An Investigation of WO3/V2O5/TiO2 Catalysts: Effects of WO3 on Morphology, Thermal Stability, and Activity for the Catalytic Oxidation of Dimethyl Sulfide
Abstract
:1. Introduction
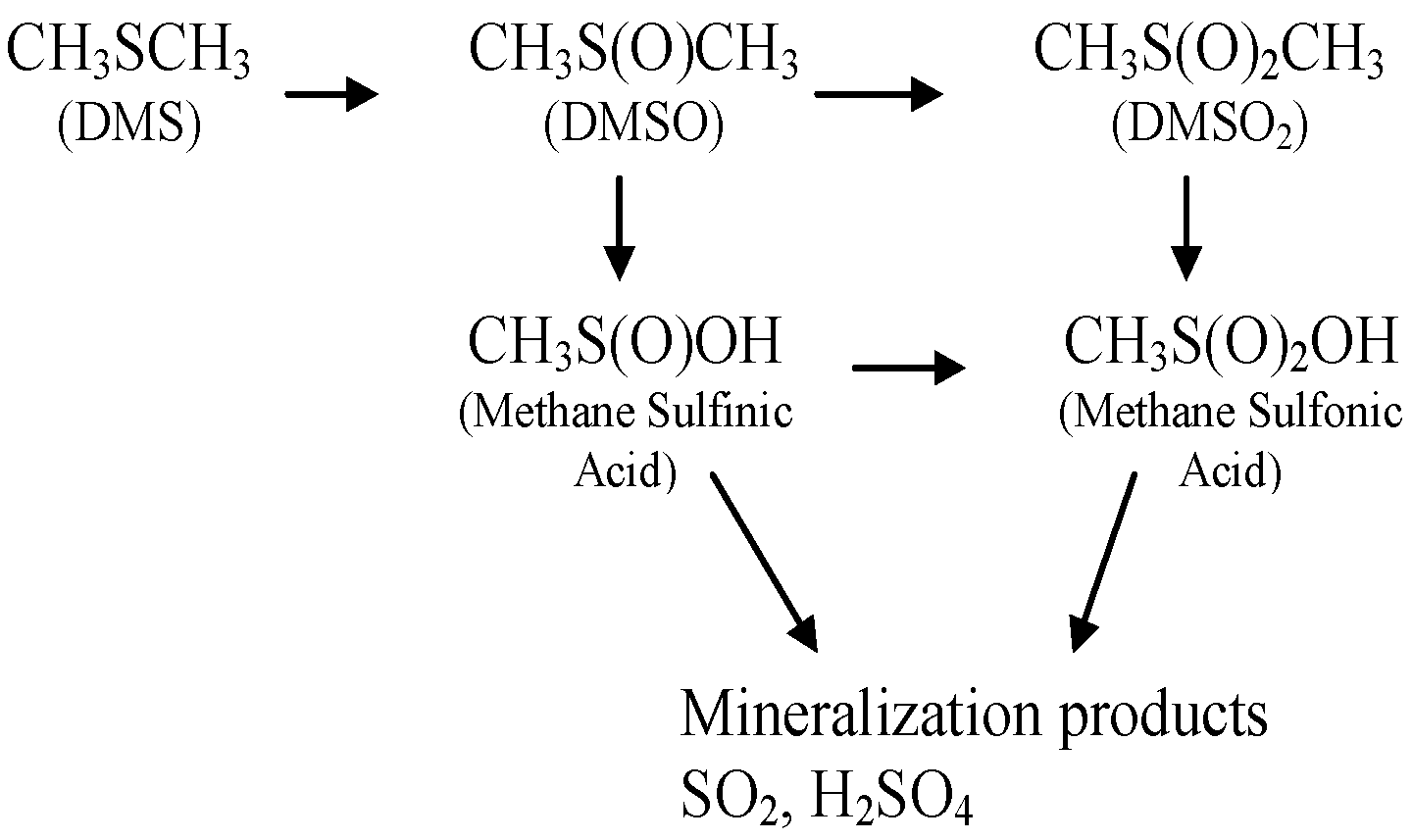
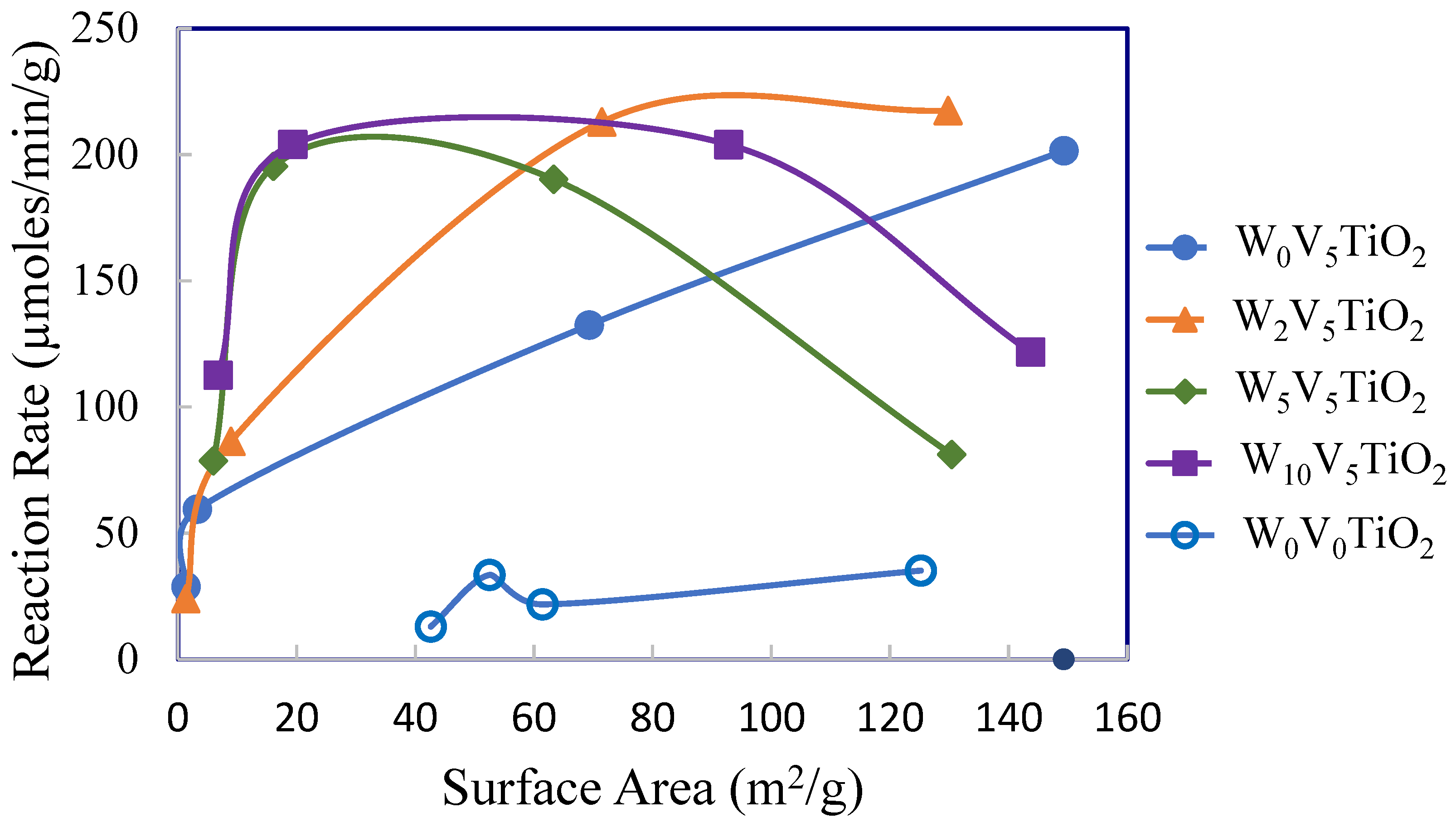
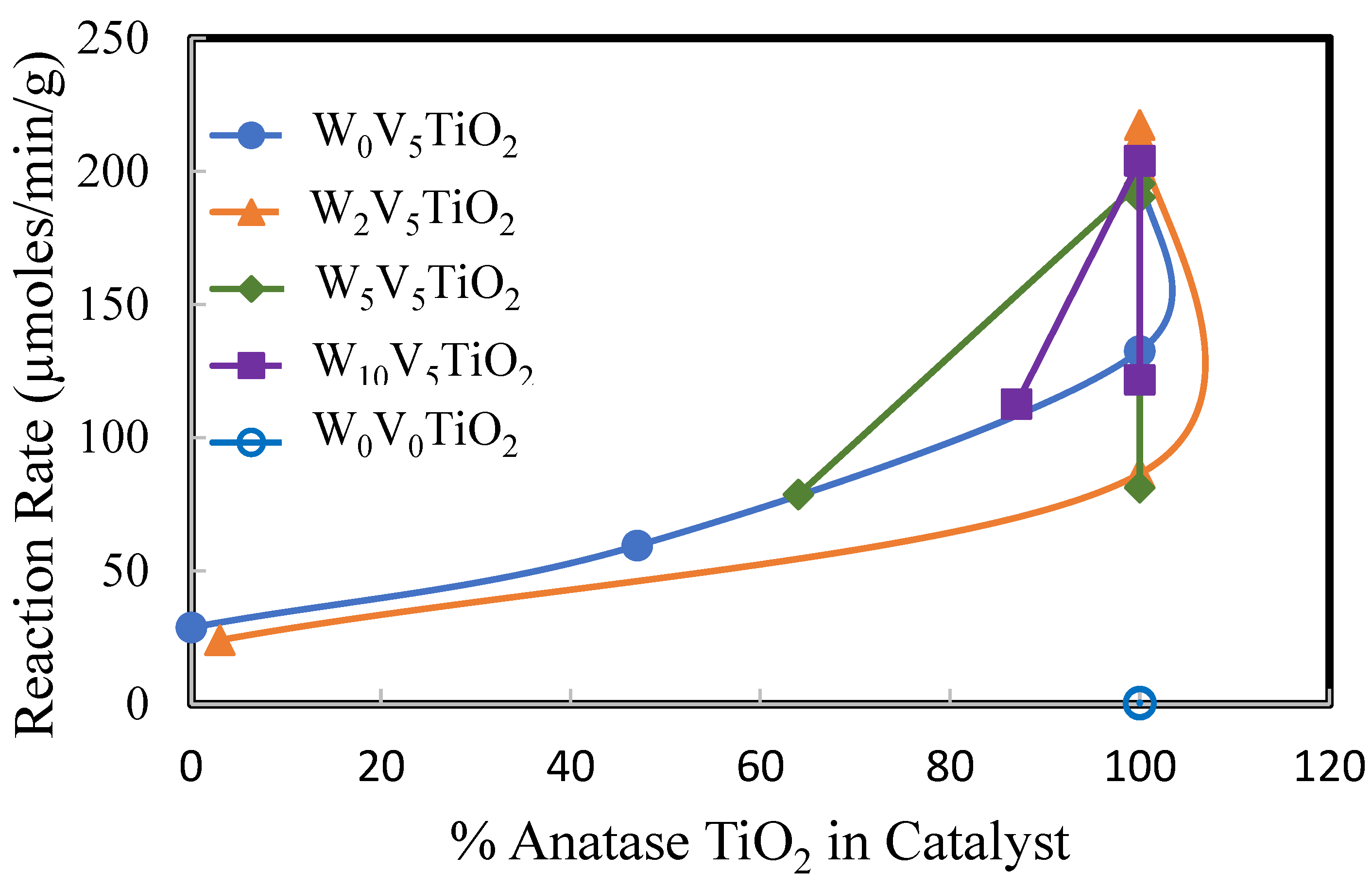
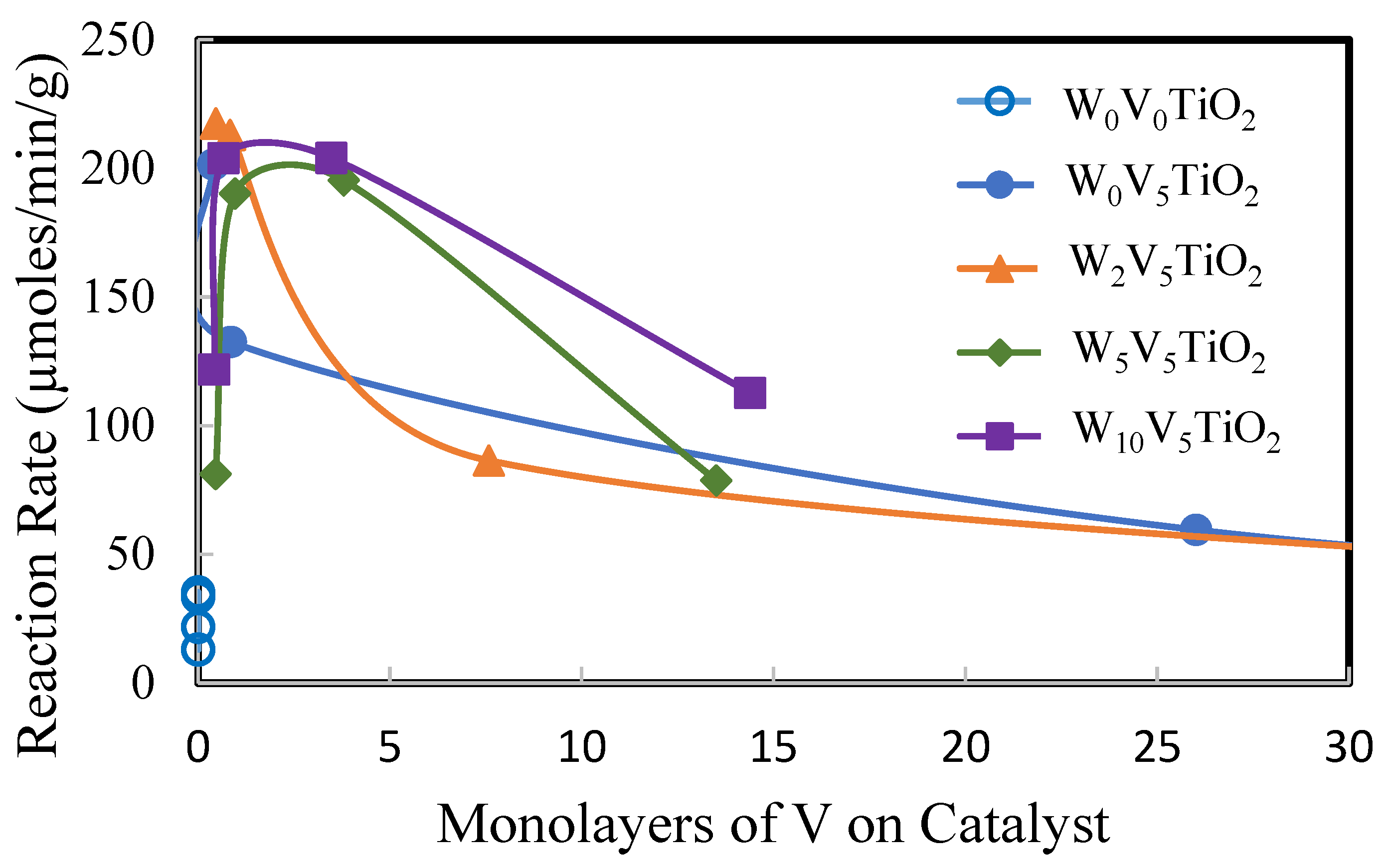
2. Results
2.1. Catalyst Characterization
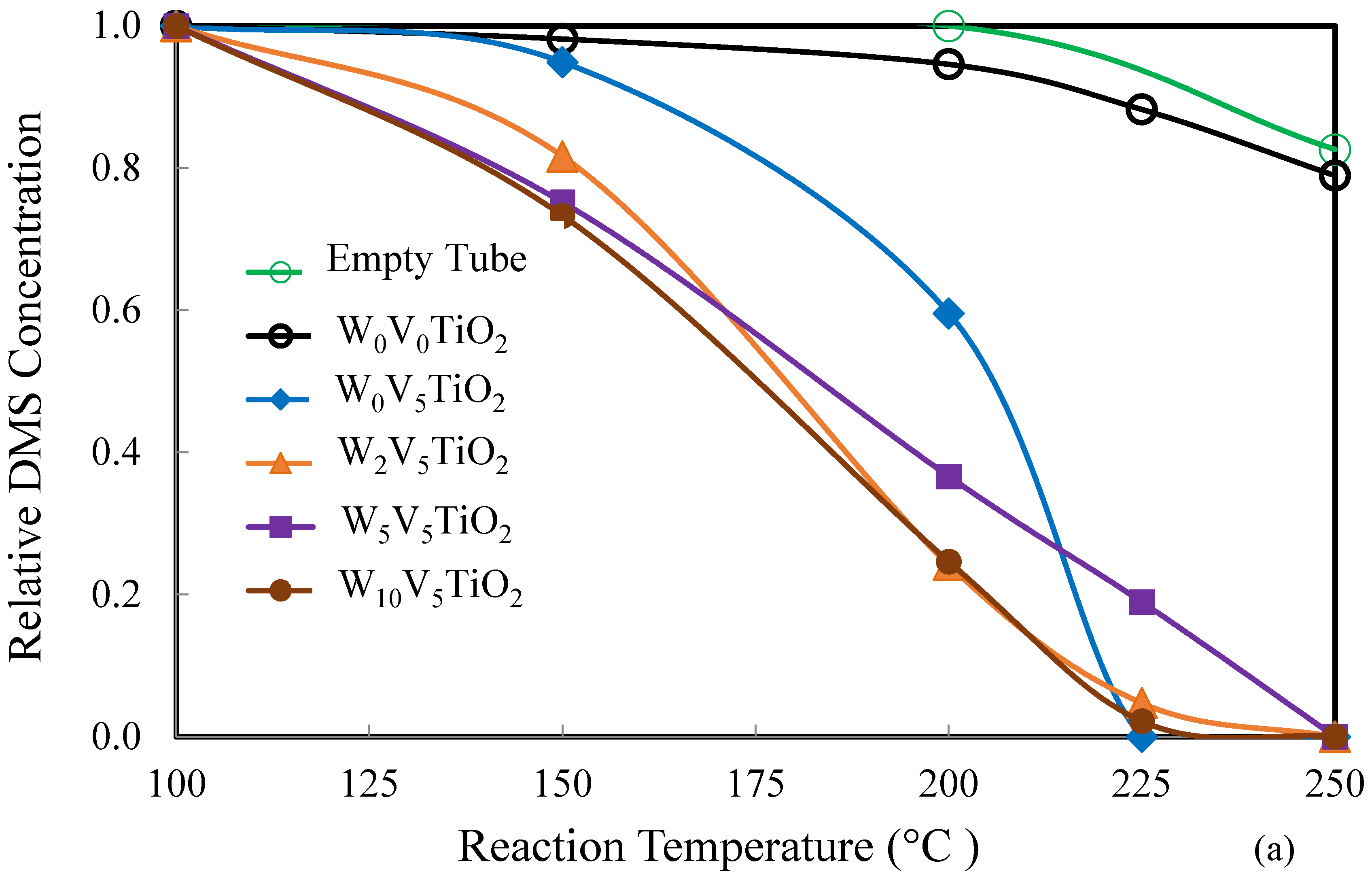
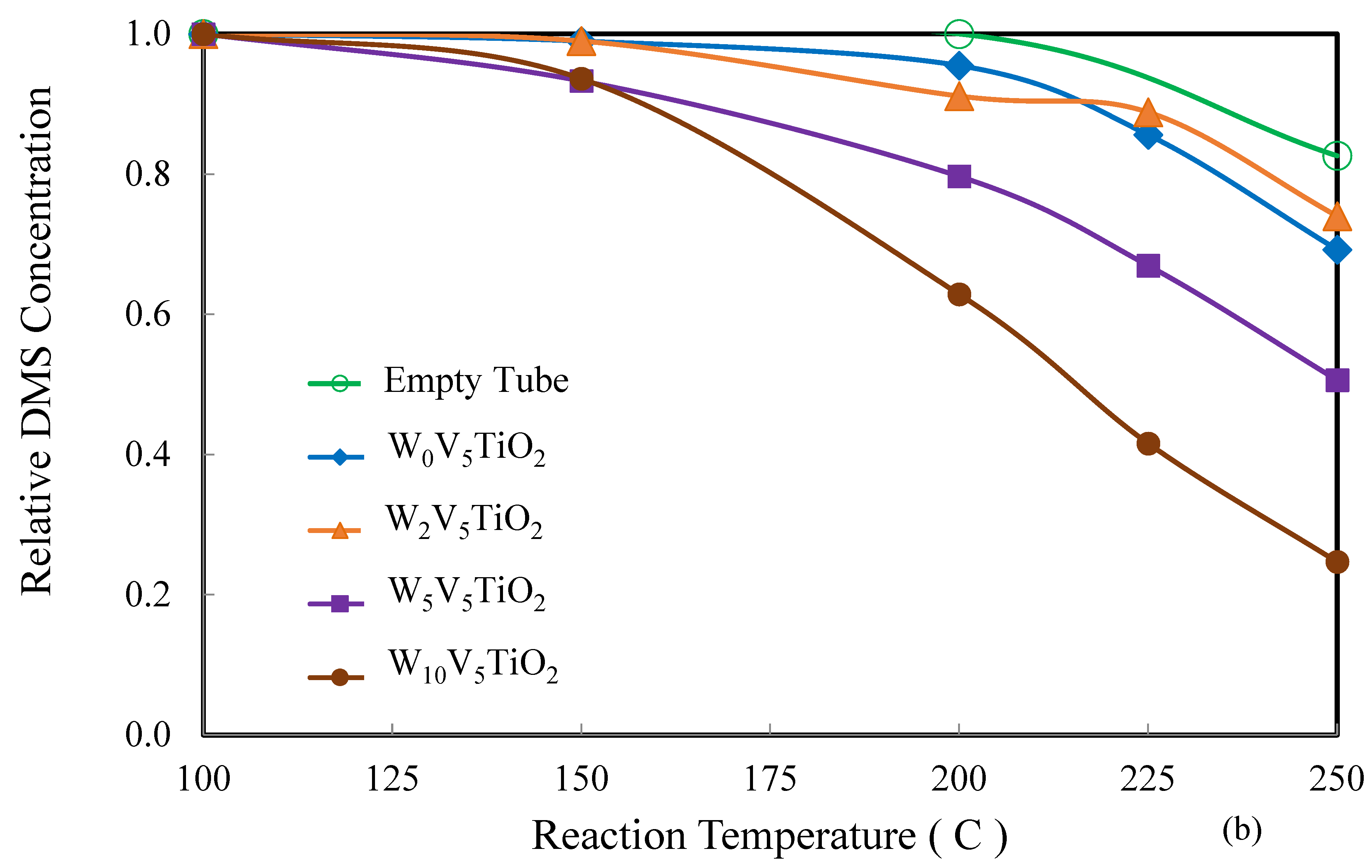
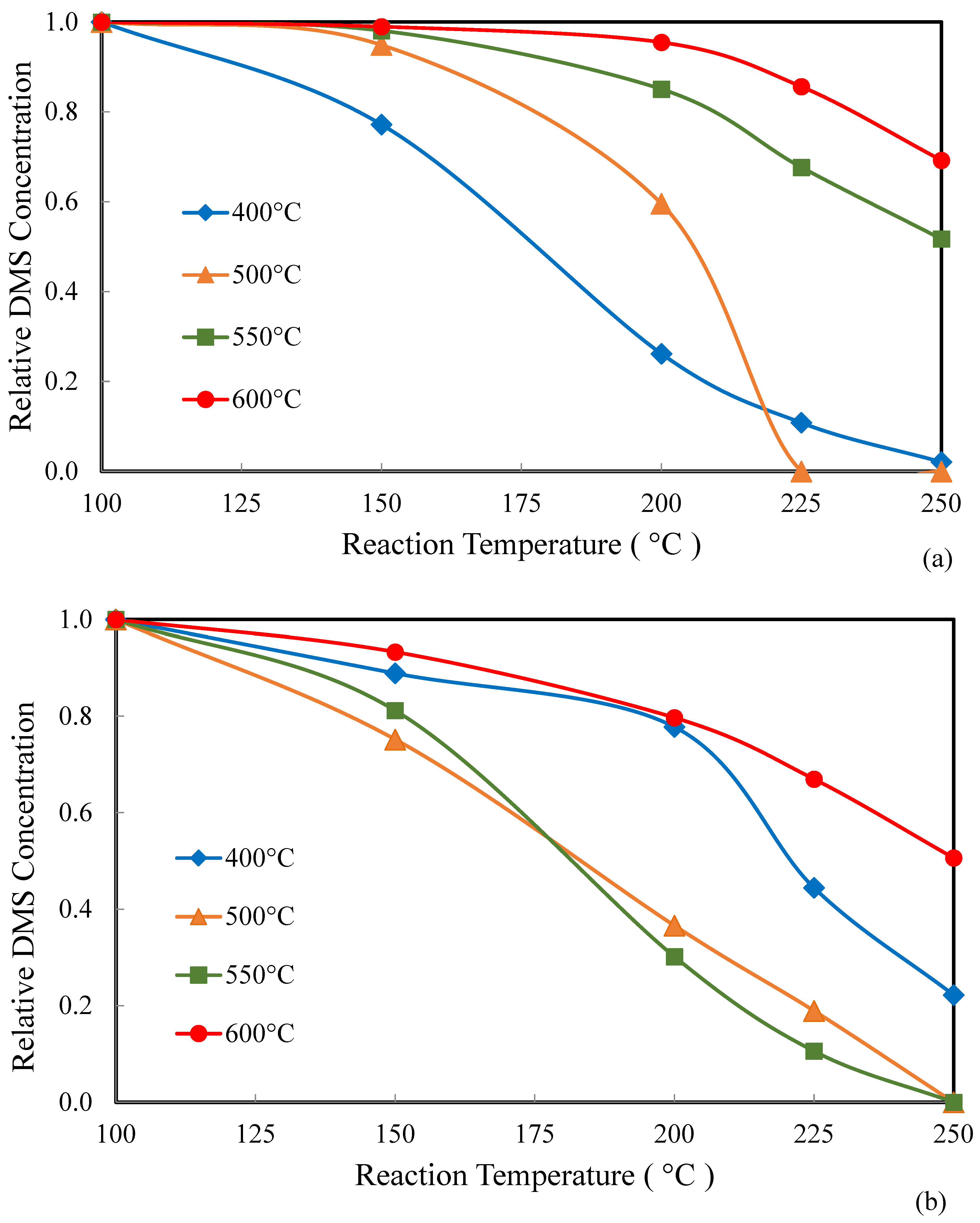
2.2. Catalysis
3. Discussion
4. Materials and Methods
4.1. Materials Used
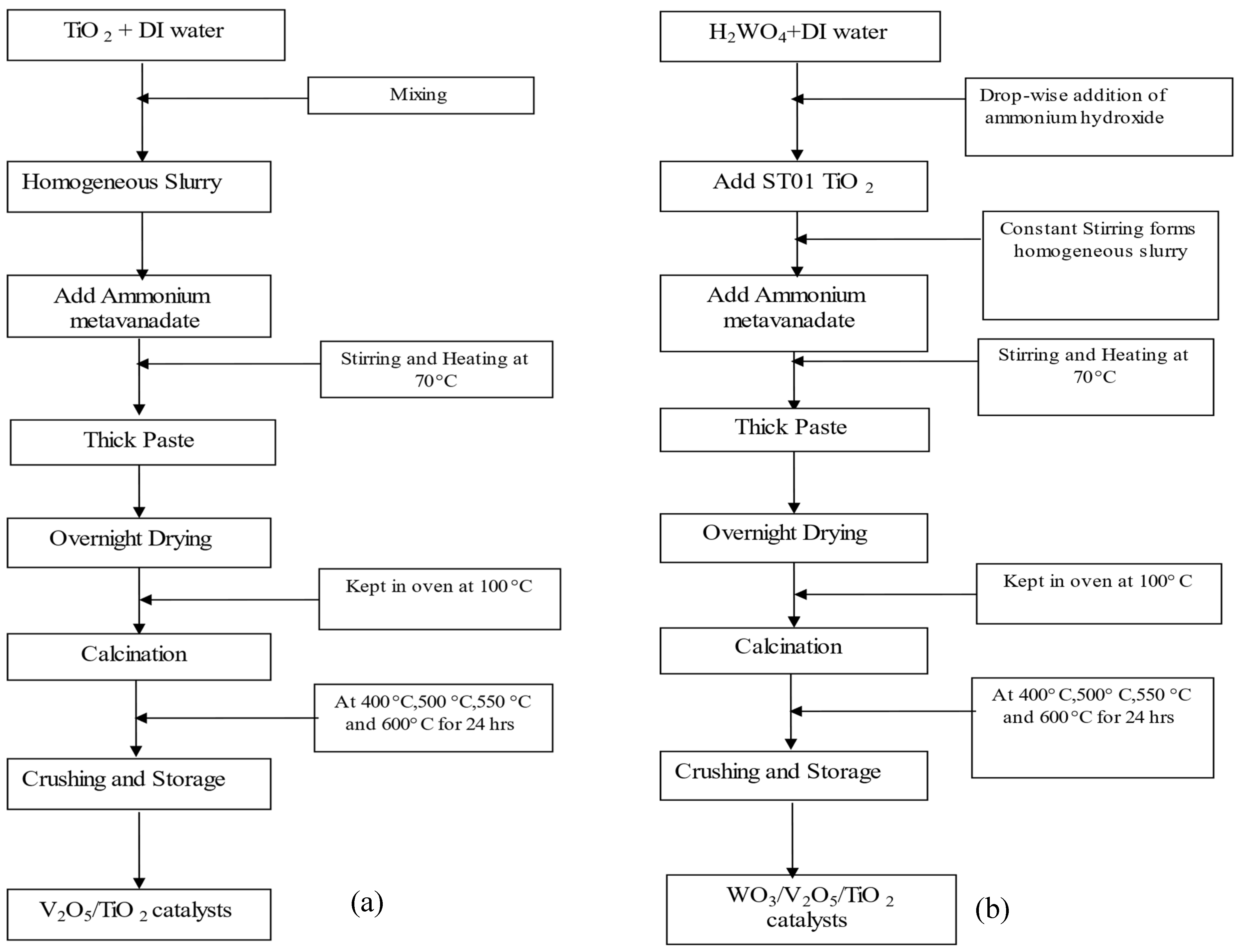
4.2. Synthesis of Catalysts
4.3. Catalyst Characterization
4.3.1. Scanning Electron Microscope (SEM)
4.3.2. Transmission Electron Microscope (TEM)
4.3.3. Raman Spectrometry (Raman)
4.3.4. Energy Dispersive X-ray Analysis (EDX)
4.3.5. BET Surface Area
4.3.6. X-ray Diffraction
4.3.7. Thermal Gravimetric Analyzer (TGA)
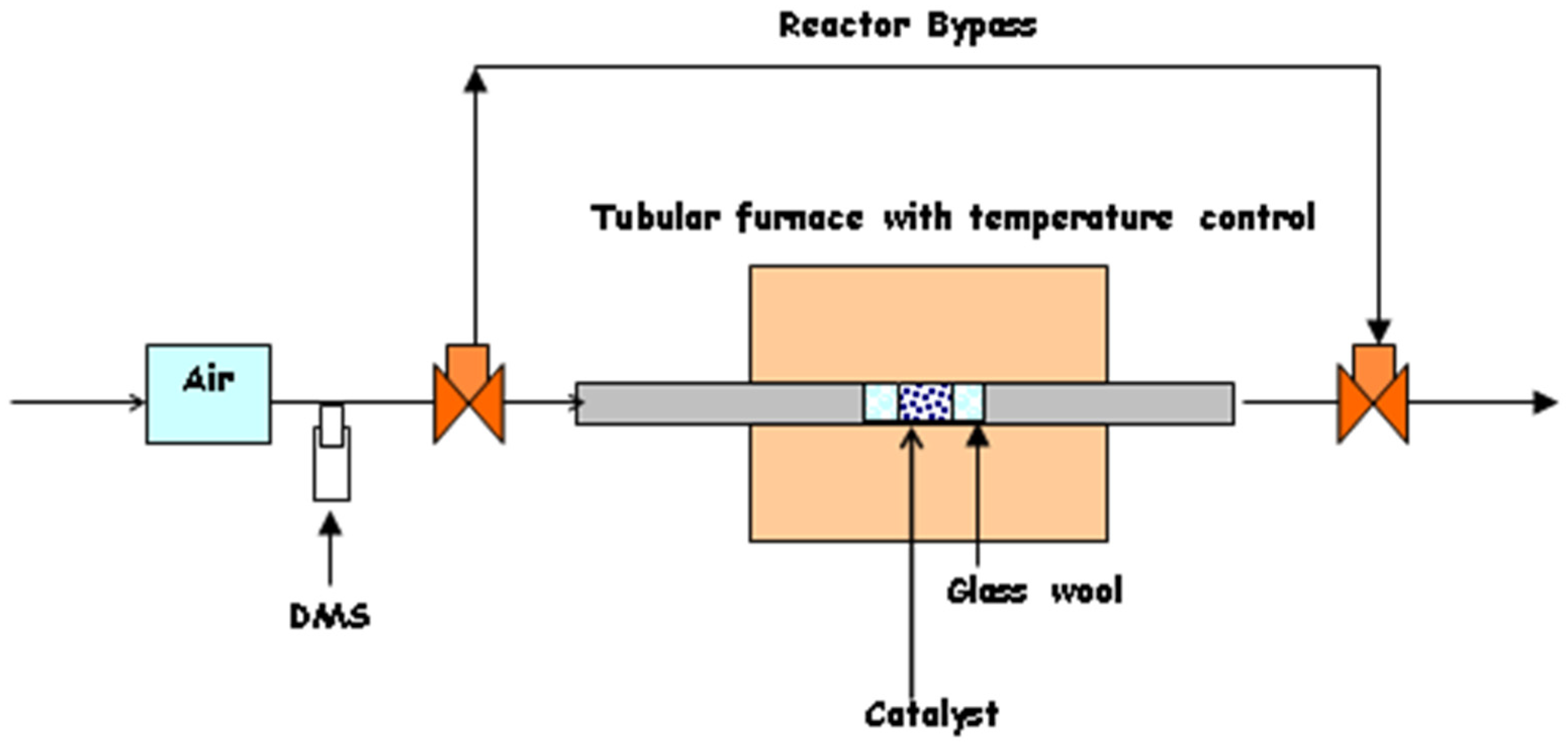
4.4. Catalyst Performance
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BET | Brunauer–Emmett–Teller surface area analysis method |
| DMS | Dimethyl sulfide |
| EDX | Energy dispersion X-ray spectroscopy |
| FTIR | Fourier transform infrared spectroscopy |
| SEM | Scanning electron microscope |
| VOCs | Volatile organic compounds |
| WxV5TiO2 | WO3/V2O5/TiO2 catalysts with x = mass ratio W/Ti; 5 = mass ratio V/Ti |
| XRD | X-ray diffraction |
References
- Fernandes, D.M.; Scofield, C.F.; Neto, A.A.; Cardoso, M.J.B.; Zotin, F.M.Z. Thermal deactivation of Pt/Rh commercial automotive catalysts. Chem. Eng. J. 2010, 160, 85–92. [Google Scholar] [CrossRef]
- Madia, G.; Elsener, M.; Koebel, M.; Raimondi, F.; Wokaun, A. Thermal stability of vanadia-tungsta-titania catalysts in the SCR process. Appl. Catal. B-Environ. 2002, 39, 181–190. [Google Scholar] [CrossRef]
- Nova, I.; Acqua, L.D.; Lietti, L.; Giamello, E.; Forzatti, P. Study of thermal deactivation of a de-NOx commercial catalyst. Appl. Catal. B Environ. 2001, 35, 31–42. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Imagawa, H.; Takahashi, N.; Tanaka, T.; Matsunaga, S.; Shinjoh, H. Improved NOx storage-reduction catalysts using Al2O3 and ZrO2-TiO2 nanocomposite support for thermal stability and sulfur durability. Appl. Catal. B Environ. 2009, 92, 23–29. [Google Scholar] [CrossRef]
- Phil, H.H.; Reddy, M.P.; Kumar, P.A.; Ju, L.K.; Hyo, J.S. SO2 resistant antimony promoted V2O5/TiO2 catalyst for NH3-SCR of NOx at low temperatures. Appl. Catal. B-Environ. 2008, 78, 301–308. [Google Scholar] [CrossRef]
