W/O/W Multiple Emulsified Microcapsules Based on Biopolymer Soybean Isolate Proteins: Improving Tannic Acid’s Biocompatibility and Sustained-Release Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of the Volume Ratio Between the Outer Water Phase (W2) and the W1/O Emulsion on the Multiple Structure and Stability of TA Composite Emulsion
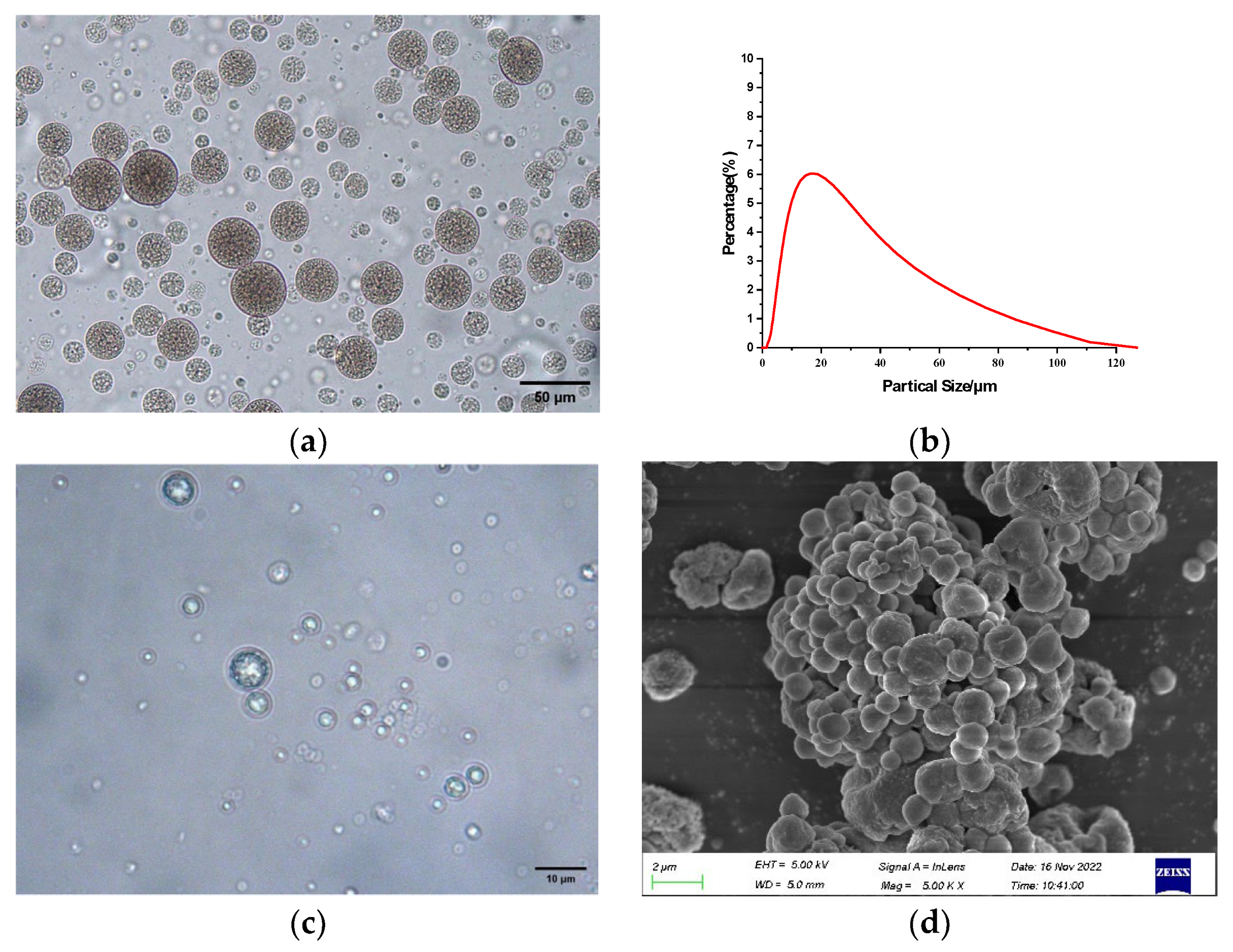
2.2. Internal Structure, Surface Morphology, and Particle Size Distribution of Microcapsules
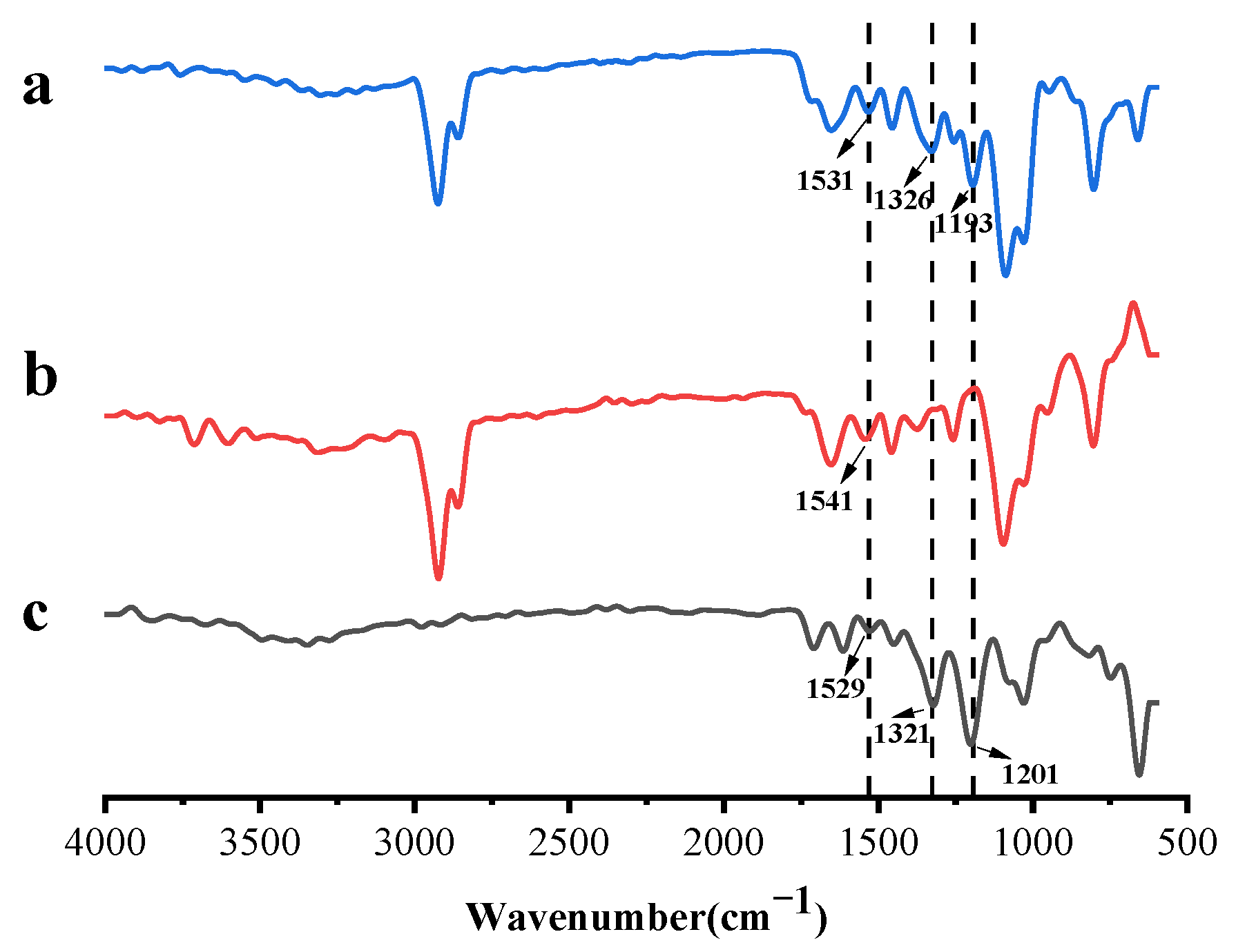
2.3. Fourier Transform Infrared (FTIR) Spectroscopy
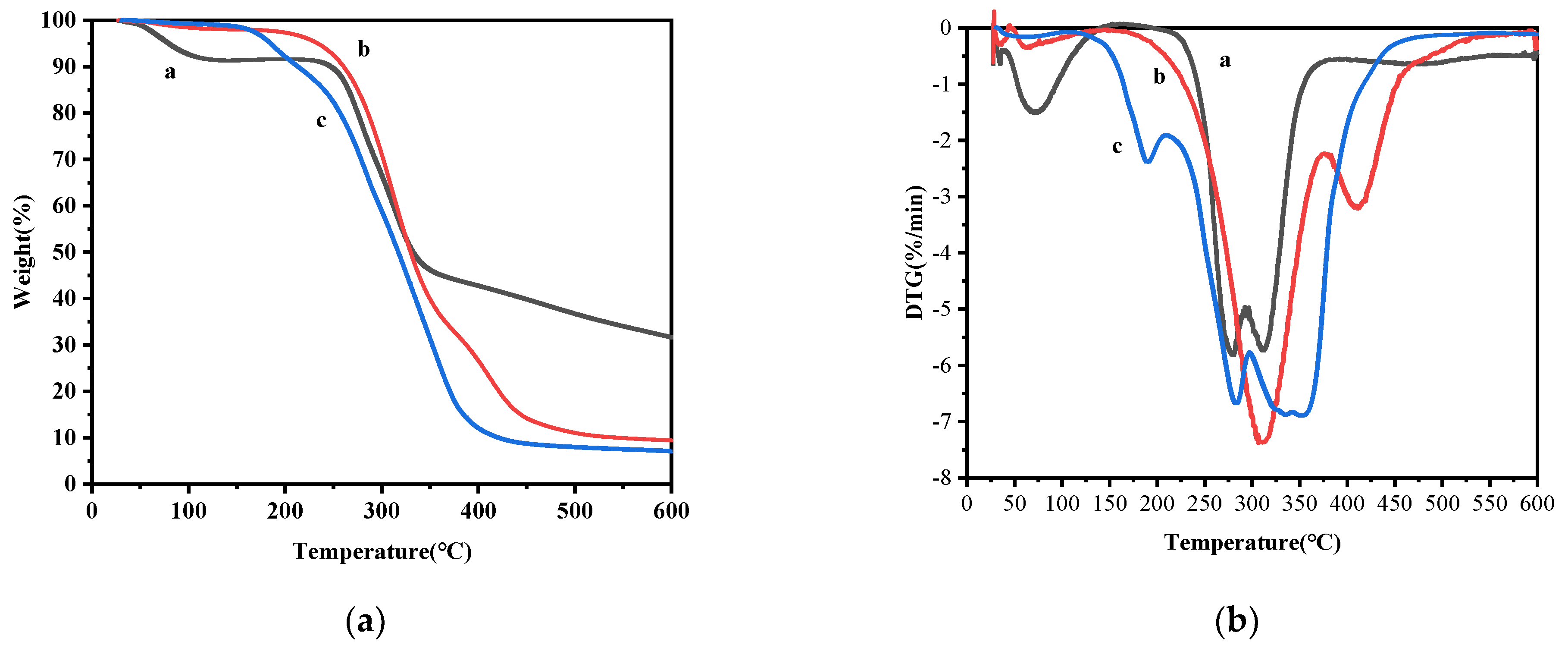
2.4. Thermal Stability Analysis
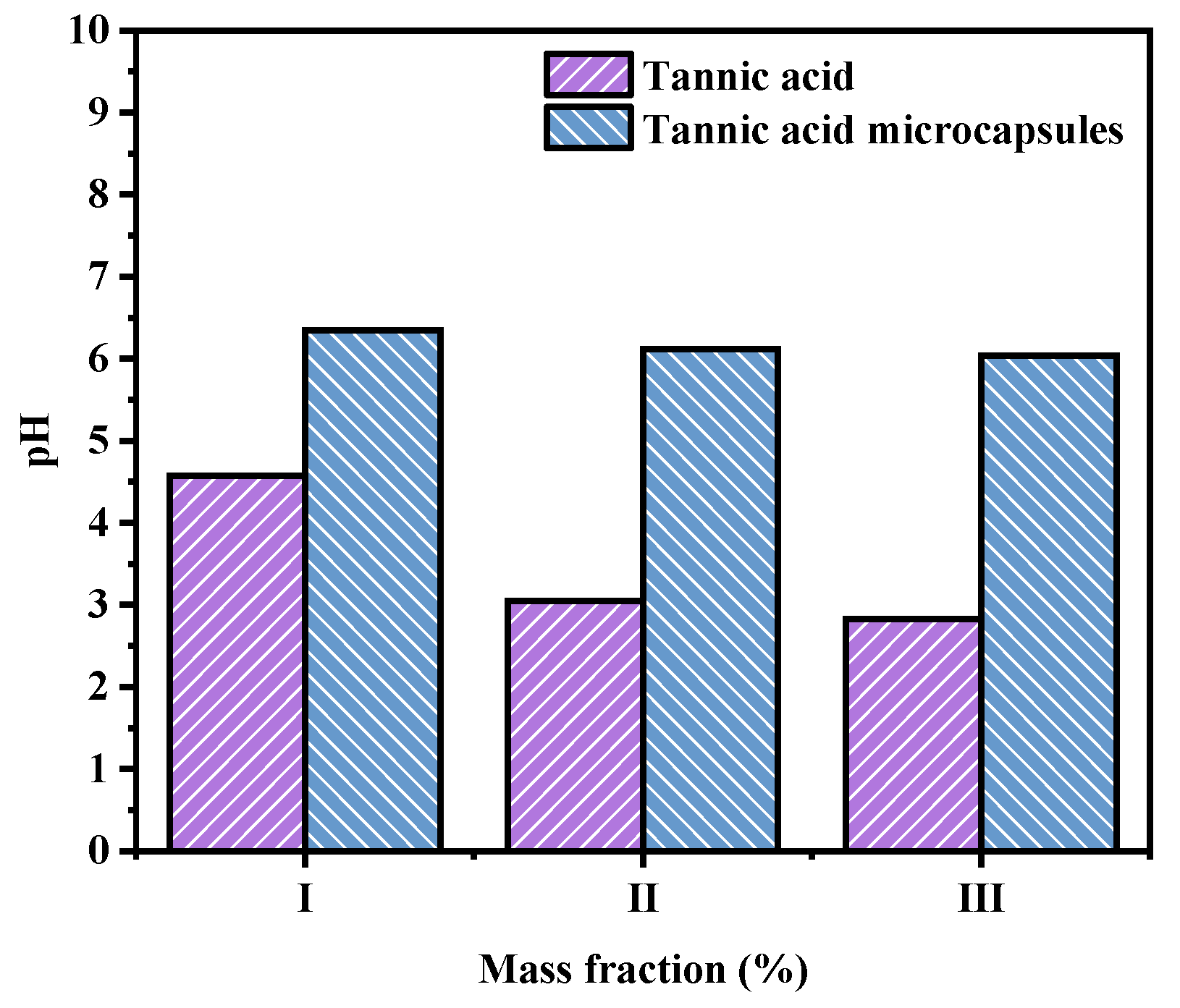
2.5. Effect of TA Microcapsules on Solution pH
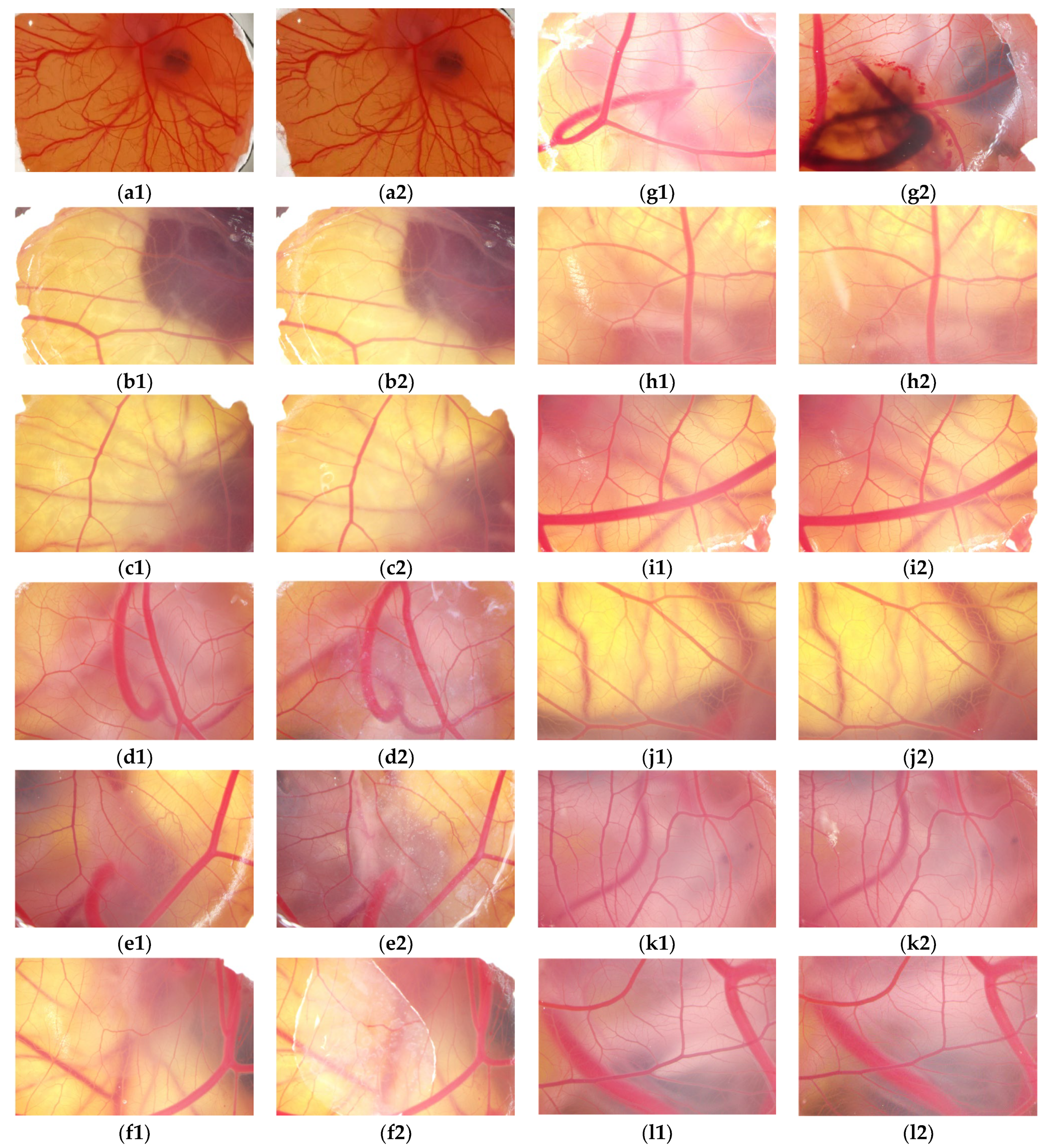
2.6. The HET-CAM Test of TA and TA Microcapsules
2.7. The STE Test of TA and TA Microcapsules
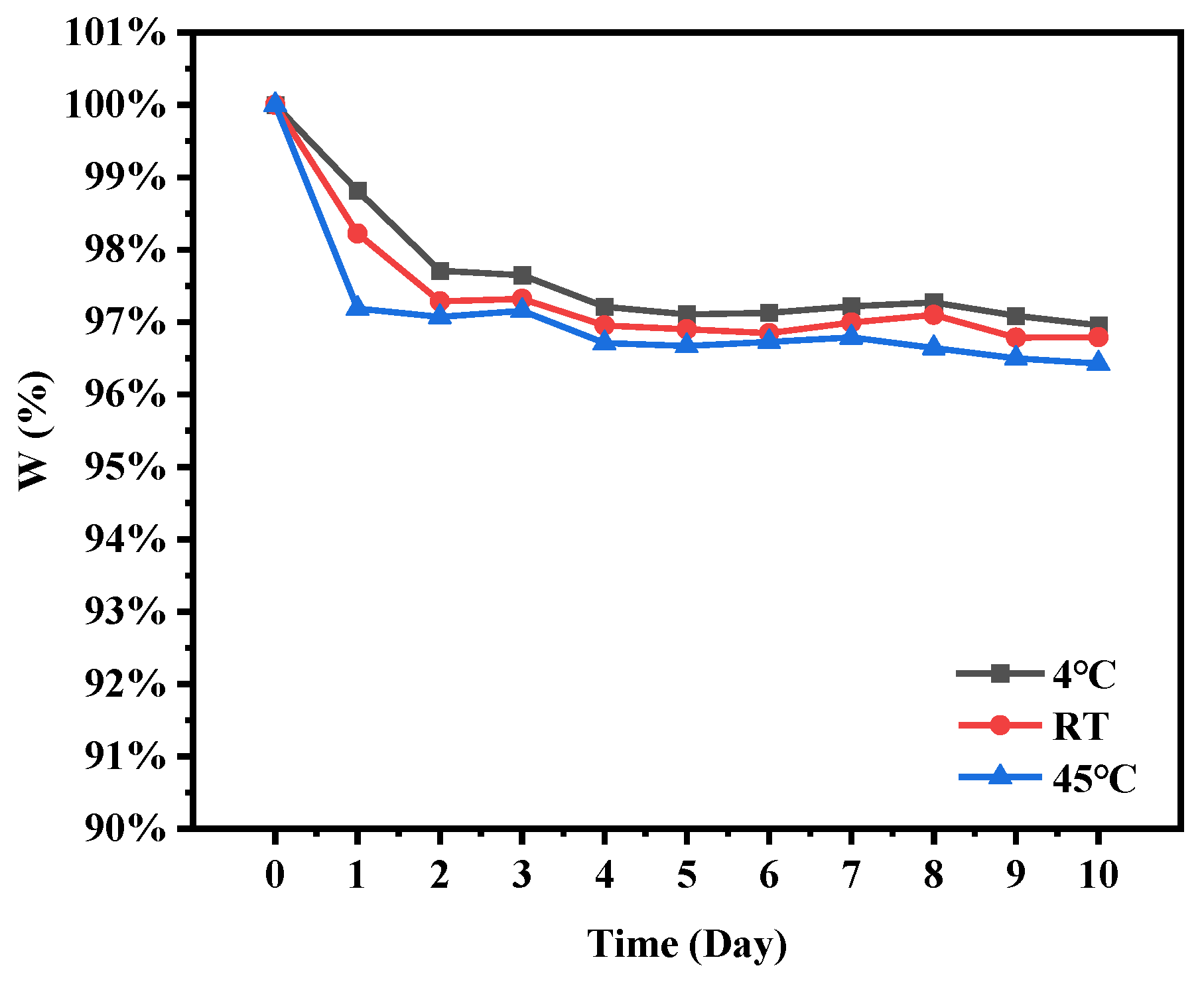
2.8. Storage Stability of Microcapsules
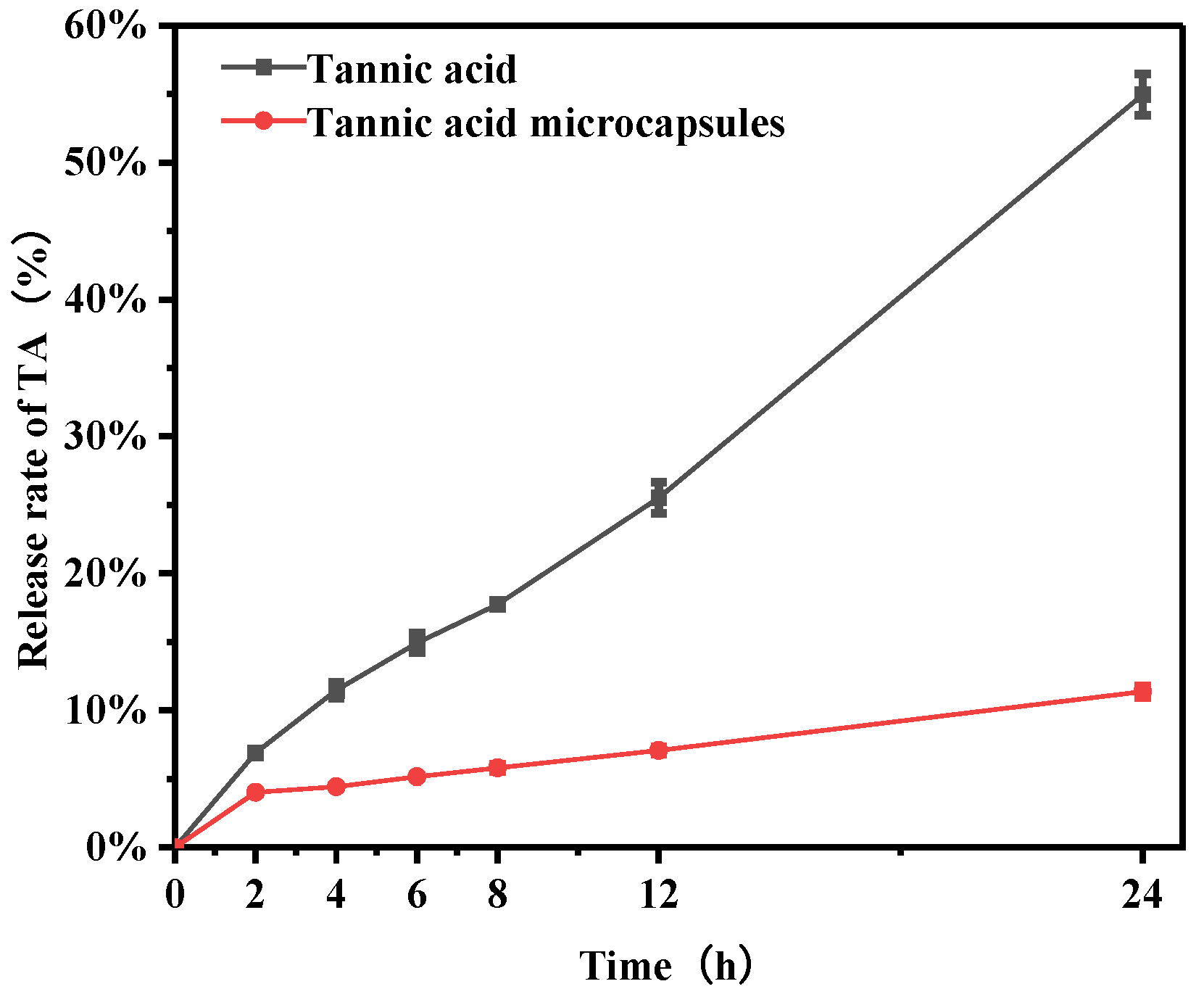
2.9. In Vitro Release Behavior Experiment
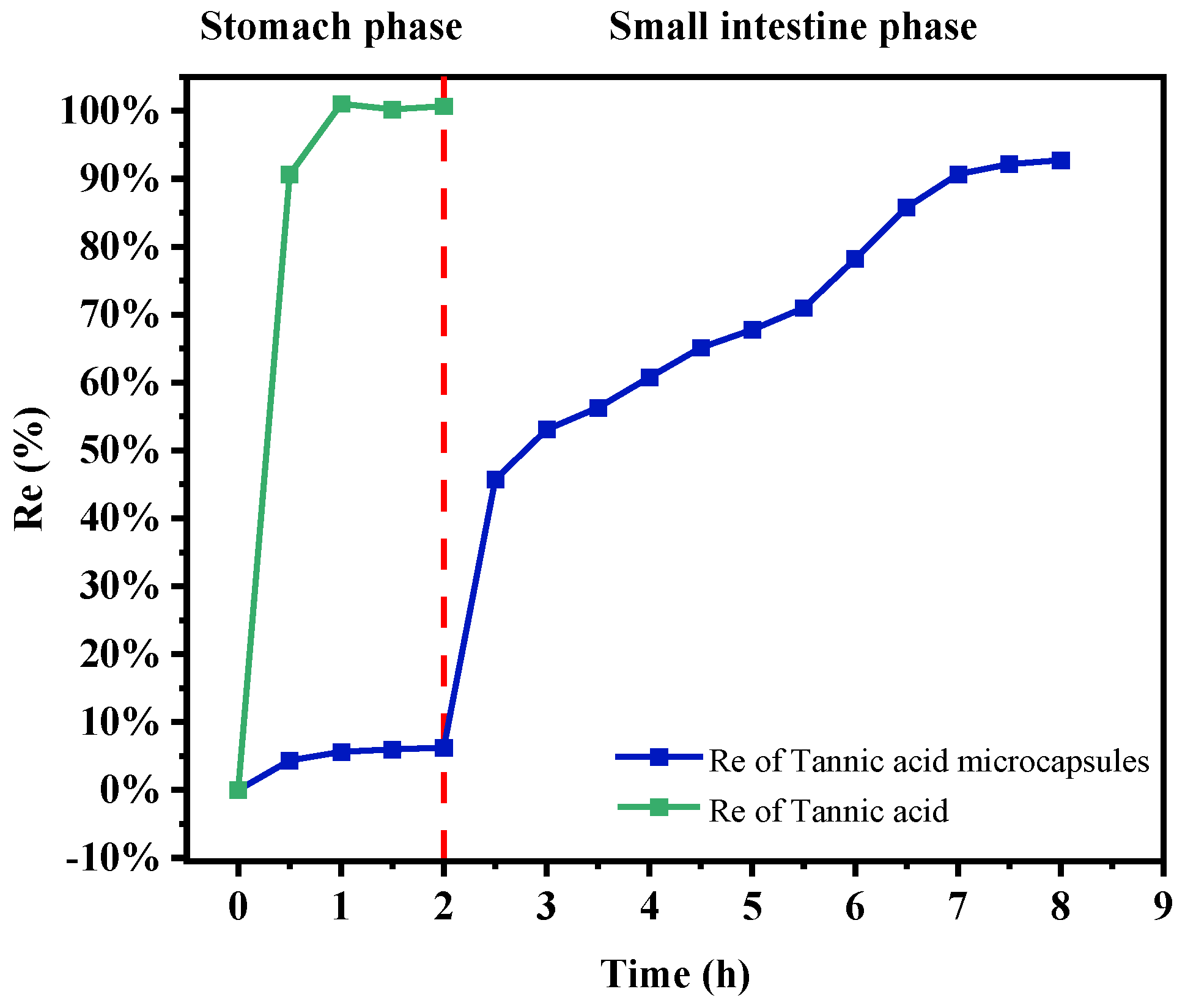
2.10. In Vitro Simulated Digestion and Release of TA Microcapsules
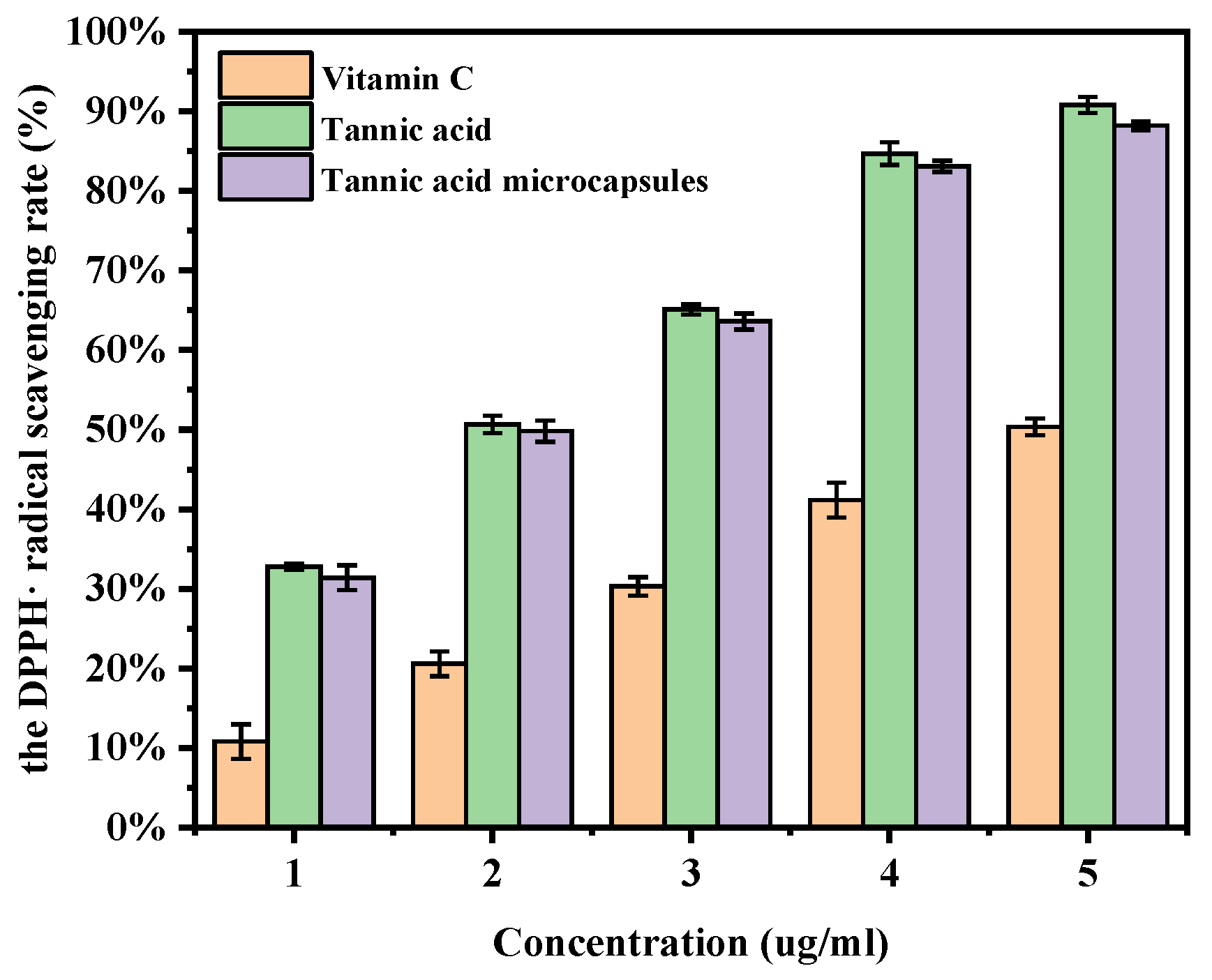
2.11. DPPH Radical Scavenging Assay
3. Materials and Methods
3.1. Experimental Materials
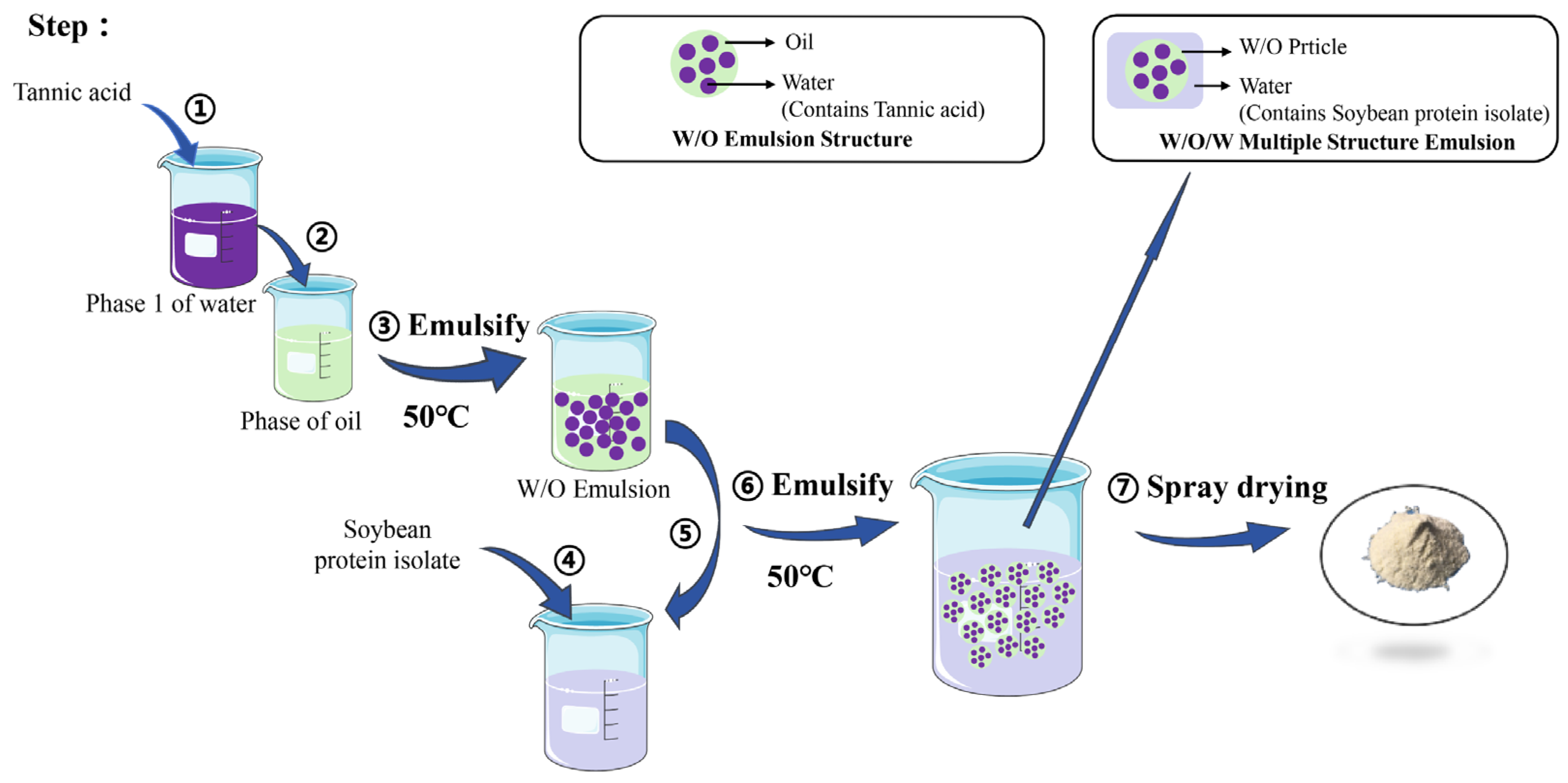
3.2. Preparation of Multiple Emulsified TA Microcapsules
3.3. Effect of the Volume Ratio Between the Outer Water Phase (W2) and the W1/O Emulsion on the Multiple Structure and Stability of TA Composite Emulsion
3.4. Determination of the Encapsulation Efficiency
3.5. Internal Structure, Surface Morphology, and Particle Size Distribution of Microcapsules
3.6. FTIR Characterization
3.7. Thermogravimetric Analysis (TGA)
3.8. Measurement of System pH Changes
3.9. The HET-CAM Test
3.10. Short Time Exposure In Vitro Test Method (STE)
3.11. Storage Stability of Microcapsules
3.12. In Vitro Release Behavior Test
3.13. In Vitro Simulated Digestion of Tannic Acid Microcapsules
3.14. DPPH Radical Scavenging Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baldwin, A.; Booth, B.W. Biomedical applications of tannic acid. J. Biomater. Appl. 2022, 36, 1503–1523. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yoshida, N.; Yasoshima, M.; Kojima, Y. Effect of tannic acid on skin barrier function. Exp. Dermatol. 