Development of Multilayer Magnetic Janus Sub-Micrometric Particles for Lipase Catalysis in Pickering Emulsion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
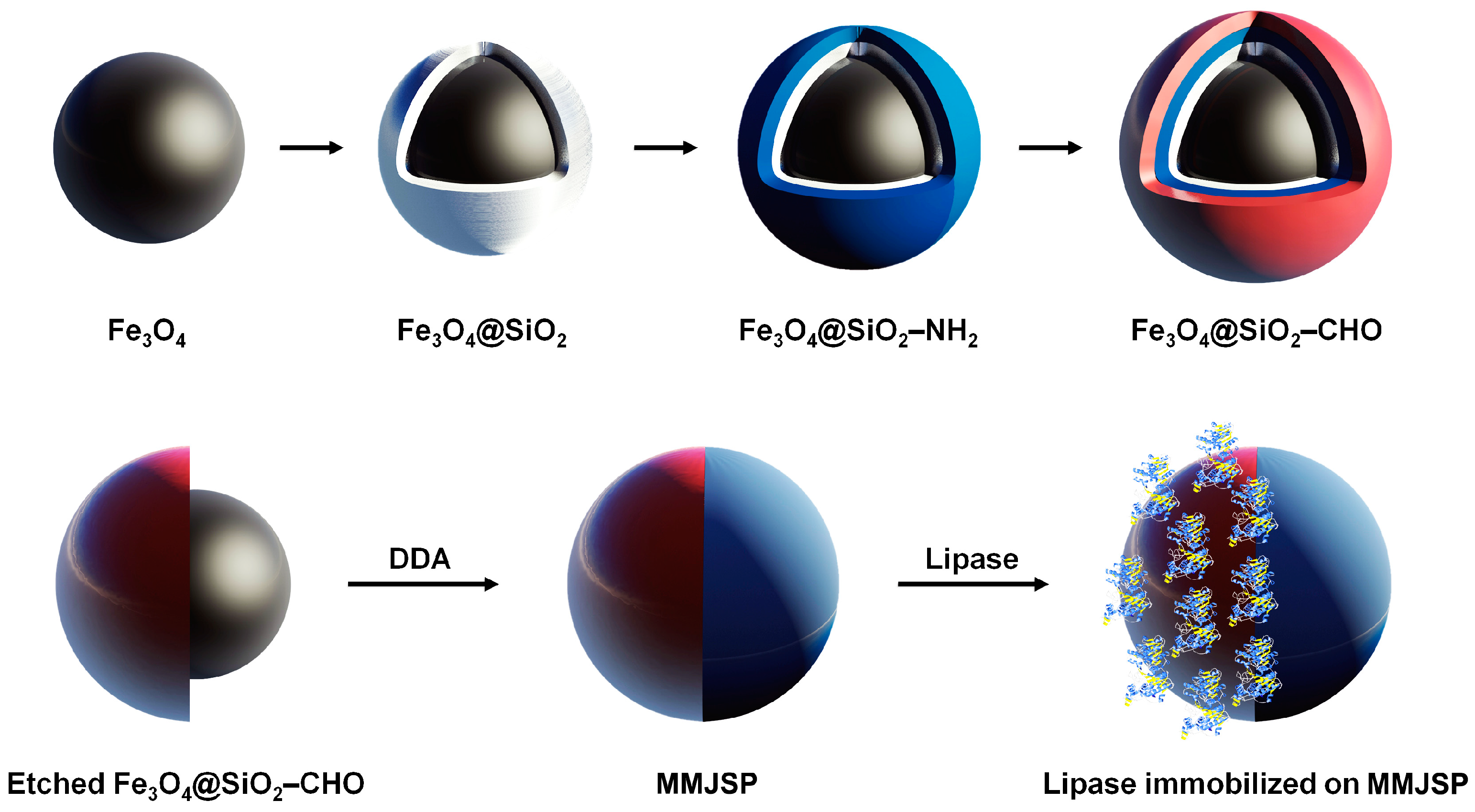
2.2. Synthesis of Paramagnetic Core-Shell Structured Nanobeads
2.3. Synthesis of Multilayer Magnetic Janus Sub-Micrometric Particles
2.4. Characterization
2.5. Enzyme Immobilization
2.6. Enzyme Activity Assay
2.7. Catalytic Efficiency Testing
2.8. Stability and Reusability Testing of Immobilized Enzymes
3. Results and Discussion
3.1. Characterization of Multilayer Magnetic Janus Sub-Micrometric Particles
3.2. Characterization of Pickering Emulsions
3.3. Catalytic Performance Testing
3.4. Stability Testing
3.5. Reusability Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, H.; Zou, Z.; Dai, L.; Liu, D.; Du, W. Ordered Macro–Microporous ZIF-8 with Different Macropore Sizes and Their Stable Derivatives for Lipase Immobilization in Biodiesel Production. ACS Sustain. Chem. Eng. 2022, 10, 14503–14514. [Google Scholar] [CrossRef]
- Ming, J.; Sun, Y.; Chen, Y.; Wang, Q.; Li, J. Novel lipase reactor based on discontinuous interfaces in hydrogel-organogel hybrid gel: A preliminary exploration. J. Agric. Food Chem. 2023, 71, 2113–2123. [Google Scholar] [CrossRef]
- Sun, T.; Dong, Z.; Wang, J.; Huang, F.-H.; Zheng, M.-M. Ultrasound-assisted interfacial immobilization of lipase on hollow mesoporous silica spheres in a pickering emulsion system: A hyperactive and sustainable biocatalyst. ACS Sustain. Chem. Eng. 2020, 8, 17280–17290. [Google Scholar] [CrossRef]
- Breger, J.C.; Susumu, K.; Lasarte-Aragonés, G.; Díaz, S.A.; Brask, J.; Medintz, I.L. Quantum dot lipase biosensor utilizing a custom-synthesized peptidyl-ester substrate. ACS Sens. 2020, 5, 1295–1304. [Google Scholar] [CrossRef]
- Liang, K.; Dong, W.; Gao, J.; Liu, Z.; Zhou, R.; Shu, Z.; Duan, M. The Conformational Transitions and Dynamics of Burkholderia cepacia Lipase Regulated by Water–Oil Interfaces. J. Chem. Inf. Model. 2023, 63, 3854–3864. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Fuentes, I.; Gonzalez-Salinas, S.; Favate, J.; Mejaes, J.; Zushida, K.; Nishi, A.; Hevi, C.; Goldsmith, N.; Buyske, S.; et al. Dopamine release and dopamine-related gene expression in the amygdala are modulated by the gastrin-releasing peptide in opposite directions during stress-enhanced fear learning and extinction. Mol. Psychiatry 2024, 1–14. [Google Scholar] [CrossRef]
- Zhang, S.; Deng, Q.; Shangguan, H.; Zheng, C.; Shi, J.; Huang, F.; Tang, B. Design and preparation of carbon nitride-based amphiphilic Janus N-doped carbon/MoS2 nanosheets for interfacial enzyme nanoreactor. ACS Appl. Mater. Interfaces 2020, 12, 12227–12237. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Dai, L.; Liu, D.; Du, W. Single-Crystalline Ordered Nanoporous Metal–Organic Frameworks for Lipase Immobilization and Structured Lipid Production. ACS Appl. Nano Mater. 2024, 7, 17140–17150. [Google Scholar] [CrossRef]