- Huang, Z.G.; Zhu, Z.P.; Liu, Z.Y. Combined effect of H2O and SO2 on V2O5/AC catalysts for NO reduction with ammonia at lower temperatures. Appl. Catal. B-Environ. 2002, 39, 361–368. [Google Scholar] [CrossRef]
- Klimczak, M.; Kern, P.; Heinzelmann, T.; Lucas, M.; Claus, P. High-throughput study of the effects of inorganic additives and poisons on NH3-SCR catalysts Part I: V2O5-WO3/TiO2 catalysts. Appl. Catal. B-Environ. 2010, 95, 39–47. [Google Scholar] [CrossRef]
- Tang, F.S.; Xu, B.L.; Shi, H.H.; Qiu, J.H.; Fan, Y.N. The poisoning effect of Na+ and Ca2+ ions doped on the V2O5/TiO2 catalysts for selective catalytic reduction of NO by NH3. Appl. Catal. B-Environ. 2010, 94, 71–76. [Google Scholar] [CrossRef]
- Nicosia, D.; Czekaj, I.; Krocher, O. Chemical deactivation of V2O5/WO3-TiO2 SCR catalysts by additives and impurities from fuels lubrication oils and urea solution—Part II. Characterization study of the effect of alkali and alkaline earth metals. Appl. Catal. B-Environ. 2008, 77, 228–236. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Jensen, A.D.; Johnsson, J.E.; Thogersen, J.R. Deactivation of V2O5-WO3-TiO2 SCR catalyst at biomass fired power plants: Elucidation of mechanisms by lab- and pilot-scale experiments. Appl. Catal. B-Environ. 2008, 83, 186–194. [Google Scholar] [CrossRef]
- Yan, S.; Shan, W.; Shi, X.; He, G.; Lian, Z.; Yu, Y.; Shan, Y.; Liu, J.; He, H. The way to enhance the thermal stability of V2O5-based catalysts for NH3-SCR. Catal. Today 2020, 355, 408–414. [Google Scholar] [CrossRef]
- Feng, Z.; He, R.; Zhang, Q.; Han, X.; Yue, Y.; Qian, G.; Zhang, J. Enhanced SO2-Resistance Ability of Ce-Modified TiO2-Wrapped V2O5 Catalyst. Energy Fuels 2025, 39, 5886–5896. [Google Scholar] [CrossRef]
- Szymaszek, A.; Samojeden, B.; Motak, M. The Deactivation of Industrial SCR Catalysts—A Short Review. Energies 2020, 13, 3870. [Google Scholar] [CrossRef]
- Dong, C.; Yao, J.; Shi, J.; Han, L.; Qin, H.; Zhang, Z.; Zhang, Q.; Wang, J. Resource utilization strategy based on the deactivation mechanism of V2O5-WO3/TiO2 catalyst. Fuel 2025, 381 Pt B, 133311. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A-Gen. 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Alemany, L.J.; Lietti, L.; Ferlazzo, N.; Forzatti, P.; Busca, G.; Giamello, E.; Bregani, F. Reactivity and physicochemical characterization of V2O5-WO3/TiO2 De-NOx catalysts. J. Catal. 1995, 155, 117–130. [Google Scholar] [CrossRef]
