Iodide/Nickel Co-Catalyzed Manganese-Mediated Denitrogenative Cross-Electrophile Coupling of Benzotriazinones with Alkyl Sulfonates
Abstract
1. Introduction
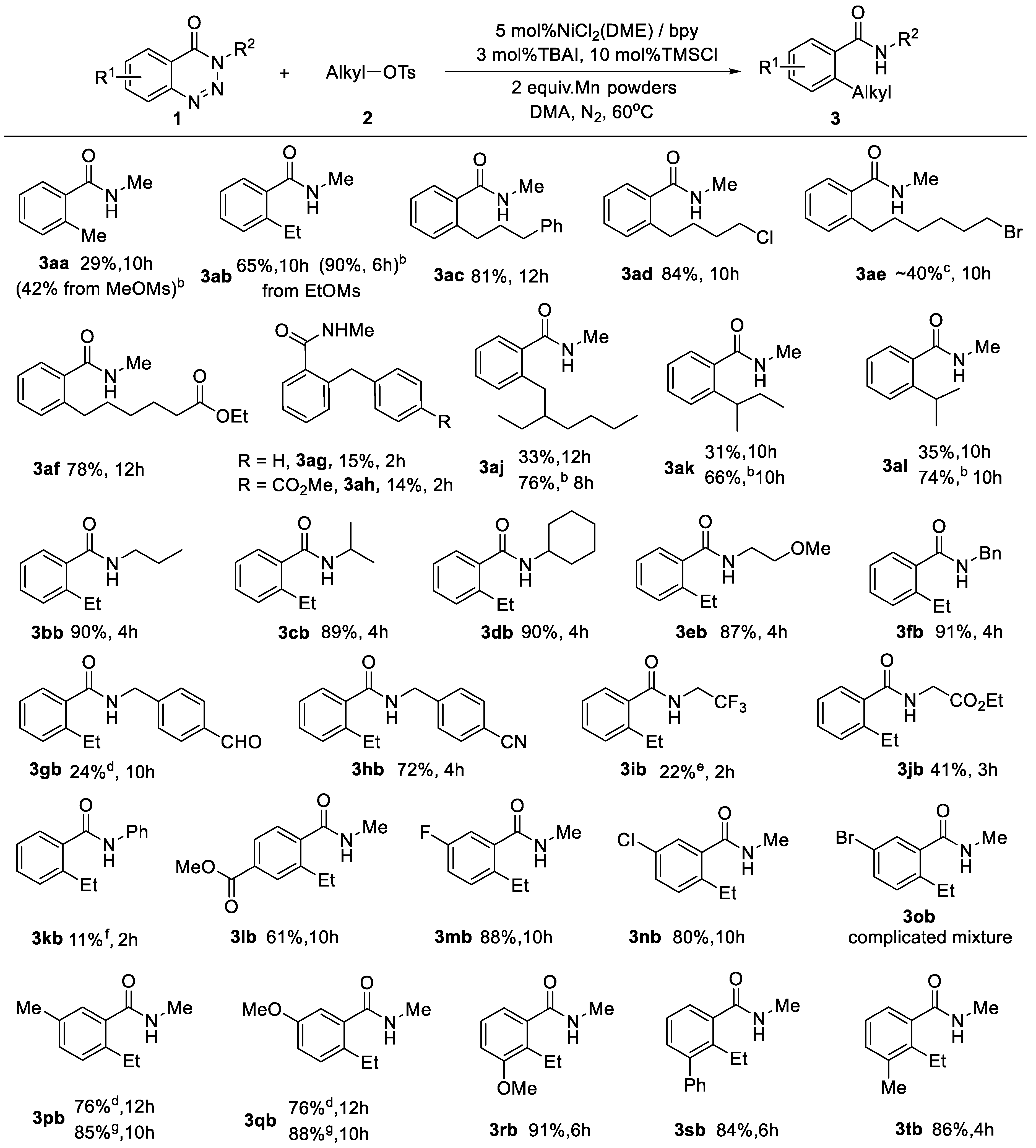
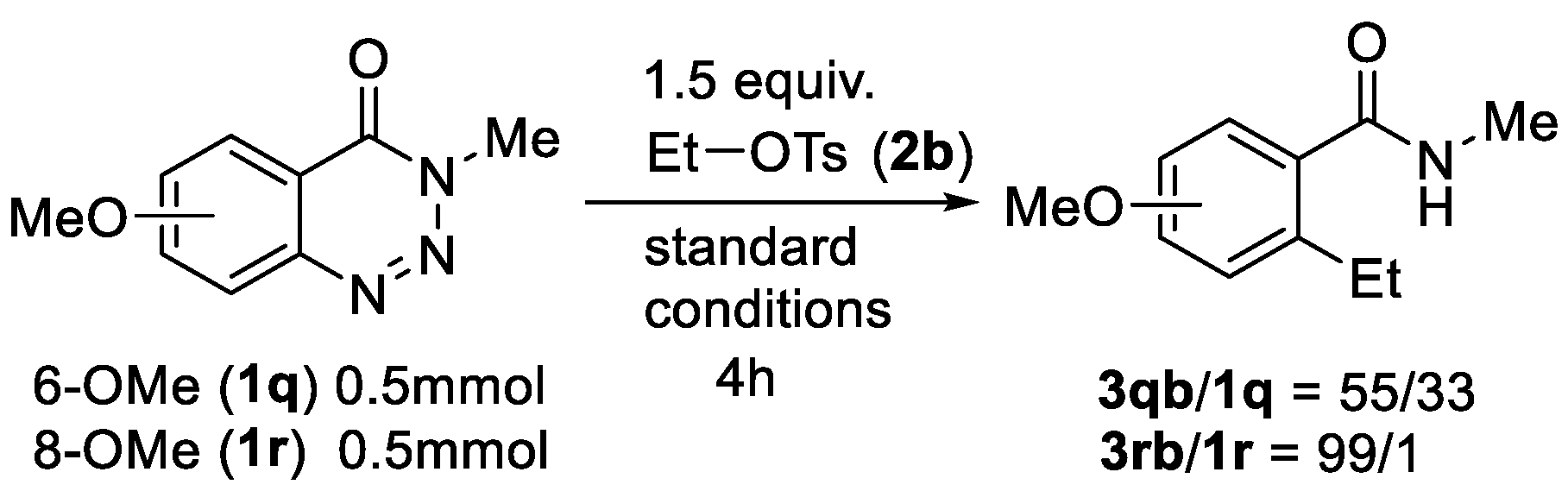
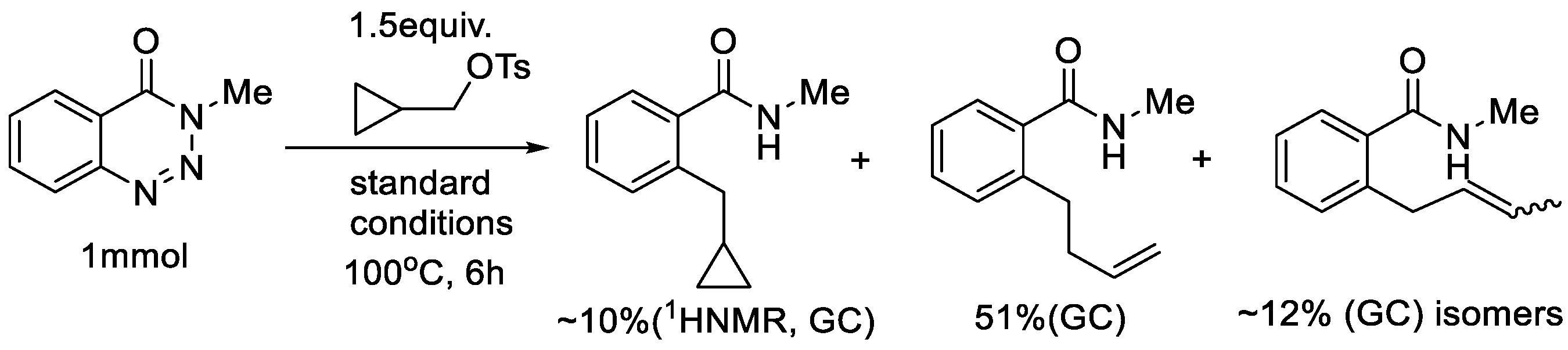
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Iodide/Nickel Co-Catalyzed Manganese-Mediated Denitrogenative Cross-Electrophile Coupling of Benzotriazinones with Alkyl Sulfonates
3.3. Procedure for Gram-Scale Synthesis of 2-Ethyl-N,3-Dimethylbenzamide (3tb)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knappke, C.E.I.; Grupe, S.; Gartner, D.; Corpet, M.; Gosmini, C.; Jacobi von Wangelin, A. Reductive cross-coupling reactions between two electrophiles. Chem. Eur. J. 2014, 20, 6828–6842. [Google Scholar] [CrossRef] [PubMed]
- Moragas, T.; Correa, A.; Martin, R. Metal-catalyzed reductive coupling reactions of organic halides with carbonyl-type compounds. Chem. Eur. J. 2014, 20, 8242–8258. [Google Scholar] [CrossRef] [PubMed]
- Weix, D.J. Methods and mechanisms for cross-electrophile coupling of Csp2 halides with alkyl electrophiles. Acc. Chem. Res. 2015, 48, 1767–1775. [Google Scholar] [CrossRef]
- Richmond, E.; Moran, J. Recent advances in nickel catalysis enabled by stoichiometric metallic reducing agents. Synthesis 2018, 50, 499–513. [Google Scholar] [CrossRef]
- Pang, X.; Peng, X.; Shu, X.-Z. Reductive cross-coupling of vinyl electrophiles. Synthesis 2020, 52, 3751–3763. [Google Scholar] [CrossRef]
- Diccianni, J.; Lin, Q.; Diao, T. Mechanisms of nickel-catalyzed coupling reactions and applications in alkene functionalization. Acc. Chem. Res. 2020, 53, 906–919. [Google Scholar] [CrossRef]
- Liu, J.; Ye, Y.; Sessler, J.L.; Gong, H. Cross-electrophile couplings of activated and sterically hindered halides and alcohol derivatives. Acc. Chem. Res. 2020, 53, 1833–1845. [Google Scholar] [CrossRef]
- Pang, X.; Su, P.-F.; Shu, X.-Z. Reductive cross-coupling of unreactive electrophiles. Acc. Chem. Res. 2022, 55, 2491–2509. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Shu, X.-Z. Reductive deoxygenative functionalization of alcohols by first-row transition metal catalysis. Chin. J. Chem. 2023, 41, 1637–1652. [Google Scholar] [CrossRef]
