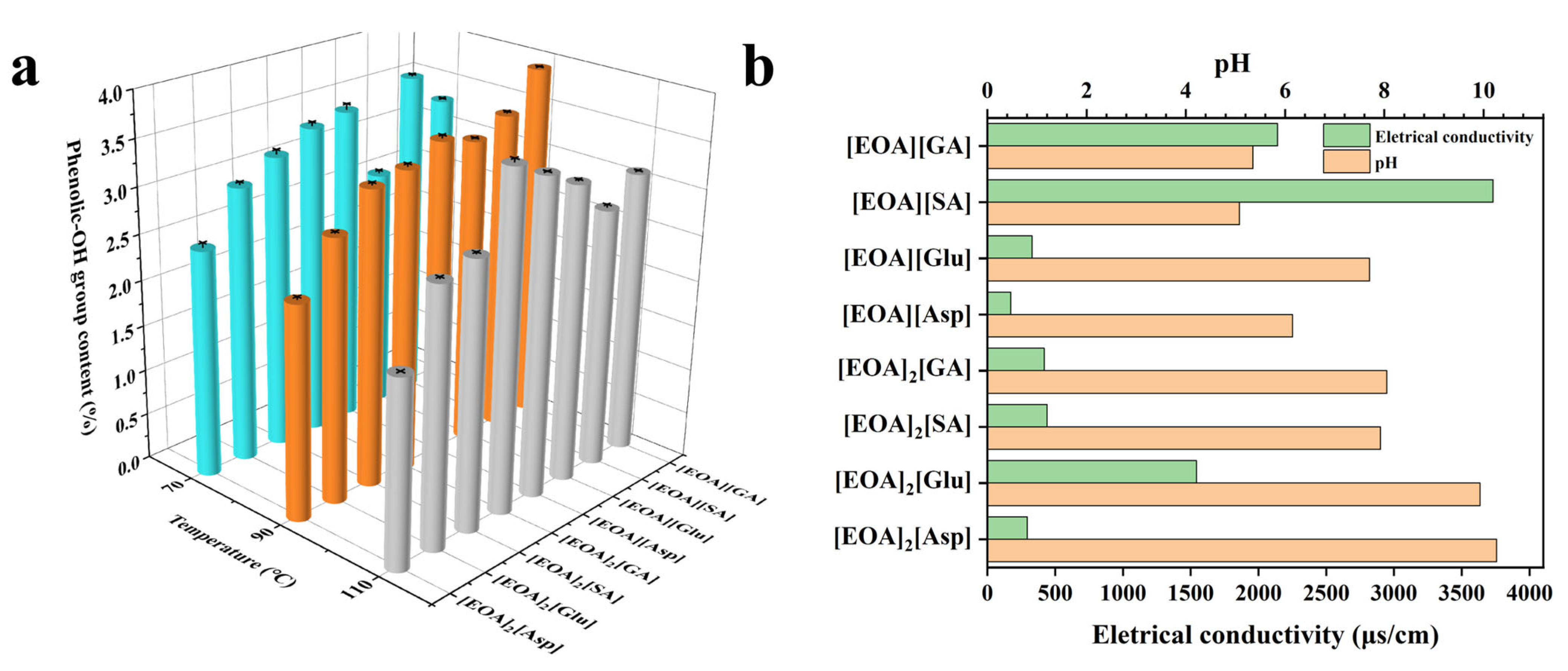
2.1. Preparation and Characterization of DAPILs
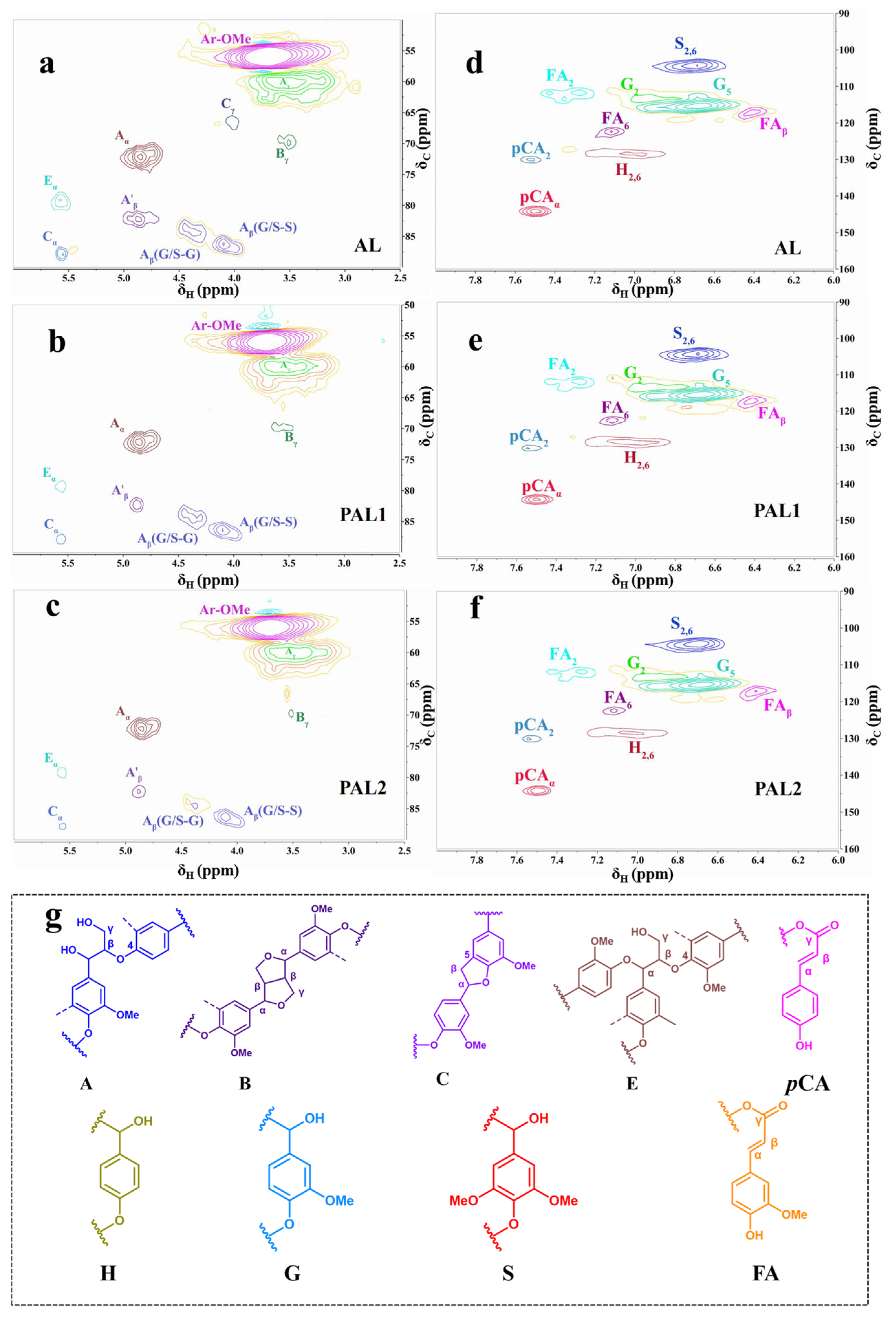
2.2. Demethylation and Structural Characterization of Lignin
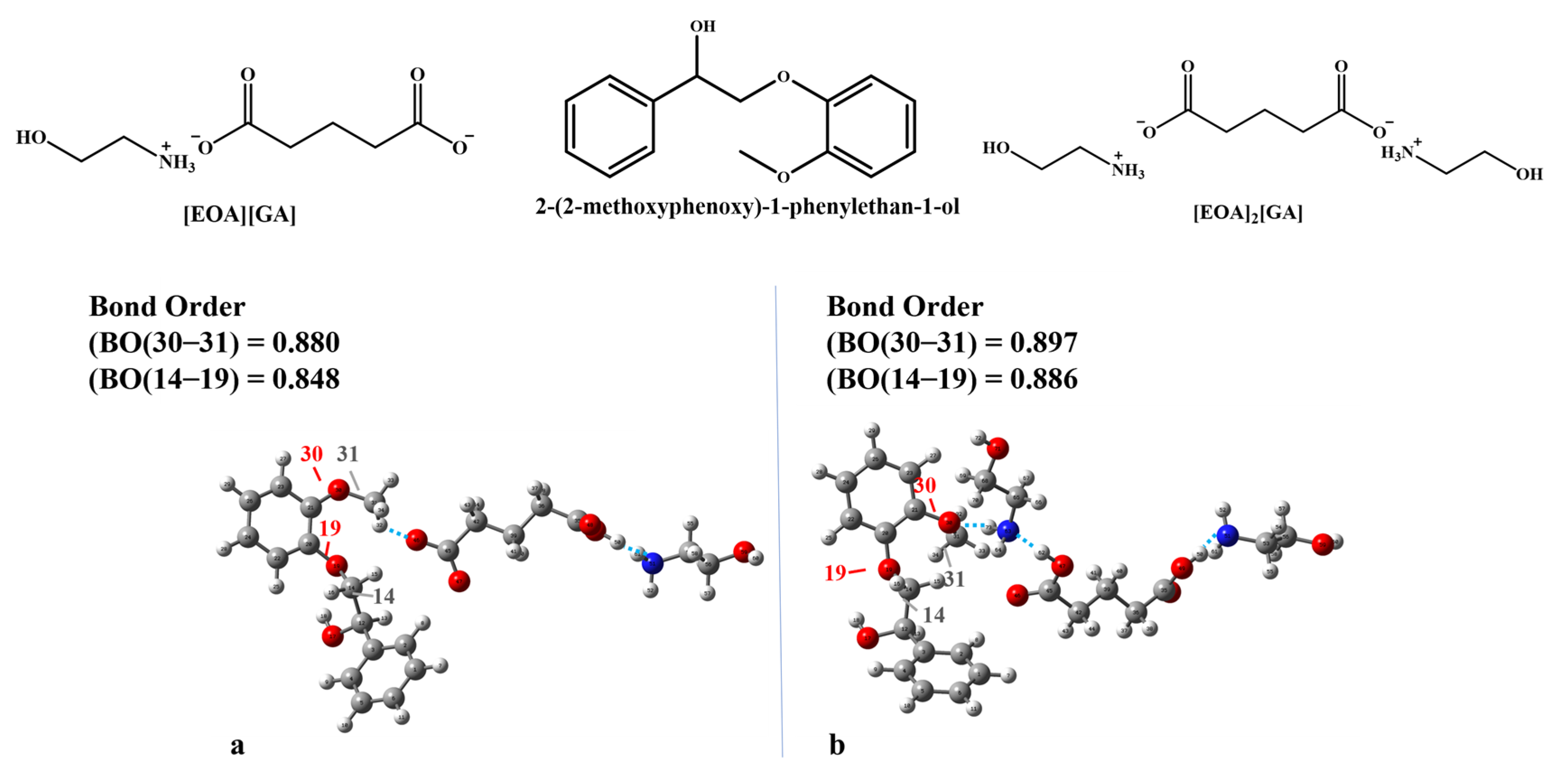
2.3. Molecular Computation
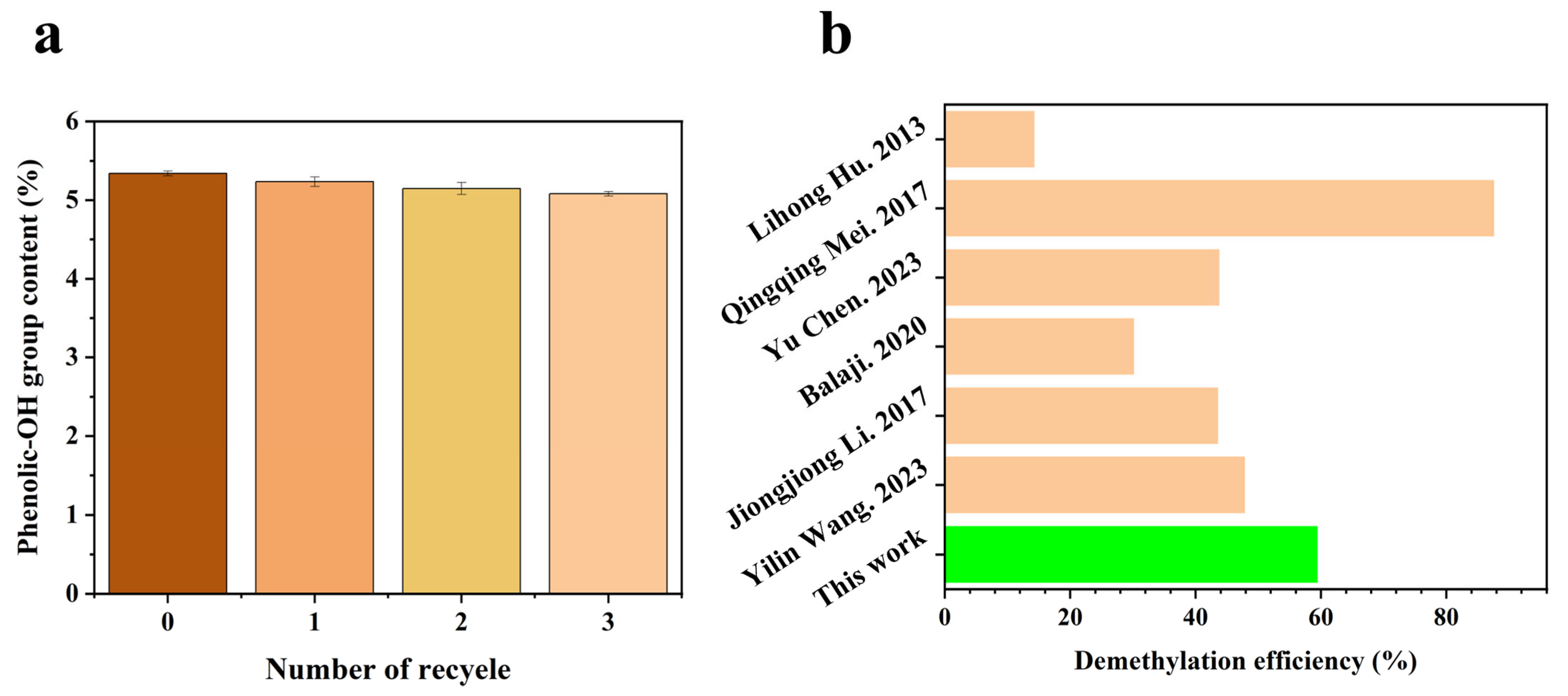
2.4. DAPIL Cycle Performance
3. Materials and Methods
3.1. Materials
3.2. Lignin Purification Treatment
3.3. Preparation of DAPILs
3.4. Demethylation of Purified Lignin in ILs and Recovery of DAPILs
3.5. Measurement of Ph-OH in Lignin Species by Ultraviolet Differential Photometry
3.6. Characterization of Lignin by Fourier Transform Infrared Spectroscopy
3.7. Gel Permeation Chromatography (GPC) Analysis of Lignin
3.8. Elemental Analysis
3.9. 1H NMR of Lignin and DAPILs
3.10. 2D HSQC NMR
3.11. DAPILs Conductivity Test
3.12. Determination of the pH Value of DAPILs
3.13. Meyer Bond Orders
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spiridon, I. Extraction of lignin and therapeutic applications of lignin-derived compounds. A review. Environ. Chem. Lett. 2020, 18, 771–785. [Google Scholar] [CrossRef]
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Pulkki, R.; Xu, C. Global lignin supply overview and kraft lignin potential as an alternative for petroleum-based polymers. Renew. Sustain. Energy Rev. 2020, 123, 109768. [Google Scholar] [CrossRef]
- Gan, M.J.; Niu, Y.Q.; Qu, X.J.; Zhou, C.H. Lignin to value-added chemicals and advanced materials: Extraction, degradation, and functionalization. Green Chem. 2022, 24, 7705–7750. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, H.; Chen, X.; Liu, T.; Zheng, Y.; Dai, L.; Si, C. Green and ultrastrong polyphenol lignin-based vitrimer adhesive with photothermal conversion property, wide temperature adaptability, and solvent resistance. Chem. Eng. J. 2023, 477, 147216. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Hao, J.-H.; Huang, H.-F.; Zhang, J.-P.; Liu, X.-C.; Lan, H.-Q.; Zhou, A.L.; Ge, S.-L.; Xuan, S.-H.; Liu, Z.-C.; Yang, J. A novel approach for characterization of lignin structure in tobacco via fast 2D HSQC NMR spectroscopy with non-uniform sampling. Ind. Crops Prod. 2025, 223, 120236. [Google Scholar] [CrossRef]
- Zhen, X.; Li, H.; Xu, Z.; Wang, Q.; Xu, J.; Zhu, S.; Wang, Z.; Yuan, Z. Demethylation, phenolation, and depolymerization of lignin for the synthesis of lignin-based epoxy resin via a one-pot strategy. Ind. Crops Prod. 2021, 173, 114135. [Google Scholar] [CrossRef]
