The High Interfacial Activity of Betaine Surfactants Triggered by Nonionic Surfactant: The Vacancy Size Matching Mechanism of Hydrophobic Groups
Abstract
1. Introduction
2. Results and Discussion
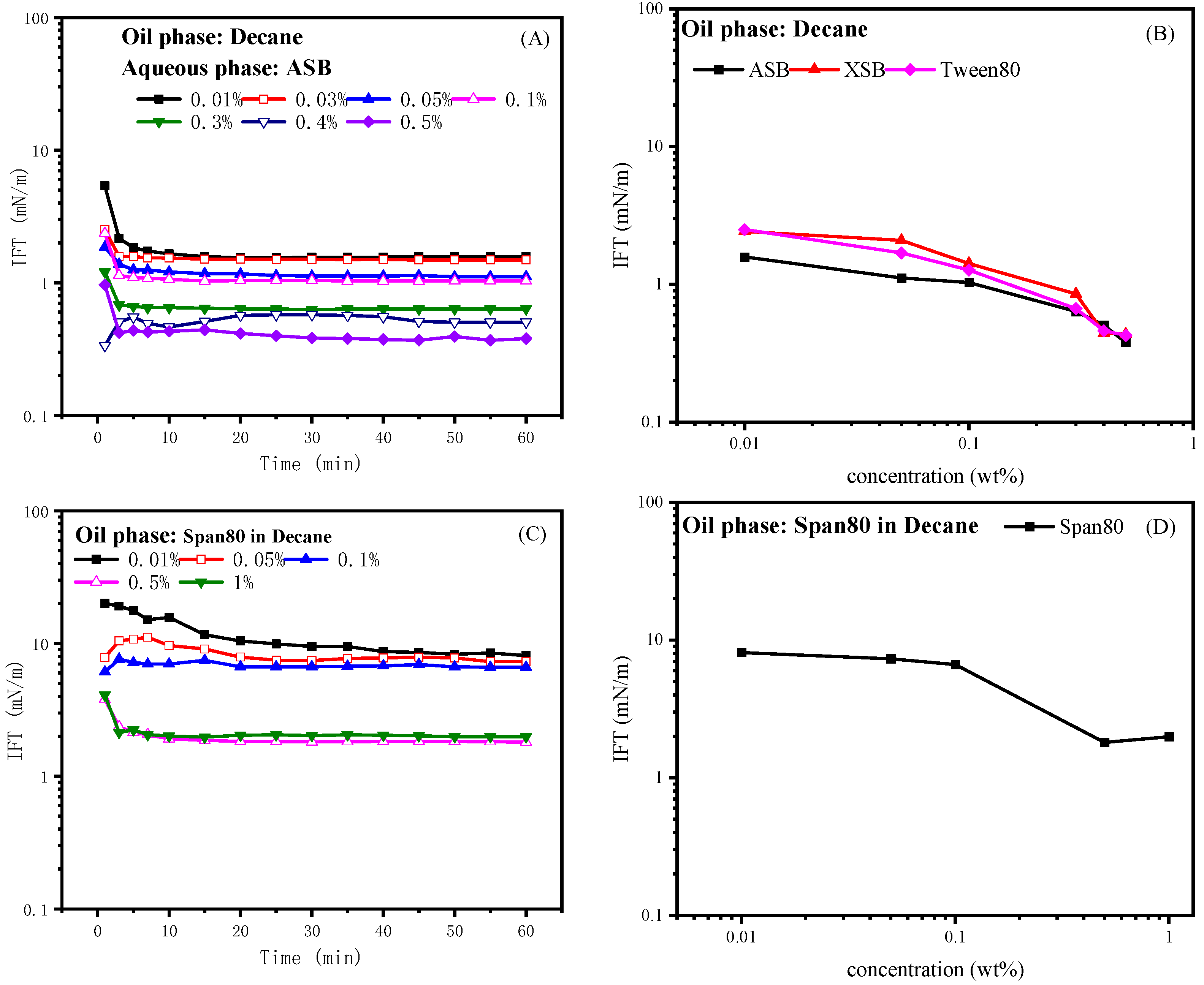
2.1. Effect of Surfactant Concentration on IFT
2.2. Effect of Oil-Soluble Nonionic Surfactants on the IFT of Betaine
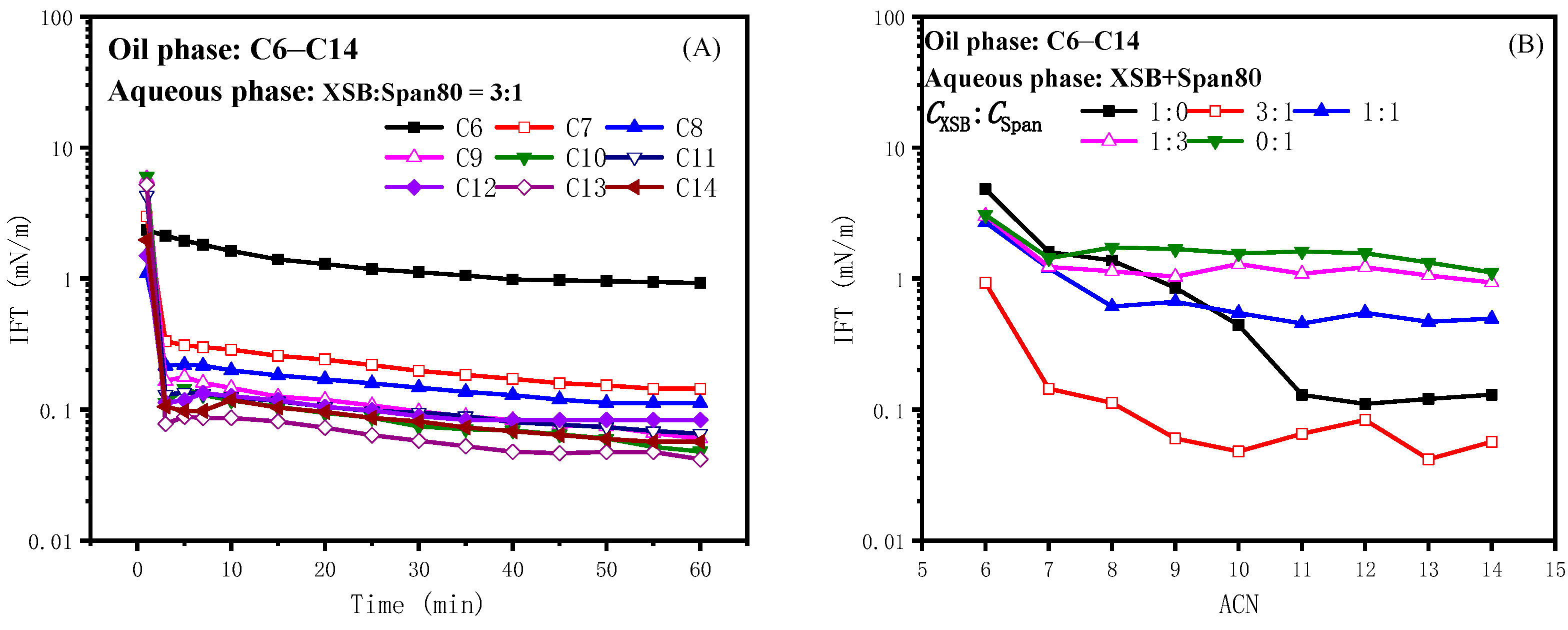
2.2.1. Effect of Span80 on the IFT of Betaine ASB
2.2.2. Effect of Span80 on the IFT of Betaine XSB
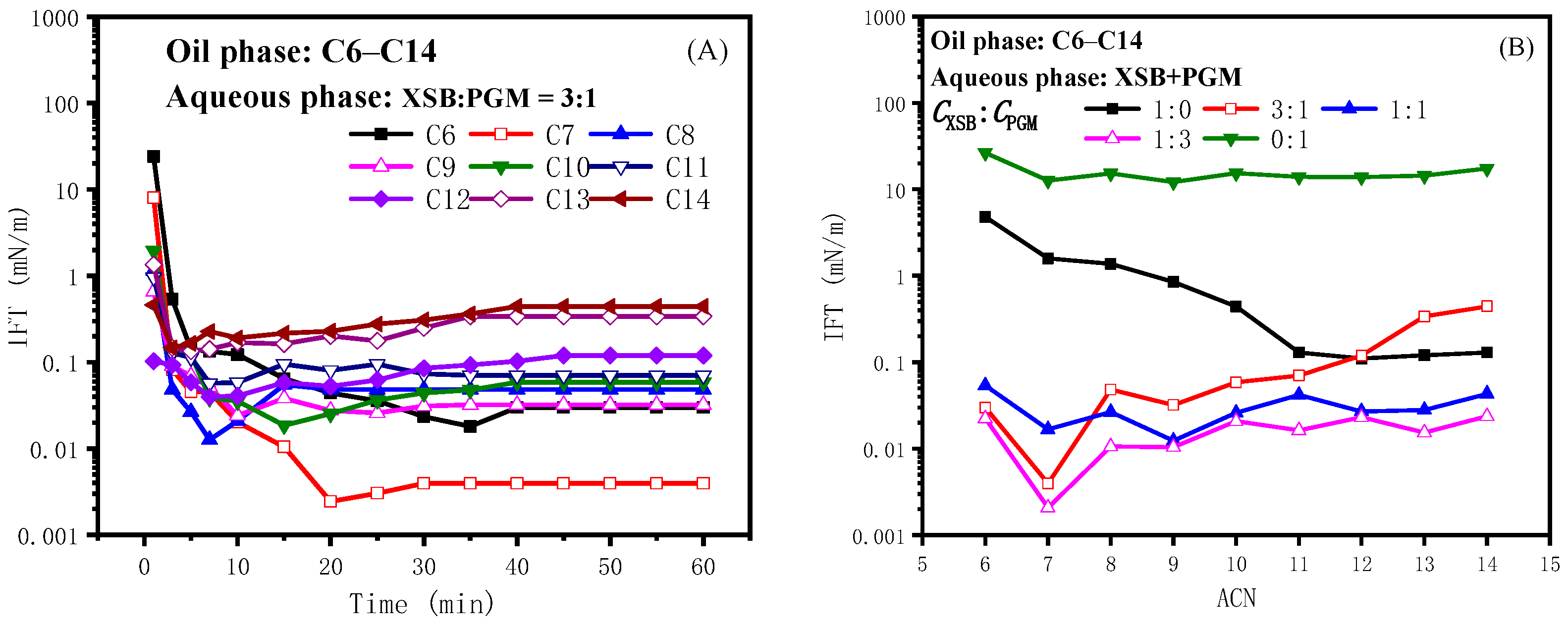
2.2.3. Effect of Propylene Glycol Monostearate on the IFT of Betaine XSB
2.2.4. Synergistic Mechanism of Oil-Soluble Surfactants with Betaine ASB and XSB
2.3. Effect of Water-Soluble Nonionic Surfactant on IFT of Betaine
2.3.1. Effect of Tween80 on IFT of Betaine ASB
2.3.2. Effect of Tween80 on the IFT of Betaine XSB
3. Materials and Methods
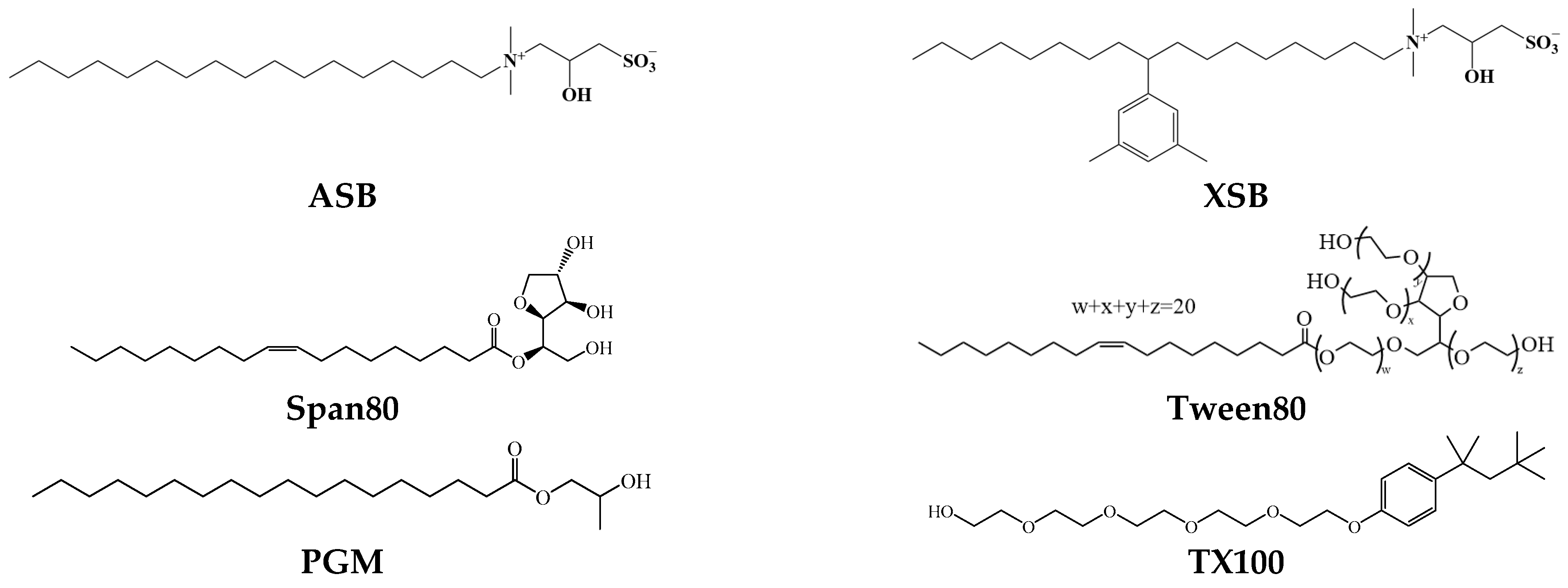
3.1. Materials
3.2. Apparatus and Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.B.; Wang, Z.Q.; Hu, Z.M.; Xu, B.Q.; Li, Y.L.; Pu, W.-F.; Zhao, J.-Z. A review of in situ upgrading technology for heavy crude oil. Petroleum 2021, 7, 117–122. [Google Scholar] [CrossRef]
- Bashir, A.; Haddad, A.S.; Rafati, R. A review of fluid displacement mechanisms in surfactant-based chemical enhanced oil recovery processes: Analyses of key influencing factors. Pet. Sci. 2022, 19, 1211–1235. [Google Scholar] [CrossRef]
- Tackie-Otoo, B.N.; Mohammed, M.A.A.; Yekeen, N.; Negash, B.M. Alternative chemical agents for alkalis, surfactants and polymers for enhanced oil recovery: Research trend and prospects. J. Pet. Sci. Eng. 2020, 187, 106828. [Google Scholar] [CrossRef]
- Dubey, S.; Majumder, S.K. Stability analysis of CO2 microbubble for CO2 sequestration and mobility control in enhanced oil recovery. Chem. Eng. J. 2024, 500, 156595. [Google Scholar] [CrossRef]
