Antibody Aggregate Removal by Multimodal Chromatography
Abstract
1. Introduction
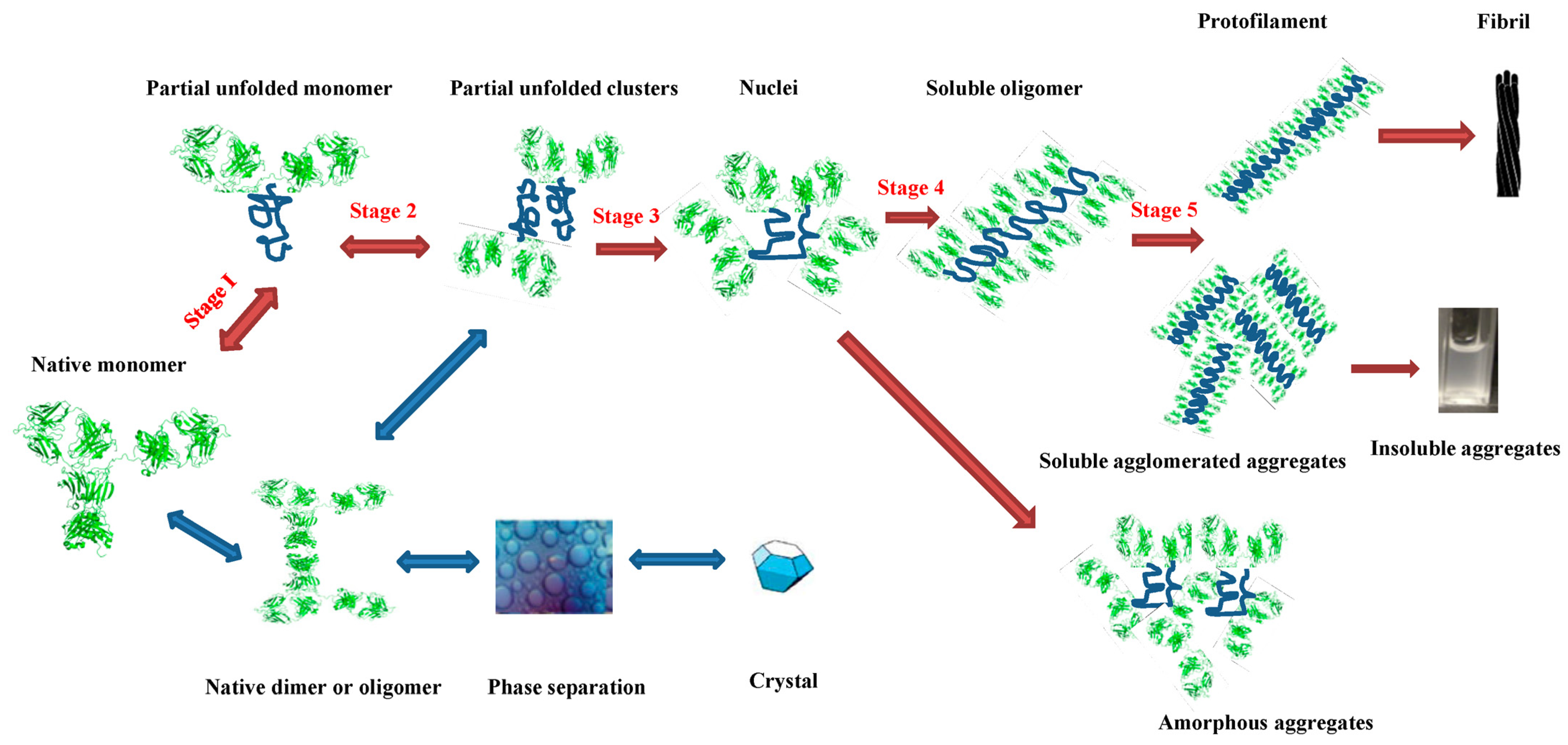
2. Aggregate Formation
3. Aggregate Removal Using Single-Mode Chromatography
3.1. Affinity Chromatography
3.2. Ion-Exchange Chromatography
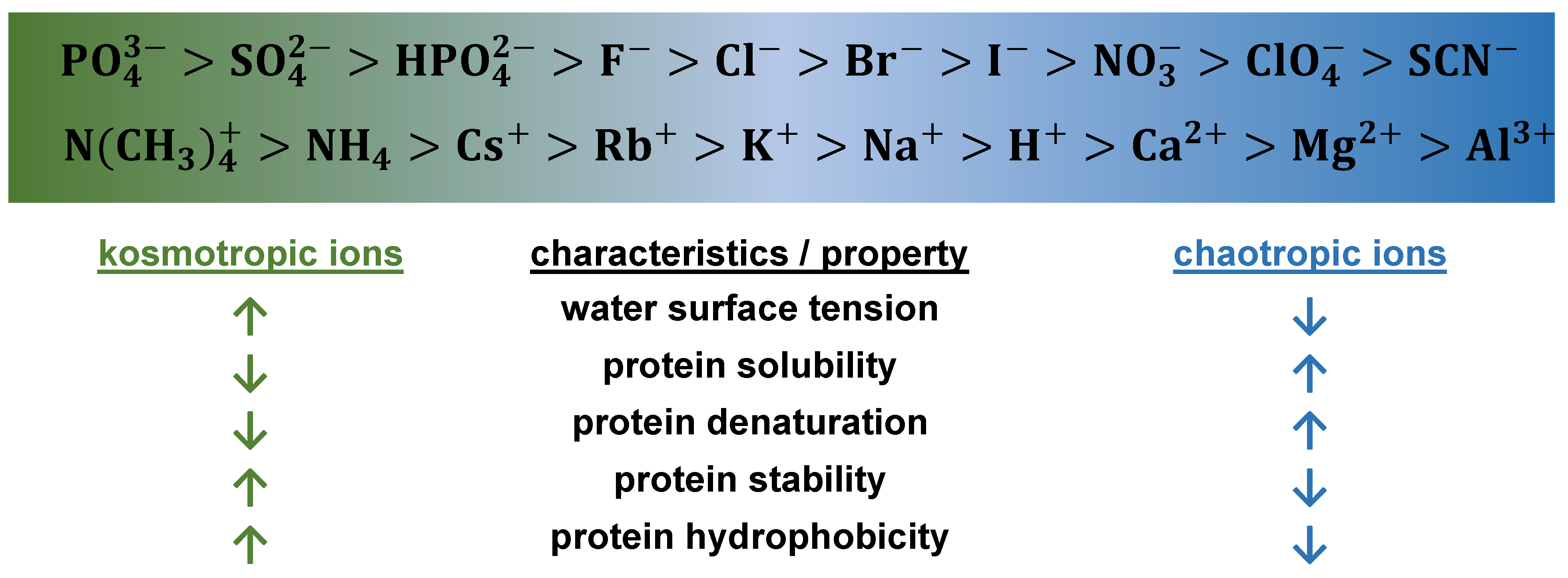
3.3. Hydrophobic Chromatography
4. Aggregate Removal Using Multimodal Chromatography
4.1. Multimodal Chromatography: Basic Concepts
4.2. Commercially Available Multimodal Adsorbents
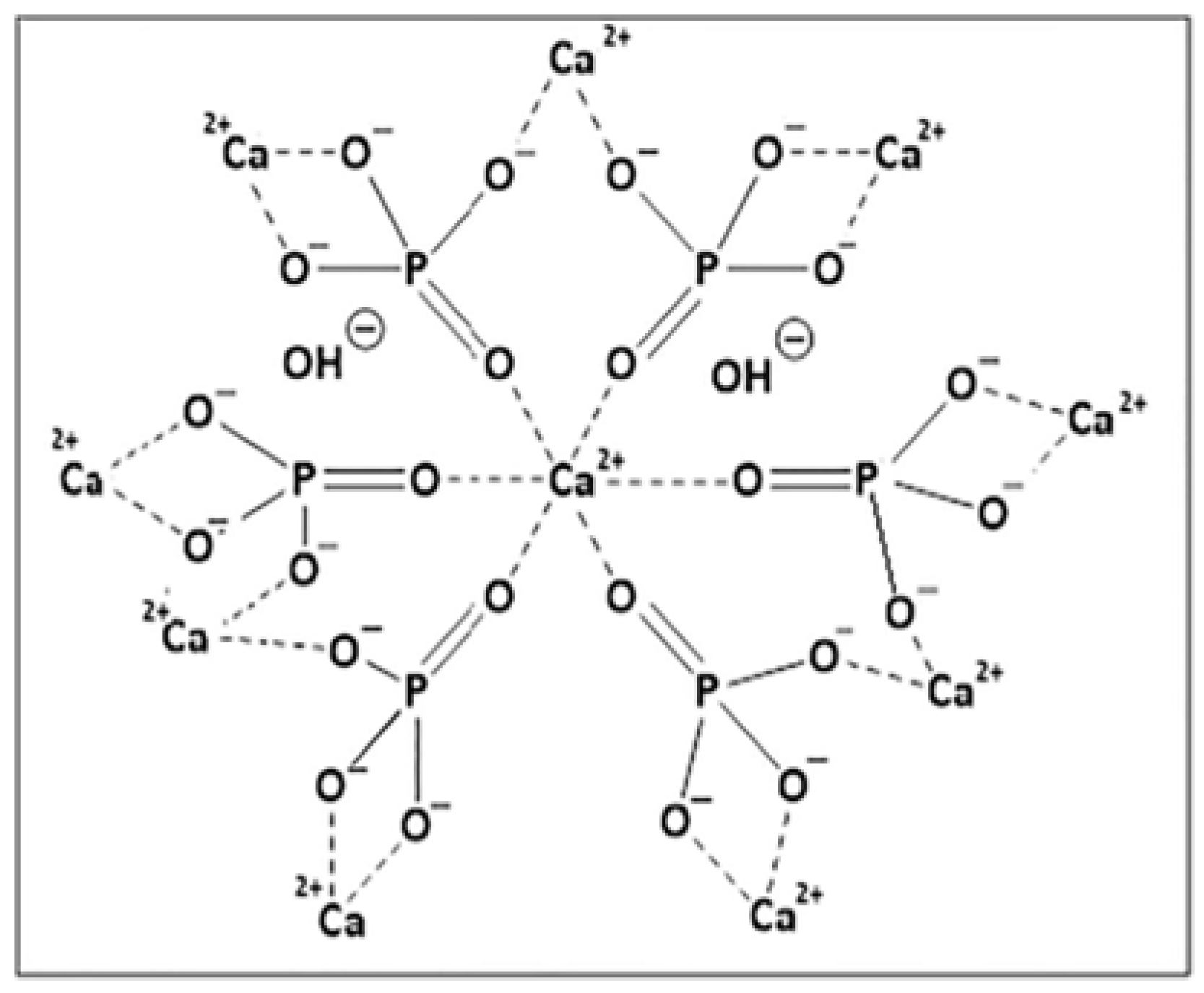
4.2.1. Hydroxyapatite Resins
| Adsorbent | Operational Mode | Position | Product | Reference |
|---|---|---|---|---|
| CHT type I | Batch | Polishing step | Monomeric IgG (aggregates selectivity 4.3) | [122] |
| CHT type II | Batch | Polishing step | Monomeric IgG (aggregates selectivity 5.8) | [122] |
| MEP HyperCel | Bind-elute | Capture step | mAbs with yield in interval 89–96% | [129] |
| Capto Adhere | Bind-elute | Polishing step | Monomeric IgG with 92% recovery | [130] |
| Nuvia aPrime 4A | Batch | Polishing step | Monomeric mAb with >92% purity | [131] |
| HEA HyperCel | Flowthrough | Post CEX | Monomeric mAb with 98.8% purity and 92% recovery | [132] |
| PPA HyperCel | Bind-elute | Capture step | mAb with 93% yield | [133] |
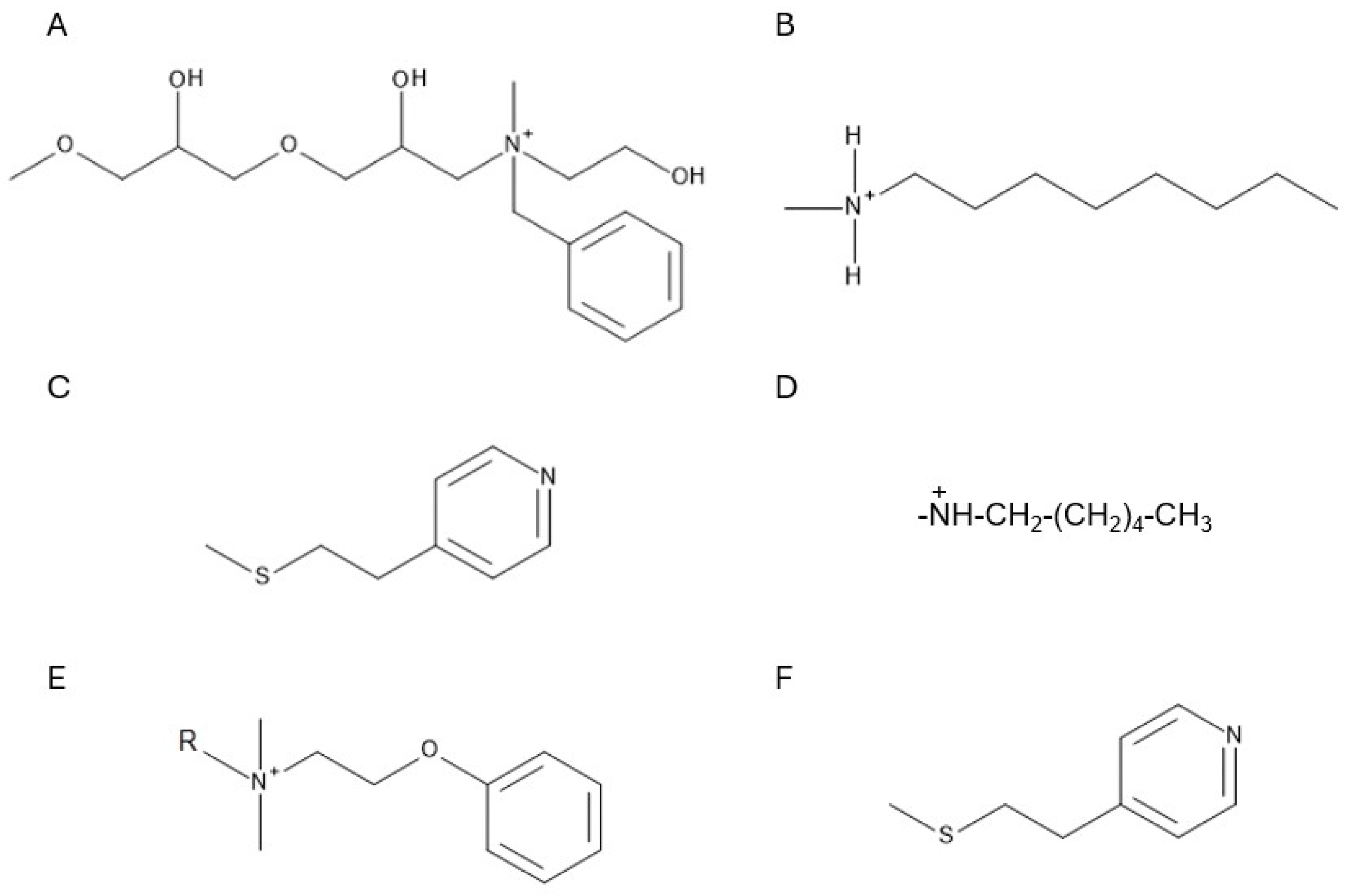
4.2.2. Multimodal Ion Exchangers
Multimodal Anion Exchangers
Multimodal Cation Exchangers
4.3. Non-Commercial Multimodal Adsorbents
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Behring, N.; Kitasato, N. Ueber das Zustandekommen der Diphtherie-Immunität und der Tetanus-Immunität bei Thieren. DMW—Dtsch. Med. Wochenschr. 1890, 16, 1113–1114. [Google Scholar] [CrossRef]