- Jablonskis, A.; Arshanitsa, A.; Arnautov, A.; Telysheva, G.; Evtuguin, D. Evaluation of Ligno Boost™ softwood kraft lignin epoxidation as an approach for its application in cured epoxy resins. Ind. Crops Prod. 2018, 112, 225–235. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Li, H.; Ying, S.; Ding, Z.; Wang, Y.; Fang, T.; Wei, C.; Ma, C.; Sun, B.; et al. Unlocking the potential of ionic liquid-functionalized aqueous electrolytes for aqueous ammonium-bromine/ion batteries. Energy Storage Mater. 2024, 70, 103553. [Google Scholar] [CrossRef]
- Ramzan, H.; Usman, M.; Nadeem, F.; Shahzaib, M.; Ur Rahman, M.; Singhania, R.R.; Jabeen, F.; Patel, A.K.; Qing, C.; Liu, S.; et al. Depolymerization of lignin: Recent progress towards value-added chemicals and biohydrogen production. Bioresour. Technol. 2023, 386, 129492. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, H.; Wang, X.; Liu, C.; Han, Y.; Zhai, S.; Du, H. Boosting lignin-based photocatalyst with photocorrosion resistance for efficient H2O2 production via hetero-interfacial π-π stacking channels. Carbon Ener. 2025, 7, e666. [Google Scholar] [CrossRef]
- Singh, M.; Lee, S.C.; Won, K. Lignin phenolation by graft copolymerization to boost its reactivity. Int. J. Biol. Macromol. 2024, 266 Pt 2, 131258. [Google Scholar] [CrossRef]
- Jiang, X.; Tian, Z.; Ji, X.; Ma, H.; Yang, G.; He, M.; Dai, L.; Xu, T.; Si, C. Alkylation modification for lignin color reduction and molecular weight adjustment. Int. J. Biol. Macromol. 2022, 201, 400–410. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Z.; Guo, L.; Zhai, H.; Ren, H. Formation of high carbohydrate and acylation condensed lignin from formic acid-acetic acid-H2O biorefinery of corn stalk rind. Ind. Crops Prod. 2021, 161, 113165. [Google Scholar] [CrossRef]
- Shan, X.; Zhao, Y.; Bo, S.; Yang, L.; Xiao, Z.; An, Q.; Zhai, S. Magnetic aminated lignin/CeO(2)/Fe(3)O(4) composites with tailored interfacial chemistry and affinity for selective phosphate removal. Sci. Total Environ. 2021, 796, 148984. [Google Scholar] [CrossRef] [PubMed]
- Dehne, L.; Vila Babarro, C.; Saake, B.; Schwarz, K.U. Influence of lignin source and esterification on properties of lignin-polyethylene blends. Ind. Crops Prod. 2016, 86, 320–328. [Google Scholar] [CrossRef]
- Wu, R.; Wang, X.; Zhang, Y.; Fu, Y.; Qin, M. Efficient removal of surface-deposited pseudo-lignin and lignin droplets by isothermal phase separation during hydrolysis. Bioresour. Technol. 2022, 345, 126533. [Google Scholar] [CrossRef]
