Functionalized Carbon Nanotubes: Emerging Nanomaterials for Enhanced Cancer Diagnosis and Imaging
Abstract
1. Introduction
2. Cancer and Carbon Nanotubes: Overview
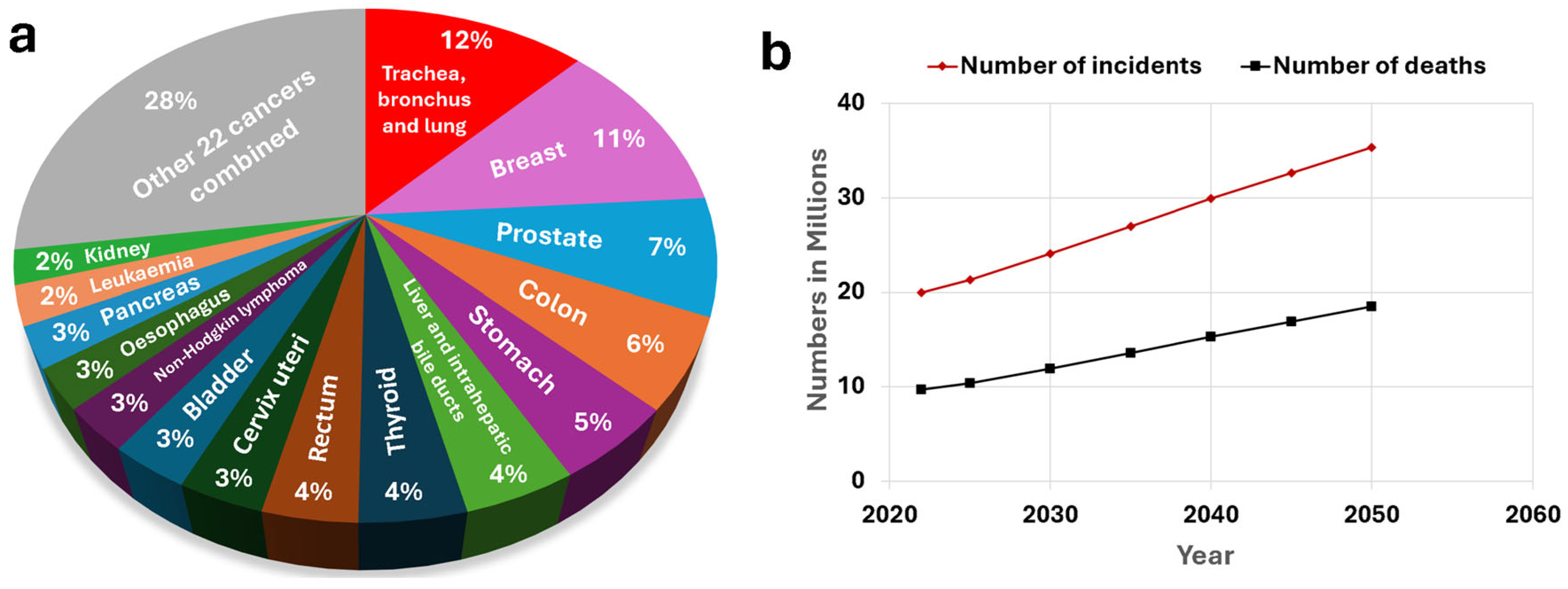
2.1. Cancer
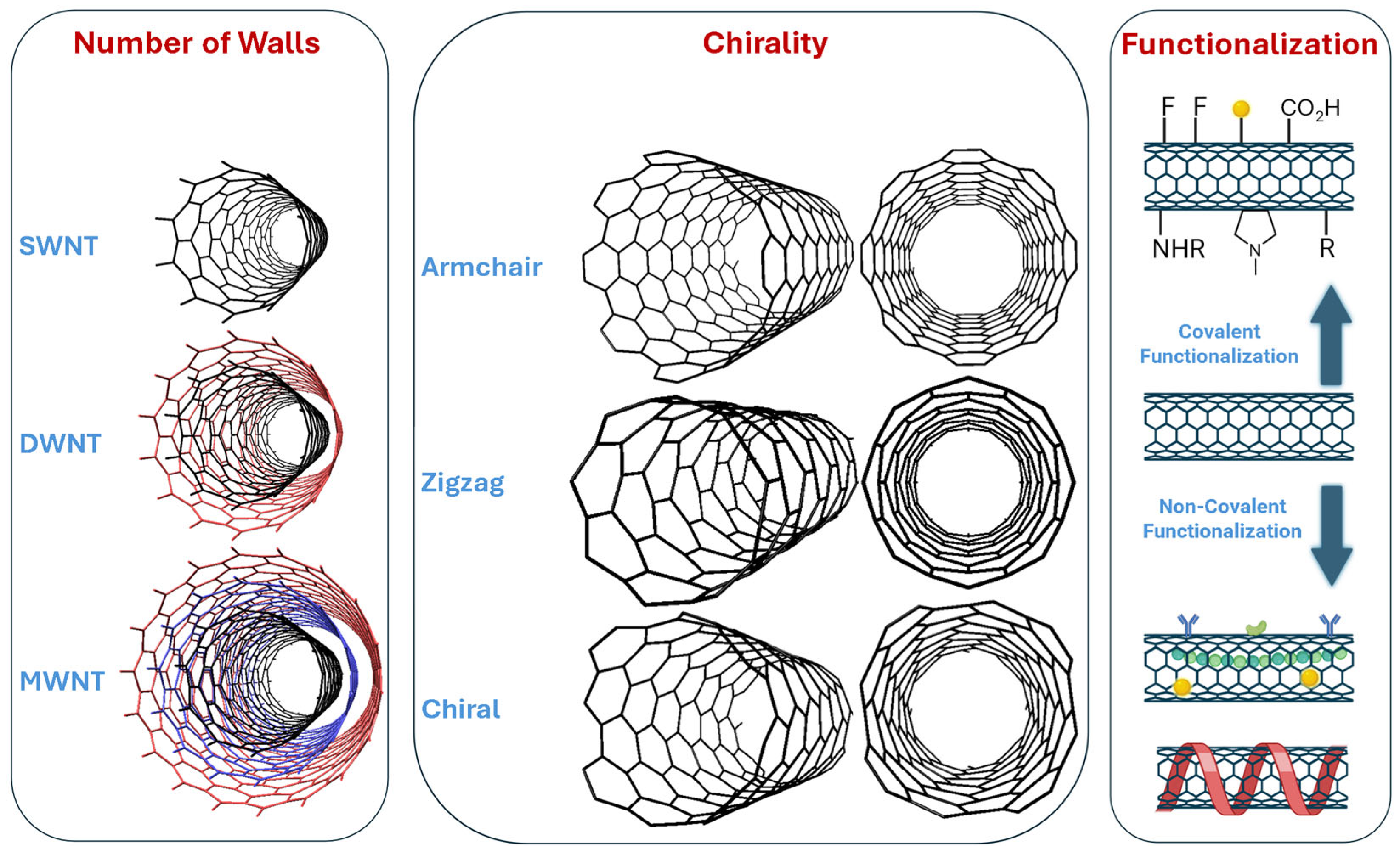
2.2. Carbon Nanotubes
3. Functionalization Strategies for CNTs
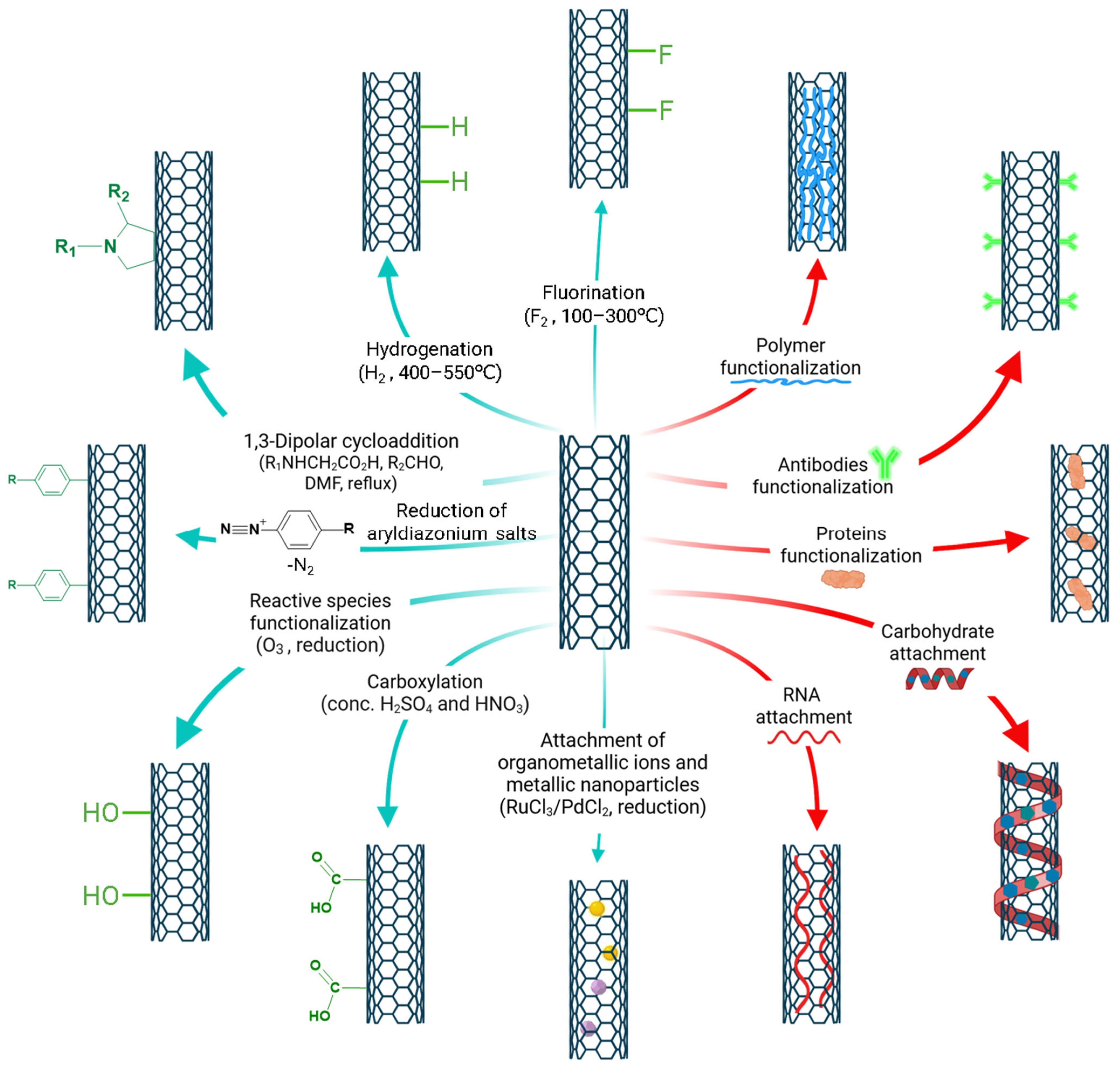
3.1. Covalent Functionalization
3.1.1. Fluorination
3.1.2. Hydrogenation
3.1.3. 1,3-Dipolar Cycloadditions
3.1.4. Reduction of Aryldiazonium Salts
3.1.5. Reactive Species Functionalization
3.1.6. Organometallic Ions
3.1.7. Carboxylation and Further Derivation
3.1.8. Attachment of Metallic Nanoparticles
3.2. Non-Covalent Functionalization
3.2.1. Polymer Functionalized CNTs
3.2.2. Antibodies Functionalized CNTs
3.2.3. Proteins Functionalized CNTs
3.2.4. Carbohydrate Attachment
3.2.5. RNA Attachment
| Type of Functionalization | Functionality Method | Example | Benefit | Application | Reference |
|---|---|---|---|---|---|
| Covalent Functionalization | Fluorination | Introduction of fluorine atoms to CNTs | Improves dispersion; enhances electronic properties; and increases reactivity | Drug delivery, photothermal therapy | [51,53,56] |
| Hydrogenation | Attachment of hydrogen atoms | Modifies electrical conductivity and improves catalytic activity. Works with cisplatin for anticancer properties. | Drug delivery, fuel cells | [19,58,59] | |
| 1,3-Dipolar cycloadditions | Reaction with azomethine ylides | Enhances bioconjugation and allows precise functionalization | Cancer treatment, photodynamic therapy | [62,66] | |
| Reduction of aryldiazonium salts | Arylation via diazonium salt chemistry | Enables attachment of functional groups for improved targeting | Drug delivery, biosensors | [24,72] | |
| Reactive species functionalization | Functionalization using reactive oxygen species | Enhances surface reactivity and interactions with biomolecules | Sensors, catalysis, medical applications | [30] | |
| Organometallic ions | Coordination with transition metals | Improves electronic and catalytic properties | Biosensors, imaging | [81,85,86] | |
| Carboxylation and further derivation | Carboxyl (-COOH) groups on CNTs | Enhances solubility; allows further modifications | Drug delivery, biosensors | [30,90] | |
| Amidation and esterification of carboxylic groups | Reaction of carboxylated CNTs with amines/esters | Improves biocompatibility and drug-loading efficiency | Drug delivery, biosensors | [90,91] | |
| Ionic functionalization of carboxylic groups | Functionalization with ionic groups | Enhances dispersibility in aqueous solutions; improves targeting | Drug delivery, diagnostics | [90] | |
| Attachment of metallic nanoparticles | Deposition of Au, Ag, and Pt nanoparticles | Improves imaging contrast; enhances targeted therapy | Imaging, cancer treatment | [47,94] | |
| Non-Covalent Functionalization | Surfactant functionalization | Use of anionic (SDS, NaDDBS), cationic (CTAB, MATMAC), or non-ionic (Triton X-100) surfactants | Enhances dispersibility and stability in biological systems | Drug delivery, antimicrobial applications | [75] |
| Polymer wrapping | Wrapping CNTs with conjugated polymers | Stabilizes CNTs in biological environments; improves targeting | Drug delivery, biosensors | [102] | |
| Polymer encapsulation | Encapsulation in biocompatible polymers | Improves biocompatibility; enables controlled drug release | Drug delivery, regenerative medicine | [109] | |
| Polymer absorption | Adsorption of functional polymers onto CNTs | Enhances stability; provides controlled release properties | Drug delivery, biosensors | [112] | |
| RNA attachment | Conjugation of RNA to CNTs | Enables gene silencing; improves targeted drug delivery | Cancer therapy, genetic engineering | [120,122] | |
| Protein attachment | Functionalization of CNTs with proteins | Enhances biocompatibility; enables targeted therapy | Cancer treatment, biosensors | [27] | |