- Gong, Y.; Hu, J.; Qiu, C.; Gong, H. Insights into recent nickel-catalyzed reductive and redox C-C coupling of electrophiles, C(sp3)-H bonds and alkenes. Acc. Chem. Res. 2024, 57, 1149–1162. [Google Scholar] [CrossRef]
- Lei, J.; Yu, S.; Xu, Z.-G. Cross-electrophile couplings (XECs) between similar electrophile reagents. Chin. J. Chem. 2024, 42, 3518–3532. [Google Scholar] [CrossRef]
- Ehehalt, L.E.; Beleh, O.M.; Priest, I.C.; Mouat, J.M.; Olszewski, A.K.; Ahern, B.N.; Cruz, A.R.; Chi, B.K.; Castro, A.J.; Kang, K.; et al. Cross-electrophile coupling: Principles, methods, and applications in synthesis. Chem. Rev. 2024, 124, 13397–13569. [Google Scholar] [CrossRef]
- Everson, D.A.; Shrestha, R.; Weix, D.J. Nickel-catalyzed reductive cross-coupling of aryl halides with alkyl halides. J. Am. Chem. Soc. 2010, 132, 920–921. [Google Scholar] [CrossRef] [PubMed]
- Everson, D.A.; Jones, B.A.; Weix, D.J. Replacing conventional carbon nucleophiles with electrophiles: Nickel-catalyzed reductive alkylation of aryl bromides and chlorides. J. Am. Chem. Soc. 2012, 134, 6146–6159. [Google Scholar] [CrossRef]
- Yu, X.; Yang, T.; Wang, S.; Xu, H.; Gong, H. Nickel-catalyzed reductive cross-coupling of unactivated alkyl halides. Org. Lett. 2011, 13, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qian, Q.; Gong, H. Nickel-catalyzed reductive coupling of aryl halides with secondary alkyl bromides and allylic acetate. Org. Lett. 2012, 14, 3352–3355. [Google Scholar] [CrossRef]
- Durandetti, M.; Nédélec, J.-Y.; Périchon, J. Nickel-catalyzed direct electrochemical cross-coupling between aryl halides and activated alkyl halides. J. Org. Chem. 1996, 61, 1748–1755. [Google Scholar] [CrossRef]
- Durandetti, M.; Gosmini, C.; Périchon, J. Ni-catalyzed activation of α-chloroesters: A simple method for the synthesis of α-arylesters and β-hydroxyesters. Tetrahedron 2007, 63, 1146–1153. [Google Scholar] [CrossRef]
- Li, Z.; Sun, W.; Wang, X.; Li, L.; Zhang, Y.; Li, C. Electrochemically enabled, nickel-catalyzed dehydroxylative cross-coupling of alcohols with aryl halides. J. Am. Chem. Soc. 2021, 143, 3536–3543. [Google Scholar] [CrossRef]