- Grzybowska-Swierkosz, B. Vanadia-titania catalysts for oxidation of o-xylene and other hydrocarbons. Appl. Catal. A-Gen. 1997, 157, 263–310. [Google Scholar] [CrossRef]
- Busca, G.; Baldi, M.; Pistarino, C.; Gallardo Amores, J.M.; Sanchez Escribano, V.; Finocchio, E.; Romezzano, G.; Bregani, F.; Toledo, G.P. Evaluation of V2O5-WO3-TiO2 and alternative SCR catalysts in the abatement of VOCs. Catal. Today 1999, 53, 525–533. [Google Scholar] [CrossRef]
- Finocchio, E.; Baldi, M.; Busca, G.; Pistarino, C.; Romezzano, G.; Bregani, F.; Toledo, G.P. A study of the abatement of VOC over V2O5-WO3-TiO2 and alternative SCR catalysts. Catal. Today 2000, 59, 264–268. [Google Scholar] [CrossRef]
- Debecker, D.P.; Delaigle, R.; Eloy, P.; Gaigneaux, E.M. Abatement of model molecules for dioxin total oxidation on V2O5-WO3/TiO2 catalysts: The case of substituted oxygen-containing VOC. J. Mol. Catal. A Chem. 2008, 289, 38–43. [Google Scholar] [CrossRef]
- Albonetti, S.; Blasioli, S.; Bonelli, R.; Mengou, J.E.; Scire, S.; Trifiro, F. The role of acidity in the decomposition of 1,2-dichlorobenzene over TiO2-based V2O5/WO3 catalysts. Appl. Catal. A Gen. 2008, 341, 18–25. [Google Scholar] [CrossRef]
- Lazar, L.; Koser, H.; Balasanian, I.; Bandrabur, F. Catalytic destruction of aromatic VOCs on SCR-DeNOx commercial catalyst. Environ. Eng. Manag. J. 2007, 6, 13–20. Available online: https://www.researchgate.net/publication/298904939_Catalytic_destruction_of_aromatic_VOCs_on_SCR-DeNOx_commercial_catalyst#fullTextFileContent (accessed on 28 May 2025). [CrossRef]
- Besselmann, S.; Loffler, E.; Muhler, M. On the role of monomeric vanadyl species in toluene adsorption and oxidation on V2O5/TiO2 catalysts: A Raman and in situ DRIFTS study. J. Mol. Catal. A Chem. 2000, 162, 401–411. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Kiwi-Minsker, L.; Renken, A. Transient kinetics of toluene partial oxidation over V/Ti oxide catalysts. Catal. Today 2000, 61, 271–277. [Google Scholar] [CrossRef]
- Dias, C.R.; Portela, M.F.; Banares, M.A.; Galan-Fereres, M.; Lopez-Granados, M.; Pena, M.A.; Fierro, J.L.G. Selective oxidation of o-xylene over ternary V-Ti-Si catalysts. Appl. Catal. A Gen. 2002, 224, 141–151. [Google Scholar] [CrossRef]
- del Val, S.; Lopez-Granados, M.; Fierro, J.L.G.; Santamaria-Gonzalez, J.; Jimenez-Lopez, A.; Blasco, T. α-TiP-supported vanadium oxide catalysts: Influence of calcinations pretreatments on structure and performance for o-xylene oxidation. J. Catal. 2001, 204, 466–478. [Google Scholar] [CrossRef]