| Antibodies attachment | Conjugation of antibodies to CNTs | Enables specific targeting of cancer cells; enhances biosensor performance | Cancer detection, immunotherapy | [105] | |
| Carbohydrate attachment | Functionalization with sugars like hyaluronic acid | Improves cellular recognition; enables targeted drug delivery | Cancer therapy, biosensors | [48] |
4. Carbon Nanotubes in Cancer Diagnosis
4.1. Overview
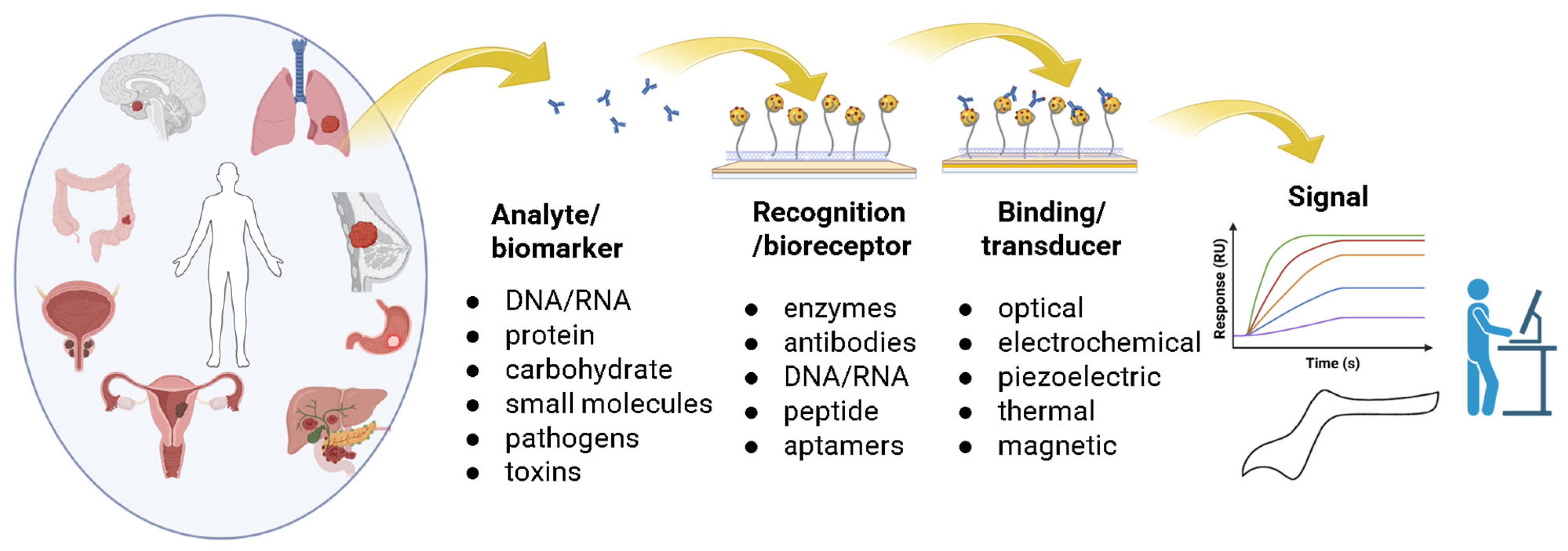
4.2. CNT-Biosensors for Selective Cancer Detection
4.2.1. Electrochemical Biosensors
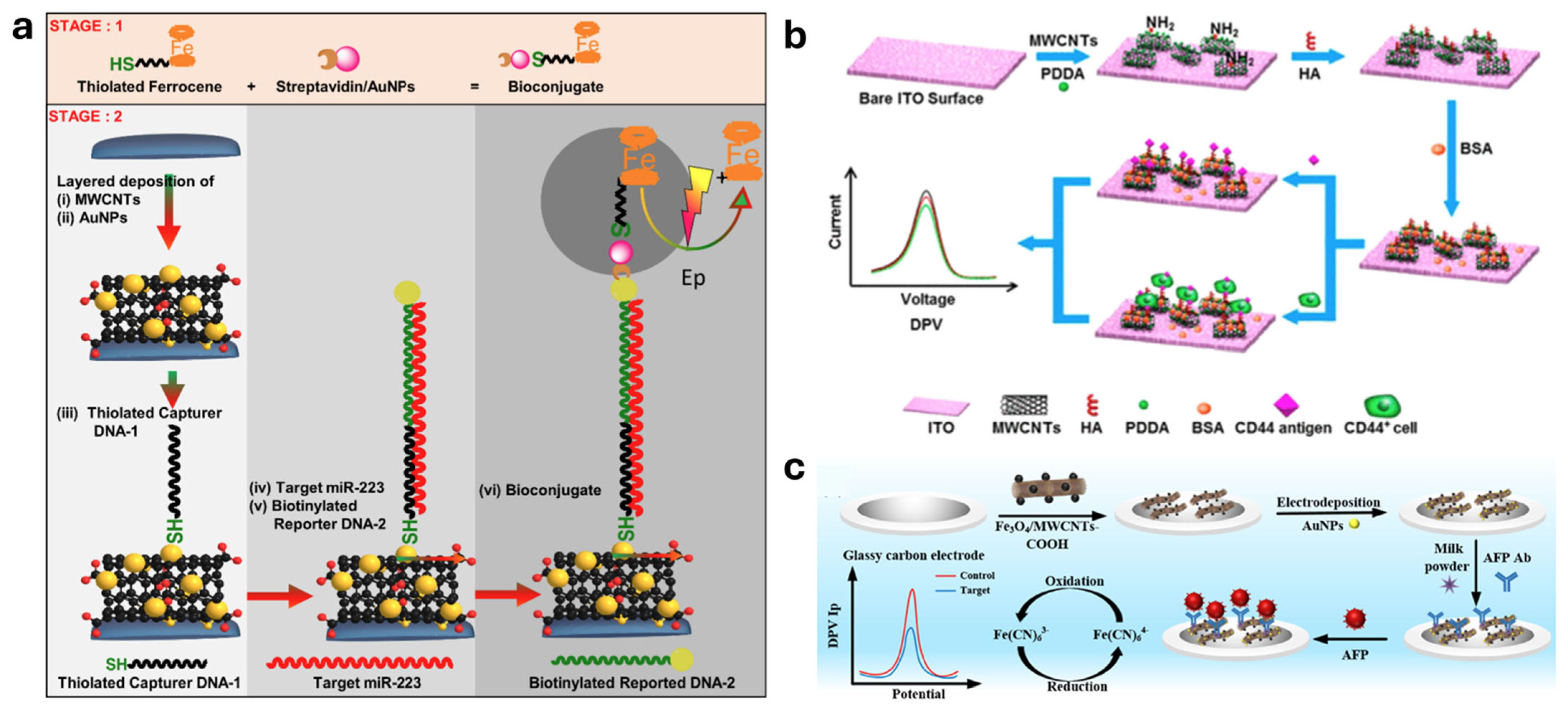
4.2.2. Field-Effect Transistor (FET) Sensors
4.2.3. Optical Sensors
| Type of Biosensor | Type of CNT Used | Type of Cancer/Biomarker/Molecule Detected | Type of Recorded Signal | Detection Limit | Ref. |
|---|---|---|---|---|---|
| Electrochemical | Functionalized MWCNT (f-MWCNT) | Lung cancer/CKAP4 | Change in current or potential (implied) | 6.25 pg/mL | [152] |
| Gold nanoparticle (AuNPs) functionalized MWCNT | Non-small cell lung cancer (NSCLC)/miR-223 | Change in current or potential (implied) | 0.73 pM | [153] | |
| MWCNTs | Lung and breast cancers/CD44 (cancer cells) | Electrochemical impedance spectroscopy (EIS) | 5.94 pg/mL | [154] | |
| MWCNTs-COOH functionalized with Fe3O4 and AuNPs | Liver cancer/alpha-fetoprotein (AFP) | Electrochemical signal | 1.09034 pg/mL | [158] | |
| CNTs combined with conducting polymer | Liver cancer/alpha-fetoprotein (AFP) | Cyclic voltammetry (CV) | 0.20 μM | [159] | |
| MWCNTs modified with gold nanoparticles (GNPs) | Breast cancer/HER2 | Differential pulse voltammetry (DPV) | 4.4 Pg/mL | [160] | |
| Field-Effect Transistor (FET) | Metal carbide@carbon nanotubes (MC@CNTs) | Liver cancer/exosomal microRNA-122 | Change in electrical conductivity/transconductance | 0.12 fM | [163] |
| CNT | Non-small cell lung cancer (NSCLC) cells/adenosine receptor activity/adenosine | Electrochemical transduction (implied through hybridization with CNT-FET) | 1 fM | [162] | |
| CNT functionalized with specific antibodies | Lung cancer/carcinoembryonic antigen (CEA) | Change in electrical conductivity (implied) | 72 ag/mL | [164] | |
| Semiconductor CNT functionalized with peptide nucleic acid (PNA) probes | Breast cancer/BRCA1 gene | Change in electrical conductivity (implied) | 1.38 aM | [165] | |
| CNT-FET coated with fibronectin | Breast cancer/exosomal miRNA21 | Change in electrical conductivity (implied) | 0.87 aM | [166] | |
| High-purity semiconductor CNT modified with enzymatic cascade reactors | Prostate cancer/sarcosine | Enhanced signal transduction (change in electrical conductivity implied) | 105 zM | [167] | |
| Optical | CNTs in a CaF2 prism/Cu/CNT/HfSe2 multilayer structure | Colorectal cancer/refractive index difference between healthy and cancerous tissue | Shift in resonance angle | Maximum sensitivity of 387.60 deg/RIU (for colorectal cancer detection) | [157] |
| MWCNTs functionalized with silane | Chronic Lymphocytic Leukemia (CLL)/CLL cells | Photocurrent and photoconductive response | Limit of detection of 27 cells/mL | [173] | |
| SWCNTs (as quenchers) | Ovarian cancer/HE4; prostate cancer/PSMA (among others not specific to one cancer in this description) | Fluorescence increases upon analyte binding | For HE4: could discriminate between mice injected with 10 pmol and controls | [176] |
4.3. CNTs in Cancer Imaging Modalities
4.3.1. CNTs in Fluorescence Imaging
4.3.2. CNT-Enhanced MRI
4.3.3. CNTs in Computed Tomography (CT)
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; Xia, F.; Lin, R. Global Burden of Cancer and Associated Risk Factors in 204 Countries and Territories, 1980–2021: A Systematic Analysis for the GBD 2021. J. Hematol. Oncol. 2024, 17, 119. [Google Scholar] [CrossRef] [PubMed]
- Cancer Key Facts. Available online: https://www.who.int/Health-Topics/Cancer#tab=tab_1 (accessed on 9 April 2025).