- The Antibody Society. Antibody Therapeutics in Late-Stage Clinical Studies; The Antibody Society: Framingham, MA, USA, 2024. [Google Scholar]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.-J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of Therapeutic Antibodies for the Treatment of Diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, J.; Nagraik, T.; Wohlenberg, O.J.; Ruhl, S.; Bahnemann, J.; Scheper, T.; Solle, D. Stress-Induced Increase of Monoclonal Antibody Production in CHO Cells. Eng. Life Sci. 2022, 22, 427–436. [Google Scholar] [CrossRef]
- Whitfield, K. In Vitro and In Vivo Monoclonal Antibody Production. Available online: https://pivotalscientific.com/scientific-library/in-vitro-and-in-vivo-monoclonal-antibody-production/ (accessed on 28 May 2025).
- Dhara, V.G.; Naik, H.M.; Majewska, N.I.; Betenbaugh, M.J. Recombinant Antibody Production in CHO and NS0 Cells: Differences and Similarities. BioDrugs 2018, 32, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Shire, S.J. Monoclonal Antibodies: Meeting the Challenges in Manufacturing, Formulation, Delivery and Stability of Final Drug Product; Woodhead Publishing Series in Biomedicine; Woodhead Publishing: Cambridge, UK; Waltham, MA, USA; Kidlington, UK, 2015; ISBN 978-0-08-100296-4. [Google Scholar]
- Ascoli, C.A.; Aggeler, B. Overlooked Benefits of Using Polyclonal Antibodies. BioTechniques 2018, 65, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Atlas of Immunology. Available online: https://www.routledge.com/Atlas-of-Immunology/Cruse-Lewis/p/book/9781439802687 (accessed on 31 May 2024).