- Makedonski, L.; Nikolov, V.; Anastasov, A.; Stancheva, M. Effect of calcinations temperature on the properties of V2O5-TiO2 (anatase) catalysts in methanol oxidation. React. Kinet. Catal. Lett. 2004, 81, 21–25. [Google Scholar] [CrossRef]
- Badlani, M.; Wachs, I.E. Methanol: A “smart” chemical probe molecule. Catal. Lett. 2001, 75, 137–149. [Google Scholar] [CrossRef]
- Wang, Q.; Madix, R.J. Partial oxidation of methanol to formaldehyde on a model supported monolayer vanadia catalysts: Vanadia on TiO2(110). Surf. Sci. 2002, 496, 51–63. [Google Scholar] [CrossRef]
- Deo, G.; Wachs, I.E. Reactivity of supported vanadium oxide catalysts: The partial oxidation of methanol. J. Catal. 1994, 146, 323–334. [Google Scholar] [CrossRef]
- Forzatti, P.; Tronconi, E.; Elmi, A.S.; Busca, G. Methanol oxidation over vanadia-based catalysts. Appl. Catal. A Gen. 1997, 157, 387–408. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, N.; Almquist, C.B. An investigation of the thermal stability and performance of wet-incipient WO3/ V2O5/TiO2 catalysts and a comparison with flame aerosol catalysts of similar composition for the gas-phase oxidation of methanol. Appl. Catal. B Environ. 2006, 69, 101–114. [Google Scholar] [CrossRef]
- Burgess, L.; Gibson, A.G.; Furstein, S.J.; Wachs, I.E. Converting waste gases from pulp mills into value-added chemicals. Environ. Prog. 2002, 21, 137–142. [Google Scholar] [CrossRef]
- Jacoby, M. Catalytic cleanup at the pulp mill. Chem. Eng. News 2002, 80, 39–40. [Google Scholar] [CrossRef]
- Sahle-Demessie, E.; Almquist, C.B.; Devulapelli, V.G. Pilot-scale study on ozone-enhanced catalytic oxidation of waste gas emissions from the pulp and paper industry. Environ. Prog. Sustain. Energy 2011, 30, 268–277. [Google Scholar] [CrossRef]
- Sahle-Demessie, E.; Devulapelli, V.G. Oxidation of methanol and total reduced sulfur compounds with ozone over V2O5/TiO2 catalyst: Effect of humidity. Appl. Catal. A-Gen. 2009, 361, 72–80. Available online: https://api.semanticscholar.org/CorpusID:94761234 (accessed on 28 May 2025). [CrossRef]
- Bordado, J.C.M.; Gomes, J.F.P. Characterization of non-condensable sulphur containing gases from Kraft pulp mills. Chemosphere 2001, 44, 1011–1016. [Google Scholar] [CrossRef]
- Lin, B. Collecting and Burning Non-Condensable Gases. Available online: https://www.tappi.org/content/events/08kros/manuscripts/3-6.pdf (accessed on 19 May 2025).