- Cancer Today. Available online: https://gco.iarc.who.int/today/ (accessed on 2 March 2025).
- Cancer Tomorrow. Available online: https://gco.iarc.who.int/tomorrow/ (accessed on 2 March 2025).
- Zhang, Z.; Wang, J.; Hou, L.; Zhu, D.; Xiao, H.-J.; Wang, K. Graphene/Carbohydrate Polymer Composites as Emerging Hybrid Materials in Tumor Therapy and Diagnosis. Int. J. Biol. Macromol. 2025, 287, 138621. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, M.M.; Almuhanna, A.; Alhiyafi, J. Mammography Image-Based Diagnosis of Breast Cancer Using Machine Learning: A Pilot Study. Sensors 2022, 22, 203. [Google Scholar] [CrossRef] [PubMed]
- Bui, C.N.; Hong, S.; Suh, M.; Jun, J.K.; Jung, K.W.; Lim, M.C.; Choi, K.S. Effect of Pap Smear Screening on Cervical Cancer Stage at Diagnosis: Results from the Korean National Cancer Screening Program. J. Gynecol. Oncol. 2021, 32, e81. [Google Scholar] [CrossRef]
- Biosensors Based on Graphene, Graphene Oxide and Graphynes for Early Detection of Cancer; Jain, P., Verma, C., Raman, A.P.S., Kumari, K., Singh, P., Eds.; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-00-349136-1. [Google Scholar]
- Alwabel, S.A.M.; Alwadai, M.S.M.; AlQahtani, H.A.M.; Almutairi, F.S.B.; Almutairi, M.A.M.; Otaibi, F.S.J.A. Novel Drug Delivery Systems: Advancements And Challenges. J. Popul. Ther. Clin. Pharmacol. 2022, 29, 2573–2576. [Google Scholar] [CrossRef]
- Bargahi, N.; Ghasemali, S.; Jahandar-Lashaki, S.; Nazari, A. Recent Advances for Cancer Detection and Treatment by Microfluidic Technology, Review and Update. Biol. Proced. Online 2022, 24, 5. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Devireddy, R.; Gartia, M.R. Surface Plasmon Resonance (SPR) Sensor for Cancer Biomarker Detection. Biosensors 2023, 13, 396. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Zou, Y.; Li, Z. Self-Powered Sensors for Biomarker Detection. Sens. Diagn. 2023, 2, 1097–1122. [Google Scholar] [CrossRef]
- Yu, S.-Q.; Li, P.; Li, H.-J.; Shang, L.-J.; Guo, R.; Sun, X.-M.; Ren, Q.-Q. Highly Sensitive Detection of Hydrogen Peroxide in Cancer Tissue Based on 3D Reduced Graphene Oxide–MXene–Multi-Walled Carbon Nanotubes Electrode. Biosensors 2024, 14, 261. [Google Scholar] [CrossRef]
- Chandradoss, S.D.; Schirle, N.T.; Szczepaniak, M.; MacRae, I.J.; Joo, C. A Dynamic Search Process Underlies MicroRNA Targeting. Cell 2015, 162, 96–107. [Google Scholar] [CrossRef]
- Chen, C. Identification of a Fully Human Antibody VH Domain Targeting Anaplastic Lymphoma Kinase (ALK) With Applications in ALK-Positive Solid Tumor Immunotherapy. Antibodies 2024, 13, 39. [Google Scholar] [CrossRef]
- Kamazani, F.M.; Nematalahi, F.S.; Siadat, S.D.; Pornour, M.; Sheikhpour, M. A Success Targeted Nano Delivery to Lung Cancer Cells With Multi-Walled Carbon Nanotubes Conjugated to Bromocriptine. Sci. Rep. 2021, 11, 24419. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, M.; Pan, Y.; Xie, D.; Hong, C.; Li, J.; Ma, X.; Xu, H.; Li, H.; Chen, T.; et al. Sequential Targeting Biomimetic Nano Platform for Enhanced Mild Photothermal Therapy and Chemotherapy of Tumor. Comput. Struct. Biotechnol. J. 2023, 21, 2780–2791. [Google Scholar] [CrossRef]
- Bohunicky, B.; Mousa, S.A. Biosensors: The New Wave in Cancer Diagnosis. Nanotechnol. Sci. Appl. 2010, 4, 1–10. [Google Scholar] [CrossRef]
- Gao, S.; Xu, B.; Sun, J.; Zhang, Z. Nanotechnological Advances in Cancer: Therapy a Comprehensive Review of Carbon Nanotube Applications. Front. Bioeng. Biotechnol. 2024, 12, 1351787. [Google Scholar] [CrossRef] [PubMed]
- Carbon Nanotubes; Topics in applied physics; Dresselhaus, M.S., Dresselhaus, G., Avouris, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 80, ISBN 978-3-540-41086-7. [Google Scholar]
- Chadar, R.; Afzal, O.; Alqahtani, S.M.; Kesharwani, P. Carbon Nanotubes as an Emerging Nanocarrier for the Delivery of Doxorubicin for Improved Chemotherapy. Colloids Surf. B Biointerfaces 2021, 208, 112044. [Google Scholar] [CrossRef] [PubMed]
- Elhissi, A.M.A.; Ahmed, W.; Hassan, I.U.; Dhanak, V.R.; D′Emanuele, A. Carbon Nanotubes in Cancer Therapy and Drug Delivery. J. Drug Deliv. 2012, 2012, 837327. [Google Scholar] [CrossRef]
- Shabnum, S.S.; Siranjeevi, R.; Raj, C.K.; Nivetha, P.; Benazir, K. A Comprehensive Review on Recent Progress in Carbon Nanotubes for Biomedical Application. Environ. Qual. Manag. 2025, 34, e70040. [Google Scholar] [CrossRef]
- Kumar, S.; Ansari, A.; Basu, M.; Ghosh, S.; Begam, S.; Ghosh, M.K. Carbon Nanotubes in Cancer Diagnosis and Treatment: Current Trends and Future Perspectives. Adv. Ther. 2025, 8, 2400283. [Google Scholar] [CrossRef]
- Rahmanifar, E.; Saheb, V.; Yoosefian, M. Carbon Nanotube Coated with Tryptophan as a pH-Sensitive Nanocarrier in the Delivery and Smart Release of the Anticancer Drug Topotecan. J. Mol. Liq. 2025, 419, 126767. [Google Scholar] [CrossRef]
- Contreras, L.; Villarroel, I.; Torres, C.; Rozas, R. Doxorubicin Encapsulation in Carbon Nanotubes Having Haeckelite or Stone–Wales Defects as Drug Carriers: A Molecular Dynamics Approach. Molecules 2021, 26, 1586. [Google Scholar] [CrossRef] [PubMed]
- Papi, R.M.; Tasioulis, K.S.; Kechagioglou, P.V.; Papaioannou, M.A.; Andriotis, E.G.; Kyriakidis, D.A. Carbon Nanotube-Mediated Delivery of PTEN Variants: In Vitro Antitumor Activity in Breast Cancer Cells. Molecules 2024, 29, 2785. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, K.T.; Nunes, A.; Methven, L.; Ali-Boucetta, H.; Li, S.; Toma, F.M.; Herrero, M.A.; Al-Jamal, W.T.; ten Eikelder, H.M.M.; Foster, J.; et al. Degree of Chemical Functionalization of Carbon Nanotubes Determines Tissue Distribution and Excretion Profile. Angew. Chem. 2012, 124, 6495–6499. [Google Scholar] [CrossRef]
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized Carbon Nanotubes: Synthesis, Properties and Applications in Water Purification, Drug Delivery, and Material and Biomedical Sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef]
- AbouAitah, K.; Abdelaziz, A.M.; Higazy, I.M.; Swiderska-Sroda, A.; Hassan, A.M.E.; Shaker, O.G.; Szałaj, U.; Stobinski, L.; Malolepszy, A.; Lojkowski, W. Functionalized Carbon Nanotubes for Delivery of Ferulic Acid and Diosgenin Anticancer Natural Agents. ACS Appl. Bio Mater. 2024, 7, 791–811. [Google Scholar] [CrossRef]
- John, B.K.; John, N.; Mathew, J.; Mathew, B. Carbon Nanotubes for Bioimaging. In Carbon Nanotubes for Biomedical Applications and Healthcare; Apple Academic Press: Point Pleasant, NJ, USA, 2024; ISBN 978-1-00-339639-0. [Google Scholar]
- Acharya, R.; Patil, T.V.; Dutta, S.D.; Lee, J.; Ganguly, K.; Kim, H.; Randhawa, A.; Lim, K.-T. Single-Walled Carbon Nanotube-Based Optical Nano/Biosensors for Biomedical Applications: Role in Bioimaging, Disease Diagnosis, and Biomarkers Detection. Adv. Mater. Technol. 2024, 9, 2400279. [Google Scholar] [CrossRef]
- Chou, H.-T.; Wang, T.-P.; Lee, C.-Y.; Tai, N.-H.; Chang, H.-Y. Photothermal Effects of Multi-Walled Carbon Nanotubes on the Viability of BT-474 Cancer Cells. Mater. Sci. Eng. C 2013, 33, 989–995. [Google Scholar] [CrossRef]
- Farbod, M.; Sharif, L.; Rezatofighi, S.E. Photothermal Performance of Carbon Nanotubes in the Visible and Near-Infrared Regions: Applications in Water Evaporation and Destroying Cancer Cells. Colloid Polym. Sci. 2025, 303, 25–31. [Google Scholar] [CrossRef]
- Murmu, M.; Dey, D.; Murmu, N.C.; Banerjee, P. Carbon Nanotubes. In Functionalized Carbon Nanotubes for Biomedical Applications; John Wiley & Sons, Ltd.: Beverly, MA, USA, 2023; pp. 49–73. ISBN 978-1-119-90508-0. [Google Scholar]
