Modified Polyethylene Oxide Solid-State Electrolytes with Poly(vinylidene fluoride-hexafluoropropylene)
Abstract
1. Introduction
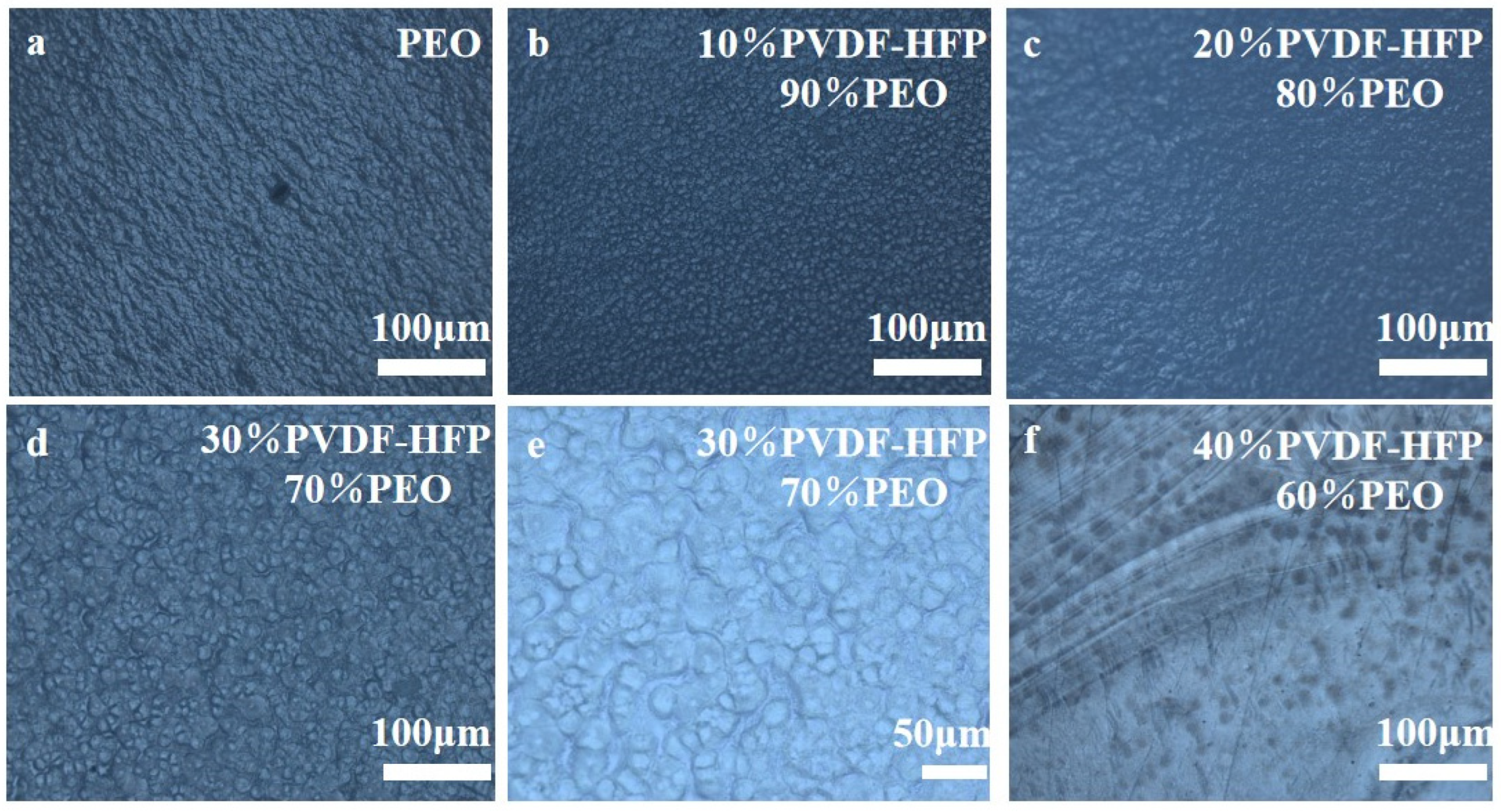
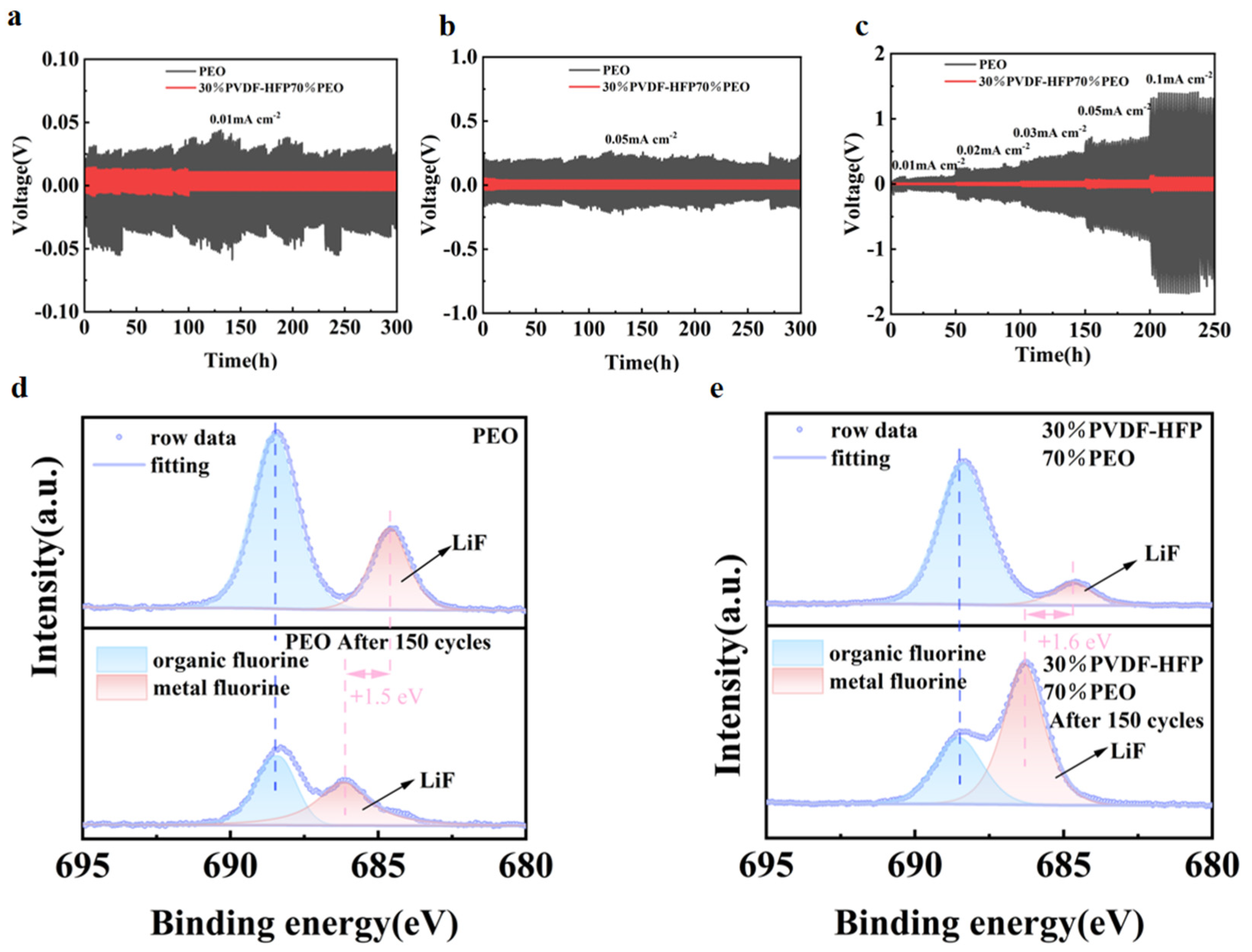
2. Results and Discussion
3. Experimental
3.1. Materials
3.2. Blending of PEO and P(VDF-HFP)
3.3. Preparation of the Cathode
3.4. Characterization and Electrochemical Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, R.; Liao, W.; Fan, S.; Chen, D.; Tang, J.; Yang, Y.; Liu, C. Regulating Interfacial Li-Ion Transport via an Integrated Corrugated 3D Skeleton in Solid Composite Electrolyte for All-Solid-State Lithium Metal Batteries. Adv. Sci. 2022, 9, 2104506. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zhang, T.; Zhou, D.; Sun, P.; Lu, Z.; Bian, H.; Dang, J.; Gao, H.; Qian, Y.; Li, W.; et al. Charge Transfer of Interfacial Catalysts for Hydrogen Energy. ACS Mater. Lett. 2022, 4, 967–977. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, Z.; Xiang, J.; Yang, X.; Shen, X.; Song, F. Recent Advances in Transition Metal Nitrides for Hydrogen Electrocatalysis in Alkaline Media: From Catalyst Design to Application. Front. Chem. 2022, 10, 1073175. [Google Scholar] [CrossRef] [PubMed]
- Schnell, J.; Knoerzer, H.; Imbsweiler, A.J.; Reinhart, G. Solid versus Liquid-A Bottom-Up Calculation Model to Analyze the Manufacturing Cost of Future High-Energy Batteries. Energy Technol. 2020, 8, 1901237. [Google Scholar] [CrossRef]
- Amici, J.; Romanin, S.; Alidoost, M.; Versaci, D.; Francia, C.; Smeacetto, F.; Bodoardo, S. UV-Cured Methacrylate Based Polymer Composite Electrolyte for Metallic Lithium Batteries. J. Electroanal. Chem. 2019, 837, 103–107. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, C.; Jiang, H.; Zheng, M.; Wu, Q.-H.; Dong, Q. Improving the Electrochemistry Performance of Layer LiNi0.5Mn0.3Co0.2O2 Material at 4.5 V Cutoff Potential Using Lithium Metaborate. Electrochim. Acta 2017, 243, 105–111. [Google Scholar] [CrossRef]