- Ji, D.; Wang, Y.; Peng, J.; Yuan, D.; Li, Z.; Ji, D.; Wu, H. Recent Advances in Depolymerization and High-Value Utilization of Lignin: A Review. Ind. Eng. Chem. Res. 2024, 63, 19916–19935. [Google Scholar] [CrossRef]
- Danlu, Z.; Xu, Z.; Sinong, W.; Yan, X.; Qiqi, D.; Fengxia, Y.; Peng, W.; Chuanfu, L.; Wu, L. Electrochemical oxidation of lignin model compounds over metal oxyhydroxides on nickel foam. Green Chem. 2024, 26, 7759–7768. [Google Scholar] [CrossRef]
- Hu, L.; Pan, H.; Zhou, Y.; Hse, C.-Y.; Liu, C.; Zhang, B.; Xu, B. Chemical Groups and Structural Characterization of Lignin via Thiol-Mediated Demethylation. J. Wood Chem. Technol. 2013, 34, 122–134. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Du, B.; Yan, S.; Li, W.; Chen, X.; Sun, R. Preparation of activated lignin with high hydroxyl content using lewis acid as demethylation reagent. Int. J. Biol. Macromol. 2022, 222 Pt B, 2571–2580. [Google Scholar] [CrossRef]
- Wang, H.; Eberhardt, T.L.; Wang, C.; Gao, S.; Pan, H. Demethylation of Alkali Lignin with Halogen Acids and Its Application to Phenolic Resins. Polymers 2019, 11, 1771. [Google Scholar] [CrossRef] [PubMed]
- Venkatesagowda, B.; Dekker, R.F.H. Microbial demethylation of lignin: Evidence of enzymes participating in the removal of methyl/methoxyl groups. Enzym. Microb. Technol. 2021, 147, 109780. [Google Scholar] [CrossRef]
- Ávila, M.I.; Alonso-Doncel, M.M.; Cueto, J.; Briones, L.; Gómez-Pozuelo, G.; Escola, J.M.; Serrano, D.P.; Peral, A.; Botas, J.A. Production of high value-added phenolic compounds through lignin catalytic pyrolysis over ion-exchanged hierarchical ZSM-5 and Beta zeolites. Catal. Today 2025, 456, 115343. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, W.; Miao, H.; Cui, Y.; Chen, Y.; Wang, Z.; Xiao, G.; Xue, B.; Jiang, W. Exploration of microwave absorptivity and catalysis of CMF@Co-MoS2 for microwave-initiated depolymerization of lignin. Chem. Eng. J. 2024, 501, 157591. [Google Scholar] [CrossRef]
- Mei, Q.; Liu, H.; Shen, X.; Meng, Q.; Liu, H.; Xiang, J.; Han, B. Selective Utilization of the Methoxy Group in Lignin to Produce Acetic Acid. Angew. Chem. Int. Ed. 2017, 56, 14868–14872. [Google Scholar] [CrossRef]
- Bomon, J.; Bal, M.; Achar, T.K.; Sergeyev, S.; Wu, X.; Wambacq, B.; Lemière, F.; Sels, B.F.; Maes, B.U.W. Efficient demethylation of aromatic methyl ethers with HCl in water. Green Chem. 2021, 23, 1995–2009. [Google Scholar] [CrossRef]
