Microplastic-Mediated Heavy Metal Uptake in Lettuce (Lactuca sativa L.): Implications for Food Safety and Agricultural Sustainability
Abstract
1. Introduction
2. Results
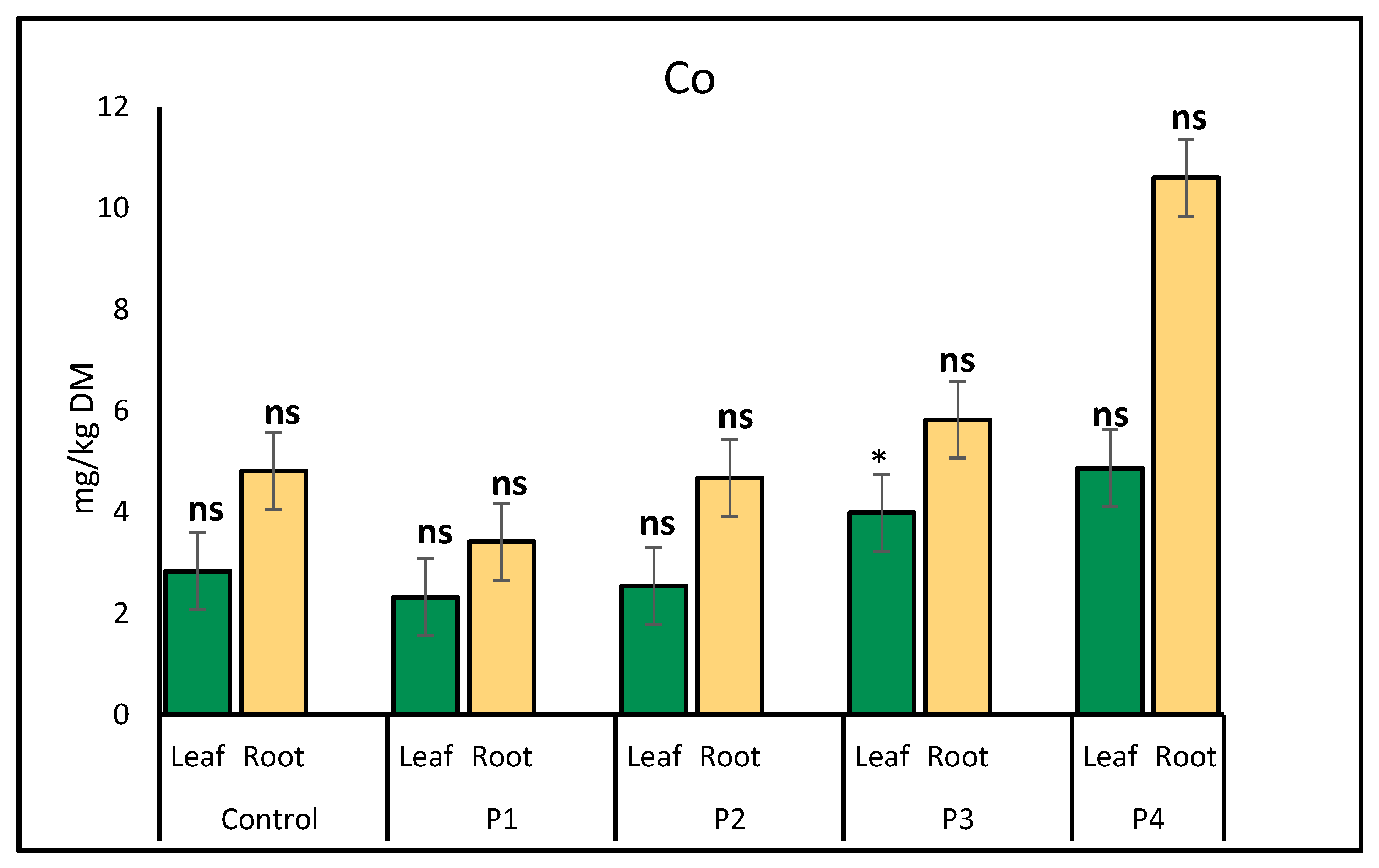
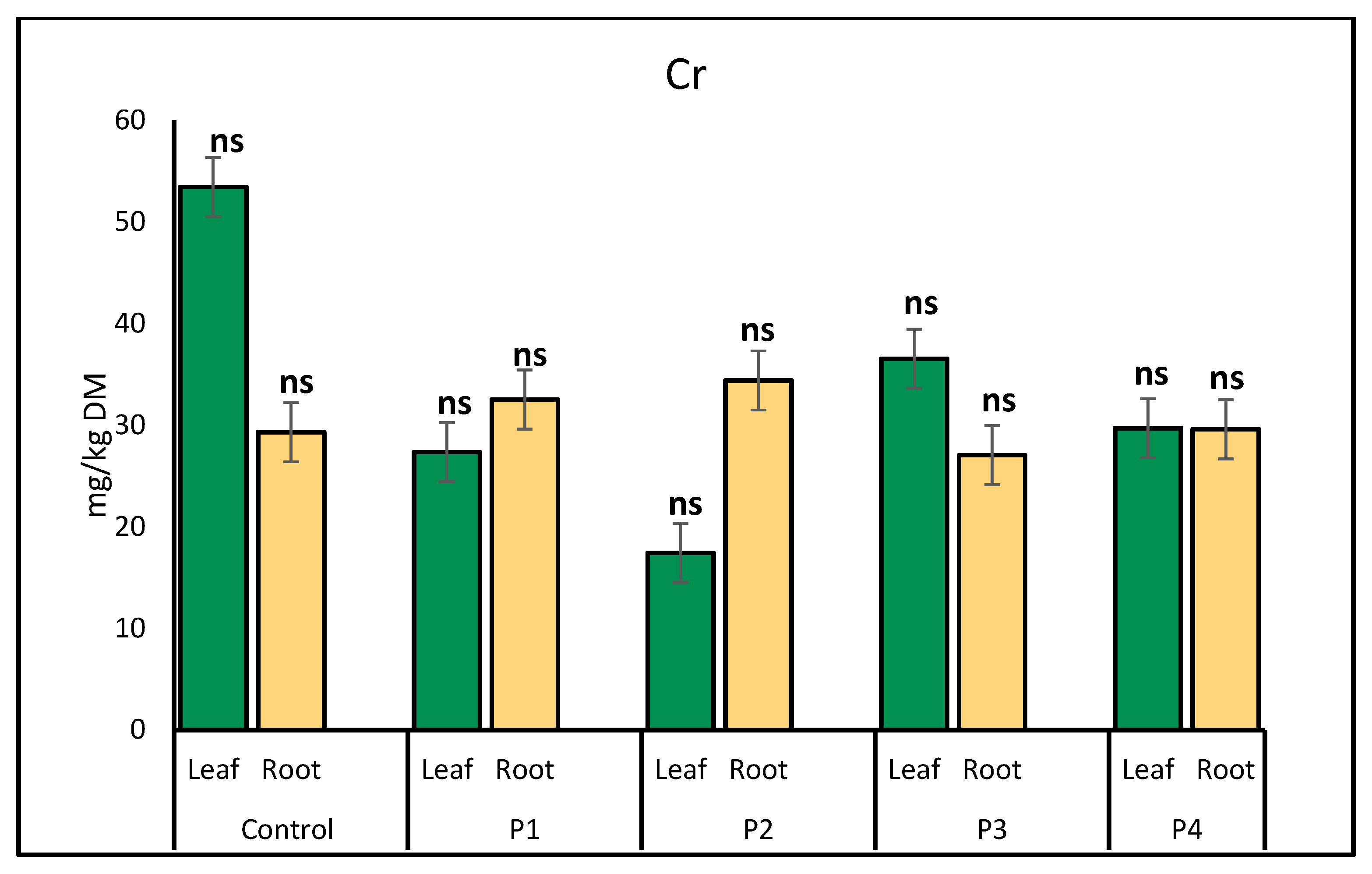
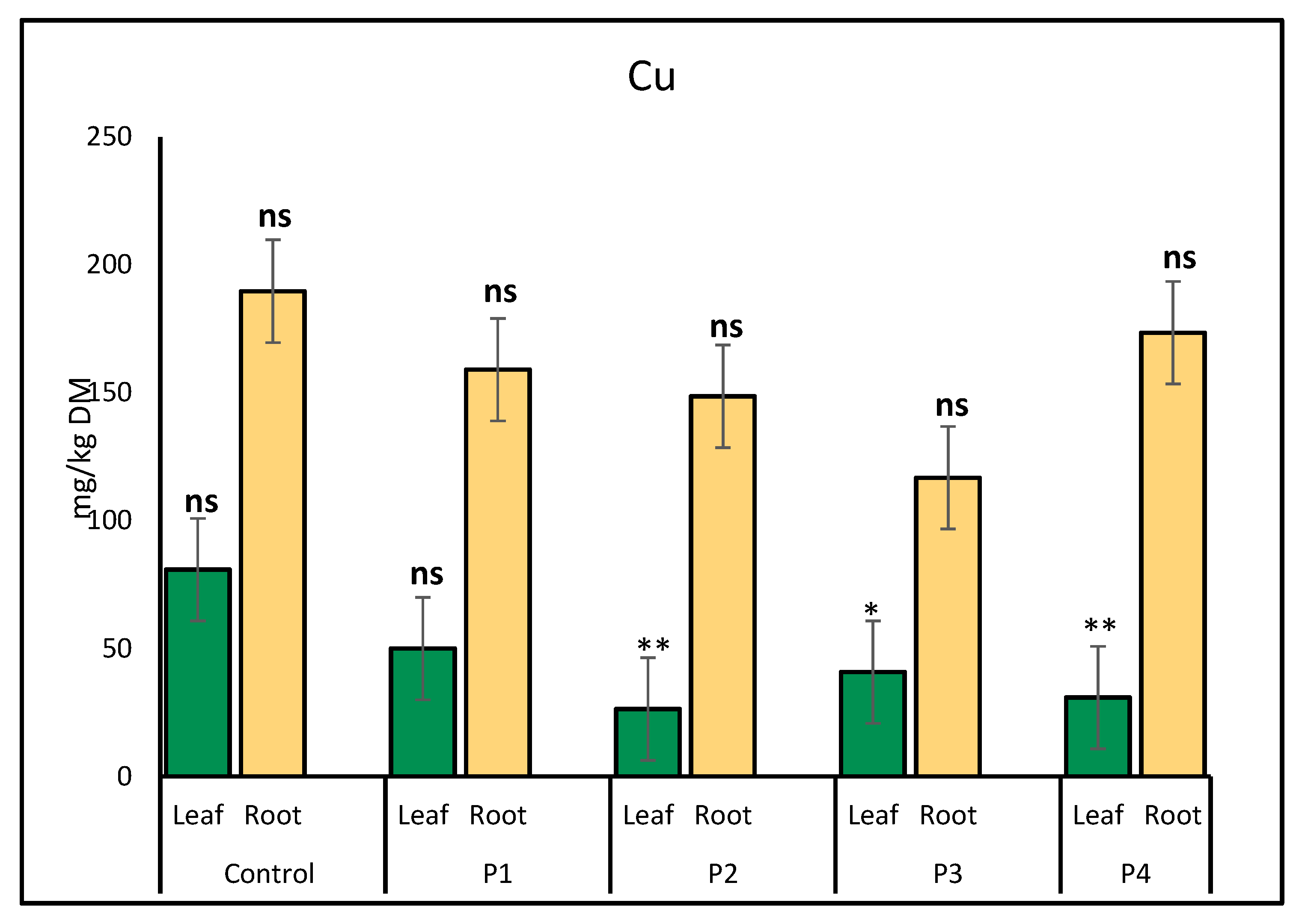
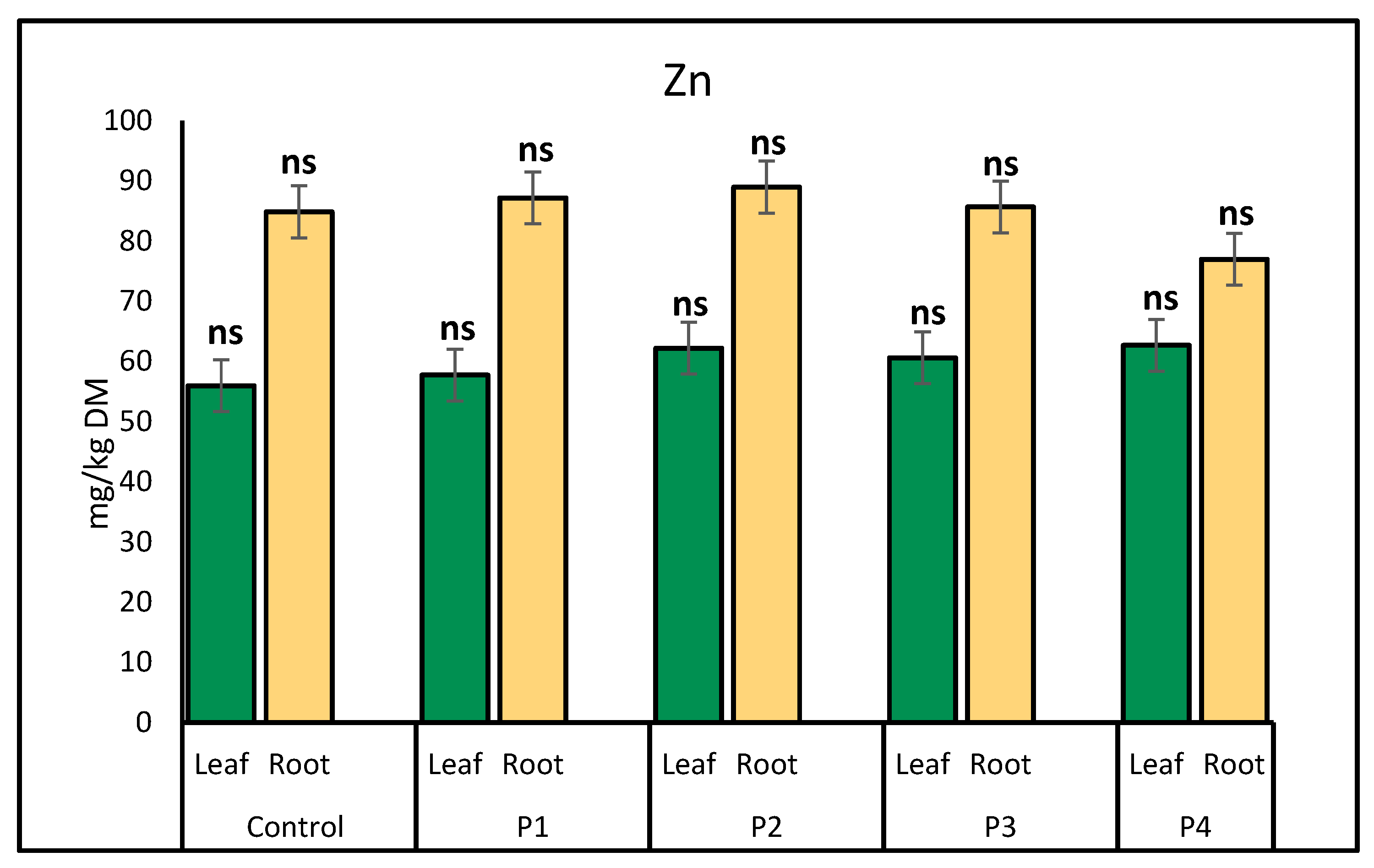
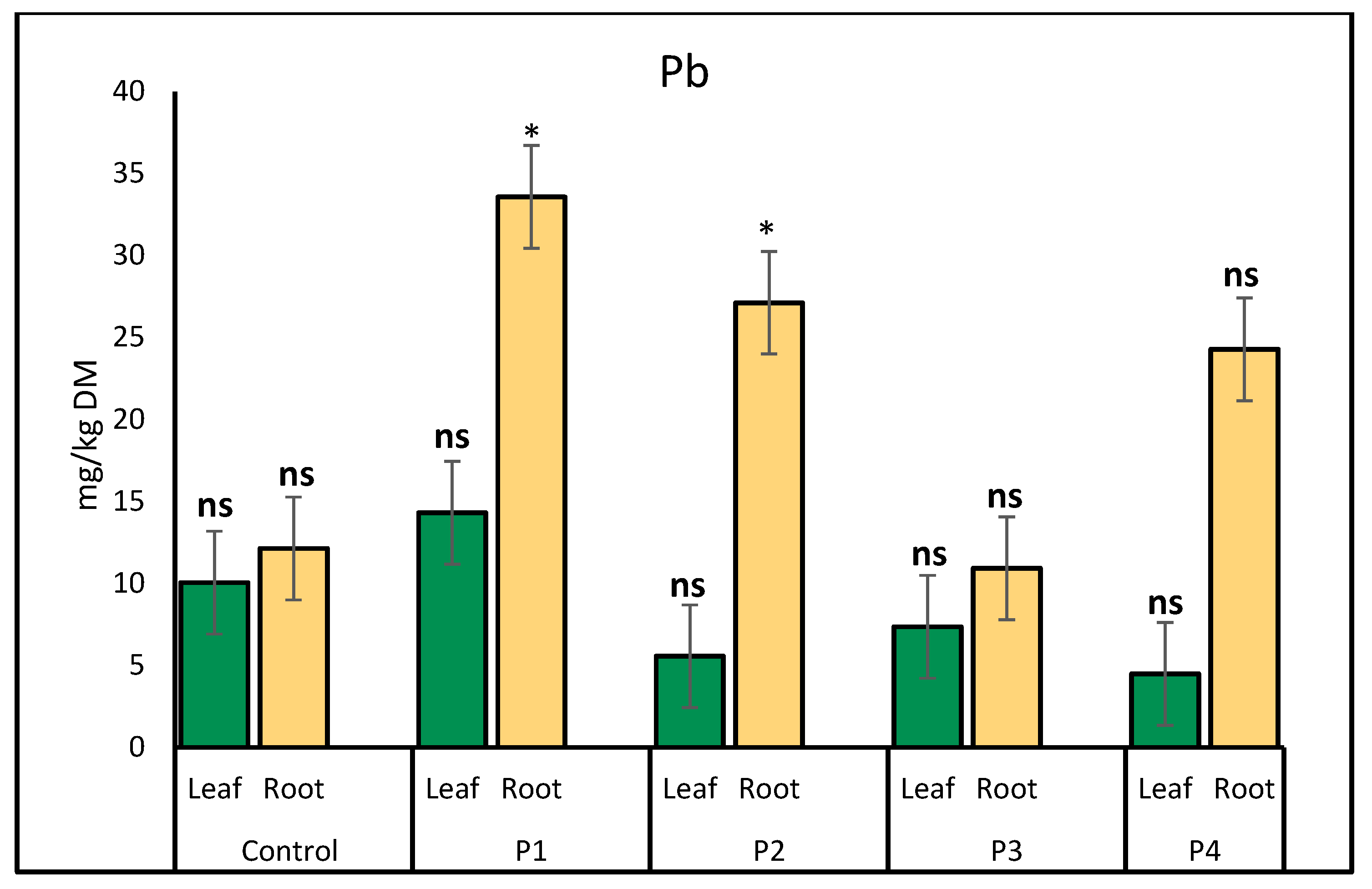
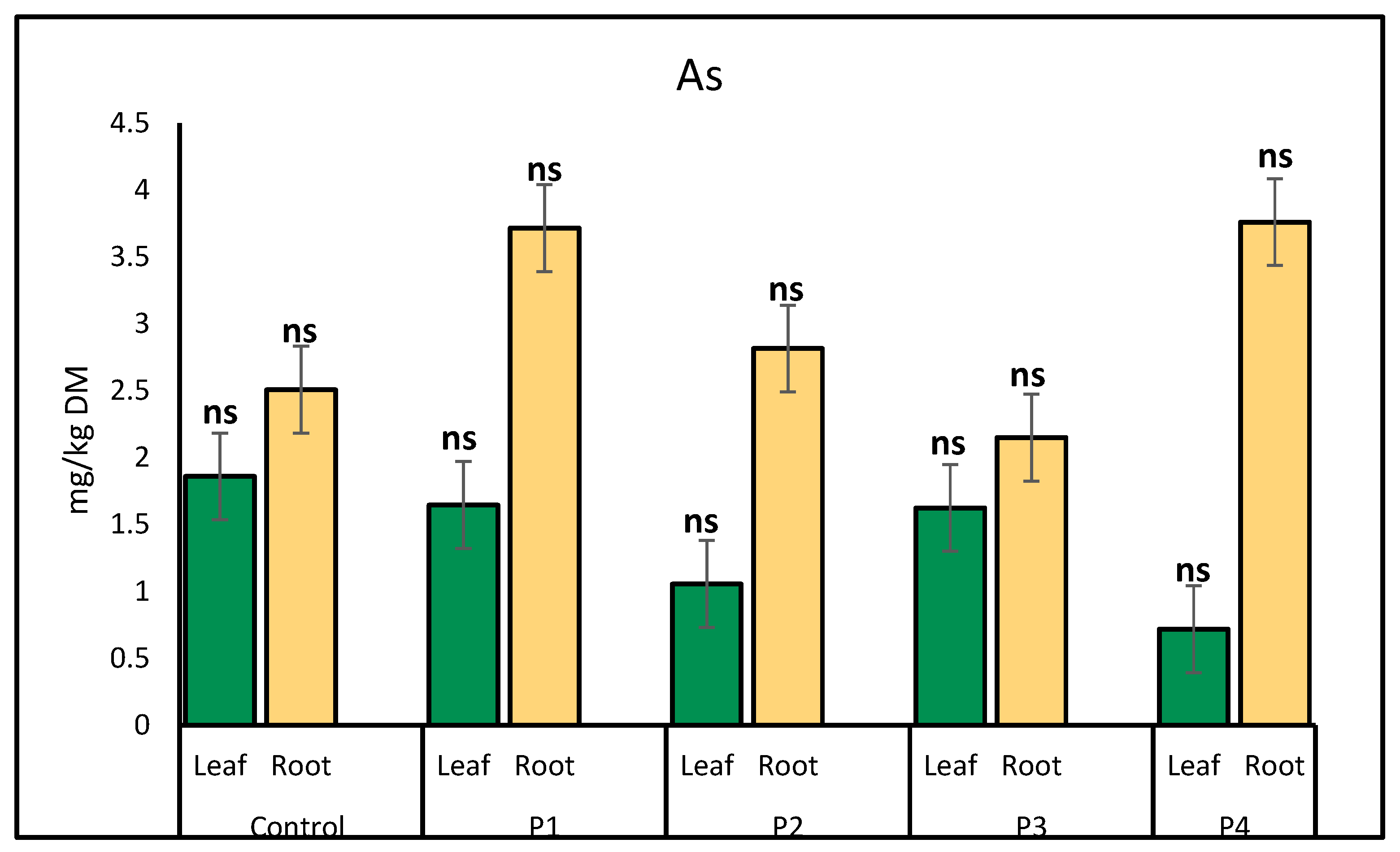
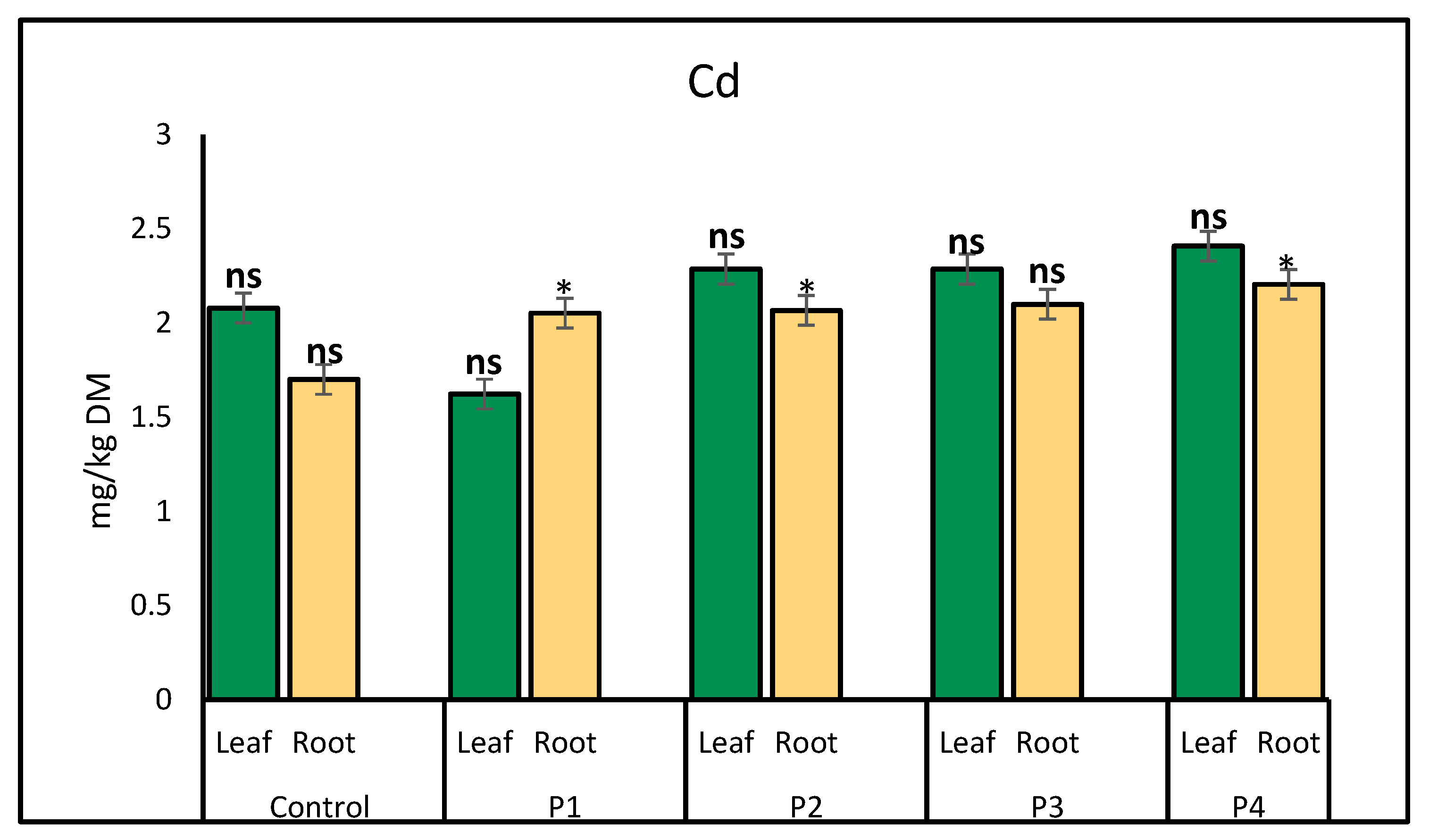
2.1. Heavy Metal Uptake by Lettuce
2.2. Root Growth and Biomass Responses
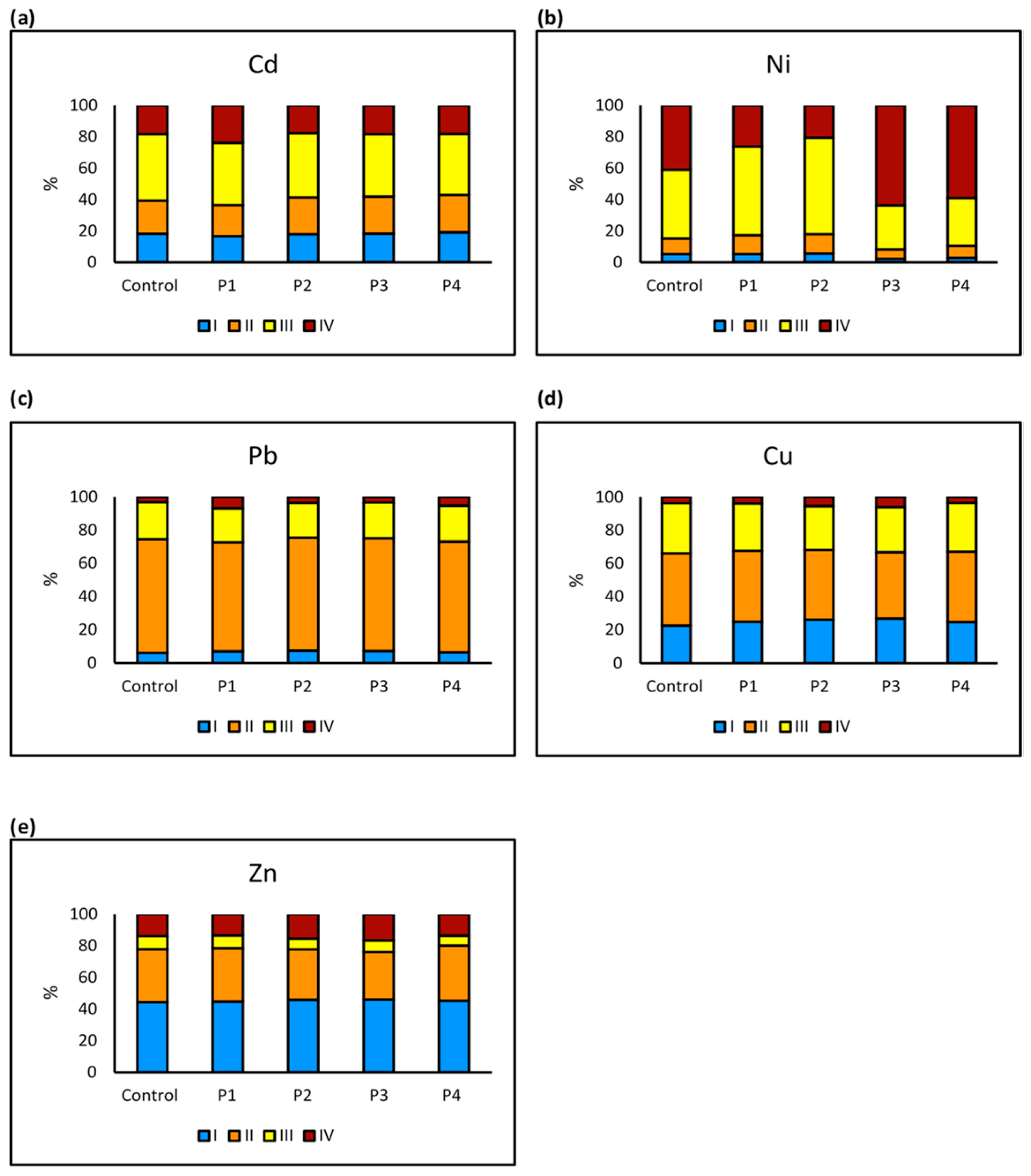
2.3. Metal Fractionation in Soil
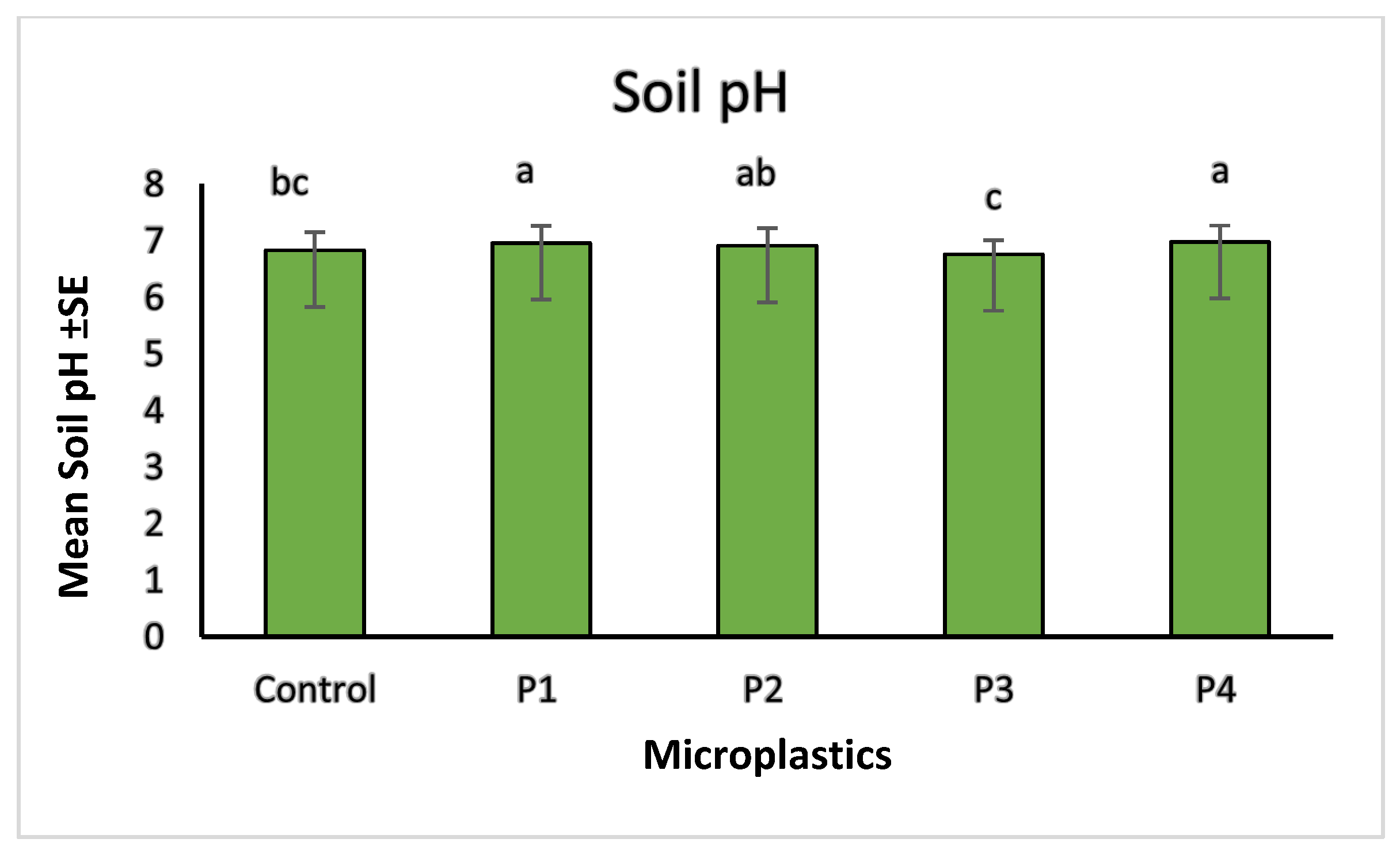
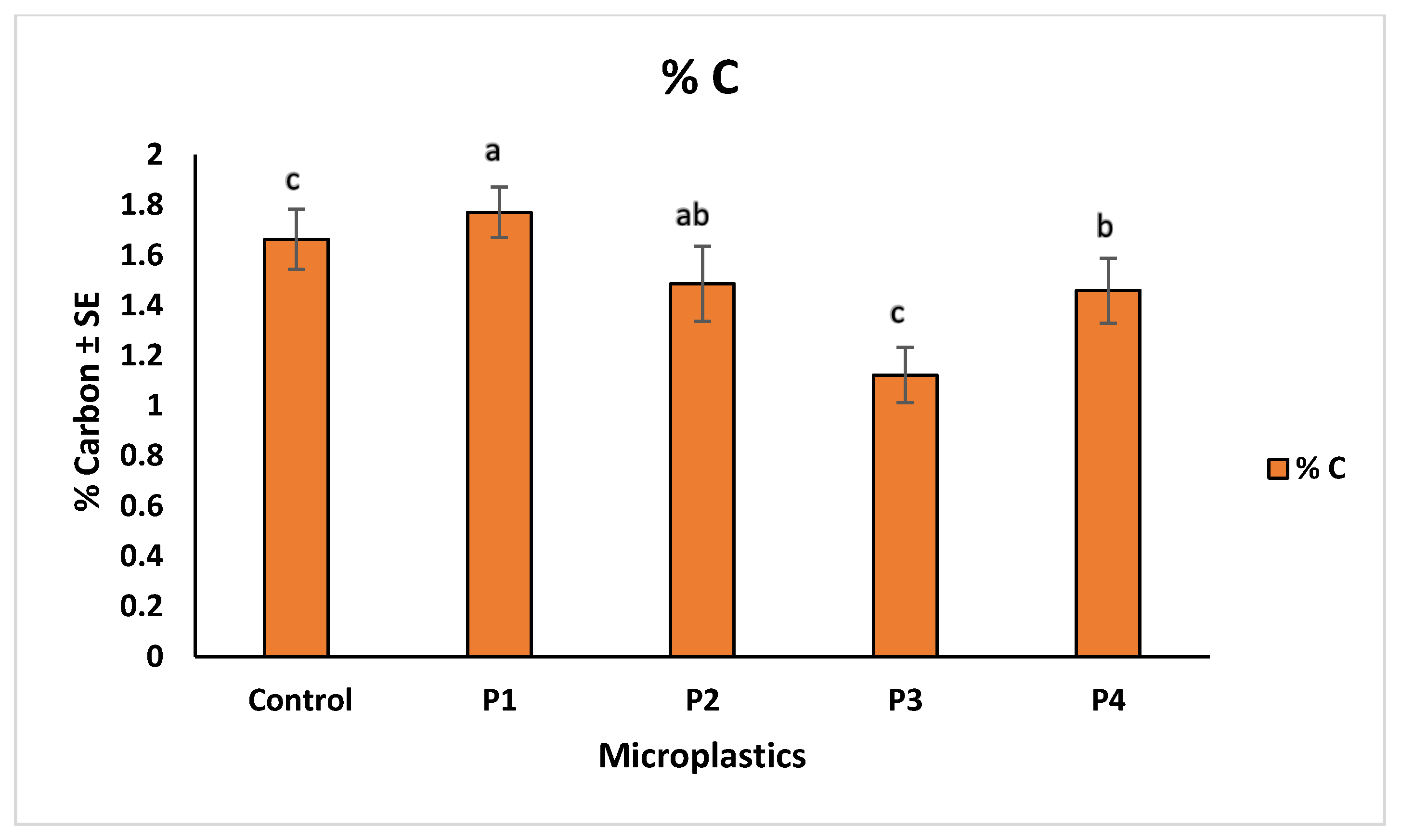
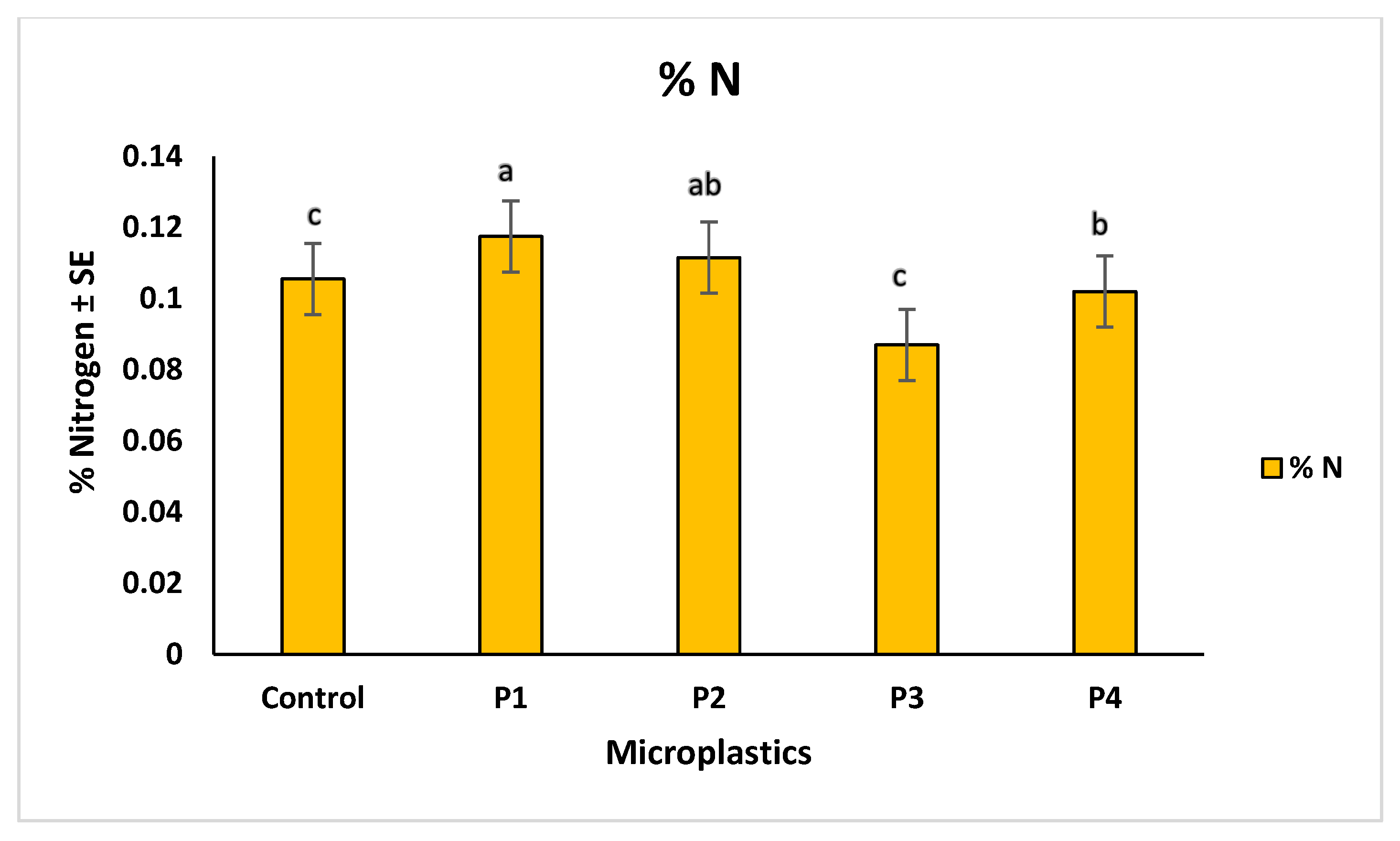
2.4. Soil Properties and Heavy Metal Correlations
3. Discussion
4. Materials and Methods
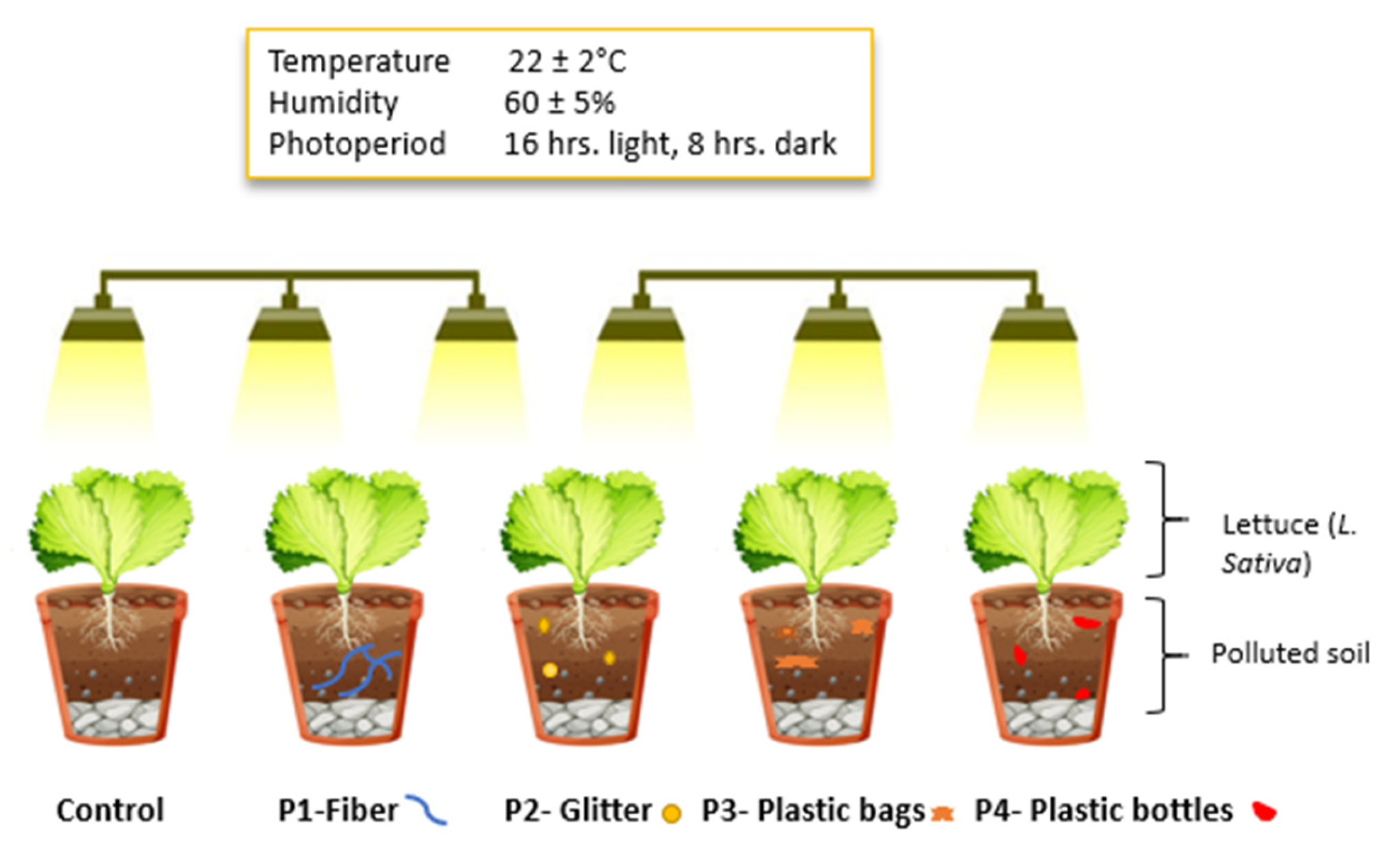
4.1. Plant Material and Pot Experimental Design
4.2. Soil Preparation and Microplastic Treatments
4.3. Plant Growth and Sampling
4.4. Heavy Metal Analysis
4.5. Sequential Extraction of Heavy Metals in Soil
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MP | Microplastic |
| PE | Polyethylene |
| PEs | Polyester fibers |
| LDPE | Low-Density Polyethylene |
| HDPE | High-Density Polyethylene |
| PET | Polyethylene Terephthalate |
| L. sativa | Lactuca sativa (scientific name for lettuce) |
| U.S. | United States |
| TOC | Total Organic Carbon |
| C/N or C:N | Carbon-to-Nitrogen ratio |
| TN | Total Nitrogen |
| RCBD | Randomized Complete Block Design |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| MP-AES | Microwave Plasma—Atomic Emission Spectrometry |
| HNO₃ | Nitric Acid |
| H₂O₂ | Hydrogen Peroxide |
| BCR | Bureau Communautaire de Référence (Community Bureau of Reference) |
| Fe-Mn | Iron–Manganese |
| SPSS | Statistical Package for the Social Sciences |
| ANOVA | Analysis of Variance |
| USA | United States of America |
| JRC | Joint Research Centre |
| P1 | Fiber |
| P2 | Glitter |
| P3 | Plastic Bags |
| P4 | Plastic Bottles |
| As | Arsenic |
| Cd | Cadmium |
| Co | Cobalt |
| Cr | Chromium |
| Cu | Copper |
| Ni | Nickel |
| Pb | Lead |
| Zn | Zinc |
| p-value | Probability value |
| %C | Percent Carbon |
| %N | Percent Nitrogen |
| pH | Potential of Hydrogen |
References
- Elgarahy, A.M.; Akhdhar, A.; Elwakeel, K.Z. Microplastics prevalence, interactions, and remediation in the aquatic environment: A critical review. J. Environ. Chem. Eng. 2021, 9, 106224. [Google Scholar] [CrossRef]