- Kamoun, E.A.; Elsabahy, M.; Mohamed Elbadry, A.M.; Abdelazim, E.B.; Mohsen, A.A.; Aleem, M.A.; Gao, H.; Eissa, N.G.; Elghamry, I.; Salim, S.A. Recent Progress of Polymer-Based Biosensors for Cancer Diagnostic Applications: Natural versus Synthetic Polymers. ACS Omega 2025, 10, 8816–8831. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories From 2020 to 2050. JAMA Oncol. 2023, 9, 465. [Google Scholar] [CrossRef]
- Yu, M.-F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes Under Tensile Load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y.; Achiba, Y. Optical Properties of Single-Wall Carbon Nanotubes. Synth. Met. 1999, 103, 2555–2558. [Google Scholar] [CrossRef]
- Issi, J.-P.; Langer, L.; Heremans, J.; Olk, C.H. Electronic Properties of Carbon Nanotubes: Experimental Results. Carbon 1995, 33, 941–948. [Google Scholar] [CrossRef]
- Hone, J.; Llaguno, M.C.; Biercuk, M.J.; Johnson, A.T.; Batlogg, B.; Benes, Z.; Fischer, J.E. Thermal Properties of Carbon Nanotubes and Nanotube-Based Materials. Appl. Phys. A Mater. Sci. Process. 2002, 74, 339–343. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical Conductivity of Individual Carbon Nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cazorla-Amorós, D.; Linares-Solano, A.; Delpeux, S.; Frackowiak, E.; Szostak, K.; Béguin, F. High Surface Area Carbon Nanotubes Prepared by Chemical Activation. Carbon 2002, 40, 1614–1617. [Google Scholar] [CrossRef]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Charlier, J.C.; Hernández, E. Electronic, Thermal and Mechanical Properties of Carbon Nanotubes. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2065–2098. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Bhattacharya, R.; Moin, A.; Hagbani, T.A.; Abdallah, M.H.; Danish Rizvi, S.M.; Khafagy, E.-S.; Hussain, T.; Gangadharappa, H.V. Dual Targeting Multiwalled Carbon Nanotubes for Improved Neratinib Delivery in Breast Cancer. RSC Adv. 2023, 13, 24309–24318. [Google Scholar] [CrossRef]
- Li, R.; Bao, Z.; Wang, P.; Deng, Y.; Fan, J.; Zhu, X.; Xia, X.; Song, Y.; Yao, H.; Li, D. Gelatin-Functionalized Carbon Nanotubes Loaded With Cisplatin for Anti-Cancer Therapy. Polymers 2023, 15, 3333. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, X.; Lin, Y.; Sang, Z.; Chen, D. Hyaluronic Acid Modified Carbon Nanotubes Using for Photothermal Therapy by Promoting Apoptosis of Nasopharyngeal Carcinoma Cells. Front. Bioeng. Biotechnol. 2023, 11, 1229852. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Hashemi, N.; Asghariha, Z.; Hosseini, M.S.; Danafar, H. SWCNTs functionalized with gold nanoradiosensitizers as radiosensitizers for enhanced radiotherapy in breast cancer. Results Chem. 2024, 14, 102113. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Li, S.; Liu, J.; Tang, N. Mechanism of Elemental Superdoping of Low-Dimensional Carbon Materials. Chem. Mater. 2024, 36, 8369–8377. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wang, Z.; Lai, W.; Zhang, X.; Wang, X.; Liu, X. Investigation of the Dispersion Behavior of Fluorinated MWCNTs in Various Solvents. Phys. Chem. Chem. Phys. 2017, 19, 21565–21574. [Google Scholar] [CrossRef]
- Raudsepp, R. Boron and Fluorine Co-Doped Graphene/Few-Walled Carbon Nanotube Composite as Highly Active Electrocatalyst for Oxygen Reduction Reaction. Chemnanomat 2024, 10, e202300546. [Google Scholar] [CrossRef]
- Ahmad, Y.; Dubois, M.; Guerin, K.; Hamwi, A.; Flahaut, E. High Energy Density of Primary Lithium Batteries Working with Sub-Fluorinated Few Walled Carbon Nanotubes Cathode. J. Alloys Compd. 2017, 726, 852–859. [Google Scholar] [CrossRef]
- Ami, T. Highly Fluorinated Nanospace in Porous Organic Salts With High Water Stability/Capability and Proton Conductivity. Angew. Chem. 2024, 136, e202407484. [Google Scholar] [CrossRef]
- Uchiyama, H. Carrier Doping in Semiconducting Carbon Nanotubes With Fluorosumanenes. J. Phys. Chem. C 2024, 128, 17668–17673. [Google Scholar] [CrossRef]
- Wang, D.; Peng, J.; Huang, Y.; Sun, L.; Liu, M.; Li, H.; Chao, M.; Gong, P.; Liu, Z.; You, J. Rational Construction of Fluorescence Turn-Off Fluorinated Carbon Fiber/Ag Composites and Their Anticancer and Antibacterial Activities. ACS Appl. Bio Mater. 2021, 4, 1749–1759. [Google Scholar] [CrossRef]
- Uniyal, A.; Srivastava, G.; Sarkar, P.; Kumar, M.; Singh, S.; Taya, S.A.; Muduli, A.; Pal, A. Fluorinated Graphene and CNT-Based Surface Plasmon Resonance Sensor for Detecting the Viral Particles of SARS-CoV-2. Phys. B Condens. Matter 2023, 669, 415282. [Google Scholar] [CrossRef]
- Yan, J.-Y. Mechanism of NCNTs Growth on Foamed Nickel and Thus-Prepared PS Hydrogenation High-Performance Carrier NCNTs@FN. Langmuir 2024, 40, 6786–6805. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W. Curvature-Switched Activity of Carbon Nanotube-Supported Single Atom Catalysts for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2024, 12, 16476–16481. [Google Scholar] [CrossRef]
- Tang, H.; Kojima, T.; Kazumi, K.; Fukami, K.; Sakaguchi, H. Platinum nanoparticles bonded with carbon nanotubes for high-performance ampere-level all-water splitting. ACS Omega 2024, 9, 21378–21387. [Google Scholar] [CrossRef]
- Duraia, E.M. Efficient Eco-Friendly Synthesis of Carbon Nanotubes Over Graphite Nanosheets From Yellow Corn: A One-Step Green Approach. Sci. Rep. 2024, 14, 16405. [Google Scholar] [CrossRef] [PubMed]
- Tomasini, M. Computational Insights Into the Regioselectivity of 1,3-Dipolar Cycloadditions Inside Carbon Nanotubes. J. Phys. Chem. C 2024, 128, 14961–14971. [Google Scholar] [CrossRef]
- Pacholak, P. Ethynyl-Substituted Benzosiloxaboroles: The Role of C(π)⋯B Interactions in Their Crystal Packing and Use in Cu(i)-Catalyzed 1,3-Dipolar Cycloaddition. RSC Adv. 2024, 14, 16069–16082. [Google Scholar] [CrossRef]
- Rebelo, S.L.H.; Laia, C.A.; Szefczyk, M.; Guedes, A.; Silva, A.M.; Freire, C. Hybrid Zn-Β-Aminoporphyrin–Carbon Nanotubes: Pyrrolidine and Direct Covalent Linkage Recognition, and Multiple-Photo Response. Molecules 2023, 28, 7438. [Google Scholar] [CrossRef]
- Fukuura, S. Roles of Carbon Nanotube Confinement in Modulating Regioselectivity of 1,3-Dipolar Cycloadditions. J. Phys. Chem. A 2023, 127, 6962–6973. [Google Scholar] [CrossRef]
- Ma, J.; Wang, G.; Ding, X.; Wang, F.; Zhu, C.; Rong, Y. Carbon-Based Nanomaterials as Drug Delivery Agents for Colorectal Cancer: Clinical Preface to Colorectal Cancer Citing Their Markers and Existing Theranostic Approaches. ACS Omega 2023, 8, 10656–10668. [Google Scholar] [CrossRef]
- Ding, K.; Zhu, Y.; Lang, Y.; Zhu, L.; Zhang, T.-T.; Zhang, R.; Li, Q.; Xie, B.; Ding, L.; Shang, L.; et al. Multiwalled Carbon Nanotubes-Reprogrammed Macrophages Facilitate Breast Cancer Metastasis via NBR2/TBX1 Axis. ACS Nano 2024, 18, 11103–11119. [Google Scholar] [CrossRef]
- Adamus-Grabicka, A.A.; Hikisz, P.; Sikora, J. Nanotechnology as a Promising Method in the Treatment of Skin Cancer. Int. J. Mol. Sci. 2024, 25, 2165. [Google Scholar] [CrossRef]
- Zhang, G.; Fu, Y.; Xiang, J.; Guan, C.; Sang, Z.; Ding, C. Nickel-Catalyzed Cross-Coupling of Aryl Diazonium Salts with Aryl Bromides. Org. Lett. 2024, 26, 6687–6691. [Google Scholar] [CrossRef]
- Han, H. 1,3-Dipolar Cycloaddition of Polycyclic Aromatic Azomethine Ylides and Alkynylbenziodoxoles for Synthesis of Functional Dibenzoullazines. Chin. J. Chem. 2024, 42, 1079–1083. [Google Scholar] [CrossRef]
- Kvasovs, N.; Gevorgyan, V. Contemporary Methods for Generation of Aryl Radicals. Chem. Soc. Rev. 2021, 50, 2244–2259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Z.; Zhang, Y. The Application of Carbon Nanotubes in Target Drug Delivery Systems for Cancer Therapies. Nanoscale Res. Lett. 2011, 6, 555. [Google Scholar] [CrossRef] [PubMed]