- Birch, J.R.; Racher, A.J. Antibody Production. Adv. Drug Deliv. Rev. 2006, 58, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Prabakaran, P.; Chen, W.; Zhu, Z.; Feng, Y.; Dimitrov, D. Antibody Aggregation: Insights from Sequence and Structure. Antibodies 2016, 5, 19. [Google Scholar] [CrossRef]
- Vázquez-Rey, M.; Lang, D.A. Aggregates in Monoclonal Antibody Manufacturing Processes. Biotechnol. Bioeng. 2011, 108, 1494–1508. [Google Scholar] [CrossRef]
- Van der Kant, R.; Karow-Zwick, A.R.; Van Durme, J.; Blech, M.; Gallardo, R.; Seeliger, D.; Aßfalg, K.; Baatsen, P.; Compernolle, G.; Gils, A.; et al. Prediction and Reduction of the Aggregation of Monoclonal Antibodies. J. Mol. Biol. 2017, 429, 1244–1261. [Google Scholar] [CrossRef]
- Leiske, D.L.; Shieh, I.C.; Tse, M.L. A Method to Measure Protein Unfolding at an Air–Liquid Interface. Langmuir 2016, 32, 9930–9937. [Google Scholar] [CrossRef]
- Wolfrum, S.; Weichsel, U.; Siedler, M.; Weber, C.; Peukert, W. Monitoring of Flow-Induced Aggregation and Conformational Change of Monoclonal Antibodies. Chem. Ing. Tech. 2017, 89, 987–994. [Google Scholar] [CrossRef]
- Arosio, P.; Barolo, G.; Müller-Späth, T.; Wu, H.; Morbidelli, M. Aggregation Stability of a Monoclonal Antibody During Downstream Processing. Pharm. Res. 2011, 28, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Rima, S.; Morbidelli, M. Aggregation Mechanism of an IgG2 and Two IgG1 Monoclonal Antibodies at Low pH: From Oligomers to Larger Aggregates. Pharm. Res. 2013, 30, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Barnett, G.V.; Pathak, J.A.; Roberts, C.J.; Sarangapani, P.S. Protein Aggregation, Particle Formation, Characterization & Rheology. Curr. Opin. Colloid Interface Sci. 2014, 19, 438–449. [Google Scholar] [CrossRef]
- Wang, W.; Nema, S.; Teagarden, D. Protein Aggregation—Pathways and Influencing Factors. Int. J. Pharm. 2010, 390, 89–99. [Google Scholar] [CrossRef]
- Wang, W.; Singh, S.; Zeng, D.L.; King, K.; Nema, S. Antibody Structure, Instability, and Formulation. J. Pharm. Sci. 2007, 96, 1–26. [Google Scholar] [CrossRef]
- Perico, N.; Purtell, J.; Dillon, T.M.; Ricci, M.S. Conformational Implications of an Inversed pH-Dependent Antibody Aggregation. J. Pharm. Sci. 2009, 98, 3031–3042. [Google Scholar] [CrossRef]
- Sahin, E.; Weiss, W.F.; Kroetsch, A.M.; King, K.R.; Kessler, R.K.; Das, T.K.; Roberts, C.J. Aggregation and pH–Temperature Phase Behavior for Aggregates of an IgG2 Antibody. J. Pharm. Sci. 2012, 101, 1678–1687. [Google Scholar] [CrossRef]
- Bee, J.S.; Davis, M.; Freund, E.; Carpenter, J.F.; Randolph, T.W. Aggregation of a Monoclonal Antibody Induced by Adsorption to Stainless Steel. Biotechnol. Bioeng. 2010, 105, 121–129. [Google Scholar] [CrossRef]
- Perevozchikova, T.; Nanda, H.; Nesta, D.P.; Roberts, C.J. Protein Adsorption, Desorption, and Aggregation Mediated by Solid-Liquid Interfaces. J. Pharm. Sci. 2015, 104, 1946–1959. [Google Scholar] [CrossRef]
- Guo, J.; Carta, G. Unfolding and Aggregation of Monoclonal Antibodies on Cation Exchange Columns: Effects of Resin Type, Load Buffer, and Protein Stability. J. Chromatogr. A 2015, 1388, 184–194. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, S.; Carta, G. Unfolding and Aggregation of a Glycosylated Monoclonal Antibody on a Cation Exchange Column. Part I. Chromatographic Elution and Batch Adsorption Behavior. J. Chromatogr. A 2014, 1356, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, C.; Chennamsetty, N.; Xu, X.; Li, Z.J. Insights in Understanding Aggregate Formation and Dissociation in Cation Exchange Chromatography for a Structurally Unstable Fc-Fusion Protein. J. Chromatogr. A 2016, 1460, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, R.; Nguyen, T.; Macneil, S.; Jones, L.; Crampton, S.; Vunnum, S. Cation Exchange Surface-Mediated Denaturation of an Aglycosylated Immunoglobulin (IgG1). J. Chromatogr. A 2012, 1251, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Carta, G. Unfolding and Aggregation of a Glycosylated Monoclonal Antibody on a Cation Exchange Column. Part II. Protein Structure Effects by Hydrogen Deuterium Exchange Mass Spectrometry. J. Chromatogr. A 2014, 1356, 129–137. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Xu, X.; Mills, J.; Jin, W.; Ghose, S.; Li, Z.J. Hydrophobic Property of Cation-Exchange Resins Affects Monoclonal Antibody Aggregation. J. Chromatogr. A 2020, 1631, 461573. [Google Scholar] [CrossRef]
- Roberts, J.A.; Carta, G. Protein Adsorption and Separation with Monomodal and Multimodal Anion Exchange Chromatography Resins. Part I. Multicomponent Adsorption Properties and Frontal Chromatography. J. Chem. Technol. Biotechnol. 2022, 97, 3292–3305. [Google Scholar] [CrossRef]
- Roberts, J.A.; Carta, G. Protein Adsorption and Separation with Monomodal and Multimodal Anion Exchange Chromatography Resins. Part II. Mechanisms of Protein Aggregation on the Chromatographic Surface. J. Chem. Technol. Biotechnol. 2023, 98, 357–368. [Google Scholar] [CrossRef]
- Muca, R.; Antos, D. Protein Association on Multimodal Chromatography Media. J. Chromatogr. A 2023, 1691, 463827. [Google Scholar] [CrossRef]
- Shukla, A.A.; Wolfe, L.S.; Mostafa, S.S.; Norman, C. Evolving Trends in mAb Production Processes. Bioeng. Transl. Med. 2017, 2, 58–69. [Google Scholar] [CrossRef]
- Shukla, A.A.; Hubbard, B.; Tressel, T.; Guhan, S.; Low, D. Downstream Processing of Monoclonal Antibodies—Application of Platform Approaches. J. Chromatogr. B 2007, 848, 28–39. [Google Scholar] [CrossRef]
- Kelley, B. Industrialization of mAb Production Technology: The Bioprocessing Industry at a Crossroads. mAbs 2009, 1, 443–452. [Google Scholar] [CrossRef]
- Kelley, B. Very Large Scale Monoclonal Antibody Purification: The Case for Conventional Unit Operations. Biotechnol. Prog. 2007, 23, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Vasan, S.; Ghosh, R.; Hale, G.; Cui, Z. Separation of Monoclonal Antibody Alemtuzumab Monomer and Dimers Using Ultrafiltration. Biotechnol. Bioeng. 2005, 90, 422–432. [Google Scholar] [CrossRef]
- McDonald, P.; Victa, C.; Carter-Franklin, J.N.; Fahrner, R. Selective Antibody Precipitation Using Polyelectrolytes: A Novel Approach to the Purification of Monoclonal Antibodies. Biotechnol. Bioeng. 2009, 102, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, P. Technology Trends in Antibody Purification. J. Chromatogr. A 2012, 1221, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, N.; Vogg, S.; Müller-Späth, T.; Morbidelli, M. Purification of Human Monoclonal Antibodies and Their Fragments. In Human Monoclonal Antibodies; Steinitz, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1904, pp. 163–188. ISBN 978-1-4939-8957-7. [Google Scholar]
- Arora, I. Chromatographic Methods for the Purification of Monoclonal Antibodies and Their Alternatives: A Review. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 475–481. [Google Scholar]
- Hage, D.S. 1—Chromatography. In Principles and Applications of Clinical Mass Spectrometry; Rifai, N., Horvath, A.R., Wittwer, C.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–32. ISBN 978-0-12-816063-3. [Google Scholar]
- Hage, D.S.; Anguizola, J.A.; Bi, C.; Li, R.; Matsuda, R.; Papastavros, E.; Pfaunmiller, E.; Vargas, J.; Zheng, X. Pharmaceutical and Biomedical Applications of Affinity Chromatography: Recent Trends and Developments. J. Pharm. Biomed. Anal. 2012, 69, 93–105. [Google Scholar] [CrossRef]
- Magdeldin, S. (Ed.) Affinity Chromatography, 1st ed.; IntechOpen: London, UK, 2012; ISBN 978-953-51-0325-7. [Google Scholar]
- Hahn, R.; Schlegel, R.; Jungbauer, A. Comparison of Protein A Affinity Sorbents. J. Chromatogr. B 2003, 790, 35–51. [Google Scholar] [CrossRef]
- Marichal-Gallardo, P.A.; Álvarez, M.M. State-of-the-art in Downstream Processing of Monoclonal Antibodies: Process Trends in Design and Validation. Biotechnol. Prog. 2012, 28, 899–916. [Google Scholar] [CrossRef]
