Abstract
Zinc oxide (ZnO) and iron oxide (IO) nanoparticles have been identified as promising candidates for biomedical applications, based on their unique physicochemical properties. The association of these nanoparticles in a single system creates a bimodal entity, allowing the excellent luminescent properties of ZnO quantum dots to be combined with the contrast agent of IO for magnetic resonance imaging (MRI). The present study focuses on the luminescent and MRI properties of a new poly(lactic-co-glycolic acid) (PLGA) nanocarrier system formulation containing ZnO NPs and IO NPs in different nominal ratios. Microscopic analysis (TEM and SEM) reveals a circular morphology with IO and ZnO NPs. The average diameter of the particles was determined to be 220 nm, as measured by DLS. The luminescence results indicate that the PLGA system shows strong emission in the visible range, and the MRI analysis shows a high r2 relaxivity of 171 mM−1 s−1 at 7T. The optimized formulation, exhibiting a molar ratio of Fe:Zn ranging from 1:10 to 1:13 (mol:mol), demonstrates superior fluorescence and MRI performance, underscoring the significance of nanoparticle composition in bimodal imaging applications. The systems evaluated demonstrate no toxicity in the THP-1 cells for doses of up to 128 µg mL−1, with efficient labeling after 4 h of incubation, yielding images of strong luminescence and T2 contrast. The PLGA:ZnO:IO system demonstrates considerable potential as a bimodal platform for diagnostic imaging.
1. Introduction
Quantum dots (QDs) or semiconductor materials stand out for their ability to produce high-resolution images due to their unique luminescent and electrical properties resulting from quantum confinement. QDs are particularly useful in diagnostic imaging, due to their ability to enhance image contrast through fluorescence, in combination with dedicated contrast agents for magnetic resonance imaging (MRI) [1,2,3,4,5]. They have also been used in photodynamic therapy [6,7,8], controlled drug delivery [9,10,11], and cell markers [12,13].
The toxicity of QDs is dependent on their physicochemical properties and environmental conditions, such as charge, size, concentration, and surface coating [14]. If the QDs’ core is based on metals such as cadmium and selenium, which have high quantum yields, their use is limited as they are toxic (e.g., CdTe QDs and CdSe/ZnS QDs) [14,15,16]; this does not seem to be the case for zinc-based QDs.
Zinc oxide is a semiconductor employed frequently due to its biocompatibility, chemical stability, biodegradability, biosafety, and excellent optical and electrical properties. Its versatility enables applications in chemical detection, biosensors, photocatalysts, and other biomedical uses [17]. Its characteristics include a wide band gap of 3.37 eV and a significant exciton binding energy of 60 meV at room temperature (RT), which contribute to its efficient light absorption and emission properties [18,19]. Furthermore, research has been conducted on its application in the domain of fluorescence imaging for the detection of tumor cells, resulting in a high fluorescence yield [17,20,21].
MRI techniques can also employ nanoparticle-based contrast agents, such as superparamagnetic iron oxide nanoparticles (SPIONs). SPIONs, in their magnetite form, exhibit superparamagnetic properties that make them responsive to the external magnetic fields used in MRI [22]. This enables a strong interaction with the magnetic fields of the MRI equipment, resulting in a high-contrast image signal. In addition to its magnetic properties, iron oxide finds application in cancer treatment through magnetic hyperthermia, nanocatalysis, and the generation of reactive oxygen species to induce cancer cell apoptosis [23].
These two compounds, iron oxide nanoparticles (IO NPs) and zinc oxide nanoparticles (ZnO NPs), are interesting due to their unique physicochemical properties, low cost, and the non-toxicity of the zinc and iron elements [24]. IO stands out for its magnetic properties [25,26,27,28], and the ZnO NP is known for its luminescent properties [29,30,31,32]. However, studies investigating the concomitant use of ZnO and IO as bimodal imaging agents are limited [33,34]. In Gupta et al. [33], a core (IO) shell (ZnO) structure is reported, along with its biocompatibility and capacity to label cells. In Lai et al. [34], research was carried out on the in vivo biosynthesis of ZnO and IO nanoclusters in Alzheimer models. Indeed, after the administration of iron(II) chloride and zinc gluconate, the formation and accumulation of metallic nanoclusters in the brains of mice were detected using fluorescence and magnetic resonance imaging.
To the best of our knowledge, no studies have proposed a bimodal imaging system based on ZnO and IO within a polymeric system, such as poly(Lactic-co-Glycolic Acid) (PLGA), which promotes stability and biocompatibility, while maintaining reduced toxicity of inorganic compounds and promoting vectorization for specific targeting, with potential application as a theranostic drug [35]. However, there are reports on ZnO-encapsulated PLGA, especially for antimicrobial activity [36,37,38,39,40], and IO-encapsulated PLGA for photodynamic therapy and drug delivery for diseases such as cancer [41,42,43,44,45,46]. The objective was to develop a biocompatible and stable PLGA system that contains both nanoparticles, maintaining their fluorescence and magnetic properties to provide a bimodal contrast agent (Figure 1). A simple emulsion and solvent evaporation method was developed, and an extensive study was conducted to optimize the system. The interaction between the two nanoparticles, based on their magnetic and luminescent properties, was investigated. Finally, a study assessed the cellular labeling and potential toxicity. This study utilized high-quality images via fluorescence and MRI to provide comprehensive insights into the system’s performance.
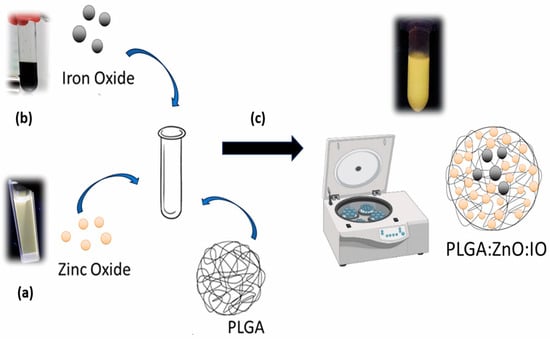
Figure 1.
Synthesis scheme of PLGA particles containing ZnO NPs and SPIONs. (a) sol-gel method for ZnO NPs, adapted from Spanhel and Anderson [47]; (b) co-precipitation method for IO NPs, adapted from Babes et al. (1999) [48]; and (c) PLGA particles produced by a simple solvent evaporation emulsion reaction, adapted from Luque-Michel et al. (2021) [45].
2. Results and Discussion
The nominal ratio between PLGA, ZnO, and IO was assessed to ensure that the resulting product exhibits favorable luminescent and magnetic characteristics. For instance, a sample with a nominal PLGA:ZnO:IO ratio of 10:1:1 (w:w:w, mg) indicates the intended use of 10 mg PLGA, 1 mg ZnO, and 1 mg IO.
During the experiments, interactions between ZnO, iron oxide, and PLGA influenced the luminescence and magnetic intensity of the materials. These interactions were analyzed, providing insights into the system’s stability.
2.1. Stability Assessment of the PLGA System: Hydrodynamic Diameter Distribution Analysis
The particle size distribution of the PLGA systems was determined using the intensity-weighted mean hydrodynamic diameter (d.nm) (Table 1). The stability in the PVA and T80 surfactant systems, at concentrations of 0.4% and 0.3%, respectively, was evaluated on the day of synthesis and 15 days post-synthesis.

Table 1.