- Waggett, A.; Pfaendtner, J. Hydrophobic Residues Promote Interfacial Activation of Candida rugosa Lipase: A Study of Rotational Dynamics. Langmuir 2024, 40, 18262–18271. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Sha, Y.; Deng, J.; Wu, J.; Yang, P.; Zou, F.; Ying, H.; Zhuang, W. Water-mediated active conformational transitions of lipase on organic solvent interfaces. Int. J. Biol. Macromol. 2024, 277, 134056. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, R.; Di, S.; Mao, X.; Huang, W.-C. Switchable CO2-Responsive Janus Nanoparticle for Lipase Catalysis in Pickering Emulsion. J. Agric. Food Chem. 2024, 72, 9967–9973. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Huang, X.-J.; Xu, Z.-K. Utilization of a biphasic oil/aqueous cellulose nanofiber membrane bioreactor with immobilized lipase for continuous hydrolysis of olive oil. Cellulose 2014, 21, 407–416. [Google Scholar] [CrossRef]
- Zhang, N.; Bittner, J.P.; Fiedler, M.; Beretta, T.; De María, P.D.; Jakobtorweihen, S.; Kara, S. Unraveling alcohol dehydrogenase catalysis in organic–aqueous biphasic systems combining experiments and molecular dynamics simulations. ACS Catal. 2022, 12, 9171–9180. [Google Scholar] [CrossRef]
- Kuang, L.; Zhang, Q.; Li, J.; Tian, H. Preparation of lipase–electrospun SiO2 nanofiber membrane bioreactors and their targeted catalytic ability at the macroscopic oil–water interface. J. Agric. Food Chem. 2020, 68, 8362–8369. [Google Scholar] [CrossRef]
- Schröder, A.; Corstens, M.N.; Ho, K.K.; Schroën, K.; Berton-Carabin, C.C. Pickering Emulsions Emulsion-Based Systems for Delivery of Food Active Compounds: Formation, Application, Health and Safety; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 29–67. [Google Scholar]
- Yin, C.; Chen, X.; Zhang, H.; Xue, Y.; Dong, H.; Mao, X. Pickering emulsion biocatalysis: Bridging interfacial design with enzymatic reactions. Biotechnol. Adv. 2024, 72, 108338. [Google Scholar] [CrossRef]
- Fu, L.; Ma, Q.; Liao, K.; An, J.; Bai, J.; He, Y. Application of Pickering emulsion in oil drilling and production. Nanotechnol. Rev. 2021, 11, 26–39. [Google Scholar] [CrossRef]
- Wu, J.; Ma, G.H. Recent studies of Pickering emulsions: Particles make the difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Liu, B.; Wang, R.; Huang, Y.; Luo, J.; Li, Y. The enhanced fatty acids flavor release for low-fat cheeses by carrier immobilized lipases on O/W Pickering emulsions. Food Hydrocoll. 2021, 116, 106651. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Y.; Wang, J.; Huang, F.; Zheng, M. Magnetic switchable pickering interfacial biocatalysis: One-pot cascade synthesis of phytosterol esters from high-acid value oil. ACS Sustain. Chem. Eng. 2021, 9, 12070–12078. [Google Scholar] [CrossRef]
- Murray, B.S. Pickering emulsions for food and drinks. Curr. Opin. Food Sci. 2019, 27, 57–63. [Google Scholar] [CrossRef]
- Öz, Y.; Sürmeli, Y.; Şanlı-Mohamed, G. Enhanced thermostability of the immobilized thermoalkalophilic esterase onto magnetic-cornstarch nanoparticle. Biotechnol. Appl. Biochem. 2022, 69, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Tercan, Ç.; Sürmeli, Y.; Şanlı-Mohamed, G. Thermoalkalophilic recombinant esterase entrapment in chitosan/calcium/alginate-blended beads and its characterization. J. Chem. Technol. Biotechnol. 2021, 96, 2257–2264. [Google Scholar] [CrossRef]