- An, Q.; Zhou, T.; Wen, C.; Yan, C. Sources of microplastic in the environment. In Microplastics in Terrestrial Environments: Emerging Contaminants and Major Challenges; Springer: Cham, Switzerland, 2020; pp. 143–159. [Google Scholar]
- Jin, T.; Tang, J.; Lyu, H.; Wang, L.; Gillmore, A.B.; Schaeffer, S.M. Activities of microplastics (MPs) in agricultural soil: A review of MPs pollution from the perspective of agricultural ecosystems. J. Agric. Food Chem. 2022, 70, 4182–4201. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Maranho, M.A.K.C.; Lima, D.S.C.; Fausto, A.P.E.R. The Role of Microplastic Pollution in Modifying Soil Properties: An Overview of Microplastic Behavior in Agricultural Soils. Environ. Sci. Technol. 2018, 52, 13256–13267. [Google Scholar]
- Jadhav, B.; Medyńska-Juraszek, A. Microplastic and nanoplastic in crops: Possible adverse effects to crop production and contaminant transfer in the food chain. Plants 2024, 13, 2526. [Google Scholar] [CrossRef]
- Kajal, S.; Thakur, S. Coexistence of microplastics and heavy metals in soil: Occurrence, transport, key interactions and effect on plants. Environ. Res. 2024, 262, 119960. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wang, T.; Li, H. Interaction of microplastics with heavy metals in soil: Mechanisms, influencing factors and biological effects. Sci. Total Environ. 2024, 918, 170281. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Jadhav, B. Influence of different microplastic forms on pH and mobility of Cu2+ and Pb2+ in soil. Molecules 2022, 27, 1744. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.; Zhang, Y. Effects of Microplastics and Their Adsorption of Cadmium as Vectors on the Cladoceran Moina monogolica Daday: Implications for Plastic-Ingesting Organisms. J. Hazard. Mater. 2020, 400, 123239. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Li, M. Effects of Microplastics on the Uptake and Accumulation of Heavy Metals in Plants: A Review. Ecotoxicol. Environ. Saf. 2025, 256, 114872. [Google Scholar]
- Verla, A.W.; Enyoh, C.E.; Verla, E.N.; Nwarnorh, K.O. Microplastic–toxic chemical interaction: A review study on quantified levels. Sci. Total Environ. 2019, 1, 1400. [Google Scholar]
- Liu, J.; Chen, H.; Zhao, L. How Aging Microplastics Influence Heavy Metal Environmental Fate and Bioavailability: A Critical Review. Environ. Pollut. 2025, 321, 121013. [Google Scholar]