- Lan, W.; Luterbacher, J.S. Preventing Lignin Condensation to Facilitate Aromatic Monomer Production. Chimia 2019, 73, 591–598. [Google Scholar] [CrossRef]
- Jain, M.; El Seoud, O.; Kailasa, S.K.; Malek, N.I. Recent advances in synthesis, properties and applications of pH, light-responsive and functionalized surface active ionic liquids. J. Ionic Liquids 2022, 2, 100046. [Google Scholar] [CrossRef]
- Sun, W.; Xu, J.; Li, L.; Liu, Z.; Xiao, Z.; An, Q.; Huang, J. Production of 5,5′(oxy-bis(methylene)) bis-2-furfural from 5-hydroxymethylfurfural through the hydrogen-bonding catalysis of ionic liquids. Mol. Catal. 2025, 573, 114842. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H.; Yuan, X.; Sha, Y.; Li, J.; Dong, T.; Song, Y.; Zhang, S. Regulating robust interphase using a functional ionic liquid additive with bi-electrode affinity to stabilize the high-voltage lithium-rich lithium metals batteries. Chem. Eng. J. 2023, 464, 142578. [Google Scholar] [CrossRef]
- Jheng, L.-C.; Chen, W.-Y.; Huang, G.-L.; Zhao, Z.-L.; Hsu, S.L.-C.; Ko, W.-C. Enhanced hydroxide conductivity and dimensional stability in quaternized polybenzimidazole-based nanocomposite membranes containing ionic liquid-impregnated covalent organic framework for anion exchange membrane fuel cells. Int. J. Hydrogen Energy 2025, 130, 108–118. [Google Scholar] [CrossRef]
- Kurc, B.; Gross, X.; Piglowska, M.; Kolodziejczak-Radzimska, A.; Klapiszewski, L. Lignin activation with selected ionic liquids based on kinetic and thermodynamic analyses. Int. J. Biol. Macromol. 2025, 305 Pt 2, 141144. [Google Scholar] [CrossRef]
- Szalaty, T.J.; Klapiszewski, Ł.; Jesionowski, T. Recent developments in modification of lignin using ionic liquids for the fabrication of advanced materials–A review. J. Mol. Liq. 2020, 301, 112417. [Google Scholar] [CrossRef]
- Cao, K.; Yang, F.; Wan, H.; Duan, X.; Shi, J.; Sun, Z. A selective oxidative depolymerization of larch lignin to ethyl vanillate by multifunctional catalysts combining alkaline ionic liquid and polyoxometalates with hydrogen peroxide. Int. J. Biol. Macromol. 2025, 295, 139642. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Konda, N.V.S.N.M.; Parthasarathi, R.; Dutta, T.; Valiev, M.; Xu, F.; Simmons, B.A.; Singh, S. One-pot integrated biofuel production using low-cost biocompatible protic ionic liquids. Green Chem. 2017, 19, 3152–3163. [Google Scholar] [CrossRef]