- Arakawa, T.; Kita, Y.; Sato, H.; Ejima, D. Stress-Free Chromatography: Affinity Chromatography. Curr. Pharm. Biotechnol. 2009, 10, 456–460. [Google Scholar] [CrossRef]
- Bian, N.; Holstein, M. Methods of Increasing Protein Purity Using Protein A Based Chromatography. U.S. Patent 10,472,389, 12 November 2019. [Google Scholar]
- Ramos-de-la-Peña, A.M.; González-Valdez, J.; Aguilar, O. Protein A Chromatography: Challenges and Progress in the Purification of Monoclonal Antibodies. J. Sep. Sci. 2019, 42, 1816–1827. [Google Scholar] [CrossRef]
- Linhult, M.; Gülich, S.; Gräslund, T.; Simon, A.; Karlsson, M.; Sjöberg, A.; Nord, K.; Hober, S. Improving the Tolerance of a Protein A Analogue to Repeated Alkaline Exposures Using a Bypass Mutagenesis Approach. Proteins Struct. Funct. Bioinform. 2004, 55, 407–416. [Google Scholar] [CrossRef]
- Weinberg, J.; Zhang, S.; Kirkby, A.; Shachar, E.; Carta, G.; Przybycien, T. Chemical Modification of Protein a Chromatography Ligands with Polyethylene Glycol. II: Effects on Resin Robustness and Process Selectivity. J. Chromatogr. A 2018, 1546, 89–96. [Google Scholar] [CrossRef]
- Zhao, W.-W.; Liu, F.-F.; Shi, Q.-H.; Dong, X.-Y.; Sun, Y. Biomimetic Design of Affinity Peptide Ligands for Human IgG Based on Protein A-IgG Complex. Biochem. Eng. J. 2014, 88, 1–11. [Google Scholar] [CrossRef]
- Huang, H.; Dong, X.; Sun, Y.; Shi, Q. Biomimetic Affinity Chromatography for Antibody Purification: Host Cell Protein Binding and Impurity Removal. J. Chromatogr. A 2023, 1707, 464305. [Google Scholar] [CrossRef] [PubMed]
- Denizli, A.; Arica, Y. Protein A-Immobilized Microporous Polyhydroxyethylmethacrylate Affinity Membranes for Selective Sorption of Human-Immunoglobulin-G from Human Plasma. J. Biomater. Sci. Polym. Ed. 2000, 11, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, N.; Daxbacher, A.; Hahn, R. Rapid Purification of mAb Using Protein a Membranes Yielding High HCP Clearance. J. Chromatogr. B 2024, 1232, 123989. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Nakanishi, K.; Matsuno, R. Ion-Exchange Chromatography of Proteins; CRC Press: Boca Raton, FL, USA, 1988; ISBN 978-0-429-17379-0. [Google Scholar]
- Grodzki, A.C.; Berenstein, E. Antibody Purification: Ion-Exchange Chromatography. In Immunocytochemical Methods and Protocols; Oliver, C., Jamur, M.C., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 588, pp. 27–32. ISBN 978-1-58829-463-0. [Google Scholar]
- Rathore, A.S.; Hebbi, V. Ion Exchange Chromatographic Methods for Purification of Therapeutic Antibodies Therapeutic Antibodies. In Therapeutic Antibodies: Methods and Protocols; Houen, G., Ed.; Springer: New York, NY, USA, 2022; pp. 179–186. ISBN 978-1-07-161450-1. [Google Scholar]
- Zhao, G.; Li, Y.; Wu, Y.; Li, S.; Chen, X.; Zhang, W.; Xie, J. Chapter 24—Applications of Ion-Exchange Chromatography for the Purification of Antibodies. In Ion-Exchange Chromatography and Related Techniques; Nesterenko, P.N., Poole, C.F., Sun, Y., Eds.; Handbooks in Separation Science; Elsevier: Amsterdam, The Netherlands, 2024; pp. 505–520. ISBN 978-0-443-15369-3. [Google Scholar]
- Bai, L.; Burman, S.; Gledhill, L. Development of Ion Exchange Chromatography Methods for Monoclonal Antibodies. J. Pharm. Biomed. Anal. 2000, 22, 605–611. [Google Scholar] [CrossRef]
- Fekete, S.; Beck, A.; Veuthey, J.-L.; Guillarme, D. Ion-Exchange Chromatography for the Characterization of Biopharmaceuticals. J. Pharm. Biomed. Anal. 2015, 113, 43–55. [Google Scholar] [CrossRef]
- Yigzaw, Y.; Hinckley, P.; Hewig, A.; Vedantham, G. Ion Exchange Chromatography of Proteins and Clearance of Aggregates. Curr. Pharm. Biotechnol. 2009, 10, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Fekete, S.; Beck, A.; Guillarme, D. Characterization of Cation Exchanger Stationary Phases Applied for the Separations of Therapeutic Monoclonal Antibodies. J. Pharm. Biomed. Anal. 2015, 111, 169–176. [Google Scholar] [CrossRef]
- Ishihara, T.; Yamamoto, S. Optimization of Monoclonal Antibody Purification by Ion-Exchange Chromatography. J. Chromatogr. A 2005, 1069, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Suda, E.J.; Thomas, K.E.; Pabst, T.M.; Mensah, P.; Ramasubramanyan, N.; Gustafson, M.E.; Hunter, A.K. Comparison of Agarose and Dextran-Grafted Agarose Strong Ion Exchangers for the Separation of Protein Aggregates. J. Chromatogr. A 2009, 1216, 5256–5264. [Google Scholar] [CrossRef]
- Stone, M.T.; Cotoni, K.A.; Stoner, J.L. Cation Exchange Frontal Chromatography for the Removal of Monoclonal Antibody Aggregates. J. Chromatogr. A 2019, 1599, 152–160. [Google Scholar] [CrossRef]
- Xu, Z.; Li, J.; Zhou, J.X. Process Development for Robust Removal of Aggregates Using Cation Exchange Chromatography in Monoclonal Antibody Purification with Implementation of Quality by Design. Prep. Biochem. Biotechnol. 2012, 42, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Kluters, S.; Frech, C.; Von Hirschheydt, T.; Schaubmar, A.; Neumann, S. Solvent Modulation Strategy for Superior Antibody Monomer/Aggregate Separation in Cation Exchange Chromatography. J. Chromatogr. B 2015, 1006, 37–46. [Google Scholar] [CrossRef]
- Strube, J.; Ditz, R.; Kornecki, M.; Huter, M.; Schmidt, A.; Thiess, H.; Zobel-Roos, S. Process Intensification in Biologics Manufacturing. Chem. Eng. Process. Process Intensif. 2018, 133, 278–293. [Google Scholar] [CrossRef]
- Nadar, S.; Shooter, G.; Somasundaram, B.; Shave, E.; Baker, K.; Lua, L.H.L. Intensified Downstream Processing of Monoclonal Antibodies Using Membrane Technology. Biotechnol. J. 2021, 16, 2000309. [Google Scholar] [CrossRef]
- Yang, X.; Merenda, A.; AL-Attabi, R.; Dumée, L.F.; Zhang, X.; Thang, S.H.; Pham, H.; Kong, L. Towards next Generation High Throughput Ion Exchange Membranes for Downstream Bioprocessing: A Review. J. Membr. Sci. 2022, 647, 120325. [Google Scholar] [CrossRef]
- Knudsen, H.L.; Fahrner, R.L.; Xu, Y.; Norling, L.A.; Blank, G.S. Membrane Ion-Exchange Chromatography for Process-Scale Antibody Purification. J. Chromatogr. A 2001, 907, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Madadkar, P.; Sadavarte, R.; Butler, M.; Durocher, Y.; Ghosh, R. Preparative Separation of Monoclonal Antibody Aggregates by Cation-Exchange Laterally-Fed Membrane Chromatography. J. Chromatogr. B 2017, 1055–1056, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Schwellenbach, J.; Taft, F.; Villain, L.; Strube, J. Preparation and Characterization of High Capacity, Strong Cation-Exchange Fiber Based Adsorbents. J. Chromatogr. A 2016, 1447, 92–106. [Google Scholar] [CrossRef]
- Winderl, J.; Neumann, E.; Hubbuch, J. Exploration of Fiber-Based Cation Exchange Adsorbents for the Removal of Monoclonal Antibody Aggregates. J. Chromatogr. A 2021, 1654, 462451. [Google Scholar] [CrossRef]
- Eriksson, K.; Belew, M. Hydrophobic Interaction Chromatography. In Methods of Biochemical Analysis; Janson, J., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 165–181. ISBN 978-0-471-74661-4. [Google Scholar]
- Naseem, B.; Arif, I.; Jamal, M.A. Kosmotropic and Chaotropic Behavior of Hydrated Ions in Aqueous Solutions in Terms of Expansibility and Compressibility Parameters. Arab. J. Chem. 2021, 14, 103405. [Google Scholar] [CrossRef]
- Queiroz, J.A.; Tomaz, C.T.; Cabral, J.M.S. Hydrophobic Interaction Chromatography of Proteins. J. Biotechnol. 2001, 87, 143–159. [Google Scholar] [CrossRef]
- McCue, J.T. Chapter 25: Theory and Use of Hydrophobic Interaction Chromatography in Protein Purification Applications. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 463, pp. 405–414. ISBN 978-0-12-374536-1. [Google Scholar]
- Fanali, S.; Haddad, P.R.; Poole, C.; Lloyd, D.K. Liquid Chromatography: Fundamentals and Instrumentation; George Newnes Ltd.: London, UK, 2013; ISBN 978-0-12-415867-2. [Google Scholar]
- Vajda, J.; Müller, E. Hydrophobic Interaction Chromatography for the Purification of Antibodies. In Process Scale Purification of Antibodies; Gottschalk, U., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 155–180. ISBN 978-1-119-12691-1. [Google Scholar]