- Yin, J.; Wei, X.X.; Hu, F.T.; Cheng, C.K.; Song, M.Y.; Zhuang, G.Q.; Ma, A.Z. Alternative stable microbiome state triggered by the introduction of functional microbes in oil reservoirs drives sustainable microbial enhanced oil recovery. Chem. Eng. J. 2023, 475, 146073. [Google Scholar] [CrossRef]
- Gomaa, S.; Salem, K.G.; El-hoshoudy, A.N. Enhanced heavy and extra heavy oil recovery: Current status and new trends. Petroleum 2024, 10, 399–410. [Google Scholar] [CrossRef]
- Zhao, M.W.; Liu, K.W.; Meng, X.J.; Ma, Z.F.; Dai, C.L. Review on principles, influence and applications of nanomaterials in enhancing oil recovery. Fuel 2024, 371, 131985. [Google Scholar] [CrossRef]
- Massarweh, O.; Abushaikha, A.S. The use of surfactants in enhanced oil recovery: A review of recent advances. Energy Rep. 2020, 6, 3150–3178. [Google Scholar] [CrossRef]
- Wang, C.G.; Pan, Y.; Xu, Z.C.; Zhang, L.; Zhang, L.; Yang, S.C. Ultralow interfacial tension achieved by extended anionic surfactants with a short hydrophobic chain. J. Mol. Liq. 2024, 400, 124514. [Google Scholar] [CrossRef]
- Rahman, A.F.A.; Arsad, A.; Sidek, A.; Abdurrahman, M. Wettability alteration and interfacial tension reduction on high salinity of anionic/nonionic surfactant. Mater. Today Proc. 2024, 110, 82–86. [Google Scholar] [CrossRef]
- Ma, L.X.; Xu, P.; Wang, L.; Xia, K.; Du, H.; Gao, R.T.; Chen, Z.-J. Study on anionic-nonionic mixed surfactant for enhanced oil recovery in a hypersaline reservoir. R. Soc. Chem. 2024, 14, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.W.; Zhu, Y.W.; Liu, T.; Xu, X.R.; Yang, J.Y. Synthesis of a novel borate ester Anion-Nonionic surfactant and its application in viscosity reduction and emulsification of heavy crude oil. Fuel 2023, 333, 126453. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, F.J.; Li, Y.; Liu, C.; Zhu, L.Y.; Yao, E.D. Performance evaluation of oil-displacement viscoelastic zwitterionic surfactant fracturing fluid. J. Mol. Liq. 2023, 377, 121545. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, Z.H.; Xiao, C.M.; Gao, M.; Han, L.; Liu, Q.C.; Zhang, L.; Zhang, Q.; Zhang, L. The synergism of improving interfacial properties between betaine and crude oil for enhanced oil recovery. J. Mol. Liq. 2023, 383, 122046. [Google Scholar] [CrossRef]
- Lv, W.F.; Zhou, Z.H.; Zhang, Q.; Zhang, X.J.; Zhang, L. Study on the mechanism of surfactant flooding: Effect of betaine structure. Adv. Geo-Energy Res. 2023, 10, 146–158. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zhang, Q.; Liu, Y.; Wang, H.Z.; Cai, H.Y.; Zhang, F.; Tian, M.Z.; Liu, Z.-Y.; Zhang, L.; Zhang, L. Effect of Fatty Acids on Interfacial Tensions of Novel Sulfobetaines Solutions. Energy Fuels 2014, 28, 1020–1027. [Google Scholar] [CrossRef]
- Zheng, D.D.; Zhou, Z.H.; Zhang, Q.; Zhang, L.; Zhu, Y.; Zhang, L. Effect of inorganic alkalis on interfacial tensions of novel betaine solutions against crude oil. J. Pet. Sci. Eng. 2017, 152, 602–610. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, Z.H.; Zhang, Q.; Zhang, F.; Ma, G.Y.; Zhang, L.; Zhang, L. Effect of Electrolyte on Synergism for Reducing Interfacial Tension between Betaine and Petroleum Sulfonate. Energy Fuels 2020, 34, 3188–3198. [Google Scholar] [CrossRef]
- Zhong, Q.L.; Zhou, Z.H.; Zhang, Q.; Ma, D.S.; Luan, H.X.; Zhang, L.; Ma, G.-Y.; Zhang, L. Studies on Interfacial Tensions of Ionic Surfactant and Alkyl Sulfobetaine Mixed Solutions. Energy Fuels 2018, 32, 8202–8209. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zheng, Y.C.; Tian, F.Q.; Liu, H.; Lu, X.-B.; Yi, X.; Wang, Z.-L. Performance of extended surfactant and its mixture with betaine surfactant for enhanced oil recovery in sandstone reservoirs with low permeability. J. Mol. Liq. 2023, 391, 123228. [Google Scholar] [CrossRef]