- Lian, J.; Wu, Y.; Zhang, Y.; Xiong, X.; Zhao, M. Impact of Polystyrene Microplastics on Lettuce (Lactuca sativa L.): Growth Inhibition and Accumulation. Environ. Pollut. 2020, 263 Pt B, 114425. [Google Scholar]
- Shi, R.; Lian, Y.; Zeb, A. Foliar Exposure to Microplastics Disrupts Lettuce Metabolism and Negatively Interferes with Symbiotic Microbial Communities. Plant Physiol. Biochem. 2025, 223, 109823. [Google Scholar] [CrossRef] [PubMed]
- Noumedem, J.A.K.; Djeussi, D.E.; Hritcu, L.; Mihasan, M.; Kuete, V. Lactuca sativa. In Medicinal Spices and Vegetables from Africa; Academic Press: Cambridge, MA, USA, 2017; pp. 437–449. [Google Scholar]
- Wang, F.; Zhang, S.; Shao, W.; Zhu, L.; Li, R.; Tian, H. Effects of microplastics on soil properties: Current knowledge and future perspectives. J. Hazard. Mater. 2022, 424, 127531. [Google Scholar] [CrossRef]
- Han, X.; Wang, S.; Yu, X.; Vogt, R.D.; Feng, J.; Zhai, L.; Ma, W.; Zhu, L.; Lu, X. Kinetics and Size Effects on Adsorption of Cu(II), Cr(III), and Pb(II) Onto Polyethylene, Polypropylene, and Polyethylene Terephthalate Microplastic Particles. Front. Mar. Sci. 2021, 8, 785146. [Google Scholar] [CrossRef]
- Bodzek, M.; Pohl, A.; Rosik-Dulewska, C. Microplastics in wastewater treatment plants: Characteristics, occurrence and removal technologies. Water 2024, 16, 3574. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Q.; Liu, Y. Influencing Mechanisms of Microplastics Existence on Soil Heavy Metal Bioavailability and Plant Uptake. Sci. Total Environ. 2025, 870, 162345. [Google Scholar]
- Ulhassan, Z.; Abbas, T.; Ali, S.; Rizwan, M.; Ahmad, M.I.; Rinklebe, J. Uptake and translocation mechanisms of metals/metalloids in plants through soil and water. In Metals Metalloids Soil Plant Water Systems; Academic Press: Cambridge, MA, USA, 2022; pp. 1–28. [Google Scholar]
- Medyńska-Juraszek, A.; Kołodyńska, D.; Jadhav, B. The Effects of Rabbit-Manure-Derived Biochar Co-Application with Compost on the Availability and Heavy Metal Uptake by Green Leafy Vegetables. Agronomy 2022, 12, 2552. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.; Li, D. Microplastic Types and Their Effect on Heavy Metal Bioaccumulation in Agricultural Systems. Environ. Sci. Technol. 2023. [Google Scholar]