Stability study of PLGA suspensions after particle synthesis and following a 15-day storage period in suspension. DLS analysis.
The diameter of PLGA-IO NPs with varying IO ratios exhibited no significant changes following a 15-day storage, as indicated in Table 1. Additionally, the hydrodynamic diameter of the PLGA:IO particles exhibited uniformity across varying IO ratios, with an average measurement of 150 nm. Particle uniformity was corroborated by the polydispersity index (PDI) values. Even after a 15-day storage period at 4 °C, the particles exhibited stability in suspension. The observed diameter of PLGA particles falls within the optimal range of 100 to 300 nm for effective cell incorporation [49].
The average diameter of PLGA:ZnO particles is approximately 220 nm. This diameter is greater than that of PLGA:IO particles (~150 nm), with a statistically significant difference (p < 0.05). This observation indicates that there are distinct interaction behaviors associated with the loading of ZnO NPs into PLGA. The diameter remained consistent after 15 days of storage at 4° C, indicating robust stability under these conditions.
For PLGA:ZnO:IO, the diameter of the particles ranged from 190 to 250 nm; this is a range similar to that of PLGA:ZnO synthesized without IO (211 to 250 nm). Over a 15-day storage period at 4 °C, formulations with higher IO content in PLGA:ZnO:IO exhibited particle growth, possibly due to interparticle interactions leading to aggregation. The increase in IO NPs may promote aggregation, likely due to a decrease in surface charge between IO particles at neutral pH during the PLGA formulation process [50]. Consequently, it is essential to determine the appropriate IO NP concentration to prevent excessive aggregation.
On the other hand, when the amount of IO was kept constant and the amount of ZnO was increased, no significant increase in the diameter of the PLGA particles was observed. Unlike IO NPs, ZnO NPs remain stable when the suspension pH is in the range of 7–8 [51]. Furthermore, although an increase in NP concentration can influence the particle aggregation, the amount of ZnO used in the formulations was not sufficient to induce an increase in PLGA particle diameter.
2.2. Characterization of the PLGA System: Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM) Analysis
The morphology of the NPs was analyzed by TEM, and the elemental analysis of iron and zinc elements in the PLGA systems was conducted by a combination of SEM and EDS analysis (Figure 2).

Figure 2.
TEM images of PLGA particles (a–f). (a) PLGA unloaded; (b) PLGA:IO 10:1; (c) PLGA:ZnO 10:6; (d) PLGA:ZnO:IO 10:6:1; (e) PLGA:ZnO 10:8; and (f) PLGA:ZnO:IO 10:8:1. Gaussian distribution curve representing the NP diameter calculation based on the presence of ZnO and IO NPs as determined by TEM analysis; (g) SEM image of the PLGA:ZnO:IO 10:6:1 sample. Energy-dispersive X-ray spectroscopy (EDS) analysis is presented, showing the detection of the elements O-K, C-K, Zn-K, and Fe-K. The corresponding EDS spectrum and elemental mapping are displayed, confirming the distribution and composition of these elements within the sample.
Additionally, PLGA particles containing only ZnO or IO NPs manifested as black dots, a phenomenon not observed in the image of unloaded PLGA. The diameter of the ZnO and IO NP was calculated by utilizing the Gaussian curve (Figure 2), and the resulting data are presented in Table 2.

Table 2.
The average diameter of ZnO and IO was analyzed by TEM and calculated using a Gaussian curve, while the zeta potential of the PLGA systems in water was measured before the lyophilization process.
The ZnO NPs present in the PLGA:ZnO 10:6 and 10:8 formulations have an average diameter of 4.73 nm and 4.56 nm, respectively. These results are consistent with those previously reported for ZnO NPs [52]. In the PLGA:IO 10:1 formulation, the IO NPs exhibited an average diameter of 7.33 nm, with the TEM analysis of isolated IO NPs revealing a comparable diameter of 8.07 nm (Figure S1).
Although there is no significant difference in the average diameters of the NPs observed in PLGA 10:8:1 or 10:6:1 (4.64 ± 0.14 nm and 4.03 ± 0.10 nm, respectively) compared to PLGA:ZnO 10:8 or 10:6 (4.73 ± 0.16 nm and 4.56 ± 0.16 nm, respectively), it can be noted that the formulations with IO (PLGA:ZnO:IO 10:8:1 and 10:6:1) exhibit a broader distribution, with NPs larger than 6 nm, which is consistent with the PLGA:IO 10:1 sample, while the formulations without IO (PLGA:ZnO 10:8 and 10:6) show a narrower distribution.
These results suggest that the NPs were successfully incorporated into the PLGA system. Elemental analysis through SEM images followed by EDS was performed to confirm the incorporation of ZnO and IO NPs. Furthermore, the integrated SEM and EDS analysis (Figure 2g) revealed that zinc and iron elements were predominantly situated within the PLGA:ZnO:IO particles, indicating their efficient incorporation into the polymeric matrix. These findings complement and reinforce the TEM results.
The surface charge was also studied using zeta potential (ZP) before the lyophilization (Table 2). The results indicate that the IO NP, in the presence of the ZnO NP, caused an increase in the zeta potential value of the PLGA particles. This increase in zeta potential may contribute to enhanced colloidal stability within the system, which can be attributed to the inhibition of particle aggregation.
2.3. PLGA Particles Incorporating Zinc Oxide and Iron Oxide Nanoparticles: Analysis of Luminescent Properties
The spectra demonstrating luminescence (emission and absorption) are presented in Figure 3. The luminescent properties of the PLGA system were adjusted based on the excitation (absorption) and emission spectra of pristine ZnO synthesized in previous work by our team [53]. The synthesized ZnO NP, with a diameter ranging from 3 to 4 nm, exhibits a peak emission at ~540 nm when excited at 343 nm. The excitation wavelength of ZnO typically varies around 350 nm, with a decrease in emission capability at longer wavelengths, generally starting from ~365 nm. This variation in the emission spectrum is attributed to the diameter of the produced ZnO NPs [54].
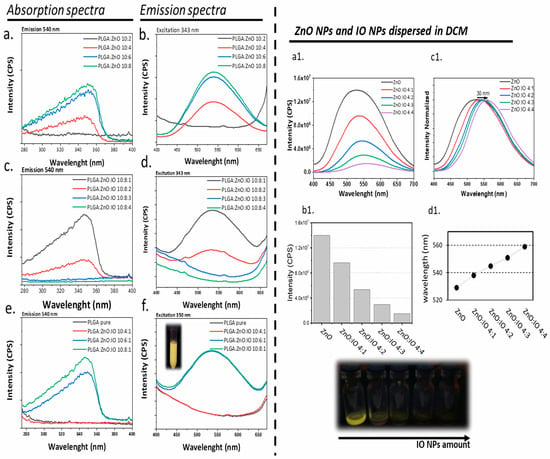
Figure 3.
Fluorescence spectra of PLGA samples: absorption spectra and emission spectra for PLGA:ZnO samples (a,b), PLGA:ZnO:IO samples with the same quantity of ZnO and varying amounts of IO (c,d), and PLGA:ZnO:IO samples with the same quantity of IO and varying amounts of ZnO (e,f). The insets (a1–d1) highlight the fluorescence assay of ZnO NPs and IO NPs dispersed in dichloromethane (DCM), with a constant quantity of ZnO and varying amounts of IO: (a1) emission spectra upon excitation at 343 nm, (b1) maximum emission intensity as a function of IO concentration, (c1) normalized emission spectra, and (d1) maximum emission wavelength as a function of IO concentration.