- Xie, H.; Guo, J.; Wang, Y.-Q.; Wang, K.; Guo, P.; Su, P.-F.; Wang, X.; Shu, X.-Z. Radical dehydroxylative alkylation of tertiary alcohols by Ti catalysis. J. Am. Chem. Soc. 2020, 142, 16787–16794. [Google Scholar] [CrossRef]
- Dong, Z.; MacMillan, D.W.C. Metallaphotoredox-enabled deoxygenative arylation of alcohols. Nature 2021, 598, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, S.; Wang, Y.; Guo, P.; Shu, X.-Z. Ti-catalyzed reductive dehydroxylative vinylation of tertiary alcohols. ACS Catal. 2022, 12, 1018–1023. [Google Scholar] [CrossRef]
- Intermaggio, N.E.; Millet, A.; Davis, D.L.; MacMillan, D.W.C. Deoxytrifluoromethylation of alcohols. J. Am. Chem. Soc. 2022, 144, 11961–11968. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Kang, T.; Li, F.; Liao, H.; Yan, Y.; Dong, J.; Li, G.; Xue, D. Ni-catalyzed deoxygenative cross-coupling of alcohols with aryl chlorides via an organic photoredox process. ACS Catal. 2024, 14, 14089–14097. [Google Scholar] [CrossRef]
- Lin, Q.; Ma, G.; Gong, H. Ni-catalyzed formal cross-electrophile coupling of alcohols with aryl halides. ACS Catal. 2021, 11, 14102–14109. [Google Scholar] [CrossRef]
- Guo, P.; Wang, K.; Jin, W.-J.; Xie, H.; Qi, L.; Liu, X.-Y.; Shu, X.-Z. Dynamic kinetic cross-electrophile arylation of benzyl alcohols by nickel catalysis. J. Am. Chem. Soc. 2021, 143, 513–523. [Google Scholar] [CrossRef]
- Ma, W.-Y.; Han, G.-Y.; Kang, S.; Pang, X.; Liu, X.-Y.; Shu, X.-Z. Cobalt-catalyzed enantiospecific dynamic kinetic cross-electrophile vinylation of allylic alcohols with vinyl triflates. J. Am. Chem. Soc. 2021, 143, 15930–15935. [Google Scholar] [CrossRef]
- Chi, B.K.; Widness, J.K.; Gilbert, M.M.; Salgueiro, D.C.; Garcia, K.J.; Weix, D.J. In-situ bromination enables formal cross-electrophile coupling of alcohols with aryl and alkenyl halides. ACS Catal. 2022, 12, 580–586. [Google Scholar] [CrossRef]
- Ahern, B.N.; Weix, D.J. One-pot chlorination and cross-electrophile coupling of alcohols with aryl chlorides. Org. Lett. 2025, 27, 1164–1169. [Google Scholar] [CrossRef]