2017, 27, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Bouki, E.; Dimitriadis, V.K.; Kaloyianni, M.; Dailianis, S. Antioxidant and pro-oxidant challenge of tannic acid in mussel hemocytes exposed to cadmium. Mar. Environ. Res. 2013, 85, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yuan, S.; Yang, F.; Yu, H.; Xie, Y.; Guo, Y.; Yao, W. Characterization of the interaction between boscalid and tannic acid and its effect on the antioxidant properties of tannic acid. J. Food Sci. 2023, 88, 1325–1335. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, L.; Yang, F.; Tong, W.; Jia, R.; Zou, Y.; Yin, L.; Li, L.; He, C.; Liang, X.; et al. Tannic Acid Accelerates Cutaneous Wound Healing in Rats Via Activation of the ERK 1/2 Signaling Pathways. Adv. Wound Care 2019, 8, 341–354. [Google Scholar] [CrossRef]
- Sharma, V.; Paramasivam, G.; Vergaelen, M.; Hoogenboom, R.; Sundaramurthy, A. Tannic Acid-Stabilized Self-Degrading Temperature-Sensitive Poly(2-n-propyl-2-oxazoline)/Gellan Gum Capsules for Lipase Delivery. ACS Appl. Bio Mater. 2021, 4, 7134–7146. [Google Scholar] [CrossRef]
- Ghasemian, M.; Kazeminava, F.; Naseri, A.; Mohebzadeh, S.; Abbaszadeh, M.; Kafil, H.S.; Ahmadian, Z. Recent progress in tannic acid based approaches as a natural polyphenolic biomaterial for cancer therapy: A review. Biomed. Pharmacother. 2023, 166, 115328. [Google Scholar] [CrossRef]
- Youness, R.A.; Kamel, R.; Elkasabgy, N.; Shao, P.; Farag, M.A. Recent Advances in Tannic Acid (Gallotannin) Anticancer Activities and Drug Delivery Systems for Efficacy Improvement; A Comprehensive Review. Molecules 2021, 26, 1486. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, Z.; Yang, X. Whey protein-tannic acid conjugate stabilized high internal phase Pickering emulsions: Interfacial stability based on covalent crosslinking. Colloids Surf. A Physicochem. Eng. Asp. 2023, 672, 131690. [Google Scholar] [CrossRef]
- Chen, L.; Gnanaraj, C.; Arulselvan, P.; El-Seedi, H.; Teng, H. A review on advanced microencapsulation technology to enhance bioavailability of phenolic compounds: Based on its activity in the treatment of Type 2 Diabetes. Trends Food Sci. Technol. 2019, 85, 149–162. [Google Scholar] [CrossRef]
- Farha, A.K.; Yang, Q.-Q.; Kim, G.; Li, H.-B.; Zhu, F.; Liu, H.-Y.; Gan, R.-Y.; Corke, H. Tannins as an alternative to antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, B. Nature-Inspired One-Step Green Procedure for Enhancing the Antibacterial and Antioxidant Behavior of a Chitin Film: Controlled Interfacial Assembly of Tannic Acid onto a Chitin Film. J. Agric. Food Chem. 2016, 64, 5736–5741. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, I.; Costa, R.; Madureira, S.; Borges, S.; Oliveira, A.L.; Pintado, M.; Baptista-Silva, S. Tannic Acid Tailored-Made Microsystems for Wound Infection. Int. J. Mol. Sci. 2023, 24, 4826. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Duan, X.; Sun, H.; Fan, X.; Wang, H.; Wang, W. Encapsulation of TA in edible nanofibrous mat improves antioxidant efficiency and their modulation of fatty acids profile in flaxseed oil. Int. J. Food Sci. Technol. 2022, 57, 7862–7871. [Google Scholar] [CrossRef]
- Liang, X.; Cao, K.; Li, W.; Li, X.; McClements, D.J.; Hu, K. Tannic acid-fortified zein-pectin nanoparticles: Stability, properties, antioxidant activity, and in vitro digestion. Food Res. Int. 2021, 145, 110425. [Google Scholar] [CrossRef]
- Tao, X.; Shi, H.; Cao, A.; Cai, L. Influence of polyphenol-metal ion-coated ovalbumin/sodium alginate composite nanoparticles on the encapsulation of kaempferol/tannin acid. Int. J. Biol. Macromol. 2022, 209, 1288–1297. [Google Scholar] [CrossRef]
- Wang, N.; Tian, X.; Cheng, B.; Guang, S.; Xu, H. Calcium alginate/silk fibroin peptide/Bletilla striata polysaccharide blended microspheres loaded with tannic acid for rapid wound healing. Int. J. Biol. Macromol. 2022, 220, 1329–1344. [Google Scholar] [CrossRef]
- Mohammadzadeh, V.; Mahmoudi, E.; Ramezani, S.; Navaeian, M.; Taheri, R.A.; Ghorbani, M. Design of a novel tannic acid enriched hemostatic wound dressing based on electrospun polyamide-6/hydroxyethyl cellulose nanofibers. J. Drug Deliv. Sci. Technol. 2023, 86, 104625. [Google Scholar] [CrossRef]
- Sahiner, N.; Sengel, S.B. Tannic acid decorated poly(methacrylic acid) micro and nanoparticles with controllable tannic acid release and antioxidant properties. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 30–38. [Google Scholar] [CrossRef]
- Salehipour, M.; Nikpour, S.; Rezaei, S.; Mohammadi, S.; Rezaei, M.; Ilbeygi, D.; Hosseini-Chegeni, A.; Mogharabi-Manzari, M. Safety of metal–organic framework nanoparticles for biomedical applications: An in vitro toxicity assessment. Inorg. Chem. Commun. 2023, 152, 110655. [Google Scholar] [CrossRef]