The behavior of the emission spectra varies with the concentration of ZnO in the PLGA polymer system. Therefore, 343 nm is a suitable excitation wavelength for evaluating ZnO luminescence, as it avoids excitation loss regardless of NP diameter or concentration. Notably, the luminescence intensity increases with increasing ZnO concentration in the polymer system (Figure 3b). The PLGA:ZnO 10:8 sample exhibits the highest luminescence intensity, although it is similar to PLGA:ZnO 10:6, suggesting a possible plateau in luminescence intensity. No luminescence was observed in the PLGA:ZnO 10:2 sample, and a low luminescence intensity of the PLGA:ZnO 10:4 sample was observed, despite several attempts, suggesting that there is a minimum concentration of ZnO required for the luminescent properties to manifest. Based on these observations, the minimum and maximum concentrations of ZnO and IO in the PLGA preparation were evaluated (Figure 3c–f).
If the amount of ZnO affects the luminescence intensity of PLGA systems, the presence and amount of IO also impact the luminescence, as shown in Figure 3c,d. PLGA:ZnO:IO 10:8:1 shows robust luminescence intensity. However, doubling the amount of IO in the formulation process to produce the PLGA:ZnO:IO 10:8:2 sample reduced the luminescence intensity. At higher IO amounts, luminescence becomes negligible. Consequently, the optimal IO nominal concentration for assessing luminescence in the current PLGA:ZnO:IO samples is determined by a quantity of 1 mg.
In the analysis of Figure 3e,f, where the ZnO concentration is varied while the IO concentration is kept constant, the emission remains constant for the PLGA:ZnO:IO 10:6:1 and 10:8:1 samples, but not for the PLGA:ZnO:IO 10:4:1 sample. This latter formulation was synthesized several times, consistently yielding the same result. The last one was synthesized multiple times, consistently producing the same result. Therefore, a minimum of 6 mg of ZnO is necessary to impart luminescent properties to this material when a nominal amount of 1 mg of IO is present in the PLGA particle preparation. An initial amount of ZnO NPs between 6 and 8 mg can ensure a luminescence plateau in samples containing 1 mg of IO.
Regarding the luminescent properties and ICP results of different Zn and Fe concentrations (Table 3), the quantified ZnO content for PLGA:ZnO:IO at 10:6:1 and 10:8:1 was 50.01 ± 0.34 mM and 45.57 ± 0.42 mM, respectively. These values indicate a saturation point for ZnO incorporation in the PLGA matrix, which is consistent with the observed luminescence intensity plateau. In formulations containing only ZnO, the luminescence intensity increased with the nominal ZnO concentration, as expected. However, in systems co-loaded with ZnO and IO, the presence of IO NPs interfered with the luminescent response. According to the ICP data, the IO:ZnO ratio shifted from 1:10 to 1:13 as the formulation changed from PLGA:ZnO:IO 10:6:1 to 10:8:1. While absorption intensity (Figure 3e) increased with higher ZnO content relative to IO, this variation had no significant effect on emission intensity at the excitation wavelength of 343 nm.

Table 3.
Relaxivity (r2) of PLGA:ZnO:IO samples, followed by ICP results of Zn and Fe elements and their ratio.
The results show that increasing the nominal concentration of IO significantly affects the material’s luminescent properties. To investigate the interaction between IO and ZnO NPs under controlled conditions, we conducted a study using different nominal concentrations of IO while keeping the nominal concentration of ZnO dispersed in DCM (Figure 3a1–d1). These results allow us to draw two important conclusions. First, an increase in IO concentration can reduce the luminescence intensity of the dispersion. Second, as the IO concentration increases, there is a 30 nm “red shift” in the maximum emission wavelength. Consequently, the luminescence properties of the ZnO NP can be influenced not only by its concentration in the PLGA particles, but also by the presence of the IO NP.
This phenomenon is attributed to a specific property of iron in fluorescent compounds known as the quenching effect, where the reduction in luminescence intensity occurs due to several factors. In the case of iron oxide and zinc oxide, this is facilitated by the energy transfer process [55]. The fluorescence of the molecule is quenched due to an energy transfer from the lowest excited singlet state to another electronic state of the metal, resulting in a loss of fluorescence [56].
2.4. PLGA Particles Incorporating Zinc Oxide and Iron Oxide: Analysis of Magnetic Resonance Imaging Properties
Magnetic resonance imaging (MRI) was used to evaluate the magnetic properties of PLGA particles containing ZnO and IO. Samples of PLGA:ZnO:IO 10:6:1 and 10:8:1 were analyzed as they exhibited the highest luminescence properties. The MRI images are shown in Figure 4.

Figure 4.
MRI characterization of PLGA samples: (a) T2-weighted (TE = 64 ms and TR = 2000 ms) and T1-weighting images (TE = 8 ms and TR = 400 ms) of PLGA samples acquired at 7T. The concentration of the samples is fixed at 2.5 mg mL−1; (b) T2 and T1 values of the PLGA samples (n = 3, mean ± SD); (c) relaxivity calculation for PLGA samples containing IO and ZnO:IO. The calculation was made based on the concentration of iron in each sample based on ICP results.
To calculate the relaxivity for each sample, the concentration of Fe was measured by ICP (Table 3). The relaxivity value (r) was determined using the relaxivity linearity plot (Figure 4c), according to Equation (1):
where 1/T is the relaxation rate (inverse of the relaxation time T) in s−1, r is the relaxivity, is the iron concentration in mM, and 1/T0 is the intercept, representing the relaxation in the absence of iron. The relaxivity values are summarized in Table 3.
As shown in Figure 4a,b, an effect on the T2-weighted image is observed when IO is present (samples of PLGA:IO 10:1, PLGA:ZnO:IO 10:6:1, and PLGA:ZnO:IO 10:8:1), with the most significant effect observed for the PLGA containing only IO. Quantitative analysis of these images confirms a reduction in T2 values for PLGA:IO 10:1 (36 ± 1 ms) and PLGA:ZnO:IO 10:6:1 and 10:8:1 (50 ± 1 ms and 106 ± 1 ms, respectively) compared to unloaded PLGA (823 ± 25 ms). On the T1-weighted images, a very limited effect was observed, as is commonly seen with NPs [57,58]. The T2 and T1 results related to different concentrations of iron are shown in Table S1 and Figure S2.
The r2 results (Figure 4c) demonstrate high relaxivity, which is probably associated with aggregation of IO NPs [57]. Interestingly, the presence of ZnO NPs negatively impacts the PLGA system’s relaxivity. This may be related to the organization of IO NPs within the PLGA particle, where the ZnO NP seems to limit the aggregation of IO, as observed in the TEM and SEM images presented in Figure 2 (Section 2.2). The addition of ZnO NPs can act as a spacer, reducing the dipolar interactions between the magnetic moments of IO NPs [59].
According to the ICP results shown in Table 3, it can be observed that the amount of zinc is approximately ten times higher than that of iron in moles. Additionally, this ratio increases with the nominal concentration of ZnO in the formulation (Fe:Zn, 1:10 to 1:13). Since the ratios can affect the aggregation state of the iron oxide NPs, this may explain why the r2 value of the PLGA:ZnO:IO 10:8:1 formulation is lower than that of the PLGA:ZnO:IO 10:6:1 formulation.