- Bouaicha, O.; Mimmo, T.; Tiziani, R.; Praeg, N.; Polidori, C.; Lucini, L.; Vigani, G.; Terzano, R.; Sanchez-Hernandez, J.C.; Illmer, P.; et al. Microplastics make their way into the soil and rhizosphere: A review of the ecological consequences. Rhizosphere 2022, 22, 100542. [Google Scholar] [CrossRef]
- Hesterberg, D.; Luthy, R.G. Copper mobilization and immobilization along an organic matter and redox gradient in contaminated soils. Environ. Sci. Technol. 2019, 52, 13698–13707. [Google Scholar]
- Wang, F.; Wang, X.; Song, N. Polyethylene microplastics increase cadmium uptake in lettuce (Lactuca sativa L.) by altering the soil microenvironment. Sci. Total Environ. 2021, 784, 147133. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, C.H.; Kögel-Knabner, A.W. Microplastics in Agricultural Soils: Increasing Awareness and Research Needs. Sci. Total Environ. 2019. [Google Scholar]
- Horton, A.A.; Galloway, P.J.; Clark, C.M.L. Heavy Metals in Agricultural Soils and the Role of Microplastics in Altering Their Bioavailability and Uptake in Plants. Environ. Sci. Technol. 2021. [Google Scholar]
- Bethanis, J.; Golia, E.E. Revealing the Combined Effects of Microplastics, Zn, and Cd on Soil Properties and Metal Accumulation by Leafy Vegetables: A Preliminary Investigation by a Laboratory Experiment. Soil Syst. 2023, 7, 65. [Google Scholar] [CrossRef]
- Roy, T.; Dey, T.K.; Jamal, M. Microplastics Increase Cadmium Absorption and Impair Nutrient Uptake and Growth in Red Amaranth (Amaranthus tricolor L.) in the Presence of Cadmium and Biochar. BMC Plant Biol. 2024, 24, 608. [Google Scholar] [CrossRef]
- Li, H.; Liu, L. Short-Term Effects of Polyethylene and Polypropylene Microplastics on Soil Phosphorus and Nitrogen Availability. Chemosphere 2022, 291, 132984. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Copper and cobalt accumulation in plants: A critical assessment of the current state of knowledge. New Phytol. 2017, 213, 537–551. [Google Scholar]
- Lehmann, A.; Leifheit, E.F.; Feng, L.; Bergmann, J.; Wulf, A.; Rillig, M.C. Microplastic fiber and drought effects on plants and soil are only slightly modified by arbuscular mycorrhizal fungi. Soil Ecol. Lett. 2020, 4, 32–44. [Google Scholar] [CrossRef]
- Sun, Q.; Li, R.; Chen, X. Interactions Between Microplastics and Heavy Metals in Agricultural Soils: Effects on Bioaccumulation in Plants and Soil Properties. Environ. Toxicol. Chem. 2022. [Google Scholar]