- Nkosi, F.P.; Cuevas, I.; Valvo, M.; Mindemark, J.; Mahun, A.; Abbrent, S.; Brus, J.; Kobera, L.; Edstrom, K. Understanding Lithium-Ion Conductivity in NASICON-Type Polymer-in-Ceramic Composite Electrolytes. ACS Appl. Energ. Mater. 2024, 7, 4609–4619. [Google Scholar] [CrossRef]
- Chen, B.; Qian, H.; Xu, J.; Qin, L.; Wu, Q.-H.; Zheng, M.; Dong, Q. Study on SnO2/Graphene Composites with Superior Electrochemical Performance for Lithium-Ion Batteries. J. Mater. Chem. A 2014, 2, 9345–9352. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Chen, Y.-T.; Yang, H.; Bao, W.; Sreenarayanan, B.; Doux, J.-M.; Li, W.; Lu, B.; Ham, S.-Y.; Sayahpour, B.; et al. Carbon-Free High-Loading Silicon Anodes Enabled by Sulfide Solid Electrolytes. Science 2021, 373, 1494–1499. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Wu, S.-H.; Chang, J.-K.; Jose, R.; Yang, C.-C. Solvent-Free Semi-Interpenetrating Composite Polymer Electrolyte Based on Dual Li-Salt for Solid-State Lithium Batteries. J. Energy Storage 2025, 110, 115335. [Google Scholar] [CrossRef]
- Kim, K.J.; Rupp, J.L.M. All Ceramic Cathode Composite Design and Manufacturing towards Low Interfacial Resistance for Garnet-Based Solid-State Lithium Batteries. Energy Environ. Sci. 2020, 13, 4930–4945. [Google Scholar] [CrossRef]
- Sharafi, A.; Kazyak, E.; Davis, A.L.; Yu, S.; Thompson, T.; Siegel, D.J.; Dasgupta, N.P.; Sakamoto, J. Surface Chemistry Mechanism of Ultra-Low Interfacial Resistance in the Solid-State Electrolyte Li7La3Zr2O12. Chem. Mater. 2017, 29, 7961–7968. [Google Scholar] [CrossRef]
- Huang, Z.; Pang, W.; Liang, P.; Jin, Z.; Grundish, N.; Li, Y.; Wang, C.-A. A Dopamine Modified Li6.4La3Zr1.4Ta0.6O12/PEO Solid-State Electrolyte: Enhanced Thermal and Electrochemical Properties. J. Mater. Chem. A 2019, 7, 16425–16436. [Google Scholar] [CrossRef]
- Tang, X.; Lv, S.; Jiang, K.; Zhou, G.; Liu, X. Recent Development of Ionic Liquid-Based Electrolytes in Lithium-Ion Batteries. J. Power Sources 2022, 542, 231792. [Google Scholar] [CrossRef]