2.5. PLGA Particles Incorporating Zinc Oxide and Iron Oxide: Analysis of Cytotoxicity and Cellular Labelling
THP-1 cells, a human monocytic cell line capable of differentiating into highly phagocytic macrophages, were used in this study. The cytotoxicity assay was performed with unloaded PLGA particles, as well as those loaded with IO, ZnO, or both, evaluating their effects as a function of particle concentration and incubation time (Figure 5). None of the tested systems induced a statistically significant reduction in cell viability, regardless of the dose or incubation period.
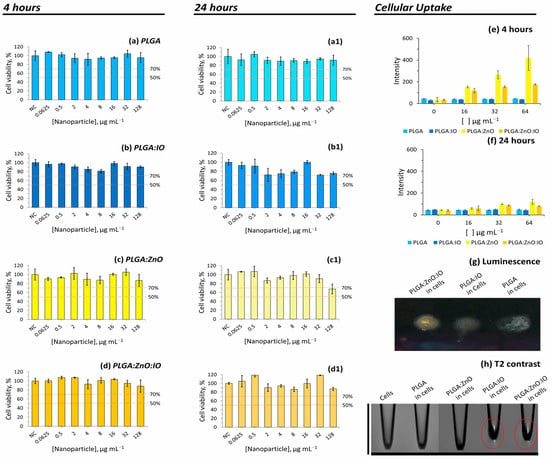
Figure 5.
Resazurin cytotoxicity assay of PLGA particles: cell viability versus concentration of PLGA samples after incubating for 4 h (a–d) and 24 h (a1–d1) (n = 3, mean ± SD) (NC = negative control). Kruskal–Wallis followed by Dunn’s test compared with NC (p > 0.05); uptake of PLGA particles containing ZnO and IO NPs by cells assessed through luminescence assay at incubation times of 4 h (e) and 24 h (f), with an excitation wavelength of 343 nm and an emission wavelength of 540 nm (n = 3, mean ± SD). UV chamber luminescence (λex = 245 nm) of uptake cells is shown in (g), and uptake of cells assessed by MRI test using T2 contrast (TR = 3000 ms and TE = 7.5 ms) is shown in (h).
A cellular labelling assay was conducted to evaluate the ability of PLGA samples to mark THP-1 cells (Figure 5e–h). The labelling efficiency was evaluated after 4 h (Figure 5e) and 24 h (Figure 5f) of incubation, using luminescence testing based on the properties of ZnO.
After the 4 h incubation period, only the cells incubated with ZnO NP-containing PLGA were detectable using luminescence (Figure 5g), in a concentration-dependent manner (Figure 5e). The PLGA and PLGA-IO samples were used as controls to demonstrate the absence of fluorescence in cells without ZnO.
On the other hand, increasing the incubation time up to 24 h led to a reduction in luminescence (Figure 5e–g). One hypothesis to explain this observation is that, over time, the particles undergo cellular metabolism, where the acidic pH dissolves the luminescent ZnO, thus accounting for the loss of luminescence [60]. A study conducted by Lallo et al. (2022) revealed that ZnO behaves differently in more acidic pH environments, leading to its dissolution [53]. Another study by Cai et al. (2017) revealed that the endocytosis of tumor cells can dissolve ZnO when in contact with the lower pH of the cellular metabolism [61].
The cellular uptake assessed using MRI after 4h incubation (Figure 5h) showed that PLGA particles containing IO, such as PLGA:IO and PLGA:ZnO:IO, provided a strong T2 contrast, showing that the double-labelled PLGA particles were also detectable using MRI. These results indicate that this approach could be promising for biomedical imaging applications.
3. Materials and Methods
3.1. Materials
Zinc acetate dihydrate C4H6O4Zn.2H2O, iron (II) chloride hexahydrate 98%, iron (III) chloride hexahydrate 97%, tetramethylammonium hydroxide pentahydrate 97% (TMAOH), oleic acid 90%, perchloric acid, poly(lactic-co-glycolic acid) polymer (Resomer RG 503H, PLGA 50:50), Tween 80 (Polysorbate 80) (T80), triethylamine, dichloromethane (DCM), and polyvinylalkohol 20–98 (PVA) were purchased from Sigma-Aldrick® (Saint Louis, MO, USA); lithium hydroxide monohydrate LiOH.H2O Chemicals™ (Ribeirão Preto, Brazil); ethanol 99.5%(Qhemis, São Paulo, Brazil); ethyl acetate 99.8% Fisher Chemical™ (Loughborough, UK). The water Milli-Q was obtained by an ultrapurification process in Merck™ Millipore (Trosly-Breuil, France).
3.2. Synthesis
3.2.1. Zinc Oxide NPs
ZnO NPs were obtained by a sol-gel method according to Spanhel and Anderson (1991) [47], with some modifications. It consists of 2 main stages: (1) Formation of acetyl zinc acetate precursor: Zinc acetate hexahydrate and 50 mL of ethyl alcohol were added in a refluxing system at 98 °C for 2 h, obtaining a solution of 0.1 M. After this step, the solution was cooled at RT and diluted to a final concentration of 0.05 M. (2) Formation of the ZnO colloidal suspension: the colloidal suspension of ZnO was carried out by a hydrolysis and condensation reaction, in high power ultrasound (frequency 37, power 70%), at 60 °C, for 60 min, using lithium hydroxide (LiOH) as a hydrolysis agent. The 1:1 molar ratio of Zn:OH was used.
The coating method with oleic acid was performed following the procedure described by Manaia et al. (2015) [62], with some modifications. For that, 75 μL of oleic acid was added to 10 mL of the pre-prepared QD ethanolic suspension. The final concentration of oleic acid was 18.57 mM. The reaction was made at 30 min, up to 60 °C with stirring at 400 rpm. The suspension was stored overnight at RT (25 °C). The final suspension was washed 3 times with 10 mL of ethanol and centrifuged at 2000 rpm at RT. The centrifuged sample was diluted in DCM to a final concentration of 40 mg mL−1. The ZnO NPs were characterized by X-ray diffraction (XRD) and absorbance measurements, with detailed results presented in Figure S3.
3.2.2. Superparamagnetic Iron Oxide NPs (SPIONs)
The synthesis of IO NPs was made according to Babes et al. (1999) [48], with some modifications. Solutions of 0.2 mL of FeCl2.4H2O 0.314 M and FeCl3.6H2O 0.666 M were added dropwise in 2 mL of 1 M TMAOH solution, magnetically stirred at RT for 5 min. Then, the solution was transferred to a 75 °C water bath, and 75 μL of oleic acid was added, stirred for 15 min, and stirred for 15 min at RT. The final solution was neutralized using 2 mL of HCLO4 1 M, then washed 10 times with pure water and 3 times with ethanol PA. The final product was dried and dispersed in DCM in a concentration of 40 mg mL−1.
3.2.3. PLGA Particles
The synthesis of the PLGA system dispersed in water was conducted following the procedure of a simple solvent evaporation emulsion reaction made by Luque-Michel et al. (2021) [45], with some modifications. Initially, 10 mg of PLGA (Resomer RG 503H) was dissolved in 0.8 mL of a mixture of triethylamine and acetyl acetate solution (1:1000). Subsequently, 0.2 mL of the contrast agent in DCM was added at the appropriate concentration. This solution was added dropwise into 2 mL of T80 1% under continuous agitation at RT. The mixture was sonicated using an ultrasonic probe system at 20 W for 20 s. The resulting emulsion was added dropwise to a solution of T80 0.3% and PVA 0.4%, and the mixture was kept under constant agitation for 1.5 h with the flask uncapped. The dispersion was then centrifuged and washed three times with pure water. The final product was dispersed in water containing trehalose 37% (a nominal amount based on trehalose:PLGA, w:w).