- Luo, Q.; Li, C.; Zhao, W.; Ding, W.; Liu, Y.; Xiao, W.; Liu, H.; Pang, X.; Sun, J. Lignin Demethylated by Protic Ionic Liquid as a Novel and Sustainable Chrome-Free Tanning Agent for Eco-Leather Production. ACS Sustain. Chem. Eng. 2024, 12, 9682–9694. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, C.; Cui, Y.; Ye, J.; He, B.; Liu, X.; Sun, J. Efficient demethylation of lignin for polyphenol production enabled by low-cost bifunctional protic ionic liquid under mild and halogen-free conditions. Chem. Eng. J. 2022, 443, 136486. [Google Scholar] [CrossRef]
- Zhu, G.; Ye, D.; Chen, X.; Wu, Y.; Yang, Z.; Mai, Y.; Liao, B.; Chen, J. Lignin-derived polyphenols with enhanced antioxidant activities by chemical demethylation and their structure-activity relationship. Int. J. Biol. Macromol. 2023, 237, 124030. [Google Scholar] [CrossRef]
- Sun, M.; Wang, X.; Ni, S.; Jiao, L.; Bian, H.; Dai, H. Structural modification of alkali lignin into higher performance energy storage materials: Demethylation and cleavage of aryl ether bonds. Ind. Crops Prod. 2022, 187, 115441. [Google Scholar] [CrossRef]
- Wang, K.; Wang, C.; Dong, Q.; Wang, K.; Qian, J.; Liu, Z.; Huang, F. Enhancing corrosion and abrasion resistances simultaneously of epoxy resin coatings by novel hyperbranched amino-polysiloxanes. Prog. Org. Coat. 2024, 196, 108680. [Google Scholar] [CrossRef]
- Li, J.-F.; Li, Z.-M.; Xu, J.-B.; Guo, C.-Y.; Fang, G.-W.; Zhou, Y.; Sun, M.-S.; Tao, D.-J. Demethylation of Waste Alkali Lignin for Rapid and Efficient Ammonia Adsorption. Ind. Eng. Chem. Res. 2024, 63, 3282–3289. [Google Scholar] [CrossRef]
- Gao, C.; Li, M.; Zhu, C.; Hu, Y.; Shen, T.; Li, M.; Ji, X.; Lyu, G.; Zhuang, W. One-pot depolymerization, demethylation and phenolation of lignin catalyzed by HBr under microwave irradiation for phenolic foam preparation. Compos. Part B Eng. 2021, 205, 108530. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, F.; Li, L.; Wang, H.; Jia, Z.; Sun, Y.; Jiang, E.; Xu, X. Promotion of biomass pyrolytic saccharification and lignin depolymerization via nucleophilic reagents quenching of the carbonium ions. Bioresour. Technol. 2022, 363, 127876. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, T.; Wu, S.; Zhang, X. Demethylation of a methoxy group to inhibit repolymerization during alkaline lignin pyrolysis. Fuel 2021, 286, 119394. [Google Scholar] [CrossRef]
- Wang, Z.; Abbas, A.; Sun, H.; Jin, H.; Jia, T.; Liu, J.; She, D. Amination-modified lignin recovery of aqueous phosphate for use as binary slow-release fertilizer. Int. J. Biol. Macromol. 2023, 242 Pt 2, 124862. [Google Scholar] [CrossRef]