- Kim, K.J.; Balaish, M.; Wadaguchi, M.; Kong, L.; Rupp, J.L.M. Solid-State Li-Metal Batteries: Challenges and Horizons of Oxide and Sulfide Solid Electrolytes and Their Interfaces. Adv. Energy Mater. 2021, 11, 2002689. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, Z.; Zhou, W.; Liu, Q.; Wu, J.-F.; Liu, T.-H.; Xu, C.; Liu, J. 4.2V Polymer All-Solid-State Lithium Batteries Enabled by High-Concentration PEO Solid Electrolytes. Energy Storage Mater. 2023, 57, 171–179. [Google Scholar] [CrossRef]
- Wu, F.; Liu, L.; Wang, S.; Xu, J.; Lu, P.; Yan, W.; Peng, J.; Wu, D.; Li, H. Solid State Ionics-Selected Topics and New Directions. Prog. Mater. Sci. 2022, 126, 100921. [Google Scholar] [CrossRef]
- Shen, J.; Lei, Z.; Wang, C. An Ion Conducting ZIF-8 Coating Protected PEO Based Polymer Electrolyte for High Voltage Lithium Metal Batteries. Chem. Eng. J. 2022, 447, 137503. [Google Scholar] [CrossRef]
- Wang, J.; Yamada, Y.; Sodeyama, K.; Watanabe, E.; Takada, K.; Tateyama, Y.; Yamada, A. Fire-Extinguishing Organic Electrolytes for Safe Batteries. Nat. Energy 2018, 3, 22–29. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, J.; Cai, W.; Lai, Y.; Song, J.; Yu, J.; Ding, B. Elastic and Well-Aligned Ceramic LLZO Nanofiber Based Electrolytes for Solid-State Lithium Batteries. Energy Storage Mater. 2019, 23, 306–313. [Google Scholar] [CrossRef]
- Marzantowicz, M.; Dygas, J.R.; Krok, F.; Tomaszewska, A.; Zukowska, G.Z.; Florjanczyk, Z.; Zygadlo-Monikowska, E. Phase Segregation Phenomena in Poly(Ethylene Oxide):LiN(CF3SO2)2 Electrolyte Studied by Local Raman Spectroscopy. Electrochim. Acta 2010, 55, 5446–5452. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, J.; Dong, S.; Li, X.; Cui, G. Intermolecular Chemistry in Solid Polymer Electrolytes for High-Energy-Density Lithium Batteries. Adv. Mater. 2019, 31, 1902029. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Wang, A.; Liu, X.; Chen, J.; Zeng, Q.; Zhang, L.; Liu, W.; Zhang, L. Covalently Linked Metal-Organic Framework (MOF)-Polymer All-Solid-State Electrolyte Membranes for Room Temperature High Performance Lithium Batteries. J. Mater. Chem. A 2018, 6, 17227–17234. [Google Scholar] [CrossRef]