3.3. Characterization
3.3.1. Evaluation of Luminescent Properties
The photoluminescence properties were evaluated by measuring the spectra of emission by photoluminescence (PL) and the excitation spectra by photoluminescence excitation (PLE). The spectra were recorded in the UV–visible range on a spectrophotometer Fluorolog™ 3 at RT, equipped with a double excitation monochromator, and a Hamamatsu™ R928 photomultiplier with a Xe lamp (450 W). The wavelength of emission and excitation was based on the previous wavelength test.
3.3.2. Transmission Electron Microscopy
TEM images were obtained with a JEOL Electron Microscope (model JEM-1400; SUPER TWIN) (JOEL Ltd., Tokyo, Japan) operated at 600 kV of 60 magnitude. The samples dispersed in water were deposited on carbon grids. Image analysis was performed by ImageJ software (version 1.53t, National Institute of Health, Bethesda, MD, USA) with 20 and 50 nm scale bars.
3.3.3. Scanning Electron Microscopy and Energy-Dispersive X-Ray Spectroscopy (EDS)
SEM was carried out to evaluate the visual aspects of the nanocrystals. The samples were carbon-coated to about 15 nm thickness using a Sample Sputter Coater SCD 050™ (Bal-Tec, Wallruf, Germany) for 70 s. After that, SEM pictures were taken using a scanning electron microscope SM300™ (TOPCON, Singapore) instrument.
Additionally, the elemental composition was determined using energy-dispersive X-ray spectroscopy (EDS) attached to the SEM. EDS spectra were obtained from selected areas of the samples, enabling qualitative and semi-quantitative identification of the elements present in the NP. The data were analyzed using JSM-IT500HR (JEOL ltd., Tokyo, Japan), providing information on the elemental distribution across the particle.
3.3.4. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
Iron and zinc concentrations were measured using inductively coupled plasma mass spectrometry (ICP-MS), following the protocol established in collaboration with the Institute of Chemical Sciences of the University of Rennes (ISCR).
3.3.5. Evaluation of Particle Diameter Distribution and Zeta Potential
Particle diameter distribution and zeta potential measurements were performed using a Malvern Dynamic Light Scattering (DLS) system (Malvern™, Malvern, UK). The samples were diluted in distilled water to achieve an optimal count rate (>300 kcps) and attenuation level (attn) between 6 and 9, following the equipment’s operational parameters. The dilution factor was adjusted empirically to ensure reliable measurements. All measurements were conducted at 25 °C, and the results were collected in triplicate.
3.3.6. Magnetic Resonance Imaging
The magnetic resonance imaging (MRI) was carried out using a 7T scanner (Biospec 70/20 Avance III, Bruker™, Wissembourg, France) equipped with a BGA12S gradient system (675 mT/m) applying a magnetic field of 7 Tesla at room temperature. Emission and reception were ensured by a 72 mm diameter resonator. Sets of spin echo sequences weighted in T1 or T2 were acquired using variable repetition times (from 200 to 10,000 ms), with a fixed echo time of 8 ms and a fixed repetition time of 2000 ms for variable echo times (ranging from 8 to 200 ms). In both cases, images were acquired with a field of view = 6 cm × 4 cm, a matrix = 256 × 192, and a slice thickness of 2 mm.
3.4. Biological Analysis
3.4.1. Cytotoxicity Assay Using Resazurin Test
The cell uptake assay was made using the THP-1 human monocytic cell line, which was obtained from ATCC, catalog number TIB-202. The differentiation of THP-1 cells into macrophage-like cells was induced by adding phorbol 12-myristate-13-acetate (PMA, Sigma-Aldrich™, Burlington, MA, USA) at a concentration of 100 nM at the time of seeding (time 0). The cells were incubated with PMA for 48 h before further experiments.
The cells were plated in a 96-well plate with a seeding density of 8.5 × 103 cells per well. After 24 h, PLGA NPs were introduced at concentrations ranging from 0.078 to 128 μg mL−1, followed by a 4 h and 24 h incubation period. Subsequently, the cells were washed and allowed to grow for an additional 24 h before the addition of 20 μL of a 25 μg mL−1 resazurin solution in phosphate-buffered saline (PBS). After 4 h, the wells were analyzed using the EnSpire™ microplate reader (PerkinElmer, Waltham, MA, USA) at 570 nm wavelength excitation, and the baseline emission was corrected at 590 nm. The percentage of viable cells was calculated using Equation (2):
where viable cells (%) represent the percentage of living cells in the sample, O.D. sample is the optical density of the sample with cells, O.D. media is the optical density of the cell-free medium, and O.D. cells is the optical density of a sample with a known number of viable cells. The analyses were conducted in triplicate. A statistical analysis was carried out using the Kruskal–Wallis test followed by Dunn’s test, to compare the viability between the concentrations of PLGA samples and the control, as well as between different incubation times. A significance level of 0.05 was considered for all analyses. Data processing and statistical tests were conducted using GraphPad Prism 9.
3.4.2. Cellular Uptake
The cellular uptake assay was performed using the TPH-1 cell line. The cells were seeded onto a 24-well plate with a seeding density of 8.5 × 103 cells per well. The concentration between 16 and 64 µg mL−1 was tested according to the cytotoxicity assay for the different samples. Incubation times were fixed at 4 and 24 h. After the incubation, the cells were washed to remove the NPs not captured by the cells. Fluorescence analyses were carried out using EnSpire™ microplate reader (PerkinElmer, Waltham, MA, USA), with excitation at 343 nm and emission at 540 nm. MRI analyses of the cells were performed according to the procedures outlined in Section 3.3.6, following a 4 h incubation period.
4. Conclusions
In this study, we present a nanoscale bimodal contrast agent for fluorescence and magnetic resonance imaging, formulated using poly(lactic-co-glycolic acid) (PLGA) encapsulating zinc oxide (ZnO) and iron oxide (IO) nanoparticles. Before the biological assessment on THP-1 cells, an extensive investigation into the interaction of these oxides and their impact on imaging properties was conducted. Our findings demonstrate the potential of PLGA:ZnO:IO particles for biomedical applications; this is attributed to their combined luminescent and magnetic properties. This bifunctional nanosystem holds promise for advanced imaging modalities in biomedical research and clinical diagnostics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30081818/s1, Figure S1. TEM analyses of IO NPs; Table S1. Results of T2 and T1 in different concentrations of PLGA systems; Figure S2. Relaxivity values (r1 and r2); Figure S3. Characterization of ZnO NP [63,64,65].