- Siddiqui, N.A.; Ziauddin, A. Emission of non-condensable gases from a pulp and paper mill: A case study. J. Ind. Pollut. Control 2011. Available online: https://www.icontrolpollution.com/articles/emission-of-noncondensable-gases-froma-pulp-and-paper-mill--a-case-study-.php?aid=37415 (accessed on 19 May 2025).
- Barnes, I.; Hjorth, J.; Mihalopoulos, N. Dimethyl sulfide and dimethyl sulfoxide and their oxidation in the atmosphere. Chem. Rev. 2006, 106, 940–975. [Google Scholar] [CrossRef]
- Sahle-Demessie, E.; Devulapelli, V.G. Vapor phase oxidation of dimethyl sulfide with ozone over V2O5/TiO2 catalyst. Appl. Catal. B Environ. 2008, 84, 408–419. [Google Scholar] [CrossRef]
- Hwang, C.-L.; Tai, N.-H. Vapor phase oxidation of dimethyl sulfide with ozone over ion-exchanged zeolites. Appl. Catal. A Gen. 2011, 393, 251–256. [Google Scholar] [CrossRef]
- Kastner, J.R.; Buquoi, Q.; Ganagavaram, R.; Das, K.C. Catalytic ozonation of gaseous reduced sulfur compounds using wood fly ash. Environ. Sci. Technol. 2005, 39, 1835–1842. [Google Scholar] [CrossRef]
- Barcellos da Rosa, M.; Behnke, W.; Zetzsch, C. Study of the heterogeneous reaction of O3 with CH3SCH3 using the wetted-wall flow tube technique. Atmos. Chem. Phys. 2003, 3, 1665–1673. [Google Scholar] [CrossRef]
- Hakoda, T.; Chowdhury, M.A.Z.; Shimada, A.; Hirota, K. Oxidation of dimethyl sulfide in air using electron-beam irradiation, and enhancement of its oxidation via an MnO2 catalyst. Plasma Chem. Plasma Process. 2009, 29, 549–557. [Google Scholar] [CrossRef]
- Ch, H.; Lee, W.T.; Horng, K.H.; Tseng, T.K. The catalytic incineration of (CH3)2S over a Pt/Al2O3 catalyst. J. Hazard. Mater. 2001, B82, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Kastner, J.R.; Das, K.C.; Melear, N.D. Catalytic oxidation of gaseous reduced sulfur compounds using coal fly ash. J. Hazard. Mater. 2002, B95, 81–90. [Google Scholar] [CrossRef]
- Demeertere, K.; Dewulf, J.; De Witte, B.; Van Lngenhove, H. Titanium dioxide mediated heterogeneous photocatalytic degradation of gaseous dimethyl sulfide: Parameter study and reaction pathways. Appl. Catal. B Environ. 2005, 60, 93–106. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, N.; Ayllon, J.A.; Domenech, X.; Peral, J. TiO2 deactivation during the gas-phase photocatalytic oxidation of dimethyl sulfide. Appl. Catal. B Environ. 2004, 52, 69–77. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Savinov, E.V.; Davydov, L.; Smirniotis, P.G. Photocatalytic destruction of gaseous diethyl sulfide over TiO2. Appl. Catal. B Environ. 2001, 32, 11–24. [Google Scholar] [CrossRef]
- Niwa, M.; Habuta, Y.; Okumura, K.; Katada, N. Solid acidity of metal oxide monolayer and its role in catalytic reactions. Catal. Today 2003, 87, 213–218. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase-Rutile Mixtures with an X-ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Dherad, S.; Tifouti, L.; Crocoll, M.; Weisweiler, W. Effect of vanadia and tungsten loadings on the physical and chemical characteristics of V2O5-WO3/TiO2 catalysts. J. Mol. Catal. A Chem. 2004, 208, 257–265. [Google Scholar] [CrossRef]
- Amores, J.M.G.; Escribano, V.S.; Busca, G. Anatase crystal growth and phase transformation to rutile in high area TiO2, MoO3-TiO2 and other TiO2-supported oxide catalysts systems. J. Mater. Chem. 1995, 5, 1245–1249. [Google Scholar] [CrossRef]
- Huang, Q.W.; Zhu, L.H.; Xu, J.; Wang, P.L.; Gu, H.; Cheng, Y.B. Effect of V2O5 on sintering behavior, microstructure and dielectric properties of textured Sr0.4Ba0.6Nb2O6 ceramics. J. Eur. Ceram. Soc. 2005, 25, 957–962. [Google Scholar] [CrossRef]
- Balikdjian, J.P.; Davidson, A.; Launay, S.; Eckert, H.; Che, M. Sintering and phase transformation of V-loaded anatase materials containing bulk and surface V species. J. Phys. Chem. B 2005, 104, 8931–8939. [Google Scholar] [CrossRef]
- Reddy, B.M.; Reddy, E.P.; Mehdi, S. Phase transformation study of titania in V2O5/TiO2 and MoO3/TiO2 catalysts by X-ray diffraction analysis. Mater. Chem. Phys. 1994, 36, 276–281. [Google Scholar] [CrossRef]
- Banares, M.A.; Alemany, L.J.; Jimenez, M.C.; Larrubia, M.A.; Delgado, F.; Granados, M.L.; Martinez-Arias, A.; Blasco, J.M.; Fierro, J.L.G. The role of vanadium oxide on the titania transformation under thermal treatments and surface vanadium states. J. Solid-State Chem. 1996, 124, 69–76. [Google Scholar] [CrossRef]
- Reed, J.S. Principles of Ceramics Processing, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1995; pp. 594–618. [Google Scholar]
- Yang, H.; Zhang, D.; Wang, L. Synthesis and characterization of tungsten oxide-doped titania nanocrystallites. Mater. Lett. 2002, 57, 674–678. [Google Scholar] [CrossRef]
- Perego, C.; Revel, R.; Durupthy, O.; Cassaignon, S.; Jolivet, J.-P. Thermal stability of TiO2-anatase: Impact of nanoparticles morphology on kinetic phase transformation. Solid State Sci. 2010, 12, 989–995. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Phase transformation of nanocrystalline anatase-to-rutile via combined interface and surface nucleation. J. Mater. Res. 2000, 15, 437–448. [Google Scholar] [CrossRef]
- Orendorz, A.; Brodyanski, A.; Lösch, J.; Bai, L.H.; Chen, Z.H.; Le, Y.K.; Ziegler, C.; Gnaser, H. Phase transformation and particle growth in nanocrystalline anatase TiO2 films analyzed by X-ray diffraction and Raman spectroscopy. Surf. Sci. 2007, 601, 4390–4394. [Google Scholar] [CrossRef]
- Rodella, C.B.; Nascente, P.A.P.; Franco, R.W.A.; Magon, C.J.; Mastelaro, V.R.; Florentino, A.O. Surface characterization of V2O5/TiO2 catalytic system. Phys. Status Solidi (A) 2001, 187, 161–169. [Google Scholar] [CrossRef]
- Rodella, C.B.; Nascente, P.A.P.; Mastelaro, V.R.; Zucci, M.R.; Franco, R.W.A.; Magon, C.J.; Donoso, P.; Florentino, A.O. Chemical and structural characterization of V2O5/TiO2 catalysts. J. Vac. Sci. Technol. A 2001, 19, 1158–1163. [Google Scholar] [CrossRef]
- Went, G.T.; Leu, L.-J.; Bell, A.T. Quantitative structural analysis of dispersed vanadia species in TiO2(Anatase)-supported V2O5. J. Catal. 1992, 134, 479–491. [Google Scholar] [CrossRef]
- Stacey, P.; Hall, S.; Stagg, S.; Clegg, F.; Sammon, C. Raman spectroscopy and X-ray diffraction responses when measuring health-related micrometre and nanometre particle size fractions of crystalline quartz and the measurement of quartz in dust samples from the cutting and polishing of natural and artificial stones. J. Raman Spectrosc. 2021, 52, 1095–1107. [Google Scholar] [CrossRef]
- Curcio, A.L.; de Godoy, M.P.F.; De Giovanni Rodrigues, A. Raman spectroscopy as a method for structural characterization of ZnO-based systems at the nanoscale. Appl. Nanosci. 2024, 14, 269–275. [Google Scholar] [CrossRef]
- Available online: https://www.malvernpanalytical.com/en/products/measurement-type/phase-quantification (accessed on 19 May 2025).
- Loffler, S.; Auer, E.; Weil, M.; Lugstein, A.; Bertagnolli, E. Impact of growth temperature on the crystal habits, forms, and structures of VO2 nanocrystals. Appl. Phys. A 2011, 102, 201–204. [Google Scholar] [CrossRef]
- Cristallo, G.; Roncari, E.; Rinaldo, A.; Trifiro, F. Study of anatase-rutile transition phase in monolithic catalyst V2O5/TiO2 and V2O5-WO3/TiO2. Appl. Catal. A Gen. 2001, 209, 249–256. [Google Scholar] [CrossRef]
- Haber, J.; Kozlowska, A.; Kozłowski, R. The structure and redox properties of vanadium oxide surface compounds. J. Catal. 1986, 102, 52–63. [Google Scholar] [CrossRef]
- Saleh, R.Y.; Wachs, I.E.; Chan, S.S.; Chersich, C.C. Interaction of V2O5 with TiO2/(anatase): Catalyst evolution with calcination temperature and o-xylene oxidation. J. Catal. 1986, 98, 102–114. Available online: https://www.lehigh.edu/operando/Publications/1986%20Interaction%20of%20V2O5%20with%20TiO2%20-%20Cat%20evo%20caln%20temp.pdf (accessed on 28 May 2025). [CrossRef]
- Allieta, M.; Coduri, M.; Naldoni, A. Black TiO2 and Oxygen Vacancies: Unraveling the Role in the Thermal Anatase-to-Rutile Transformation. Appl. Nano 2024, 5, 72–83. [Google Scholar] [CrossRef]
- Galizia, P.; Maizza, G.; Galassi, C. Heating rate dependence of anatase to rutile transformation. Process. Appl. Ceram. 2016, 10, 235–241. [Google Scholar] [CrossRef]
- Hanaor, D.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Jung, H.; Lim, Y.H.; Pophali, A.; Lee, E.; Kim, H.; Kim, H.S.; Suh, J.; Choi, J.-S.; Kim, S.; Bang, J.; et al. Promotional effect of WO3 in V2O5–WO3/TiO2 on low-temperature activity in the catalytic oxidation of 1,2-dichloroethane. Chem. Eng. J. 2024, 494, 153029. [Google Scholar] [CrossRef]
- Photocatalytic Titanium Dioxide, ST Series Information, Ishihara Sangyo Kaisha, Ltd. Available online: https://www.iskweb.co.jp/eng/products/functional05.html (accessed on 18 April 2025).
- Available online: https://www.agilent.com/store/en_US/Prod-125-7032/125-7032 (accessed on 19 May 2025).