- Pei, H.H.; Liu, Y.; Shan, J.L.; Zhao, J.W.; Zhang, J.; Wu, Y.H.; Zhang, G.C. Enhancement of in-situ emulsification performance through the synergistic effects of zwitterionic and nonionic-anionic surfactants to improve heavy oil recovery. J. Mol. Liq. 2024, 410, 125562. [Google Scholar] [CrossRef]
- Zhong, Q.L.; Cao, X.L.; Zhu, Y.W.; Ma, B.D.; Xu, Z.C.; Zhang, L.; Ma, G.Y.; Zhang, L. Studies on interfacial tensions of betaine and anionic-nonionic surfactant mixed solutions. J. Mol. Liq. 2020, 311, 113262. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Z.; Wang, C.Y.; Shi, L.; Hou, J.; Zhang, L.L. Effect of nonionic surfactants on the synergistic interaction between asphaltene and resin: Emulsion phase inversion and stability. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 132056. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, Z.Q.; Song, X.W.; Zhang, J.C.; Zhang, L.; Zhang, L.; Zhao, S. Dynamic interfacial tensions of binary nonionic–anionic and nonionic surfactant mixtures at water–alkane interfaces. Fuel 2014, 135, 91–98. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zheng, Y.C.; Mu, J.; Chang, S.T.; Sarsenbekuly, B.; Yang, H.-B.; Kang, W.L. Interactions and oil recovery performance of a prepared extended amphoteric surfactant combining with a nonionic surfactant under high salinity condition. J. Mol. Liq. 2024, 414, 126077. [Google Scholar] [CrossRef]
- Zhao, R.H.; Huang, H.Y.; Wang, H.Y.; Zhang, J.C.; Zhang, L.; Zhang, L.; Zhao, S. Effect of Organic Additives and Crude Oil Fractions on Interfacial Tensions of Alkylbenzene Sulfonates. J. Dispers. Sci. Technol. 2013, 34, 623–631. [Google Scholar] [CrossRef]