Author Contributions
Conceptualization, T.W.L.B., L.L. and L.A.C.; methodology, T.W.L.B., L.L., I.V. and L.A.C.; software, T.W.L.B. and L.L.; validation, T.W.L.B. and L.L.; formal analysis, T.W.L.B., L.L., I.V., L.S. and N.G.d.F.; investigation, T.W.L.B., L.L. and L.A.C.; resources, L.L. and L.A.C.; data curation, T.W.L.B., L.L. and I.V.; writing—original draft preparation, T.W.L.B., L.S. and N.G.d.F.; writing—review and editing, T.W.L.B., L.L., M.P.S. and L.A.C.; visualization, T.W.L.B., L.L. and L.A.C.; supervision, L.L. and L.A.C.; project administration, L.L. and L.A.C.; funding acquisition, L.L. and L.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support of CAPES-finance code 001 (Grant number: 88887.529995/2020-00), CAPES-Print code 41/2017 (Grant number: 88887.696991/2022-00), CNPQ (Grant number: 301994/2022-6).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors would like to express their gratitude to CAPES, CAPES Print, and CNPQ for funding the project, as well as to the MINT laboratories and the University of Angers for their support and col-laboration. We would like to also thank Prism platform for providing access to the MRI facility, SCIAM facility and especially Romain Mallet for the TEM images acquisition, Bertrand Lefeuvre for the ICP analysis at Institut des Sciences Chimiques of Rennes (Fr), and Marina Paiva Abuçafy for the SEM images acquisition at Chemical Institute of Unesp—Araraquara.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DCM | Dichloromethane |
| EDS | Energy-Dispersive Spectroscopy |
| IO | Iron Oxide |
| MRI | Magnetic Resonance Imaging |
| NC | Negative Control |
| NP | Nanoparticle |
| PDI | Polydispersity Index |
| PLGA | Poly(lactic-co-glycolic acid) |
| PVA | Polyvinyl Alcohol |
| RT | Room Temperature |
| SEM | Scanning Electronic Microscopy |
| SPION | Superparamagnetic Iron Oxide Nanoparticles |
| T80 | Tween 80 |
| TEM | Transmission Electronic Microscopy |
| TE | Echo Time |
| THP-1 | (Human monocytic cell line) |
| TR | Repetition Time |
| ZnO | Zinc Oxide |
References
- Yang, D.; Wei, X.; Piao, Z.; Cui, Z.; He, H.; Wen, Z.; Zhang, W.; Wang, L.; Mei, S.; Guo, R. Constructing Bimodal Nanoprobe Based on Gd:AgInS2/ZnS Quantum Dots for Fluorometric/Magnetic Resonance Imaging in Mesenchymal Stem Cells. J. Mater. Sci. Technol. 2023, 148, 116–122. [Google Scholar] [CrossRef]
- Molaei, M.J. Turmeric-Derived Gadolinium-Doped Carbon Quantum Dots for Multifunctional Fluorescence Imaging and MRI Contrast Agent. J. Lumin. 2023, 257, 119692. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Z.; Li, S.; Lin, S.; Zhang, L.; Meng, Z.; Zhang, X.; Sun, S.K. Non-Invasive Diagnosis of Acute Kidney Injury Using Mn-Doped Carbon Dots-Based Magnetic Resonance Imaging. Biomater. Sci. 2023, 11, 4289–4297. [Google Scholar] [CrossRef]
- Jin, L.; Bai, W.; Yu, S.; Zhang, J. One-Pot Preparation of Mn3O4/GSH/CdTe Quantum Dots Complex for T1-Weighted MRI/Fluorescence Detection of H3PO4. Talanta 2023, 263, 124713. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zang, L.Y.; Gao, H.Y.; Peng, J.; Zheng, D.Y.; Liu, C.; Liu, X.J.; Cheng, D.B.; Zhu, C.N. Cu-In-S/ZnS:Gd3+ Quantum Dots with Isolated Fluorescent and Paramagnetic Modules for Dual-Modality Imaging in Vivo. Colloids Surf. B Biointerfaces 2023, 223, 113158. [Google Scholar] [CrossRef]
- Ren, C.; Hu, D.; Cui, Y.; Chen, P.; Xu, X.; Cheng, J.; He, T. Ag-Doped InP/ZnS Quantum Dots for Type-I Photosensitizers. Chem. Commun. 2023, 59, 2311–2314. [Google Scholar] [CrossRef]
- Liu, F.; Lin, J.; Luo, Y.; Xie, D.; Bian, J.; Liu, X.; Yue, J. Sialic Acid-Targeting Multi-Functionalized Silicon Quantum Dots for Synergistic Photodynamic and Photothermal Cancer Therapy. Biomater. Sci. 2023, 11, 4009–4021. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Wu, X.; Bo, B.; Qin, J.; Zhang, Y.; Wu, X.; Li, X.; Wang, Y.; Peng, H. Synthesis of NIR-II Fluorescent Hybrid Ag2S/ZnPc Nanoparticles for in Vitro Photodynamic Therapy. ChemNanoMat 2023, 9, e202200570. [Google Scholar] [CrossRef]
- Singh, B.; Singh, S.; Gautam, A.; Sutherland, A.; Pal, K. Preparation and Characterization of PLA Microspheres as Drug Delivery System for Controlled Release of Cetirizine with Carbon Dots as Drug Carrier. Polym. Bull. 2023, 80, 5741–5757. [Google Scholar] [CrossRef]
- Kurniawan, D.; Mathew, J.; Rahardja, M.R.; Pham, H.P.; Wong, P.C.; Rao, N.V.; Ostrikov, K.; Chiang, W.H. Plasma-Enabled Graphene Quantum Dot Hydrogels as Smart Anticancer Drug Nanocarriers. Small 2023, 19, 2206813. [Google Scholar] [CrossRef]
- Mohammed-Ahmed, H.K.; Nakipoglu, M.; Tezcaner, A.; Keskin, D.; Evis, Z. Functionalization of Graphene Oxide Quantum Dots for Anticancer Drug Delivery. J. Drug Deliv. Sci. Technol. 2023, 80, 104199. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, P.; Li, J.; Li, N.; Xu, D.; Wu, R.; Shen, H.; Li, L.S. Establishment of a Ca(II) Ion-Quantum Dots Fluorescence Signal Amplification Sensor for High-Sensitivity Biomarker Detection. Anal. Chim. Acta 2023, 1237, 340534. [Google Scholar] [CrossRef] [PubMed]
- Magdy, G.; Elmansi, H.; Belal, F.; El-Deen, A.K. Doped Carbon Dots as Promising Fluorescent Nanosensors: Synthesis, Characterization, and Recent Applications. Curr. Pharm. Des. 2022, 29, 415–444. [Google Scholar] [CrossRef] [PubMed]
- Hardman, R. A Toxicologic Review of Quantum Dots: Toxicity Depends on Physicochemical and Environmental Factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef]
- Winnik, F.M.; Maysinger, D. Quantum Dot Cytotoxicity and Ways to Reduce It. Acc. Chem. Res. 2013, 46, 672–680. [Google Scholar] [CrossRef]
- Hoshino, A.; Hanada, S.; Yamamoto, K. Toxicity of Nanocrystal Quantum Dots: The Relevance of Surface Modifications. Arch. Toxicol. 2011, 85, 707–720. [Google Scholar] [CrossRef]