- Duan, J.; Du, Y.-F.; Pang, X.; Shu, X.-Z. Ni-catalyzed cross-electrophile coupling between vinyl/aryl and alkyl sulfonates: Synthesis of cycloalkenes and modification of peptides. Chem. Sci. 2019, 10, 8706–8712. [Google Scholar] [CrossRef]
- Liang, Z.; Xue, W.; Lin, K.; Gong, H. Nickel-catalyzed reductive methylation of alkyl halides and acid chlorides with methyl p-tosylate. Org. Lett. 2014, 16, 5620–5623. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J.; Gong, H. Nickel-catalyzed methylation of aryl halides/tosylates with methyl tosylate. Chem. Commun. 2017, 53, 10180–10183. [Google Scholar] [CrossRef]
- Komeyama, K.; Michiyuki, T.; Osaka, I. Nickel/cobalt-catalyzed C(sp3)-C(sp3) cross-coupling of alkyl halides with alkyl tosylates. ACS Catal. 2019, 9, 9285–9291. [Google Scholar] [CrossRef]
- Anka-Lufford, L.L.; Prinsell, M.R.; Weix, D.J. Selective cross-coupling of organic halides with allylic acetates. J. Org. Chem. 2012, 77, 9989–10000. [Google Scholar] [CrossRef]
- He, R.-D.; Bai, Y.; Han, G.-Y.; Zhao, Z.-Z.; Pang, X.; Pan, X.; Liu, X.-Y.; Shu, X.-Z. Reductive alkylation of alkenyl acetates with alkyl bromides by nickel catalysis. Angew. Chem. Int. Ed. 2022, 61, e202114556. [Google Scholar] [CrossRef] [PubMed]
- Konev, M.O.; Hanna, L.E.; Jarvo, E.R. Intra- and intermolecular nickel-catalyzed reductive cross-electrophile coupling reactions of benzylic esters with aryl halides. Angew. Chem. Int. Ed. 2016, 55, 6730–6733. [Google Scholar] [CrossRef]
- Tao, X.; Chen, Y.; Guo, J.; Wang, X.; Gong, H. Preparation of α-amino acids via Ni-catalyzed reductive vinylation and arylation of α-pivaloyloxy glycine. Chem. Sci. 2021, 12, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-B.; Li, C.-L.; Jin, W.-J.; Guo, P.; Shu, X.-Z. Reductive coupling of benzyl oxalates with highly functionalized alkyl bromides by nickel catalysis. Chem. Sci. 2018, 9, 4529–4534. [Google Scholar] [CrossRef]
- Gao, M.; Sun, D.; Gong, H. Ni-catalyzed reductive C-O bond arylation of oxalates derived from α-hydroxy esters with aryl halides. Org. Lett. 2019, 21, 1645–1648. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, H.; Sessler, J.L.; Gong, H. Zn-mediated fragmentation of tertiary alkyl oxalates enabling formation of alkylated and arylated quaternary carbon centers. J. Am. Chem. Soc. 2019, 141, 820–824. [Google Scholar] [CrossRef]