- Brigodiot, C.; Marsiglia, M.; Dalmazzone, C.; Schroen, K.; Colin, A. Studying surfactant mass transport through dynamic interfacial tension measurements: A review of the models, experiments, and the contribution of microfluidics. Adv. Colloid Interface Sci. 2024, 331, 103239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, L.; Cao, X.L.; Song, X.W.; Jin, Z.Q.; Zhang, L.; Zhao, S. Effect of Electrolytes on Interfacial Tensions of Alkyl Ether Carboxylate Solutions. Energy Fuels 2013, 27, 3122–3129. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, Z.H.; Shangguan, Y.N.; Han, L.; Wang, L.L.; Fan, W.; Zhang, Q.; Zhang, L.; Ma, G.Y.; Zhang, L. Effect of bivalent cations on the interfacial tensions of extended anionic surfactant solutions. J. Mol. Liq. 2022, 349, 118162. [Google Scholar] [CrossRef]
- Sheng, S.S.; Cao, X.L.; Zhu, Y.W.; Jin, Z.Q.; Zhang, L.; Zhu, Y.; Zhang, L. Structure-activity relationship of anionic-nonionic surfactant for reducing interfacial tension of crude oil. J. Mol. Liq. 2020, 313, 112772. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, L.; Zhao, S.; Yu, J.Y. Studies of synergism/antagonism for lowering dynamic interfacial tensions in surfactant/alkali/acidic oil systems, part 2: Synergism/antagonism in binary surfactant mixtures. J. Colloid Interface Sci. 2002, 251, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.S.; Zhou, Z.H.; Han, L.; Chen, X.; He, H.J.; Zhang, Q.; Xu, Z.C.; Gong, Q.T.; Zhang, L.; Ma, G.Y.; et al. The mechanism for lowering interfacial tension by extended surfactant containing ethylene oxide and propylene oxide groups. J. Mol. Liq. 2022, 359, 119364. [Google Scholar] [CrossRef]
- Bian, S.W.; Liu, P.; Mao, Z.Q.; Huang, W.H.; Zhu, Y.W.; Zhang, L.; Hou, Y.; Zhang, L. Studying the factors determining the ultralow interfacial tensions of betaine solutions against crude oil. Colloids Surf. A Physicochem. Eng. Asp. 2024, 686, 133453. [Google Scholar] [CrossRef]