- Alrushaid, N.; Khan, F.A.; Al-Suhaimi, E.A.; Elaissari, A. Nanotechnology in Cancer Diagnosis and Treatment. Pharmaceutics 2023, 15, 1025. [Google Scholar] [CrossRef]
- Billon, J.; Omelchuk, A.; Shkirskiy, V.; Dabos-Seignon, S.; Alévêque, O.; Levillain, E.; Breton, T.; Gautier, C. An Innovative Method for Controlled Synthesis of Bicomponent Monolayer Films Obtained by Reduction of Diazonium. Nanoscale 2023, 15, 19213–19218. [Google Scholar] [CrossRef]
- Hilmer, A.J.; McNicholas, T.P.; Lin, S.; Zhang, J.; Wang, Q.H.; Mendenhall, J.D.; Song, C.; Heller, D.A.; Barone, P.W.; Blankschtein, D.; et al. Role of Adsorbed Surfactant in the Reaction of Aryl Diazonium Salts With Single-Walled Carbon Nanotubes. Langmuir 2012, 28, 1309–1321. [Google Scholar] [CrossRef]
- Talyzin, A.V.; Luzan, S.M.; Anoshkin, I.V.; Nasibulin, A.G.; Jiang, H.; Kauppinen, E.I.; Mikoushkin, V.M.; Shnitov, V.V.; Marchenko, D.; Noréus, D. Hydrogenation, Purification, and Unzipping of Carbon Nanotubes by Reaction With Molecular Hydrogen: Road to Graphane Nanoribbons. ACS Nano 2011, 5, 5132–5140. [Google Scholar] [CrossRef]
- Bhowmick, R.; Rajasekaran, S.; Friebel, D.; Beasley, C.; Jiao, L.; Ogasawara, H.; Dai, H.; Clemens, B.M.; Nilsson, A. Hydrogen Spillover in Pt-Single-Walled Carbon Nanotube Composites: Formation of Stable C–H Bonds. J. Am. Chem. Soc. 2011, 133, 5580–5586. [Google Scholar] [CrossRef]
- Larijani, M.M.; Safa, S. Increase of Hydrogen Storage Capacity of CNTs by Using Transition Metal, Metal Oxide-CNT Nanocomposites. Acta Phys. Pol. A 2014, 126, 732–736. [Google Scholar] [CrossRef]
- Zhornik, E.V.; Baranova, L.A.; Emel’yanova, V.P.; Volotovsky, I.D. The Generation of Reactive Oxygen Species and Induction of Lipid Peroxidation in Human Lymphocytes Under the Influence of Carbon Nanotubes. In Proceedings of the 2010 International Kharkov Symposium on Physics and Engineering of Microwaves, Millimeter, and Submillimeter Waves, Kharkiv, Ukraine, Kharkiv, Ukraine, 21–26 June 2010. [Google Scholar] [CrossRef]
- Szymański, T.; Kempa, M.; Giersig, M.; Rybka, J.D. Carbon Nanotubes Interference With Luminescence-Based Assays. Materials 2020, 13, 4270. [Google Scholar] [CrossRef]
- Gu, X.; Qi, W.; Xu, X.; Sun, Z.; Zhang, L.; Liu, W.; Pan, X.; Su, D. Covalently Functionalized Carbon Nanotube Supported Pd Nanoparticles for Catalytic Reduction of 4-Nitrophenol. Nanoscale 2014, 6, 6609–6616. [Google Scholar] [CrossRef]
- Bayazit, M.K.; Coleman, K.S. Probing the Selectivity of Azomethine Imine Cycloaddition to Single-Walled Carbon Nanotubes by Resonance Raman Spectroscopy. Chem. Asian J. 2012, 7, 2925–2930. [Google Scholar] [CrossRef]
- Veerakumar, P.; Vinothkumar, V.; Chen, S.-M.; Sangili, A.; Lin, K.-C. Ultrafine Rhenium–Ruthenium Nanoparticles Decorated on Functionalized Carbon Nanotubes for the Simultaneous Determination of Antibiotic (Nitrofurantoin) and Anti-Testosterone (Flutamide) Drugs. J. Mater. Chem. C 2021, 9, 15949–15966. [Google Scholar] [CrossRef]
- Safarkhani, M.; Moghaddam, S.S.; Taghavimandi, F.; Bagherzadeh, M.; Fatahi, Y.; Park, U.; Radmanesh, F.; Huh, Y.S.; Rabiee, N. Bioengineered Smart Nanocarriers for Breast Cancer Treatment: Adorned Carbon-Based Nanocomposites with Silver and Palladium Complexes for Efficient Drug Delivery. ACS Omega 2024, 9, 1183–1195. [Google Scholar] [CrossRef]
- Saghatchi, F.; Mohseni-Dargah, M.; Akbari-Birgani, S.; Saghatchi, S.; Kaboudin, B. Cancer Therapy and Imaging Through Functionalized Carbon Nanotubes Decorated with Magnetite and Gold Nanoparticles as a Multimodal Tool. Appl. Biochem. Biotechnol. 2020, 191, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, Z.; Pang, W.; Huang, S.; Deng, M.; Yao, J.; Huang, Q.; Jin, M.; Shui, L. Integrated Biosensor Array for Multiplex Biomarkers Cancer Diagnosis via in-Situ Self-Assembly Carbon Nanotubes with an Ordered Inverse-Opal Structure. Biosens. Bioelectron. 2024, 262, 116528. [Google Scholar] [CrossRef]
- Mo, F.; Qiu, D.; Zhang, L.; Wang, J. Recent Development of Aryl Diazonium Chemistry for the Derivatization of Aromatic Compounds. Chem. Rev. 2021, 121, 5741–5829. [Google Scholar] [CrossRef]
- Sonam; Shinde, V.N.; Kumar, A. KPF6-Mediated Esterification and Amidation of Carboxylic Acids. J. Org. Chem. 2022, 87, 2651–2661. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Z.; Yu, F.; Thakkar, M.; Mitra, S. Variation in Chemical, Colloidal and Electrochemical Properties of Carbon Nanotubes with the Degree of Carboxylation. J. Nanopart Res. 2017, 19, 16. [Google Scholar] [CrossRef]
- DanDan, W.; Luo, L.; Deng, D.; Gong, G.; Qin, J.; Cai, K.; Gu, Y.; Mei, Y. Electrochemical Immunosensor for the Determination of Nuclear Matrix Protein 22 in Urine Using Carboxylated MWCNTs and Zeolitic Imidazolate Framework Modified with AgNPs. ACS Appl. Nano Mater. 2023, 6, 18328–18336. [Google Scholar] [CrossRef]
- Chattaraj, A.; Mishra, Y.; Aljabali, A.A.A.; Mishra, V. Development and Evaluation of Folic Acid Conjugated Curcumin-Loaded Functionalized Multiwalled Carbon Nanotubes for Enhanced Efficacy in Ovarian Cancer Treatment. Carbon Trends 2025, 19, 100464. [Google Scholar] [CrossRef]
- Zuo, D. Highly Efficient Esterification of Carboxylic Acids With O–H Nucleophiles Through Acid/Iodide Cooperative Catalysis. Org. Biomol. Chem. 2024, 22, 6181–6188. [Google Scholar] [CrossRef]
- Yu, P. Electrochemical Decarboxylative Cross-Coupling With Nucleophiles. Chem. A Eur. J. 2024, 30, e202402124. [Google Scholar] [CrossRef]
- Naief, M.F.; Khalaf, Y.H.; Mohammed, A.M. Novel Photothermal Therapy Using Multi-Walled Carbon Nanotubes and Platinum Nanocomposite for Human Prostate Cancer PC3 Cell Line. J. Organomet. Chem. 2022, 975, 122422. [Google Scholar] [CrossRef]
- Shi, J.; Ma, R.; Wang, L.; Zhang, J.; Liu, R.; Li, L.; Liu, Y.; Hou, L.; Yu, X.; Gao, J.; et al. The Application of Hyaluronic Acid-Derivatized Carbon Nanotubes in Hematoporphyrin Monomethyl Ether-Based Photodynamic Therapy for in Vivo and in Vitro Cancer Treatment. Int. J. Nanomed. 2013, 8, 2361–2373. [Google Scholar] [CrossRef]
- Song, T. Electrospun Polyimide Nanofiber-based Separators Containing Carboxyl Groups for Lithium-ion Batteries. J. Appl. Polym. Sci. 2024, 141, e55721. [Google Scholar] [CrossRef]
- Yang, E. Harnessing Coordination-Assisted Surface Functionalization for Ligand-Induced Growth of Ultrafine Metal Nanoparticles on MXene. Adv. Funct. Mater. 2024, 34, 2408444. [Google Scholar] [CrossRef]
- Scaglione, N. Tailored Carbon Dioxide Capacity in Carboxylate-Based Ionic Liquids. Faraday Discuss. 2024, 253, 233–250. [Google Scholar] [CrossRef]
- Briesemeister, M. PVC/CNT Electrospun Composites: Morphology and Thermal and Impedance Behavior. Polymers 2024, 16, 2867. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Q.P. The Mott–Schottky Co2P/Co Heterocatalyst Encapsulated by N,p-Doped Graphene/Carbon Nanotubes as High-Efficiency Trifunctional Electrocatalysts for Cable-Type Flexible Zn–air Batteries and Water Splitting. J. Mater. Chem. A 2024, 12, 1185–1199. [Google Scholar] [CrossRef]
- Tajima, T. Photocatalytic Ammonia Decomposition Using Dye-Encapsulated Single-Walled Carbon Nanotubes. Catalysts 2024, 14, 715. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, H.; Hong, L.; Nakashima, N.; Yang, C. Modulation for Redox States of Single-Walled Carbon Nanotubes: Effect of Wrapping Conjugated Polymers. Chem. Asian J. 2024, 20, e202400879. [Google Scholar] [CrossRef]