- Azeem, I.; Wang, Q.; Adeel, M.; Shakoor, N.; Zain, M.; Li, Y.; Azeem, K.; Nadeem, M.; Zhu, G.; Yukui, R. Assessing the combined impacts of microplastics and nickel oxide nanomaterials on soybean growth and nitrogen fixation potential. J. Hazard. Mater. 2024, 480, 136062. [Google Scholar] [CrossRef] [PubMed]
- Ammara, S.; Rafiq, M.T.; Aziz, R.; Feng, Y.; Mehmood, S.; Taneez, M.; Suhaib, M.; Asif, F. Nickel uptake in leafy greens from contaminated soil: An investigation into phytoavailability and health risk assessment using in vitro digestion model. Environ. Monit. Assess. 2024, 196, 171. [Google Scholar] [CrossRef] [PubMed]
- Colzi, I.; Renna, L.; Bianchi, E.; Castellani, M.B.; Coppi, A.; Pignattelli, S.; Loppi, S.; Gonnelli, C. Impact of microplastics on growth, photosynthesis and essential elements in Cucurbita pepo L. J. Hazard. Mater. 2022, 423, 127238. [Google Scholar] [CrossRef]
- Moreno-Caselles, J.; Perez-Espinosa, A.; Murcia, P.; Moral, R.; Gómez Lucas, I. Cobalt-induced stress in soilless lettuce cultivation: I. Effect on yield and pollutant accumulation. Acta Hortic. 1998, 458, 239–242. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hossain, M.S.; Khan, M.A. Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Chemosphere 2020, 260, 127652. [Google Scholar]
- Zhou, L.; Zhang, M.; Wang, H. Microplastics in Agricultural Soils: Effects on Soil Properties, Microbial Communities, and Heavy Metal Uptake in Crops. Environ. Pollut. 2021. [Google Scholar]
- Qi, L.; Luo, Y.; Liu, J. Impact of Microplastics on Soil Microbial Activity and Organic Matter Content: Consequences for Metal Bioavailability. Soil Biol. Biochem. 2022. [Google Scholar]
- Medyńska-Juraszek, A.; Jadhav, B. Biochar Amendments in Soils and Heavy Metal Tolerance in Crop Plants. In Biochar for Environmental Management: Science, Technology and Applications; Hossain, M.A., Sarkar, B., Ok, Y.S., Bolan, N., Saint-Carlier, S., Awasthi, M.K., Kumar, M., Oleszczuk, P., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 387–412. [Google Scholar]
- Chen, G.; Fu, Q.; Tan, X.; Yang, H.; Luo, Y.; Shen, M.; Gu, Y. Speciation and Release Risk of Heavy Metals Bonded on Simulated Naturally-Aged Microplastics Prepared from Artificially Broken Macroplastics. Environ. Pollut. 2022, 295, 118695. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Zhang, Y.; Fan, P.; Xi, B.; Tan, W. Metal type and aggregate microenvironment govern the response sequence of speciation transformation of different heavy metals to microplastics in soil. Sci. Total Environ. 2021, 752, 141956. [Google Scholar] [CrossRef]