- Ortiz-Casas, B.; Galdámez-Martínez, A.; Gutiérrez-Flores, J.; Baca Ibañez, A.; Kumar Panda, P.; Santana, G.; de la Vega, H.A.; Suar, M.; Gutiérrez Rodelo, C.; Kaushik, A.; et al. Bio-Acceptable 0D and 1D ZnO Nanostructures for Cancer Diagnostics and Treatment. Mater. Today 2021, 50, 533–569. [Google Scholar] [CrossRef]
- Norek, M. Approaches to Enhance UV Light Emission in ZnO Nanomaterials. Curr. Appl. Phys. 2019, 19, 867–883. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Zhang, G.; Bian, H.; Zhao, M.; Ma, S. Optical Properties and the Band-Gap Variation in Diverse Zn1-XSnxO Nanostructures. Superlattices Microstruct. 2018, 123, 349–357. [Google Scholar] [CrossRef]
- Ibraheem, S.; Kadhim, A.A.; Kadhim, K.A.; Kadhim, I.A.; Jabir, M. Zinc Oxide Nanoparticles as Diagnostic Tool for Cancer Cells. Int. J. Biomater. 2022, 2022, 2807644. [Google Scholar] [CrossRef]
- Wanas, W.; Abd El-Kaream, S.A.; Ebrahim, S.; Soliman, M.; Karim, M. Cancer Bioimaging Using Dual Mode Luminescence of Graphene/FA-ZnO Nanocomposite Based on Novel Green Technique. Sci. Rep. 2023, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M. Magnetic Resonance Imaging and Iron-Oxide Nanoparticles in the Era of Personalized Medicine. Nanotheranostics 2023, 7, 424–449. [Google Scholar] [CrossRef]
- Liu, M.; Ye, Y.; Ye, J.; Gao, T.; Wang, D.; Chen, G.; Song, Z. Recent Advances of Magnetite (Fe3O4)-Based Magnetic Materials in Catalytic Applications. Magnetochemistry 2023, 9, 110. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Toxicity of Metal Compounds: Knowledge and Myths. Organometallics 2017, 36, 4071–4090. [Google Scholar] [CrossRef]
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-Mediated Drug Delivery Systems for Anticancer Agents: An Overview and Perspectives. Int. J. Nanomed. 2021, 16, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barahona, I.; Muñoz-Hernando, M.; Ruiz-Cabello, J.; Herranz, F.; Pellico, J. Iron Oxide Nanoparticles: An Alternative for Positive Contrast in Magnetic Resonance Imaging. Inorganics 2020, 8, 28. [Google Scholar] [CrossRef]
- Calsolaro, F.; Garello, F.; Cavallari, E.; Magnacca, G.; Trukhan, M.V.; Valsania, M.C.; Cravotto, G.; Terreno, E.; Martina, K. Amphoteric β-Cyclodextrin Coated Iron Oxide Magnetic Nanoparticles: New Insights into Synthesis and Application in MRI. Nanoscale Adv. 2025, 7, 155–168. [Google Scholar] [CrossRef]
- Lu, K.; Zhang, R.; Wang, H.; Li, C.; Yang, Z.; Xu, K.; Cao, X.; Wang, N.; Cai, W.; Zeng, J.; et al. PEGylated Ultrasmall Iron Oxide Nanoparticles as MRI Contrast Agents for Vascular Imaging and Real-Time Monitoring. ACS Nano 2025, 19, 3519–3530. [Google Scholar] [CrossRef]
- Sreenan, B.; Kafil, V.; Hunt, T.; Shin, S.H.R.; Brennan, A.A.; Thallapally, P.K.; Tal-Gan, Y.; Zhu, X. Luminescent ZnO-Carbon Hybrid Nanomaterials: Synthesis, Characterization, Emission Mechanism, and Applications. ACS Appl. Opt. Mater. 2025, 3, 698–711. [Google Scholar] [CrossRef]
- Babayevska, N.; Kustrzyńska, K.; Przysiecka, Ł.; Jarek, M.; Jancelewicz, M.; Iatsunskyi, I.; Dydak, K.; Skupin-Mrugalska, P.; Janiszewska, E. Doxorubicin and ZnO-Loaded Gadolinium Oxide Hollow Spheres for Targeted Cancer Therapy and Bioimaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 333, 125903. [Google Scholar] [CrossRef]
- Mehto, A.; Shukla, P. Bioimaging Potential: Comparative Study of ZnO Nanoparticles Synthesized via Green and Chemical Routes. Next Nanotechnol. 2025, 7, 100118. [Google Scholar] [CrossRef]
- Verma, A.K.; Lakshmi, G.B.V.S.; Dhiman, T.K.; Hashmi, S.Z.H.; Kumar, A.; Solanki, P.R. Optical Tuning of Polymer Functionalized Zinc Oxide Quantum Dots as a Selective Probe for the Detection of Antibiotics. Sci. Rep. 2025, 15, 1648. [Google Scholar] [CrossRef]
- Gupta, J.; Hassan, P.A.; Barick, K.C. Core-Shell Fe3O4@ZnO Nanoparticles for Magnetic Hyperthermia and Bio-Imaging Applications. AIP Adv. 2021, 11, 25207. [Google Scholar] [CrossRef]
- Lai, L.; Jiang, X.; Han, S.; Zhao, C.; Du, T.; Rehman, F.U.; Zheng, Y.; Li, X.; Liu, X.; Jiang, H.; et al. In Vivo Biosynthesized Zinc and Iron Oxide Nanoclusters for High Spatiotemporal Dual-Modality Bioimaging of Alzheimer’s Disease. Langmuir 2017, 33, 9018–9024. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef]
- Burmistrov, D.; Serov, D.; Grigorieva, D.; Simakin, A. Physicochemical, Antibacterial, and Cytotoxic Properties of Composite Materials Based on Biodegradable Poly (Lactic-Co-Glycolic Acid) Functionalized with ZnO Nanoparticles. BIO Web Conf. 2023, 57, 02005. [Google Scholar] [CrossRef]
- Ostovar, S.; Pourmadadi, M.; Zaker, M.A. Co-Biopolymer of Chitosan/Carboxymethyl Cellulose Hydrogel Improved by Zinc Oxide and Graphene Quantum Dots Nanoparticles as PH-Sensitive Nanocomposite for Quercetin Delivery to Brain Cancer Treatment. Int. J. Biol. Macromol. 2023, 253, 127091. [Google Scholar] [CrossRef]
- Li, S.; Yin, J.; Xu, L. Batch Fabrication and Characterization of ZnO/PLGA/PCL Nanofiber Membranes for Antibacterial Materials. Fibers Polym. 2022, 23, 1225–1234. [Google Scholar] [CrossRef]
- Stanković, A.; Sezen, M.; Milenković, M.; Kaišarević, S.; Andrić, N.; Stevanović, M. PLGA/Nano-ZnO Composite Particles for Use in Biomedical Applications: Preparation, Characterization, and Antimicrobial Activity. J. Nanomater. 2016, 2016, 9425289. [Google Scholar] [CrossRef]
- Mozaffari, A.; Mirzapour, S.M.; Rad, M.S.; Ranjbaran, M. Cytotoxicity of PLGA-Zinc Oxide Nanocomposite on Human Gingival Fibroblasts. J. Adv. Periodontol. Implant. Dent. 2023, 15, 27–33. [Google Scholar] [CrossRef]
- Ayyanaar, S.; Kesavan, M.P. Magnetic Iron Oxide Nanoparticles@lecithin/Poly (l-Lactic Acid) Microspheres for Targeted Drug Release in Cancer Therapy. Int. J. Biol. Macromol. 2023, 253, 127480. [Google Scholar] [CrossRef] [PubMed]