- Xie, L.; Wehling, R.L.; Ciftci, O.; Zhang, Y. Formation of complexes between tannic acid with bovine serum albumin, egg ovalbumin and bovine beta-lactoglobulin. Food Res. Int. 2017, 102, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.F.; Nascimento, J.M.D.; Sobral, R.; Amorim, E.L.C.; Silva, R.O.; Leite, A.C.L. Tannic acid as a precipitating agent of human plasma proteins. Eur. J. Pharm. Sci. 2019, 138, 105018. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, X.; Du, Y.; Tang, Z.; Duan, X.; Sun, Z.; Zhao, J.; Xu, W.; Wang, Y.; Tang, Y. Enhancement of oral bioavailability of celastrol by chitosan microencapsulated porous starch carriers. Int. J. Biol. Macromol. 2024, 282, 137167. [Google Scholar] [CrossRef]
- Pasban, A.; Mousavi, S.F.; Abdollahi, S.; Hesarinejad, M.A. Evaluating the potential of soy protein isolate/alginate hydrogel as polyphenolic liposome carrier during gastrointestinal tract: A case study on sumac extract. LWT 2024, 211, 116883. [Google Scholar] [CrossRef]
- Mohsen, S.; Bakr, M.M.; ElDegwy, M.A.; Abouhussein, D.M.N.; Fares, A.R.; ElMeshad, A.N. Pomegranate extract-loaded surfactant-free zein nanoparticles as a promising green approach for hepatic cancer: Optimization and in vitro cytotoxicity. Future J. Pharm. Sci. 2024, 10, 73. [Google Scholar] [CrossRef]
- Tampucci, S.; Tofani, G.; Chetoni, P.; Di Gangi, M.; Mezzetta, A.; Paganini, V.; Burgalassi, S.; Pomelli, C.S.; Monti, D. Sporopollenin Microcapsule: Sunscreen Delivery System with Photoprotective Properties. Pharmaceutics 2022, 14, 2041. [Google Scholar] [CrossRef]
- Jafari, Y.; Sabahi, H.; Rezayan, A.H. Stability and loading properties of Curcumin encapsulated in saffron pollen. Food Chem. Adv. 2025, 7, 101009. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Li, X.; Cao, Q.; Ma, F.; Li, Y.; Xie, D.; Ma, H.; Zhang, B.; Li, X.; et al. Preparation and characteristics of soy protein isolate—Sea buckthorn flavone emulsion and their effects of on quality and heterocyclic amines of roasted mutton granules. Int. J. Biol. Macromol. 2024, 281, 136181. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Korma, S.A.; Han, W.; Karim, A.; Sharif, H.R.; Ali, A.; Yaqoob, S.; Khalifa, S.A.; Cacciotti, I. Influence of diverse natural biopolymers on the physicochemical characteristics of borage seed oil-peppermint oil loaded W/O/W nanoemulsions entrapped with lycopene. Nanotechnology 2021, 32, 505302. [Google Scholar] [CrossRef]
- Castro-Criado, D.; Jiménez-Rosado, M.; Perez-Puyana, V.; Romero, A. Soy Protein Isolate as Emulsifier of Nanoemulsified Beverages: Rheological and Physical Evaluation. Foods 2023, 12, 507. [Google Scholar] [CrossRef]