- Kurák, T.; Polakovič, M. Adsorption Performance of a Multimodal Anion-Exchange Chromatography Membrane: Effect of Liquid Phase Composition and Separation Mode. Membranes 2022, 12, 1173. [Google Scholar] [CrossRef]
- Fekete, S.; Veuthey, J.-L.; Beck, A.; Guillarme, D. Hydrophobic Interaction Chromatography for the Characterization of Monoclonal Antibodies and Related Products. J. Pharm. Biomed. Anal. 2016, 130, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Baca, M.; De Vos, J.; Bruylants, G.; Bartik, K.; Liu, X.; Cook, K.; Eeltink, S. A Comprehensive Study to Protein Retention in Hydrophobic Interaction Chromatography. J. Chromatogr. B 2016, 1032, 182–188. [Google Scholar] [CrossRef]
- Ghose, S.; Tao, Y.; Conley, L.; Cecchini, D. Purification of Monoclonal Antibodies by Hydrophobic Interaction Chromatography under No-Salt Conditions. mAbs 2013, 5, 795–800. [Google Scholar] [CrossRef]
- Ren, J.; Yao, P.; Chen, J.; Jia, L. Salt-Independent Hydrophobic Displacement Chromatography for Antibody Purification Using Cyclodextrin as Supermolecular Displacer. J. Chromatogr. A 2014, 1369, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Aller, M.; Guillarme, D.; Beck, A.; Fekete, S. Practical Method Development for the Separation of Monoclonal Antibodies and Antibody-Drug-Conjugate Species in Hydrophobic Interaction Chromatography, Part 1: Optimization of the Mobile Phase. J. Pharm. Biomed. Anal. 2016, 118, 393–403. [Google Scholar] [CrossRef]
- Cusumano, A.; Guillarme, D.; Beck, A.; Fekete, S. Practical Method Development for the Separation of Monoclonal Antibodies and Antibody-Drug-Conjugate Species in Hydrophobic Interaction Chromatoraphy, Part 2: Optimization of the Phase System. J. Pharm. Biomed. Anal. 2016, 121, 161–173. [Google Scholar] [CrossRef]
- Lu, Y.; Williamson, B.; Gillespie, R. Recent Advancement in Application of Hydrophobic Interaction Chromatography for Aggregate Removal in Industrial Purification Process. Curr. Pharm. Biotechnol. 2009, 10, 427–433. [Google Scholar] [CrossRef] [PubMed]
- McCue, J.T.; Engel, P.; Thömmes, J. Effect of Phenyl Sepharose Ligand Density on Protein Monomer/Aggregate Purification and Separation Using Hydrophobic Interaction Chromatography. J. Chromatogr. A 2009, 1216, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Bresolin, I.R.A.P.; Lingg, N.; Bresolin, I.T.L.; Jungbauer, A. Hydrophobic Interaction Chromatography as Polishing Step Enables Obtaining Ultra-Pure Recombinant Antibodies. J. Biotechnol. 2020, 324, 100020. [Google Scholar] [CrossRef]
- Hall, T.; Kelly, G.M.; Emery, W.R. Use of Mobile Phase Additives for the Elution of Bispecific and Monoclonal Antibodies from Phenyl Based Hydrophobic Interaction Chromatography Resins. J. Chromatogr. B 2018, 1096, 20–30. [Google Scholar] [CrossRef]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and Challenges for the next Generation of Antibody–Drug Conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Ducry, L.; Stump, B. Antibody-Drug Conjugates: Linking Cytotoxic Payloads to Monoclonal Antibodies. Bioconjug. Chem. 2010, 21, 5–13. [Google Scholar] [CrossRef]
- Matsuda, Y. Current Approaches for the Purification of Antibody–Drug Conjugates. J. Sep. Sci. 2022, 45, 27–37. [Google Scholar] [CrossRef]
- Matsuda, Y.; Leung, M.; Okuzumi, T.; Mendelsohn, B. A Purification Strategy Utilizing Hydrophobic Interaction Chromatography to Obtain Homogeneous Species from a Site-Specific Antibody Drug Conjugate Produced by AJICAPTM First Generation. Antibodies 2020, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.M.; Ghosh, R. Simultaneous Removal of Leached Protein-A and Aggregates from Monoclonal Antibody Using Hydrophobic Interaction Membrane Chromatography. J. Membr. Sci. 2012, 390–391, 263–269. [Google Scholar] [CrossRef]
- Ebert, S.; Fischer, S. Efficient Aggregate Removal from Impure Pharmaceutical Active Antibodies. BioProcess Int. 2011, 9, 36–42. [Google Scholar]
- Azevedo, A.M.; Rosa, P.A.J.; Ferreira, I.F.; Aires-Barros, M.R. Integrated Process for the Purification of Antibodies Combining Aqueous Two-Phase Extraction, Hydrophobic Interaction Chromatography and Size-Exclusion Chromatography. J. Chromatogr. A 2008, 1213, 154–161. [Google Scholar] [CrossRef]
- Carta, G.; Jungbauer, A. Protein Chromatography: Process Development and Scale-Up, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2020; ISBN 978-3-527-34666-0. [Google Scholar]
- Halan, V.; Maity, S.; Bhambure, R.; Rathore, A.S. Multimodal Chromatography for Purification of Biotherapeutics—A Review. Curr. Protein Pept. Sci. 2018, 20, 4–13. [Google Scholar] [CrossRef]
- Pinto, I.F.; Aires-Barros, M.R.; Azevedo, A.M. Multimodal Chromatography: Debottlenecking the Downstream Processing of Monoclonal Antibodies. Pharm. Bioprocess. 2015, 3, 263–279. [Google Scholar] [CrossRef]
- Kurák, T.; Molnár, T.; Polakovič, M. Adsorption of Recombinant Human Erythropoietin and Protein Impurities on a Multimodal Chromatography Membrane. Chem. Pap. 2019, 73, 1805–1811. [Google Scholar] [CrossRef]
- Yang, Y.; Geng, X. Mixed-Mode Chromatography and Its Applications to Biopolymers. J. Chromatogr. A 2011, 1218, 8813–8825. [Google Scholar] [CrossRef]
- Kallberg, K.; Johansson, H.; Bulow, L. Multimodal Chromatography: An Efficient Tool in Downstream Processing of Proteins. Biotechnol. J. 2012, 7, 1485–1495. [Google Scholar] [CrossRef]
- Gagnon, P. Purification of Monoclonal Antibodies by Mixed-Mode Chromatography. In Process Scale Purification of Antibodies; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 181–197. ISBN 978-1-119-12694-2. [Google Scholar]
- Stein, D.; Thom, V.; Hubbuch, J. Streamlined Process Development Procedure Incorporating the Selection of Various Stationary Phase Types Established in a mAb Aggregate Reduction Study with Different Mixed Mode Ligands. Biotechnol. Prog. 2022, 38, e3230. [Google Scholar] [CrossRef]
- Multimodal Chromatography. Available online: https://cdn.cytivalifesciences.com/api/public/content/digi-16870-pdf (accessed on 28 May 2025).
- Zhao, G.; Dong, X.-Y.; Sun, Y. Ligands for Mixed-Mode Protein Chromatography: Principles, Characteristics and Design. J. Biotechnol. 2009, 144, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, X. Mixed-Mode Chromatography in Pharmaceutical and Biopharmaceutical Applications. J. Pharm. Biomed. Anal. 2016, 128, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Moats, W.A. Effect of the Silica Support of Bonded Reversed-Phase Columns on Chromatography of Some Antibiotic Compounds. J. Chromatogr. A 1986, 366, 69–78. [Google Scholar] [CrossRef]
- Hartmann, E.; Chen, Y.; Mant, C.T.; Jungbauer, A.; Hodges, R.S. Comparison of Reversed-Phase Liquid Chromatography and Hydrophilic Interaction/Cation-Exchange Chromatography for the Separation of Amphipathic Alpha-Helical Peptides with L- and D-Amino Acid Substitutions in the Hydrophilic Face. J. Chromatogr. A 2003, 1009, 61–71. [Google Scholar] [CrossRef]
- Gudhka, R.B.; Roush, D.J.; Cramer, S.M. A Thermodynamic Evaluation of Antibody-Surface Interactions in Multimodal Cation Exchange Chromatography. J. Chromatogr. A 2020, 1628, 461479. [Google Scholar] [CrossRef]
- Tiselius, A.; Hjertén, S.; Levin, Ö. Protein Chromatography on Calcium Phosphate Columns. Arch. Biochem. Biophys. 1956, 65, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, X.; Cabanne, C. Chapter 22—Separation of Proteins by Mixed-Mode Chromatography. In Ion-Exchange Chromatography and Related Techniques; Nesterenko, P.N., Poole, C.F., Sun, Y., Eds.; Handbooks in Separation Science; Elsevier: Amsterdam, The Netherlands, 2024; pp. 461–472. ISBN 978-0-443-15369-3. [Google Scholar]
- Sogami, M.; Tamura, Y.; Imai, Y.; Shinagawa, Y. Studies on Gamma-Ray Irradiated Bovine Plasma Albumin Solution. J. Biochem. 1959, 46, 1251–1253. [Google Scholar]
- Available online: https://www.bio-rad.com/en-sk/product/cht-ceramic-hydroxyapatite-type-i-media?ID=7b9e4566-4a07-482a-8082-62dfcb1dd0b1 (accessed on 6 March 2024).