- Jazbia, S.; Chen, Y.; Shah, A.H.; Da, Y.; Zhou, G.; Sun, Q. Microplastic Driving Changes in the Soil Microbes and Lettuce Growth Under the Influence of Heavy Metals Contaminated Soil. Front. Plant Sci. 2024, 15, 1427166. [Google Scholar]
- Binda, G.; Carnati, S.; Spanu, D.; Bellasi, A.; Hurley, R.; Bettinetti, R.; Monticelli, D.; Pozzi, A.; Nizzetto, L. Selection of the Optimal Extraction Protocol to Investigate the Interaction Between Trace Elements and Environmental Plastic. J. Hazard. Mater. 2023, 452, 131330. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Zhou, T.; Wen, C.; Yan, C. The Effects of Microplastics on Heavy Metals Bioavailability in Soils: A Meta-analysis. J. Hazard. Mater. 2023, 460, 132369. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, F.J. Organic matter-micronutrient reactions in soil. Micronutr. Agric. 1991, 4, 145–186. [Google Scholar]
- ur Rehman, M.Z.; Rizwan, M.; Rauf, A.; Ayub, M.A.; Ali, S.; Qayyum, M.F.; Waris, A.A.; Naeem, A.; Sanaullah, M. Split application of silicon in cadmium (Cd) spiked alkaline soil plays a vital role in decreasing Cd accumulation in rice (Oryza sativa L.) grains. Chemosphere 2019, 226, 454–462. [Google Scholar] [CrossRef]
- Shi, J.; Wu, Q.; Zheng, C.; Yang, J. The interaction between particulate organic matter and copper, zinc in paddy soil. Environ. Pollut. 2018, 243, 1394–1402. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Chen, J.; Shi, X. Microplastics in Agricultural Soils: Effects on Soil Properties, Plant Growth, and Heavy Metal Uptake. Environ. Pollut. 2023, 291, 118128. [Google Scholar]
- Mbachu, O.; Ugwoke, P.E.; Osakwe, O.O. The rise of artificial soil carbon inputs: Reviewing microplastic pollution effects in the soil environment. Sci. Total Environ. 2021, 780, 146569. [Google Scholar] [CrossRef]
- Roy, T.; Dey, T.K.; Jamal, M. Microplastic/nanoplastic toxicity in plants: An imminent concern. Environ. Monit. Assess. 2023, 195, 27. [Google Scholar] [CrossRef]
- Khalil, H.A.; Hossain, S.; Rosamah, E.; Azli, N.; Saddon, N.; Davoudpoura, Y.; Islam, N.; Dungani, R. The role of soil properties and its interaction towards quality plant fiber: A review. Renew. Sustain. Energy Rev. 2015, 43, 1006–1015. [Google Scholar] [CrossRef]