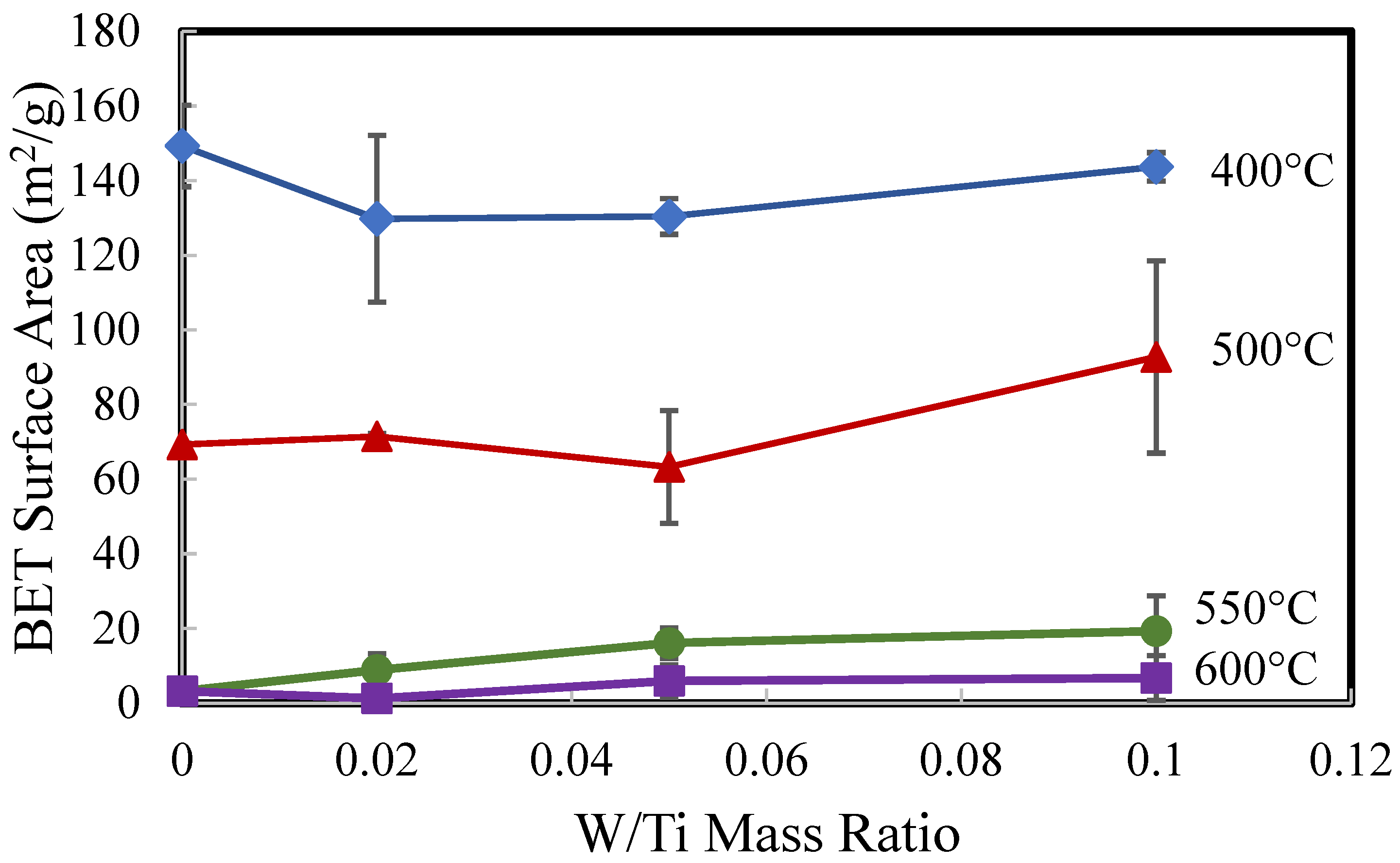
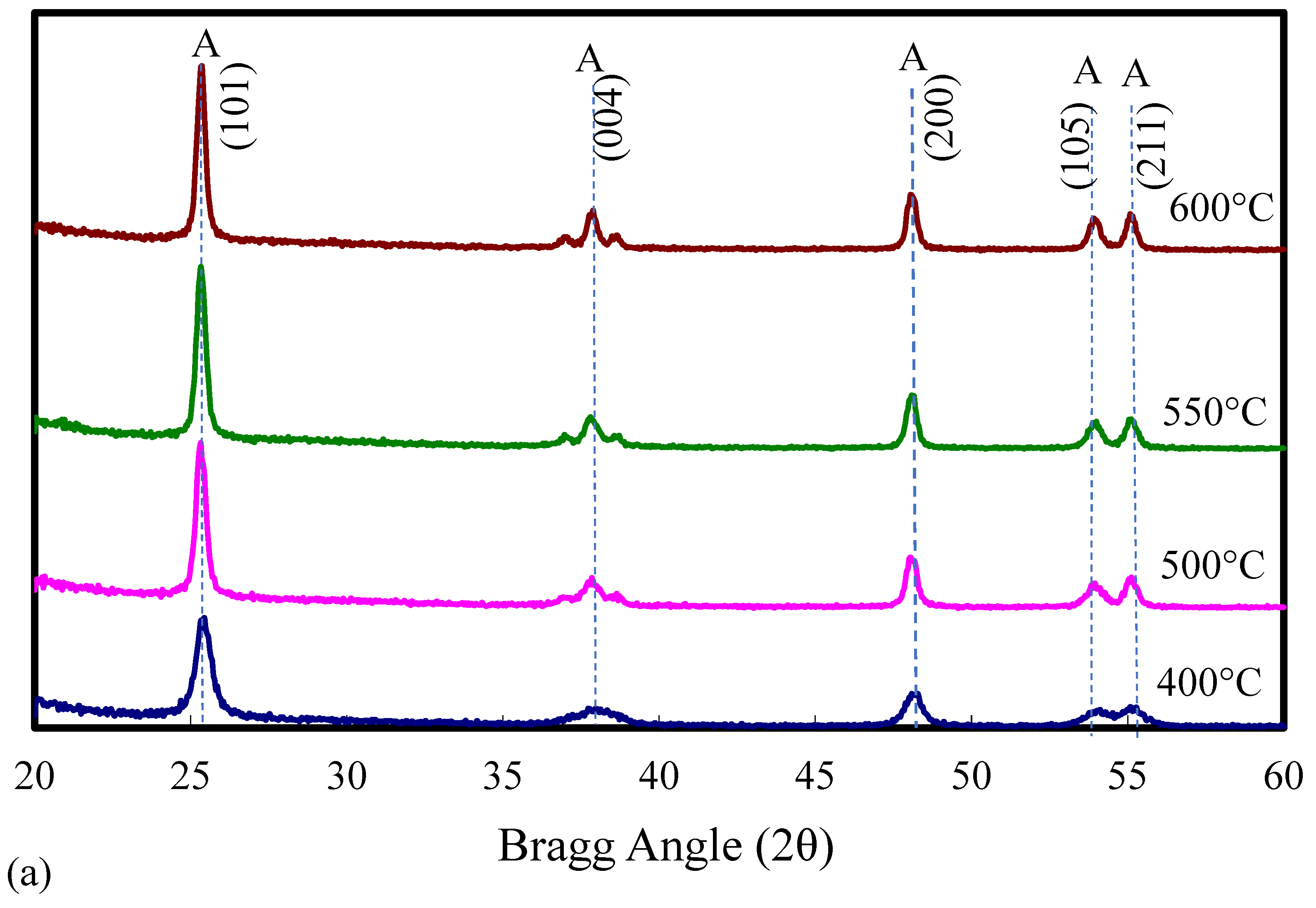
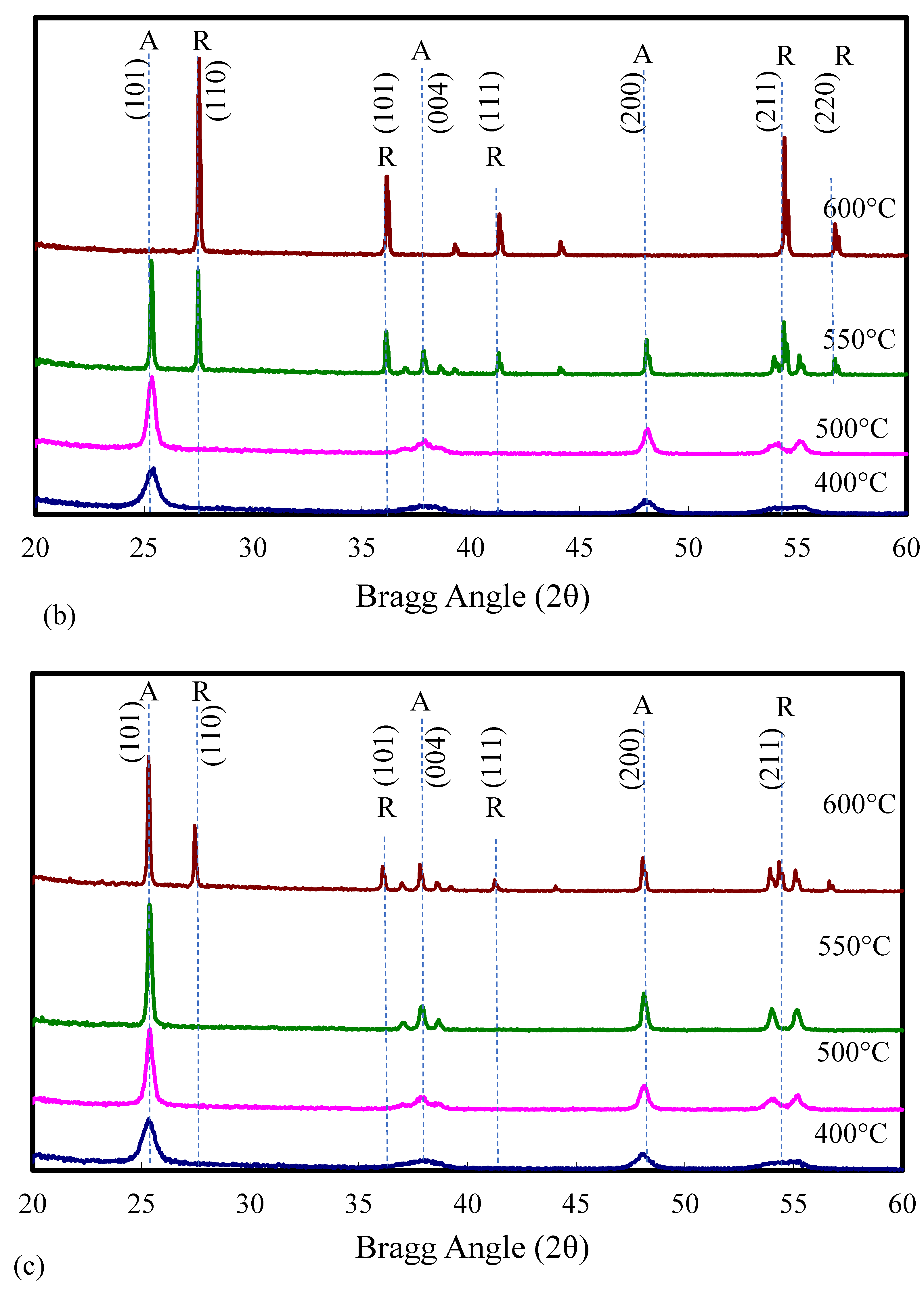
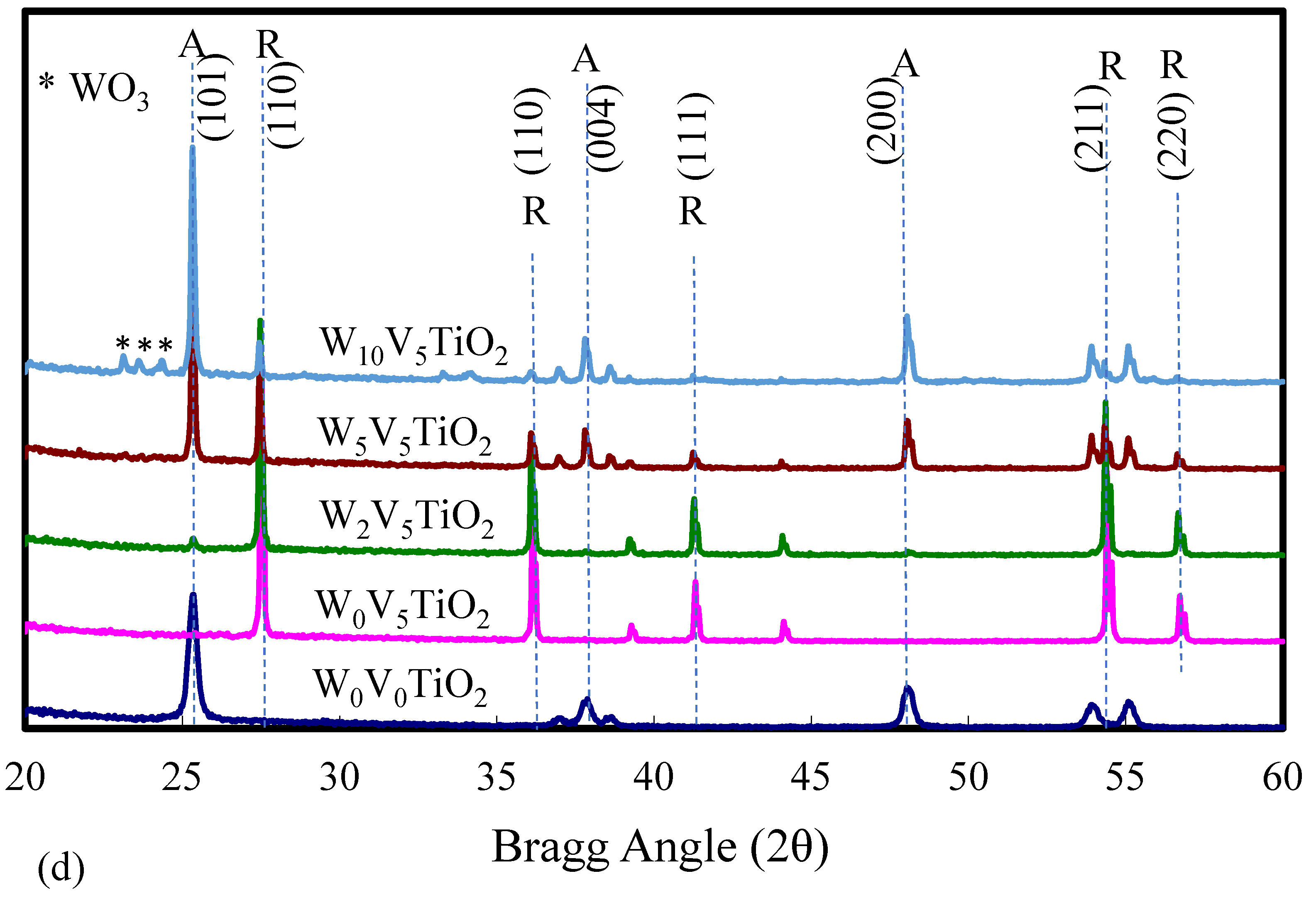
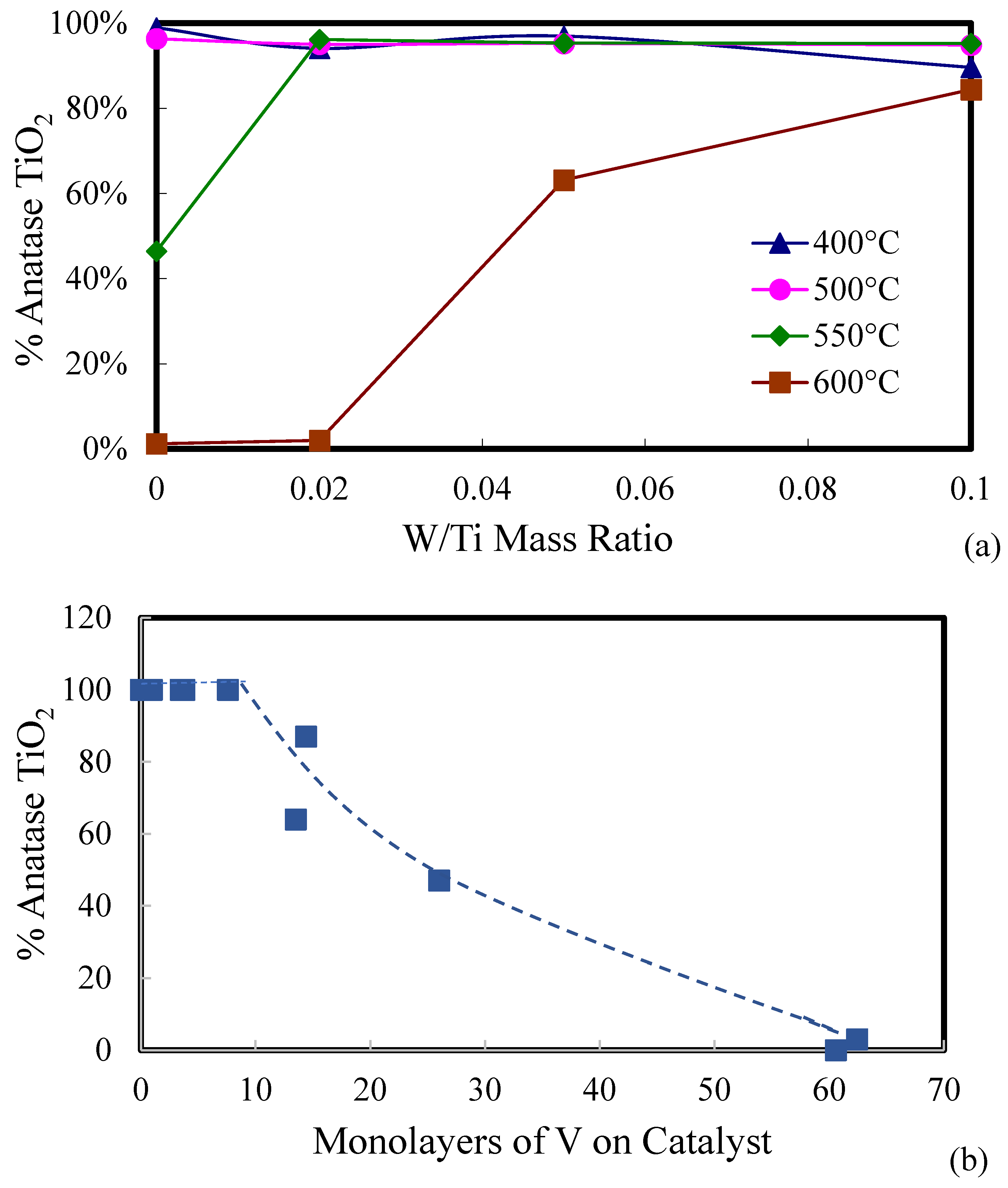
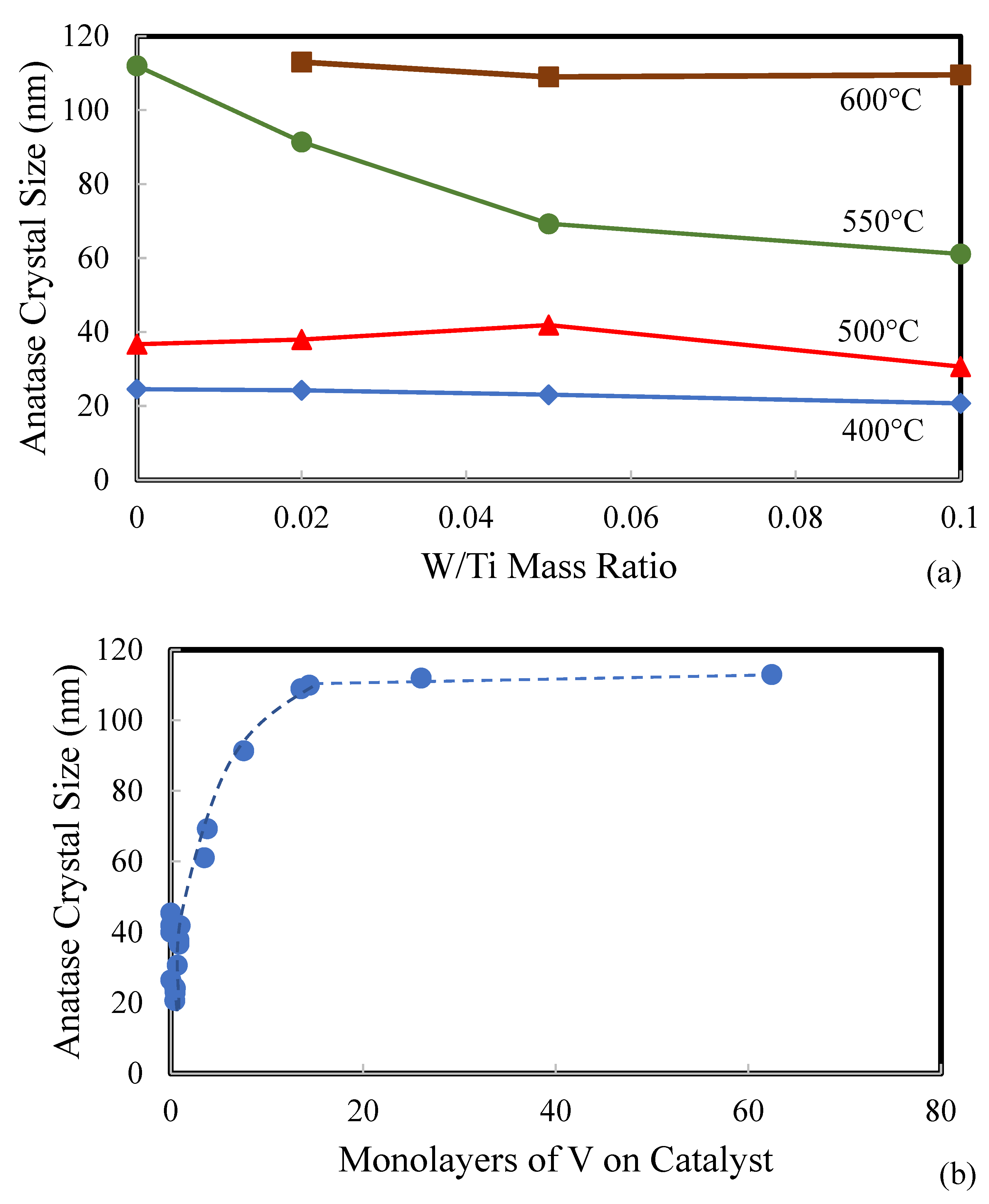
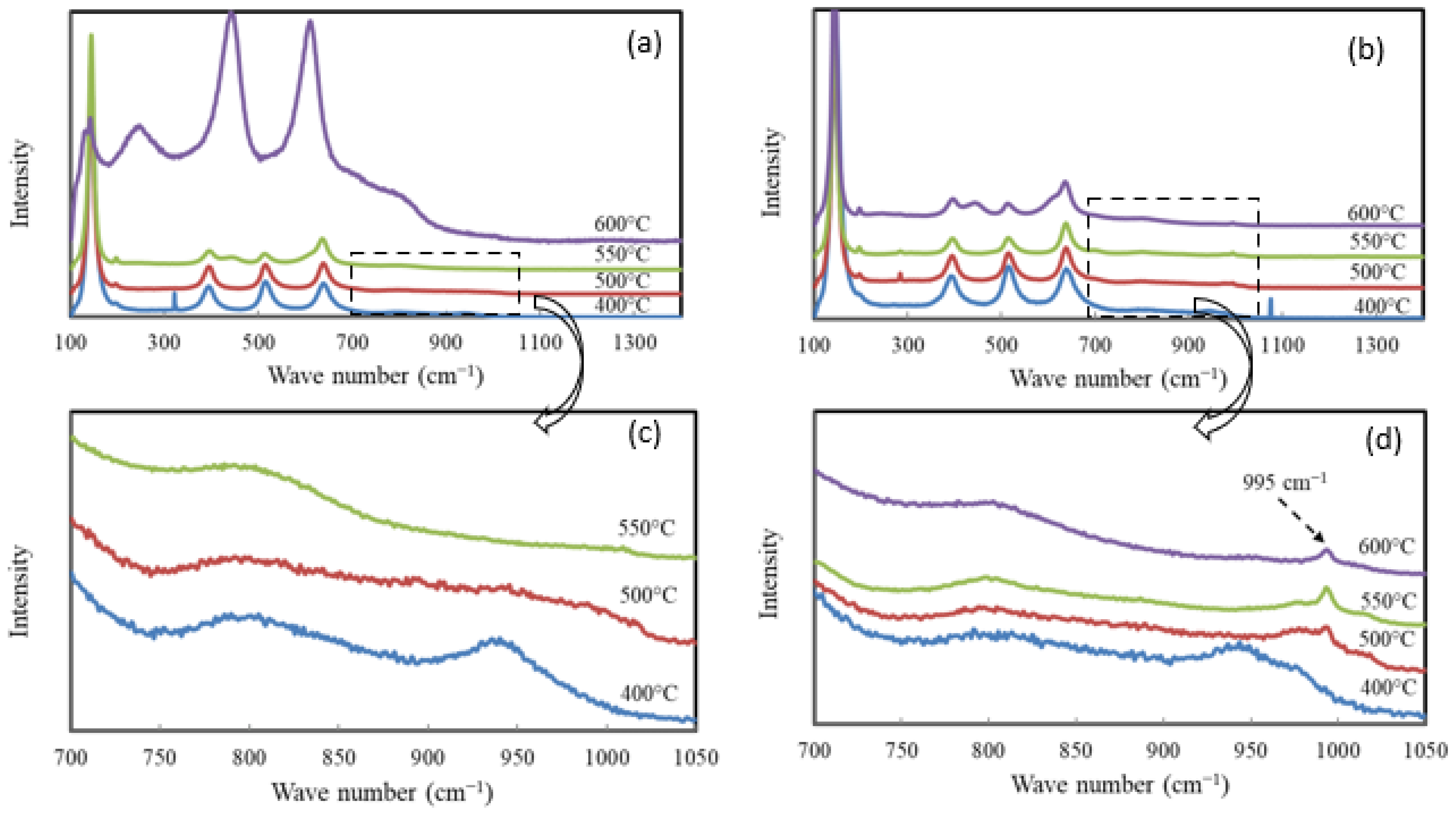

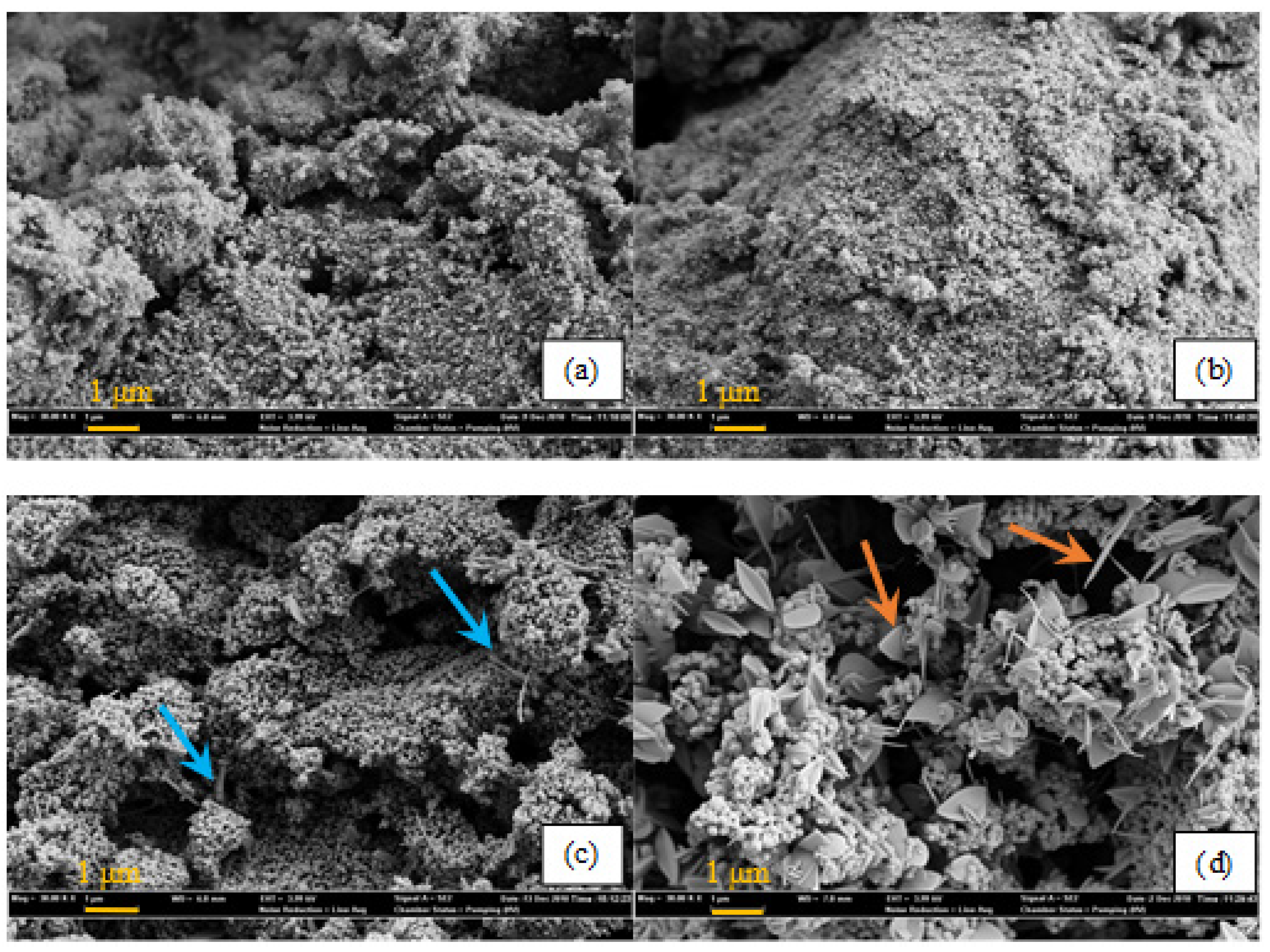
| Catalyst | Catalyst Composition 1 | Calcination Temperature (°C) | XRD Results | BET Area (m2/g) | Pore Size 4 (nm) | Monolayers 5 of V on TiO2 | ||
|---|---|---|---|---|---|---|---|---|
| V/Ti Mass Ratio | W/Ti Mass Ratio | Percent Anatase 2 (%) | Anatase Crystal Size 3 (nm) | |||||
| W0V0TiO2 | 0 | 0 | 400 | 100 | 26.5 | 125 ± 8.2 | 17.4 | 0 |
| 500 | 100 | 40.1 | 61.4 ± 13.5 | 23.2 | 0 | |||
| 550 | 100 | 42.0 | 52.5 ± 12 | 25.3 | 0 | |||
| 600 | 100 | 45.5 | 42.6 ± 7.6 | 26.2 | 0 | |||
| W0V5TiO2 | 0.05 | 0 | 400 | 100 | 24.5 | 149 ± 10.9 | 14.7 | 0.40 ± 0.03 |
| 500 | 100 | 36.7 | 69.3 ± 0.4 | 25.6 | 0.85 ± 0.01 | |||
| 550 | 47 | 112 | 3.2 ± 2.5 | 51.2 | 26.0 ± 20.1 | |||