- O’Kennedy, R.; Murphy, C.; Devine, T. Technology Advancements in Antibody Purification. Antib. Technol. J. 2016, 6, 17–32. [Google Scholar] [CrossRef]
- Cummings, L.J.; Snyder, M.A.; Brisack, K. Chapter 24—Protein Chromatography on Hydroxyapatite Columns. In Methods in Enzymology, 2nd ed.; Burgess, R.R., Deutscher, M.P., Eds.; Guide to Protein Purification; Academic Press: Cambridge, MA, USA, 2009; Volume 463, pp. 387–404. [Google Scholar]
- Ng, P.; He, J.; Cohen, A. How CHTTM Ceramic Hydroxyapatite Works; Bio-Rad Laboratories, Inc.: Hercules, CA, USA, 2006. [Google Scholar]
- Wang, Y.; Carta, G. Competitive Binding of Monoclonal Antibody Monomer-Dimer Mixtures on Ceramic Hydroxyapatite. J. Chromatogr. A 2019, 1587, 136–145. [Google Scholar] [CrossRef]
- Gagnon, P. Improved Antibody Aggregate Removal by Hydroxyapatite Chromatography in the Presence of Polyethylene Glycol. J. Immunol. Methods 2008, 336, 222–228. [Google Scholar] [CrossRef]
- Gagnon, P.; Beam, K. Antibody Aggregate Removal by Hydroxyapatite Chromatography. Curr. Pharm. Biotechnol. 2009, 10, 440–446. [Google Scholar] [CrossRef]
- Chen, J.; Tetrault, J.; Zhang, Y.; Wasserman, A.; Conley, G.; DiLeo, M.; Haimes, E.; Nixon, A.E.; Ley, A. The Distinctive Separation Attributes of Mixed-Mode Resins and Their Application in Monoclonal Antibody Downstream Purification Process. J. Chromatogr. A 2010, 1217, 216–224. [Google Scholar] [CrossRef]
- Gagnon, P. Hydrogen Bond Chromatography. Genetic Engineering and Biotechnology News, 28 September 2018. Available online: https://www.genengnews.com/topics/bioprocessing/hydrogen-bond-chromatography/ (accessed on 28 May 2025).
- Rebula, L.; Raspor, A.; Bavčar, M.; Štrancar, A.; Leskovec, M. CIM Monolithic Chromatography as a Useful Tool for Endotoxin Reduction and Purification of Bacteriophage Particles Supported with PAT Analytics. J. Chromatogr. B 2023, 1217, 123606. [Google Scholar] [CrossRef]
- Hydrogen Bond Chromatography—A New Tool for Enhanced Separation of Proteins from Virus Particles and Other Very Large Biologics. Available online: https://www.bioprocessintl.com/sponsored-content/hydrogen-bond-chromatography-a-new-tool-for-enhanced-separation-of-proteins-from-virus-particles-and-other-very-large-biologics (accessed on 15 May 2025).
- Ghose, S.; Hubbard, B.; Cramer, S.M. Evaluation and Comparison of Alternatives to Protein A Chromatography. J. Chromatogr. A 2006, 1122, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, T.; Li, Y. A Parallel Demonstration of Different Resins’ Antibody Aggregate Removing Capability by a Case Study. Protein Expr. Purif. 2019, 153, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Konrad, S.; Graf, T.; Falkenstein, R. The Effect of Kosmotropic Salts on Aggregate Removal from Monoclonal Antibodies in Mixed-Mode Anion Exchange Chromatography. Curr. Res. Biotechnol. 2023, 6, 100139. [Google Scholar] [CrossRef]
- Kateja, N.; Kumar, D.; Godara, A.; Kumar, V.; Rathore, A.S. Integrated Chromatographic Platform for Simultaneous Separation of Charge Variants and Aggregates from Monoclonal Antibody Therapeutic Products. Biotechnol. J. 2017, 12, 1700133. [Google Scholar] [CrossRef] [PubMed]
- Pezzini, J.; Joucla, G.; Gantier, R.; Toueille, M.; Lomenech, A.-M.; Le Sénéchal, C.; Garbay, B.; Santarelli, X.; Cabanne, C. Antibody Capture by Mixed-Mode Chromatography: A Comprehensive Study from Determination of Optimal Purification Conditions to Identification of Contaminating Host Cell Proteins. J. Chromatogr. A 2011, 1218, 8197–8208. [Google Scholar] [CrossRef]
- Gao, D.; Wang, L.-L.; Lin, D.-Q.; Yao, S.-J. Evaluating Antibody Monomer Separation from Associated Aggregates Using Mixed-Mode Chromatography. J. Chromatogr. A 2013, 1294, 70–75. [Google Scholar] [CrossRef]
- Mi, X.; Wang, S.-C.; Winters, M.A.; Carta, G. Protein Adsorption on Core-Shell Resins for Flow-through Purifications: Effect of Protein Molecular Size, Shape, and Salt Concentration. Biotechnol. Prog. 2023, 39, e3300. [Google Scholar] [CrossRef]
- Ranjini, S.S.; Bimal, D.; Dhivya, A.P.; Vijayalakshmi, M.A. Study of the Mechanism of Interaction of Antibody (IgG) on Two Mixed Mode Sorbents. J. Chromatogr. B 2010, 878, 1031–1037. [Google Scholar] [CrossRef]
- Wang, R.-Z.; Lin, D.-Q.; Tong, H.-F.; Lu, H.-L.; Yao, S.-J. Evaluation of Mixed-Mode Chromatographic Resins for Separating IgG from Serum Albumin Containing Feedstock. J. Chromatogr. B 2013, 936, 33–41. [Google Scholar] [CrossRef]
- Pezzini, J.; Cabanne, C.; Gantier, R.; Janakiraman, V.N.; Santarelli, X. A Comprehensive Evaluation of Mixed Mode Interactions of HEA and PPA HyperCelTM Chromatographic Media. J. Chromatogr. B 2015, 976–977, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Burton, S.C.; Harding, D.R.K. Hydrophobic Charge Induction Chromatography: Salt Independent Protein Adsorption and Facile Elution with Aqueous Buffers. J. Chromatogr. A 1998, 814, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, E. Antibody Separation by Hydrophobic Charge Induction Chromatography. Trends Biotechnol. 2002, 20, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhang, Y.; Meng, J. Protein–Ligand Interactions for Hydrophobic Charge-Induction Chromatography: A QCM-D Study. Appl. Surf. Sci. 2022, 572, 151420. [Google Scholar] [CrossRef]
- Bak, H.; Thomas, O.R.T. Evaluation of Commercial Chromatographic Adsorbents for the Direct Capture of Polyclonal Rabbit Antibodies from Clarified Antiserum. J. Chromatogr. B 2007, 848, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.-F.; Lin, D.-Q.; Yuan, X.-M.; Yao, S.-J. Enhancing IgG Purification from Serum Albumin Containing Feedstock with Hydrophobic Charge-Induction Chromatography. J. Chromatogr. A 2012, 1244, 116–122. [Google Scholar] [CrossRef]
- Gagnon, P. IgG Aggregate Removal by Charged-Hydrophobic Mixed Mode Chromatography. Curr. Pharm. Biotechnol. 2009, 10, 434–439. [Google Scholar] [CrossRef]
- Chmielowski, R.A.; Meissner, S.; Roush, D.; Linden, T.O.; Glowacki, E.; Konietzko, J.; Nti-Gyabaah, J. Resolution of Heterogeneous Charged Antibody Aggregates via Multimodal Chromatography: A Comparison to Conventional Approaches. Biotechnol. Prog. 2014, 30, 636–645. [Google Scholar] [CrossRef]