- Khan, Z.I.; Ashfaq, A.; Ahmad, K.; Batool, A.I.; Aslam, M.; Ahmad, T.; Mehmood, N.; Noorka, I.R.; Gaafar, A.-R.Z.; Elshikh, M.S.; et al. Cobalt Uptake by Food Plants and Accumulation in Municipal Solid Waste Materials Compost-amended Soil: Public Health Implications. Biol. Trace Elem. Res. 2024, 202, 4302–4313. [Google Scholar] [CrossRef]
- Balkhair, K.S.; Ashraf, M.A. Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J. Biol. Sci. 2016, 23, S32–S44. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; Magni, S.; Binelli, A. A global perspective on microplastic bioaccumulation in marine organisms. Ecol. Indic. 2023, 149, 110179. [Google Scholar] [CrossRef]
- Wen, X.; Yin, L.; Zhou, Z.; Kang, Z.; Sun, Q.; Zhang, Y.; Long, Y.; Nie, X.; Wu, Z.; Jiang, C. Microplastics can affect soil properties and chemical speciation of metals in yellow-brown soil. Ecotoxicol. Environ. Saf. 2022, 243, 113958. [Google Scholar] [CrossRef]
- Kinigopoulou, S.; Drosou, C.; Kalogerakis, N. Microplastics as carriers of inorganic and organic contaminants in the environment: A review of recent progress. J. Mol. Liq. 2022, 350, 118580. [Google Scholar] [CrossRef]
- Selonen, S.; Dolar, A.; Kokalj, A.J.; Skalar, T.; Dolcet, L.P.; Hurley, R.; van Gestel, C.A. Exploring the impacts of plastics in soil-The effects of polyester textile fibers on soil invertebrates. Sci. Total Environ. 2020, 700, 134451. [Google Scholar] [CrossRef]
- ASTM D6400. Available online: https://store.astm.org/d6400-21.html (accessed on 25 May 2025).
- EN13432. Available online: https://docs.european-bioplastics.org/publications/bp/EUBP_BP_En_13432.pdf (accessed on 25 May 2025).
- Brazauskienė, D.M.; Paulauskas, V.; Sabienė, N. Speciation of Zn, Cu, and Pb in the soil depending on soil texture and fertilization with sewage sludge compost. J. Soils Sediments 2008, 8, 184–192. [Google Scholar] [CrossRef]
- Durmaz, B.; Sanin, F.D. Effect of carbon to nitrogen ratio on the physical and chemical properties of activated sludge. Environ. Technol. 2003, 24, 1331–1340. [Google Scholar] [CrossRef]
- Daud, N.H.; Jayaraman, S.; Mohamed, R. Methods Paper: An improved surface sterilization technique for introducing leaf, nodal and seed explants of Aquilaria malaccensis from field sources into tissue culture. Aspac. J. Mol. Biol. Biotechnol. 2012, 20, 55–58. [Google Scholar]