- Chen, S.; Fan, L.; Chen, X.; Guo, Z.; Zhang, B. Microencapsulation of Lonicera caerulea pomace extract by spray drying: Characterization and stability studies. LWT—Food Sci. Technol. 2025, 223, 117778. [Google Scholar] [CrossRef]
- Chao Song, Z.; Zhang, H.; Fei Niu, P.; Shi, L.S.; Yan Yang, X.; Hong Meng, Y.; Yu Wang, X.; Gong, T.; Rong Guo, Y. Fabrication of a novel antioxidant emulsifier through tuning the molecular interaction between soy protein isolates and young apple polyphenols. Food Chem. 2023, 420, 136110. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.R.; Pulikkal, A.K. Nano emulsions stabilized by natural emulsifiers: A comprehensive review on feasibility, stability and bio-applicability. J. Drug Deliv. Sci. Technol. 2024, 92, 105303. [Google Scholar] [CrossRef]
- Guo, Q.; Li, S.; Du, G.; Chen, H.; Yan, X.; Chang, S.; Yue, T.; Yuan, Y. Formulation and characterization of microcapsules encapsulating carvacrol using complex coacervation crosslinked with tannic acid. LWT—Food Sci. Technol. 2022, 165, 113683. [Google Scholar] [CrossRef]
- Raj, R.; Kahraman, R.; Shakoor, A.; Montemor, F.; Taryba, M. Tannic Acid-Loaded Hydroxyapatite Carriers for Corrosion Protection of Polyolefin-Coated Carbon Steel. Appl. Sci. 2022, 12, 10263. [Google Scholar] [CrossRef]
- Heidari, F.; Akbarzadeh, I.; Nourouzian, D.; Mirzaie, A.; Bakhshandeh, H. Optimization and characterization of tannic acid loaded niosomes for enhanced antibacterial and anti-biofilm activities. Adv. Powder Technol. 2020, 31, 4768–4781. [Google Scholar] [CrossRef]
- Yang, G.; Huang, X.; Cai, J.; Zhang, Q. Curing mechanism of triglycidylamine crosslinked soybean protein adhesive analyzed by Fourier transform infrared, second derivative infrared and two-dimensional correlation spectroscopy. Int. J. Adhes. Adhes. 2021, 107, 102825. [Google Scholar] [CrossRef]
- Hu, W.; Chen, C.; Wang, Y.; He, W.; He, Z.; Chen, J.; Li, Z.; Li, J.; Li, W. Development of high internal phase emulsions with noncovalent crosslink of soy protein isolate and tannic acid: Mechanism and application for 3D printing. Food Chem. 2023, 427, 136651. [Google Scholar] [CrossRef]
- Nam, S.; Easson, M.W.; Condon, B.D.; Hillyer, M.B.; Sun, L.; Xia, Z.; Nagarajan, R. A reinforced thermal barrier coat of a Na–tannic acid complex from the view of thermal kinetics. RSC Adv. 2019, 9, 10914–10926. [Google Scholar] [CrossRef]