- Wu, J.-F.; Guo, X. MOF-Derived Nanoporous Multifunctional Fillers Enhancing the Performances of Polymer Electrolytes for Solid-State Lithium Batteries. J. Mater. Chem. A 2019, 7, 2653–2659. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Y.; Chen, D.; Dong, L.; Guang, Z.; Liu, J.; Yuan, B.; Yang, M.; Dong, Y.; Li, Q.; et al. Hydroxyapatite Functionalization of Solid Polymer Electrolytes for High-Conductivity Solid-State Lithium-Ion Batteries. Mater. Today Energy 2021, 20, 100694. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Liu, D.; Gao, Y.; Wang, Y.; Bu, H.; Li, M.; Zhang, Y.; Gao, G.; Ding, S. A Composite Solid Polymer Electrolyte Incorporating MnO2 Nanosheets with Reinforced Mechanical Properties and Electrochemical Stability for Lithium Metal Batteries. J. Mater. Chem. A 2020, 8, 2021–2032. [Google Scholar] [CrossRef]
- Han, L.; Wang, J.; Mu, X.; Wu, T.; Liao, C.; Wu, N.; Xing, W.; Song, L.; Kan, Y.; Hu, Y. Controllable Magnetic Field Aligned Sepiolite Nanowires for High Ionic Conductivity and High Safety PEO Solid Polymer Electrolytes. J. Colloid Interface Sci. 2021, 585, 596–604. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Feng, X.; Zhang, Z.; Gao, H.; Huang, J. Silica-Assisted Cross-Linked Polymer Electrolyte Membrane with High Electrochemical Stability for Lithium-Ion Batteries. J. Colloid Interface Sci. 2021, 594, 115335. [Google Scholar] [CrossRef]
- Mery, A.; Rousselot, S.; Lepage, D.; Dolle, M. A Critical Review for an Accurate Electrochemical Stability Window Measurement of Solid Polymer and Composite Electrolytes. Materials 2021, 14, 3840. [Google Scholar] [CrossRef]
- Zhu, P.; Yan, C.; Dirican, M.; Zhu, J.; Zang, J.; Selvan, R.K.; Chung, C.-C.; Jia, H.; Li, Y.; Kiyak, Y.; et al. Li0.33La0.557TiO3 Ceramic Nanofiber-Enhanced Polyethylene Oxide-Based Composite Polymer Electrolytes for All-Solid-State Lithium Batteries. J. Mater. Chem. A 2018, 6, 4279–4285. [Google Scholar] [CrossRef]