- Gong, C.; Ding, Y.; Lu, H.-C.; Bu, D.-L.; Wang, L.-H.; Xiong, T.; Zhang, Z.-X. Simultaneous determination of 28 elements including rare earth elements by ICP-MS with five-acid dissolution. Rock Miner. Anal. 2021, 40, 340–348. [Google Scholar]
- Villen-Guzman, M.; Cerrillo-Gonzalez, M.d.M.; Paz-Garcia, J.M.; Vereda-Alonso, C.; Gomez-Lahoz, C.; Rodriguez-Maroto, J.M. Sequential Extraction Procedure: A Versatile Tool for Environmental Research. Detritus 2020, 13, 23–28. [Google Scholar] [CrossRef]

| Microplastic Treatment | Root Length (cm) | Root Biomass (gm) |
|---|---|---|
| Control | 9.20 ± 1.31 ab | 0.48 ± 0.02 a |
| P1 (Fiber) | 10.30 ± 3.41 a | 0.37 ± 0.27 a |
| P2 (Glitter) | 9.62 ± 1.44 ab | 0.43 ± 0.15 a |
| P3 (Plastic Bags) | 7.53 ± 2.48 ab | 0.39 ± 0.01 a |
| P4 (Plastic Bottles) | 7.12 ± 1.27 b | 0.34 ± 0.06 a |
| Soil Properties | Heavy Metal | ||||||
|---|---|---|---|---|---|---|---|
| Cd | Co | Cr | Cu | Zn | Pb | As | |
| Soil pH | 0.887 * | 0.666 | 0.298 | −0.095 | −0.455 | 0.623 | 0.618 |
| C:N ratio | −0.680 | −0.200 | −0.030 | 0.900 * | −0.201 | 0.136 | 0.419 |
| Characteristics | Values |
|---|---|
| Texture | loamy sand (87% sand, 6% silt, 7% clay). |
| pH | 6.83 |
| TOC | 1.66% |
| TN | 0.10% |
| C/N | 15.8 |
| Total Cu | 354.6 mg/kg |
| Total Pb | 147.4 mg/kg |
| Total Ni | 18.8 mg/kg |
| Total Zn | 68.5 mg/kg |
| Total Cd | 13.0 mg/kg |
| Total Cr | 30.0 mg/kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadhav, B.; Medyńska-Juraszek, A. Microplastic-Mediated Heavy Metal Uptake in Lettuce (Lactuca sativa L.): Implications for Food Safety and Agricultural Sustainability. Molecules 2025, 30, 2370. https://doi.org/10.3390/molecules30112370
Jadhav B, Medyńska-Juraszek A. Microplastic-Mediated Heavy Metal Uptake in Lettuce (Lactuca sativa L.): Implications for Food Safety and Agricultural Sustainability. Molecules. 2025; 30(11):2370. https://doi.org/10.3390/molecules30112370
Chicago/Turabian StyleJadhav, Bhakti, and Agnieszka Medyńska-Juraszek. 2025. "Microplastic-Mediated Heavy Metal Uptake in Lettuce (Lactuca sativa L.): Implications for Food Safety and Agricultural Sustainability" Molecules 30, no. 11: 2370. https://doi.org/10.3390/molecules30112370
APA StyleJadhav, B., & Medyńska-Juraszek, A. (2025). Microplastic-Mediated Heavy Metal Uptake in Lettuce (Lactuca sativa L.): Implications for Food Safety and Agricultural Sustainability. Molecules, 30(11), 2370. https://doi.org/10.3390/molecules30112370