- Liu, Z.; Zeng, J.; Dong, Z.; Chen, Y.; Yang, H.; Chen, H. Insight into the mechanism of lignin amination pretreatment on lignin structure and its pyrolysis property for lignin valorization. Chem. Eng. J. 2024, 499, 156386. [Google Scholar] [CrossRef]
- Ahadyani, N.; Abdollahi, M. Phenolation, amination and cross-linking of lignin: Synthesis and characterization of functionalized lignin. Polym. Bull. 2023, 81, 8643–8661. [Google Scholar] [CrossRef]
- Chen, K.; Kang, Q.K.; Li, Y.; Wu, W.Q.; Zhu, H.; Shi, H. Catalytic Amination of Phenols with Amines. J. Am. Chem. Soc. 2022, 144, 1144–1151. [Google Scholar] [CrossRef]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef]
- Da Costa Sousa, L.; Foston, M.; Bokade, V.; Azarpira, A.; Lu, F.; Ragauskas, A.J.; Ralph, J.; Dale, B.; Balan, V. Isolation and characterization of new lignin streams derived from extractive-ammonia (EA) pretreatment. Green Chem. 2016, 18, 4205–4215. [Google Scholar] [CrossRef]
- Shi, T.; Xu, L.; Wang, Y.-N.; Liu, S.-C.; Liu, Z.-H.; Zhao, G.-J.; Li, B.-Z.; Yuan, Y.-J. Aminated and amidated structures introduced by ethylenediamine pretreatment endow lignin with bright fluorescence. Green Chem. 2022, 24, 9040–9054. [Google Scholar] [CrossRef]
- Li, J.; Henriksson, G.; Gellerstedt, G. Lignin depolymerization/repolymerization and its critical role for delignification of aspen wood by steam explosion. Bioresour. Technol. 2007, 98, 3061–3068. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. A review on lignin-based epoxy resins: Lignin effects on their synthesis and properties. Int. J. Biol. Macromol. 2023, 229, 778–790. [Google Scholar] [CrossRef]
- Wen, J.-L.; Xue, B.-L.; Xu, F.; Sun, R.-C.; Pinkert, A. Unmasking the structural features and property of lignin from bamboo. Ind. Crops Prod. 2013, 42, 332–343. [Google Scholar] [CrossRef]
- You, T.; Zhang, L.; Guo, S.; Shao, L.; Xu, F. Unraveling the Structural Modifications in Lignin of Arundo donax Linn. during Acid-Enhanced Ionic Liquid Pretreatment. J. Agric. Food Chem. 2015, 63, 10747–10756. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef]
- Senftle, T.P.; Hong, S.; Islam, M.M.; Kylasa, S.B.; Zheng, Y.; Shin, Y.K.; Junkermeier, C.; Engel-Herbert, R.; Janik, M.J.; Aktulga, H.M.; et al. The ReaxFF reactive force-field: Development, applications and future directions. npj Comput. Mater. 2016, 2, 2057–3960. [Google Scholar] [CrossRef]