- Freitag, K.M.; Walke, P.; Nilges, T.; Kirchhain, H.; Spranger, R.J.; van Wuellen, L. Electrospun-Sodiumtetrafluoroborate-Polyethylene Oxide Membranes for Solvent-Free Sodium Ion Transport in Solid State Sodium Ion Batteries. J. Power Sources 2018, 378, 610–617. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Li, Y.; Yang, M.; Chen, L.; Li, H.; Wu, F. Long-Life Lithium-Metal All-Solid-State Batteries and Stable Li Plating Enabled by In Situ Formation of Li3PS4 in the SEI Layer. Adv. Mater. 2022, 34, 2203281. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lai, C.; Chen, K.; Wu, Q.; Gu, Y.; Wu, C.; Li, C. Dual Fluorination of Polymer Electrolyte and Conversion-Type Cathode for High-Capacity All-Solid-State Lithium Metal Batteries. Nat. Commun. 2022, 13, 7914. [Google Scholar] [CrossRef]
- Liu, W.; Li, G.; Yu, W.; Gao, L.; Shi, D.; Ju, J.; Deng, N.; Kang, W. Asymmetric Organic-Inorganic Bi-Functional Composite Solid-State Electrolyte for Long Stable Cycling of High-Voltage Lithium Battery. Energy Storage Mater. 2023, 63, 103005. [Google Scholar] [CrossRef]
- Zheng, X.; Wei, J.; Lin, W.; Ji, K.; Wang, C.; Chen, M. Bridging Li7La3Zr2O12 Nanofibers with Poly(Ethylene Oxide) by Coordination Bonds to Enhance the Cycling Stability of All-Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 5346–5354. [Google Scholar] [CrossRef]
- Shen, J.; Liu, S.; Bian, D.; Chen, Z.; Pan, H.; Yang, C.; Tian, W.; Li, Y.; Kong, L.; Quan, H.; et al. Efficient Nanoarchitectonics of Solid-Electrolyte-Interface for High-Performance All-Solid-State Lithium Metal Batteries via Mild Fluorination on Polyethylene Oxide. Electrochim. Acta 2023, 456, 142482. [Google Scholar] [CrossRef]
- Ni’mah, Y.L.; Muhaiminah, Z.H.; Suprapto, S. Increase of Solid Polymer Electrolyte Ionic Conductivity Using Nano-SiO2 Synthesized from Sugarcane Bagasse as Filler. Polymers 2021, 13, 4240. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Borodin, A.; Endres, F. Ionic Liquid and Polymer Coated Garnet Solid Electrolytes for High-Energy Solid-State Lithium Metal Batteries. Energy Technol. 