- Hadzieva, J.; Mladenovska, K.; Simonoska Crcarevska, M.; Glavaš Dodov, M.; Dimchevska, S.; Geškovski, N.; Grozdanov, A.; Popovski, E.; Petruševski, G.; Chachorovska, M.; et al. Lactobacillus casei loaded Soy Protein-Alginate Microparticles prepared by Spray-Drying. Food Technol. Biotechnol. 2017, 55, 173–186. [Google Scholar] [CrossRef]
- Jiang, K.; Lei, Z.; Yi, M.; Lv, W.; Jing, M.; Feng, Q.; Tan, H.; Chen, Y.; Xiao, H. Improved performance of soy protein adhesive with melamine–urea–formaldehyde prepolymer. RSC Adv. 2021, 11, 27126–27134. [Google Scholar] [CrossRef] [PubMed]
- Boachie, R.T.; Okagu, O.D.; Abioye, R.; Huttmann, N.; Oliviero, T.; Capuano, E.; Fogliano, V.; Udenigwe, C.C. Lentil Protein and Tannic Acid Interaction Limits in Vitro Peptic Hydrolysis and Alters Peptidomic Profiles of the Proteins. J. Agric. Food Chem. 2022, 70, 6519–6529. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.; Arken, A.; Wali, A.; Gao, Y.; Aisa, H.A.; Yili, A. Effect of phenolic compound-protein covalent conjugation on the physicochemical, anti-inflammatory, and antioxidant activities of silk sericin. Process Biochem. 2022, 117, 101–109. [Google Scholar] [CrossRef]
- Pi, X.; Liu, J.; Sun, Y.; Ban, Q.; Cheng, J.; Guo, M. Protein modification, IgE binding capacity, and functional properties of soybean protein upon conjugation with polyphenols. Food Chem. 2022, 405, 134820. [Google Scholar] [CrossRef]
- Wu, J.; Ding, X.; Zhang, J.; Chen, W. Online Determination of Colloidal Properties of Tannin Solutions under Microwave Irradiation using a Modified Zetasizer. Anal. Lett. 2018, 52, 879–891. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Gautier, C.; Cheynier, V.; Imberty, A. Interactions between flavan-3-ols and poly(L-proline) studied by isothermal titration calorimetry: Effect of the tannin structure. J. Agric. Food Chem. 2007, 55, 9235–9240. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, L.; Kong, F.; Yu, L. Design and preparation of poly(tannic acid) nanoparticles with intrinsic fluorescence: A sensitive detector of picric acid. Chem. Eng. J. 2021, 416, 129090. [Google Scholar] [CrossRef]
- Liang, X.; Cheng, W.; Liang, Z.; Zhan, Y.; McClements, D.J.; Hu, K. Co-Encapsulation of Tannic Acid and Resveratrol in Zein/Pectin Nanoparticles: Stability, Antioxidant Activity, and Bioaccessibility. Foods 2022, 11, 3478. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, S.; Wang, Y.; Wang, X.; Xu, N.; Jiang, L. Stability and in vitro digestion simulation of soy protein isolate-vitamin D3 nanocomposites. LWT 2020, 117, 108647. [Google Scholar] [CrossRef]