- Senturk, F.; Cakmak, S. Fabrication of Curcumin-Loaded Magnetic PEGylated-PLGA Nanocarriers Tagged with GRGDS Peptide for Improving Anticancer Activity. MethodsX 2023, 10, 102229. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.D.; de Souza Junior, F.G.; Pinto, J.C.; Filho, S.T.; Pal, K.; dos Santos Pyrrho, A.; da Costa, R.C.; da Cunha, B.P.; da Silveira Maranhão, F.; de Almeida, T.M. Evaluation of Hyperthermic Potential and Acute Toxicity of PLGA-PEG/Magnetite Microspheres Loaded with Oxaliplatin Using Mice as a Test System. Macromol. React. Eng. 2023, 17, 2300005. [Google Scholar] [CrossRef]
- Gupta, A.; Niveria, K.; Chandpa, H.H.; Singh, M.; Kumar, V.; Meena, J. Stimuli Responsive Magnetic-Silica-Poly Lactic-Co-Glycolic Acid Hybrid Nanoparticles for Targeted Cancer Chemo-Immunotherapy. Drug Deliv. Transl. Res. 2024, 14, 2712–2726. [Google Scholar] [CrossRef]
- Luque-Michel, E.; Lemaire, L.; Blanco-Prieto, M.J. SPION and Doxorubicin-Loaded Polymeric Nanocarriers for Glioblastoma Theranostics. Drug Deliv. Transl. Res. 2021, 11, 515–523. [Google Scholar] [CrossRef]
- Luque-Michel, E.; Sebastian, V.; Larrea, A.; Marquina, C.; Blanco-Prieto, M.J. Co-Encapsulation of Superparamagnetic Nanoparticles and Doxorubicin in PLGA Nanocarriers: Development, Characterization and in Vitro Antitumor Efficacy in Glioma Cells. Eur. J. Pharm. Biopharm. 2019, 145, 65–75. [Google Scholar] [CrossRef]
- Spanhel, L.; Anderson, M.A. Semiconductor Clusters in the Sol-Gel Process: Quantized Aggregation, Gelation, and Crystal Growth in Concentrated ZnO Colloids. J. Am. Chem. Soc. 1991, 113, 2826–2833. [Google Scholar] [CrossRef]
- Babes, L.; Denizot, B.; Tanguy, G.; Le Jeune, J.J.; Jallet, P. Synthesis of Iron Oxide Nanoparticles Used as MRI Contrast Agents: A Parametric Study. J. Colloid. Interface Sci. 1999, 212, 474–482. [Google Scholar] [CrossRef]
- Chiu, H.I.; Samad, N.A.; Fang, L.; Lim, V. Cytotoxicity of Targeted PLGA Nanoparticles: A Systematic Review. RSC Adv. 2021, 11, 9433–9449. [Google Scholar] [CrossRef]
- Baalousha, M. Aggregation and Disaggregation of Iron Oxide Nanoparticles: Influence of Particle Concentration, PH and Natural Organic Matter. Sci. Total Environ. 2009, 407, 2093–2101. [Google Scholar] [CrossRef]
- Mohd Omar, F.; Abdul Aziz, H.; Stoll, S. Aggregation and Disaggregation of ZnO Nanoparticles: Influence of PH and Adsorption of Suwannee River Humic Acid. Sci. Total Environ. 2014, 468–469, 195–201. [Google Scholar] [CrossRef]
- Lallo da Silva, B.; Caetano, B.L.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Increased Antibacterial Activity of ZnO Nanoparticles: Influence of Size and Surface Modification. Colloids Surf. B Biointerfaces 2019, 177, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Lallo da Silva, B.; Garcia, M.M.; Oshiro-Junior, J.A.; Sato, M.R.; Caetano, B.L.; Chiavacci, L.A. Modified Zinc Oxide Nanoparticles against Multiresistant Enterobacteriaceae: Stability, Growth Studies, and Antibacterial Activity. J. Sol-Gel Sci. Technol. 2022, 101, 244–255. [Google Scholar] [CrossRef]
- Caetano, B.L.; Silva, M.N.; Santilli, C.V.; Briois, V.; Pulcinelli, S.H. Unified ZnO Q-Dot Growth Mechanism from Simultaneous UV–Vis and EXAFS Monitoring of Sol-Gel Reactions Induced by Different Alkali Base. Opt. Mater. 2016, 61, 92–97. [Google Scholar] [CrossRef]
- Ashley, J.; Manikova, P. Fluorescent Sensors. In Fundamentals of Sensor Technology: Principles and Novel Designs; Woodhead Publishing: Cambridge, UK, 2023; pp. 147–161. [Google Scholar] [CrossRef]
- Liang, L.; Chen, J.; Liu, X. Lanthanide-Doped Upconversion Nanomaterials. In Comprehensive Inorganic Chemistry III, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1–10, pp. 439–485. [Google Scholar] [CrossRef]
- Ragheb, R.R.T.; Kim, D.; Bandyopadhyay, A.; Chahboune, H.; Bulutoglu, B.; Ezaldein, H.; Criscione, J.M.; Fahmy, T.M. Induced Clustered Nanoconfinement of Superparamagnetic Iron Oxide in Biodegradable Nanoparticles Enhances Transverse Relaxivity for Targeted Theranostics. Magn. Reson. Med. 2013, 70, 1748–1760. [Google Scholar] [CrossRef]
- Geraldes, C.F.G.C. Rational Design of Magnetic Nanoparticles as T1–T2 Dual-Mode MRI Contrast Agents. Molecules 2024, 29, 1352. [Google Scholar] [CrossRef]
- Fleutot, S.; Nealon, G.L.; Pauly, M.; Pichon, B.P.; Leuvrey, C.; Drillon, M.; Gallani, J.-L.; Guillon, D.; Donnio, B.; Begin-Colin, S. Spacing-Dependent Dipolar Interactions in Dendronized Magnetic Iron Oxide Nanoparticle 2D Arrays and Powders. Nanoscale 2013, 5, 1507. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Rupasinghe, T.; Wu, C.M.; Grassian, V.H. Dissolution of ZnO Nanoparticles at Circumneutral PH: A Study of Size Effects in the Presence and Absence of Citric Acid. Langmuir 2012, 28, 396–403. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Yan, H.; Du, D.; Lin, Y. PH-Responsive ZnO Nanocluster for Lung Cancer Chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 5739–5747. [Google Scholar] [CrossRef]
- Manaia, E.B.; Kaminski, R.C.K.; Caetano, B.L.; Briois, V.; Chiavacci, L.A.; Bourgaux, C. Surface Modified Mg-Doped ZnO QDs for Biological Imaging. Eur. J. Nanomed. 2015, 7, 109–120. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Visible Light Photocatalytic Activity of Fe3+-Doped ZnO Nanoparticle Prepared via Sol-Gel Technique. Chemosphere 2013, 91, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Sekar, A.D.; Muthukumar, H.; Chandrasekaran, N.I.; Matheswaran, M. Photocatalytic Degradation of Naphthalene Using Calcined Fe–ZnO/ PVA Nanofibers. Chemosphere 2018, 205, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Krobthong, S.; Wongrerkdee, S.; Pimpang, P.; Moungsrijun, S.; Sujinnapram, S.; Nilphai, S.; Rungsawang, T.; Wongrerkdee, S. ZnO Nanoparticles Coprecipitation with Aluminum and Copper Ions for Efficient Photocatalytic Degradation of Commercial Glyphosate. Integr. Ferroelectr. 2022, 222, 69–83. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).