- He, R.-D.; Li, C.-L.; Pan, Q.-Q.; Guo, P.; Liu, X.-Y.; Shu, X.-Z. Reductive coupling between C–N and C–O electrophiles. J. Am. Chem. Soc. 2019, 141, 12481–12486. [Google Scholar] [CrossRef] [PubMed]
- Doraghi, F.; Karimian, S.; Larijani, B.; Mahdavi, M. Recent advances in synthesis and transformations of 1,2,3-benzotriazinones. J. Organomet. Chem. 2024, 1013, 123156. [Google Scholar] [CrossRef]
- Wang, F.; Tong, Y.; Zou, G. Nickel-catalyzed, manganese-assisted denitrogenative cross-electrophile-coupling of benzotriazinones with alkyl halides for ortho-alkylated benzamides. Org. Lett. 2022, 24, 5741–5745. [Google Scholar] [CrossRef]
- Wang, H.; Ding, W.; Zou, G. Mechanoredox/nickel co-catalyzed cross electrophile coupling of benzotriazinones with alkyl (pseudo)halides. J. Org. Chem. 2023, 88, 12891–12901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hong, Y.; Zou, G. Liquid-assisted grinding enables efficient Ni-catalyzed, Mn-mediated denitrogenative cross-electrophile coupling of benzotriazinones with benzyl chlorides. Molecules 2025, 30, 1060. [Google Scholar] [CrossRef]
- Xü, M.; Xü, K.; Zou, G. Improved palladium catalysis in Suzuki-type coupling of benzotriazinones for o-aryl/alkenyl benzamides. Adv. Synth. Catal. 2024, 366, 2623–2628. [Google Scholar] [CrossRef]
- Wang, F.; Hong, Y.; Zhang, X.; Zou, G. A sequential olefin hydroboration/Suzuki coupling for denitrogenative alkylation of benzotriazinones with α-olefins. J. Organomet. Chem. 2024, 1022, 123383. [Google Scholar] [CrossRef]
- Lin, T.; Wang, Y.-E.; Cui, N.; Li, M.; Wang, R.; Bai, J.; Fan, Y.; Xiong, D.; Xue, F.; Walsh, P.J.; et al. Nickel-catalyzed cross-electrophile coupling of 1,2,3-Benzotriazin-4(3H)-ones with aryl bromides. J. Org. Chem. 2022, 87, 16567–16577. [Google Scholar] [CrossRef]
- Barreiro, E.J.; Kummerle, A.E.; Fraga, C.A. The methylation effect in medicinal chemistry. Chem. Rev. 2011, 111, 5215–5246. [Google Scholar] [CrossRef]