- Oppermann, A.K.L.; Noppers, J.M.E.; Stieger, M.; Scholten, E. Effect of outer water phase composition on oil droplet size and yield of (w(1)/o/w(2)) double emulsions. Food Res. Int. 2018, 107, 148–157. [Google Scholar] [CrossRef]
- Neumann, S.M.; Scherbej, I.; van der Schaaf, U.S.; Karbstein, H.P. Investigations on the influence of osmotic active substances on the structure of water in oil emulsions for the application as inner phase in double emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 56–62. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, W.; Li, X.; Pan, G.; Chen, N.; Xie, Q.; Wang, X. Preparation of pH Sensitive Bacteriostatic W/O/W Emulsion Microcapsules. J. Biomater. Sci. Polym. Ed. 2023, 34, 2060–2075. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Li, H.; Tang, Q.; Wang, X.; Li, Y.; Li, L. Preparation and characterization of glucoamylase microcapsules prepared by W/O/W type complex coacervation freeze drying. J. Food Sci. 2022, 88, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.A.; Namli, I.; Gulec, K.; Kiyan, H.T. Diclofenac sodium loaded PLGA nanoparticles for inflammatory diseases with high anti-inflammatory properties at low dose: Formulation, characterization and in vivo HET-CAM analysis. Microvasc. Res. 2020, 130, 103991. [Google Scholar] [CrossRef]
- Campos, P.; Benevenuto, C.G.; Calixto, L.S.; Melo, M.O.; Pereira, K.C.; Gaspar, L.R. Spirulina, Palmaria Palmata, Cichorium Intybus, and Medicago Sativa extracts in cosmetic formulations: An integrated approach of in vitro toxicity and in vivo acceptability studies. Cutan. Ocul. Toxicol. 2019, 38, 322–329. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, T. Preparation of Natural Composite Microcapsules Containing Orchid Black Currant Fragrance and its Sustained-Release Properties on Hair Bundle. J. Polym. Environ. 2021, 30, 136–150. [Google Scholar] [CrossRef]
- Yu, N.; Luo, Z.; Ma, F.; Li, J.; Yang, P.; Li, G.; Li, J. Cationic Gelatin Cross-Linked with Transglutaminase and Its Electrospinning in Aqueous Solution. Langmuir 2023, 39, 3668–3677. [Google Scholar] [CrossRef]
- Dong, S.; Hu, S.-M.; Yu, S.-J.; Zhou, S.; Zhou, T. Soybean protein isolate/chitosan complex-rutin microcapsules. Int. J. Biol. Macromol. 2023, 243, 125323. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Zhang, H. Ultrasound-assisted extraction and antioxidant activity of polysaccharides from Tenebrio molitor. Sci. Rep. 2024, 14, 28526. [Google Scholar] [CrossRef]