- He, X. Mixed-Mode Chromatography for mAb S Aggregate Removal: Comparison of CHTTM Ceramic Hydroxyapatite, Capto Adhere, and Capto Adhere ImpRes; Bulletin 6749; Bio-Rad Laboratories, Inc.: Hercules, CA, USA, 2015. [Google Scholar]
- Dutta, A.K.; Fedorenko, D.; Tan, J.; Costanzo, J.A.; Kahn, D.S.; Zydney, A.L.; Shinkazh, O. Continuous Countercurrent Tangential Chromatography for Mixed Mode Post-Capture Operations in Monoclonal Antibody Purification. J. Chromatogr. A 2017, 1511, 37–44. [Google Scholar] [CrossRef]
- Koley, S.; Altern, S.H.; Vats, M.; Han, X.; Jang, D.; Snyder, M.A.; Belisle, C.; Cramer, S.M. Evaluation of Guanidine-Based Multimodal Anion Exchangers for Protein Selectivity and Orthogonality. J. Chromatogr. A 2021, 1653, 462398. [Google Scholar] [CrossRef]
- O’Connor, E.; Aspelund, M.; Bartnik, F.; Berge, M.; Coughlin, K.; Kambarami, M.; Spencer, D.; Yan, H.; Wang, W. Monoclonal Antibody Fragment Removal Mediated by Mixed Mode Resins. J. Chromatogr. A 2017, 1499, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Maria, S.; Joucla, G.; Garbay, B.; Dieryck, W.; Lomenech, A.-M.; Santarelli, X.; Cabanne, C. Purification Process of Recombinant Monoclonal Antibodies with Mixed Mode Chromatography. J. Chromatogr. A 2015, 1393, 57–64. [Google Scholar] [CrossRef]
- Nadar, S.; Somasundaram, B.; Charry, M.; Billakanti, J.; Shave, E.; Baker, K.; Lua, L.H.L. Design and Optimization of Membrane Chromatography for Monoclonal Antibody Charge Variant Separation. Biotechnol. Prog. 2022, 38, e3288. [Google Scholar] [CrossRef] [PubMed]
- Osuofa, J.; Henn, D.; Zhou, J.; Forsyth, A.; Husson, S.M. High-Capacity Multimodal Anion-Exchange Membranes for Polishing of Therapeutic Proteins. Biotechnol. Prog. 2021, 37, e3129. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Yang, H.; Cao, W.; Liu, Q. Separation of Bispecific Antibody Related Impurities with Mixed-Mode Chromatography. Process Biochem. 2023, 132, 110–120. [Google Scholar] [CrossRef]
- Arakawa, T.; Ponce, S.; Young, G. Isoform Separation of Proteins by Mixed-Mode Chromatography. Protein Expr. Purif. 2015, 116, 144–151. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, X.; Chen, T.; Wang, Y.; Li, Y. Removal of Half Antibody, Hole-Hole Homodimer and Aggregates During Bispecific Antibody Purification Using MMC ImpRes Mixed-Mode Chromatography. Protein Expr. Purif. 2020, 167, 105529. [Google Scholar] [CrossRef]
- Arakawa, T.; Kurosawa, Y.; Storms, M.; Maruyama, T.; Okumura, C.J.; Kita, Y. Capto MMC Mixed-Mode Chromatography of Murine and Rabbit Antibodies. Protein Expr. Purif. 2016, 127, 105–110. [Google Scholar] [CrossRef]
- Kaleas, K.A.; Tripodi, M.; Revelli, S.; Sharma, V.; Pizarro, S.A. Evaluation of a Multimodal Resin for Selective Capture of CHO-Derived Monoclonal Antibodies Directly from Harvested Cell Culture Fluid. J. Chromatogr. B 2014, 969, 256–263. [Google Scholar] [CrossRef]
- Joucla, G.; Le Sénéchal, C.; Bégorre, M.; Garbay, B.; Santarelli, X.; Cabanne, C. Cation Exchange Versus Multimodal Cation Exchange Resins for Antibody Capture from CHO Supernatants: Identification of Contaminating Host Cell Proteins by Mass Spectrometry. J. Chromatogr. B 2013, 942–943, 126–133. [Google Scholar] [CrossRef]
- Nascimento, A.; Pinto, I.F.; Chu, V.; Aires-Barros, M.R.; Conde, J.P.; Azevedo, A.M. Studies on the Purification of Antibody Fragments. Sep. Purif. Technol. 2018, 195, 388–397. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, L.; Wang, Y.; Li, Y. Processing of High-Salt-Containing Protein A Eluate Using Mixed-Mode Chromatography in Purifying an Aggregation-Prone Antibody. Protein Expr. Purif. 2019, 164, 105458. [Google Scholar] [CrossRef] [PubMed]
- Vajda, J.; Mueller, E.; Bahret, E. Dual Salt Mixtures in Mixed Mode Chromatography with an Immobilized Tryptophan Ligand Influence the Removal of Aggregated Monoclonal Antibodies. Biotechnol. J. 2014, 9, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wan, Y.; Duan, J.; Li, Y. Evaluating a New Mixed-Mode Resin Diamond MMC Mustang Using Capto MMC ImpRes as a Benchmark. Protein Expr. Purif. 2021, 186, 105930. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Q.; Xian, M.; Nian, R.; Xu, F. Application of Enhanced Electronegative Multimodal Chromatography as the Primary Capture Step for Immunoglobulin G Purification. AMB Express 2018, 8, 93. [Google Scholar] [CrossRef]
- Koehnlein, W.; Holzgreve, A.; Schwendner, K.; Skudas, R.; Schelter, F. Purification of Hydrophobic Complex Antibody Formats Using a Moderately Hydrophobic Mixed Mode Cation Exchange Resin. J. Chromatogr. A 2023, 1687, 463696. [Google Scholar] [CrossRef]
- DiLeo, M.; Ley, A.; Nixon, A.E.; Chen, J. Choices of Capture Chromatography Technology in Antibody Manufacturing Processes. J. Chromatogr. B 2017, 1068–1069, 136–148. [Google Scholar] [CrossRef]
- He, X.M.; Voß, C.; Li, J. Exploring the Unique Selectivity of Hydrophobic Cation Exchanger Nuvia cPrime for the Removal of a Major Process Impurity: A Case Study with IgM. Curr. Protein Pept. Sci. 2019, 20, 65–74. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Q.; Lin, D.; Yao, S. Protein Adsorption Behavior and Immunoglobulin Separation with a Mixed-Mode Resin Based on p-Aminohippuric Acid. J. Sep. Sci. 2014, 37, 2474–2480. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Roush, D.; Cramer, S.M. The Effect of pH on Antibody Retention in Multimodal Cation Exchange Chromatographic Systems. J. Chromatogr. A 2020, 1617, 460838. [Google Scholar] [CrossRef]
- Arakawa, T.; Tomioka, Y.; Nakagawa, M.; Sakuma, C.; Kurosawa, Y.; Ejima, D.; Tsumoto, K.; Akuta, T. Non-Affinity Purification of Antibodies. Antibodies 2023, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, Y.; Tong, H.; Liu, Y.; Sun, L.; Wang, Y.; Xiao, L. Preparation and Evaluation of Dextran-Grafted Mixed-Mode Chromatography Adsorbents. J. Chromatogr. A 2019, 1599, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-L.; Pi, W.-Y.; Xie, W.-F.; Gu, J.-W.; Wang, Y.-J.; Xiao, L.; Li, Y.-J.; Li, J.-F. Improved Antibody Adsorption Performance of Phenyl-Based Mixed-Mode Adsorbents by Adjusting the Functional Group of Ligand. Biochem. Eng. J. 2021, 176, 108092. [Google Scholar] [CrossRef]