- Schönherr, H.; Cernak, T. Profound methyl effects in drug discovery and a call for new C-H methylation reactions. Angew. Chem. Int. Ed. 2013, 52, 12256–12267. [Google Scholar] [CrossRef]
- Pinheiro, P.d.S.M.; Franco, L.S.; Fraga, C.A.M. The magic methyl and its tricks in drug discovery and development. Pharmaceuticals 2023, 16, 1157–1179. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G.; Figdore, P.E. Relative reactivities of methyl iodide and methyl tosylate with transition-metal nucleophiles. J. Am. Chem. Soc. 1980, 102, 1541–1547. [Google Scholar] [CrossRef]
- Cao, J.; Ding, W.; Zou, G. Tetrabutylammonium bromide (TBAB)-promoted, Pd/Cu-catalyzed Sonogashira coupling of N-tosyl aryltriazenes. Org. Lett. 2024, 26, 4576–4580. [Google Scholar] [CrossRef]
- Dawson, G.A.; Spielvogel, E.H.; Diao, T. Nickel-catalyzed radical mechanisms: Informing cross-coupling for synthesizing non-canonical biomolecules. Acc. Chem. Res. 2023, 56, 3640–3653. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Spielvogel, E.H.; Diao, T. Carbon-centered radical capture at nickel(II) complexes: Spectroscopic evidence, rates, and selectivity. Chem 2023, 9, 1295–1308. [Google Scholar] [CrossRef]
- Ju, L.; Hu, C.T.; Diao, T. Strategies for promoting reductive elimination of bi- and bis-oxazoline ligated organonickel complexes. Organometallics 2022, 41, 1748–1753. [Google Scholar] [CrossRef]
- Wu, K.; Doyle, A.G. Parameterization of phosphine ligands demonstrates enhancement of nickel catalysis via remote steric effects. Nat. Chem 2017, 9, 779–784. [Google Scholar] [CrossRef]
- Newman-Stonebraker, S.H.; Smith, S.R.; Borowski, J.E.; Peters, E.; Gensch, T.; Johnson, H.C.; Sigman, M.S.; Doyle, A.G. Univariate classification of phosphine ligation state and reactivity in cross-coupling catalysis. Science 2021, 374, 301–308. [Google Scholar] [CrossRef]
- Sato, T.; Yoshida, T.; Al Mamari, H.H.; Ilies, L.; Nakamura, E. Manganese-catalyzed directed methylation of C(sp2)-H bonds at 25 °C with high catalytic turnover. Org. Lett. 2017, 19, 5458–5461. [Google Scholar] [CrossRef]