- Das, S.; Roy, S.; Dinda, S.C.; Bose, A.; Mahapatra, C.; Basu, B.; Prajapati, B. Carbon Nanotubes in Brain Targeted Drug Delivery: A Comprehensive Review. Results Chem. 2025, 15, 102206. [Google Scholar] [CrossRef]
- Niidome, Y.; Matsumoto, H.; Hamano, R.; Kato, K.; Fujigaya, T.; Shiraki, T. Polymer Wrapping State Changes at Defect Sites of Locally Functionalized Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2024, 128, 5146–5155. [Google Scholar] [CrossRef]
- Mal, S.; e Souza, L.D.; Allard, C.; David, C.; Blais-Ouellette, S.; Gaboury, L.; Tang, N.; Martel, R. Duplex Phenotype Detection and Targeting of Breast Cancer Cells Using Nanotube Nanoprobes and Raman Imaging. ACS Appl. Bio Mater. 2023, 6, 1173–1184. [Google Scholar] [CrossRef]
- Minu, S. Dodecyl Surfactants Induced Crystalline Modifications in Polyamide-6 Nanofibers. ACS Appl. Polym. Mater. 2024, 6, 10891–10905. [Google Scholar] [CrossRef]
- Gonçalves, M. Polydimethylsiloxane Surface Modification of Microfluidic Devices for Blood Plasma Separation. Polymers 2024, 16, 1416. [Google Scholar] [CrossRef]
- Sui, N. Photoprogrammed Multifunctional Optoelectronic Synaptic Transistor Arrays Based on Photosensitive Polymer-Sorted Semiconducting Single-Walled Carbon Nanotubes for Image Recognition. Adv. Sci. 2024, 11, e2401794. [Google Scholar] [CrossRef]
- Mousavi, S.M. Recent Advances in Bioactive Carbon Nanotubes Based on Polymer Composites for Biosensor Applications. Chem. Biodivers. 2024, 21, e202301288. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.M. Shape-Memory Polymers Based on Carbon Nanotube Composites. Micromachines 2024, 15, 748. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, Y.; Yang, M.; Sun, L.; Tao, Y.; Yang, C.-A. Sulfur-Containing Polymer/Carbon Nanotube Composite Cathode Materials for High-Energy Lithium–Sulfur Batteries. New J. Chem. 2024, 48, 621–630. [Google Scholar] [CrossRef]
- Panahizadeh, V. Optimization of Impact Strength and Elastic Modulus of Polyamide-Based Nanocomposites: Using Particle Swarm Optimization Method. J. Elastomers Plast. 2024, 56, 244–261. [Google Scholar] [CrossRef]
- Pietrzak, Ł. The Electromagnetic Shielding Properties of Biodegradable Carbon Nanotube–Polymer Composites. Electronics 2024, 13, 2169. [Google Scholar] [CrossRef]
- Choi, H.R.; Kim, K. Theranostics for Triple-Negative Breast Cancer. Diagnostics 2023, 13, 272. [Google Scholar] [CrossRef]
- Suleman, M.; Murshed, A.; Imran, K.; Khan, A.; Ali, Z.; Albekairi, N.A.; Wei, D.; Yassine, H.M.; Crovella, S. Abrogation of ORF8–IRF3 Binding Interface With Carbon Nanotube Derivatives to Rescue the Host Immune System Against SARS-CoV-2 by Using Molecular Screening and Simulation Approaches. BMC Chem. 2024, 18, 99. [Google Scholar] [CrossRef]
- Rida, J.F. The Employment of Carbon Nanotubes in Biomedical Applications. Scalable Comput. Pract. Exp. 2024, 25, 4283–4300. [Google Scholar] [CrossRef]
- Saini, S.; Bhattacharjee, A.K.; Gouda, G.M. Switching Behavior of the Composite Low Dimensional Structural Hybrids of Carbon After UV Exposure. In Proceedings of the Iop Conference Series Materials Science and Engineering, Mangalore, India, 21–23 September 2024. [Google Scholar] [CrossRef]
- Thakur, C.K.; Martins, F.G.; Karthikeyan, C.; Bhal, S.; Kundu, C.N.; Hari Moorthy, N.S.; Sousa, S.F. In Silico-Guided Discovery and in Vitro Validation of Novel Sugar-Tethered Lysinated Carbon Nanotubes for Targeted Drug Delivery of Doxorubicin. J. Mol. Model. 2024, 30, 261. [Google Scholar] [CrossRef]
- Pandey, R.P.; Dhiman, R.; Bazad, N.; Mukerjee, R.; Vidić, J.; Leal, É.; Raj, V.S.; Chang, C.-M. Enhancing Breast Cancer Therapy: Nanocarrier-Based Targeted Drug Delivery. Preprints 2023. [Google Scholar] [CrossRef]
- Son, K.H.; Hong, J.H.; Lee, J.W. Carbon Nanotubes as Cancer Therapeutic Carriers and Mediators. Int. J. Nanomed. 2016, 11, 5163–5185. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, M.; Bunka, D.H.J.; Nadal, P.; Stockley, P.G.; O’Sullivan, C.K. Selection of 2′F-Modified RNA Aptamers against Prostate-Specific Antigen and Their Evaluation for Diagnostic and Therapeutic Applications. Anal. Bioanal. Chem. 2013, 405, 9149–9157. [Google Scholar] [CrossRef]
- Alidori, S.; Asqiriba, K.; Londero, P.; Bergkvist, M.; Leona, M.; Scheinberg, D.A.; McDevitt, M.R. Deploying RNA and DNA with Functionalized Carbon Nanotubes. J. Phys. Chem. C 2013, 117, 5982–5992. [Google Scholar] [CrossRef]
- Mahor, A.; Singh, P.P.; Bharadwaj, P.; Sharma, N.; Yadav, S.; Rosenholm, J.M.; Bansal, K.K. Carbon-Based Nanomaterials for Delivery of Biologicals and Therapeutics: A Cutting-Edge Technology. C 2021, 7, 19. [Google Scholar] [CrossRef]
- Cygert, S.; Pastuszak, K.; Górski, F.; Sieczczyński, M.; Juszczyk, P.; Rutkowski, A.; Lewalski, S.; Różański, R.; Jopek, M.A.; Jassem, J.; et al. Platelet-Based Liquid Biopsies Through the Lens of Machine Learning. Cancers 2023, 15, 2336. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Liu, C.; Zhang, B.; Yang, F.; Xu, J.; Long, J.; Jin, C.; Fu, D.; Ni, Q.; Yu, X. Carbon Nanotubes in Cancer Diagnosis and Therapy. Biochim. Biophys. Acta BBA Rev. Cancer 2010, 1806, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Jaishree, V.; Gupta, P.D. Nanotechnology: A Revolution in Cancer Diagnosis. Ind. J. Clin. Biochem. 2012, 27, 214–220. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Yang, M.; Liu, X.; Fang, Y.; Xiao, Q.; Zhang, Y. MX/MWCNTs Composite Material Aids New Strategy for HBV-DNA Electrochemical Biosensor: Achieving Multi-Level Signal Amplification and Unlabeled Detection. Adv. Compos. Hybrid. Mater. 2025, 8, 127. [Google Scholar] [CrossRef]
- Surana, K.; Ahire, E.; Aher, P.; Laddha, U.; Talele, S.; Mahajan, S.; Kshirsagar, S.; Gajbhiye, S. Role of Carbon Nanotube in Biosensor Developments. In Carbon Nanotubes for Biomedical Applications and Healthcare; Apple Academic Press: Point Pleasant, NJ, USA, 2024; ISBN 978-1-00-339639-0. [Google Scholar]
- Nagaraju, K.; Reddy, R.; Reddy, N. A Review on Protein Functionalized Carbon Nanotubes. J. Appl. Biomater. Funct. Mater. 2015, 13, 301–312. [Google Scholar] [CrossRef]
- Li, R.; Wu, R.; Zhao, L.; Qin, H.; Wu, J.; Zhang, J.; Bao, R.; Zou, H. In Vivo Detection of Magnetic Labeled Oxidized Multi-Walled Carbon Nanotubes by Magnetic Resonance Imaging. Nanotechnology 2014, 25, 495102. [Google Scholar] [CrossRef]
- Li, X.; Fan, Y.; Watari, F. Current Investigations into Carbon Nanotubes for Biomedical Application. Biomed. Mater. 2010, 5, 022001. [Google Scholar] [CrossRef] [PubMed]
- Meredith, J.R. Biomedical Applications of Carbon-Nanotube Composites. Front. Biosci. 2013, 5, 610–621. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, S. Cancer Targeting and Diagnosis: Recent Trends with Carbon Nanotubes. Nanomaterials 2022, 12, 2283. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of Carbon Nanotubes for Biomedical Applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Advances in Drug Delivery Nanosystems Using Graphene-Based Materials and Carbon Nanotubes. Materials 2021, 14, 1059. [Google Scholar] [CrossRef] [PubMed]
- Augustine, S.; Singh, J.; Srivastava, M.; Sharma, M.; Das, A.; Malhotra, B.D. Recent Advances in Carbon Based Nanosystems for Cancer Theranostics. Biomater. Sci. 2017, 5, 901–952. [Google Scholar] [CrossRef]
- Sinha, A.; Dhanjai; Jain, R.; Zhao, H.; Karolia, P.; Jadon, N. Voltammetric Sensing Based on the Use of Advanced Carbonaceous Nanomaterials: A Review. Microchim. Acta 2018, 185, 89. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Ramasamy, R. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef]