| 600 | 0 | 0.0 | 1.3 ± 1 | 38.6 | 60.6 ± 43.9 | |||
| W2V5TiO2 | 0.05 | 0.02 | 400 | 100 | 24.2 | 130 ± 22.3 | 14.9 | 0.46 ± 0.08 |
| 500 | 100 | 38.0 | 71.5 ± 0.8 | 25.8 | 0.83 ± 0.01 | |||
| 550 | 100 | 91.4 | 8.9 ± 4.4 | 49.6 | 7.58 ± 3.75 | |||
| 600 | 3 | 113 | 1.3 ± 1.0 | 43.6 | 62.4 ± 46.5 | |||
| W5V5/TiO2 | 0.05 | 0.05 | 400 | 100 | 23.0 | 130 ± 4.8 | 16.2 | 0.45 ± 0.02 |
| 500 | 100 | 41.9 | 63.3 ± 15.1 | 25.3 | 0.96 ± 0.23 | |||
| 550 | 100 | 69.3 | 16.1 ± 4.1 | 39.8 | 3.79 ± 0.97 | |||
| 600 | 64 | 109 | 5.95 ± 4.3 | 37.3 | 13.5 ± 9.82 | |||
| W10V5/TiO2 | 0.05 | 0.10 | 400 | 100 | 20.7 | 144 ± 3.8 | 14.8 | 0.41 ± 0.01 |
| 500 | 100 | 30.7 | 92.8 ± 25.7 | 22.2 | 0.66 ± 0.18 | |||
| 550 | 100 | 61.1 | 19.3 ± 9.4 | 47.4 | 3.47 ± 1.69 | |||
| 600 | 87 | 110 | 6.76 ± 6.0 | 42.2 | 14.4 ± 12.8 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, G.; Sahle-Demessie, E.; Almquist, C.B. An Investigation of WO3/V2O5/TiO2 Catalysts: Effects of WO3 on Morphology, Thermal Stability, and Activity for the Catalytic Oxidation of Dimethyl Sulfide. Molecules 2025, 30, 2436. https://doi.org/10.3390/molecules30112436
Sharma G, Sahle-Demessie E, Almquist CB. An Investigation of WO3/V2O5/TiO2 Catalysts: Effects of WO3 on Morphology, Thermal Stability, and Activity for the Catalytic Oxidation of Dimethyl Sulfide. Molecules. 2025; 30(11):2436. https://doi.org/10.3390/molecules30112436
Chicago/Turabian StyleSharma, Gaytri, Endalkachew Sahle-Demessie, and Catherine B. Almquist. 2025. "An Investigation of WO3/V2O5/TiO2 Catalysts: Effects of WO3 on Morphology, Thermal Stability, and Activity for the Catalytic Oxidation of Dimethyl Sulfide" Molecules 30, no. 11: 2436. https://doi.org/10.3390/molecules30112436
APA StyleSharma, G., Sahle-Demessie, E., & Almquist, C. B. (2025). An Investigation of WO3/V2O5/TiO2 Catalysts: Effects of WO3 on Morphology, Thermal Stability, and Activity for the Catalytic Oxidation of Dimethyl Sulfide. Molecules, 30(11), 2436. https://doi.org/10.3390/molecules30112436