- Havlik, S.E.; Simmons, J.M.; Winton, V.J.; Johnson, J.B. Nickel-mediated decarbonylative cross-coupling of phthalimides with in situ generated diorganozinc reagents. J. Org. Chem. 2011, 76, 3588–3593. [Google Scholar] [CrossRef]
- Nozawa-Kumada, K.; Ono, K.; Kurosu, S.; Shigeno, M.; Kondo, Y. Copper-catalyzed aerobic benzylic C(sp3)-H lactonization of 2-alkylbenzamides via N-centered radicals. Org. Biomol. Chem. 2022, 20, 5948–5952. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Zhu, R.; Bai, J.; Shen, Y.; Pan, M.; Li, W. Synthesis of ortho-methylated benzamides via palladium-catalyzed denitrogenative cross-coupling reaction of [1–3]-benzotriazin-4(3H)-ones with DABAL-Me3. Org. Lett. 2023, 25, 5443–5447. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ilies, L.; Yoshikai, N.; Nakamura, E. Cobalt-catalyzed coupling of alkyl Grignard reagent with Benzamide and 2-phenylpyridine derivatives through directed C-H bond activation under air. Org. Lett. 2011, 13, 3232–3234. [Google Scholar] [CrossRef] [PubMed]
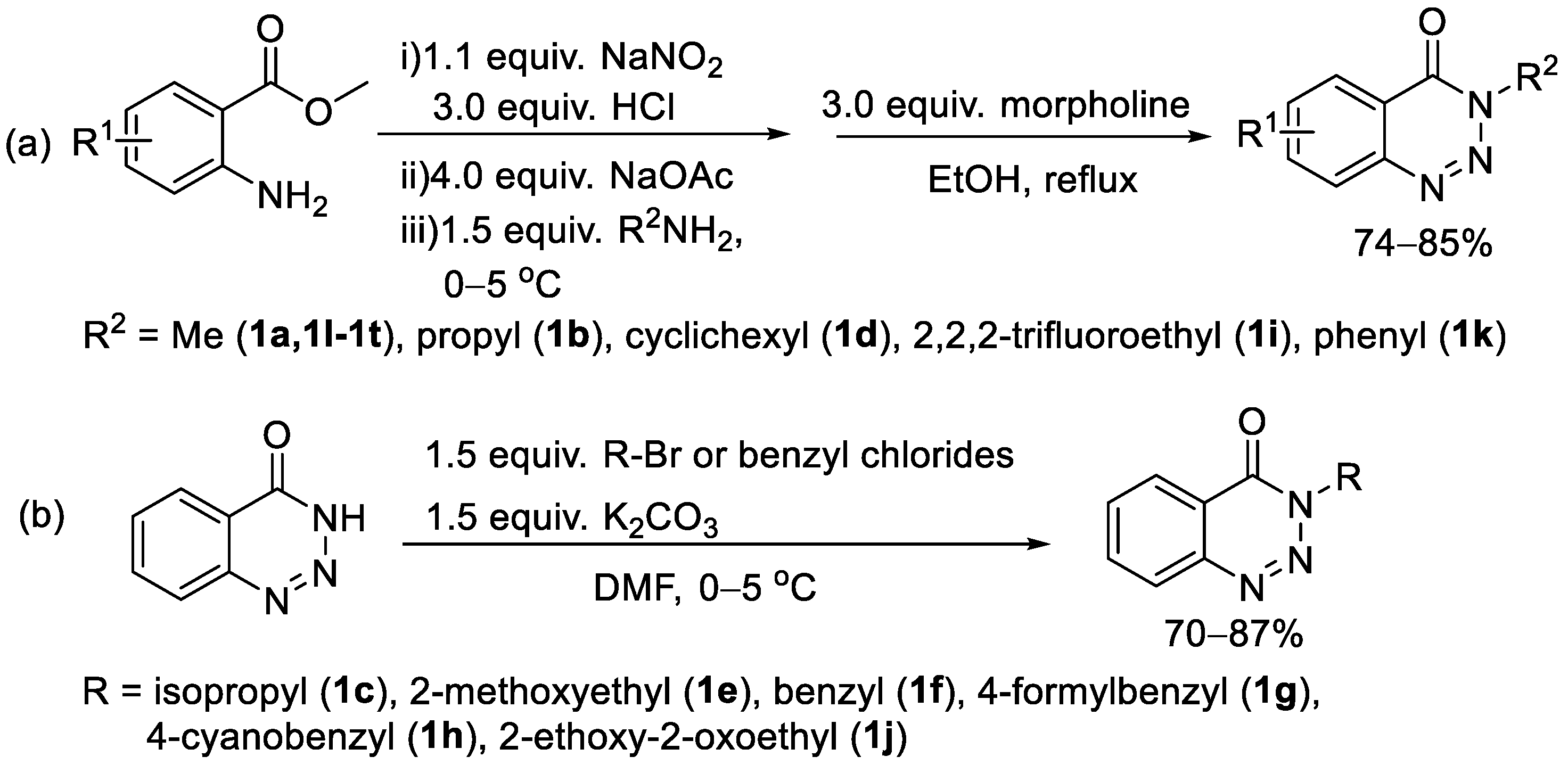

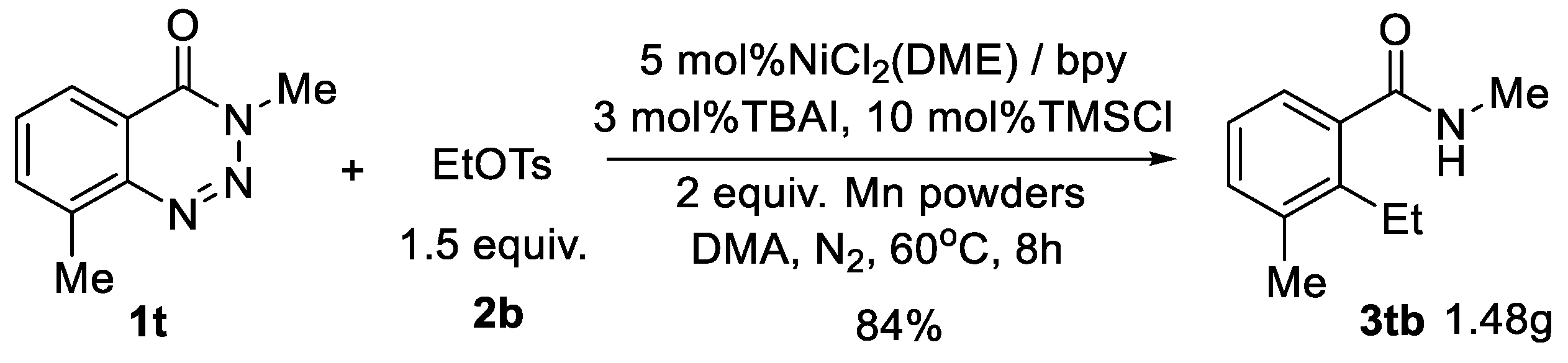

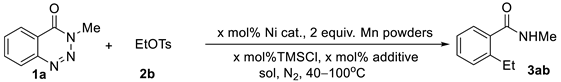 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Cat. (mol%) | mol% TMSCl | Add. (mol%) | Sol. | T (℃) | Time (h) | Yield (%) b |
| 1 | NiCl2(bpy) (10) | 25 | KI (10) | DMA | 100 | 2 | 76 |
| 2 | NiCl2(bpy) (10) | 25 | NaI (10) | DMA | 100 | 2 | 74 |
| 3 | NiCl2(bpy) (10) | 25 | / | DMA | 100 | 4 | 20 |
| 4 | NiCl2(bpy) (10) | 25 | KBr (10) | DMA | 100 | 4 | 36 |
| 5 | NiCl2(bpy) (10) | 25 | TBAB (10) | DMA | 100 | 4 | 41 |
| 6 | NiCl2(bpy) (10) | 25 | TBAI (10) | DMA | 100 | 1 | 86 |
| 7 | NiCl2(bpy) (10) | 25 | TBAI (3) | DMA | 100 | 1 | 83 |
| 8 | NiCl2(bpy) (10) | 25 | TBAI (1) | DMA | 100 | 3 | 77 |
| 9 | NiCl2(bpy) (10) | 25 | TBAI (3) | DMA | 80 | 2 | 85 |
| 10 | NiCl2(bpy) (10) | 25 | TBAI (3) | DMA | 60 | 4 | 86 |
| 11 | NiCl2(bpy) (10) | 25 | TBAI (3) | DMA | 40 | 10 | 56 c |
| 12 | NiCl2(bpy) (5) | 25 | TBAI (3) | DMA | 60 | 10 | 86 |
| 13 | NiCl2(bpy) (3) | 25 | TBAI (3) | DMA | 60 | 14 | 74 c |
| 14 | NiCl2/bpy (5) | 25 | TBAI (3) | DMA | 60 | 10 | 85 |
| 15 | Ni(OAc)2/bpy (5) | 25 | TBAI (3) | DMA | 60 | 12 | 70 c |