2022, 10, 2100907. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, A.; Liu, Q.; Zhang, M.; Li, Q.; Li, F. Effect of Liquid Crystal Ionomer Intercalated Montmorillonite Nanocomposites on PEO/PLA Solid Polymer Electrolytes. Ionics 2018, 24, 3805–3813. [Google Scholar] [CrossRef]
- Shen, X.; Li, R.; Ma, H.; Peng, L.; Huang, B.; Zhang, P.; Zhao, J. Enhancing Li Transport Kinetics of PEO-Based Polymer Electrolyte with Mesoporous Silica-Derived Fillers for Lithium-Ion Batteries+. Solid State Ion. 2020, 354, 115412. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, B.; Lu, Y.; Shen, F.; Zhao, B.; Han, X. Robust and High Thermal-Stable Composite Polymer Electrolyte Reinforced by PI Nanofiber Network. Nanotechnology 2021, 32, 495401. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Shokrieh, A.; Li, C.; Zhang, B.; Amin, K.; Mao, L.; Wei, Z. PVDF-HFP Layer with High Porosity and Polarity for High-Performance Lithium Metal Anodes in Both Ether and Carbonate Electrolytes. Nano Energy 2022, 95, 107009. [Google Scholar] [CrossRef]
- Shao, Z.; Zhang, X.; Liu, J.; Liu, X.; Zhang, C. Electrospinning of Highly Bi-Oriented Flexible Piezoelectric Nanofibers for An-isotropic-Responsive Intelligent Sensing. Small Methods 2023, 7, 2300701. [Google Scholar] [CrossRef] [PubMed]




| Temperature (°C) | 25 °C | 30 °C | 35 °C | 40 °C | 45 °C | 50 °C | 55 °C | 60 °C |
|---|---|---|---|---|---|---|---|---|
| Electrolyte | ||||||||
| PEO | 3.86 × 10−4 | 4.43 × 10−4 | 6.77 × 10−4 | 8.13 × 10−4 | 1.46 × 10−3 | 1.69 × 10−3 | 2.42 × 10−3 | 3.18 × 10−3 |
| 10%P(VDF-HFP)90%PEO | 1.64 × 10−3 | 1.93 × 10−3 | 2.23 × 10−3 | 3.25 × 10−3 | 3.85 × 10−3 | 4.77 × 10−3 | 6.53 × 10−3 | 7.87 × 10−3 |
| 20%P(VDF-HFP)80%PEO | 1.92 × 10−3 | 2.31 × 10−3 | 2.87 × 10−3 | 3.79 × 10−3 | 4.84 × 10−3 | 5.76 × 10−3 | 6.79 × 10−3 | 7.95 × 10−3 |
| 30%P(VDF-HFP)70%PEO | 8.89 × 10−3 | 1.68 × 10−2 | 2.35 × 10−2 | 2.56 × 10−2 | 2.76 × 10−2 | 2.98 × 10−2 | 3.23 × 10−2 | 3.71 × 10−2 |