- An, L.; Si, C.; Wang, G.; Sui, W.; Tao, Z. Enhancing the solubility and antioxidant activity of high-molecular-weight lignin by moderate depolymerization via in situ ethanol/acid catalysis. Ind. Crops Prod. 2019, 128, 177–185. [Google Scholar] [CrossRef]
- Zhu, Y.; Han, Z.; Fu, L.; Liu, C.; Zhang, D. Cleavage of the β–O–4 bond in a lignin model compound using the acidic ionic liquid 1-H-3-methylimidazolium chloride as catalyst: A DFT mechanistic study. J. Mol. Model. 2018, 24, 322. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, J.; Wang, W.; Lin, L.; Lv, R.; Zhang, S.; Ma, J. Demethylation of lignin with mild conditions and preparation of green adhesives to reduce formaldehyde emissions and health risks. Int. J. Biol. Macromol. 2023, 242 Pt 1, 124462. [Google Scholar] [CrossRef]
- Venkatesagowda, B.; Dekker, R.F.H. Enzymatic demethylation of Kraft lignin for lignin-based phenol-formaldehyde resin applications. Biomass Conv. Biorefin. 2019, 10, 203–225. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhang, S.; Gao, Q.; Li, J.; Zhang, W. Fast Curing Bio-Based Phenolic Resins via Lignin Demethylated under Mild Reaction Condition. Polymers 2017, 9, 428. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Shen, R.; Li, Y.; Ma, G.; Qi, S.; Wu, W.; Jin, Y.; Jiang, B. A mild iodocyclohexane demethylation for highly enhancing antioxidant activity of lignin. J. Bioresour. Bioprod. 2023, 8, 306–317. [Google Scholar] [CrossRef]
- Nakasu, P.Y.S.; Clarke, C.J.; Rabelo, S.C.; Costa, A.C.; Brandt-Talbot, A.; Hallett, J.P. Interplay of Acid–Base Ratio and Recycling on the Pretreatment Performance of the Protic Ionic Liquid Monoethanolammonium Acetate. ACS Sustain. Chem. Eng. 2020, 8, 7952–7961. [Google Scholar] [CrossRef]
- Dias, R.M.; Vilas-Boas, S.M.; da Costa, M.C. Unveiling the ability of protic and aprotic ionic liquids to dissolve and modify Kraft lignin. Sep. Purif. Technol. 2024, 350, 127977. [Google Scholar] [CrossRef]
- Ge, M.; Fang, T.; Zhou, G.; Li, C.; Li, Y.; Liu, X. Insight into the dual effect of water on lignin dissolution in ionic liquids. Int. J. Biol. Macromol. 2022, 205, 178–184. [Google Scholar] [CrossRef]
- Mancera, C.; Ferrando, F.; Salvadó, J.; El Mansouri, N.E. Kraft lignin behavior during reaction in an alkaline medium. Biomass Bioenergy 2011, 35, 2072–2079. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, W.; Zeng, J.; Gong, N.; Ying, G.; Li, P.; Wang, B.; Xu, J.; Gao, W.; Chen, K. Acid-catalyzed phenolation of lignin with tea polyphenol: Enhancing uv resistance and oxidation resistance for potential applications. Int. J. Biol. Macromol. 2024, 267, 131462. [Google Scholar] [CrossRef]