- Tîlmaciu, C.-M.; Morris, M.C. Carbon Nanotube Biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef]
- Ding, S.; Cargill, A.; Das, S.; Medintz, I.; Claussen, J. Biosensing with Förster Resonance Energy Transfer Coupling between Fluorophores and Nanocarbon Allotropes. Sensors 2015, 15, 14766–14787. [Google Scholar] [CrossRef]
- Gillen, A.J.; Boghossian, A.A. Non-Covalent Methods of Engineering Optical Sensors Based on Single-Walled Carbon Nanotubes. Front. Chem. 2019, 7, 612. [Google Scholar] [CrossRef] [PubMed]
- Chik, M.W.; Hussain, Z.; Zulkefeli, M.; Tripathy, M.; Kumar, S.; Majeed, A.B.A.; Byrappa, K. Polymer-Wrapped Single-Walled Carbon Nanotubes: A Transformation toward Better Applications in Healthcare. Drug Deliv. Transl. Res. 2019, 9, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Jeong, J.W.; Kim, Y.A.; Chang, M. Carbon Nanomaterials as Versatile Platforms for Biosensing Applications. Micromachines 2020, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; He, S.; Nag, A.; Wong, J.W.C. A Review of the Use of Carbon Nanotubes and Graphene-Based Sensors for the Detection of Aflatoxin M1 Compounds in Milk. Sensors 2021, 21, 3602. [Google Scholar] [CrossRef]
- Pan, M.; Yin, Z.; Liu, K.; Du, X.; Liu, H.; Wang, S. Carbon-Based Nanomaterials in Sensors for Food Safety. Nanomaterials 2019, 9, 1330. [Google Scholar] [CrossRef]
- Ahmad, I.; Sead, F.F.; Kanjariya, P.; Kumar, A.; Rajivm, A.; Shankhyan, A.; Jaidka, S.; Kumar, H.; Aminov, Z. Nanomaterial Sensors for Enhanced Detection of Serotonin. Clin. Chim. Acta 2025, 569, 120160. [Google Scholar] [CrossRef]
- Govindasamy, M.; Chen, S.-M.; Mani, V.; Devasenathipathy, R.; Umamaheswari, R.; Joseph Santhanaraj, K.; Sathiyan, A. Molybdenum Disulfide Nanosheets Coated Multiwalled Carbon Nanotubes Composite for Highly Sensitive Determination of Chloramphenicol in Food Samples Milk, Honey and Powdered Milk. J. Colloid Interface Sci. 2017, 485, 129–136. [Google Scholar] [CrossRef]
- Yari, A.; Shams, A. Silver-Filled MWCNT Nanocomposite as a Sensing Element for Voltammetric Determination of Sulfamethoxazole. Anal. Chim. Acta 2018, 1039, 51–58. [Google Scholar] [CrossRef]
- An, J.; Zhang, M.; Fu, Y.; Zhang, Q.; Si, Y.; Zhang, Y.; Fang, Y.; Zhang, D. Emerging Electrochemical Biosensors for Lung Cancer-Associated Protein Biomarker and miRNA Detection. Int. J. Biol. Macromol. 2024, 280, 135972. [Google Scholar] [CrossRef]
- Zheng, R.; Wu, A.; Li, J.; Tang, Z.; Zhang, J.; Zhang, M.; Wei, Z. Progress and Outlook on Electrochemical Sensing of Lung Cancer Biomarkers. Molecules 2024, 29, 3156. [Google Scholar] [CrossRef]
- Noreen, S.; Ishaq, I.; Saleem, M.H.; Ali, B.; Muhammad, A.; Syed, M.A.; Iqbal, J. Electrochemical Biosensing in Oncology: A Review Advancements and Prospects for Cancer Diagnosis. Cancer Biol. Ther. 2025, 26, 2475581. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Kumar, Y.; Kumar, N.; Yadav, B.K.; Sharma, N.; Chandra, R.; Kumar, S. Nanoengineered Multiwalled Carbon Nanotube for Lung Cancer Diagnosis. J. Mol. Struct. 2025, 1320, 139629. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; George, M.; Neppolian, B.; Das, J. Electrochemical Detection of Non-Small Cell Lung Cancer (NSCLC) Mir-223 Biomarker Employing Gold/MWCNT Nanocomposite–Based Sandwich Platform. J. Solid State Electrochem. 2025, 29, 669–680. [Google Scholar] [CrossRef]
- Zhang, R.; Rejeeth, C.; Xu, W.; Zhu, C.; Liu, X.; Wan, J.; Jiang, M.; Qian, K. Label-Free Electrochemical Sensor for CD44 by Ligand-Protein Interaction. Anal. Chem. 2019, 91, 7078–7085. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Bae, M.; Cho, E.; Kim, K.S.; Lee, J.-H. Plasmonic Biosensors in Cancer-Associated miRNA Detection. Biosensors 2025, 15, 165. [Google Scholar] [CrossRef]
- Saadh, M.J.; Saeed, T.N.; Alfarttoosi, K.H.; Sanghvi, G.; Roopashree, R.; Thakur, V.; Lakshmi, L.; Aminov, Z.; Taher, W.M.; Alwan, M.; et al. Plasmonic Nanoparticles: Enhancing Early Breast Cancer Detection Through Biosensors. Plasmonics 2025, 20, 1–18. [Google Scholar] [CrossRef]
- Karki, B.; Pal, A.; Dhiman, G.; Ahmed, M.Z. Ultra-Sensitive Early Detection of Colorectal Cancer Using Surface Plasmon Resonance Sensor: Theoretical Analysis. Microchim. Acta 2025, 192, 126. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, G.; Yang, X. Electrochemical Immunosensor Based on Fe3O4/MWCNTs-COOH/AuNPs Nanocomposites for Trace Liver Cancer Marker Alpha-Fetoprotein Detection. Talanta 2023, 259, 124492. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, W.; Lin, G.; Wu, Q.; Xu, M.; Huang, X.; Luo, J.; Zhu, Y.; Liu, X. Long Conducting and Water-Compatible Polymer/Carbon Nanotubes Nanocomposite with “Beads-on-a-String” Structure as a Highly Effective Electrochemical Sensing Material. ACS Sustain. Chem. Eng. 2019, 7, 3556–3566. [Google Scholar] [CrossRef]
- Makableh, Y.; Athamneh, T.; Ajlouni, M.; Hijazi, S.; Alnaimi, A. Enhanced Response and Selective Gold Nanoparticles/Carbon Nanotubes Biosensor for the Early Detection of HER2 Biomarker. Sens. Actuators Rep. 2023, 5, 100158. [Google Scholar] [CrossRef]
- Kim, J.P.; Lee, B.Y.; Lee, J.; Hong, S.; Sim, S.J. Enhancement of Sensitivity and Specificity by Surface Modification of Carbon Nanotubes in Diagnosis of Prostate Cancer Based on Carbon Nanotube Field Effect Transistors. Biosens. Bioelectron. 2009, 24, 3372–3378. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Jeong, J.-Y.; Hong, S. Highly Sensitive Real-Time Monitoring of Adenosine Receptor Activities in Nonsmall Cell Lung Cancer Cells Using Carbon Nanotube Field-Effect Transistors. ACS Appl. Mater. Interfaces 2024, 16, 2101–2109. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, Y.; Tao, J.; Liu, G.; Li, B.; Teng, Y.; Xu, J.; Feng, L.; You, Z. Miniaturized and Portable Device for Noninvasive, Ultrasensitive and Point-of-Care Diagnosis by Engineered Metal-Carbide-Based Field Effect Transistor. Chem. Eng. J. 2025, 506, 160264. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Wei, T.; Wang, K.; Zhao, Z.; Cao, J.; Liu, Y.; Zhang, Z. Carbon Nanotube Field-Effect Transistor Biosensor with an Enlarged Gate Area for Ultra-Sensitive Detection of a Lung Cancer Biomarker. ACS Appl. Mater. Interfaces 2023, 15, 27299–27306. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Y.; Cai, B.; Li, J.; Zhang, G. Probe-Screened Carbon Nanotube Field-Effect Transistor Biosensor to Enhance Breast Cancer-Related Gene Assay. In Proceedings of the SPIE Third International Conference on Biomedical and Intelligent Systems (IC-BIS 2024), Nanchang, China, 26–28 April 2024; Volume 13208, pp. 26–35. [Google Scholar]
- Li, T.; Liang, Y.; Li, J.; Yu, Y.; Xiao, M.-M.; Ni, W.; Zhang, Z.; Zhang, G.-J. Carbon Nanotube Field-Effect Transistor Biosensor for Ultrasensitive and Label-Free Detection of Breast Cancer Exosomal miRNA21. Anal. Chem. 2021, 93, 15501–15507. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, X.; Dong, B.; Liu, Y.; Wei, D. Enzymatic Cascade Reactors on Carbon Nanotube Transistor Detecting Trace Prostate Cancer Biomarker. Biosens. Bioelectron. 2024, 263, 116603. [Google Scholar] [CrossRef]
- Murshid, N.; Keogh, D.; Kitaev, V. Optimized Synthetic Protocols for Preparation of Versatile Plasmonic Platform Based on Silver Nanoparticles with Pentagonal Symmetries. Part. Part. Syst. Charact. 2014, 31, 178–189. [Google Scholar] [CrossRef]