- Liu, X.; Niu, J.; Shen, J.; Chu, H.; Wang, C.; Wei, Y. Boronate Affinity/Hydrophobic Charge Induction Synergistic Adsorption for Improving Purification of Antibody. Chem. Eng. J. 2024, 483, 149216. [Google Scholar] [CrossRef]
- Aoyama, S.; Matsumoto, Y.; Mori, C.; Sota, K. Application of Novel Mixed Mode Chromatography (MMC) Resins Having a Hydrophobic Modified Polyallylamine Ligand for Monoclonal Antibody Purification. J. Chromatogr. B 2022, 1191, 123072. [Google Scholar] [CrossRef]
- LeBarre, J.P.; Chu, W.; Altern, S.H.; Kocot, A.J.; Bhandari, D.; Barbieri, E.; Sly, J.; Crapanzano, M.; Cramer, S.M.; Phillips, M.; et al. Mixed-Mode Size-Exclusion Silica Resin for Polishing Human Antibodies in Flow-through Mode. J. Chromatogr. A 2024, 1720, 464772. [Google Scholar] [CrossRef]
- Shekhawat, L.K.; Markle, T.; Esfandiarfard, K.; Theel, E.K.; Maloisel, J.-L.; Malmquist, G. Next Generation Multimodal Chromatography Resins via an Iterative Mapping Approach: Chemical Diversity, High-Throughput Screening, and Chromatographic Modelling. J. Chromatogr. A 2023, 1699, 464018. [Google Scholar] [CrossRef]
- Liu, T.; Lin, D.-Q.; Lu, H.-L.; Yao, S.-J. Preparation and Evaluation of Dextran-Grafted Agarose Resin for Hydrophobic Charge-Induction Chromatography. J. Chromatogr. A 2014, 1369, 116–124. [Google Scholar] [CrossRef]
- Xia, H.-F.; Lin, D.-Q.; Wang, L.-P.; Chen, Z.-J.; Yao, S.-J. Preparation and Evaluation of Cellulose Adsorbents for Hydrophobic Charge Induction Chromatography. Ind. Eng. Chem. Res. 2008, 47, 9566–9572. [Google Scholar] [CrossRef]
- Gao, D.; Yao, S.-J.; Lin, D.-Q. Preparation and Adsorption Behavior of a Cellulose-Based, Mixed-Mode Adsorbent with a Benzylamine Ligand for Expanded Bed Applications. J. Appl. Polym. Sci. 2008, 107, 674–682. [Google Scholar] [CrossRef]
- Liu, T.; Lin, D.-Q.; Zhang, Q.-L.; Yao, S.-J. Characterization of Immunoglobulin Adsorption on Dextran-Grafted Hydrophobic Charge-Induction Resins: Cross-Effects of Ligand Density and pH/Salt Concentration. J. Chromatogr. A 2015, 1396, 45–53. [Google Scholar] [CrossRef]
- Lu, H.-L.; Lin, D.-Q.; Gao, D.; Yao, S.-J. Evaluation of Immunoglobulin Adsorption on the Hydrophobic Charge-Induction Resins with Different Ligand Densities and Pore Sizes. J. Chromatogr. A 2013, 1278, 61–68. [Google Scholar] [CrossRef]
- Liu, T.; Lin, D.-Q.; Wu, Q.-C.; Zhang, Q.-L.; Wang, C.-X.; Yao, S.-J. A Novel Polymer-Grafted Hydrophobic Charge-Induction Chromatographic Resin for Enhancing Protein Adsorption Capacity. Chem. Eng. J. 2016, 304, 251–258. [Google Scholar] [CrossRef]
- Liu, T.; Lin, D.-Q.; Wang, C.-X.; Yao, S.-J. Poly(Glycidyl Methacrylate)-Grafted Hydrophobic Charge-Induction Agarose Resins with 5-Aminobenzimidazole as a Functional Ligand. J. Sep. Sci. 2016, 39, 3130–3136. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-M.; Lin, D.-Q.; Yao, S.-J. Adsorption of IgG and BSA on Two Chromatographic Resins—Poly(Ethylenimine)-4FF Resin and Tetrapeptide-Poly(Ethylenimine)-4FF Resin. J. Chem. Eng. Data 2018, 63, 4418–4424. [Google Scholar] [CrossRef]
- Tong, H.-F.; Lin, D.-Q.; Chu, W.-N.; Zhang, Q.-L.; Gao, D.; Wang, R.-Z.; Yao, S.-J. Multimodal Charge-Induction Chromatography for Antibody Purification. J. Chromatogr. A 2016, 1429, 258–264. [Google Scholar] [CrossRef]
- Wang, J.; Sproul, R.T.; Anderson, L.S.; Husson, S.M. Development of Multimodal Membrane Adsorbers for Antibody Purification Using Atom Transfer Radical Polymerization. Polymer 2014, 55, 1404–1411. [Google Scholar] [CrossRef]
- Wang, J.; Jenkins, E.W.; Robinson, J.R.; Wilson, A.; Husson, S.M. A New Multimodal Membrane Adsorber for Monoclonal Antibody Purifications. J. Membr. Sci. 2015, 492, 137–146. [Google Scholar] [CrossRef]
- Fan, J.; Sripada, S.A.; Pham, D.N.; Linova, M.Y.; Woodley, J.M.; Menegatti, S.; Boi, C.; Carbonell, R.G. Purification of a Monoclonal Antibody Using a Novel High-Capacity Multimodal Cation Exchange Nonwoven Membrane. Sep. Purif. Technol. 2023, 317, 123920. [Google Scholar] [CrossRef]
- Ma, N.; Yao, D.; Yang, H.; Yin, J.; Wang, H.; Zhang, Y.; Meng, J. Surface Modification of Cellulose Membranes to Prepare a High-Capacity Membrane Adsorber for Monoclonal Antibody Purification via Hydrophobic Charge-Induction Chromatography. Ind. Eng. Chem. Res. 2018, 57, 13235–13246. [Google Scholar] [CrossRef]
- Pham, D.N.; Linova, M.Y.; Smith, W.K.; Brown, H.; Elhanafi, D.; Fan, J.; Lavoie, J.; Woodley, J.M.; Carbonell, R.G. Novel Multimodal Cation-Exchange Membrane for the Purification of a Single-Chain Variable Fragment from Pichia pastoris Supernatant. J. Chromatogr. A 2024, 1718, 464682. [Google Scholar] [CrossRef] [PubMed]
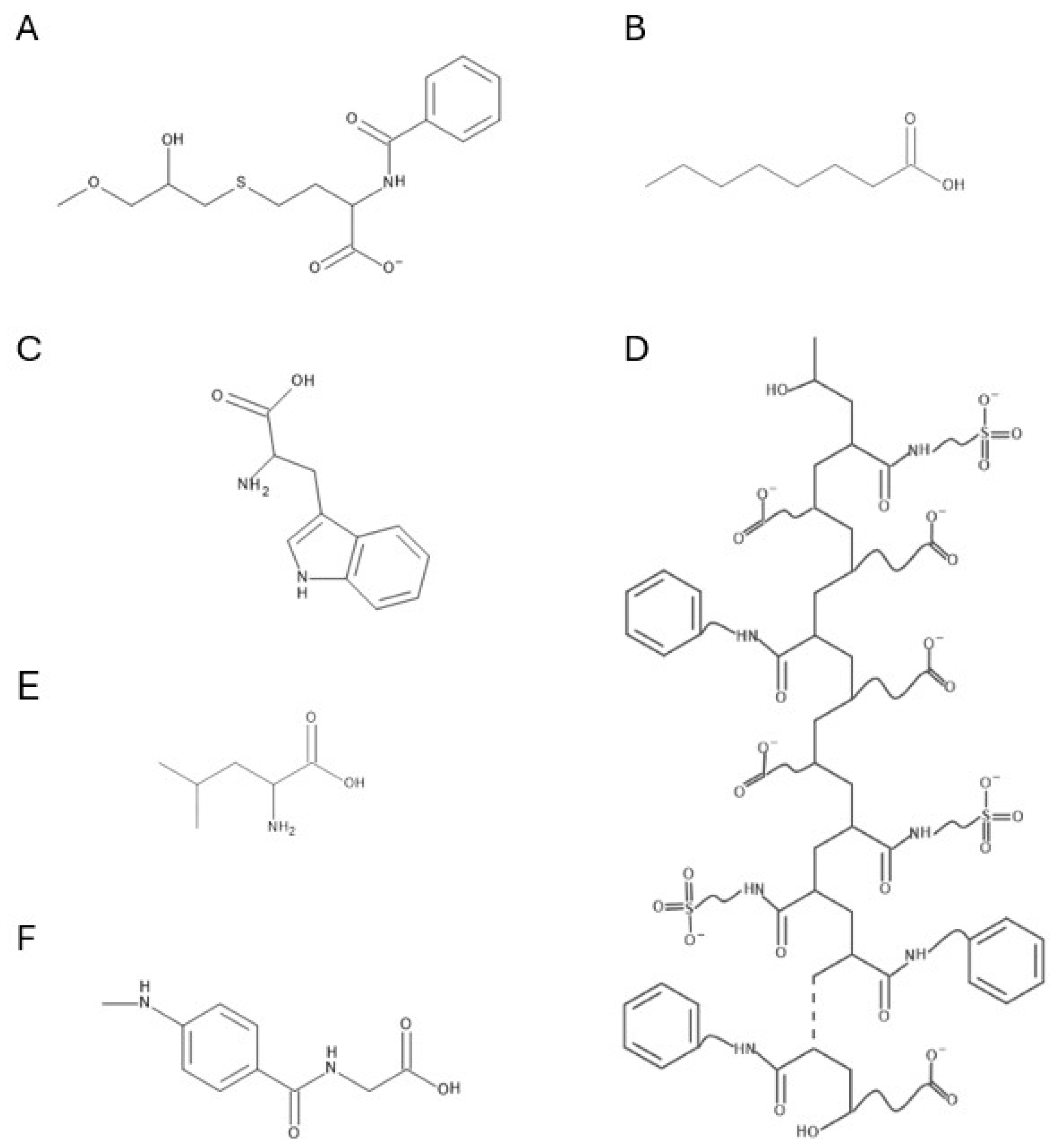
| Adsorbent | Operational Mode | Position | Product | Reference |
|---|---|---|---|---|
| Capto MMC | Bind-elute | Capture step | Monomeric IgG with >99% purity and 82% yield | [157] |
| Toyopearl MX-Trp-650M | Bind-elute | Polishing step | Monomeric mAb with 90% yield and aggregate removal factor 6.5 (from original 15%) | [161] |
| Diamond MMC | Bind-elute | Polishing step | Monomeric bAb with 98.8% purity and 55.2% yield | [162] |
| Eshmuno HCX | Bind-elute | Capture step | Monomeric IgG with purity > 99.5% and recovery 94.6% | [163] |
| Eshmuno CMX | Bind-elute | Polishing step | Monomeric IgG with >97% purity and 70% recovery | [164] |
| Nuvia cPrime | Flowthrough | Capture step | Dynamic binding capacity 55 mg/mL | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupčíková, V.; Molnár, T.; Kurák, T.; Polakovič, M. Antibody Aggregate Removal by Multimodal Chromatography. Molecules 2025, 30, 2363. https://doi.org/10.3390/molecules30112363
Rupčíková V, Molnár T, Kurák T, Polakovič M. Antibody Aggregate Removal by Multimodal Chromatography. Molecules. 2025; 30(11):2363. https://doi.org/10.3390/molecules30112363
Chicago/Turabian StyleRupčíková, Veronika, Tomáš Molnár, Tomáš Kurák, and Milan Polakovič. 2025. "Antibody Aggregate Removal by Multimodal Chromatography" Molecules 30, no. 11: 2363. https://doi.org/10.3390/molecules30112363
APA StyleRupčíková, V., Molnár, T., Kurák, T., & Polakovič, M. (2025). Antibody Aggregate Removal by Multimodal Chromatography. Molecules, 30(11), 2363. https://doi.org/10.3390/molecules30112363