| 16 | Ni(acac)2/bpy (5) | 25 | TBAI (3) | DMA | 60 | 12 | 68 c |
| 17 | NiCl2(DME)/bpy (5) | 25 | TBAI (3) | DMA | 60 | 10 | 89 |
| 18 | NiCl2(PPh3)2(5) d | 25 | TBAI (3) | DMA | 60 | 14 | trace |
| 19 | NiCl2(DME)/bpy (3) | 25 | TBAI (3) | DMA | 60 | 14 | 85 |
| 20 | NiCl2(DME)/dtbbpy(3) | 25 | TBAI (3) | DMA | 60 | 14 | 26 c |
| 21 | NiCl2(DME)/phen (3) | 25 | TBAI (3) | DMA | 60 | 14 | 71 c |
| 22 | NiCl2(DME)/bpy (5) | 25 | TBAI (3) | DMF | 60 | 12 | 74 |
| 23 | NiCl2(DME)/bpy (5) | 25 | TBAI (3) | Sol.e | 60 | 12 | NR |
| 24 | NiCl2(DME)/bpy (5) | 50 | TBAI (3) | DMA | 60 | 8 | 65 c |
| 25 | NiCl2(DME)/bpy (5) | 10 | TBAI (3) | DMA | 60 | 10 | 88 |
| 26 | NiCl2(DME)/bpy (5) | 5 | TBAI (3) | DMA | 60 | 12 | 83 c |
| 27 | NiCl2(DME)/bpy (5) | / | TBAI (3) | DMA | 60 | 12 | 36 c |
| 28 | NiCl2(DME)/bpy (5) | 10 | TBAI (3) | DMA | 60 | 10 | 72 c,f |
| 29 | NiCl2(DME)/bpy (5) | 10 | TBAI (3) | DMA | 60 | 8 | 89 g |
| 30 | NiCl2(DME)/bpy (5) | 10 | TBAI (3) | DMA | 60 | 10 | 86 h |
| 31 | NiCl2(DME)/bpy (5) | 10 | TBAI (3) | DMA | 60 | 10 | 81 i |
| 32 | NiCl2(DME)/bpy (5) | 10 | TBAI (3) | DMA | 60 | 8 | NR j |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Zhang, X.; Zou, G. Iodide/Nickel Co-Catalyzed Manganese-Mediated Denitrogenative Cross-Electrophile Coupling of Benzotriazinones with Alkyl Sulfonates. Molecules 2025, 30, 2397. https://doi.org/10.3390/molecules30112397
Hong Y, Zhang X, Zou G. Iodide/Nickel Co-Catalyzed Manganese-Mediated Denitrogenative Cross-Electrophile Coupling of Benzotriazinones with Alkyl Sulfonates. Molecules. 2025; 30(11):2397. https://doi.org/10.3390/molecules30112397
Chicago/Turabian StyleHong, Yingying, Xuanxuan Zhang, and Gang Zou. 2025. "Iodide/Nickel Co-Catalyzed Manganese-Mediated Denitrogenative Cross-Electrophile Coupling of Benzotriazinones with Alkyl Sulfonates" Molecules 30, no. 11: 2397. https://doi.org/10.3390/molecules30112397
APA StyleHong, Y., Zhang, X., & Zou, G. (2025). Iodide/Nickel Co-Catalyzed Manganese-Mediated Denitrogenative Cross-Electrophile Coupling of Benzotriazinones with Alkyl Sulfonates. Molecules, 30(11), 2397. https://doi.org/10.3390/molecules30112397