- Cathcart, N.; Murshid, N.; Campbell, P.; Kitaev, V. Selective Plasmonic Sensing and Highly Ordered Metallodielectrics via Encapsulation of Plasmonic Metal Nanoparticles with Metal Oxides. ACS Appl. Nano Mater. 2018, 1, 6514–6524. [Google Scholar] [CrossRef]
- Ahmed Taha, B.; Kadhim, A.C.; Addie, A.J.; Haider, A.J.; Azzahrani, A.S.; Raizada, P.; Rustagi, S.; Chaudhary, V.; Arsad, N. Advancing Cancer Diagnostics through Multifaceted Optical Biosensors Supported by Nanomaterials and Artificial Intelligence: A Panoramic Outlook. Microchem. J. 2024, 205, 111307. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Optical Biosensors: An Exhaustive and Comprehensive Review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef]
- Špringer, T.; Bocková, M.; Slabý, J.; Sohrabi, F.; Čapková, M.; Homola, J. Surface Plasmon Resonance Biosensors and Their Medical Applications. Biosens. Bioelectron. 2025, 278, 117308. [Google Scholar] [CrossRef] [PubMed]
- Kala, K.; Kumar, U.S.; Devi, K.Y.; Kapali, B.S.C.; Devi, N.B. Optical Biosensor Manufacturing for Chronic Lymphocytic Leukemia Biomarker Detection with-Walled Carbon Nano Tubes-Based Multi Electrodes. Surf. Interfaces 2023, 42, 103371. [Google Scholar] [CrossRef]
- Ackermann, J.; Metternich, J.T.; Herbertz, S.; Kruss, S. Biosensing with Fluorescent Carbon Nanotubes. Angew. Chem. Int. Ed. 2022, 61, e202112372. [Google Scholar] [CrossRef]
- Kruss, S.; Hilmer, A.J.; Zhang, J.; Reuel, N.F.; Mu, B.; Strano, M.S. Carbon Nanotubes as Optical Biomedical Sensors. Adv. Drug Deliv. Rev. 2013, 65, 1933–1950. [Google Scholar] [CrossRef]
- Cohen, Z.; Williams, R.M. Single-Walled Carbon Nanotubes as Optical Transducers for Nanobiosensors In Vivo. ACS Nano 2024, 18, 35164–35181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Wu, F.; Yuan, P.; Chi, C.; Zhou, N. Magnetic and Fluorescent Carbon Nanotubes for Dual Modal Imaging and Photothermal and Chemo-Therapy of Cancer Cells in Living Mice. Carbon 2017, 123, 70–83. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Pn, N.; Alexander, K.; Zavabeti, A.; Sherrell, P.C.; Ivanova, E.P.; Adhikari, B.; Naebe, M.; Bhargava, S.K. Fluorescent Nanocarbons: From Synthesis and Structure to Cancer Imaging and Therapy. Adv. Mater. 2024, 36, 2312474. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, Y.; Xue, Y.; Zhang, W.; Sun, X.; Xu, X.; Liu, C. Nanomaterials for Ultrasound Imaging- Guided Sonodynamic Therapy. Technol. Cancer Res. Treat. 2024, 23, 15330338241263197. [Google Scholar] [CrossRef]
- Hendler-Neumark, A.; Wulf, V.; Bisker, G. In Vivo Imaging of Fluorescent Single-Walled Carbon Nanotubes within C. elegans Nematodes Near-Infrared Window. Mater. Today Bio 2021, 12, 100175. [Google Scholar] [CrossRef]
- Kojima, K.; Iizumi, Y.; Zhang, M.; Okazaki, T. Streptavidin-Conjugated Oxygen-Doped Single-Walled Carbon Nanotubes as Near-Infrared Labels for Immunoassays. Langmuir 2022, 38, 1509–1513. [Google Scholar] [CrossRef]
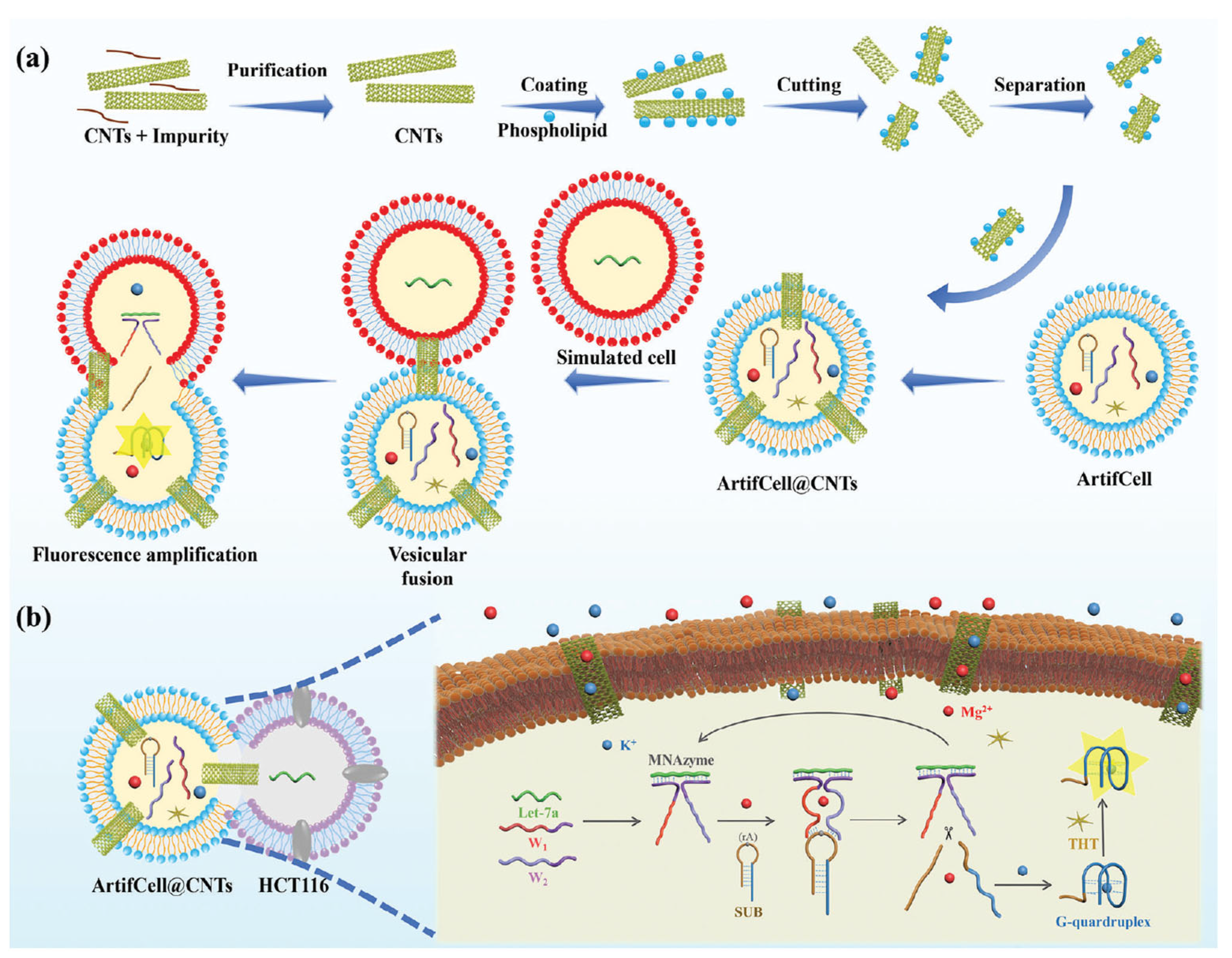
- Wu, J.; Zeng, X.; Wang, L.; Zhang, J.; Cui, S.; Ma, Z.; Pan, R.; Liu, C.; Kong, D.; Song, J.; et al. Embedding Carbon Nanotubes in Artificial Cells Enhances Probe Transfer. Adv. Mater. 2025, 37, 2418271. [Google Scholar] [CrossRef] [PubMed]
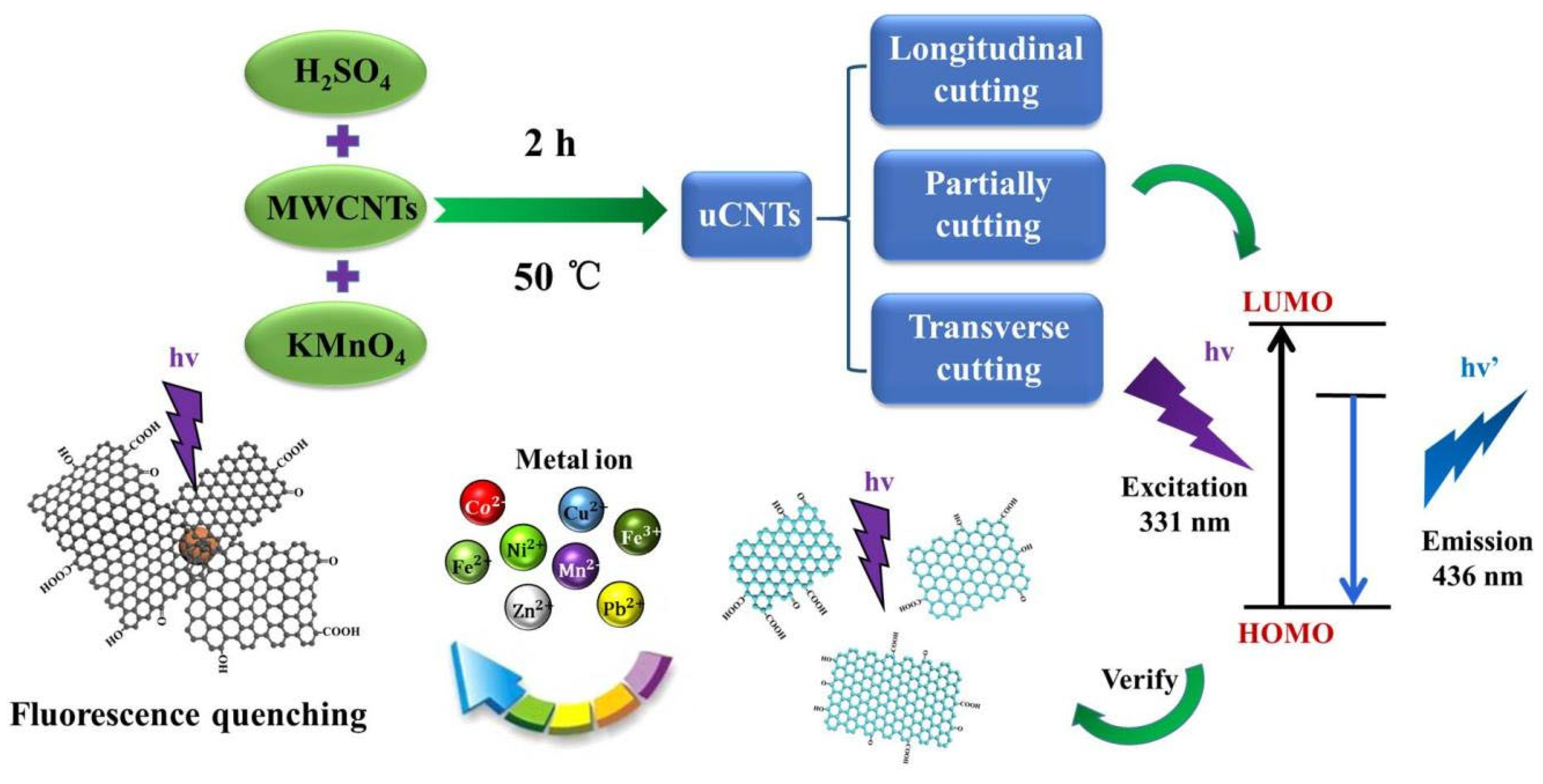
- Chen, M.; Qi, X.; Zhang, W.; Yang, N.; Yang, D.; Wang, Y.; Zhang, L.; Yang, W.; Huang, L.; Zhang, M.; et al. Self-Photoluminescence of Unzipped Multi-Walled Carbon Nanotubes. Nanomaterials 2021, 11, 1632. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Wu, X.; Ferreira, J.S.; Kim, M.; Powell, L.R.; Kwon, H.; Groc, L.; Wang, Y.; Cognet, L. Fluorescent Sp3 Defect-Tailored Carbon Nanotubes Enable NIR-II Single Particle Imaging in Live Brain Slices at Ultra-Low Excitation Doses. Sci. Rep. 2019, 10, 5286. [Google Scholar] [CrossRef] [PubMed]
- Mehri-Kakavand, G.; Hasanzadeh, H.; Jahanbakhsh, R.; Abdollahi, M.; Nasr, R.; Bitarafan-Rajabi, A.; Jadidi, M.; Darbandi-Azar, A.; Emadi, A. Gdn3+@CNTs-PEG versus Gadovist®: In Vitro Assay. Oman Med. J. 2019, 34, 147–155. [Google Scholar] [CrossRef]
- Wolski, P.; Nieszporek, K.; Panczyk, T. Multimodal, pH Sensitive, and Magnetically Assisted Carrier of Doxorubicin Designed and Analyzed by Means of Computer Simulations. Langmuir 2018, 34, 2543–2550. [Google Scholar] [CrossRef]
- Glória, J.; Brito, W.; Gandarilla, A.; Larrude, D.; Carlos, J.; Araújo, F.; Almeida, M.E.; Manzato, L.; Mariúba, L.A.M. Solubilization, Characterization, and Protein Coupling Analysis to Multiwalled Carbon Nanotubes. High Perform. Polym. 2021, 33, 338–344. [Google Scholar] [CrossRef]
- Aher, P.; Surana, K.; Ahire, E.; Talele, S.; Talele, G.; Mahajan, S.; Kshirsagar, S. Carbon Nanotube: A Promising Role in Biomedical Imaging. In Carbon Nanotubes for Biomedical Applications and Healthcare; Apple Academic Press: Point Pleasant, NJ, USA, 2024; ISBN 978-1-00-339639-0. [Google Scholar]
- Kim, W.; Ahn, J.S.; Ryu, J. Carbon Nanotube Emitter X-Ray Source for High-Resolution Micro-Computed Tomography. In The Medical Imaging 2023: Physics of Medical Imaging; Fahrig, R., Sabol, J.M., Yu, L., Eds.; SPIE: San Diego, CA, USA, 2023; p. 177. [Google Scholar]
- Puett, C.; Inscoe, C.; Hartman, A.; Calliste, J.; Franceschi, D.K.; Lu, J.; Zhou, O.; Lee, Y.Z. An Update on Carbon Nanotube-Enabled X-Ray Sources for Biomedical Imaging. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1475. [Google Scholar] [CrossRef]
- Kim, W.; Jung, J.; Rajpoot, S.; Chung, W.-K.; Ahn, J.S.; Lee, B.-N.; Ryu, J. Development of High-Speed Micro Computed Tomography System Based on Carbon Nanotube Emitter x-Ray Source. In The Medical Imaging 2022: Physics of Medical Imaging; Zhao, W., Yu, L., Eds.; SPIE: San Diego, CA, US, 2022; p. 103. [Google Scholar]
- Jo, B. Optimizing Image Quality in Carbon Nanotube-Based Rectilinear Digital Tomosynthesis System via Projection Number Variation. J. Magn. 2024, 29, 480–486. [Google Scholar] [CrossRef]
- Dillon, O.; Reynolds, T.; O’Brien, R.T. X-Ray Source Arrays for Volumetric Imaging during Radiotherapy Treatment. Sci. Rep. 2023, 13, 9776. [Google Scholar] [CrossRef]
- Choi, J.; Lee, H.; Jang, J.; Sharma, M.B.; Jung, J.; Yeo, S.J.; Kim, S.H.; Kong, M.; Lee, S.H.; Ryu, J. Innovative CNT X-Ray Source-Based 3D Hemispherical Multi-Beam Tomosynthesis for Medical Imaging. In The Medical Imaging 2024: Physics of Medical Imaging; Fahrig, R., Sabol, J.M., Li, K., Eds.; SPIE: San Diego, CA, USA, 2024; p. 143. [Google Scholar]
- Zulkifli, N.A.; Ismail, N.H.; Jaafar, M.; Othman, A.R. Effect of Carbon Nanotubes on the Flexural Properties and Flammability of Carbon Fibre/Epoxy Multi-scale Composites. Mater. Werkst 2022, 53, 1544–1550. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohani, A.P.; Elosta, M.; Maksoud, M.; Murshid, N. Functionalized Carbon Nanotubes: Emerging Nanomaterials for Enhanced Cancer Diagnosis and Imaging. Molecules 2025, 30, 2364. https://doi.org/10.3390/molecules30112364
Lohani AP, Elosta M, Maksoud M, Murshid N. Functionalized Carbon Nanotubes: Emerging Nanomaterials for Enhanced Cancer Diagnosis and Imaging. Molecules. 2025; 30(11):2364. https://doi.org/10.3390/molecules30112364
Chicago/Turabian StyleLohani, Anish Prasad, Mohamed Elosta, Mahmoud Maksoud, and Nimer Murshid. 2025. "Functionalized Carbon Nanotubes: Emerging Nanomaterials for Enhanced Cancer Diagnosis and Imaging" Molecules 30, no. 11: 2364. https://doi.org/10.3390/molecules30112364
APA StyleLohani, A. P., Elosta, M., Maksoud, M., & Murshid, N. (2025). Functionalized Carbon Nanotubes: Emerging Nanomaterials for Enhanced Cancer Diagnosis and Imaging. Molecules, 30(11), 2364. https://doi.org/10.3390/molecules30112364