- Yu, X.; Chen, S.; Wang, W.; Deng, T.; Wang, H. Empowering alkali lignin with high performance in pickering emulsion by selective phenolation for the protection and controlled-release of agrochemical. Clean. Prod. 2022, 339, 130769. [Google Scholar] [CrossRef]
- Tarannum, N.; Singh, M. Selective Recognition and Detection of L-Aspartic Acid by Molecularly Imprinted Polymer in Aqueous Solution. Am. J. Anal. Chem. 2011, 2, 909–918. [Google Scholar] [CrossRef][Green Version]
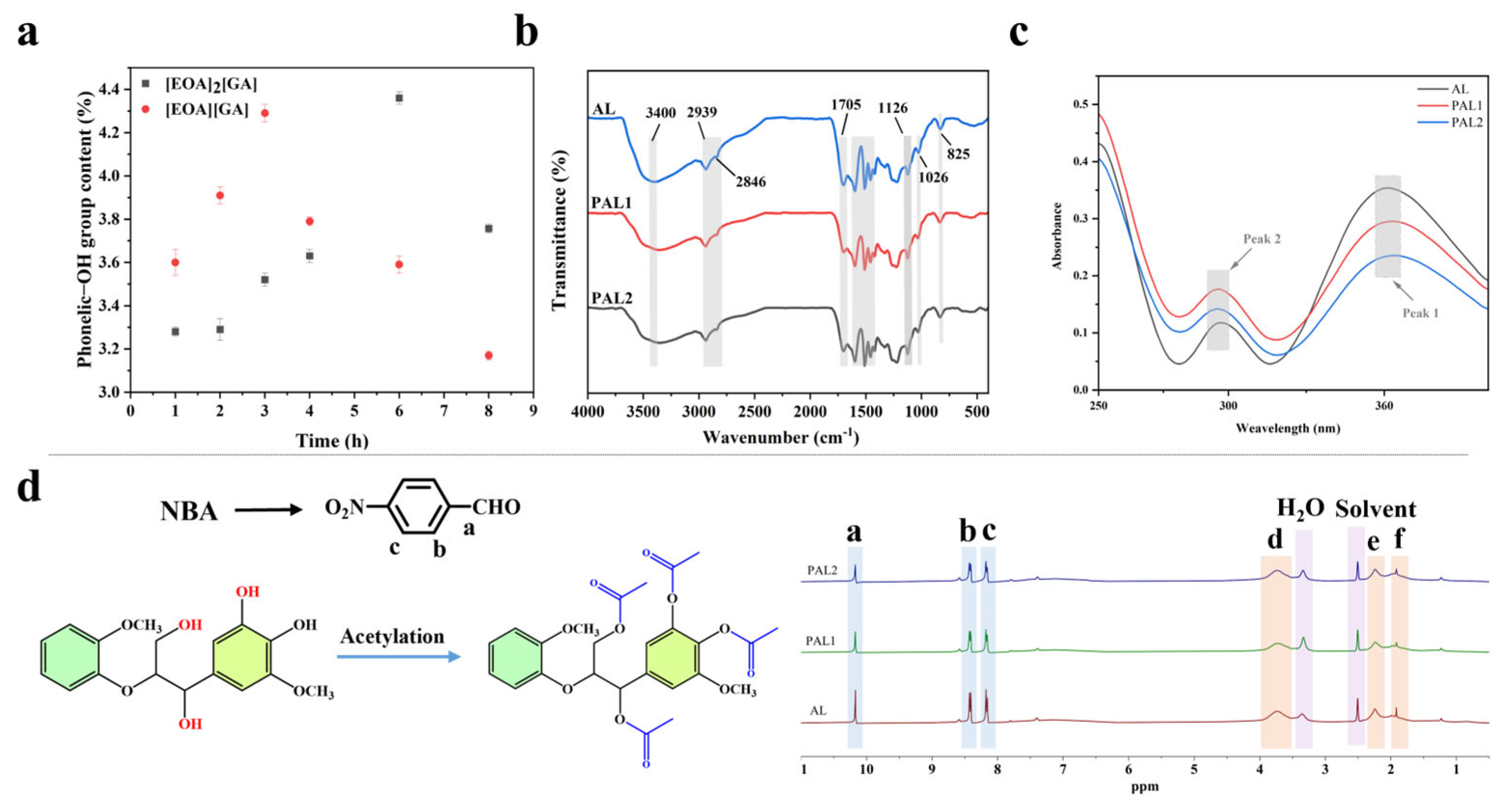
| Sample | M/mg | Mi/mg | Chemical Shift | FAr-OH (mmol/g) | FMeO (mmol/g) | ||
|---|---|---|---|---|---|---|---|
| Ii δ10.3–10.1 | Iar-OH δ2.46–2.11 | IMeO δ4.0–3.71 | |||||
| AL | 40 | 5 | 1 | 4.05 | 8.86 | 0.50 | 1.11 |
| PAL1 | 40 | 5 | 1 | 4.68 | 3.64 | 0.58 | 0.45 |
| PAL2 | 40 | 5 | 1 | 4.93 | 5.93 | 0.61 | 0.61 |
| Lignin Sample | Inter-Unit Linkages (/100 Ara) | Lignin Aromatic Units (%) | S/G | ||||
|---|---|---|---|---|---|---|---|
| β-O-4′ | β-β′ | β-5′ | S | G | H | ||
| AL | 36.14 | 12.04 | 7.22 | 66.46 | 17.3 | 19.23 | 3.66 |
| PAL1 | 23.52 | 9.41 | 4.7 | 44.77 | 26.86 | 28.35 | 1.66 |
| PAL2 | 28.91 | 9.6 | 4.81 | 51.51 | 25.75 | 22.72 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Xiao, X.; Luo, Q.; Zhao, W.; Xiao, W.; Xiao, L.-P.; Tong, Y.; Zhai, S.; Sun, J. Finely Designing Dicarboxylic Acid-Based Protic Ionic Liquids System for Tailoring Lignin Structure via Demethylation Strategy. Molecules 2025, 30, 2445. https://doi.org/10.3390/molecules30112445
Li C, Xiao X, Luo Q, Zhao W, Xiao W, Xiao L-P, Tong Y, Zhai S, Sun J. Finely Designing Dicarboxylic Acid-Based Protic Ionic Liquids System for Tailoring Lignin Structure via Demethylation Strategy. Molecules. 2025; 30(11):2445. https://doi.org/10.3390/molecules30112445
Chicago/Turabian StyleLi, Cheng, Xinyu Xiao, Qizhen Luo, Wanting Zhao, Wenzhe Xiao, Ling-Ping Xiao, Yao Tong, Shangru Zhai, and Jian Sun. 2025. "Finely Designing Dicarboxylic Acid-Based Protic Ionic Liquids System for Tailoring Lignin Structure via Demethylation Strategy" Molecules 30, no. 11: 2445. https://doi.org/10.3390/molecules30112445
APA StyleLi, C., Xiao, X., Luo, Q., Zhao, W., Xiao, W., Xiao, L.-P., Tong, Y., Zhai, S., & Sun, J. (2025). Finely Designing Dicarboxylic Acid-Based Protic Ionic Liquids System for Tailoring Lignin Structure via Demethylation Strategy. Molecules, 30(11), 2445. https://doi.org/10.3390/molecules30112445