- Olarte-Plata, J.D.; Gabriel, J.; Albella, P.; Bresme, F. Spatial control of heat flow at the nanoscale using Janus particles. ACS Nano 2021, 16, 694–709. [Google Scholar] [CrossRef]
- Luo, G.; Guo, Y.; Yang, S.; Liu, Y.; Yang, C.; Miao, X.; Liu, S.; Bai, Y. Amphiphilic Janus Nanoparticles with Enhanced Sealing Properties as Follower Sealants. ACS Appl. Nano Mater. 2024, 7, 11704–11714. [Google Scholar] [CrossRef]
- Jiang, Y.; Chakroun, R.; Gu, P.; Gröschel, A.H.; Russell, T.P. Soft polymer Janus nanoparticles at liquid–liquid interfaces. Angew. Chem. 2020, 132, 12851–12855. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, Y.-L.; Zhao, Y.; Li, C.Y.; Mukhopadhyay, A.; Sun, Z.-Y.; Koynov, K.; Butt, H.J. Brownian diffusion of individual janus nanoparticles at water/oil interfaces. ACS Nano 2020, 14, 10095–10103. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, B.; Zhang, M.; Yang, H. Interfacial Effects of Catalysis in Pickering Emulsions. J. Phys. Chem. Lett. 2024, 15, 8973–8983. [Google Scholar] [CrossRef]
- Wei, C.; Zhou, J.; Liu, T.; Zhao, W.; Zhang, X.-E.; Men, D. Self-assembled enzymatic nanowires with a “dry and wet” interface improve the catalytic performance of januvia transaminase in organic solvents. ACS Catal. 2021, 12, 372–382. [Google Scholar] [CrossRef]
- Wang, W.; Guo, N.; Huang, W.; Zhang, Z.; Mao, X. Immobilization of chitosanases onto magnetic nanoparticles to enhance enzyme performance. Catalysts 2018, 8, 401. [Google Scholar] [CrossRef]
- Khosroshahi, M.E.; Ghazanfari, L. Synthesis and functionalization of SiO2, coated Fe3O4, nanoparticles with amine groups based on self-assembly. Mater. Sci. Eng. C 2012, 32, 1043–1049. [Google Scholar] [CrossRef]
- Sonmez, M.; Georgescu, M.; Alexandrescu, L.; Gurau, D.; Ficai, A.; Ficai, D.; Andronescu, E. Synthesis and applications of Fe3O4/SiO2 core-shell materials. Curr. Pharm. Des. 2015, 21, 5324–5335. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Han, T.; Sun, D.; Shan, D.; Liu, Z.; Liang, F. Multifunctional Fe3O4@SiO2 Janus Particles. Acta Chim. Sin. 2020, 78, 954. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Chen, X.; Huang, W.-C.; Di, S.; Luo, J. Development of Multilayer Magnetic Janus Sub-Micrometric Particles for Lipase Catalysis in Pickering Emulsion. Molecules 2025, 30, 2429. https://doi.org/10.3390/molecules30112429
Wang W, Chen X, Huang W-C, Di S, Luo J. Development of Multilayer Magnetic Janus Sub-Micrometric Particles for Lipase Catalysis in Pickering Emulsion. Molecules. 2025; 30(11):2429. https://doi.org/10.3390/molecules30112429
Chicago/Turabian StyleWang, Wei, Xiangyao Chen, Wen-Can Huang, Simiao Di, and Jie Luo. 2025. "Development of Multilayer Magnetic Janus Sub-Micrometric Particles for Lipase Catalysis in Pickering Emulsion" Molecules 30, no. 11: 2429. https://doi.org/10.3390/molecules30112429
APA StyleWang, W., Chen, X., Huang, W.-C., Di, S., & Luo, J. (2025). Development of Multilayer Magnetic Janus Sub-Micrometric Particles for Lipase Catalysis in Pickering Emulsion. Molecules, 30(11), 2429. https://doi.org/10.3390/molecules30112429