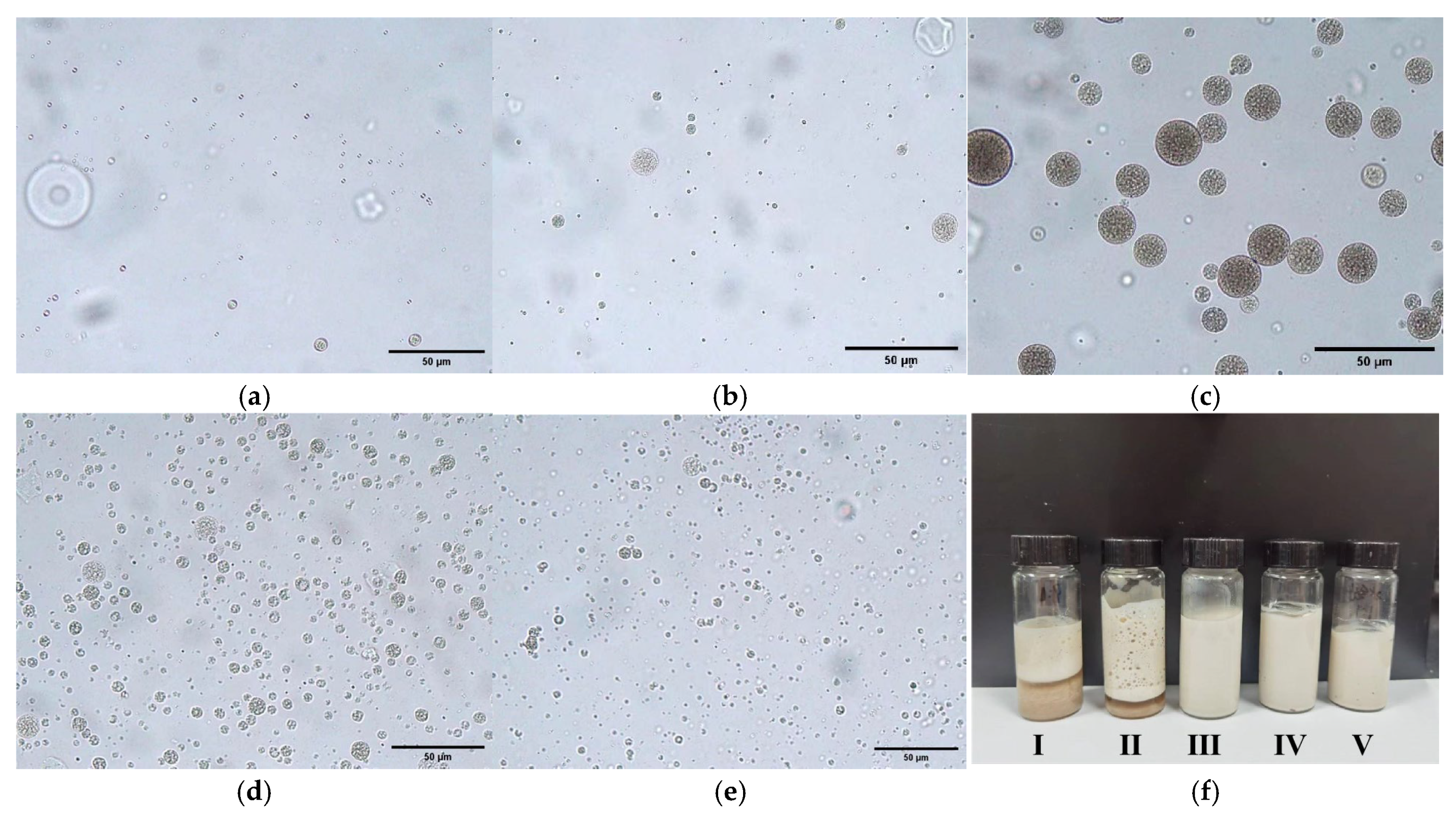
| Multiple Emulsions | I | II | III | IV | V |
|---|---|---|---|---|---|
| Ratio of outer water phase (W2) and W1/O emulsion | 9:1 | 4:1 | 7:3 | 3:2 | 1:1 |
| Stability | layered, broken | layered, broken | stable | stable | stable |
| Sample | Decomposition Stage | Tonset (°C) | Tpeak (°C) |
|---|---|---|---|
| TA | 30–130 °C | 30 | 75 |
| 200–375 °C | 200 | 280–310 | |
| Blank microcapsules | 50–150 °C | 50 | - |
| 200–360 °C | 200 | 310 | |
| 360–500 °C | 360 | 408 | |
| TA microcapsules | 50–150 °C | 50 | - |
| 150–250 °C | 150 | 175 | |
| 250–500 °C | 250 | 288–350 |
| Group | Concentration (%) | STE: Cell Viability (%) | |||
|---|---|---|---|---|---|
| First | Second | Third | Average ± SD | ||
| Negative control | - | 104.53 | 104.95 | 102.97 | 104.15 ± 1.05 |
| Positive control | - | 57.22 | 58.42 | 64.05 | 59.90 ± 3.65 |
| TA | 5 | 62.09 | 65.87 | 68.82 | 65.59 ± 3.37 |
| 2.5 | 78.33 | 84.81 | 88.44 | 83.86 ± 5.12 | |
| 0.5 | 99.71 | 83.49 | 110.01 | 97.74 ± 13.37 | |
| TA microcapsules corresponding amount of TA | 5 | 94.95 | 95.53 | 93.46 | 94.65 ± 1.07 |
| 2.5 | 104.63 | 97.33 | 102.69 | 101.55 ± 3.78 | |
| 0.5 | 102.58 | 100.38 | 98.92 | 100.63 ± 1.84 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Yan, R.; Zhang, S.; Lu, Y. W/O/W Multiple Emulsified Microcapsules Based on Biopolymer Soybean Isolate Proteins: Improving Tannic Acid’s Biocompatibility and Sustained-Release Performance. Molecules 2025, 30, 2373. https://doi.org/10.3390/molecules30112373
Zhang S, Yan R, Zhang S, Lu Y. W/O/W Multiple Emulsified Microcapsules Based on Biopolymer Soybean Isolate Proteins: Improving Tannic Acid’s Biocompatibility and Sustained-Release Performance. Molecules. 2025; 30(11):2373. https://doi.org/10.3390/molecules30112373
Chicago/Turabian StyleZhang, Suning, Ruman Yan, Siyu Zhang, and Yina Lu. 2025. "W/O/W Multiple Emulsified Microcapsules Based on Biopolymer Soybean Isolate Proteins: Improving Tannic Acid’s Biocompatibility and Sustained-Release Performance" Molecules 30, no. 11: 2373. https://doi.org/10.3390/molecules30112373
APA StyleZhang, S., Yan, R., Zhang, S., & Lu, Y. (2025). W/O/W Multiple Emulsified Microcapsules Based on Biopolymer Soybean Isolate Proteins: Improving Tannic Acid’s Biocompatibility and Sustained-Release Performance. Molecules, 30(11), 2373. https://doi.org/10.3390/molecules30112373