- Song, X.W.; Dong, L.F.; Cao, X.L.; Xu, Z.C.; Wang, C.; Zhang, L.; Zhang, L.; Zhao, S. Dynamic interfacial tensions of p-(n-lauryl)-benzyl polyoxyethylene ether carboxybetaine solutions. J. Pet. Sci. Eng. 2014, 124, 27–34. [Google Scholar] [CrossRef]






| Cl− | Na+ | Ca2+ | Mg2+ | TDS |
|---|---|---|---|---|
| 11,699 | 6921 | 413 | 102 | 19,135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Zhao, J.; Han, L.; Wu, Q.; Zhang, Q.; Zhang, B.; Yue, R.; Yan, F.; Zhou, Z.; Ding, W. The High Interfacial Activity of Betaine Surfactants Triggered by Nonionic Surfactant: The Vacancy Size Matching Mechanism of Hydrophobic Groups. Molecules 2025, 30, 2413. https://doi.org/10.3390/molecules30112413
Li G, Zhao J, Han L, Wu Q, Zhang Q, Zhang B, Yue R, Yan F, Zhou Z, Ding W. The High Interfacial Activity of Betaine Surfactants Triggered by Nonionic Surfactant: The Vacancy Size Matching Mechanism of Hydrophobic Groups. Molecules. 2025; 30(11):2413. https://doi.org/10.3390/molecules30112413
Chicago/Turabian StyleLi, Guoqiao, Jinyi Zhao, Lu Han, Qingbo Wu, Qun Zhang, Bo Zhang, Rushan Yue, Feng Yan, Zhaohui Zhou, and Wei Ding. 2025. "The High Interfacial Activity of Betaine Surfactants Triggered by Nonionic Surfactant: The Vacancy Size Matching Mechanism of Hydrophobic Groups" Molecules 30, no. 11: 2413. https://doi.org/10.3390/molecules30112413
APA StyleLi, G., Zhao, J., Han, L., Wu, Q., Zhang, Q., Zhang, B., Yue, R., Yan, F., Zhou, Z., & Ding, W. (2025). The High Interfacial Activity of Betaine Surfactants Triggered by Nonionic Surfactant: The Vacancy Size Matching Mechanism of Hydrophobic Groups. Molecules, 30(11), 2413. https://doi.org/10.3390/molecules30112413