| 40%P(VDF-HFP)60%PEO | 9.01 × 10−4 | 1.16 × 10−3 | 1.37 × 10−3 | 2.79 × 10−3 | 3.53 × 10−3 | 4.00 × 10−3 | 4.52 × 10−3 | 4.70 × 10−3 |
| Polymer Solid Electrolyte | ΔV (V) | Ι0 (μA) | Ιs (μA) | R0 (Ω) | Rs (Ω) | tLi+ |
|---|---|---|---|---|---|---|
| PEO | 0.01 | 36.60 | 12.90 | 150.64 | 221.37 | 0.22 |
| 30%P(VDF-HFP)70%PEO | 0.01 | 40.75 | 24.36 | 124.03 | 139.21 | 0.45 |
| Solid Electrolyte | T (°C) | Ionic Conductivity (S cm−1) | Ref. | |
|---|---|---|---|---|
| 1 | F-PEO/LiTFSI | 60 | 3.32 × 10−4 | [36] |
| 2 | PEO/NaClO4/Nano-SiO2 | 60 | 1.18 × 10−6 | [37] |
| 3 | PEO-IL/LiTFSI/Ga-LLZO | 25 | 5.7 × 10−4 | [38] |
| 4 | PEO/PLA/LiClO4/MMT | 25 | 1.05 × 10−5 | [39] |
| 5 | PEO/LiTFSI/SBA-LiIL | 60 | 4.3 × 10−4 | [40] |
| 6 | PEO/LiTFSI/PI | 60 | 4.2 × 10−4 | [41] |
| 7 | 70%PEO/LiTFSI/30%P(VDF-HFP) | 25 60 | 8.89 × 10−3 3.71 × 10−2 | This paper |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Huang, W.; Hu, T.; Huang, H.; Zhu, C.; Chen, Z.; Fan, X.; Wu, Q.; Li, Y. Modified Polyethylene Oxide Solid-State Electrolytes with Poly(vinylidene fluoride-hexafluoropropylene). Molecules 2025, 30, 2422. https://doi.org/10.3390/molecules30112422
Yan J, Huang W, Hu T, Huang H, Zhu C, Chen Z, Fan X, Wu Q, Li Y. Modified Polyethylene Oxide Solid-State Electrolytes with Poly(vinylidene fluoride-hexafluoropropylene). Molecules. 2025; 30(11):2422. https://doi.org/10.3390/molecules30112422
Chicago/Turabian StyleYan, Jinwei, Wen Huang, Tangqi Hu, Hai Huang, Chengwei Zhu, Zhijie Chen, Xiaohong Fan, Qihui Wu, and Yi Li. 2025. "Modified Polyethylene Oxide Solid-State Electrolytes with Poly(vinylidene fluoride-hexafluoropropylene)" Molecules 30, no. 11: 2422. https://doi.org/10.3390/molecules30112422
APA StyleYan, J., Huang, W., Hu, T., Huang, H., Zhu, C., Chen, Z., Fan, X., Wu, Q., & Li, Y. (2025). Modified Polyethylene Oxide Solid-State Electrolytes with Poly(vinylidene fluoride-hexafluoropropylene). Molecules, 30(11), 2422. https://doi.org/10.3390/molecules30112422









